> Medicine Group. 2020 Dec 22;1(8):431-438. doi: 10.37871/jbres1175.
Pharmacodynamics of Remdesivir: How to Improve for COVID-19 Treatment
Ashok Chakraborty1#* and Anil Diwan2#
2Nanoviricides, Inc., 1 Controls Dr. Shelton, CT 06484, USA
#Both the authors contributed equally
- Remdesivir
- COVID-19
- Pharmacodynamics
- Pharmacokinetics
- Antivirals
Potential clinical benefit in SARS-CoV-2 infection upon remdesivir treatment has been established. Recently, FDA has granted full approval for the clinical use of remdesivir for COVID therapy. However, the efficacy of remdesivir alone or in combination with other antivirals is still open to research, especially in terms of benefits vs. risk ratio. We here review remdesivir therapy based on a search for relevant pharmacological evidences with regards to the Pharmacokinetics (PK) and Pharmacodynamics (PD) of appropriate antiviral compounds against COVID-19 alone or in combination with other potential therapies. Drug–Drug Interactions (DDIs), if any in case of combo treatments have also been taken into consideration. We found promising in vitro evidence for efficacy of remdesivir, in combination with (hydroxy) chloroquine and/or favipiravir against SARS-CoV-2 infection in cell culture studies. However, clinical trial results from these combinations were not in line with the promising in vitro data, and therefore limit the use of such combinations in practice. Remdesivir and antibody therapies have also been used clinically either in combination or in sequential application. However, there are no substantive evaluable clinical data on these uses as of now. Additionally, some other drug combinations with remdesivir have been proposed in this article for future improvement in therapies.
Drugs active against SARS‑CoV‑2
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that causes COVID-19 was declared as a global pandemic on 11 March 2020, by the WHO, however, the evidence for therapies against this virus is as yet inadequate. Various medical teams are prescribing drugs for this collection of ailments based on some mechanistic data but with limited clinical findings in support of their activity. The clinical utility of preclinically validated dosing regimens relies heavily on the available Pharmacokinetic (PK) data used in the computer simulations performed to support such use for the particular drug [1].
Therapeutic agents available for COVID-19 can have other treatment challenges, particularly drug-drug interactions. Several compounds that have been proposed for the treatment of SARS-CoV-2 are affected by the Cytochrome P450 (CYP)-metabolizing system as an either substrate, enzyme inhibitor or enzyme inducer [2]. Therefore, the dosing requirements of concomitant SARS-CoV-2 or other supportive drug therapies should be evaluated properly based on the above–mentioned possible drug-drug interaction, at least for non-novel compounds. For example, Lopinavir/Ritonavir (LPV/r), a strong inhibitor of CYP3A4 and CYP2D6, can metabolize hydroxychloroquine (an antiviral against SARS-CoV-2) and therefore exhibited a potential toxicity in an animal model [2].
Remdesivir, formerly known as GS-5734, is a nucleotide analogue that is claimed to have been originally developed as a treatment against Ebola [3]. This drug can also inhibit corona virus replication by inhibiting RNA polymerases (RdRp4). This compound has shown broad antiviral activity in vitro against Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1), and SARS-CoV-2 [4-6].
Animal studies
In animal studies, remdesivir has been found effective in protecting rhesus monkeys from MERS-CoV infection, when given prior to infection [7]. It also protected African green monkeys from Nipah virus, a cause of fatal encephalitis; and also rhesus monkeys from Ebola virus [8,9]. A randomized, well-marked, controlled animal study with 12 rhesus monkeys infected with SARS-CoV-2 reported that an attenuation of respiratory symptoms and reduction in lung damage with remdesivir administered 12 hours after virus infection [10].
SARS-CoV-2 virus enters host cells by binding to and fusing with cell membrane receptor, ACE-2, followed by membrane fusion. Once inside, the virus uses the host cell’s machinery to replicate, using the virus’s RNA Dependent RNA Polymerase (RdRp) for making genome and transcript copies. Among the different strains of the corona virus, this non-structural protein is unique in structure, and thus making it a potentially useful drug target. Sofosbuvir, a synthetic analogue of nucleosides and nucleotides which inhibits RdRp has led to a successful treatment for hepatitis C infection [11]. Based on these facts, remdesivir was approved by FDA in various clinical trials for the treatment of COVID-19 infected people [12].
However, efficacy of remdesivir in vitro or in animals does not match with the clinical outcomes in humans. Further, remdesivir has some side effects. In the Ebola trial, the side effects of Remdesivir (RDV) were possible liver damage demonstrated in increased liver enzyme levels in plasma. Similar increases in liver enzymes in three U.S. COVID-19 patients were also documented after remdesivir treatment. Other typical antiviral drug side effects include Nausea and Vomiting [13]; and also affects kidney and mitochondria [14,15].
We searched for other compounds or drugs that could be used in conjunction with remdesivir to potentiate its effect against SARS-CoV-2 and that could minimize the side effects of remdesivir.
Search methodology
Drugs of interest were identified from literature search as listed in the (Table 1), and their PK/PD characteristics were reviewed (Table 2). Searches of the PubMed and Embase databases (no date limits) were performed to identify the clinical trials with those drugs of interest. Retrospective clinical studies, and animal and/or in vitro studies on the drug therapies are summarized in table 3. In addition, information on Drug–Drug Interactions (DDIs) were also noted [16]. The influence of extracorporeal support treatments on antiviral PK might also be necessary for critically ill patients with COVID-19, and is depicted in table3.
| Table 1: Some prospective Antivirals against COVID-19. | ||||
| Generic name | Mode of action | Study phase | Approved doses (mg) | Route of Administration |
| Remdesivir | Viral RNA Polymerase Inhibitor | Phase III/IV | 200 mg on day 1, followed by 100mg/Day on days 2-10 | IV |
| Chloroquine | Inhibits endosome-mediated viral entry; and pH-dependent steps in viral replication [36] | Phase III/IV | 600 mg/12 hr on day 1, followed by 300mg bid on days 2-5. OR: 500 mg/12 hr over 5 days [37] | PO or IV |
| Hydroxychloro- quine | Same as Chloroquine | Phase III/IV | 400 mg/day for 5 days, OR 400 mg/ 12 hr on day 1 followed by 200 mg/12 hr on days 2-5 [37] | PO |
| Lopinavir (LPV) / Ritonavir |
HIV: Protease inhibitor | Phase IV | LPV: 400 mg/12 hr Rito: 100 mg/12 hr [38] |
PO |
| Favipiravir | Viral RNA Polymerase Inhibitor | Phase III | Under study | PO, IV [39] |
| Ribavirin | Multiple possible mechanisms | Phase II | 500 mg/12 h or 500 mg/8h IV [40] | Aerosol, PO or IV |
| IFN-a1b | Adjuvant treatment: Enhancement of phagocytic/ cytotoxic mechanisms |
Early Phase I | 10 g/12 hr | Nebulized |
| IFN-a | Same as IFN-α1β | Not applicable | 5 million IU/12 h [41] | Nebulized |
| IFN- β1β | Same as IFN-α1β | Phase II | 25 g SC inj. alternate day for 3 days | SC |
| Camostat | Blocks interaction between the S1 protein and SARS-CoV-2 target cell | Phase I/II/III | 200 mg/12 or 8 hr | PO |
| Nafamostat | Same as Camostat | Phase II | 20-50 mg IV [42] | IV |
| PO: Oral; IV: Intravenous; SC: Subcutaneous | ||||
| Table 2: PK/PD of Some Antivirals against COVID-19. | |||||
| Drug/ Generic name |
IC50 | Type of study | EC50/EC90 (µM) For COVID-19 |
Blood Concentrations | Remarks |
| Remdesivir | EC50 EC90 |
In vitro (vero E6 cells) | 0.77 [6] 23.15 [43] 1.76 [6] |
10 µM in non-human primates was reached after a dose of 10 mg/kg IV. | Treatment outcome is similar to CoVID-19 patients [40] |
| Chloroquine | EC50 EC90 |
In vitro (vero E6 cells) | 1.13 -7.36 [6] 6.9 [6] |
A concentration of 6.9 µM is achievable in patients after a 500 mg dose [6,45] | Adverse effects were found with 600 mg/12 h for 10 days compared to 450 mg/12h on day 1 and once daily between days 2 and 5 [46] |
| Hydroxychloro quine | EC50 | In vitro (vero E6 cells) | 0.72 [37] 4.51 - 12.96 [20] |
Conc. >1.49 µM (>500 ng/mL) achievable following a 6 mg/kg/day dose [51] | Treatment outcomes were no different from standard care of hospitalized COVID patients [52] |
| Lopinavir/ Ritonavir |
EC50 | In vitro (vero E6 cells) |
LPV: 26.63 [43] | LPV Cmax average 12.72 µM and Ritonavir Cmax average 0.7 µM [49] | Treatment outcomes were no different from standard care of hospitalized patients with COVID-19 [50] |
| Favipiravir | EC50 | In vitro (vero E6 cells) | 61.88 [6] >100 [43] |
Conc. of 1190±478 µM were achieved after 1 h of 400 mg dose in non-human primates [51] | Faster viral clearance and radiological improvement was reported in patients received Favipiravir compared to Lopinavir/ Ritonavir [52] |
| Ribavarin | EC50 | In vitro (vero E6 cells) | 109.50 [6] >100 [43] |
Conc. range between 25 and 10.65 µM achieved with a ribavirin dose of 400-600 mg/12 h [53] | |
| IFN- β1β | EC50 EC90 |
Not available | Not available | Conc. 240 UI/mL following 8 million IU SC [54] | |
| Camostat | EC50 EC90 |
In vitro (Calu-3 cells) | 0.087 – 1 [55,56] 5 [55] |
Conc. 589 µM was achieved 12 h after Camostat 40 mg IV administration in humans [57] | |
| Nafamostat | EC50 | In vitro (Calu-3 cells) | 0.005 [56] | Conc. 41, 116, and 174 uM were achieved 12 h after Nafamostat 10, 20 and 40 µM, IV, administration in humans, respectively [58] | |
List of potential antiviral agents for the treatment of COVID-19
The general information for some key drugs against COVID-19 are summarized in table 1. Most agents were originally approved for the treatment of other viral infections, except hydroxy-chloroquine that has been used for over 60 years primarily for malaria treatment, and is also approved for systemic lupus erythematosus [17].
Pharmacokinetics/Pharmacodynamics (PK/PD) of combo antiviral agents for the treatment of COVID‑19
For a drug to be clinical effective, it’s in vivo concentrations in tissues of interest and potentially in the blood stream as well, should exceed its antiviral effectiveness or inhibitory concentration values. Table 2 summarizes the viral inhibition data of some of the agents recommended for SARS-CoV-2. The available data are based on in vitro studies in various cell lines. The EC50 values for other viruses are compared against SARS-CoV-2, while EC90 is usually preferred only when the EC90 is nine-fold higher than EC50 [18].
The combo drug effect of remdesivir and ribavirin were tested on MERS-CoV and SARS-CoV. The EC50 values were higher in SARS compared to MERS, indicating that a higher dose of the drug would be needed to treat COVID-19 than for MERS. However, the EC50 value of chloroquine was within the same range for SARS-CoV-2 and MERS. Since EC50 is not a time-dependent parameter, this data may not be reliable in an in vitro target (i.e. as a clinical parameter) [19]. Furthermore, other studies have found that while chloroquine was though more potent than hydroxychloroquine, both result in an increased mortality when used in COVID-19 [20]. These drugs have cardiac side effects which are likely to be responsible for the increased mortality.
Several Interferons (IFNs), including IFN-α, PegIFN-α2β, IFN-α1β and IFN-β1β, have been examined for the treatment of COVID-19 as an adjuvant therapy with other anti-COVID-19 drugs. However, the drug efficacy and PK/PD targets for COVID-19 are not available, only some data on plasma concentrations are available in the literature (Table 2).
Search for any Drug-Drug Interaction (DDI) during combination of SARS‑CoV‑2 antiviral agents
LPV/r plus ribavirin therapy resulted in a reduction in mortality, Acute Respiratory Distress Syndrome (ARDS), and viral shedding in the treatment of SARS (Table 3). Another HIV protease inhibitor, nelfinavir, exhibited good activity against SARS [21,22], but are less effective against MERS [23]. Combination of LPV/r, ribavirin and IFN resulted in a shorter duration of viral shedding and hospital stay when compared with LPV/r alone. Randomized trials involving these drugs are yet, to be known.
| Table 3: Drug-Drug Interactions of some prospective antivirals against COVID-19. | ||||
| Combination of Drugs | Pharmacodynamic Rationality | Effects of drug-drug Interactions | Types of Evidences | Therapeutic Comments |
| Ribavarin + Lopinavir [59] | Inhibition of Viral RNA synthesis and their replication | Increased risk of liver toxicity | Retrospective clinical data [21,60] In vivo animal data [61] |
Monitoring for Liver toxicity |
| Chloroquine + Lopinavir | Inhibition of Viral entry and their replication | Increased risk QTc prolongation. Inhibition of CYP3A-mediated metabolism of chloroquine by ritonavir |
Retrospective clinical data: No data yet In vivo animal data, or any in vitro data: Not yet |
Monitoring for ECG, and for increased toxicity. Dose reduction of chloroquine might be necessary in such a case. |
| Favipiravir + Interferon |
Inhibition of viral RNA synthesis and immune modulation | No data | Clinical trials: No data In vivo animal data, or any in vitro data: Not yet |
None |
| Interferon + Ribavirin | Immune modulation and Inhibition of viral RNA synthesis | No data | Clinical trials: Ongoing [62] In vivo and in vitro data: Synergistic antiviral effect were described elsewhere [61-64]. |
|
| LPV/r +Interferon +Ribavirin |
Immune modulation, Inhibition of viral RNA synthesis, plus inhibition of replication | Level of severity: Major | Clinical trials: The combination group had a significantly shorter median time from start of study treatment to negative naso-pharyngeal swab, and shorter duration of hospitalization than the control group [65] In vivo and in vitro data: Not yet |
Monitoring for Liver toxicity |
The newer investigational antiviral agents, remdesivir and favipiravir, appear to have a lower potential for DDIs; however, the main concern with their use is the decrease in the drug concentrations if co-administered with CYP-enzyme inducers. A comprehensive and evolving DDI database has been created by the University of Liverpool and this should be consulted for potential DDIs, if needed [2].
Improvement of Antiviral activity of Remdesivir against SAR-CoV-2
FDA authorizes second COVID-19 treatment drug to be used in combination with remdesivir [24]. Baricitinib is a Janus Kinase Inhibitor (JAK), which blocks the activity of one or more of a specific family of enzymes, interfering with the pathway that leads to inflammation. Baricitinib is FDA-approved oral medication for severely active rheumatoid arthritis. In a clinical trial of hospitalized patients with COVID-19, baricitinib, in combination with remdesivir, was shown to reduce recovery time from 8 to 7 days compared to placebo and remdesivir (18 days of recovery) [25]. The safety and effectiveness of this investigational therapy for use in the treatment of COVID-19 continues to be evaluated.
Concerns exists that Angiotensin-Converting Enzyme Inhibitors (ACE-Is) and Angiotensin Receptor Blockers (ARBs) may increase susceptibility to coronavirus SARS CoV [26]. There is also widespread speculation about the potential benefits of ACE-Is and ARBs, based on biological plausibility arguments and animal data and small clinical studies on patients with other viral respiratory infections [27].
A new study by researchers at Germany’s Jülich Research Centre reports that a hexapeptide can inhibit the aggregation and activation of the Spike (S) protein of SARS-CoV-2. In vitro experiments have shown that this peptide prevented the infection of cells in culture by SARS-CoV-2. Additionally, it has been found that this peptide also prevents another coronavirus, h-CoV-NL63, from replicating inside the cells. Further, the hexapeptide is also highly specific for the S protein, and therefore can be considered for development as a potential drug either alone or in combination with remdesivir [28].
Remdesivir is a RNA polymerase inhibitor and it inhibits infection of SARS-CoV-2 virus in a human cell line, in vitro [6]. In an animal model of SARS-CoV and MERS-CoV infections, Remdesivir also showed decreased viral load and improved pulmonary function [5]. These data, together with the human safety profile data from intravenous remdesivir therapy of Ebola virus disease are the basis for the use of remdesivir intravenously for COVID-19 therapy in humans [3,29]. After intravenous administration, in two critically ill Chinese patients with severe adult respiratory distress syndrome, remdesivir showed a peak at the end of infusion and a half-life of 1 h, while GS-441524 (a plasma metabolite of remdesivir, that also has antiviral activity though very limited, compared to remdesivir) reached a peak 1 h after infusion and then remained detectable until the next remdesivir administration [30]. Similar data have been shown in rhesus monkeys infected with SARS-CoV-2, where the intravenous administration of remdesivir which was converted into the nucleoside analogue GS-441524, reached and sustained to its EC90 values [6,9,29,31].
Remdesivir is metabolized by non-specific esterases in the blood stream as well as inside cells to GS-441524. While remdesivir contains a 5’-phosphate, and thus would become rapidly converted to the active triphosphate nucleotide analog inside cells via non-specific pyrophosphorylases and kinases, the metabolite GS-441524 must go through the rate-limiting slow step of first phosphorylation with a nuclcoside kinase. GS-441524 therefore exhibits substantially weaker activity compared to remdesivir in vitro. Thus conversion to weakly active form in vitro potentially limits the clinical activity of remdesivir and accounts for its poor clinical response compared to its significantly strong in vitro efficacy. Thus, improvement in PK/PD of remdesivir in terms of protection from esterase metabolism may significantly improve its clinical efficacy.
These preliminary observations improve knowledge about the PK and use of remdesivir for treatment of COVID-19 patients. However, further studies, with greater numbers of patients, and clinical trials, are needed to confirm these preliminary results, along with other strategies to strengthen the combo effects of remdesivir and other novel candidates. In particular, WHO recently suggested that retrospective analysis of remdesivir treatment did not show significant benefits, in contrast to what was observed in controlled clinical trials [32]. Remdesivir (Veklury, Gilead) is supplied as a lyophilized solid containing 100 mg remdesivir and 600 mg SBECD (sulfobutylether-β-cyclodextrin) which requires to be redissolved in WFI water by the Pharmacist, and injected into infusion fluid (saline) preferably through a sterile filter [https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=98b7e6bf-2668-4a61-a874-194eb674b15c&version=7#!]. It is likely that the redissolution may not be complete, and filtration may be removing undissolved remdesivir, thus reducing the dose applied, especially in the hands of inexperienced personnel during this pandemic. This could explain the discrepancy between the remdesivir controlled clinical trials and the WHO datasets. Cyclodextrins form colloids at high concentrations and allow insoluble drugs to be held in the colloid. However, the colloid dilutes out into the bloodstream quickly and would lead to falling out of the API if it is not held by a true cage-binding mechanism. Thus, in addition to protecting remdesivir from metabolism, keeping it in an encapsulated form physiologically is essential if its full potential (as observed in cell culture studies) is to be realized clinically.
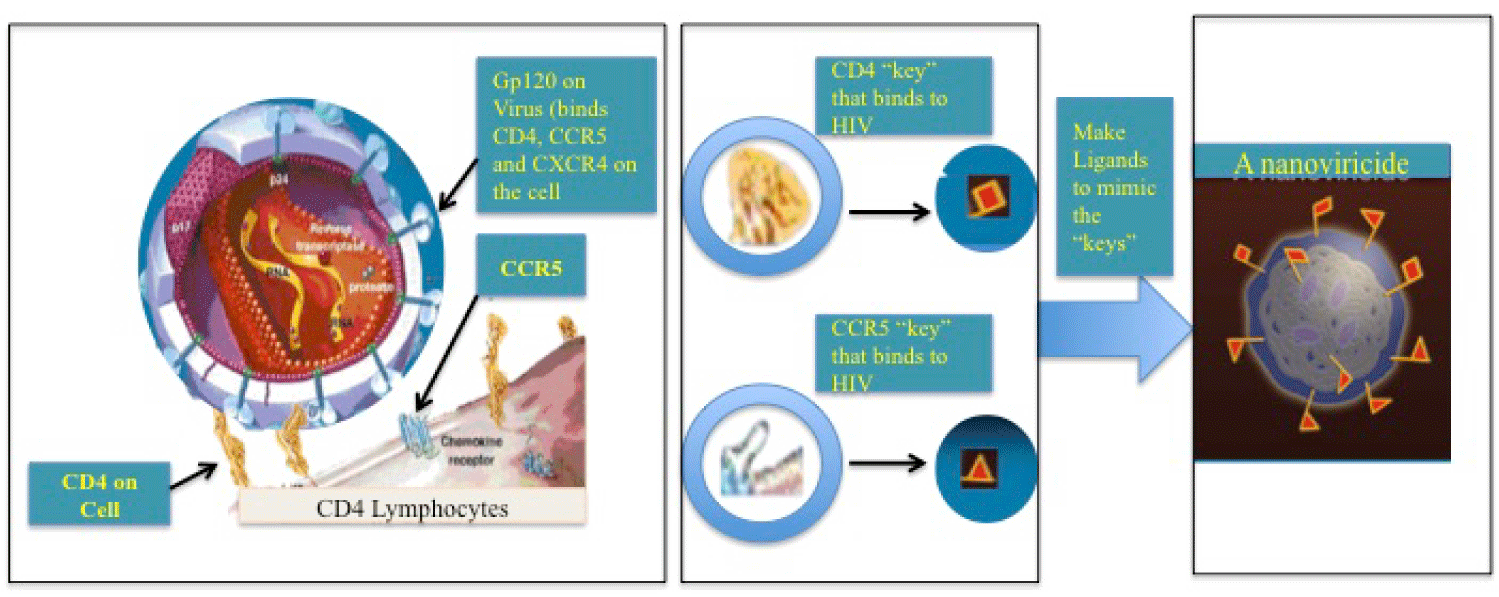
NanoViricides, Inc. at Shelton, CT, are working to protect the remdesivir by encapsulation in a polymer to guard from degradation in the blood stream and also for slow release to minimize the side effects [33]. Besides, NanoViricides, Inc. has also developed a platform technology such that a when a virus encounters our nanoviricide® bio-mimetic polymer, the virus particle would bind to and would get engulfed into the polymeric nanoviricide, acting like a “Venus-fly-trap”, and the virus particle would get destroyed in the process (Figure 1). Using a plug-and-play approach, we can change the virus binding ligand portion of this nanomedicine to attack a different virus. We have already tested several drug candidates for broad-spectrum anti-coronavirus effectiveness in cell culture studies. One of the coronavirus strains (h-CoV-NL63) that we studied, uses the same cell surface receptor ACE2 (angiotensin converting enzyme-2) that is shared by SARS-CoV-2 and SARS-CoV-1. Out of our several test drug candidates, one drug showed as much as 15-times more effectiveness than favipiravir against two different coronaviruses (h-CoV-NL63 and HCoV-229E) in this study. Safety and Tolerability of that anti-coronavirus drug candidate was studied in an animal model, and found to be safe and well tolerated. There were no clinical signs of immune or allergic reactions such as itching, biting, twitching, rough coat, etc. Further, there were no observable changes in any organs including large intestine or colon on post mortem in gross histology. This non-GLP safety/tolerability study was conducted under GLP-like conditions by AR BioSystems, Inc., Odessa, Tampa, FL. Further microscopic histology and blood work analyses are in progress [34].
In summary, promising therapeutic options for COVID-19 are now emerging, while vaccines are being approved at a rapid rate. Continued evolution and emergence of distinctly different lineages and variants of the SARS-CoV-2 virus such as the lineage B.1.1.7, also called Variant of Concern VOC-202012/01 in the UK, and of 501.V2, among others, raises severe concerns regarding the ability of vaccines based on the original SARS-CoV-2 to remain effective. Same concern exists for the efficacy of antibody based drugs. The need for an effective treatment for infected patients thus looms large. The results from Randomized Controlled Trial (RCT) for remdesivir are encouraging and provide some direction for the treatment of COVID-19 patients [18]. Further to this, it is highly likely that one or more other agents mentioned in this review, in combination may emerge as a prophylactic or as an early treatment option to decrease the viral shedding, transmission, and/ or to reduce disease progression.
From a PK/PD perspective, drug development should not focus on the discovery of new treatment options alone, but should also help to investigate common key aspects, particularly the following.
- The optimal time point to start antiviral therapy, and required duration of therapy.
- The role of the individualization of therapy based on dose adaptation and Therapeutic Drug Monitoring (TDM) in the treatment of COVID-19.
In brief, this comparative analysis found that remdesivir treatment is associated with significantly lower mortality and higher recovery than any other standard treatment without remdesivir in patients with severe COVID-19 [35]. Improvement in its clinical effectiveness may be possible if its PK/PD characteristics can be improved, for example, if it can be protected from initial metabolism in the blood stream. Further ongoing research will reveal more information in the near future. Meanwhile, public health measures continue to be the best tools for containment of the COVID-19 epidemic worldwide. Given the rate of variant emergence, it would not be advisable to rely on vaccination to replace these measures, at least until highly effective broad-spectrum therapeutics become available. It is advisable to avoid infection by appropriate social distancing, use of face masks, and cleanliness protocols including hand washing. Furthermore, the use of PCR-based diagnostic tests and contact tracing continue to be important for limiting the spread of infection.
Both the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The study received funding from Nanoviricides, Inc.
Both the authors contributed equally to prepare this article, read and approved the final manuscript.
We would like to thank our colleagues for their help during writing this article.
- Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL; International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014 Jun;14(6):498-509. doi: 10.1016/S1473-3099(14)70036-2. Epub 2014 Apr 24. PMID: 24768475; PMCID: PMC4181663.
- Liverpool Uo. Covid-19 drug interactions. 2020.
- Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ; PALM Writing Group, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J; PALM Consortium Study Team. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019 Dec 12;381(24):2293-2303. doi: 10.1056/NEJMoa1910993. Epub 2019 Nov 27. PMID: 31774950.
- Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020 Apr 10;295(15):4773-4779. doi: 10.1074/jbc.AC120.013056. Epub 2020 Feb 24. PMID: 32094225; PMCID: PMC7152756.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun 28;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. PMID: 28659436; PMCID: PMC5567817.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269-271. doi: 10.1038/s41422-020-0282-0. Epub 2020 Feb 4. PMID: 32020029; PMCID: PMC7054408.
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020 Mar 24;117(12):6771-6776. doi: 10.1073/pnas.1922083117. Epub 2020 Feb 13. PMID: 32054787; PMCID: PMC7104368.
- Lo MK, Feldmann F, Gary JM, Jordan R, Bannister R, Cronin J, Patel NR, Klena JD, Nichol ST, Cihlar T, Zaki SR, Feldmann H, Spiropoulou CF, de Wit E. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med. 2019 May 29;11(494):eaau9242. doi: 10.1126/scitranslmed.aau9242. PMID: 31142680; PMCID: PMC6732787.
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016 Mar 17;531(7594):381-5. doi: 10.1038/nature17180. Epub 2016 Mar 2. Erratum in: ACS Chem Biol. 2016 May 20;11(5):1463. PMID: 26934220; PMCID: PMC5551389.
- Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Kwe Yinda C, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv [Preprint]. 2020 Apr 22:2020.04.15.043166. doi: 10.1101/2020.04.15.043166. Update in: Nature. 2020 Jun 9;: PMID: 32511319; PMCID: PMC7239049.
- Xie Y, Ogah CA, Jiang X, Li J, Shen J. Nucleoside Inhibitors of Hepatitis C Virus NS5B Polymerase: A Systematic Review. Curr Drug Targets. 2016;17(13):1560-76. doi: 10.2174/1389450117666151209123751. PMID: 26648061.
- https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/.
- https://www.rxlist.com/consumer_remdesivir_rdv/drugs-condition.htm.
- Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, Nigwekar S, Rhee EP, Sise ME. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19. J Am Soc Nephrol. 2020 Jul;31(7):1384-1386. doi: 10.1681/ASN.2020050589. Epub 2020 Jun 8. PMID: 32513665; PMCID: PMC7351006.
- Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020 Sep;54:1-7. doi: 10.1016/j.mito.2020.06.008. Epub 2020 Jun 20. PMID: 32574708.
- Elsevier. Clinical Pharmacology. https://www.clinicalpharmacology com/. Accessed 17 July 2020.
- Lei ZN, Wu ZX, Dong S, Yang DH, Zhang L, Ke Z, Zou C, Chen ZS. Chloroquine and hydroxychloroquine in the treatment of malaria and repurposing in treating COVID-19. Pharmacol Ther. 2020 Dec;216:107672. doi: 10.1016/j.pharmthera.2020.107672. Epub 2020 Sep 8. PMID: 32910933; PMCID: PMC7476892.
- Zeitlinger M, Koch BCP, Bruggemann R, De Cock P, Felton T, Hites M, Le J, Luque S, MacGowan AP, Marriott DJE, Muller AE, Nadrah K, Paterson DL, Standing JF, Telles JP, Wölfl-Duchek M, Thy M, Roberts JA; PK/PD of Anti-Infectives Study Group (EPASG) of the European Society of Clinical Microbiology, Infectious Diseases (ESCMID). Pharmacokinetics/Pharmacodynamics of Antiviral Agents Used to Treat SARS-CoV-2 and Their Potential Interaction with Drugs and Other Supportive Measures: A Comprehensive Review by the PK/PD of Anti-Infectives Study Group of the European Society of Antimicrobial Agents. Clin Pharmacokinet. 2020 Oct;59(10):1195-1216. doi: 10.1007/s40262-020-00924-9. PMID: 32725382; PMCID: PMC7385074.
- Fan J, Zhang X, Liu J, Yang Y, Zheng N, Liu Q, Bergman K, Reynolds K, Huang SM, Zhu H, Wang Y. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis. 2020 May 21:ciaa623. doi: 10.1093/cid/ciaa623. Epub ahead of print. PMID: 32435791; PMCID: PMC7314136.
- Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020 Mar 18;6:16. doi: 10.1038/s41421-020-0156-0. PMID: 32194981; PMCID: PMC7078228.
- Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004 Mar;59(3):252-6. doi: 10.1136/thorax.2003.012658. PMID: 14985565; PMCID: PMC1746980.
- Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A, Tamamura H, Fujii N, Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004 Jun 4;318(3):719-25. doi: 10.1016/j.bbrc.2004.04.083. PMID: 15144898; PMCID: PMC7111005.
- Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, Hayden FG, Yuen KY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013 Dec;67(6):606-16. doi: 10.1016/j.jinf.2013.09.029. Epub 2013 Oct 3. PMID: 24096239; PMCID: PMC7112612.
- FDA NEWS RELEASE. Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19. Nov 19 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19
- Medical News Today. Remdesivir and baricitinib shortened recovery time from COVID-19. Jocelyn Solis-Moreira. January 2, 2021 — Fact checked by Mary Cooke, Ph.D. https://www.medicalnewstoday.com/articles/remdesivir-and-baricitinib-shortened-recovery-time-from-covid-19.
- Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810.
- Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020; 41(19):1801-1803. https://doi.org/10.1093/eurheartj/ehaa235.
- Peter EK and Schug A. The Inhibitory Effect of a Coronavirus Spike Protein Fragment with ACE2 . Biophysical Journal. 2020 Dec;119:1-10.
- Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, Hsueh PR. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020 Apr;55(4):105933. doi: 10.1016/j.ijantimicag.2020.105933. Epub 2020 Mar 6. PMID: 32147516; PMCID: PMC7135364.
- Albarello F, Pianura E, Di Stefano F, Cristofaro M, Petrone A, Marchioni L, Palazzolo C, Schininà V, Nicastri E, Petrosillo N, Campioni P, Eskild P, Zumla A, Ippolito G; COVID 19 INMI Study Group. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: An uncommon radiological presentation. Int J Infect Dis. 2020 Apr;93:192-197. doi: 10.1016/j.ijid.2020.02.043. Epub 2020 Feb 26. PMID: 32112966; PMCID: PMC7110436.
- Tempestilli M, Caputi P, Avataneo V, Notari S, Forini O, Scorzolini L, Marchioni L, Ascoli Bartoli T, Castilletti C, Lalle E, Capobianchi MR, Nicastri E, D’Avolio A, Ippolito G, Agrati C; COVID 19 INMI Study Group. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother. 2020 Oct 1;75(10):2977-2980. doi: 10.1093/jac/dkaa239. PMID: 32607555; PMCID: PMC7337789.
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2020 Dec 2:NEJMoa2023184. doi: 10.1056/NEJMoa2023184. Epub ahead of print. PMID: 33264556; PMCID: PMC7727327.
- https://www.accesswire.com/615993/NanoViricides-Has-Engaged-Calvert-Labs-for-Safety-Pharmacology-Studies-of-Its-Drug-for-the-Treatment-of-COVID-19.
- NanoViricides Develops Highly Effective Broad-Spectrum Drug Candidates Against Coronaviruses. Published: May 12, 2020 at 7:15 a.m. ET. https://www.marketwatch.com/press-release/nanoviricidesdevelops-highly-effective-broad-spectrum-drug-candidates-againstcoronaviruses-2020-05-12.
- Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, Wang S, Walunas TL, Swaminathan S, Slim J, Chin B, De Wit S, Ali SM, Soriano Viladomiu A, Robinson P, Gottlieb RL, Tsang TYO, Lee IH, Haubrich RH, Chokkalingam AP, Lin L, Zhong L, Bekele BN, Mera-Giler R, Gallant J, Smith LE, Osinusi AO, Brainard DM, Hu H, Phulpin C, Edgar H, Diaz-Cuervo H, Bernardino JI. Remdesivir for Severe COVID-19 versus a Cohort Receiving Standard of Care. Clin Infect Dis. 2020 Jul 24:ciaa1041. doi: 10.1093/cid/ciaa1041. Epub ahead of print. PMID: 32706859; PMCID: PMC7454434.
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003 Nov;3(11):722-7. doi: 10.1016/s1473-3099(03)00806-5. PMID: 14592603; PMCID: PMC7128816.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Jul 28;71(15):732-739. doi: 10.1093/cid/ciaa237. PMID: 32150618; PMCID: PMC7108130.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020 May 7;382(19):1787-1799. doi: 10.1056/NEJMoa2001282. Epub 2020 Mar 18. PMID: 32187464; PMCID: PMC7121492.
- Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019 Apr;32(2):176-186. doi: 10.1097/QCO.0000000000000532. PMID: 30724789; PMCID: PMC6416007.
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58-60. doi: 10.5582/ddt.2020.01012. PMID: 32147628.
- Jin YH, Cai L, Cheng ZS, Hong C, Tong D, Fan YP, Fang C, Huang DI, Huang L, Huang Q, Han Y, Hu BO, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma Lu, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4.
- Midgley I, Hood AJ, Proctor P, Chasseaud LF, Irons SR, Cheng KN, Brindley CJ, Bonn R. Metabolic fate of 14C-camostat mesylate in man, rat and dog after intravenous administration. Xenobiotica. 1994 Jan;24(1):79-92. doi: 10.3109/00498259409043223. PMID: 8165824.
- Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020 Jun;178:104786. doi: 10.1016/j.antiviral.2020.104786. Epub 2020 Apr 3. PMID: 32251767; PMCID: PMC7127386.
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-1578. doi: 10.1016/S0140-6736(20)31022-9. Epub 2020 Apr 29. Erratum in: Lancet. 2020 May 30;395(10238):1694. PMID: 32423584; PMCID: PMC7190303.
- Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983 Jul 18;75(1A):40-5. doi: 10.1016/0002-9343(83)91269-x. PMID: 6869410.
- Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG; CloroCovid-19 Team. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020 Apr 24;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. PMID: 32330277.
- Mok CC, Penn HJ, Chan KL, Tse SM, Langman LJ, Jannetto PJ. Hydroxychloroquine Serum Concentrations and Flares of Systemic Lupus Erythematosus: A Longitudinal Cohort Analysis. Arthritis Care Res (Hoboken). 2016 Sep;68(9):1295-302. doi: 10.1002/acr.22837. Epub 2016 Jul 27. PMID: 26749299.
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 Jun 18;382(25):2411-2418. doi: 10.1056/NEJMoa2012410. Epub 2020 May 7. PMID: 32379955; PMCID: PMC7224609.
- Dickinson L, Boffito M, Back D, Else L, von Hentig N, Davies G, Khoo S, Pozniak A, Moyle G, Aarons L. Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies. Antimicrob Agents Chemother. 2011 Jun;55(6):2775-82. doi: 10.1128/AAC.00887-10. Epub 2011 Mar 21. PMID: 21422211; PMCID: PMC3101471.
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020 May 7;382(19):1787-1799. doi: 10.1056/NEJMoa2001282. Epub 2020 Mar 18. PMID: 32187464; PMCID: PMC7121492.
- Bixler SL, Bocan TM, Wells J, Wetzel KS, Van Tongeren SA, Dong L, Garza NL, Donnelly G, Cazares LH, Nuss J, Soloveva V, Koistinen KA, Welch L, Epstein C, Liang LF, Giesing D, Lenk R, Bavari S, Warren TK. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antiviral Res. 2018 Mar;151:97-104. doi: 10.1016/j.antiviral.2017.12.021. Epub 2017 Dec 28. PMID: 29289666.
- Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020 Oct;6(10):1192-1198. doi: 10.1016/j.eng.2020.03.007. Epub 2020 Mar 18. PMID: 32346491; PMCID: PMC7185795.
- Fuchs EJ, Kiser JJ, Hendrix CW, Sulkowski M, Radebaugh C, Bushman L, Ray ML, Andrade A. Plasma and intracellular ribavirin concentrations are not significantly altered by abacavir in hepatitis C virus-infected patients. J Antimicrob Chemother. 2016 Jun;71(6):1597-600. doi: 10.1093/jac/dkw009. Epub 2016 Feb 10. PMID: 26869690; PMCID: PMC4867100.
- Hegen H, Auer M, Deisenhammer F. Pharmacokinetic considerations in the treatment of multiple sclerosis with interferon-β. Expert Opin Drug Metab Toxicol. 2015;11(12):1803-19. doi: 10.1517/17425255.2015.1094055. Epub 2015 Sep 30. PMID: 26419922.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627.
- Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother. 2020 May 21;64(6):e00754-20. doi: 10.1128/AAC.00754-20. PMID: 32312781; PMCID: PMC7269515.
- Midgley I, Hood AJ, Proctor P, Chasseaud LF, Irons SR, Cheng KN, Brindley CJ, Bonn R. Metabolic fate of 14C-camostat mesylate in man, rat and dog after intravenous administration. Xenobiotica. 1994 Jan;24(1):79-92. doi: 10.3109/00498259409043223. PMID: 8165824.
- Cao YG, Zhang M, Yu D, Shao JP, Chen YC, Liu XQ. A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: more accurate evaluation for pharmacokinetic study. Anal Bioanal Chem. 2008 Jun;391(3):1063-71. doi: 10.1007/s00216-008-2054-4. Epub 2008 Apr 6. PMID: 18392693.
- ClinicalTrials.gov. The efficacy of lopinavir plus ritonavir and arbidol against novel coronavirus infection (ELACOI). https://www.clini caltr ialsg ov/ct2/show/NCT04 25288 5?cond=Coronaviru s&draw=3&rank=12.
- Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, Yuen KY. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003 Dec;9(6):399-406. PMID: 14660806.
- Park SY, Lee JS, Son JS, Ko JH, Peck KR, Jung Y, Woo HJ, Joo YS, Eom JS, Shi H. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019 Jan;101(1):42-46. doi: 10.1016/j.jhin.2018.09.005. Epub 2018 Sep 18. PMID: 30240813; PMCID: PMC7114948.
- Zeng YM, Xu XL, He XQ, Tang SQ, Li Y, Huang YQ, Harypursat V, Chen YK. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin Med J (Engl). 2020 May 5;133(9):1132-1134. doi: 10.1097/CM9.0000000000000790. PMID: 32149772; PMCID: PMC7213617.
- Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004 Sep;31(1):69-75. doi: 10.1016/j.jcv.2004.03.003. PMID: 15288617; PMCID: PMC7128415.
- Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006 Sep;3(9):e343. doi: 10.1371/journal.pmed.0030343. PMID: 16968120; PMCID: PMC1564166.
- Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AK, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CC, Zhang RR, Fung AY, Yan EY, Leung KH, Ip JD, Chu AW, Chan WM, Ng AC, Lee R, Fung K, Yeung A, Wu TC, Chan JW, Yan WW, Chan WM, Chan JF, Lie AK, Tsang OT, Cheng VC, Que TL, Lau CS, Chan KH, To KK, Yuen KY. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 May 30;395(10238):1695-1704. doi: 10.1016/S0140-6736(20)31042-4. Epub 2020 May 10. PMID: 32401715; PMCID: PMC7211500.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.