Medicine Group . 2022 April 16;3(4):335-343. doi: 10.37871/jbres1445.
Analysis of Vitamin D and its Correlation with Interleukin-6 in COVID-19 Patients at Mary Begg Health Services, Zambia: Implication for Patient Management
Misheck Chileshe1*, Mwamba Mulamba1, Vernon Julius2, Mwenya Kwangu3 and Stephen Mwisiya Mubita2,4
2Mary Begg Health Services, 56 Chintu Avenue, Northrise, Ndola, Zambia
3Copperbelt University, Micheal Chilufya Sata School of Medicine, Department of Medical Basic Sciences, Ndola, Zambia
4Micropath Diagnostics & Mobile Services Limited, Corner of Luangwa and Kopa Road, 52 Kopa Road, Northrise, Ndola, Zambia
- COVID-19
- Vitamin D
- Interleukin-6
- Intensive care
- Correlation
- Zambia
Abstract
Introduction: The emergence of SARS COV-2 and Coronavirus Disease 2019 (COVID-19) came without any known medication or treatment, thereby raising concerns for drug (therapeutics) research and development. Vitamin D is a potent immunomodulator with a proven protective effect against respiratory viral infections, and because of this, many studies have been carried out to evaluate the effects of vitamin D on COVID-19 infection, however, with varying conclusions.
Objective: To assess serum vitamin D levels and their correlation with IL-6 and other clinical characteristics among COVID-19 patients attended to at Mary Begg Health Services (MBHS).
Methods: This cross-sectional study was conducted among COVID-19 patients at MBHS. The study included 33 confirmed severe patients admitted to the intensive care unit, 45 patients with mild symptoms, and 45 healthy controls. The Kruskal–Wallis test was used to compare the median serum vitamin D levels among the three groups, and Spearman’s correlations were performed to assess the correlation between serum vitamin D, IL-6, and clinical characteristics of the patients.
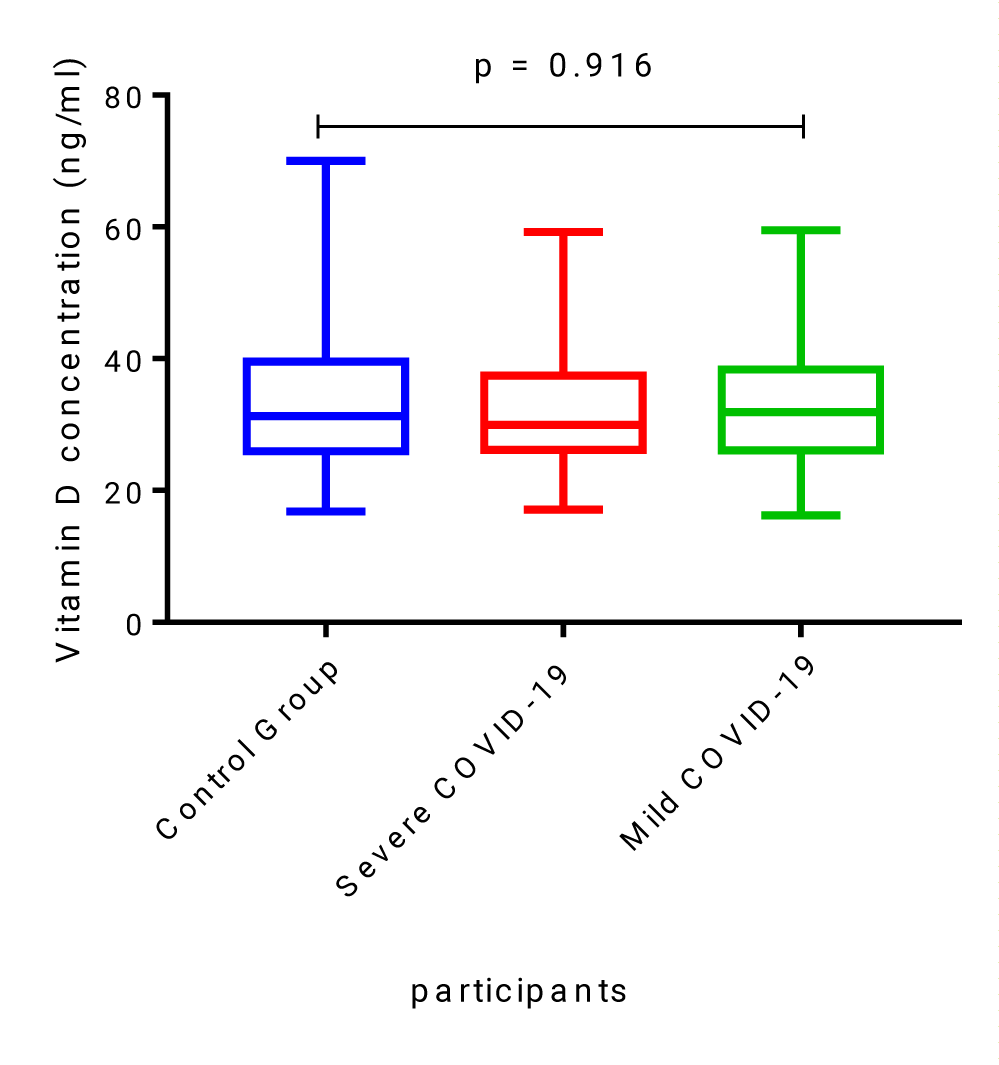
Results: The majority of COVID-19 patients in this study had optimal levels of vitamin D 44/78 (56.4%), with vitamin D deficiency being observed in only 6/78 (7.7%). Vitamin D levels in the control group were not significantly different when compared to levels measured in severe and mild COVID-19 patients, median [IQR], 31.33 ng/ml [25.9-39.56] compared to 29.97 ng/ml [26.19-37.45] and 31.9 ng/ml [26.12-38.34], p = 0.916, respectively. Severe COVID-19 patients admitted to the intensive care unit were older and had median higher IL-6 levels (43.67 ± 11.86 years vs. 33.89 ± 13.38 years; p = 0.001 and 27.56 pg/ml [13.13-47.81] vs. 8.34 pg/ml [5.1-21.63]; p = 0.0003, respectively) than patients with mild disease. A significant negative correlation between vitamin D and IL-6 (r = - 0.42; p = 0.016) was found in severe COVID-19 patients.
Conclusion: A negative (inverse) correlation between serum vitamin D and IL-6 was found in this study. Therefore, patients with severe COVID-19 might benefit from vitamin D supplementation, which would help to downregulate the cytokine storm and hence reduced disease severity.
Introduction
Since December 2019, the numbers of patients with fever, dry cough, normal, or decreased white blood cell counts who were initially diagnosed as having “Fever of Unknown Origin with pneumonia” had continuously increased in Wuhan [1,2]. The causative agent was rapidly identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), an infectious agent with not only strong human‐to‐human transmission but also causes severe pneumonia and death [3,4]. What started as a pandemic in one city turned out to be a global pandemic that has impacted all our lives [5]. This emergence of SARS COV-2 and Coronavirus Disease 2019 (COVID-19) came without any known medication, treatment and/or management, thereby raising concerns for drug (therapeutics) research and development. While this is the case, the role of vitamin D, a known immunomodulator with the potential to suppress proinflammatory reactions in COVID-19 [6-8], has been less investigated in the Zambian context.
It is widely accepted that SARS-CoV-2 human transmissibility and pathogenesis depend on the interaction of the virus and Angiotensin-Converting Enzyme-2 Receptor (ACE-2) through viral attachment using the transmembrane spike glycoprotein (S-protein) receptor-binding domain [9,10]. Once inside the host cell, SARS-CoV-2 is a potent inducer of inflammatory cytokines. The “cytokine storm” or “cytokine cascade” is the postulated mechanism for organ damage. The virus activates immune cells and induces the secretion of inflammatory cytokines and chemokines into pulmonary vascular endothelial cells that are responsible for the observed clinical manifestations [11-13].
One of the important molecules involved in this “cytokine storm” is interleukin-6 (IL-6), a proinflammatory cytokine [8,14]. Interestingly, vitamin D, a group of fat-soluble vitamins, has been shown to have an inhibitory effect on IL-6 and other proinflammatory cytokines, such as IL-10 and tumor necrosis factor-α (TNF-α) [15-17]. This is achieved by vitamin D blunting the T-helper cell type 1 (Th1) immune response and favoring T-helper cell type 2 (Th2) and regulatory T-cell responses [18]. This leads to a decrease in proinflammatory cytokines and an increase in anti-inflammatory cytokines. By downregulating proinflammatory cytokines and upregulating anti-inflammatory cytokines, vitamin D may be capable of preventing severe complications related to COVID-19 and other viral illnesses [18,19].
Vitamin D is a potent immunomodulator that has a protective effect against respiratory viral infections, and many studies have been carried out to evaluate the effects of vitamin D on COVID-19 infection. Some studies have shown that people with vitamin D deficiency have had severe disease and/or poorer outcomes [3,15,16,20-22]. Furthermore, several studies have demonstrated an association between vitamin D and IL-6 [15,20,23,24], but other studies have not established such associations [14,25,26]. This shows that there are inconsistencies in the literature regarding the association between vitamin D, IL-6, and disease severity in COVID-19 patients. However, most, if not all, of these studies have been conducted in Western countries and rarely in African countries. To the best of our knowledge, there were no published studies about the concentration of vitamin D in COVID-19 patients and its correlation to IL-6 and other clinical factors in Zambia, an African country, by the time of this study. Therefore, the current study aimed at assessing serum vitamin D levels and their correlation with IL-6 and other clinical characteristics among COVID-19 patients attended to at Mary Begg Health Services (MBHS). IL-6 was studied as a cytokine of interest because it has been documented to be a robust marker for predicting disease prognosis, deterioration of clinical profile and is significantly associated with mortality in comparison to other proinflammatory cytokines [27,28].
Materials and Methods
Study design, sample size and sampling
This was a retrospective cross-sectional study conducted at Mary Begg health services, Kansanshi Mine Hospital, Solwezi, in the northwestern part of Zambia between May 4th and December 21, 2021. The sample size and sampling of participants were done conveniently based on the number of patients that had mild and severe COVID-19 during the study period and had complete laboratory, clinical and demographic information.
Definition of mild and severe COVID-19
In this study, mild and severe COVID-19 diseases were defined based on the World Health Organisation (WHO) clinical spectrum of SARS-CoV-2 infection [29,30] as;
- Mild COVID-19 patients: those individuals who presented with any of the various signs and symptoms of COVID-19 such as fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell but who did not have shortness of breath, dyspnea, or abnormal chest imaging. These were not admitted to the hospital.
- Severe COVID-19 patients: these were individuals with confirmed COVID-19 and had oxygen saturation (SpO2) < 90% on room air, signs of pneumonia and signs of severe respiratory distress (in adults, accessory muscle use, inability to complete full sentences, respiratory rate > 30 breaths/minute). These were admitted to the intensive care unit (ICU) and received oxygen therapy.
Participants (patients) and blood sampling
Vitamin D, IL-6 concentration, lymphocyte, and monocyte counts were determined in blood samples of 78 confirmed COVID-19. Of these patients, 33 were admitted to the intensive care unit on oxygen. SARS-CoV-2 infection was confirmed using Real-Time Polymerase Chain Reaction (RT-PCR). Both male and female patients over the age of 18 were included in the study. Lymphocyte and monocyte counts were obtained retrospectively from the Complete Blood Count (CBC) results stored electronically, which were performed as part of routine tests on patient presentation to the hospital during their COVID-19 clinical scrutiny. During the same period, serum samples were collected and archived as frozen samples at -20°C pending measurement for serum vitamin D and IL-6 concentrations.
A control group composed of 45 healthy participants matched for age and sex with the severe COVID-19 group. All patients had normal lymphocyte counts, monocyte counts, kidney function tests, liver function tests and CRP levels of less than 10 mg/L. The control group had no history of COVID-19 infection in the previous 1 year, as confirmed by the electronic records and upon RT-PCR. All the participants were of the same race, lived in the same geographic location, were non-obese and had no chronic illness. Further, all the names of the participants in this group were checked against the registers for those who received vitamin D and C supplements and those who were found on the list were not included. Additionally, for the control group, the specimens were collected during the day to avoid reduced concentrations observed during the nighttime [31].
Confirmation of SARS-CoV-2 infection
All patients were diagnosed and confirmed to be positive for COVID-19 (SARS-CoV-2 infection) by Real-Time Polymerase Chain Reaction (RT-PCR). Nasopharyngeal swabs were taken, and RT-PCR analysis was performed. A COVID-19 (SARS-CoV-2) nucleic acid test kit (eDiagnosis®, Wuhan EasyDiagnosis Biomedicine Co., Ltd, China) was used to obtain cDNA and amplify the nucleocapsid protein (N) and open reading frame 1 ab (ORF1ab) genes with the RNaseP gene as an internal control according to the manufacturer’s instructions.
Measurement of vitamin D (25-Hydroxyvitamin D)
Vitamin D levels were measured in archived serum samples using a commercially available Elecsys Vitamin D total II assay on a Cobas e411 immunoanalyzer (Roche Diagnostics, Germany) according to the manufacturer’s instructions. The measuring range was 3-100 ng/ml. Vitamin D deficiency was defined as 25‑hydroxyvitamin D of < 20 ng/mL, vitamin D insufficiency as a concentration between 20‑29.9 ng/mL, and optimal level as 25‑hydroxyvitamin D ≥ 30 ng/mL [32].
Measurement of interleukin (IL)-6
Serum collected using standard sampling tubes was used to measure IL-6 using the commercially available Elecsys IL-6 immunoassay on a Cobas e411 immunoanalyzer (Roche Diagnostics, Germany) according to the manufacturer’s protocol. The measuring range was 1.5-5000 pg/ml with a normal reference range up to 7 pg/mL (95th percentile).
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows (version 21.0) and GraphPad Prism 7 (version 7.0, for Windows). Data distribution was assessed using the Shapiro–Wilk test. Vitamin D, IL-6, lymphocyte count, monocyte count, and duration of admission were not normally distributed. To compare the median vitamin D levels among the control group, severe COVID-19 patients and mild COVID-19 patients, the Kruskal–Wallis test was performed. To compare the median vitamin D, IL-6, lymphocyte counts, and monocyte counts between COVID-19 patients admitted to the ICU and those with mild symptoms, the Mann–Whitney U test was performed, and unpaired Student’s t test was performed for data that were normally distributed. Categorical variables were compared using the chi-square test. Correlations were assessed using Spearman’s correlations. Data are presented as the mean ± standard deviation, median [IQR], or frequency and percentages. All statistical tests were two-tailed at a significance level of p ≤ 0.05.
Ethics considerations
This study was approved by the Tropical Disease Research Centre (TDRC) Ethics Review Committee, IRB registration number 00002911, study protocol TRC/C4/02/2022. Clearance to conduct the study was given by the National Health Research Authority, Zambia with reference number: NHRA000004/14/02/2022. Permission to use the data retrospectively from electronic records was granted by hospital management. Clients’ details were deidentified by allocating study IDs and were not to be presented as raw data during data presentation in any form.
Results
Clinical characteristics of participants
Overall, 78 COVID-19 patients and 45 healthy controls were involved in this study. The mean age of the patients was 38.15 years (SD = 13.37), with the majority being males, 57/78 (73.1%). There was no significant difference in the mean age between the COVID-19 patients and the control group (p = 0.139). Lymphopenia was observed in 38/78 (48.7%) of the patients, with 16/78 (20.5%) having monocytosis. The majority of the patients had optimal levels of vitamin D 44/78 (56.4%), with vitamin D deficiency being observed only in 6/78 (7.7%) patients, and a similar picture was seen in the control group (p = 0.526). Slightly less than half of the patients were admitted to the ICU, 33/77 (42.3%) (Table 1).
| Table 1: Demographics and clinical characteristics of COVID-19 patients at MBHS Kansanshi mine hospital. | |||
| Variable | COVID-19 patients (n = 78) |
Control Group (n = 45) |
p value |
| Age (mean ± SD) | 38.15 ± 13.37 | 34.15 ± 10.7 | 0.139* |
| Sex Male n (%) Female n (%) |
57 (73.1) 21 (26.9) |
24 (53.3) 21 (46.7) |
0.03** |
| Lymphocyte Status Normal n (%) Lymphopenia (< 1000 cells/µl) n (%) |
40 (51.3) 38 (48.7) |
45 (100.0) - |
- |
| Monocyte Count Normal n (%) Monocytopenia (< 200 cells/µl) Monocytosis (> 800 cells/µl) |
59 (75.6) 3 (3.8) 16 (20.5) |
45 (100.0) - - |
- |
| Vitamin D Status Optimal (≥ 30 ng/ml) n (%) Insufficient (20-29.9 ng/ml) n (%) Deficient (< 20 ng/ml) n (%) |
44 (56.4) 28 (35.9) 6 (7.7) |
28 (62.2) 12 (26.7) 5 (11.1) |
0.526** |
| Admission to ICU Yes No |
33 (42.3) 45 (57.7) |
- - |
- |
| SD: Standard Deviation; ICU: Intensive Care Unit. *Student’s t test; **Fisher’s exact test | |||
Vitamin D levels in the healthy, non-COVID-19 control group were not significantly different when compared to levels measured in severe and mild COVID-19 patients, median [IQR], 31.33 ng/ml [25.9-39.56] compared to 29.97 ng/ml [26.19-37.45] and 31.9 ng/ml [26.12-38.34], p = 0.916 (Figure 1).
Distribution of clinical characteristics of COVID-19 patients based on disease severity
A comparison of clinical characteristics between severely symptomatic COVID-19 patients admitted to the Intensive Care Unit (ICU) and those with mild symptoms without admission revealed that those who were admitted to the ICU were statistically significantly older and had significantly higher median IL-6 levels (3-fold higher) (43.67 ± 11.86 years vs. 33.89 ± 13.38 years; p = 0.001 and 27.56 pg/ml [13.13-47.81] vs. 8.34 pg/ml [5.1-21.63]; p = 0.0003, respectively). The admitted COVID-19 patients had a slightly lower median vitamin D level than those not admitted, but this difference was not statistically significant (29.97 ng/ml [17.07-37.45] vs. 31.90 [16.20-38.34]; p = 0.678). Most of the patients who were not admitted had lymphopenia, but there was no significant difference in comparison to those admitted (55.6% vs. 39.4%; p = 0.158). Furthermore, there was no significant difference in the median absolute lymphocyte and monocyte counts between the patients admitted to the ICU and those not admitted (Table 2).
| Table 2: Clinical characteristics of severely symptomatic COVID-19 patients and mildly symptomatic patients. | ||||
| Whole cohort (n = 78) |
Severe COVID-19 (n = 33) |
Mild COVID-19 (n = 45) |
p value | |
| Age, Years mean (SD) | 38.15 ± 13.37 | 43.67 (11.86) | 33.89 (13.38) | 0.001a |
| Male n (%) | 57 (73.1%) | 25 (75.8%) | 32 (71.1%) | 0.648b |
| Lymphopenia (<1000 cells/µl) n (%) | 38 (48.7%) | 13 (39.4%) | 25 (55.6%) | 0.158b |
| Monocytosis (> 800 cells/µl) n (%) | 16 (20.5%) | 8 (24.2%) | 8 (17.8%) | 0.759b |
| 25-OHD ng/ml median [IQR] | 31.44 [26.15-37.84] | 29.97 [26.19-37.45] | 31.90 [26.12-38.34] | 0.678c |
| Vitamin D insufficiency (25-OHD 20-29.9 ng/ml) N (%) | 28 (35.9%) | 15 (45.5%) | 13 (28.9%) | 0.318b |
| IL-6 pg/ml median [IQR] | 17.07 [6.27-32.03] | 27.56 [13.13-47.81] | 8.34 [5.1-21.63] | 0.0003c |
| Lymphocyte count, X 103/µl median [IQR] | 1.02 [0.69-1.52] | 1.04 [0.83-1.53] | 0.82 [0.64-1.56] | 0.271c |
| Monocyte count, X 103/µl median [IQR] | 0.53 [0.36-0.79] | 0.56 [0.31-0.84] | 0.52 [0.38-0.76] | 0.660c |
| SD: Standard Deviation; IQR = Interquartile Range; ICU: Intensive Care Unit, 25-OHD: 25-hydroxyvitamin D, IL-6: Interleukin 6. a = unpaired Student’s t test, b = Chi-square, c = Mann–Whitney U test. The p values are comparisons between the two groups, severe COVID-19 and mild COVID-19. |
||||
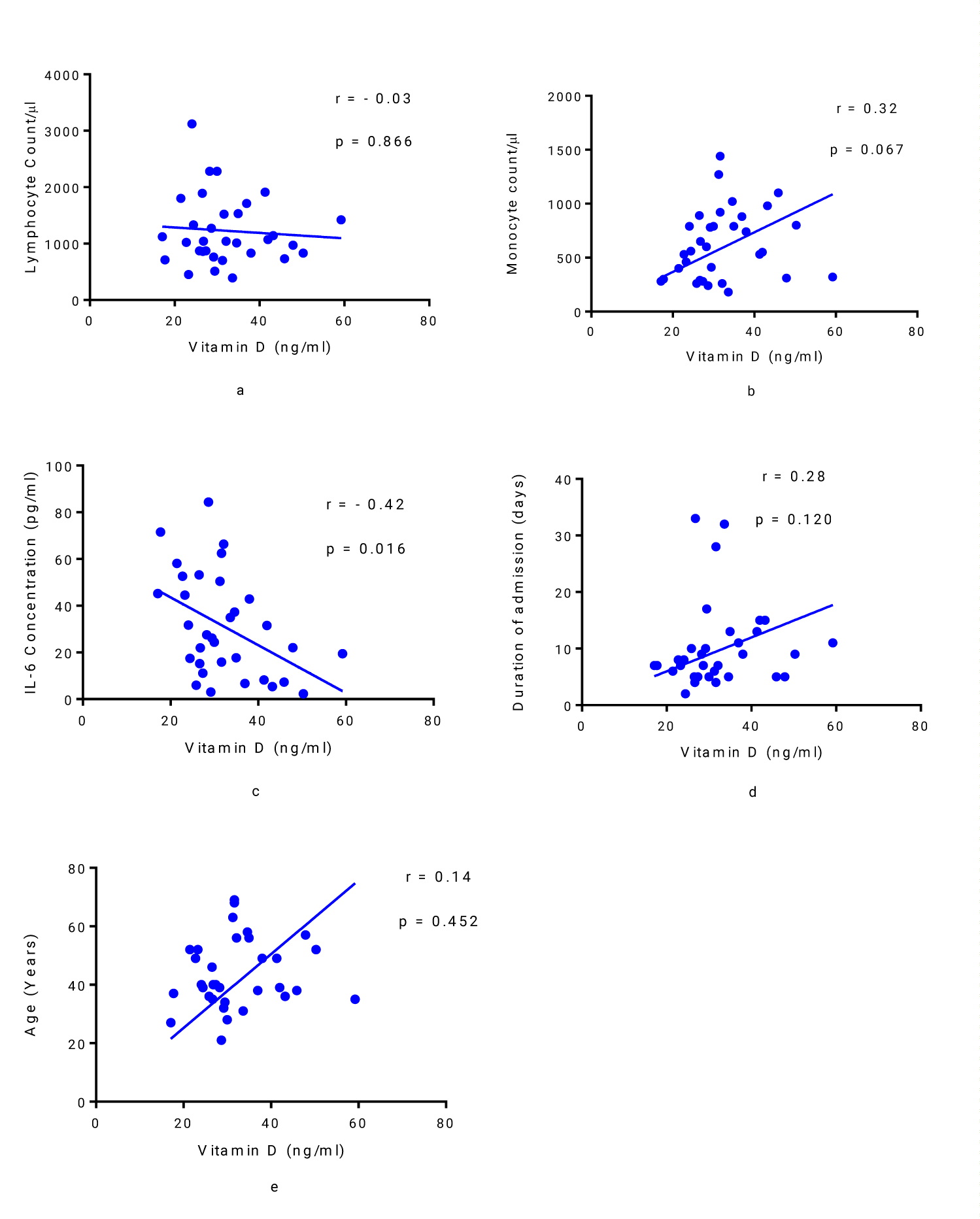
Spearman’s correlation analysis of vitamin D in relation to lymphocyte count, monocyte count, IL-6 concentration, duration of admission and age of patients with severe COVID-19 revealed a negative correlation between vitamin D levels and lymphocyte count, but the relationship was not statistically significant (r = - 0.03, p = 0.886) (Figure 2a). A statistically non-significant positive correlation was observed between vitamin D and monocyte count (r = 0.32, p = 0.067) (Figure 2b) while a statistically significant negative correlation between vitamin D and IL-6 was observed (r = - 0.42, p = 0.016) (Figure 2c). The duration of admission and age did not show any significant correlation with vitamin D (r = 0.28, p = 0.120; and r = 0.14, p = 0.452; respectively) (Figures 2 d,e).
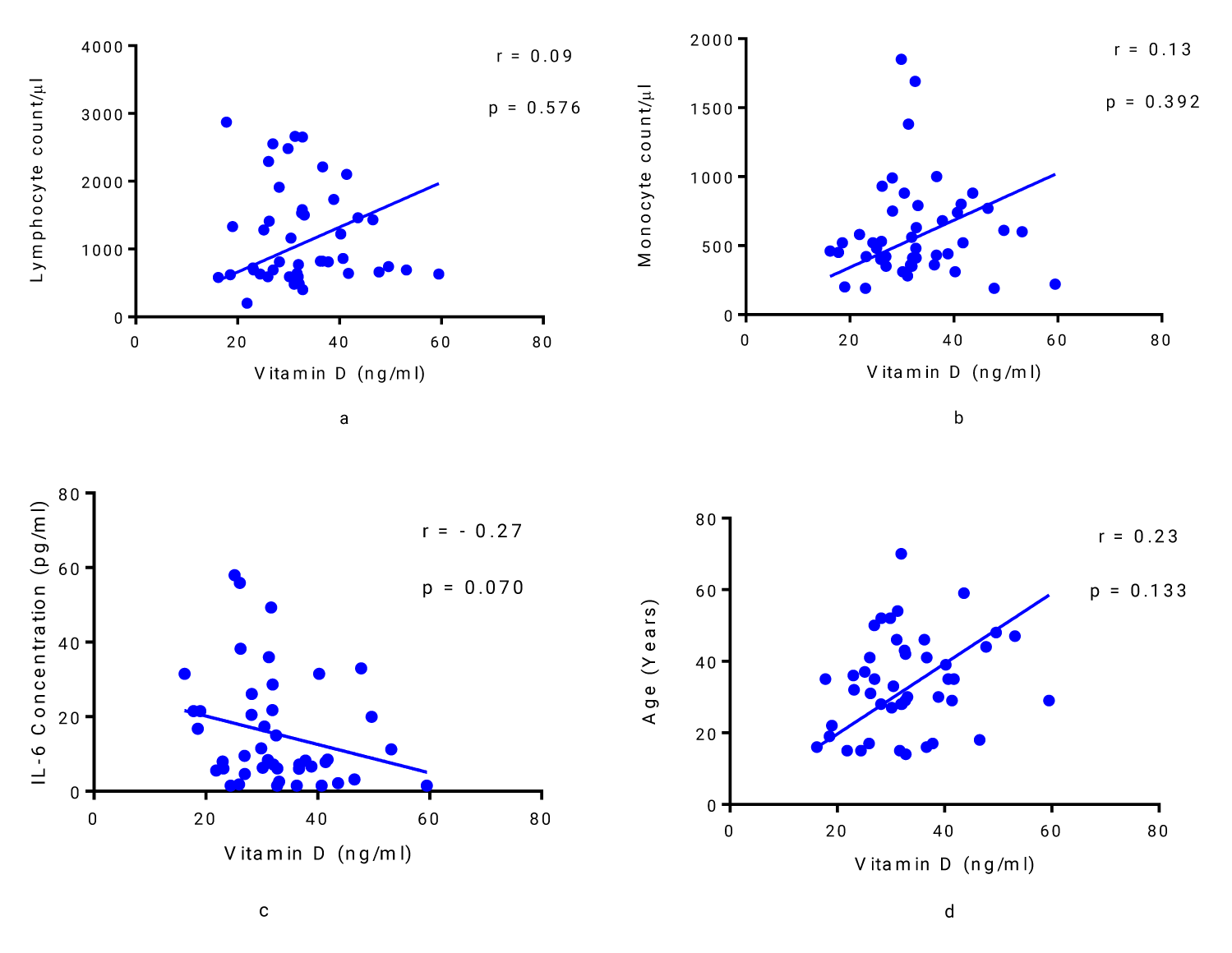
Lymphocyte and monocyte counts of the patients with mild COVID-19 were found to be positively correlated with vitamin D, although the correlation was not statistically significant (r = 0.09, p = 0.576; and r = 0.13, p = 0.392; respectively) (Figures 3 a,b). Vitamin D was found to be negatively correlated with IL-6 in COVID-19 patients with mild symptoms (Figure 3c), but this relationship was not statistically significant (r = - 0.27, p = 0.070). There was no statistically significant correlation between vitamin D and the age of patients (r = 0.23, p = 0.133) (Figure 3d).
Discussion
The clinical presentation of patients with SARS-CoV-2 infection has varied, from asymptomatic or mildly symptomatic to moderate and severe COVID-19, characterized by Acute Respiratory Distress Syndrome (ARDS) and death [20,21,33,34]. Several studies have been published regarding the potential role of vitamin D in COVID-19 infection with varying conclusions in Western countries [6,20,25,35-39]. Therefore, this study aimed to assess vitamin D levels among severe COVID-19 patients admitted to the ICU in comparison to mild patients and healthy control individuals and to further explore its link to interleukin 6 in the African setting, specifically in Zambia, at Mary Begg Health Services.
Patients with severe COVID-19 were significantly older than those with mild infection, which is in agreement with several other studies [20,23,40]. This observation in all these studies could be attributed to the disrupted immune responses that occur with aging resulting in failure to mount anti-inflammatory responses, thereby resulting in increased proinflammatory responses and severe disease [41-44]. Additionally, with increasing age, there is an increased incidence of comorbidities that might exacerbate COVID-19 disease progression and severity, as previously documented in other studies [36,45,46]. Lymphopenia was also observed in almost half of the COVID-19 patients in this study, and this is in tandem with what has been reported in other studies [47-51]. There are several postulates for this observed lymphopenia, including an inflammatory cytokine storm, which might result in the destruction of lymphocytes, lymphocyte exhaustion, SARV-CoV-2 infection interference with T-cell expansion, apoptosis of T cells and natural killer cells due to viral infection, and the virus might directly destroy the lymphatic organs. These and other possible mechanisms might contribute to the observed lymphopenia in the study outcomes [52-55].
Vitamin D has been proven to reduce the risk of getting the common cold and has been shown to have modulatory as well as regulatory roles in host defense, adaptive and innate immunity, inflammation, and epithelial repair [56,57]. Due to these properties, studies have been conducted to assess vitamin D levels in COVID-19 patients. In the present study, the majority of COVID-19 patients (both severe and mild) had optimal vitamin D levels, and further, there was no significant difference in vitamin D concentration among those with severe COVID-19 compared to mild COVID-19 patients and the control group. This is contrary to what was found in other studies conducted elsewhere in which vitamin D was found to be significantly lower (deficient) in patients with severe COVID-19 in comparison to control groups [3,7,10,15,20-22,58]. This observed difference could be explained in terms of vitamin D sources. Over 80% of vitamin D in humans comes from the conversion of the precursor molecule 7-dehydrocholesterol (provitamin D3) following exposure to Ultraviolet B (UVB) radiation with a wavelength between 290 nm and 320 nm of sunlight [59]. The majority of African countries, including Zambia, in which this study was conducted, have longer summer periods, which would translate into longer exposure to sunlight and increased synthesis of vitamin D, unlike in other settings. While it is true that melanin reduces the natural synthesis of vitamin D by absorbing photons from UVB and we would expect a higher prevalence of vitamin D deficiency in this study as the majority of participants were of dark skin tone, most of the participants had optimal levels probably due to prolonged exposure to UVB such that optimal levels of vitamin D are synthesised in addition to nutrition intake [60,61]. This would explain the higher levels of vitamin D observed in the participants in this study regardless of COVID-19 infection severity and possibly the observed low infections and death rates due to COVID-19 among people within the tropical regions of Africa [37,62]. However, there is a paucity of data showing epidemiological vitamin D concentrations and effects in the region, hence a recommendation for a large cohort study of vitamin D concentration among the people of Zambia.
A direct correlation was observed in the study between vitamin D concentration and monocytes, in both mild and severe COVID-19 although not statistically significant. This lack of statistical significance could be attributed to the small sample size and demands critical scrutiny with sound epidemiological numbers. This is because, within the monocyte/macrophage subset of white blood cells, activation of Toll-Like Receptors (TLR) leads to the stimulation of the expression of the vitamin D receptors and localized production of vitamin D from precursor 25-hydroxyvitamin D3 (25OHD) [63]. This in turn results in increased recruitment of monocytes to the site of inflammation through the cathelicidin pathway and helps to clear the pathogen [64], hence the need to explore this relationship.
Furthermore, the vitamin D concentration in severe COVID-19 patients in this study was inversely (negatively) correlated with IL-6, which is in agreement with other studies [23-25,65,66]. This inhibitory effect of vitamin D on IL-6 is due to the blunting of the T-helper cell type 1 (Th1) immune response and through the MAPK/P38 signaling pathway [18,67]. This leads to a decrease in proinflammatory cytokines and an increase in anti-inflammatory cytokines. By downregulating proinflammatory cytokines and upregulating anti-inflammatory cytokines, vitamin D may be capable of preventing severe complications related to COVID-19 and other viral illnesses [18,19].
Study limitations
The sample size was very limited. For this reason, it would not be expedient to generalize the findings in this study. The use of samples dominantly from this locality of the study site, thus, means that genetically and environmentally, this population may be different from others, and hence, more studies are advised. Potential confounders were vitamin D supplements in clients with chronic underlying conditions (comorbidities) who initially were given vitamin D prophylaxis beforehand. This confounder was managed by going back to the list of all clients who took supplementary vitamin D during the season. Only one client from those included in this study had a chronic underlying condition and possibly received vitamin D supplements, none of the rest of the participants had received vitamin D supplements. Another possible confounder could have been specimen collection time for vitamin D determination knowing its nocturnal properties. We could not control for this, but the difference may not be statistically significant for the interpretation of the data and its inference.
Conclusion
The vitamin D concentration in severe COVID-19 patients admitted to the ICU was not significantly different from that measured in mild patients and the control group. However, vitamin D was found to be significantly negatively correlated with IL-6 in patients with severe disease, and a similar picture was seen in mild COVID-19 patients, although statistical significance could not be reached. Therefore, this suggests that COVID-19 patients could benefit from vitamin D supplementation to negate the effects of IL-6 on disease severity and progression. Large-scale studies and clinical trials are recommended to further explore the beneficial effects of vitamin D on COVID-19 patients.
Conflict of Interest
The authors have no conflicts of interest associated with the material presented in this paper.
Acknowledgement
We would like to acknowledge the support received from Mary Begg Health Services’ Management. Furthermore, we would like to express our gratitude to the following biomedical technologists, Mr. Jonathan Banda, Mr. Irack Sakala, Ms. Chitalu Nachilima, and Ms. Barbara Mulunda, for their support during data collection and sample processing.
Authors’ Contributions
Conceptualization: MC, SMM. Data curation: MC. Formal analysis: MC. Methodology: MC, SMM. Project administration: MC and SMM. Writing – original draft: MC, SMM. Writing – review & editing: SMM, VJ, MM and MK. All authors approved the final version of the manuscript.
References
- Chileshe M, Mupeta G, Kasanga M, Lindizyani Mfune R, Mudenda S, Biemba M, et al. Prevalence of Adverse Events Post-COVID-19 Vaccination amongst the Adult Zambian Population. J Biomed Res Environ Sci. 2021;2(12):1315-1321. doi: 10.37871/jbres1389.
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol. 2020 Jul;95(7):834-847. doi: 10.1002/ajh.25829. Epub 2020 May 23. PMID: 32282949; PMCID: PMC7262337.
- Padhi S, Suvankar S, Panda VK, Pati A, Panda AK. Lower levels of vitamin D are associated with SARS-CoV-2 infection and mortality in the Indian population: An observational study. Int Immunopharmacol. 2020 Nov;88:107001. doi: 10.1016/j.intimp.2020.107001. Epub 2020 Sep 14. PMID: 33182040; PMCID: PMC7489890.
- Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020 Sep 1;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722. PMID: 32880651; PMCID: PMC7489852.
- Kazancioglu S, Yilmaz FM, Bastug A, Sakallı A, Ozbay BO, Buyuktarakci C, Bodur H, Yilmaz G. Lymphocyte Subset Alteration and Monocyte CD4 Expression Reduction in Patients with Severe COVID-19. Viral Immunol. 2021 Jun;34(5):342-351. doi: 10.1089/vim.2020.0166. Epub 2020 Nov 23. PMID: 33264073.
- Xu Y, Baylink DJ, Chen CS, Reeves ME, Xiao J, Lacy C, Lau E, Cao H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J Transl Med. 2020 Aug 26;18(1):322. doi: 10.1186/s12967-020-02488-5. PMID: 32847594; PMCID: PMC7447609.
- Adami G, Giollo A, Fassio A, Benini C, Bertoldo E, Bertoldo F, Orsolini G, Idolazzi L, Viapiana O, Giannini S, Passeri G, Tacconelli E, Micheletto C, Gatti D, Rossini M. Vitamin D and disease severity in coronavirus disease 19 (COVID-19). Reumatismo. 2021 Jan 18;72(4):189-196. doi: 10.4081/reumatismo.2020.1333. PMID: 33677945.
- Silberstein M. COVID-19 and IL-6: Why vitamin D (probably) helps but tocilizumab might not. Eur J Pharmacol. 2021 May 15;899:174031. doi: 10.1016/j.ejphar.2021.174031. Epub 2021 Mar 13. PMID: 33722593; PMCID: PMC7954769.
- Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020 Jul 25;5(1):128. doi: 10.1038/s41392-020-00243-2. PMID: 32712629; PMCID: PMC7381863.
- Honardoost M, Ghavideldarestani M, Khamseh ME. Role of vitamin D in pathogenesis and severity of COVID-19 infection. Arch Physiol Biochem. 2020 Oct 30:1-7. doi: 10.1080/13813455.2020.1792505. Epub ahead of print. PMID: 33125298.
- COVID-19 and social distancing. Can J Addict. 2020 Jun 3;11(2):4-6. doi: 10.1097/CXA.0000000000000081. PMID: 34192129; PMCID: PMC7309640.
- Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. medRxiv. 2020. doi: 10.1101/2020.04.08.20058578
- Pinheiro MM, Fabbri A, Infante M. Cytokine storm modulation in COVID-19: a proposed role for vitamin D and DPP-4 inhibitor combination therapy (VIDPP-4i). Immunotherapy. 2021 Jun;13(9):753-765. doi: 10.2217/imt-2020-0349. Epub 2021 Apr 28. PMID: 33906375; PMCID: PMC8080872.
- Visser MPJ, Dofferhoff ASM, van den Ouweland JMW, van Daal H, Kramers C, Schurgers LJ, Janssen R, Walk J. Corrigendum: Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front Nutr. 2022 Mar 8;9:868324. doi: 10.3389/fnut.2022.868324. Erratum for: Front Nutr. 2022 Jan 17;8:761191. PMID: 35356738; PMCID: PMC8959105.
- Bayraktar N, Turan H, Bayraktar M, Ozturk A, Erdoğdu H. Analysis of serum cytokine and protective vitamin D levels in severe cases of COVID-19. J Med Virol. 2022 Jan;94(1):154-160. doi: 10.1002/jmv.27294. Epub 2021 Aug 30. PMID: 34427934; PMCID: PMC8661791.
- Balzanelli MG, Distratis P, Lazzaro R, Cefalo A, Catucci O, Aityan SK, Dipalma G, Vimercati L, Inchingolo AD, Maggiore ME, Mancini A, Santacroce L, Gesualdo L, Pham VH, Iacobone D, Contaldo M, Serpico R, Scarano A, Lorusso F, Toai TC, Tafuri S, Migliore G, Inchingolo AM, Nguyen KCD, Inchingolo F, Tomassone D, Gargiulo Isacco C. The Vitamin D, IL-6 and the eGFR Markers a Possible Way to Elucidate the Lung-Heart-Kidney Cross-Talk in COVID-19 Disease: A Foregone Conclusion. Microorganisms. 2021 Sep 7;9(9):1903. doi: 10.3390/microorganisms9091903. PMID: 34576798; PMCID: PMC8464828.
- Ohaegbulam KC, Swalih M, Patel P, Smith MA, Perrin R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am J Ther. 2020 Sep/Oct;27(5):e485-e490. doi: 10.1097/MJT.0000000000001222. PMID: 32804682; PMCID: PMC7473790.
- Hribar CA, Cobbold PH, Church FC. Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson's Disease. Brain Sci. 2020 May 8;10(5):284. doi: 10.3390/brainsci10050284. PMID: 32397275; PMCID: PMC7287983.
- Razdan K, Singh K, Singh D. Vitamin D Levels and COVID-19 Susceptibility: Is there any Correlation? Med Drug Discov. 2020 Sep;7:100051. doi: 10.1016/j.medidd.2020.100051. Epub 2020 Jun 2. PMID: 32835212; PMCID: PMC7266578.
- Campi I, Gennari L, Merlotti D, Mingiano C, Frosali A, Giovanelli L, Torlasco C, Pengo MF, Heilbron F, Soranna D, Zambon A, Di Stefano M, Aresta C, Bonomi M, Cangiano B, Favero V, Fatti L, Perego GB, Chiodini I, Parati G, Persani L. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021 Jun 14;21(1):566. doi: 10.1186/s12879-021-06281-7. PMID: 34126960; PMCID: PMC8200788.
- Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020 Nov 19;10(1):20191. doi: 10.1038/s41598-020-77093-z. PMID: 33214648; PMCID: PMC7677378.
- Ricci A, Pagliuca A, D'Ascanio M, Innammorato M, De Vitis C, Mancini R, Giovagnoli S, Facchiano F, Sposato B, Anibaldi P, Marcolongo A, De Dominicis C, Laghi A, Muscogiuri E, Sciacchitano S. Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir Res. 2021 Mar 3;22(1):76. doi: 10.1186/s12931-021-01666-3. PMID: 33658032; PMCID: PMC7928197.
- Orrù B, Szekeres-Bartho J, Bizzarri M, Spiga AM, Unfer V. Inhibitory effects of Vitamin D on inflammation and IL-6 release. A further support for COVID-19 management? Eur Rev Med Pharmacol Sci. 2020 Aug;24(15):8187-8193. doi: 10.26355/eurrev_202008_22507. PMID: 32767348.
- Labudzynskyi D, Shymanskyy I, Veliky M. Role of vitamin D3 in regulation of interleukin-6 and osteopontin expression in liver of diabetic mice. Eur Rev Med Pharmacol Sci. 2016 Jul;20(13):2916-9. PMID: 27424994.
- Lohia P, Kapur S, Patel P, Seyoum B. Letter to the editor: Vitamin D levels in acute illness and clinical severity in COVID-19 patients. Respir Res. 2021 Apr 9;22(1):102. doi: 10.1186/s12931-021-01703-1. PMID: 33832495; PMCID: PMC8032551.
- Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ, Crespo J, Martínez-Taboada VM. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J Clin Endocrinol Metab. 2021 Mar 8;106(3):e1343-e1353. doi: 10.1210/clinem/dgaa733. PMID: 33159440; PMCID: PMC7797757.
- Shekhawat J, Gauba K, Gupta S, Purohit P, Mitra P, Garg M, Misra S, Sharma P, Banerjee M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J Clin Biochem. 2021 Jun 21;36(4):1-11. doi: 10.1007/s12291-021-00989-8. Epub ahead of print. PMID: 34177139; PMCID: PMC8216093.
- Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020 Oct 1;40:37. doi: 10.1186/s41232-020-00146-3. PMID: 33014208; PMCID: PMC7527296.
- National Institutes of Health. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). Nih. 2021;2019:1-243.
- World Health Organisation. Therapeutics and COVID-19 LIVING GUIDELINE. 3 MARCH 2022. 2022;1-109.
- Daugaard S, Garde AH, Hansen ÅM, Vistisen HT, Rejnmark L, Kolstad HA. Indoor, outdoor, and night work and blood concentrations of vitamin D and parathyroid hormone. Scand J Work Environ Health. 2018 Nov 1;44(6):647-657. doi: 10.5271/sjweh.3745. Epub 2018 Jun 17. PMID: 29909424.
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013 Jul 5;5(7):2502-21. doi: 10.3390/nu5072502. PMID: 23857223; PMCID: PMC3738984.
- Abraham J, Dowling K, Florentine S. Can Optimum Solar Radiation Exposure or Supplemented Vitamin D Intake Reduce the Severity of COVID-19 Symptoms? Int J Environ Res Public Health. 2021 Jan 16;18(2):740. doi: 10.3390/ijerph18020740. PMID: 33467131; PMCID: PMC7829816.
- Shahin W, Rabie W, Alyossof O, Alasiri M, Alfaki M, Mahmoud E, Hijazi M, Faraidi HE, Alahmari H. COVID-19 in children ranging from asymptomatic to a multi-system inflammatory disease: A single-center study. Saudi Med J. 2021 Mar;42(3):299-305. doi: 10.15537/smj.2021.42.3.20200625. PMID: 33632909; PMCID: PMC7989263.
- Peng MY, Liu WC, Zheng JQ, Lu CL, Hou YC, Zheng CM, Song JY, Lu KC, Chao YC. Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. Int J Mol Sci. 2021 May 16;22(10):5251. doi: 10.3390/ijms22105251. PMID: 34065735; PMCID: PMC8155889.
- Oristrell J, Oliva JC, Subirana I, Casado E, Domínguez D, Toloba A, Aguilera P, Esplugues J, Fafián P, Grau M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines. 2021 May 5;9(5):509. doi: 10.3390/biomedicines9050509. PMID: 34063015; PMCID: PMC8147982.
- Phosri A, Cao Y, Harada Sassa M, Harada KH. Socio-economic factors do also matter: comments on the article "Can climatic factors explain the differences in COVID-19 incidence and severity across the spanish regions?: an ecological study". Environ Health. 2021 Feb 18;20(1):17. doi: 10.1186/s12940-021-00701-6. PMID: 33602202; PMCID: PMC7891113.
- Exploring links between Vitamin D deficiency and covid-19. PLoS Pathog. 2020;16(9):1–7.
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul;32(7):1195-1198. doi: 10.1007/s40520-020-01570-8. Epub 2020 May 6. PMID: 32377965; PMCID: PMC7202265.
- Vasheghani M, Jannati N, Baghaei P, Rezaei M, Aliyari R, Marjani M. The relationship between serum 25-hydroxyvitamin D levels and the severity of COVID-19 disease and its mortality. Sci Rep. 2021 Sep 2;11(1):17594. doi: 10.1038/s41598-021-97017-9. PMID: 34475485; PMCID: PMC8413335.
- Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of "inflame-aging". Inflamm Res. 2020 Sep;69(9):825-839. doi: 10.1007/s00011-020-01372-8. Epub 2020 Jun 11. PMID: 32529477; PMCID: PMC7289226.
- Pawelec G. T-cell immunity in the aging human. Haematologica. 2014;99(5):795-797. doi: 10.3324/haematol.2013.094383
- O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, Fontanet A, Cauchemez S, Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021 Feb;590(7844):140-145. doi: 10.1038/s41586-020-2918-0. Epub 2020 Nov 2. PMID: 33137809.
- Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, Boekschoten MV, Savelkoul HFJ, De Vos P, Dekker J, Wells JM. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci Rep. 2019 Feb 5;9(1):1437. doi: 10.1038/s41598-018-35228-3. PMID: 30723224; PMCID: PMC6363726.
- Arumugam VA, Thangavelu S, Fathah Z, Ravindran P, Sanjeev AMA, Babu S, Meyyazhagan A, Yatoo MI, Sharun K, Tiwari R, Pandey MK, Sah R, Chandra R, Dhama K. COVID-19 and the world with co-morbidities of heart disease, hypertension and diabetes. J Pure Appl Microbiol. 2020;14(3):1623-1638. Doi: 10.22207/JPAM.14.3.01
- Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020 Oct;22(10):1915-1924. doi: 10.1111/dom.14124. Epub 2020 Jul 16. PMID: 32573903; PMCID: PMC7361304.
- Mardani R, Ahmadi Vasmehjani A, Zali F, Gholami A, Mousavi Nasab SD, Kaghazian H, Kaviani M, Ahmadi N. Laboratory Parameters in Detection of COVID-19 Patients with Positive RT-PCR; a Diagnostic Accuracy Study. Arch Acad Emerg Med. 2020 Apr 4;8(1):e43. PMID: 32259132; PMCID: PMC7130449.
- Seyit M, Avci E, Nar R, Senol H, Yilmaz A, Ozen M, Oskay A, Aybek H. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. 2021 Feb;40:110-114. doi: 10.1016/j.ajem.2020.11.058. Epub 2020 Dec 6. PMID: 33309506; PMCID: PMC7719281.
- Cao W, Shi L, Chen L, Xu X, Wu Z. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv. 2020. doi: 10.1101/2020.02.23.20026963
- Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020 May 24;8:36. doi: 10.1186/s40560-020-00453-4. PMID: 32483488; PMCID: PMC7245646.
- Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020 May 24;8:36. doi: 10.1186/s40560-020-00453-4. PMID: 32483488; PMCID: PMC7245646.
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020 Mar 27;5(1):33. doi: 10.1038/s41392-020-0148-4. Erratum in: Signal Transduct Target Ther. 2020 Apr 29;5(1):61. PMID: 32296069; PMCID: PMC7100419.
- Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020 May;17(5):541-543. doi: 10.1038/s41423-020-0401-3. Epub 2020 Mar 17. PMID: 32203186; PMCID: PMC7091621.
- Fathi N, Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int. 2020 Sep;44(9):1792-1797. doi: 10.1002/cbin.11403. Epub 2020 Jun 3. PMID: 32458561; PMCID: PMC7283672.
- Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020 Sep;225:31-32. doi: 10.1016/j.imlet.2020.06.013. Epub 2020 Jun 20. PMID: 32569607; PMCID: PMC7305732.
- Alipio M. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-2019). SSRN Electron J. 2020;2019(082):1-6.
- Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017 Jan;27(1). doi: 10.1002/rmv.1909. Epub 2016 Oct 7. PMID: 27714929.
- Gaudio A, Murabito AR, Agodi A, Montineri A, Castellino P, D O CoV Research. Vitamin D Levels Are Reduced at the Time of Hospital Admission in Sicilian SARS-CoV-2-Positive Patients. Int J Environ Res Public Health. 2021 Mar 27;18(7):3491. doi: 10.3390/ijerph18073491. PMID: 33801759; PMCID: PMC8036292.
- Samanta S. Vitamin D and immunomodulation in the skin: a useful affirmative nexus. Explor Immunol. 2021;90-111. doi: 10.37349/ei.2021.00009
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008 May-Jun;84(3):539-49. doi: 10.1111/j.1751-1097.2007.00226.x. PMID: 18435612; PMCID: PMC2671032.
- Islam MT, Salehi B, Karampelas O, Sharifi-Rad J, Docea AO, Martorell M, Calina D. High skin melanin content, vitamin D deficiency and immunity: Potential interference for severity of COVID-19. Farmacia. 2020;68(6):970-983. https://bit.ly/3KGa0tm
- Alipio MM. Do latitude and ozone concentration predict Covid-2019 cases in 34 countries? medRxiv. 2020;63(082):1-9. doi: 10.1101/2020.04.09.20060202
- Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009 Apr 1;182(7):4289-95. doi: 10.4049/jimmunol.0803736. PMID: 19299728; PMCID: PMC2683618.
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007 Aug 15;179(4):2060-3. doi: 10.4049/jimmunol.179.4.2060. PMID: 17675463.
- Djibo DA, Sahr F, McCutchan JA, Jain S, Araneta MRG, Brodine SK, Shaffer RA. Prevalence and Risk Factors for Human Immunodeficiency Virus (HIV) and Syphilis Infections Among Military Personnel in Sierra Leone. Curr HIV Res. 2017;15(2):128-136. doi: 10.2174/1570162X15666170517101349. PMID: 28521722.
- Cimmino G, Conte S, Morello M, Pellegrino G, Marra L, Morello A, Nicoletti G, De Rosa G, Golino P, Cirillo P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J Cardiovasc Dev Dis. 2022 Jan 13;9(1):27. doi: 10.3390/jcdd9010027. PMID: 35050236; PMCID: PMC8781542.
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012 Mar 1;188(5):2127-35. doi: 10.4049/jimmunol.1102412. Epub 2012 Feb 1. PMID: 22301548; PMCID: PMC3368346.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.