Medicine Group . 2022 June 23;3(6):703-711. doi: 10.37871/jbres1500.
Respiratory Viruses in Hospitalized Patients before and after SARS-CoV-2
Tina Ursic*, Rok Kogoj, Kaja Erzar, Monika Jevsnik Virant and Miroslav Petrovec
- Respiratory viruses
- SARS-CoV-2
- COVID-19
- Hospitalized patients
Abstract
Background: Since the emergence of COVID-19, countries have implemented combinations of non-pharmaceutical measures not only to contain the COVID-19 cases manageable for their healthcare system but also to reduce the economic burden of the disease. Among the various options, authorities have mostly adopted frequent hand disinfection and wearing surgical masks. Because these measures have long been used in medicine to prevent infections, there is reason to expect an effect not only on SARS-CoV-2 but also on other microorganisms that spread via droplet inhalation and close contact. This study investigates the epidemiology of typical respiratory viruses in Slovenian hospitalized patients before and during the ongoing SARS-CoV-2 epidemic.
Methods: Molecular testing data were analyzed using a laboratory informative system for typical respiratory viruses from 2012 to 2022 in conjunction with SARS-CoV-2 testing from March 2020 onward.
Results: First, we observed a significant decrease in the number of typical respiratory virus testing requirements after the SARS-CoV-2 outbreak; second, a change in the age structure of hospitalized patients, with fewer children and more working-age individuals during the 2020/21 season; third, complete disappearance of influenza A, B, RSV, and other enveloped respiratory viruses; and, fourth, unchanged epidemiology of rhinovirus. Finally, with the slow fading of all measures implemented in the 2020/21 season, respiratory viruses appear to be “back on the scene” despite the still ongoing SARS-CoV-2 epidemic in 2021/22.
Conclusions: Further monitoring seems to be important in the coming seasons to shed more light on future changes in respiratory viruses’ epidemiology because restrictions are bound to be loosened.
Abbreviations
COVID-19: Corona Virus Disease 2019; COPD: Chronic Obstructive Pulmonary Disease; EV: Enterovirus; FluA/B: Influenza A/B Virus; HAdV: Human Mastadenovirus; HBoV1: Human bocavirus 1; HCoV: Human Coronavirus; HMPV: Human Metapneumovirus; HRV: Human Rhinovirus; LRTI: Lower Respiratory Tract Infection; LTCF: Long-Term Care Facility; PIV1–4: Parainfluenza Viruses 1–4; RSV: Respiratory Syncytial Virus; RT-PCR: Reverse Transcription-Polymerase Chain Reaction; RVs: Respiratory Viruses; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; UK: United Kingdom; WHO: World Health Organization
Introduction
A variety of viruses play an important role in Respiratory Tract Infections (RTIs) in humans and cause significant morbidity and mortality in all age groups worldwide. Over the past decade, the molecular diagnostics of Respiratory Viruses (RVs) have improved tremendously, resulting in the development of faster and more accurate tests that can detect many viruses simultaneously. In addition, these advances have led to new insights into the prevalence and epidemiology of individual RVs in hospitalized patients. Syndromic testing has also become available in recent years, and its ability to detect the majority of RVs provides more realistic insight into the extent of RTIs caused by these pathogens.
From our longitudinal surveillance of RV infections, we have learned that in Slovenia and surrounding countries influenza A and B viruses (FluA/B) and Respiratory Syncytial Virus (RSV), along with Human Coronaviruses (HCoVs), cause regular annual winter epidemics, whereas Human Metapneumoviruses (HMPVs) cause smaller seasonal peaks of infection in the spring. Human Mastadenoviruses (HAdVs) are detected throughout the year, and Parainfluenza Viruses (PIVs) show seasonal peaks in spring, late summer, and fall [1,2]. Human Rhinoviruses (HRVs) are most commonly detected in the spring and fall, when humidity is highest, whereas Enteroviruses (EVs) are most commonly detected in the summer months [1].
After the emergence of the SARS-CoV-2 virus in December 2019 in Wuhan, Hubei Province, China, the virus spread rapidly throughout the globe and reached pandemic proportions, officially declared by WHO on March 11th, 2020. The first case of SARS-CoV-2 in Slovenia was confirmed on March 4th, 2020, and, similar to other countries around the globe, an unprecedented demand for SARS-CoV-2 testing quickly emerged. Due to the number of laboratory-confirmed cases in the following months, the Slovenian government, in collaboration with public health authorities, implemented several Non-Pharmaceutical Interventions (NPIs) in various combinations at different times during the epidemic. The NPIs included cancelation of all social activities, prohibition of gatherings outside immediate family members, prohibition of travel between Slovenian geographic regions, wearing of surgical masks indoors and outdoors in public areas, closure of all nonessential stores, mandatory hand disinfection at entrances to essential stores and residences, closing schools at all levels, encouraging online work for as many professions as possible, and quarantine for positive patients and their contacts, all to keep the number of COVID-19 cases manageable for hospitals. Because the emergence of COVID-19 in the Slovenian population occurred at the end of the regular 2019/20 RV season, no one could predict the impact of extensive SARS-CoV-2 testing, viral competition, and NPIs on other RVs in the upcoming 2020/21 RV infection season.
This study compares the epidemiology of RVs in hospitalized patients before the SARS-CoV-2 epidemic in Slovenia, in the 1st year of the SARS-CoV-2 epidemic, and in the 2nd year of the SARS-CoV-2 epidemic. In addition, it assesses whether SARS-CoV-2 had an impact on the percentage of hospitalized patients in each age group and whether the prevalence and seasonality of other RVs changed after the emergence of SARS-CoV-2.
Materials and Methods
RVs and SARS-CoV-2 testing
Routine molecular diagnostics of RVs using a respiratory virus panel, which allows detection of all RVs simultaneously, have been performed since January 2012 at IMI. Between January 2012 and November 2018, an in-house real-time RT-PCR test was used for the detection of FluA/B, RSV, HRVs, HMPV, PIV 1–4, HCoVs (229E, NL63, OC43, and HKU1), HAdVs, EV, and HBoV1, as previously described [3]. In December 2018, the diagnostics of respiratory viruses were switched to the commercially available automatic platform Respiratory Virus 16-Well Assay (AusDiagnostics, Pty Ltd, Mascot, Australia), which covers all RVs listed above and is still in use in its latest version to date. Extraction of total nucleic acids from upper respiratory tract swabs collected in viral transport medium remained unchanged during this platform switch and is still performed as previously described [3]. By specific request, mostly during the winter months, FluA/B and RSV were detected using contemporarily available GeneXpert assays (Cepheid, USA). More specifically, from January 2012 to the end of December 2014, FluA/B was detected with the GeneXpert FluA/B assay, and starting in January 2015 FluA/B and RSV were simultaneously detected with a newer version of the GeneXpert assay; the Xpert® Xpress Flu/RSV assay, and from January 2021 also the GeneXpert assay; Xpert® Xpress SARS-CoV-2/Flu/RSV was also available.
In total (from January 2012 to March 2022), results from 75,288 respiratory samples from 51,136 hospitalized patients were included in the RV part of the study. The data on the total number of hospitalized patients and outpatients tested for SARS-CoV-2 were obtained from a Laboratory Information System (LIS). The seasonal distribution and the prevalence of SARS-CoV-2 in the results section are shown only for hospitalized patients, for whom a complete RV panel was ordered from March 2021 onward.
All samples were collected from patients with RTI admitted to the Ljubljana Medical Center, the largest tertiary hospital in the country, located in the central part of Slovenia, with 2,138 beds.
Statistical analysis
Pearson’s chi-squared test was used to compare categorical variables between different age groups and seasons.
Results
Epidemiology of respiratory viruses before SARS-CoV-2 emergence, 2012–2020 seasons
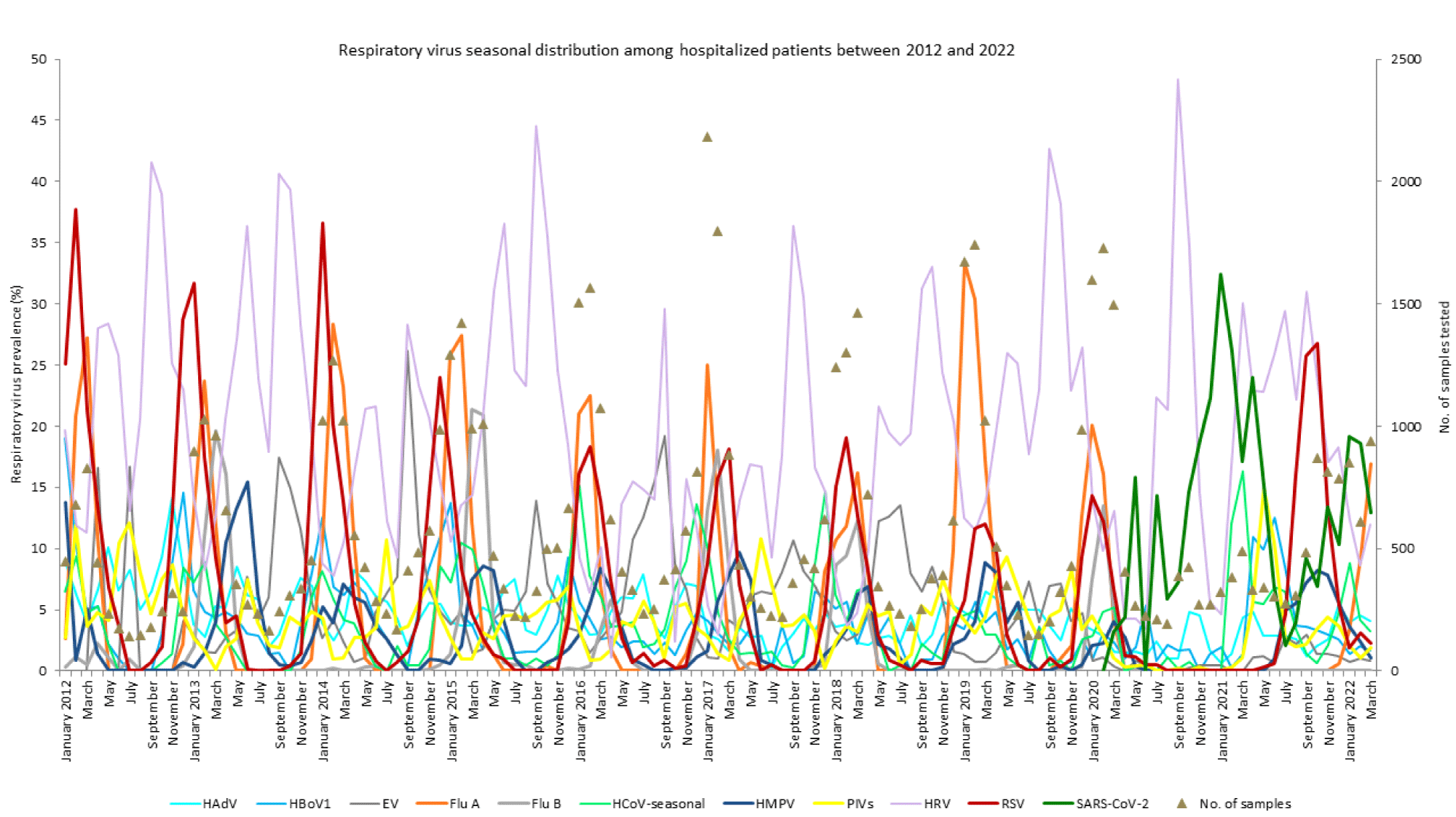
From 2012 to 2020, we received 63,684 respiratory samples from 42,800 hospitalized patients for the detection of RVs; 16,623 were tested with the GeneXpert Assay (Cepheid, USA) for FluA/B or FluA/B and RSV, and 47,061 were tested using either the in-house respiratory virus panel (3) or the Respiratory Virus 16-Well Assay (AusDiagnostics, Pty Ltd, Mascot, Australia), as shown in detail in figure 1.
The female-to-male ratio was 1:1.1 (30,288:33,396). The 9-year average (2012–2020) shows that one-fourth of the samples obtained from the hospital were from small children (0–4 years), followed by 14.9%, 12.1%, and 9.6% of samples obtained from older patients (75–84, 65–74, and 55–64 years). We obtained 9% of samples from older children (5–14 years) and from the elderly (≥ 85 years), followed by 5.7%, 5%, 5%, and 3.8% from adults and young adults (45–54, 35–44, 25–34, and 15–24 years), shown in table 1. From 2012 to 2020, the most frequently detected virus in small children (0–4 years) was HRV (25.6%), followed by RSV (24.6%) and HBoV1 (11.7%), as shown in table 1.
| Table 1: Distribution of the three most frequently detected respiratory viruses in respective age groups per different timeframes. | ||||||||||
| Age groups, years | 0–4 | 5–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥ 85 |
| Jan 2012 – Mar 2020, %; according to age | 25.9 | 9 | 3.8 | 5.0 | 5.0 | 5.7 | 9.6 | 12.1 | 14.9 | 9 |
| Viruses detected according to frequency | ||||||||||
| 1st | HRV | HRV | HRV | FluA | FluA | FluA | FluA | FluA | FluA | FluA |
| 2nd | RSV | FluB | FluA | HRV | HRV | HRV | HRV | HRV | HRV | HRV |
| 3rd | HBoV1 | FluA | FluB | FluB | FluB | FluB | RSV | FluB | RSV | RSV |
| Age groups, years | 0–4 | 5–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥ 85 |
| Apr 2019 – Mar 2020, %; according to age | 19.9 | 9.5 | 4.5 | 6.5 | 7.1 | 6.5 | 9.1 | 12.7 | 15.2 | 9 |
| Viruses detected according to frequency | ||||||||||
| 1st | HRV | HRV | HRV | HRV | HRV | HRV | HRV | FluA | FluA | FluA |
| 2nd | RSV | FluB | FluB | FluA | FluA | FluA | FluA | HRV | HRV | HRV |
| 3rd | FluA | FluA | FluA | FluB | FluB | RSV | RSV | RSV | RSV | RSV |
| Age groups, years | 0–4 | 5–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥ 85 |
| Apr 2020 – Mar 2021, %; according to age | 21.4 | 10.2 | 4.1 | 6 | 8.9 | 7.8 | 11.8 | 14 | 11 | 4.8 |
| Viruses detected according to frequency | ||||||||||
| 1st | HRV | HRV | SARS-CoV-2 | HRV | HRV | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 |
| 2nd | HAdV | SARS-CoV-2 | HRV | SARS-CoV-2 | SARS-CoV-2 | HRV | HRV | HRV | HRV | HRV |
| 3rd | HCoV | HCoV | HCoV | HCoV | HCoV | HCoV | HCoV | HCoV | HMPV | HCoV |
| Age groups, years | 0–4 | 5–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥ 85 |
| Apr 2021 – Mar 2022, %; according to age | 36.9 | 9.7 | 3.9 | 5 | 5.3 | 5.6 | 9.1 | 10.8 | 8.7 | 5 |
| Viruses detected according to frequency | ||||||||||
| 1st | HRV | HRV | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 | SARS-CoV-2 |
| 2nd | RSV | FluA | HRV | HRV | HRV | HRV | HRV | HRV | HRV | HCoV |
| 3rd | HBoV1 | SARS-CoV-2 | FluA | HCoV | HCoV | HCoV | HMPV | HMPV | HCoV | HRV |
| Chi-squared, p-value 2019/2020 versus 2020/2021 | p = 0.082 | p = 0.276 | p = 0.428 | p = 0.287 | p = 0.017 | p = 0.018 | p < 0.001 | p = 0.071 | p < 0.001 | p < 0.001 |
| Chi-squared, p-value 2012/2020 versus 2020/2021 | p < 0.001 | p = 0.030 | p = 0.363 | p = 0.020 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.001 | p < 0.001 | p < 0.001 |
| Chi-squared, p-value 2012/2020 versus 2021/2022 | p < 0.001 | p = 0.092 | p = 0.609 | p = 0.923 | p = 0.323 | p = 0.716 | p = 0.250 | p = 0.005 | p < 0.001 | p < 0.001 |
| Chi-squared, p-value 2020/2021 versus 2021/2022 | p < 0.001 | p = 0.455 | p = 0.662 | p = 0.064 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.005 | p = 0.784 |
| HRV = Human Rhinovirus; RSV = Respiratory Syncytial Virus; Flua = Influenza A Virus; Flub = Influenza B Virus; Hbov1 = Human Bocavirus 1; SARS-Cov-2 = SARS-Corona Virus 2; Hcov = Human Coronavirus (OC43, HKU1, NL63, 229E); HMPV = Human Metapneumovirus, Hadv = Human Mastadenovirus | ||||||||||
Epidemiology of respiratory viruses after SARS-CoV-2 emergence, 2020–2021 season, and comparison with previous 9 years
The situation dramatically changed after March 2020, when SARS-CoV-2 emerged in the Slovenian population, as shown in figures 1-3. Between April 2020 and March 2021, the diagnosis of typical RVs decreased significantly in comparison to previous seasons (p < 0.001, t-test) and was performed on 3,999 respiratory samples from 3,054 hospitalized patients; 111 were tested with the GeneXpert Assay (Cepheid, USA) for FluA/B and RSV, and 3,888 were tested using the Respiratory Virus 16-Well Assay (AusDiagnostics, Australia). The ratio of females versus males tested, however, remained similar; 1:1.17 (1,843:2,156). The demand for SARS-CoV-2 testing among hospitalized patients has exceeded all expectations and was significantly higher in the season from April 2020 to March 2021 (p = 0.002, t-test) (Table 2 & figure 2). However, in the 2020/21 RV season, a significantly higher percentage of hospitalized adults (35–44, 45–54, and 55–64 years; 8.9%, 7.8%, and 11.8%) was observed in comparison to the 2019/20 season (7.1%, 6.5%, and 9.1%) and even the previous 9 years (2012–2020; 5%, 5.7%, and 9.6%). Moreover, in the 2020/21 respiratory season, a significantly lower percentage of hospitalized elderly (75–84 and ≥ 85 years; 11% and 4.8%) was observed in comparison to the previous 2019/20 season (15.5% and 9%) and the even the previous 9 years (2012–2020; 14.9% and 9%), shown in table 1. A significantly lower percentage of hospitalized small children (0–4 years; 21.4%) and a significantly higher percentage of hospitalized young adults (25–34 years; 6%) was observed in the 2020/21 season in comparison to the previous 9-year average (2012–2020; 25.6% and 5%), shown in table 1.
| Table 2: Extent of typical respiratory virus testing in hospitalized patients from December 2019 to March 2022 in comparison to SARS-CoV-2. The data for SARS-CoV-2 tested hospitalized patients and outpatients were obtained from the Laboratory Information System (LIS). | ||||
| Month | <Number of hospitalized patients tested for RV panel | <Number of hospitalized patients tested for GeneXpert FluA/B & RSV or SARS-CoV-2/Flu/RSV | <Number of hospitalized patients tested for SARS-CoV-2 | <Number of all patients tested for SARS-CoV-2 |
| Dec. 2019 | <450 | <595 | <0 | <0 |
| Jan. 2020 | <653 | <1117 | <0 | <0 |
| Feb. 2020 | <770 | <1114 | <1,080 | <1,216 |
| Mar. 2020 | <1,212 | <403 | <4778 | <10,426 |
| Apr. 2020 | <352 | <69 | <6,049 | <14,111 |
| May. 2020 | <247 | <9 | <4,828 | <12,523 |
| Jun. 2020 | <222 | <3 | <2,398 | <13,563 |
| Jul. 2020 | <210 | <2 | <2,668 | <15,068 |
| Aug. 2020 | <205 | <1 | <2,879 | <14,427 |
| Sep. 2020 | <412 | <0 | <4,783 | <32,687 |
| Oct. 2020 | <451 | <3 | <9,507 | <52,920 |
| Nov. 2020 | <354 | <1 | <10,122 | <47,802 |
| Dec. 2020 | <255 | <7 | <10,540 | <45,497 |
| Jan. 2021 | <315 | <5 | <9,275 | <43,577 |
| Feb. 2021 | <369 | <5 | <6,866 | <43,789 |
| Mar. 2021 | <465 | <4 | <6,372 | <59,701 |
| Apr. 2021 | <332 | <0 | <8,287 | <44,191 |
| May. 2021 | <343 | <0 | <7,402 | <40,801 |
| Jun. 2021 | <306 | <0 | <5,915 | <25,486 |
| Jul. 2021 | <278 | <0 | <3,415 | <14,345 |
| Aug. 2021 | <307 | <0 | <2,248 | <21,329 |
| Sep. 2021 | <486 | <1 | <6,504 | <49,333 |
| Oct. 2021 | <871 | <177 | <7,592 | <55,767 |
| Nov. 2021 | <814 | <213 | <7,856 | <73,871 |
| Dec. 2021 | <785 | <188 | <7,647 | <47,026 |
| Jan. 2022 | <749 | <198 | <10,761 | <134,628 |
| Feb. 2022 | <608 | <148 | <6,145 | <18,663 |
| Mar. 2022 | <941 | <350 | <8,860 | <11,782 |
| RV panel = Respiratory Viruses Panel | ||||
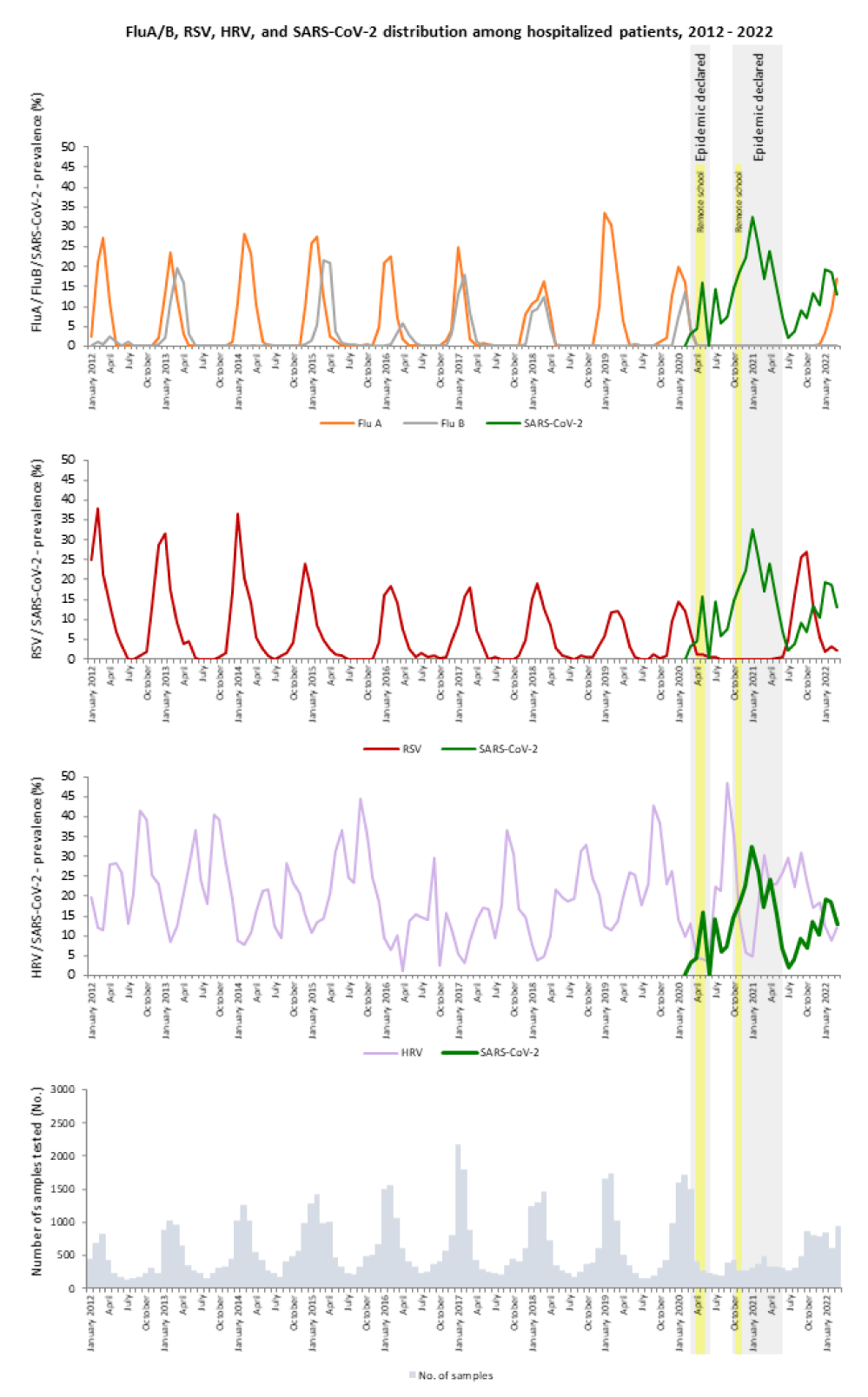
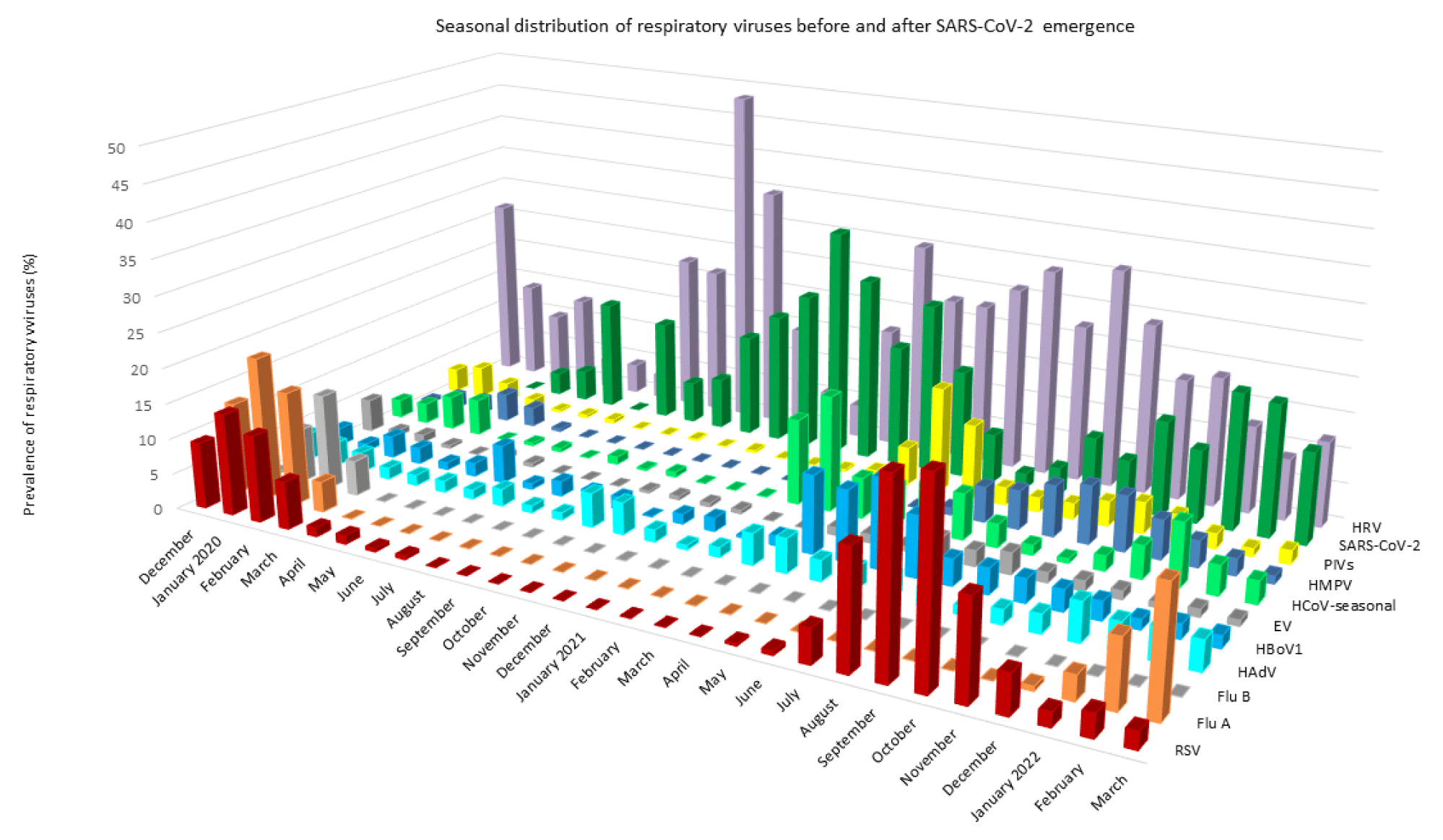
Thus, in the 2020/21 season, we observed RSV in only 10 samples (April–July 2020) and not observed a single case of FluA or FluB in either age group. However, not a single case of RSV was observed after July 2020 until the end of March 2021, as shown in figures 2 and 3. SARS CoV-2 predominated together with HRV and seasonal coronaviruses in all age groups except the youngest. Interestingly, SARS CoV-2 was detected in only four samples of small children (0–4 years).
Coexistence of respiratory viruses with SARS-CoV-2, 2021–2022 season
The last respiratory season, from April 2021 to March 2022, started after 1 year of SARS-CoV-2 emergence in Slovenia and ended after 2 years of the SARS-CoV-2 epidemic. Between April 2021 and March 2022, the diagnostics of RVs increased in comparison to the previous season (2020/21) and were performed on 7,605 respiratory samples from 5,958 hospitalized patients; 1,297 were tested with the XpertXpress SARS-CoV-2/Flu/RSV Assay (Cepheid, USA) and 6,308 were tested using the Respiratory Virus 16-Well Assay (AusDiagnostics, Australia). The ratio of females versus males tested, however, remained similar; 1:1.15 (2,775:3,183), and so was the demand for SARS-CoV-2 testing among hospitalized patients between the 2020/21 and 2021/22 seasons. However, the demand for SARS-CoV-2 testing in outpatients in the 2021/22 season was even higher (537,222) in comparison to the 2020/21 season (407,307), as shown in table 2.
In the 2021/22 respiratory season, a significantly higher percentage of hospitalized small children (0–4 years; 36.9% vs. 25.9%) and a significantly lower percentage of hospitalized elderly (65–74 years: 10.8% vs. 12.1%; 75–84 years: 8.7% vs. 14.9%; ≥ 85 years: 5% vs. 9%) was observed in comparison to the previous 9 years of seasons (2012–2020), shown in table 1. Moreover, in the 2021/22 respiratory season a significantly higher percentage of hospitalized small children (0–4 years; 36.9% vs. 21.4%) and a significantly lower percentage of hospitalized adults and elderly (35–44 years: 5.3% vs. 8.9%; 45–54 years: 5.6% vs. 7.8%; 55–64 years: 9.1% vs. 11.8%; 65–74 years: 10.8% vs. 14%; 75–84 years: 8.7% vs. 11%) was observed in comparison to the previous year, 2020/21, shown in table 1.
Thus, in the last respiratory season, 2021/22, SARS-CoV-2 predominates together with HRV and HCoV infections, as shown in figures 2 and 3 and table 1. In addition, infections caused by HMPV and PIVs were observed among hospitalized patients (Figure 3).
A respiratory syncytial virus early epidemic peak was observed between September and October 2021, which was missing in 2020/21, together with the late FluA infections, which started in March 2022. The FluA epidemic will likely peak in April 2022, as shown in figures 1-3. In the youngest age group, the three most frequently detected viruses in the last season are the same (HRVs, RSV, and HBoV1) as they were in the previous 9-year average (2012–2020), whereas in all other age groups the SARS-CoV-2 prevalence together with HRVs remains the highest.
Discussion
To start with, we would like to highlight the great value of the data presented in our study, which is the result of a professional decision in 2011 made by clinicians at the largest hospital in Slovenia together with the Institute of Microbiology and Immunology. The decision was that all patients hospitalized due to severe respiratory infections receive complete molecular respiratory virus panel testing, thus putting the diagnostics of respiratory viruses on a new higher level. The diagnostics for hospitalized patients became uniform and the processing of samples easier; moreover, this involved a new surveillance regional hospital system setup. By setting up the surveillance system for the largest hospital in Slovenia (in the central region), we were suddenly able to infer which viruses were circulating in the population.
First, from 2012 to 2020, in Slovenia, we observed more or less the same seasonal distribution of RVs among hospitalized patients, with only minor seasonal changes in the epidemiology, resulting in higher or lower peaks and early or delayed peaks for the respective RV. This study shows that with the onset of SARS-CoV-2 testing for typical respiratory viruses in hospitalized patients in Slovenia changed dramatically. Testing for respiratory viruses in the last RV season (2020/21) dropped to half of the previous demand, and specific testing for influenza alone dropped to only a few (Table 2).
Second, significant differences were observed among the youngest age group of patients hospitalized between 2012 and 2020, during the 2020/21 season, and during the last season, 2021/22. There was a drop in hospitalized patients in the youngest age group (0–4 years; from 25.9% to 21.4%), which could be explained by the preventive measures and the absence of all respiratory viruses except SARS-CoV-2, HRVs, and HCoVs in the 2020/21 season. Moreover, in the 2021/22 season, a significant increase of hospitalized patients in the youngest age group (0–4 years; from 21.4% to 36.9%) was observed, which could be explained by the naive population of small children vulnerable to severe infections due to the absence of the majority of respiratory viruses in the previous year.
Third, the percentage of hospitalized adults and elderly (age groups 25–34, 35–44, 45–54, 55–64, and 65–74 years) in 2020/21 increased significantly, whereas these percentages dropped in 2021/22 in comparison to the previous 9-year average. The patients in these age groups represent the bulk of the working population (except the age group 65–74 years), which were more susceptible to SARS-CoV-2, and they were under more stress and consequently a more vulnerable group for a severe course of the respiratory disease.
Last but not least, the percentage of hospitalized elderly patients tested (age groups 75–84 and ≥ 85 years) dropped significantly in the 2020/21 season and remained lower even in the last season, 2021/22, in comparison to the previous 9-year average (2012–2020). This could be explained by the absence of influenza and RSV in the 2020/21 season, preventive measures, and the changed LTCF operating policy due to the SARS-CoV-2 epidemic.
Although the onset of SARS-CoV-2 changed the diagnostics of hospitalized patients with respiratory disease in Slovenia in the 2020/21 season, preferentially to only SARS-CoV-2 testing, the onset of SARS-CoV-2 completely changed the seasonality and prevalence of other very important respiratory viruses, such as FluA/B and RSV, which were not detected at all either in the general Slovenian population or in hospitalized patients.
However, the SARS-CoV-2 epidemic in Slovenia together with all preventive measures taken by the government had an impact on each RV except HRV. Human rhinoviruses, a group of small viruses (family Picornaviridae) known as common cold viruses linked to exacerbations of COPD, and asthma can cause severe bronchiolitis in young children, and severe pneumonia in adults and the immune compromised; all of these seem to have been unaffected by the SARS-CoV-2 epidemic and all the associated preventive measures imposed by the government due to the epidemic. Together with SARS-CoV-2, HRV was the second most frequently detected virus in hospitalized patients in all age groups and the only virus under surveillance from 2012 with unchanged seasonality.
Changes in the seasonal circulation of several respiratory viruses are being reported from all over the world and seem to confirm our findings [4-14]. An article by Redlberger-Fritz, et al. [13] assessed the impact of nationwide SARS-CoV-2 lockdown measures in neighboring Austria on the circulation of other respiratory viruses. Their result clearly shows that a 70% reduction in mobility had a significant impact on the circulation of influenza, RSV, HMPV, and HRV as early as 1 week after the implementation of measures. Similarly, a study from Finland by Kuitunen, et al. [15] showed a remarkable decrease in pediatric Emergency Room (ER) visits due to respiratory tract infections at two hospitals after the start of the national state of emergency and lockdown. In addition, our results show that the 2019/20 influenza and RSV seasons were quite similar in prevalence to the previous season(s) but ended more rapidly. These could be explained by higher numbers of influenza infections observed in preschool and school children (0–4 and 5–14 years) in comparison to previous seasons. Although such observations coincided with the lockdown, an additional reason for generally lower ER visits was suggested by the authors; namely, fear of going to the hospital and increased treatment of minor respiratory infections at home [15]. Similar sharp decreases in the activity of influenza soon after COVID-19 non-pharmaceutical prevention methods have also already been reported in the United States, Australia, Chile, South Africa [4], Taiwan [5], Hong Kong [6], South Korea [7], and Singapore [8]. Moreover, a study from Shanghai, China by Liu, et al. [11] described a significant impact on the circulation of respiratory viruses (FluA, FluB, PIV1–3, RSV, HAdV, HMPV, and HRV), resulting in a 45.5% decrease in hospitalization of children due to LRTI. Finally, similar patterns have also been reported from New Zealand, showing significant decreases in the number of cases of influenza, RSV, HRV, EV, HAdV, HMPV, and PIV after lockdown. All papers link the observed results to lockdowns and other non-pharmaceutical interventions implemented by respective countries to limit SARS-CoV-2 spreading. Interestingly, in Japan, a decrease in influenza activity in 2020 was also observed; however, such a decrease might have also been linked to temperature or virulence and not mainly to SARS-CoV-2 preventive measures because awareness regarding measures to reduce the risk of disease transmission was high among the Japanese public since before COVID-19.
From our results, HRVs, HBoV1, HAdV, and HCoVs seem to be somewhat of an exception and less affected by anti-COVID-19 measures. A resurgence of HRV has also been reported by Takeshita, et al. [16] who reported an increased risk of HRV infection in children under 10 during the COVID-19 pandemic. Similar to our results, other enveloped respiratory viruses were strongly suppressed in the 2020/21 season, but non-enveloped viruses (HRV, Coxsackie A/B, and HAdV) retained their circulation. Moreover, HRV seemed to have gained momentum in children under 10. From their results, it seems that the consequence of infection prevention methods was a complete absence of influenza and only rare cases of SARS-CoV-2 in this age group. This caused space for HRV to thrive [16] because it is more resistant to ethanol-based disinfectants [17], which allows HRV to survive for a prolonged time on surfaces [18], compounded by the inability of surgical masks to prevent transmission of HRV infection from symptomatic individuals [19]. Such findings are also supported by the work of Poole, et al. [20] and show a drop in the rate of detection of respiratory viruses during the 2020 lockdown in Southampton, UK, with the exception of HRV. Although a lower detection rate was observed for HRV during the lockdown, a sharp increase in the incidence was detected soon after easing off the measures.
Our results are in good agreement with published data, thus showing that non-pharmaceutical interventions against COVID-19 were similarly effective in Slovenia as in other countries, with the exception of HRV. Rhinovirus seems to have endured COVID-19 restrictions because it also remained among the three respiratory viruses most often detected in hospitalized patients after the onset of SARS-CoV-2. It would seem that the nature of the virus (non-enveloped and more resistant to ethanol-based disinfectants) helped it replicate in the population, although a higher awareness of hand disinfection was achieved. These results clearly show the beneficial impact of social distancing, surgical masks, and hand disinfection on the circulation of enveloped respiratory viruses.
Last but not least, in the last season, 2021/22, all viruses returned with a delay. This could be explained by the lockdown of the country, the last time in October 2021, but after that, the country was not shut down again. After October 2021, every week there were increasingly fewer preventive measures. In the 2021/22 season, almost all viruses had already returned, which could be explained by easing preventive measures, opening schools where wearing masks is no longer mandatory, and opening the country and all the industries to their full extent.
Further monitoring thus seems to be important in the coming seasons to shed more light on future changes in respiratory viruses’ epidemiology because restrictions are bound to be loosened.
Limitations
The present study gives insight into the epidemiology of respiratory viral infections only among hospitalized patients (inpatients), and since the results cannot be extrapolated to the general population, therefore, the epidemiology of the respiratory viruses in the non-hospitalized patient population remains unknown.
Additional limitations of the study are the exclusion of the data before 2012, due to the change in the methodology of respiratory virus detection in late 2011, from a less sensitive direct immunofluorescence assay with a smaller virus set to a more sensitive real-time polymerase chain reaction with a wider range of respiratory virus targets.
Acknowledgment
Ethical approval was not required because the study was conducted using only stored data from test results obtained from specimens originally collected for diagnostic purposes. In accordance with the principles of the Declaration of Helsinki, the Oviedo Convention on Human Rights and Biomedicine, and the Slovenian Code of Medical Deontology, all data on patients’ sex, age, and RV/COVID-19 results were linked only to randomized numerical codes. Because no additional samples or data were collected, the study was considered low-risk, and additional ethical approval by the National Medical Ethics Committee was waived.
Author Contribution
The concept of the study was created by T.U., R.K., and M.P. Data interpretation and analysis were performed by T.U., K.E., and R.K. The work was drafted by T.U., R.K., M.J.V., and M.P. The manuscript was critically revised by T.U., R.K., M.J.V., and M.P.
Conflict of Interest
The authors declare no conflict of interest.
Financial Disclosure
This study was partially funded by the Slovenian Research Agency (P3-0083).
References
- Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol 2020 Mar; 7(1):83-101. doi: 10.1146/annurev-virology-012420-022445. Epub 2020 Mar 20. PMID: 32196426.
- Audi A, AlIbrahim M, Kaddoura M, et al. Seasonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front Public Health 2020 Sep; 8:567184. doi: 10.3389/fpubh.2020.567184. PMID: 33042956 PMCID: PMC7522168
- Ursic T, Miksic NG, Lusa L, et al. Viral respiratory infections in a nursing home: a six-month prospective study. BMC Infect Dis 2016 Nov; 16(1):637. doi: 10.1186/s12879-016-1962-8. PMID: 27814689 PMCID: PMC5097393
- Olsen SJ, Winn AK, Budd AP, et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic - United States, 2020-2021. MMWR Morb Mortal Wkly Rep 2021 Jul; 70(29):1013-9. doi: 10.15585/mmwr.mm7029a1. PMID: 34292924 PMCID: PMC8297694
- Kuo SC, Shih SM, Chien LH, et al. Collateral Benefit of COVID-19 Control Measures on Influenza Activity, Taiwan. Emerg Infect Dis 2020 Aug; 26(8):1928-30. doi: 10.3201/eid2608.201192. Epub 2020 Apr 27. PMID: 32339091 PMCID: PMC7392415
- Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health 2020 May; 5(5):e279-e88. doi: 10.1016/S2468-2667(20)30090-6 Epub 2020 Apr 17.PMID: 32311320 PMCID: PMC7164922
- Lee H, Lee H, Song KH, et al. Impact of Public Health Interventions on Seasonal Influenza Activity During the COVID-19 Outbreak in Korea. Clin Infect Dis 2021 Jul; 73(1):e132-e40. doi: 10.1093/cid/ciaa672. PMID: 32472687 PMCID: PMC7314207
- Soo RJJ, Chiew CJ, Ma S, et al. Decreased Influenza Incidence under COVID-19 Control Measures, Singapore. Emerg Infect Dis 2020 Aug; 26(8):1933-5. doi: 10.3201/eid2608.201229. Epub 2020 Apr 27. PMID: 32339092 PMCID: PMC7392467
- Sakamoto H, Ishikane M, Ueda P. Seasonal Influenza Activity During the SARS-CoV-2 Outbreak in Japan. JAMA 2020 May; 323(19):1969-71. doi: 10.1001/jama.2020.6173. PMID: 32275293 PMCID: PMC7149351
- Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill 2021 Jul; 26(29). doi: 10.2807/1560-7917.ES.2021.26.29.2001600. PMID: 34296675 PMCID: PMC8299743
- Liu P, Xu M, Cao L, et al. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol J 2021 Aug; 18(1):159. doi: 10.1186/s12985-021-01627-8. PMID: 34344406 PMCID: PMC8329611
- Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. medRxiv 2021 Feb; doi: 10.1101/2020.11.11.20228692. PMID: 33579926 PMCID: PMC7881137
- Redlberger-Fritz M, Kundi M, Aberle SW, et al. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol 2021 Apr; 137:104795. doi: 10.1016/j.jcv.2021.104795. Epub 2021 Mar 16. PMID: 33761423 PMCID: PMC7962988
- Kuitunen I, Artama M, Makela L, et al. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland During Early 2020. Pediatr Infect Dis J 2020 Dec; 39(12):e423-e7. doi: 10.1097/INF.0000000000002845. PMID: 32773660
- Kuitunen I, Ponkilainen VT, Launonen AP, et al. The effect of national lockdown due to COVID-19 on emergency department visits. Scand J Trauma Resusc Emerg Med 2020 Dec; 28(1):114. doi: 10.1186/s13049-020-00810-0. PMID: 33276799 PMCID: PMC7716110
- Takashita E, Kawakami C, Momoki T, et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir Viruses 2021 Jul; 15(4):488-94. doi: DOI: 10.1111/irv.12854. Epub 2021 Mar 14. PMID: 33715290 PMCID: PMC8189209
- Savolainen-Kopra C, Korpela T, Simonen-Tikka ML, et al. Single treatment with ethanol hand rub is ineffective against human rhinovirus--hand washing with soap and water removes the virus efficiently. J Med Virol 2012 Mar; 84(3):543-7. doi: 10.1002/jmv.23222. PMID: 22246844
- Winther B, McCue K, Ashe K, et al. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J Med Virol 2007 Oct; 79(10):1606-10. doi: 10.1002/jmv.20956. PMID: 17705174
- Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020 May; 26(5):676-80. doi: 10.1038/s41591-020-0843-2. Epub 2020 Apr 3. PMID: 32371934 PMCID: PMC8238571
- Poole S, Brendish NJ, Clark TW. SARS-CoV-2 has displaced other seasonal respiratory viruses: Results from a prospective cohort study. J Infect 2020; 81(6):966-72. doi: 10.1016/j.jinf.2020.11.010. Epub 2020 Nov 15. PMID: 33207254 PMCID: PMC7666810
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.