Medicine Group . 2023 February 15;4(2):218-225. doi: 10.37871/jbres1664.
Electrophysiological Study in a Patient with Visual Deficit after Severe Coronavirus 2 Pneumonia
Adrian Egea Gonzalez1*, Maria Carmen Gonzalez Gallardo2, Esther Miralles Martin1, Miguel Lopez-Alcazar1 and Jose Antonio Saez Moreno1
2Ophthalmology Service, Hospital San Cecilio de Granada
- COVID-19
- Retinopathy
- Electrophysiological examination
Abstract
We describe the electrophysiological findings on visual pathway examination in a 54-year-old female patient who suffered loss of visual acuity without cause on clinical examination. The clinical picture followed a case of Severe Pneumonia Secondary to Coronavirus 2 (SARS-CoV-2) infection that required admission to the Intensive Care Unit (ICU) for High-Flow Nasal Oxygen Therapy (HFO).
Introduction
Although initially known as a severe acute respiratory distress syndrome, the disease caused by SARS-CoV-2 involves a wide spectrum of clinical presentations. Ophthalmologically, a variety of symptoms have been described at virtually every level of injury. At retinal level, we can distinguish between vascular and inflammatory pathology (Table 1) [1].
| Table 1: COVID 19 related retinal pathology. | |
| Retinal Vascular Pathology | Retinal Inflammatory Pathology |
| Central retinal vein occlusion | Vitritis and disorders of the outer retina |
| Central retinal artery occlusion | Acute retinal necrosis |
| Acute macular neuroretinopathy | |
| Acute paracentral maculopathy | |
In the literature, more retinal manifestations have been reported but the causal relationship with SARS-CoV-2 is doubtful [1].
This paper presents the case of a patient who, after suffering severe COVID-19 pneumonia without cytokine release syndrome, suddenly developed a painless visual acuity deficit with no apparent cause on clinical examination. Although this patient was in the ICU, it was only to administer HFO without the need for sedation, mechanical ventilation or pronation. The originality of the case, however, can be found in the findings of the neurophysiological examination of the visual pathway.
Clinical Case
54-year-old female
Family history: Without relevance from an ophthalmological point of view.
Personal history: High blood pressure, lumbar canal stenosis, osteoarthritis, fibromyalgia, obesity, obstructive sleep apnoea syndrome, gastro-oesophageal reflux disease. No toxic habits. Metamizole allergy.
Self-sufficient in basic activities of daily living, she has held various jobs related to retail and sewing.
Since developing the COVID infection, she has had difficulty concentrating and memory lapses, and is being monitored by Neurology without any underlying pathology to justify these cognitive problems (although, from an iatrogenic point of view, some of the drugs described below could contribute to cognitive impairment).
Treatment at the time of acquiring coronavirus infection: alprazolam, amitriptyline, duloxetine, amlodipine, furosemide, losartan and omeprazole. Nocturnal CPAP.
Pharmacological treatment administered during her hospital stay in addition to the aforementioned outpatient treatment: bemiparin sodium, dexamethasone, prednisone, ceftriaxone, paracetamol, dextromethorphan, insulin gulisine (occasionally for hyperglycaemia).
On 4 January 2021 she was admitted to the ICU for moderate acute respiratory distress syndrome secondary to bilateral SARS-COV-2 pneumonia, confirmed by PCR in oropharyngeal mucosa. With HFO, corticosteroid treatment and antibiotic therapy, she progressed favourably and was discharged on 14 January 2021 (Table 2). At that time she noticed a slight problem with both near and far vision, which was ill defined, and so she went to an optical centre where there was no objective evidence of a correctable refractive error. On 5 March she was referred to the ophthalmology department by her GP.
| Table 2: Respiratory parameters during hospitalization. | |||
| Evolutionary phase | Respiratory parameters | ||
| Respiratory rate (breaths per minute) | O2 saturation (pulse oximetry) | fiO2 provided | |
| Admission to the ICU | 31 | 94% | 100% |
| ICU epicrisis | 25 | 96% | 36% |
| Discharged | 15-20 | 96% | No |
Ophthalmological examination at the first consultation
Visual acuity: OD 6/10 does not improve with pinhole. OI 6/7.5 does not improve with pinhole.
Pupils are isochoric and normoreactive to light and accommodation.
Preserved extrinsic ocular motility.
Biomicroscopy: Phacosclerosis. Anterior and posterior chambers good.
Fundus (pharmacological mydriasis): papilla with sharp edges and good colouration. Normal macular area. Retina without peripheral lesions.
Clinical assessment: Phacosclerosis
Action plan: Referral to Clinical Neurophysiology.
Results of neurophysiological examination of the visual pathway
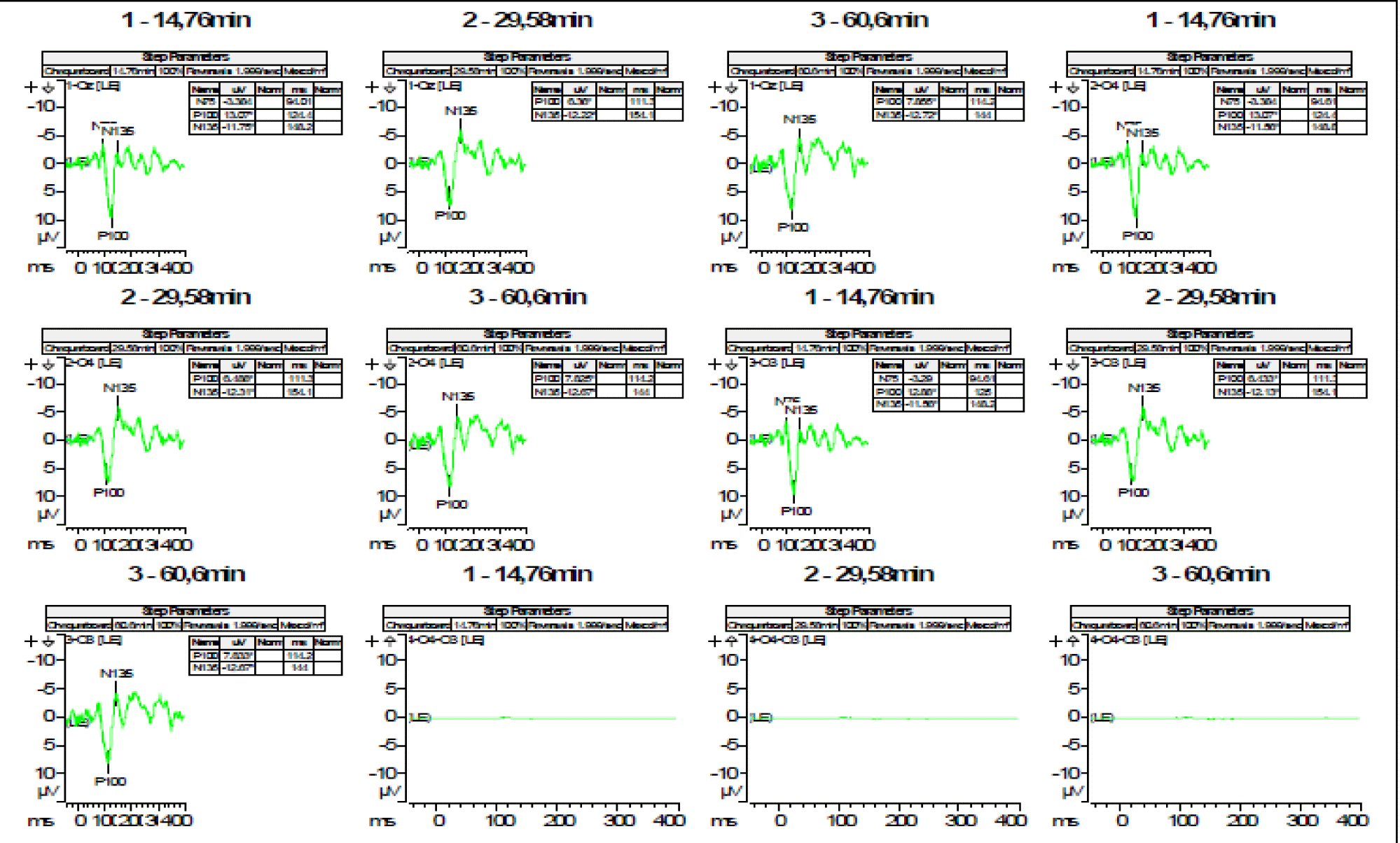
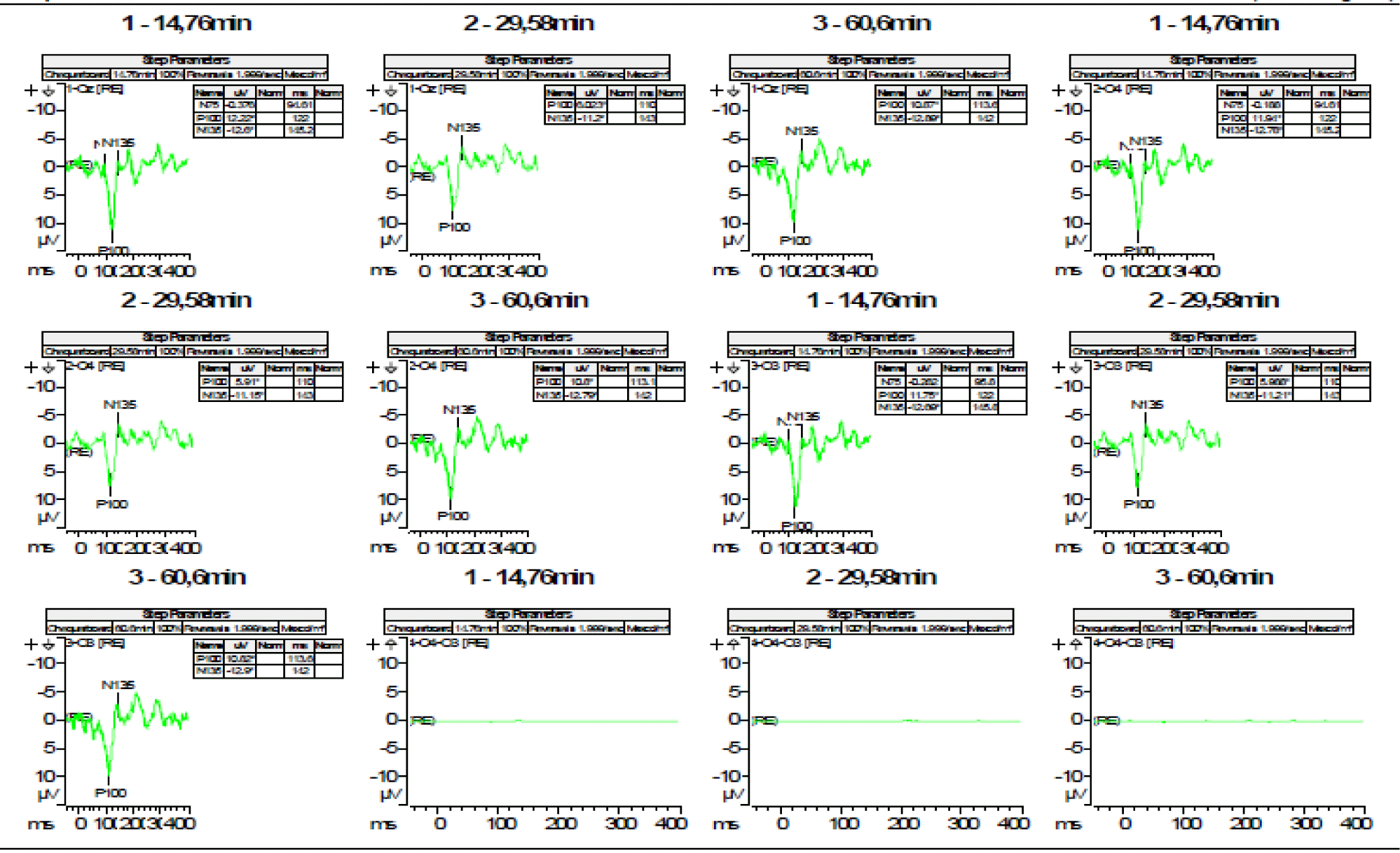
- PEVs with alternating checkerboard stimulus showing a discrete delay in the latency of the P100 wave for the 15′ arc angle. This disturbance is corrected by increasing the image size. All other parameters are normal (Figures 1,2).
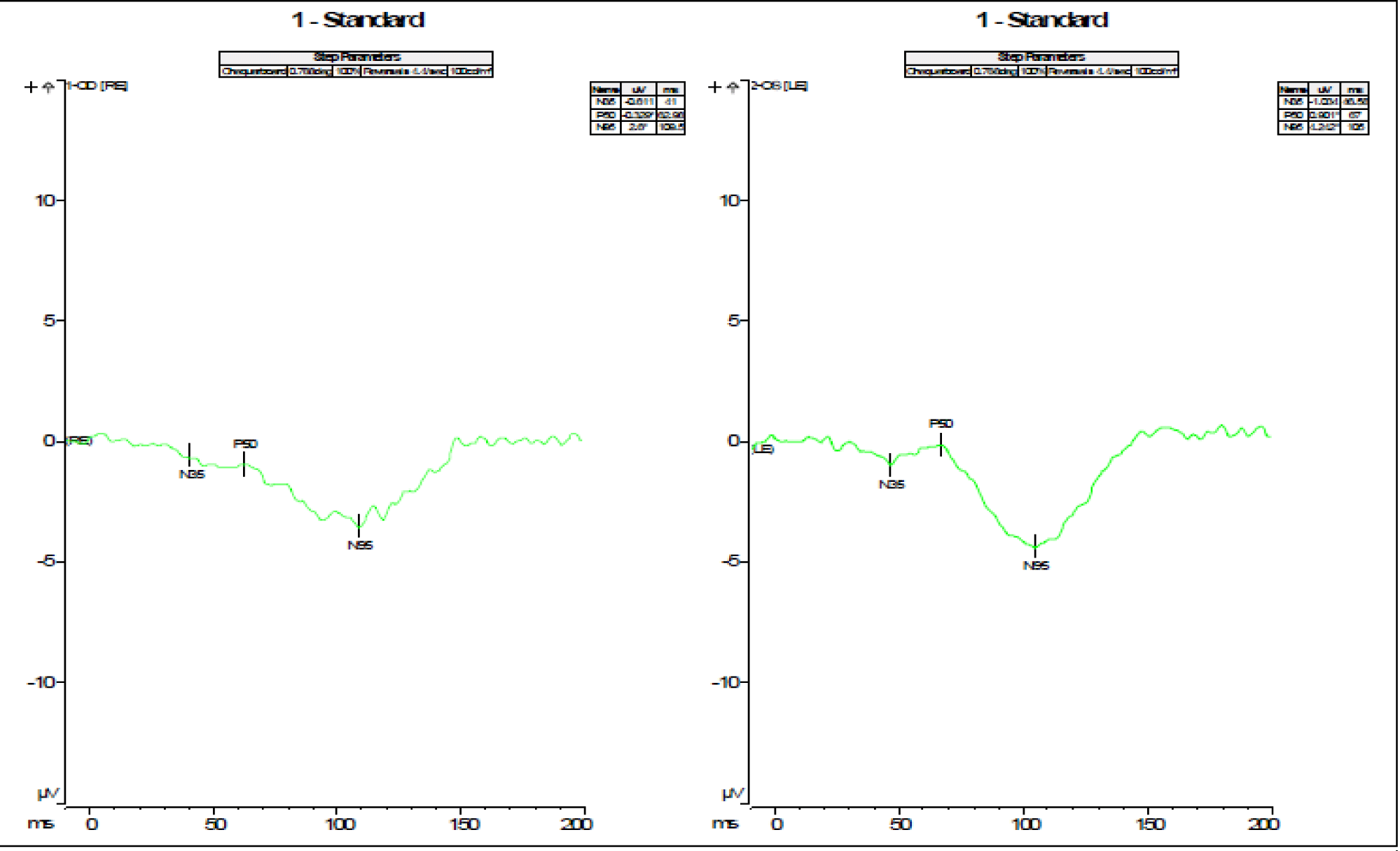
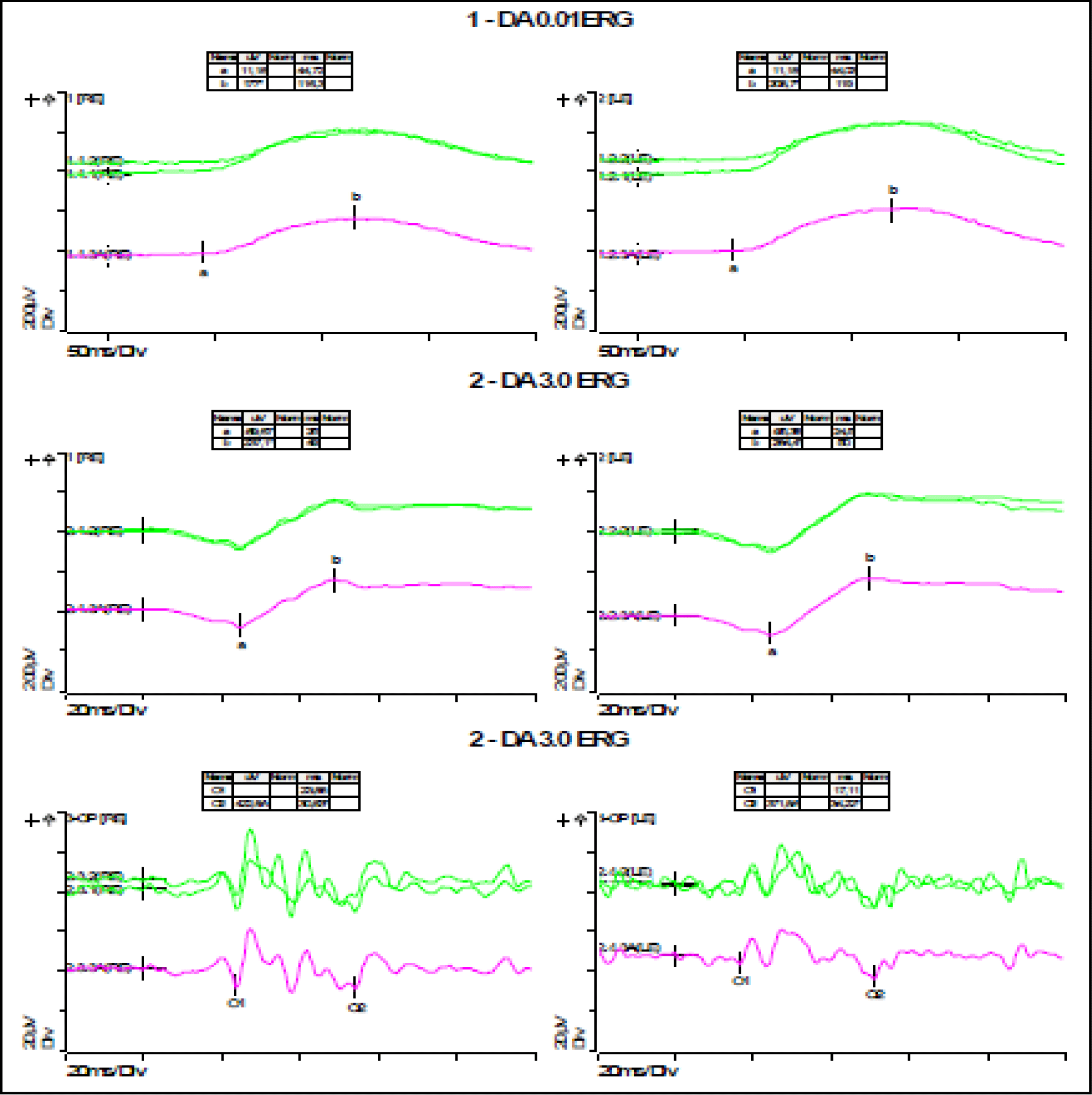
- ERG pattern with virtually no P50 potential in the right eye and very low amplitude and delayed latency in the left eye. There is also an impairment in the N95 wave (Figure 3).
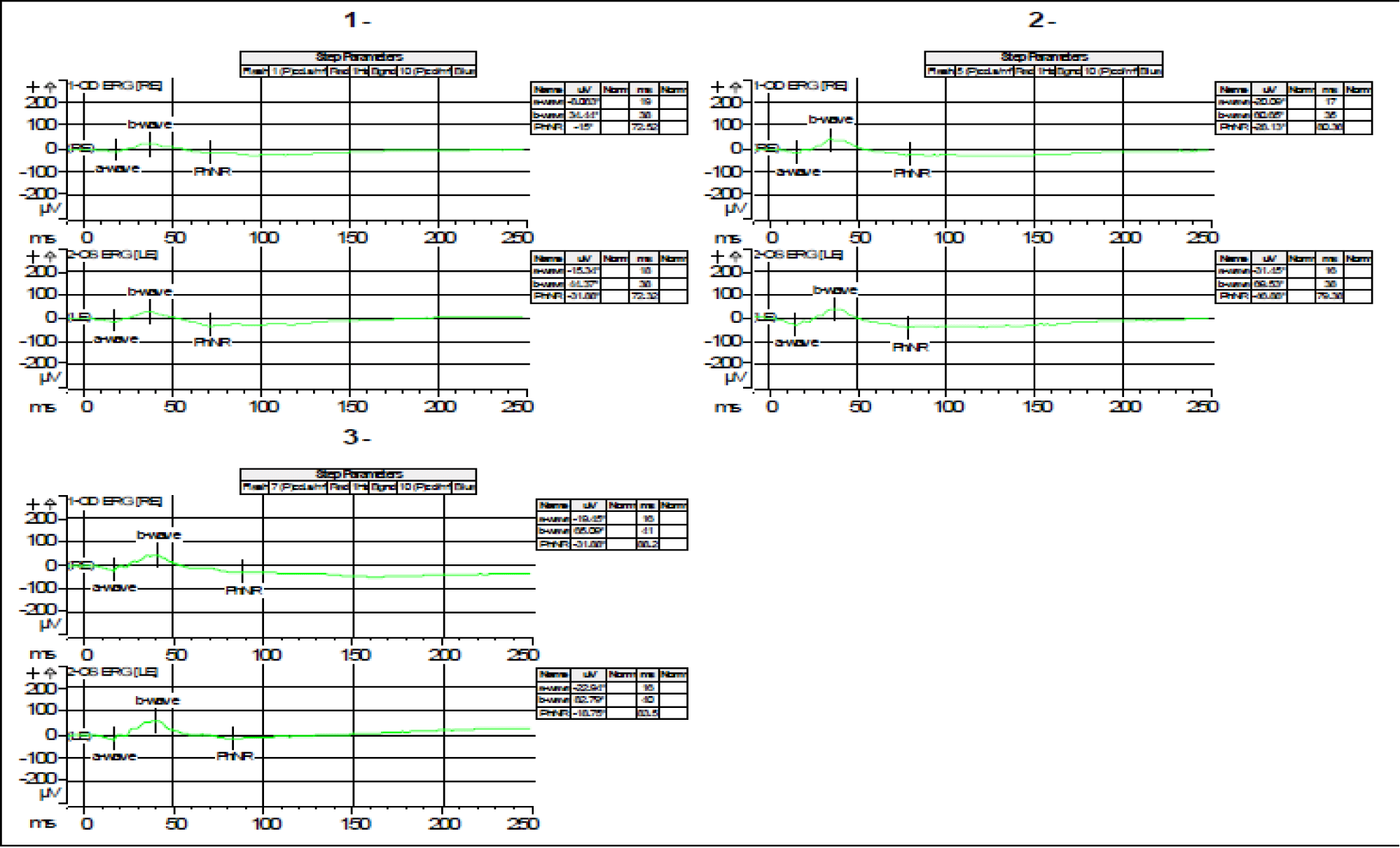
- Negative photopic response with bilaterally delayed latency (Figure 4).
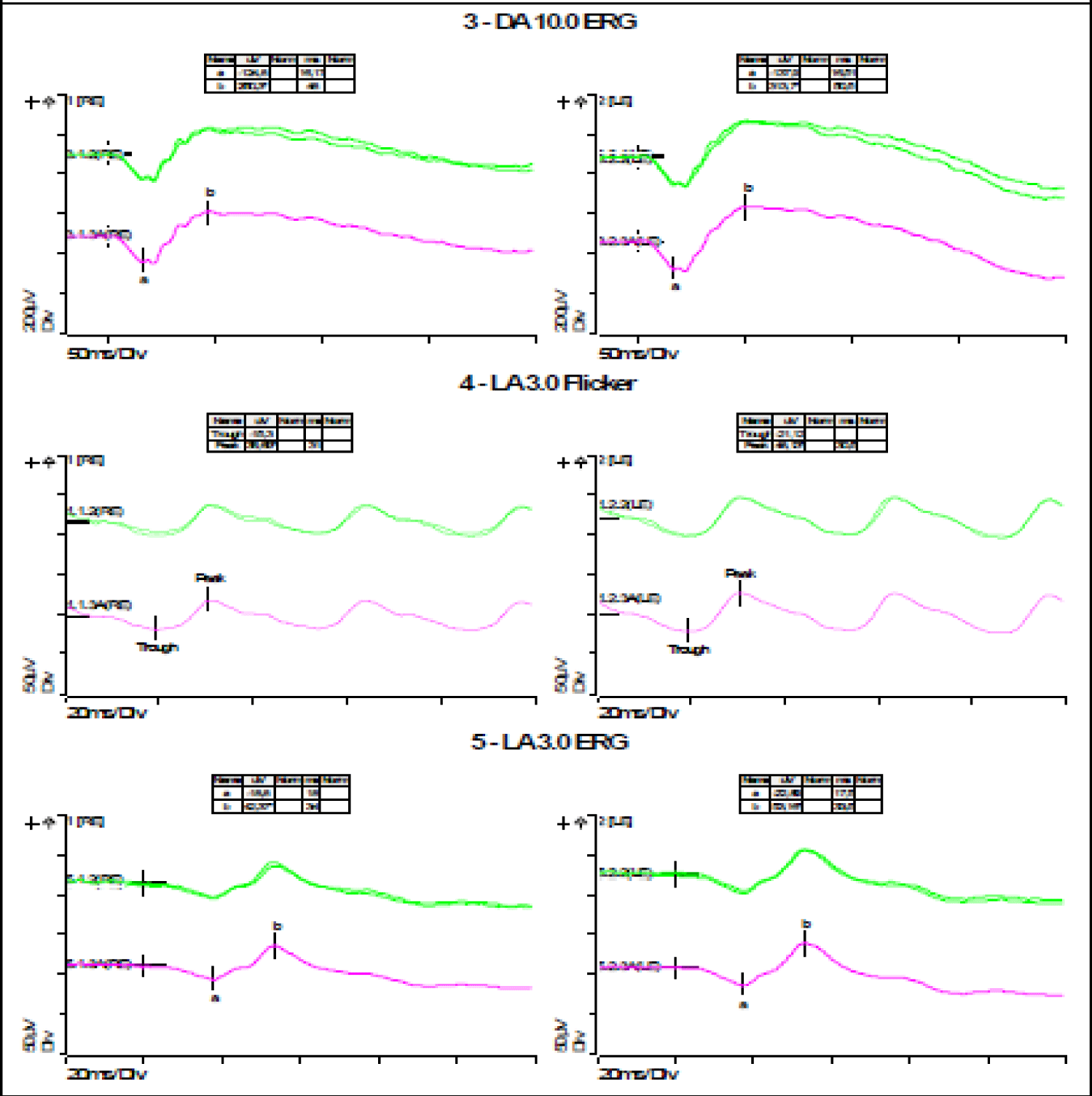
- ERG Ganzfeld without pharmacological mydriasis (Figures 5,6);
• Decrease in amplitude and delay in a- and b-wave latencies under both photopic and scotopic conditions.
• Low amplitude oscillatory potentials with bilaterally disaggregated morphology.
• Flicker at 30 Hz with low amplitude and extended culmination time bilaterally.
Conclusion
Signs of bilateral diffuse retinopathy, which is worse in the case of the right eye, mainly affecting inner retinal layers.
A follow-up visit was made to the ophthalmology department, with a clinical examination similar to the previous one. On this occasion, macular OCT was performed with the following result: both eyes; foveal profile preserved with decreased foveal notch. Decreased thickness of nuclear layers. Preserved photoreceptor line.
With the diagnosis of phacosclerosis and bilateral retinopathy, a cautious position was adopted.
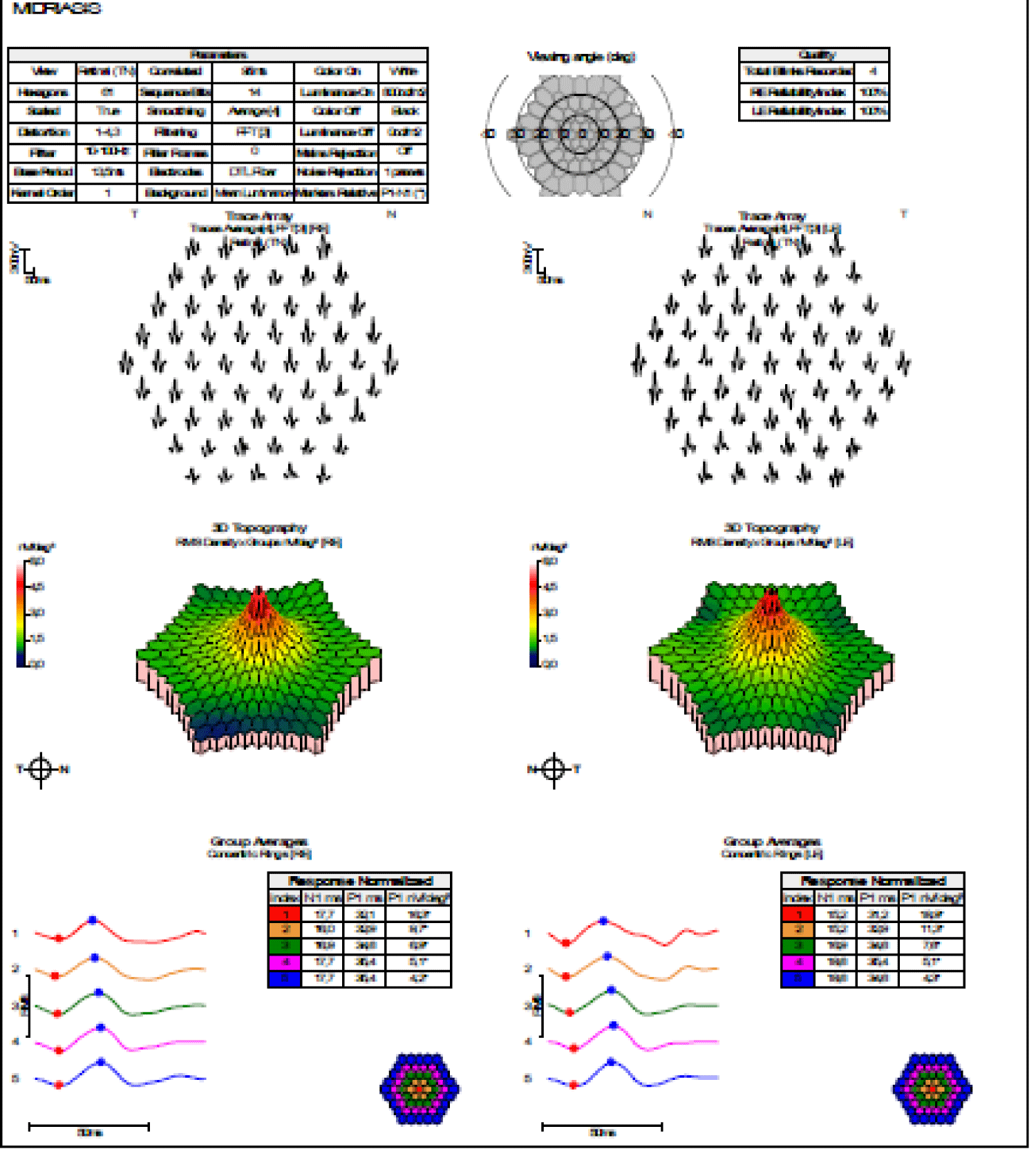
In a subsequent neurophysiological check-up seven months after the first study, the results are practically identical to the first, although the patient reports some subjective improvement. On this occasion, a multifocal electroretinogram (Figure 7) was also performed under conditions of pharmacological mydriasis, which showed a generalised low electrical density.
Discussion
The ability of various strains of coronavirus to cause retinal pathology in animal studies has been known for many years [2]. Several current studies [1,3-5] present cases of this pathology in the context of the SARS-CoV-2 pandemic, essentially based on funduscopy, radiological examination by Magnetic Resonance Imaging (MRI) and Optical Coherence Tomography (OCT). The most frequent findings among critically ill patients are related to microvascular complications [6] (haemorrhages, cotton wool spots, venous dilatation) although, as mentioned in the introduction, these are not the only findings. It should be noted that, at the time of writing this paper, we are not aware of any literature on electrophysiological examinations of the visual pathway in this type of patient.
Based on the data available to us, it is unlikely from a neurophysiological point of view that the aetiology of the retinopathy in the patient in question is SARS-CoV-2. The problem cannot be attributed to her stay in the ICU either; or due to drugs administered, both chronically and during her hospital stay (no alterations have been described in the electrophysiological tests at therapeutic doses). The ICU stay would be particularly critical in the case of a patient requiring mechanical ventilation and pronation, as retinopathy could occur secondary to impaired retinal venous drainage due to increased central venous pressure [4,7]. The patient in question did not require mechanical ventilation or pronation. However, this patient has several cardiovascular risk factors (hypertension, obesity, OSA) that predispose her to retinopathy, which affects the inner layers of the retina. One might wonder whether in an especially vulnerable individual, a situation of severe respiratory distress with hypoxaemia such as the one she suffered could trigger a latent retinal pathology.
The main benefit for this patient was to objectify functional alterations that guided the ophthalmologist in her diagnosis. We consider it useful to have two points of view at the time of diagnosis: the morphological one (OCT) and the functional one (electrophysiology). Currently, we have no treatment for this entity, but when it comes to prevention, neurophysiology tests are cheap and safe tools that could help to identify and follow patients at high risk of retinal damage due to hypoxia in order to assume more effectives treatment and surveillance measures from earlier phases.
Conclusion
In any person who has suffered SARS-CoV-2 infection with vision loss, it appears to be essential to carry out a proper clinical and OCT examination, and electrophysiological studies may be useful to determine the existence of retinal pathology and its level of damage to the retina. It is important to consider the individual patient's ophthalmological vulnerability to retinal pathology in the context of severe acute respiratory distress syndrome.
References
- Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J Ophthalmol. 2021 Mar;69(3):488-509. doi: 10.4103/ijo.IJO_297_21. PMID: 33595463; PMCID: PMC7942063.
- Vinores SA, Wang Y, Vinores MA, Derevjanik NL, Shi A, Klein DA, Detrick B, Hooks JJ. Blood-retinal barrier breakdown in experimental coronavirus retinopathy: association with viral antigen, inflammation, and VEGF in sensitive and resistant strains. J Neuroimmunol. 2001 Oct 1;119(2):175-82. doi: 10.1016/s0165-5728(01)00374-5. PMID: 11585619; PMCID: PMC7119735.
- Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V, Giacomelli A, Cigada M, Milazzo L, Ridolfo A, Faggion I, Cordier L, Oldani M, Marini S, Villa P, Rizzardini G, Galli M, Antinori S, Staurenghi G, Meroni L. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020 Oct;27:100550. doi: 10.1016/j.eclinm.2020.100550. Epub 2020 Sep 20. PMID: 32984785; PMCID: PMC7502280.
- Lecler A, Cotton F, Lersy F, Kremer S, Héran F; SFNR's COVID Study Group. Ocular MRI Findings in Patients with Severe COVID-19: A Retrospective Multicenter Observational Study. Radiology. 2021 May;299(2):E226-E229. doi: 10.1148/radiol.2021204394. Epub 2021 Feb 16. Erratum in: Radiology. 2022 Jan;302(1):E4. PMID: 33591889; PMCID: PMC7887777.
- Turker IC, Dogan CU, Guven D, Kutucu OK, Gul C. Optical coherence tomography angiography findings in patients with COVID-19. Can J Ophthalmol. 2021 Apr;56(2):83-87. doi: 10.1016/j.jcjo.2020.12.021. Epub 2021 Jan 9. PMID: 33497612; PMCID: PMC7833266.
- Pereira LA, Mansano Soares LC, Nascimento PA, Netto Cirillo LR, Sakuma HT, Da Veiga GL, et al. Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol. 2020;1-4.
- Sanghi P, Malik M, Hossain IT, Manzouri B. Ocular Complications in the Prone Position in the Critical Care Setting: The COVID-19 Pandemic. J Intensive Care Med. 2021 Mar;36(3):361-372. doi: 10.1177/0885066620959031. Epub 2020 Sep 28. PMID: 32985317
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.