Afef Dhaffouli*, Emna Ali, Sondes Guesmi, Pedro A Salazar-Carballo and Houcine Barhoumi
Volume5-Issue11
Dates: Received: 2024-09-10 | Accepted: 2024-11-19 | Published: 2024-11-21
Pages: 1503-1510
Abstract
The high adsorption capacities of Polyaniline (PANI) and Carbon Nanotubes (CNTs) for heavy metals, combined with the synergistic effect of Magnesium Oxide (MgO), were leveraged to develop a modified Glassy Carbon Electrode (GCE), referred to as MgO@PANI@CNT/GCE, for the accurate detection of Cd²+ and Pb²⺠ions. A comprehensive investigation of the electrochemical response of the MgO@PANI@CNT/GCE was carried out using Differential Pulse Voltammetry (DPV). A systematic optimisation of the electrochemical parameters, including the pH of the buffer solution, the amount of MgO@PANI@CNT on the electrode surface, and the drying time, was carried out. Under optimal conditions, the MgO@PANI@CNT/GCE sensor showed a linear response in the concentration range from 10-8 to 2.5 × 10-7 M. The lowest detectable concentrations for Cd2+ and Pb2+ were measured as 5 × 10-8 M and 2.5 × 10-8 M, respectively. The sensor exhibited excellent stability and reproducibility, and its practical utility was confirmed through the successful detection of Cd²⺠and Pb²⺠in river water samples, highlighting its effectiveness for real-world applications.
Graphical abstract
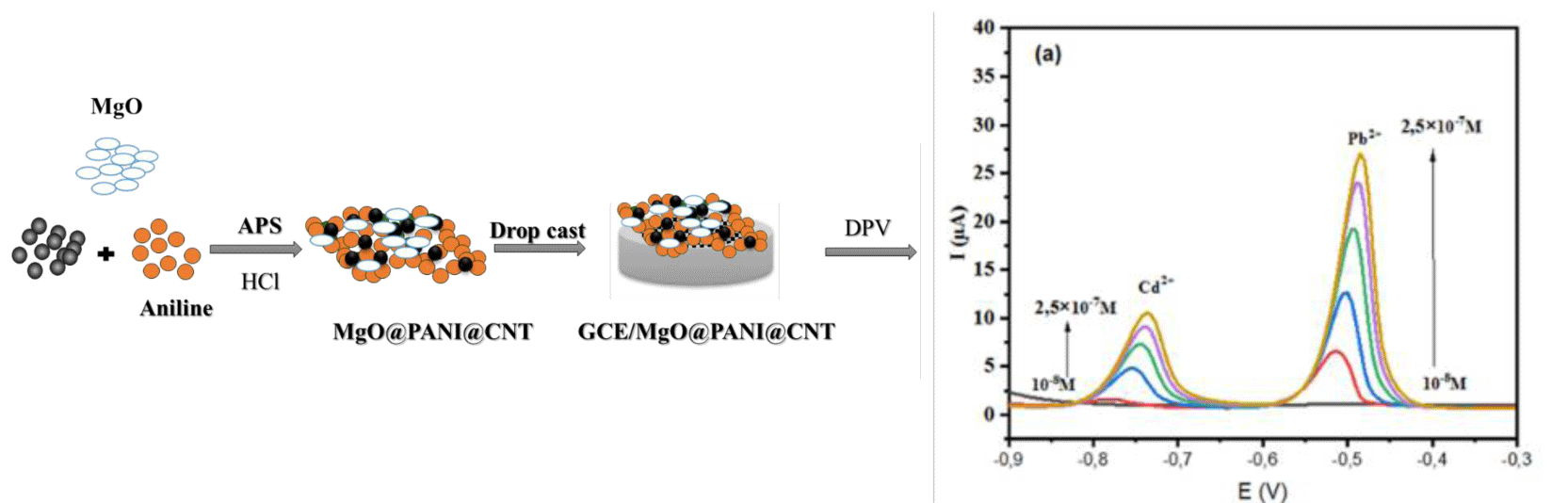
FullText HTML
FullText PDF
DOI: 10.37871/jbres2038
Certificate of Publication

Copyright
© 2024 Dhaffouli A, et al. Distributed under Creative Commons CC-BY 4.0
How to cite this article
Dhaffouli A, Ali E, Guesmi S, Salazar-Carballo PA,Barhoumi H. Electrochemical Sensor Based on Mgo@PANI@ CNT Nanocomposites for Simultaneous Detection of Cd2+ and Pb2+. J Biomed Res Environ Sci. 2024 Nov 21; 5(11): 1503-1510. doi: 10.37871/jbres2038, Article ID: JBRES2038, Available at: https://www.jelsciences.com/articles/jbres2038.pdf
Subject area(s)
References
- Cerutti S, Silva MF, Gásquez JA, Olsina RA, Martinez LD. On- linepreconcentration/determination of cadmium in drinking water on activated carbon using 8-hydroxyquinoline in a flow injection system coupled to an induc- tively coupled plasma optical emission spectrometer. Spectrochim Acta - Part B at Spectrosc. 2003;58(1):43-50. doi: 10.1016/S0584-8547(02)00215-X.
- Noh Y, Jo EJ, Mun H, Ahn YD, Kim MG. Homogeneous and selective detection of cadmium ions by forming fluorescent cadmium-protein nanoclusters. Chemosphere. 2017 May;174:524-530. doi: 10.1016/j.chemosphere.2017.02.025. Epub 2017 Feb 6. PMID: 28189897.
- Mañay N, Cousillas AZ, Alvarez C, Heller T. Lead contamination in Uruguay: the "La Teja" neighborhood case. Rev Environ Contam Toxicol. 2008;195:93-115. PMID: 18418955.
- Jarvis P, Quy K, Macadam J, Edwards M, Smith M. Intake of lead (Pb) from tap water of homes with leaded and low lead plumbing systems. Sci Total Environ. 2018 Dec 10;644:1346-1356. doi: 10.1016/j.scitotenv.2018.07.064. Epub 2018 Jul 13. PMID: 30743847.
- Gao Y, Ren X, Wu J, Hayat T, Alsaedi A, Cheng C, Chen C. Graphene oxide interactions with co-existing heavy metal cations: Adsorption, colloidal properties and joint toxicity. Environ Sci Nano. 2018;5(2):362-371.
- Vázquez-González M, Carrillo-Carrion C. Analytical strategies based on quantum dots for heavy metal ions detection. J Biomed Opt. 2014;19(10):101503. doi: 10.1117/1.JBO.19.10.101503. PMID: 24853041.
- Idris MB, Zakariyya UA. The role of some antioxidants on absorption, distribution and elimination of lead and iron: An in -vivo study. Ejbps. 2016;3:528-531 .
- Erarpat S, Özzeybek G, Chormey DS, Bakirdere S. Determination of lead at trace levels in mussel and sea water samples using vortex assisted dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Chemo- sphere. 2017;189:180-185 doi: 10.1016/j.chemosphere.2017.09.072.
- Lu YK, Sun HW, Yuan CG, Yan XP. Simultaneous determination of trace cadmium and arsenic in biological samples by hydride generation-double channel atomic fluorescence spectrometry. Anal Chem. 2002 Apr 1;74(7):1525-9. doi: 10.1021/ac0156971. PMID: 12033240.
- Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R. A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta. 2010 Feb 5;659(1-2):172-7. doi: 10.1016/j.aca.2009.11.053. Epub 2009 Nov 27. PMID: 20103121.
- Q. Zhou, Lei M, Liu Y, Wu Y, Yuan Y. Simultaneous determination of cad- mium, lead and mercury ions at trace level by magnetic solid phase extraction with Fe@Ag@Dimercaptobenzene coupled to high performance liquid chromatography. Talanta. 2017;175:194-199. doi: 10.1016/j.talanta.2017.07.043.
- Sitko R, Janik P, Zawisza B, Talik E, Margui E, Queralt I. Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal Chem. 2015 Mar 17;87(6):3535-42. doi: 10.1021/acs.analchem.5b00283. Epub 2015 Mar 2. PMID: 25707847.
- Ullah N, Mansha M, Khan I, Qurashi A. Nanomaterial-based optical chemical sen- sors for the detection of heavy metals in water: Recent advances and challenges. TrAC - Trends Anal Chem. 2018;100:155-166. doi: 10.1016/j.trac.2018.01.002.
- Pujol L, Evrard D, Groenen-Serrano K, Freyssinier M, Ruffien-Cizsak A, Gros P. Electrochemical sensors and devices for heavy metals assay in water: the French groups' contribution. Front Chem. 2014 Apr 30;2:19. doi: 10.3389/fchem.2014.00019. PMID: 24818124; PMCID: PMC4012207.
- Aragay G, Pons J, Merkoçi A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev. 2011 May 11;111(5):3433-58. doi: 10.1021/cr100383r. Epub 2011 Mar 11. PMID: 21395328.
- Zhang B, Chen J, Zhu H, Yang T, Zou M, Zhang M, Du M. Facile and green fabrication of size-controlled AuNPs/CNFs hybrids for the highly sensitive simul- taneous detection of heavy metal ions. Electrochim Acta. 2016;196:422-430. doi: 10.1016/j.electacta.2016.02.163.
- Hua K, Li X, Fang D, Yi J, Bao R, Luo Z. Electrodeposition of high-density lithium vanadate nanowires for lithium-ion battery. Appl Surf Sci. 2018;447:610-616. doi: 10.1016/j.apsusc.2018.04.043.
- Wang Y, Hu S. Applications of carbon nanotubes and graphene for electrochem- ical sensing of environmental pollutants. J Nanosci Nanotechnol. 2016;16:7852-7872. doi: 10.1166/jnn.2016.12762.
- Jasmin JP, Miserque F, Dumas E, Vickridge I, Ganem JJ, Cannizzo C, Chaussé A. XPS and NRA investigations during the fabrication of gold nanostructured functionalized screen-printed sensors for the detection of metallic pollutants. Appl Surf Sci. 2017;397:159-166. doi: 10.1016/j.apsusc.2016.11.125.
- Abd El-Haleem HS, Hefnawy A, Hassan RYA, Badawi AH, El- Sherbiny IM. Manganese dioxide-core-shell hyperbranched chitosan (MnO2-HBCs) nano-structured screen printed electrode for enzymatic glucose biosensors. RSC Adv. 2016;6(110):109185-109191. doi: 10.1039/C6RA24419J.
- Shen C, Chen C, Wen T, Zhao Z, Wang X, Xu A. Superior adsorption capacity of g-C₃N₄ for heavy metal ions from aqueous solutions. J Colloid Interface Sci. 2015 Oct 15;456:7-14. doi: 10.1016/j.jcis.2015.06.004. Epub 2015 Jun 10. PMID: 26079526.
- Pauliukaite R, Metelka R, Svancara I, Królicka A, Bobrowski A, Vytras K, Norkus E, Kalcher K. Carbon paste electrodes modified with Bi(2)O(3) as sensors for the determination of Cd and Pb. Anal Bioanal Chem. 2002 Nov;374(6):1155-8. doi: 10.1007/s00216-002-1569-3. Epub 2002 Oct 24. PMID: 12458435.
- Zheng H, Ntuli L, Mbanjwa M, Palaniyandy N, Smith S, Modibedi M, Land K, Mathe M The effect of g-C3N4 materials on Pb(II) and Cd(II) detection using disposable screen-printed sensors, Electrocatalysis. 2019;10:149-155. doi: 10.1007/s12678-018-0504-0.
- Guo S, Wu K, Gao Y, Liu L, Zhu X, Li X, Zhang F. Efficient removal of Zn(II), Pb(II), and Cd(II) in waste water based on magnetic graphitic carbon nitride materi- als with enhanced adsorption capacity. J Chem Eng Data. 2018;63:3902-3912. doi: 10.1021/acs.jced.8b00526.
- Zhu Y, Xue J, Xu T, He G, Chen H. Enhanced photocatalytic activity of mag- netic core–shell Fe3 O 4 @Bi 2 O 3 –RGO heterojunctions for quinolone antibiotics degra- dation under visible light. J Mater Sci Mater Electron. 2017;28(12):8519-8528. doi: 10.1007/s10854-017-6574-6.
- Yao XZ, Guo Z, Yuan QH, Liu ZG, Liu JH, Huang XJ. Exploiting differential electrochemical stripping behaviors of Fe3O4 nanocrystals toward heavy metal ions by crystal cutting. ACS Appl Mater Interfaces. 2014 Aug 13;6(15):12203-13. doi: 10.1021/am501617a. Epub 2014 Jul 18. PMID: 25014119.
- Zinoubi K, Majdoub H, Barhoumi H, Boufi S, Jaffrezic-Renault N. Determination of trace heavy metal ions by anodic stripping voltammetry using nanofibrillated cellulose modified electrode. Journal of Electroanalytical Chemistry. 2017;799:70-77. doi: 10.1016/j.jelechem.2017.05.039.
- Naseh MV, Khodadadi AA, Mortazavi Y, Sahraei OA, Pourfayaz F, Sedghi SM. Functionalization of carbon nanotubes using nitric acid oxidation and DBD plasma. World Acad Sci Eng Technol. 2009;49:177-179.
- Philips MF, Gopalan AI, Lee KP. Development of a novel cyano group containing electrochemically deposited polymer film for ultrasensitive simultaneous detection of trace level cadmium and lead. J Hazard Mater. 2012 Oct 30;237-238:46-54. doi: 10.1016/j.jhazmat.2012.07.069. Epub 2012 Aug 24. PMID: 22964385.
- Wang ZM, Guo HW, Liu E, Yang GC, Khun NW. Bismuth/polyaniline/glassy carbon electrodes prepared with different protocols for stripping voltammetric determination of trace Cd and Pb in solutions having surfactants. Electroanalysis. 2010;22(2):209-215. doi: 10.1002/elan.200900251.