Medicine Group . 2023 April 22;4(4):760-772. doi: 10.37871/jbres1734.
Association between Dietary Habits, Lifestyle and Migraine Attacks During Social Isolation in the COVID-19 Pandemic: A Systematic Review of Observational Studies
Luana de Oliveira Leite1,2*, Izabele Maria de Barros Lôbo1,2, Nathalia Herculano de Sousa1, Priscila Souza Capistano1, Hellen Maria Santos da Silva1, Lavínia Siqueira Pinho1, Lisiane Pereira dos Santos1, Hellen Vanessa de Carvalho Silva1, Jaqueline Araujo da Silva1 and Anoedrize Soares Oliveira Batista1
2Federal University of Bahia, School of Nutrition, Basilio da Gama St, Canela, Salvador, Bahia, Brazil
- Migraine disorder
- Headache
- Diet
- Lifestyle
- COVID-19
Abstract
Eating habits and lifestyle were the areas of life most affected by the COVID-19 pandemic, especially in patients with migraine, whose triggers for their crises are related to these factors. Thus, the aim of this study was to systematically review the association between eating habits, lifestyle and migraine attacks during social isolation in the COVID-19 pandemic. Therefore, a systematic review was carried out, developed in accordance with PRISMA and registered in PROSPERO nº CRD42022350308, with observational studies, which evaluated eating habits and lifestyle as exposure variables for the increase or alteration of migraine attacks during the pandemic of COVID-19 in adult patients diagnosed with migraine. Searches were performed in PubMed/MEDLINE, Web of Science, LILACS, and Google Scholar (gray literature) databases, and MESH and DECS database descriptors were used without language limits. 688 publications were identified, of which 11 met the inclusion criteria for data extraction, totaling, in the end, 3,256 respondents. The assessment of the methodological quality of the studies was performed using the Newcastle-Ottawa scale. The publications included were of low to moderate methodological quality, with a high risk of bias, and most found an association between lifestyle, eating habits and migraine attacks. Sleep disorders were most positively associated with migraine attacks, followed by eating habits. However, in most studies, there was no association between caffeine and migraine during the pandemic. We emphasize the need for more prospective, robust studies with better methodological quality to assess the impact of the COVID-19 pandemic on the association between eating habits, lifestyle and migraine attacks.
Introduction
The state of pandemic declared by the World Health Organization (WHO) in 2020, due to the emergence of the SARS-CoV-2 virus, caused the need for social isolation to contain the spread of the disease. In this context, the impacts of the health crisis significantly modified the way of life of the population worldwide. Since lifestyle was one of the most affected points, health and quality of life were negatively influenced after the drastic advance of the virus, especially for patients with migraines [1].
A migraine is a form of primary headache with a neurovascular and inflammatory profile, characterized by constant attacks of headache that can occur with variable frequency. Already recognized by the International Classification of Diseases (ICD), this clinical condition has a multifactorial etiology, being caused by different triggers, such as diet and lifestyle (sleep disorders, dehydration, sedentary lifestyle, alcoholism, smoking), in addition to hormonal and emotional issues, such as stress and anxiety, among others [2].
With regard to the impacts of social isolation in the COVID-19 pandemic on migraine patients, the new lifestyle has implied changes in eating habits, which can be influenced by internal and/or external issues. An increase in the consumption of more palatable foods, mostly ultra-processed foods that are rich in sugars, fats, and additives, and a reduction in the consumption of fruits, vegetables, and fiber can be observed [3]. In addition to food quality, in the pandemic context, the regularity of meals was also affected [4]. It is known that eating meals at irregular times and fasting for a long period of time can cause synaptic modification due to hypoglycemia, leading to the onset of migraines [5].
Another contributing factor is related to the change in the rhythm of life with social isolation, which contributed to a reduction in the level of physical activity and, consequently, an increase in sedentary lifestyle, which can contribute with the increase in migraine attacks [6]. In addition, assessing water intake becomes important, since low consumption can trigger a state of dehydration, which generates venous narrowing and loss of electrolytes, causing worsening of migraine attacks [7].
The change in eating habits and reduced physical activity, in association with stress and a higher level of fear and anxiety experienced during the pandemic, can be understood as factors responsible for inducing dysfunction in the circadian cycle, thus implying the physiological secretion of the hormone melatonin, which consequently leads to changes in sleep patterns [8]. It is worth noting that chronic sleep disruption is related to a predisposition to migraine attacks [9]. In addition, during the health crisis, there was a tendency to increase smoking and drinking habits [10,11], which can have a substantial effect on crises due to the presence of histamine and nicotine in alcoholic beverages and cigarettes, respectively [12].
Therefore, it appears that during the health crisis, migraine patients were widely affected due to sudden changes in lifestyle and eating habits, which are possible triggers for migraine attacks. It is therefore necessary to develop studies on this topic to expand care strategies for migraine patients. Therefore, in view of the high prevalence of migraines and the strong association with lifestyle and eating habits, this study aimed to systematically review the association between eating habits, lifestyle, and migraine attacks during social isolation in the COVID-19 pandemic, answering the following guiding question: “What is the association between eating habits, lifestyle, and migraine crises during social isolation in the COVID-19 pandemic?”.
Methodology
This is a systematic review, developed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and registered in PROSPERO (International prospective register of systematic reviews) under number CRD42022350308. The author LOL is responsible for this review; however, all authors contributed to the development of selection criteria, assessment, and risk of bias, as well as the search strategy. All authors provided comments and approved the final manuscript.
Participants, Exposure, Comparison, Outcome and Study Design (PECOS)
All observational studies that evaluated dietary habits (consumption of trigger foods) and lifestyle (regularity of meals, sleep disorders, physical activity, water intake, alcohol consumption, and smoking) were included as exposure variables for the increase or change in migraine attacks during social isolation in the COVID-19 pandemic in adult patients diagnosed with migraines. In this way, the prevalence or mean values of lifestyle and eating habits in the exposed group (meal irregularity, sleep disorders, sedentary lifestyle, smoking, low water intake, and consumption of trigger foods and alcohol) and in the unexposed group were compared regular meals, unaltered sleep pattern, physical activity, adequate water intake, low consumption of trigger foods and alcoholic beverages, and no smoking practice), or even identifying the degree of correlation between changes in eating habits (consumption of trigger foods) and lifestyle (regularity of meals, sleep disorders, physical activity, water intake, alcohol consumption, smoking) and migraine attacks (Chart 1).
| Chart 1: PECOS - criteria for inclusion and exclusion of studies. | |
| Parameters | Criteria |
| Population | Adult individuals diagnosed with migraines |
| Exposure | Irregularity of meals, sleep disorders, sedentary lifestyle, low water intake, and consumption of trigger foods and alcohol, and smoking |
| Comparator | Regular meals, unaltered sleep pattern, physical activity, adequate fluid intake, low consumption of trigger foods and alcohol, and no smoking |
| Outcomes | Increase or changes in migraine attacks (any parameter) during the COVID-19 pandemic |
| Setting or study design | Observational studies |
Inclusion and non-inclusion criteria
Studies that evaluated the association between eating habits (consumption of trigger foods), lifestyle (regularity of meals, sleep disorders, physical activity, water intake, alcohol consumption, smoking), and migraine attacks during social isolation in the COVID-19 pandemic were searched. Language restrictions have not been applied. It is noteworthy that the stipulated time was the one referring to the beginning of the COVID-19 pandemic by the WHO in March [13]. Studies included in the systematic review met the following criteria: (1) observational (cohort, case-control, or cross-sectional); (2) the population consists of adult individuals (≥ 18 years old) diagnosed with migraines; (3) exposure related to changes in eating habits (consumption of trigger foods) and lifestyle (regularity of meals, sleep disorders, physical activity, water intake, alcohol consumption, smoking); (4) the outcome being related to migraine attacks (regardless of the parameter used); and (5) provide at least the Relative Risk (RR), Prevalence Ratio (PR), or Odds Ratio (OR) with their Confidence Intervals (CIs) for categorical risk estimates, mean values and standard deviation, or the correlation coefficient with respective p-values for continuous variables. Studies in which individuals were selected by a group of chronic or high-risk diseases, in addition to interventional research, were not included, as well as clinical trials, in vitro and in vivo studies, review and/or case reports, and studies that did not assess the outcome.
Information sources
The articles were searched in electronic bibliographic databases: PubMed/MEDLINE (Medical Literature Analysis and Retrieval System Online/PubMed), Web of Science, LILACS (Scientific and Technical Literature of Latin America and the Caribbean) and Google Scholar (grey literature). To ensure literature saturation, reference lists of included studies or relevant reviews manually identified through the search were analyzed to add studies that were not indexed in the databases but would still be relevant for inclusion in this review. The authors’ personal files were also searched to ensure that all relevant material was captured. For permission of the content available in the databases, remote access was used via the Federated Academic Community (CAFe) of the Coordination for the Improvement of Higher Education Personnel (CAPES) Periodicals Portal, according to the content signed by the portal available to the University of the State of Bahia.
Search strategy
Observational studies were searched in the aforementioned databases. Language limits were not imposed on the search, but a search filter for time (start of the COVID-19 pandemic in March 2020) was used. The terms for outcome (migraine and COVID-19) and population (adults aged 18+ years with migraines) and their synonyms were used in the search strategy with the aim of including all relevant studies on this topic. Descriptors for specific outcome and population for each database are presented in appendix.
The Boolean operators “AND” and “OR” were adopted to search the databases. The use of Boolean expressions allows a comprehensive search for several studies in different databases. This form of research increases the possibility of finding relevant articles on the topic, because, when it comes to health care, the possibility of results should not be neglected [14]. Some artifices were used to construct the search, like symbols, such as truncation (*), and the use of quotation marks and parentheses according to each database. The descriptors from the Medical Subject Headings (MESH) database were selected, and sensitization was chosen with the inclusion of “entry terms”, uncontrolled vocabulary. Thus, Boolean word combinations were developed for searching in databases that use the MESH descriptors (PubMed/MEDLINE, Web of Science, and Google Scholar) (Appendix A1). As for the search in the LILACS database, the descriptors selected from the Health Sciences Descriptors (DeCS) from the Virtual Health Library (BVC) were used, and Boolean expressions of words were developed for research in this database (Appendix A2).
Data management
The results of the literature search were uploaded to Endnote Reference Management in its online version, a software program that identifies duplicates, facilitating collaboration between reviewers during the study selection process. However, the articles that the program did not detect as duplicates because they were attached to the databases in different ways, were manually eliminated by the reviewers.
Selection, extraction and synthesis of data
The articles identified in the databases, which met the eligibility criteria for the studies, were selected by five independent reviewers. Titles and abstracts were read and those that did not meet the selection criteria were excluded. All studies that were selected in the previous phase had their eligibility confirmed by reading the full article. At this stage, the primary reason for exclusion was recorded to compose the article selection flow. To guide this phase, a previously prepared standardized eligibility assessment form was used, which basically contains the established eligibility criteria (inclusion and exclusion).
As relevant studies were identified, the reviewers checked other cited articles. The differences identified were resolved by the main researcher in a consensus meeting. The research team jointly concluded which studies were ultimately selected for data synthesis. None of the review authors were blinded to journal titles or study authors or institutions. From the eligible studies, data were independently extracted using a spreadsheet prepared in the Microsoft Office® Excel program. The following items were extracted from the included studies: authors, journal, year of publication, country, sample size, gender, age or mean age, statistical approach used, exposure (consumption of trigger foods, meal irregularity, sleep disorders, physical activity, low water intake, alcohol consumption, and smoking), instruments used to assess exposure, outcome (migraine-related), instruments used to assess the outcome, and conclusions on the association between exposure and outcome. Due to the great heterogeneity of the included studies, especially regarding the tools used to assess exposures and the outcome and statistical analyses, it was not possible to perform a quantitative synthesis of the data, so a narrative synthesis was performed.
Assessment of methodological quality and risk of bias
The evaluation of the methodological quality of the studies was carried out using the Newcastle-Ottawa scale, which consists of 8 questions inserted in 3 main domains (participant selection, participant comparability, and outcome/exposure assessment). The maximum total score that each cohort study could receive for this scale was 9 stars (2 stars for comparability). In the version adapted for cross-sectional studies, there are 6 questions, and the maximum possible total score is 7. In the present systematic review, studies were classified as having methodological quality when they received 7 stars (cross-sectional studies) or 9 stars (cohort and case studies) [15]. All studies, regardless of their quality score, were included in this review.
Results
Literary search
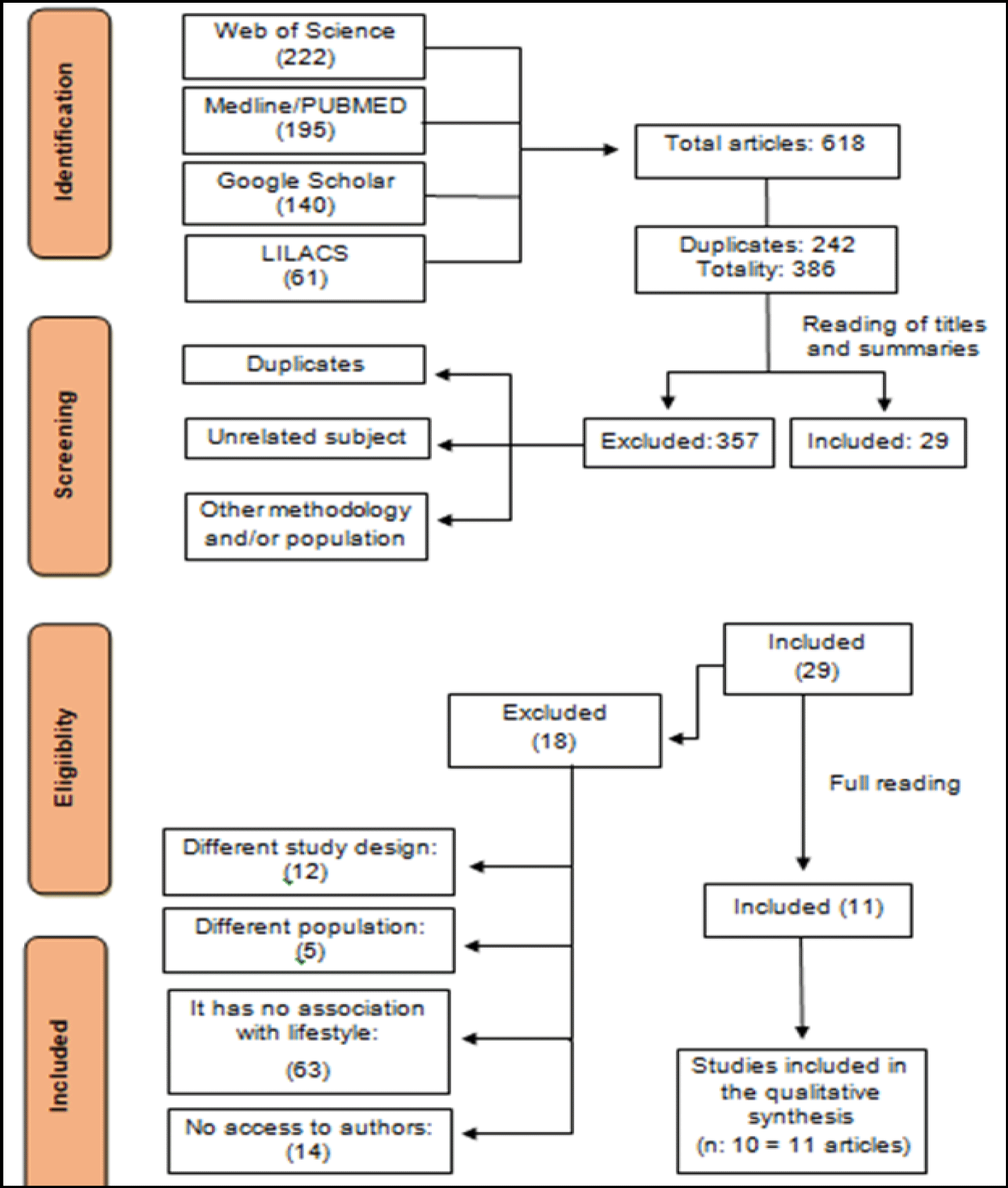
The detailed steps of the search and selection of studies, carried out between November 2021 and January 2022 and updated in April 2022, are presented in a flowchart below figure 1. After applying the search strategy, a total of 618 potentially relevant publications were identified: 195 in the PubMed/MEDLINE database, 222 in the Web of Science database, 140 in Google Scholar, and 61 in the LILACS database.
Database results were pooled and imported into Endnote Reference Management in its free online version. Two hundred and thirty-two duplicates and 386 articles were manually identified by the reviewers for reading abstracts and titles. After reading the abstracts and titles, a total of 357 studies were excluded for not meeting the inclusion criteria. Thus, 29 articles were read in full. After reading all of the full texts, 18 articles were excluded and 11 articles were selected for analysis of the theme in question, as they met the inclusion criteria, resulting in a total of 11 articles for data extraction from 10 different studies.
Characteristics of the studies
Table 1 (included as supplementary file) describes the characteristics of the studies included in this review.
Period, country of correspondence of the authors/performance of the studies, and journals: The studies included were published in English, starting in 2020, after the beginning of the SARS-CoV-2 pandemic, and studies from different continents were found, namely: Europe (n = 6) with studies by Currò CT, et al. [16], Cola F, et al. [17], Gonzalez-Martinez A, et al. [2], Di Stefano V, et al. [18], Smith M, et al. [19], and Granato A, et al. [6]; Asia (n = 3) with studies by Suzuki K, et al. [20], Al-Hashel JY, et al. [1], and Togha M, et al. [21]; and America (n = 1) with a study by Silva HMS, et al. [22]. The articles come from 11 different journals.
Study design: All included studies had a cross-sectional design.
Sample: In total, this review included 3,256 participants (including both genders), aged between 18 and 77 years. As for the sample of the studies, the total number of participants included varied between 37 [6] and 1,018 [1], with females being predominant in 9 studies (90%) that contained the details of the sample, while 1 study (10%) did not have this information. The 10 studies included did not present information on the time of the diagnosis of migraines for the participants; in contrast, in 7 studies (70%) [1,6,16-20], patients were followed up through treatment centers or outpatient clinics for migraines or used prophylactic drugs as a form of treatment.
Exposures: Most of the studies included in this review evaluated sleep disturbances, food triggers, and physical activity as exposure variables. According to table S1, sleep disturbance was assessed in most of the articles (n = 9; 90%) included. Subsequently, most also evaluated food triggers (n = 8; 80%) and physical activity (n = 7; 70%). Meanwhile, the regularity of meals (n = 4; 40%), smoking (n = 3; 30%), alcohol consumption (n = 3; 30%), and water intake (n = 2; 20%) were less studied. Exposures were assessed using non-validated instruments, such as an online questionnaire and telephone interviews but also using validated instruments, such as the Pittsburgh Sleep Quality Index (PSQI) for sleep, the International Physical Activity Questionnaire Short-Form (IPAQ-SF) (adapted) to assess physical activity, a Food Frequency Questionnaire (FFQ) (adapted) to assess eating habits, and the Insomnia Severity Index (ISI) for insomnia.
Outcomes: The migraine parameters analyzed were frequency, intensity, pain impact, pain duration, time of diagnosis, symptoms, and duration of attacks, with the frequency and intensity of attacks being the two most evaluated factors by most of the studies included in this review. The frequency of migraines was present in 90% of the studies [1,2,6,16-19,21,22]; in terms of intensity, it was evaluated in 30% of the studies [1,2,16]. However, there was no standardization in the parameters and instruments evaluated.
Association between sleep disorders and migraines
Regarding the association between sleep disorders and migraines during the health crisis, 9 studies evaluated this lifestyle factor. Seven studies found a positive association with the frequency of migraine attacks, while one showed a relationship with intensity [1,2,6,16-19,21,22]. Currò CT, et al. [16] showed a positive association regarding the impact of pain. However, the studies by Gonzalez-Martinez A, et al. [2] and Granato A, et al. [6] demonstrated no association between pain frequency and sleep parameters.
Association between consumption of trigger foods and migraines
Among the analyzed scientific studies (n = 10), the majority (n = 8) were associated with the consumption of trigger foods and migraine. As for the frequency of migraine, only 3 studies found a positive association with the consumption of trigger foods [1,18,21]. Regarding intensity, a single study showed a positive association [1]. The impact of pain, duration of attacks, and symptoms of migraines showed no association with trigger foods [6,16].
Association between physical activity and migraines
Most (n = 7) of the analyzed studies (n = 10) were associated with physical activity and migraines. A positive association was found for the frequency of migraine attacks in 3 of the studies [17,18,21]. Regarding the impact of pain, duration of attacks, and symptoms, the study by Granato A, et al. [6] found no association for migraine and physical activity and Al-Hashel JY, et al. [1] found no association as for pain intensity.
Association between regularity of meals and migraines
Among the analyzed studies (n = 10), few (n = 4) made an association with regularity of meals. As for the frequency of migraines, only two studies found a positive association with the regularity of meals [17,21]. In relation to intensity, as well as duration and impact of pain, a single studyshowed a positive association [16].
Association between alcoholism and migraines
A small number of the analyzed studies (n = 3) explored the relationship between alcoholism and migraines, with no association in all studies [6,19,22]. In these evaluated studies, the following parameters for migraine were used: daily frequency of pain [19,22], monthly frequency, impact of pain, duration of attacks, and symptoms [6].
Association between smoking and migraines
Only 3 of the analyzed studies were related to smoking and migraines. Only one study identified a positive association between smoking and the frequency and impact of migraines [16].
Association between water intake and migraines
Only 2 of the analyzed studies explored the association between water intake and migraines: Granato A, et al. [6] and Silva HMS, et al. [22]. They found no association with the parameters of migraines analyzed, namely: monthly frequency, pain impact, duration of attacks, symptoms [6], and daily frequency [22].
Assessment of methodological quality
After applying the Newcastle-Ottawa scale [15], it was possible to observe the predominance of the risk of bias related to the outcome, given that due to the pandemic and the need for social isolation, the studies carried out this determination through self-report via online questionnaires. It is also worth highlighting the fact that most of the studies used convenience samples (as volunteers), allowing risk of bias due to an unrepresentative sample, as well as using non-validated tools to determine exposure.
The low quality identified in the research by Togha M, et al. [21] may be associated with the fact that the work available was a summary published in the proceedings of the International Headache Congress (2021) (grey literature), which implied a scarce amount of exposed data that were necessary for a more accurate evaluation of methodological quality.
In short, as mentioned above, the studies that received 7 stars on the aforementioned scale would be considered as having methodological quality, a score that was not achieved by any of the analyzed studies. More studies related to the theme, with greater methodological rigor, should be encouraged. The complete risk of bias analysis of the studies selected for the review can be seen in table 2.
| Table 2: Assessment of the methodological quality of the studies included in the review. | ||||
| Reference | Selection | Comparability | Outcome | Totalª |
| Al-Hashel JY, et al. [1] | - b | ** | * | 3 |
| Cola F, et al. [17] | * | ** | * | 4 |
| Currò CT, et al. [16] | * | ** | - b | 3 |
| Di Stefano V, et al. [18] | ** | ** | - b | 4 |
| Gonzalez-Martinez A, et al. [2] | * | ** | * | 4 |
| Granato A, et al. [6] | - b | ** | - b | 2 |
| Silva HMS, et al. [22] | * | ** | - b | 3 |
| Smith M, et al. [19] | * | ** | * | 4 |
| Suzuki K, et al. [20] | *** | ** | * | 6 |
| Togha M, et al. [21] | - b | - b | - b | 0 |
| Caption: aCross-sectional studies: maximum score of 7 stars; moderate quality: 4 to 6 stars; low quality: ≤ 3 stars. bDid not score. |
||||
Discussion
The results obtained in the present review mainly found an association between sleep disorders and migraine attacks during the social isolation in the COVID-19 pandemic. Eating habits and physical activity were the other most studied factors in relation to headache attacks during the health crisis. In addition, studies originating mainly from Europe (50%) were reviewed. The propensity for research on the subject can be justified by the fact that in that continent, headaches are the second cause for “years lived with disability”, according to data from the Global Burden of Disease Study (GDB), which is capable of having a major impact on public health [23].
Discussing GBD data, it was reported that, in relation to age, young adult women (15-49 years) are the most affected by migraines. In the aforementioned research, the disease occupied the second place related to disability, only behind gynecological diseases. Such data are close to those seen in this review, where the studies that provided the mean age of the sample group showed values from 42.3 to 45.2 years for the age group, and in the analyzed populations, women also predominated. Young adults and women appear to be more prone to migraines [24-27]. Through a literature review, Delaruelle Z, et al. [27] observed that primary headaches occur more frequently in females of reproductive age. For the authors, headache patterns evolve over time and are correlated with hormonal changes throughout life, since sex hormones have effects on the nervous system and affect important brain areas.
Sleep disorders were evaluated by 90% of the studies that composed the current work, and it was possible to find positive relationships between these disorders and the frequency and intensity of migraines. In this aspect, Buse DC, et al. [28] observed that migraine subjects had a triple chance of insomnia through a prospective longitudinal cross-sectional study. Likewise, the intensity of the headache was related to a greater risk for the disorder.
According to Souza LFF, et al. [29], the COVID-19 pandemic, with the imposition of social isolation, contributed as a risk factor related to mental health, which directly impacts sleep quality. In this way, the state of reclusion and the public health situation may have acted as drivers for this factor related to migraines, in view of the occurrence of an association between migraines, anxiety, and sleep disorders [30]. As exposed by Korabelnikova EA, et al. [31], migraines and sleep disorders have a complex bidirectional relationship, both as a cause and aggravation, which is due to the sharing of neurobiological pathways. The authors reinforce that anxiety worsens this relationship, resulting in a decrease in quality of life. Thus, diagnosing and treating sleep-related comorbidities should be part of the goals included in the treatment of migraines, since better sleep quality is crucial for reducing the severity and recurrence of headache attacks [32,33].
Most studies (70%) also evaluated the association between eating habits and worsening of migraines, due to the idea that food is a trigger of migraine episodes, in addition to the context of the pandemic that influenced the emotional eating developed by some individuals in situations of stress and anxiety. This leads to an increase in the consumption of foods considered palatable and often chosen in an attempt to seek comfort, which are also known as potential triggers for migraine attacks [22]. However, only three studies identified a positive association between these food triggers and migraine attacks, which can be explained by the low methodological quality and high risk of bias in the publications included that may have contributed to the absence of further associations.
According to Finkel AG, et al. [34], dietary changes can cause chronic implications in almost all migraine subtypes regardless of the nutrient, and the characteristics in the individual’s behavior can make it possible to make choices, conscious or not, that reduce or provoke the ability of migraine triggers. Work by Di Stefano V, et al. [18] showed increased consumption of carbohydrate-rich foods and sweets in all groups of migraine patients during social distancing but also a positive correlation between increased consumption of dairy or fruit in the general group and subgroup of patients who reported a stable headache. According to the authors, the lockdown may have contributed to a lower sensitivity of migraine patients in relation to food triggers, making it difficult for migraines to worsen.
Among the food triggers, caffeinated beverages, including coffee, were the most studied in this review. Alcoholic and sugar-rich drinks were also evaluated as triggers. In most of the included articles that evaluated caffeinated beverages as migraine triggers [6,16,22], coffee consumption was not associated with pain duration, intensity, impact, or frequency of migraine attacks. However, the study by Toghaet M, et al. [21] showed a positive association between migraine attacks and consumption of caffeinated beverages. Headaches caused by vasodilation have symptom relief with caffeine, which has the property of constricting blood vessels [35]. According to Zhang, caffeine may exhibit antinociceptive actions by blocking adenosine receptors, inhibiting the synthesis of the cyclooxygenase-2 enzyme or by changing the emotional state [36].
Given the complexity of triggers for migraine attacks, including food, complementary non-pharmacological strategies or treatment alternatives aim to improve migraine control and, consequently, functional capacity with minimized adverse effects, compared to prophylactic pharmacological treatment [37]. In this context, moderate-intensity aerobic exercise is also suggested for pain modulation in prophylactic treatment, with a possible short- and long-term analgesic effect at the central and peripheral levels [37,38].
In a study by Di Stefano V, et al. [18], a significant decrease in physical activity levels during COVID-19 quarantine was observed in the entire study sample, due to the isolation imposed by the pandemic. During the period of social distancing, 28% of patients reported worsening headache, 33% reported improvement, and 39% reported a stable headache frequency. The study by Cola et al. reported that the negative impact of the pandemic on migraines implied a change in the daily routine of individuals, causing physical inactivity and irregularity of meals [17].
As for the study of Togha M, et al. [21], the reduction in physical activity was significantly more reported by individuals who had increased migraine attacks during the pandemic, and decreased hours of sleep, consumption of caffeinated beverages, and regularity of meals were also reported. Regarding the latter, prolonged fasting is one of the most cited triggers of migraines [5,39,40]. Therefore, 40% of the studies in this current review evaluated the association between migraines and meal regularity, obtaining findings of a positive association [16,17,21].
In a cross-sectional study, Curró CT, et al. [16] found that individuals who reduced the regularity of meals during social isolation had a longer duration of migraines. It is known that eating at regular times, avoiding fasting, and maintaining adequate food in quantity and quality is important to avoid migraines [41-43]. This may be related to hypoglycemia, which causes the brain to not function properly, as the organ is dependent on glucose for energy and to fulfill its functions. Unfavorable conditions increase blood flow to obtain more glucose, leading to vasodilation, which can cause headaches [39,40,43].
A higher frequency of migraine days is associated with irregular meals [17,21]. According to Cola et al., those patients who modified their eating habits during social isolation, avoiding fasting and binge eating, were in the group that had improved migraine attacks compared to the others [17]. It is already established that the modification of eating habits and behaviors, aimed at improving migraine attacks, can have a significant effect on both the reduction of days and the severity of pain, thus promoting a better quality of life for these patients [40,42], since triggering factors of the disease are associated with metabolic disorders and oxidative stress [43].
Although the investigation of this relationship was present in a small portion of the analyzed studies (30%), with an association absent in all, alcohol consumption is reported as one of the 10 biggest triggers for migraines [44], and the period of the pandemic seems to have increased its consumption, mainly related to anxiety [10,45,46]. Recent literature reviews on food triggers for migraines point to alcohol consumption as a common triggering factor for increased frequency of attacks [42,47,48]. Alcoholic beverages, especially red wine, are described as triggers for the onset of migraine attacks [49-52]. According to the scientific literature, the relationship involves the action of biogenic amines, sulfites, and phenolic flavonoids present in such beverages, their vasodilating effects, and mechanisms linked to 5-hydroxytryptamine [49,51]. In a review, Martins et al. observed that alcohol-induced headache can be immediate or delayed, and the doses needed to trigger the attacks are variable [53].
In this review, only three articles performed the analysis between migraines and smoking, with two studies reporting that smoking had no association with migraine [6,17], in disagreement with Curró CT, et al. [16], who showed a positive association with an increase in days of migraine attacks, corroborating other findings in the literature [54-57]. There are some factors that can trigger migraines in smokers and worsen the pattern of pain compared to non-smokers, such as high levels of carboxyhemoglobin in smokers. In addition, nicotine can accelerate the metabolism of some drugs, such as caffeine, propranolol, and imipramine, as well as influence the neuroendocrine increase and serotonin turnover. However, it is worth mentioning that nicotine withdrawal can also lead the individual to have headaches [54-56].
Although a migraine is a primary headache and the mechanisms of relationship between headaches and water intake are multifactorial and variable and its pathophysiology is not completely understood [7], a correlation has already been found between increased water intake and improvement in migraine severity, pain intensity, frequency, and duration of attacks. Some hypotheses are suggested, including the fact that some triggers for migraine, such as alcohol intake, sleep disturbances, and stress, are possibly affected by water balance. In addition to increasing water consumption, it reduces osmolarity and balances electrolyte concentration [58]. It is also speculated that water scarcity can cause dural venous stretching and hypertonicity, leading to traction on vascular structures and pain-sensitive meninges, and that the pain threshold in dehydrated people is lower [7]. However, in the two studies evaluated by the present review, it was not possible to identify the level of dehydration or hypohydration of the individuals, considering that the range to consider low water intake was large (between 0 and 1.5 L) and did not address other signs and symptoms of dehydration.
In summary, sleep disorders and eating habits were the main evaluated factors for the worsening of migraine attacks. Therefore, better identifying these migraine triggers, as well as others (alcohol consumption, dehydration, fasting, physical exercise) from detailed and up-to-date notes of these triggers, can help to avoid or modify them to some extent [59]. However, to assess migraines, Granato A, et al. [6], Curró CT, et al. [16] and Suzuki K, et al. [20] were the only studies that used validated instruments that are useful to measure the disability impact that migraine has on quality of life, such as the Headache Impact Test (HIT-6) and the Migraine Disability Assessment Scale (MIDAS) [60-62].
Regarding the non-association between migraine and factors such as alcoholism, smoking and water consumption, observed in some studies included in the present review, but with an association present in other studies in the scientific literature, this can be explained by the small number of studies that analyzed such correlations. In addition, it should be noted that the studies had gaps in methodological quality and a high risk of bias in terms of information, selection, and representativeness of the sample. It is also known that cross-sectional studies are limiting for identifying causal relationships and have not ensured that confounding factors are equally distributed between groups. In addition, some studies have associated a reduction in the duration of pain in the period of confinement, less contact with stressors, and the possibility of resting at the time of the migraine attack, due to being at home. Therefore, these are limitations to the evidence produced by the current review.
Other studies with greater methodological rigor that investigate triggers related to lifestyle and migraines, especially during and after the COVID-19 pandemic, investigating the repercussions generated by the quarantine period, should be encouraged so that more effective measures are formulated for the treatment of migraines. However, the importance of the findings of this review is highlighted because it is unprecedented and has fulfilled all the steps described in the methodology to avoid bias. Selection, data extraction, and quality and bias assessment were performed independently by the researchers.
Conclusion
This systematic review identified that sleep disorders, eating habits, and physical activity were the main triggers studied in relation to migraine during the period of social isolation caused by the SARS-CoV-2 virus pandemic, with changes related to sleep patterns being the most frequent were associated with worsening of migraine attacks. It is noteworthy that the frequency and intensity of migraines were the most used parameters to assess attacks. Among food triggers, no association was found between caffeine and migraine attacks in most studies. However, caution is recommended in the interpretation of these data, as the inclusion of only cross-sectional studies (with methodological limitations) compromises the quality of the evidence, as already described, emphasizing the need to develop more prospective, robust studies with better methodological quality that evaluate the repercussions of the COVID-19 pandemic on the association between eating habits, lifestyle, and migraine attacks.
Acknowledgment
The authors LOL, IMBL, LPS, ASOB, and PSC contributed substantially to the conception and design of the study, data acquisition, analysis, and interpretation. The authors HMSS, NHS, LSP, HVCS, and JAS were responsible for the search and also selected the studies. The authors IMBL, NHS, and JAS were responsible for analyzing the methodological quality and risk of bias, in addition to extracting data from the selected studies. In the stages of study selection, analysis of quality and risk of bias, and data extraction, the differences identified were resolved by the author LOL in a consensus meeting. LOL, IMBL, LPS, ASOB, PSC, HMSS, NHS, LSP, HVCS, and JAS wrote the manuscript, and LOL provided critical review of the article, as well as final approval of the version to be published.
Funding
This research has not received any funding for its realization.
Conflicts of Interest
There are no conflicts of interest related to this research.
References
- Al-Hashel JY, Ismail II. Impact of coronavirus disease 2019 (COVID-19) pandemic on patients with migraine: a web-based survey study. J Headache Pain. 2020 Sep 24;21(1):115. doi: 10.1186/s10194-020-01183-6. PMID: 32972360; PMCID: PMC7513457.
- Gonzalez-Martinez A, Planchuelo-Gómez Á, Guerrero ÁL, García-Azorín D, Santos-Lasaosa S, Navarro-Pérez MP, Odriozola-González P, Irurtia MJ, Quintas S, de Luis-García R, Gago-Veiga AB. Evaluation of the Impact of the COVID-19 Lockdown in the Clinical Course of Migraine. Pain Med. 2021 Sep 8;22(9):2079-2091. doi: 10.1093/pm/pnaa449. PMID: 33659991; PMCID: PMC8108628.
- Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, Leggeri C, Caparello G, Barrea L, Scerbo F, Esposito E, De Lorenzo A. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020 Jun 8;18(1):229. doi: 10.1186/s12967-020-02399-5. PMID: 32513197; PMCID: PMC7278251.
- Aguilar-Martínez A, Bosque-Prous M, González-Casals H, Colillas-Malet E, Puigcorbé S, Esquius L, Espelt A. Social Inequalities in Changes in Diet in Adolescents during Confinement Due to COVID-19 in Spain: The DESKcohort Project. Nutrients. 2021 May 8;13(5):1577. doi: 10.3390/nu13051577. PMID: 34066867; PMCID: PMC8151229.
- Marmura MJ. Triggers, Protectors, and Predictors in Episodic Migraine. Curr Pain Headache Rep. 2018 Oct 5;22(12):81. doi: 10.1007/s11916-018-0734-0. PMID: 30291562.
- Granato A, Furlanis G, D'Acunto L, Olivo S, Buoite Stella A, Manganotti P. Lifestyle impact on migraine during home confinement. Acta Neurol Belg. 2022 Apr;122(2):497-503. doi: 10.1007/s13760-021-01856-2. Epub 2022 Feb 11. PMID: 35146703; PMCID: PMC8831145.
- Arca KN, Halker Singh RB. Dehydration and Headache. Curr Pain Headache Rep. 2021 Jul 15;25(8):56. doi: 10.1007/s11916-021-00966-z. PMID: 34268642; PMCID: PMC8280611.
- Elmacıoğlu F, Emiroğlu E, Ülker MT, Özyılmaz Kırcali B, Oruç S. Evaluation of nutritional behaviour related to COVID-19. Public Health Nutr. 2021 Feb;24(3):512-518. doi: 10.1017/S1368980020004140. Epub 2020 Oct 19. PMID: 33070798; PMCID: PMC7737137.
- Grozeva V, Mínguez-Olaondo A, Vila-Pueyo M. Experiment in vivo: How COVID-19 Lifestyle Modifications Affect Migraine. Front Neurol. 2021 Oct 5;12:744796. doi: 10.3389/fneur.2021.744796. PMID: 34707560; PMCID: PMC8544242.
- Valente JY, Sohi I, Garcia-Cerde R, Monteiro MG, Sanchez ZM. What is associated with the increased frequency of heavy episodic drinking during the COVID-19 pandemic? Data from the PAHO regional web-based survey. Drug Alcohol Depend. 2021 Apr 1;221:108621. doi: 10.1016/j.drugalcdep.2021.108621. Epub 2021 Feb 16. PMID: 33636598; PMCID: PMC9759720.
- Malta DC, Gomes CS, Souza Júnior PRB, Szwarcwald CL, Barros MBA, Machado ÍE, Romero DE, Lima MG, Silva AGD, Prates EJS, Cardoso LSM, Damacena GN, Werneck AO, Silva DRPD, Azevedo LO. Factors associated with increased cigarette consumption in the Brazilian population during the COVID-19 pandemic. Cad Saude Publica. 2021 Apr 7;37(3):e00252220. English, Portuguese. doi: 10.1590/0102-311X00252220. PMID: 33852666.
- Iglesias HCE, Bottura R, Naves MMV. Fatores nutricionais relacionados à enxaqueca. Com Ciências Saúde. 2009; 20:229-240.
- WHO. WHO Director-General's opening remarks at the media briefing on COVID-19. In: World Health Organization. 2020.
- Pohl S, Zobel J, Moffat A. Extended Boolean retrieval for systematic biomedical reviews. Australian Computer Society. 2010;102:117-126.
- Wells GA, Wells G, Shea B, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In: Ottawa Hospital Research Institute. 2014.
- Currò CT, Ciacciarelli A, Vitale C, Vinci ES, Toscano A, Vita G, Trimarchi G, Silvestri R, Autunno M. Chronic migraine in the first COVID-19 lockdown: the impact of sleep, remote working, and other life/psychological changes. Neurol Sci. 2021 Nov;42(11):4403-4418. doi: 10.1007/s10072-021-05521-7. Epub 2021 Aug 8. PMID: 34365547; PMCID: PMC8349308.
- Schiano di Cola F, Caratozzolo S, Di Cesare M, Liberini P, Rao R, Padovani A. Migraine Monitoring in the Time of COVID-19: Triggers and Protectors During a Pandemic. Pain Med. 2021 Nov 26;22(11):2728-2738. doi: 10.1093/pm/pnab202. PMID: 34181002.
- Di Stefano V, Ornello R, Gagliardo A, Torrente A, Illuminato E, Caponnetto V, Frattale I, Golini R, Di Felice C, Graziano F, Caccamo M, Ventimiglia D, Iacono S, Matarazzo G, Armetta F, Battaglia G, Firenze A, Sacco S, Brighina F. Social Distancing in Chronic Migraine during the COVID-19 Outbreak: Results from a Multicenter Observational Study. Nutrients. 2021 Apr 19;13(4):1361. doi: 10.3390/nu13041361. PMID: 33921674; PMCID: PMC8074143.
- Smith M, Nakamoto M, Crocker J, Tiffany Morden F, Liu K, Ma E, Chong A, Van N, Vajjala V, Carrazana E, Viereck J, Liow K. Early impact of the COVID-19 pandemic on outpatient migraine care in Hawaii: Results of a quality improvement survey. Headache. 2021 Jan;61(1):149-156. doi: 10.1111/head.14030. Epub 2020 Dec 14. PMID: 33316097.
- Suzuki K, Takeshima T, Igarashi H, Imai N, Danno D, Yamamoto T, Nagata E, Haruyama Y, Mitsufuji T, Suzuki S, Ito Y, Shibata M, Kowa H, Kikui S, Shiina T, Okamura M, Tatsumoto M, Hirata K. Impact of the COVID-19 pandemic on migraine in Japan: a multicentre cross-sectional study. J Headache Pain. 2021 Jun 7;22(1):53. doi: 10.1186/s10194-021-01263-1. PMID: 34098873; PMCID: PMC8182734.
- Togha M, Martami F, Jafari E, Ebadi S. Evaluation of changes in migraine headache patterns and characteristics before and after the pandemic of COVID-19 disease. Cephalalgia. 2021;41:1-227. doi: 10.1177/033310242110340.
- Silva HMS, Sousa NH, Araujo APPQ, Sousa MGC, Santos ACJ, Pires ABJ, Cardoso ES, Leite LO. Association between eating behavior and lifestyle habits and increase in migraine attacks in university students during COVID-19 pandemic. Headache Med. 2021;12(4). doi: 10.48208/HeadacheMed.2021.50.
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z; Lifting The Burden: the Global Campaign against Headache. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020 Dec 2;21(1):137. doi: 10.1186/s10194-020-01208-0. PMID: 33267788; PMCID: PMC7708887.
- Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015 Jan;55(1):21-34. doi: 10.1111/head.12482. Erratum in: Headache. 2015 Feb;55(2):356. PMID: 25600719.
- El-Metwally A, Toivola P, AlAhmary K, Bahkali S, AlKhathaami A, Al Ammar SA, Altamimi IM, Alosaimi SM, Jawed M, Almustanyir S. The Epidemiology of Migraine Headache in Arab Countries: A Systematic Review. ScientificWorldJournal. 2020 Jun 16;2020:4790254. doi: 10.1155/2020/4790254. PMID: 32607079; PMCID: PMC7315321.
- Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, Sullman MJM, Kolahi AA, Safiri S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front Neurol. 2022 Feb 23;12:800605. doi: 10.3389/fneur.2021.800605. PMID: 35281991; PMCID: PMC8904749.
- Delaruelle Z, Ivanova TA, Khan S, Negro A, Ornello R, Raffaelli B, Terrin A, Mitsikostas DD, Reuter U; European Headache Federation School of Advanced Studies (EHF-SAS). Male and female sex hormones in primary headaches. J Headache Pain. 2018 Nov 29;19(1):117. doi: 10.1186/s10194-018-0922-7. PMID: 30497379; PMCID: PMC6755575.
- Buse DC, Reed ML, Fanning KM, Bostic R, Dodick DW, Schwedt TJ, Munjal S, Singh P, Lipton RB. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2020 Mar 2;21(1):23. doi: 10.1186/s10194-020-1084-y. PMID: 32122324; PMCID: PMC7053108.
- Souza LFF, Paineiras-Domingos LL, Melo-Oliveira MES, Pessanha-Freitas J, Moreira-Marconi E, Lacerda ACR, Mendonça VA, Sá-Caputo DDC, Bernardo-Filho M. The impact of COVID-19 pandemic in the quality of sleep by Pittsburgh Sleep Quality Index: A systematic review. Cien Saude Colet. 2021 Apr;26(4):1457-1466. doi: 10.1590/1413-81232021264.45952020. Epub 2021 Jan 17. PMID: 33886773.
- Boardman HF, Thomas E, Millson DS, Croft PR. Psychological, sleep, lifestyle, and comorbid associations with headache. Headache. 2005 Jun;45(6):657-69. doi: 10.1111/j.1526-4610.2005.05133.x. PMID: 15953298.
- Korabelnikova EA, Danilov AB, Danilov AB, Vorobyeva YD, Latysheva NV, Artemenko AR. Sleep Disorders and Headache: A Review of Correlation and Mutual Influence. Pain Ther. 2020 Dec;9(2):411-425. doi: 10.1007/s40122-020-00180-6. Epub 2020 Jul 3. PMID: 32621175; PMCID: PMC7648824.
- Vgontzas A, Pavlović JM. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache. 2018 Jul;58(7):1030-1039. doi: 10.1111/head.13358. Epub 2018 Aug 8. PMID: 30091160; PMCID: PMC6527324.
- Tiseo C, Vacca A, Felbush A, Filimonova T, Gai A, Glazyrina T, Hubalek IA, Marchenko Y, Overeem LH, Piroso S, Tkachev A, Martelletti P, Sacco S; European Headache Federation School of Advanced Studies (EHF-SAS). Migraine and sleep disorders: a systematic review. J Headache Pain. 2020 Oct 27;21(1):126. doi: 10.1186/s10194-020-01192-5. PMID: 33109076; PMCID: PMC7590682.
- Finkel AG, Yerry JA, Mann JD. Dietary considerations in migraine management: does a consistent diet improve migraine? Curr Pain Headache Rep. 2013 Nov;17(11):373. doi: 10.1007/s11916-013-0373-4. PMID: 24068338.
- Vilela DA, Lourenço KD, Tames MLS, Bahia RF, Navarro F. Análise da ausência do teor de cafeína nas rotulagens dos cafés comercializados. RBONE-Revista Brasileira de Obesidade, Nutrição e Emagrecimento. 2007;1:92-105.
- Zhang WY. A benefit-risk assessment of caffeine as an analgesic adjuvant. Drug Saf. 2001;24(15):1127-42. doi: 10.2165/00002018-200124150-00004. PMID: 11772146.
- Lemmens J, De Pauw J, Van Soom T, Michiels S, Versijpt J, van Breda E, Castien R, De Hertogh W. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain. 2019 Feb 14;20(1):16. doi: 10.1186/s10194-019-0961-8. PMID: 30764753; PMCID: PMC6734345.
- Koseoglu E, Yetkin MF, Ugur F, Bilgen M. The role of exercise in migraine treatment. J Sports Med Phys Fitness. 2015 Sep;55(9):1029-36. Epub 2014 Jun 12. PMID: 24921618.
- Silva LCS, Jesus B, Freitas AS. Dietetics and nutrition influence in the migraine. J Health Sci. 2016;18:63-69.
- Silva ACP, Matos MCP, Landim LASR, Oliveira LMN. Relationship between eating habits and triggering factors of migraine crises. Research, Society and Development. 2020. doi: 10.33448/rsd-v9i11.10541.
- Finocchi C, Sivori G. Food as trigger and aggravating factor of migraine. Neurol Sci. 2012 May;33 Suppl 1:S77-80. doi: 10.1007/s10072-012-1046-5. PMID: 22644176.
- Hindiyeh NA, Zhang N, Farrar M, Banerjee P, Lombard L, Aurora SK. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache. 2020 Jul;60(7):1300-1316. doi: 10.1111/head.13836. Epub 2020 May 25. PMID: 32449944; PMCID: PMC7496357.
- Khan J, Asoom LIA, Sunni AA, Rafique N, Latif R, Saif SA, Almandil NB, Almohazey D, AbdulAzeez S, Borgio JF. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. 2021 Jul;139:111557. doi: 10.1016/j.biopha.2021.111557. Epub 2021 May 17. PMID: 34243621.
- Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep. 2014 Oct;18(10):454. doi: 10.1007/s11916-014-0454-z. PMID: 25160711.
- Garcia-Cerde R, Valente JY, Sohi I, Falade R, Sanchez ZM, Monteiro MG. Alcohol use during the COVID-19 pandemic in Latin America and the Caribbean. Rev Panam Salud Publica. 2021 May 20;45:e52. doi: 10.26633/RPSP.2021.52. PMID: 34025727; PMCID: PMC8132959.
- Stickley A, Shirama A, Inagawa T, Sumiyoshi T. Binge drinking in Japan during the COVID-19 pandemic: Prevalence, correlates and association with preventive behaviors. Drug Alcohol Depend. 2022 May 1;234:109415. doi: 10.1016/j.drugalcdep.2022.109415. Epub 2022 Mar 21. PMID: 35381568; PMCID: PMC8934738.
- Dueland AN. Headache and Alcohol. Headache. 2015 Jul-Aug;55(7):1045-9. doi: 10.1111/head.12621. Epub 2015 Jun 29. PMID: 26121267.
- Kesserwani H. Migraine Triggers: An Overview of the Pharmacology, Biochemistry, Atmospherics, and Their Effects on Neural Networks. Cureus. 2021 Apr 1;13(4):e14243. doi: 10.7759/cureus.14243. PMID: 33954064; PMCID: PMC8088284.
- Panconesi A. Alcohol and migraine: trigger factor, consumption, mechanisms. A review. J Headache Pain. 2008 Feb;9(1):19-27. doi: 10.1007/s10194-008-0006-1. Epub 2008 Jan 30. PMID: 18231712; PMCID: PMC3476173.
- Bordini CA, Roesler C, Carvalho Dde S, Macedo DD, Piovesan É, Melhado EM, Dach F, Kowacs F, Silva Júnior HM, Souza JA, Maciel JA Jr, Carvalho JJ, Speciali JG, Barea LM, Queiroz LP, Ciciarelli MC, Valença MM, Lima MM, Vincent MB. Recommendations for the treatment of migraine attacks - a Brazilian consensus. Arq Neuropsiquiatr. 2016 Mar;74(3):262-71. doi: 10.1590/0004-282X2015021. PMID: 27050859.
- Minen MT, Begasse De Dhaem O, Kroon Van Diest A, Powers S, Schwedt TJ, Lipton R, Silbersweig D. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016 Jul;87(7):741-9. doi: 10.1136/jnnp-2015-312233. Epub 2016 Jan 5. PMID: 26733600.
- Onderwater GLJ, van Oosterhout WPJ, Schoonman GG, Ferrari MD, Terwindt GM. Alcoholic beverages as trigger factor and the effect on alcohol consumption behavior in patients with migraine. Eur J Neurol. 2019 Apr;26(4):588-595. doi: 10.1111/ene.13861. Epub 2018 Dec 18. PMID: 30565341.
- Martins LB, Menezes JF, Lima DC, Costa ABP, Teixeira AL, Oliveira DC. Migraine and the triggering food factors. Headache Medicine. 2013;4:63-69.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am. 2001 Jul;85(4):911-41. doi: 10.1016/s0025-7125(05)70351-5. PMID: 11480265.
- Vlajinac H, Šipetić S, Džoljić E, Maksimović J, Marinković J, Kostić V. Some lifestyle habits of female Belgrade university students with migraine and non-migraine primary headache. J Headache Pain. 2003;4:67-71. doi: 10.1007/s10194-003-0033-x.
- Schramm SH, Obermann M, Katsarava Z, Diener HC, Moebus S, Yoon MS. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J Headache Pain. 2013 May 7;14(1):40. doi: 10.1186/1129-2377-14-40. PMID: 23651174; PMCID: PMC3655106.
- Milde-Busch A, Blaschek A, Borggräfe I, Heinen F, Straube A, von Kries R. Associations of diet and lifestyle with headache in high-school students: results from a cross-sectional study. Headache. 2010 Jul;50(7):1104-14. doi: 10.1111/j.1526-4610.2010.01706.x. Epub 2010 Jun 7. PMID: 20533961.
- Khorsha F, Mirzababaei A, Togha M, Mirzaei K. Association of drinking water and migraine headache severity. J Clin Neurosci. 2020 Jul;77:81-84. doi: 10.1016/j.jocn.2020.05.034. Epub 2020 May 20. PMID: 32446809.
- Kelman L. Migraine pain location: a tertiary care study of 1283 migraineurs. Headache. 2005 Sep;45(8):1038-47. doi: 10.1111/j.1526-4610.2005.05185.x. PMID: 16109118.
- Houts CR, McGinley JS, Wirth RJ, Cady R, Lipton RB. Reliability and validity of the 6-item Headache Impact Test in chronic migraine from the PROMISE-2 study. Qual Life Res. 2021 Mar;30(3):931-943. doi: 10.1007/s11136-020-02668-2. Epub 2020 Oct 20. PMID: 33079313; PMCID: PMC7952287.
- Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, Cady R, Dahlöf CG, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003 Dec;12(8):963-74. doi: 10.1023/a:1026119331193. PMID: 14651415.
- Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000 Oct;88(1):41-52. doi: 10.1016/S0304-3959(00)00305-5. PMID: 11098098.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.