Medicine Group . 2023 January 27;4(1):104-116. doi: 10.37871/jbres1654.
Is Anosmia-Ageusia in COVID-19 Patients Associated with Neuro-Philic Virus Mutant and Mild Respiratory Involvement?
Duygu Zorlu1*, Ali Bulut2, Lokman Hızmalı3 and Yahya Sahin4
2Free Cardiovasculer Surgery Clınıc, Antalya, Turkey
3Ahi Evran Üniversity, Medical Faculty of Clınıc Mıcrobıology and Infectıon Disease Department, Kirsehır, Turkey
4Ahi Evran Üniversity, Medical Faculty of Emergency Department, Kirsehır, Turkey
- COVID-19
- Loss of taste and smell
- Neuro-specific SARS-CoV-2
- Anosmia-ageusia
- Neuro-philic virus mutant
- Pulmono-philic virus mutant
Abstract
Introduction and Objective: In COVID-19, sudden onset anosmia-ageusia can be observed in patients, regardless of other rhinologic symptoms or prominent nasal symptoms.
In our clinical follow-ups, it has drawn our attention that patients presenting with anosmia-ageusia have milder pulmonary symptoms and milder progression. It was thought that this group of patients were infected with neurosensitive SARS-CoV-2 and the study was planned based on this hypothesis. We present our results to contribute to the literature because our study may be a practical screening approach in patient follow-up, may provide predictions of disease progression, and isolation period can be determined.
Materials and Methods: The study was conducted in March 2020, by interviewing recorded patients via phone. Patients’ anosmia-ageusia characteristics, hospitalizations, and recorded Thoracic Computed Tomography (CT) reports were evaluated. The reports were analyzed by a single physician and CT positivity was reported and grouped as mild, moderate and severe.
Results: A total of 1438 patients were included in the study. Of the patients, 47.8% were male and 52.0% were male, while the mean age was 44.33 ∓ 16.01 years. In terms of educational levels, patients were found to be elementary (25.6%) and high school (20.7%) graduates at most.
Discussion and Conclusion: Hospitalization rates of patients presenting with anosmia-ageusia were lower and their disease progression were milder. We suggest that there are mainly neuro- or pulmonary-sensitive variants of the virus. This characteristic is of great importance for the long-term follow-up of these patients and predictions of complications. Patients presenting with a sudden development of anosmia-ageusia should be considered as positive patients. Quarantine and isolation periods should be extended in these patients, keeping in mind the prolonged RT-PCR positivity as well. Besides, it should be noted that these patients are more likely to develop central nervous system complications and they should be followed up.
Introduction
Pulmonary symptoms such as dyspnea, cough, and chest pain represent forerunner symptoms of COVID-19 and have been determinant in the disease progression and even in mortality. However, different symptom groups of extrapulmonary manifestations such as diarrhea, myocardial infarction associated with different system involvement (such as gastrointestinal, cardiovascular system) are observed as well. Current data considers small vessel “vasculitis” as the etiopathogenic cause of multi-organ involvement [1,2].
Headache, diarrhea, arthralgia, malaise/fatigue, and anosmia-ageusia are among the major extrapulmonary symptoms. Smell dysfunction has been reported in other viral infections (parainfluenza, rhinovirus, SARS, and others), but the incidence is much lower than SARS-CoV-2 infection. It has been stated that prognosis of smell and taste recovery in SARS-CoV-2 is better than other post-viral infection. The pathophysiology of post-infectious olfactory loss was hypothesized that viruses may produce an inflammatory reaction of the nasal mucosa or damage the olfactory neuroepithelium directly. However, loss of smell could be presented in COVID-19 patients without other rhinologic symptoms or significant nasal inflammation. Pathological mechanisms still remain unclear but most likely because of primary infection of non-neuronal olfactory epithelial cell types leading to olfactory neuron injuries. Increasing verified evidence and recent studies from many countries have revealed that sudden onset olfactory and taste loss could be a sign of SARS-CoV-2 infection. Anosmia or ageusia, which are characteristics symptoms, would help to provide an early diagnosis of COVID-19. Therefore, COVID-19 patients should be identified in this way and their own isolation process should be initiated [3,4].
It was remarkable in our clinical follow-ups that the pulmonary symptoms of the patients who presented with anosmia and/or ageusia were milder and their progression was more moderate. This group of patients is believed to be infected with neurosensitive SARS-CoV-2. We planned our study with this hypothesis. We present our results to contribute to the literature because our study may be a practical screening approach in patient follow-up, may provide predictions of disease progression, and isolation period can be determined.
Materials and Methods
Ethical approval (Decision number: 2021-03/35) for this study was obtained from….. University Clinical Research Ethics Committee. Informed consent was obtained from all patients who were volunteer to participate in the study.
Study population
The study was conducted with patients diagnosed with COVID-19 who were registered by the Provincial Directorate of Health. The study started in March 2021 and for the study population, 1500 patients were randomly and retrospectively screened as of this date, and these patients were interviewed via telephone. The evaluation of 1500 random patients composing the study population was carried out by interviewing to the patients on the official line of the Provincial Directorate of Health. Patient Follow-up Form named “Clinical characteristics of patients diagnosed with COVID-19” was created by the researchers to assess patients’ characteristics (Table 1, Figure 1). The questions include data on COVID-19 symptoms, diagnostic features, and drug use, as well as the demographic characteristics of the participants (Figure 2). The answers to the questions asked one-to-one to the participants from the official line were recorded on each patient form. Each patient, who were volunteered to participate in the study, was informed about the study at the phone call. Consent and phone calls were recorded. 8 patients stated that they did not want to participate in the study. 42 patients did not volunteer to participate in the study and 12 patients could not be reached from the phone numbers they gave. The data of these patients were excluded from the study. Pediatric population were also excluded from the study. A total of 1438 patients who volunteered to participate in the study were included in the study.
Patients’ laboratory and radiological examination data were obtained from existing records. Patients’ recorded Thoracic Computed Tomography (CT) reports were evaluated from a prepared patient file by a single pulmonologist. The reports were analyzed singly and the severity of CT positivity was reported and grouped as mild, moderate and severe.
Statistical analysis
Statistical analysis of the study was performed using Statistical Package for Social Sciences version 25.0 software for Windows (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp., USA). The assumption of normality for quantitative data was tested with the Kolmogorov-Smirnov and Shapiro-Wilk tests. Explanatory statistics in the study were given as mean ∓ standard deviation and n (%). For univariate analysis of variables included in the study, chi-square and independent t-test were used depending on the type of variable and the availability of assumptions. In all statistical analysis, p < 0.05 values were interpreted as statistically significant.
The sample size of the study was computed by performing power analysis. As a result of the power analysis performed by taking effect size w = 0.15, power (1-β) = 0.95, Df = 13 for the chi-square test, it was determined that the total sample size should be a minimum of 1180. When effect size d = 0.20 and power (1-β) = 0.95 in the power analysis for the independent t-test, the required minimum sample size was found as 1305. Considering these minimum sample sizes in the study, the research was conducted with a total of 1438 patients. Power analysis was performed using G*Power 3.1.9.6 (Franz Faul, Universitat Kiel, Germany).Results
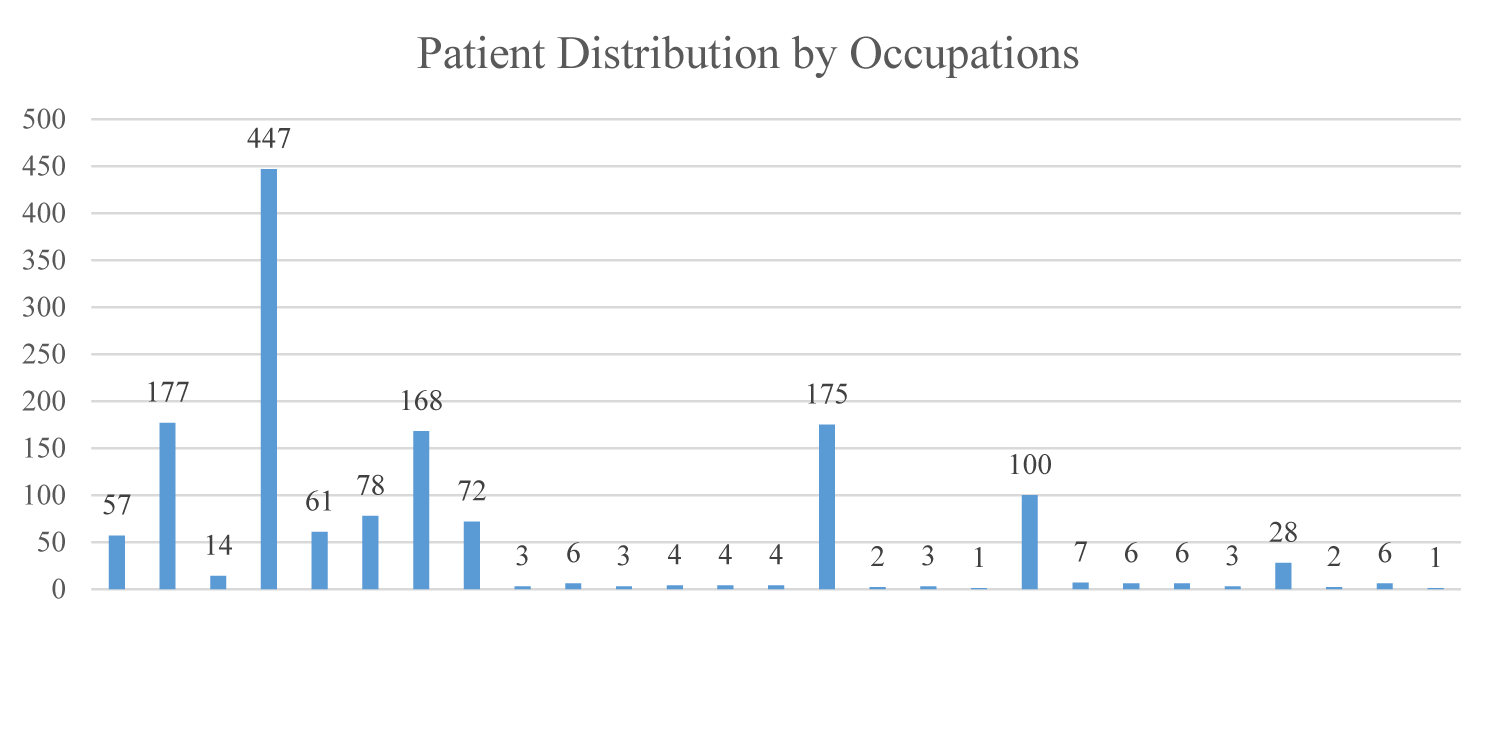
A total of 1438 patients were included in the study. Of the patients, 47.8% were male and 52.0% were male, while the mean age was 44.33 ∓ 16.01 years. In terms of educational levels, patients were found to be elementary (25.6%) and high school (20.7%) graduates at most. In terms of occupations, it was found that those who were diagnosed with COVID-19 at most were homemakers (31.1%) (Figure 1). Evaluating the comorbidities of patients diagnosed with COVID-19, it was seen that Essential Hypertension (HT) (28.0%) and Diabetes Mellitus (DM) (21.7%) were at most. 964 (67.04%) of the patients had no comorbidities. 179 (37.76%) of 474 (32.96%) patients with comorbidity have more than one comorbidities. Only 295 (62.23%) of the patients have a single comorbidity (Table 1).
| Table 1: Descriptive statistics and frequencies of variables. | ||
| Variables | N (%) | |
| Gender | ||
| Female | 688(47.8) | |
| Male | 748(52.0) | |
| Missing | 2(0.1) | |
| Educational Level | ||
| Elementary | 368(25.6) | |
| Secondary | 147(10.2) | |
| High School | 297(20.7) | |
| Associate degree | 268(18.6) | |
| Bachelor’s degree | 69(4.8) | |
| Master’s degree | 28(1.9) | |
| Illiterate | 30(2.1) | |
| Missing | 231(16.1) | |
| Occupation | ||
| Teacher | 57(4.0) | |
| Retired | 177(12.3) | |
| Farmer | 14(1.0) | |
| Homemaker | 447(31.1) | |
| Healthcare worker | 61(4.2) | |
| Student | 78(5.4) | |
| Laborer | 168(11.7) | |
| Craft | 72(5.0) | |
| Academic | 3(0.2) | |
| Military personnel | 6(0.4) | |
| Cook | 3(0.2) | |
| Caregiver | 4(0.3) | |
| Bank employee | 4(0.3) | |
| Office staff | 4(0.3) | |
| Unemployed | 175(12.2) | |
| Child development | 2(0.1) | |
| Security guard | 3(0.2) | |
| Servant | 1(0.1) | |
| Gov. servant | 100(7.0) | |
| Engineer | 7(0.5) | |
| Police | 6(0.4) | |
| Salesman | 6(0.4) | |
| Secretary | 3(0.2) | |
| Driver | 28(1.9) | |
| Technician | 2(0.1) | |
| Cleaning staff | 6(0.4) | |
| Other | 1(0.1) | |
| Chronic Diseases | ||
| HT | 200(28.0) | |
| DM | 155(21.7) | |
| Asthma | 62(8.7) | |
| COPD | 42(5.9) | |
| Liver Disease | 39(5.5) | |
| CAD | 102(14.3) | |
| Kidney Disease | 13(1.8) | |
| Thyroid Disease | 40(5.6) | |
| Neurological Diseases | 14(2.0) | |
| GIS | 6(0.8) | |
| BPH | 7(1.0)) | |
| Other Lung Diseases | 12(1.7) | |
| Cancer | 8(1.1) | |
| Rheumatic diseases | 11(1.5) | |
| Pregnant | 1(0.1) | |
| Eye Diseases | 1(0.1) | |
| Psychiatric Diseases | 2(0.1) | |
| First PCR Test Result (2020) | ||
| March | 1(0.1) | |
| April | 0(0.0) | |
| May | 0(0.0) | |
| June | 43(3.0) | |
| July | 25(1.7) | |
| August | 138(9.6) | |
| September | 491(34.1) | |
| October | 340(23.6) | |
| November | 356(24.8) | |
| December | 41(2.9) | |
| Missing | 3(0.2) | |
| Date of COVID-19 Diagnosis (2020) | ||
| June | 43(3.0) | |
| July | 26(1.8) | |
| August | 138(9.6) | |
| September | 488(33.9) | |
| October | 338(23.5) | |
| November | 357(24.8) | |
| December | 44(3.1) | |
| Missing | 4(0.2) | |
| Complaint at Admission | ||
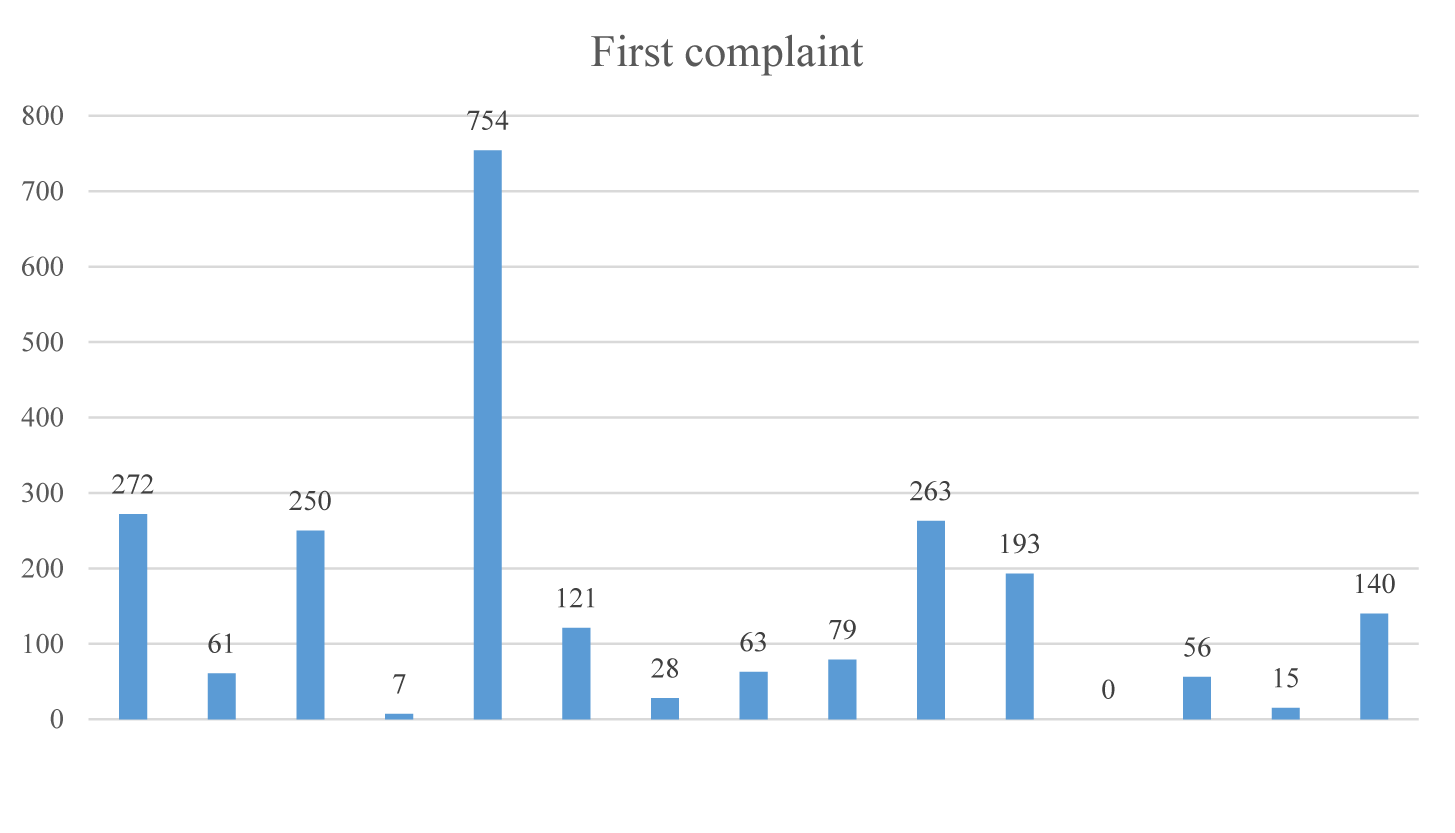
| Cough | 272(11.7) | |
| Shortness of breath | 61(2.6) | |
| Fever | 250(10.8) | |
| Abdominal pain | 7(0.3) | |
| Malaise/Fatigue | 745(32.1) | |
| Headache | 121(5.2) | |
| Nasal flow | 28(1.2) | |
| Nausea-vomiting | 63(2.7) | |
| Perspiration | 79(3.4) | |
| Arthralgia | 263(11.3) | |
| Throat ache | 193(8.3) | |
| Diarrhea | 0(0.0) | |
| Loss of taste and smell | 56(2.4) | |
| Chest pain | 15(0.6) | |
| No complaints | 140(6.0) | |
| 17 | 14(0.6) | |
| 22 | 17(0.7) | |
| Prescribing Favipiravir | ||
| Yes | 929(64.6) | |
| No | 416(28.9) | |
| Missing | 93(6.5) | |
| Use of Fvipiravir | ||
| Yes | 928(64.5) | |
| No | 457(31.8) | |
| Missing | 53(3.7) | |
| Prescribing Chloroquine | ||
| Yes | 756(52.6) | |
| No | 622(43.3) | |
| Missing | 60(4.2) | |
| Use of Chloroquine | ||
| Yes | 748(52.0) | |
| No | 617(42.9) | |
| Missing | 73(5.1) | |
| Prescribing Anticoagulants | ||
| Yes | 610(42.4) | |
| No | 746(51.9) | |
| Missing | 82(5.7) | |
| Use of Anticoagulants | ||
| Yes | 577(40.12) | |
| No | 753(52.40) | |
| Missing | 108(7.5) | |
| Dietary Supplements | ||
| Vitamin D | 68(4.6) | |
| Vitamin C | 319(21.4) | |
| Multivitamin | 87(5.8) | |
| Zinc | 8(0.5) | |
| Magnesium | 2(0.1) | |
| Propolis | 5(0.3) | |
| Black elder (Sambucus nigra) | 4(0.3) | |
| Ginger | 5(0.3) | |
| Turmeric (Curcuma longa) | 2(0.1) | |
| Did not use any supplement | 988(66.4) | |
| Presenting Symptoms | ||
| Shortness of breath | 41(2.9) | |
| Chest pain/Stinging feeling in the chest | 12(0.8) | |
| Cough | 28(1.9) | |
| Malaise/Fatigue | 440(30.6) | |
| Loss of taste and smell | 29(2.0) | |
| No presenting symptom | 803(55.8) | |
| Missing | 85(5.9) | |
| Perceptual, Concentration and Attention Deficit | ||
| Yes | 208(14.5) | |
| No | 1122(78.0) | |
| Missing | 108(7.5) | |
| Smoking Status | ||
| Yes | 230(16.0) | |
| No | 1167(81.2) | |
| Missing | 41(2.9) | |
| Cigarettes (Box per year) | 4.78∓12.21 | |
| Quitting | ||
| Yes | 86(6.0) | |
| No | 942(65.5) | |
| Missing | 410(28.5) | |
| Anosmia-Ageusia | ||
| Yes | 598(41.6) | |
| No | 838(58.3) | |
| Missing | 2(0.1) | |
| Smell and Taste Recovery | ||
| After 7 days | 197(13.7) | |
| After 10-30 days | 308(21.4) | |
| After 30-40 days | 45(3.1) | |
| Unknown/Failure to reach/missing | 818(56.9) | |
| Still on-going | 70(4.9) | |
| Inpatient Treatment | ||
| Yes | 80(5.6) | |
| No | 1345(93.5) | |
| Missing | 13(0.9) | |
| Inpatient treatment (days) | 0.59∓3.00 | |
| Length of Inpatient Treatment (days) | ||
| 0 | 1345(93.5) | |
| 1 | 4(0.3) | |
| 2 | 3(0.2) | |
| 3 | 9(0.6) | |
| 4 | 6(0.4 | |
| 5 | 14(1.0) | |
| 6 | 3(0.2) | |
| 7 | 14(1.0) | |
| 8+ | 40(2.8) | |
| Reporting Oxygen Concentration | ||
| Yes | 63(4.4) | |
| No | 1157(80.5) | |
| Missing | 218(15.2) | |
| Current use of Oxygen Concentrator | ||
| Yes | 44(3.1) | |
| No | 1321(91.9) | |
| Missing | 73(5.1) | |
| Getting Information about COVID-19 | ||
| Social media | 594(38.8) | |
| News/TV | 530(34.6) | |
| From the doctor he/she got examined | 69(4.5) | |
| From the filiation team | 98(6.4) | |
| From the family doctor | 59(3.9) | |
| Acquaintance/neighbor/relative | 54(3.5) | |
| He/she or his/her spouse is healthcare professional | 54(3.5) | |
| Provincial directorate of health | 73(4.8) | |
| CT Positivity | ||
| None (BT Normal) | 580(37.9) | |
| Mild | 238(15.5) | |
| Moderate | 73(4.8) | |
| Severe | 30(2.0) | |
| Missing | 517(33.8) | |
| HT: Essential Hypertension; DM: Diabetes Mellitus; COPD: Chronic Obstructive Pulmonary Disease; CAD: Coronary Artery Disease; GIS: Gastrointestinal System; BPH: Benign Prostatic Hyperplasia; CT: Computed Tomography. | ||
It was determined that the number of positive RT-PCR tests has increased since June 2020. The highest number of positive RT-PCR tests were seen in September 2020 (34.1%). The period when RT-PCR test results increased was September, October and November 2020. Therefore, the numbers on the date of COVID-19 diagnosis also increased in the specified months.
Patients generally applied to the health institution with more than one complaint. There were 272 (11.70%) patients with the complaint of cough. The number of patients who applied to the healthcare institution with the complaint of cough only was 41 (2.85%). There were 61 (4.24%) patients with the complaint of shortness of breath. The number of patients who applied with the complaint of shortness of breath only was 13 (0.09%). Patients were found to apply with the complaints of malaise/fatigue (32.06%) at most. The complaint that was not stated during the period of study was diarrhea. The number of patients who did not have any complaints or symptoms but having contact with someone with COVID-19 was 140 (6.02%) (Figure 2).
Of the patients, 64.60% were prescribed favipiravir while 28.92% were not. 64.5% of the patients used the prescribed drug (Favipiravir tablet) while 31.8% did not. Of the patients, 52.6% were prescribed chloroquine while 43.3% were not. 52.0% of the patients used chloroquine tablet. 610 (42.4%) patients were prescribed anticoagulants and 40.12% of them used this medication.
A significant part of patients (66.4%) reported not using dietary supplements during the pandemic. The most preferred dietary supplement was vitamin C with 21.4%. During the study period, 30.6% of patients reported that their complaints of malaise/fatigue were still ongoing, while 803 (55.8%) patients reported not. However, 14.5% of patients stated that they suffered from perceptual and concentration deficit, that they experienced “brain fog”.
The smoking rate of the patients included in the study was 16.0%. 81.2% of patients did not smoke. The rate of those who quit smoking was 6.0%, and the rate of those who did not quit was 65.5% (n = 942).
5.6% of patients had inpatient treatment, while 93.5% (1345) had outpatient treatment. The number of patients having inpatient treatment for more than 8 days was 40 (2.8%). Of the patients included in the study, 63 (4.4%) were reported oxygen concentrator in discharge, while 1157 (80.5%) did not have a reporting indication. 44 (3.1%) patients reported that they were currently using supportive care with an oxygen concentrator.
Patients stated that they got information about COVID-19 from social media (38.8%) and news/TV (34.6%) at most.
Patients’ CT findings were found normal (37.9%), mild (15.5%), moderate (4.8%) and severe (2.0%).
41.6% of patients developed anosmia and ageusia. 2 patients reported that they could not perceive smells (anosmia) but developed loss of taste (ageusia) other than for sweet. They reported that they felt the sensation of “sugar water” from every nutrition while consuming liquid food in particular. The remaining patients developed both anosmia and ageusia. 13.7% of patients regained their senses of smell and taste after 7-10 days, 21.4% regained after 10-30 days, but 4.9% continue to suffer from this problem (Table 1).
The average age of patients who developed anosmia-ageusia was lower than those who did not develop and this difference was statistically significant (p < 0.01). The association of the development of anosmia-ageusia with gender was statistically insignificant (p > 0.05). In other words, the development of anosmia-ageusia is not related to gender. The association of anosmia-ageusia with inpatient treatment was also statistically insignificant (p > 0.05) (Table 2).
| Table 2: Descriptive statistics and group comparisons regarding the development of loss of taste and smell. | ||||
| Loss of Taste and Smell | ||||
| Variables | Yes | No | p | |
| Age, (Year) | 40.36 ∓ 16.30 | 43.93 ∓ 17.56 | 0.000 | |
| Gender | ||||
| Female | 317(50.3) | 411(46.8) | 0.172 | |
| Male | 313(49.7) | 468(53.2) | ||
| Inpatient Treatment | ||||
| Yes | 29(4.9) | 51(6.1) | 0.320 | |
| No | 563(95.1) | 781(93.9) | ||
| CT Positivity | ||||
| None (BT Normal) | 255(68.0) | 339(60.5) | 0.102 | |
| Mild | 87(23.2) | 150(26.8) | ||
| Moderate | 24(6.4) | 50(8.9) | ||
| Severe | 9(2.4) | 21(3.8) | ||
| Date of COVID-19 Diagnosis | ||||
| June | 11(1.7) | 32(3.6) | 0.000 | |
| July | 6(1.0) | 22(2.5) | ||
| August | 63(10.0) | 80(9.1) | ||
| September | 188(29.8) | 324(36.9) | ||
| October | 150(23.8) | 206(23.5) | ||
| November | 187(29.7) | 193(22.0) | ||
| December | 25(4.0) | 20(2.3) | ||
| Use of Favipiravir | ||||
| Yes | 436(69.5) | 498(60.1) | 0.000 | |
| No | 191(30.5) | 331(39.9) | ||
| Use of Chloroquine | ||||
| Yes | 318(51.4) | 440(53.7) | 0.377 | |
| No | 301(48.6) | 379(46.3) | ||
| Use of Anticoagulants | ||||
| Yes | 262(42.2) | 320(41.0) | 0.646 | |
| No | 359(57.8) | 461(59.0) | ||
| Smell and Taste Recovery | ||||
| 1-7 days | 178(37.2) | 33(18.9) | 0.000 | |
| 10-30 days | 226(47.2) | 98(56.0) | ||
| 30-40 days | 27(5.6) | 21(12.0) | ||
| Still ongoing | 48(10.0) | 23(13.1) | ||
| Perceptual, Concentration and Attention Deficit | ||||
| Yes | 154(24.6) | 65(8.4) | 0.000 | |
| No | 471(75.4) | 711(91.6) | ||
| Smoking Status | ||||
| Yes | 116(18.5) | 117(13.9) | 0.017 | |
| No | 511(81.5) | 725(86.1) | ||
| CT: Computed Tomography; p: power. | ||||
Only 4.9% of these patients in this group had inpatient treatment, while 95.1% had outpatient treatment. The association of thoracic CT positivity with the development of anosmia-ageusia was also statistically insignificant (p > 0.05). 255 (68.0%) patients had normal thoracic CT finding, while only 9 (2.4%) of them had severe CT positivity. Of the patients who did not develop anosmia-ageusia, on the other hand, 339 (60.5%) had normal thoracic CT finding while 21 (3.8%) had severe CT finding.
The association of the development of anosmia-ageusia with the date of COVID-19 diagnosis was statistically significant (p < 0.01). These patients were diagnosed in September 2020 (29.8%) at most and in July 2020 (1.0%) at the least. The COVID-19 diagnosis of patients who did not develop anosmia-ageusia was made in September (36.9%) at most and in December (2.3%) at the least.
The association of the development of anosmia-ageusia with the use of favipiravir was statistically significant (p < 0.01). While 69.5% of patients who were administered favipiravir had anosmia-ageusia, 60.1 of patients were not presented with anosmia-ageusia. The association of the development of anosmia-ageusia with the use of chloroquine and anticoagulants was statistically insignificant (p > 0.05).
The association of the development of anosmia-ageusia with recovery period of other symptoms was statistically significant (p < 0.01). 10.0% of these patients reported that other complaints continued; while other complaints did not recover in 13.1% of those who did not develop anosmia-ageusia.
The association of the development of anosmia-ageusia with perceptual, concentration and attention deficit was also statistically significant (p < 0.01). While 24.6% of these patients suffered from perceptual, concentration and attention deficit, only 8.4% of those who did not develop anosmia-ageusia experienced these symptoms.
The association of the development of anosmia-ageusia with smoking was statistically significant (p < 0.05). While 18.5% of these patients were smokers, the rate of smokers in patients who did not develop anosmia-ageusia was 13.9% (Table 2).
Discussion
Loss of smell and taste can be observed in viral upper respiratory infections, while loss of smell is more common. This shows a slow and long progression and patients usually do not notice the symptoms. Its pathophysiology suggested that it may resulted from mucosal edema [3-6]. Anosmia-ageusia observed in COVID-19 is typical and characterized by sudden onset and simultaneous loss of taste and smell. In the studies performed, it was thought that the presence of olfactory neurons whose axons project through the cribriform plate of the ethmoid bone, and synapse with neurons in the central olfactory nervous system might be the related mechanism [5,7-10]. In our study, the development of anosmia-ageusia in patients was determined as 41.6%. 2 patients reported that they did not develop anosmia but perceived loss of taste (ageusia) other than for sweet. These 2 patients reported that they felt the sensation of “sugar water” from every nutrition while consuming liquid food in particular. This indicated us that, although rare, there may be anosmia-ageusia variants. This may be due to the ascending pathways of virus, neural network variation in the person, or variation in epithelial receptor levels.
13.7% of our patients regained their senses of smell and taste after 7-10 days, 21.4% regained after 10-30 days, but 4.9% continue to suffer from this problem. A similar study revealed that loss of smell in viral infections was above 60%. It was reported that a sudden, severe and isolated loss of smell occurs in 20% of viral infections. Frequency of taste disorder in affected patients has shown a high variability from 5 to 98% during the COVID-19. It was emphasized that these patients were important for early diagnosis, and they should comply with the isolation and mask-distance rules for one month [3,11-17]. Failure to check RT-PCR positivity of this group of patients in our study can be considered as an important limitation. Because we have a hypothesis that patients with anosmia-ageusia stay RT-PCR positive for longer periods. If this result is clarified by studies, it is necessary to extend the isolation and quarantine periods for these patients. RT-PCR follow-up studies with larger populations should thus be performed for patients with anosmia-ageusia.
Another study revealed that the prevalence rates of ageusia and anosmia was 6-10% in COVID-19 cases and more frequent in women population. It was stated that this finding was observed just before the onset of the disease or simultaneously with the disease. In this study, it was stated that the ageusia and anosmia continues for 7-14 days. In other studies, it was reported that the ageusia and anosmia was observed at a rate of 10-20% in COVID-19 cases [4,18-20]. In our study, ageusia and anosmia was observed at a rate of 41.6%, while this result was found to be significantly higher than the rates in similar studies in the literature. Besides, unlike that studies, no relationship between ageusia-anosmia and gender was observed in our study, and it was also seen that ageusia-anosmia was not limited to 7-14 days but can last more than 1 month; moreover, this finding may be related to the intensity of viral load.
Only 4.9% of patients who developed ageusia-anosmia were hospitalized, while 95.1% had outpatient treatment. This clinical finding confirms our hypothesis that patients with ageusia-anosmia had milder disease progression. The COVID-19 progression in patients with ageusia-anosmia was not severe enough to require hospitalization. Another study suggested that loss of smell is an independent positive prognostic factor of less severe COVID-19 infection [6,7,21] and it was also significantly associated with decreased hospitalization, intensive care unit admission, intubation, and acute respiratory distress syndrome. Similarly, smaller studies of 169 and 34 patients who received a positive COVID-19 diagnosis found an association between anosmia with outpatient care as opposed to hospitalization [7-9]. In addition, smell loss was associated with less lymphopenia and higher levels of albumin, suggesting a less severe reaction to COVID-19 in patients with smell loss than those with an intact smell [7-10]. In a study conducted with 419 (41.8%) patients, when compared with patients without smell loss, other laboratory values and inflammatory markers were not associated with smell loss among patients with COVID-19. These results did not change after adjusting for demographics and BMI and smell loss was found to be associated with decreased hospitalization and intensive care unit admission. Additionally, patients with smell loss were found to have lower intubation rates [7-9]. In our study, the relationship between blood parameters and loss of smell and taste was not evaluated and this can be considered as the limitation of the study.
In another study in the literature, a higher rate of acute smell loss was observed in COVID-19 patients with a history of preexisting smell dysfunction, allergic rhinitis, or chronic rhinosinusitis [8-12]. This also was an expected result. However, the allergic rhinitis or chronic rhinosinusitis history of patients with loss of taste and smell was not questioned in our study and this may be regarded as another limitation of our study.
COVID-19 has not been associated with persistent anosmia, and the basal rates of hyposmia and anosmia for the studied population, the prevalence of COVID-19 infection, and the individual disease stages of each infected patient could not be determined [9,10,13-16]. This may be due to the failure to compute of positive and negative predictive values. However, in a more detailed study; female gender, lower average age, higher BMI, previous history of loss of smell and preexisting allergic rhinitis and chronic rhinosinusitis were suggested to be important predictors of smell loss during COVID-19 infection [6,20-23]. In our study, too, the mean age of the patients who developed anosmia and ageusia was found to be lower than the patients who did not, while this difference was statistically significant (p < 0.01).
Anosmia and taste disturbance in SARS-CoV-2 disease: incidence and effects on COVID-19 severity and mortality, and possible pathobiological conditions - system in a review and meta-analysis; out of 32,142 COVID-19 patients from 107 studies, anosmia was reported in 12,038 patients with a prevalence of 38.2% (95% CI: 36.5%, 47.2%); whereas, dysgeusia was reported in 11,337 patients out of 30,901 COVID-19 patients from 101 studies, with prevalence of 36.6% (95% CI: 35.2%, 45.2%), worldwide. Furthermore, the prevalence of anosmia was 10.2-fold higher (OR: 10.21; 95% CI: 6.53, 15.96, p < 0.001) and that of dysgeusia was 8.6-fold higher (OR: 8.61; 95% CI: 5.26, 14.11, p < 0.001) in COVID-19 patients compared to those with other respiratory infections or COVID-19 like illness. To date, no study has assessed the association of anosmia and dysgeusia with severity and mortality of COVID-19 [24].
We evaluated the association of anosmia-ageusia with pulmonary involvement using thoracic CT and we identified that patients presenting with anosmia-ageusia had a milder progression of respiratory system symptoms and CT COVID-19 positivity; however, this correlation did not reach a statistically significant level (p = 0.102). It was found that 255 (68.0%) patients had normal thoracic CT finding, while only 9 (2.4%) of them had severe CT positivity. Of the patients who did not develop anosmia-ageusia, on the other hand, 339 (60.5%) had normal thoracic CT finding while 21 (3.8%) had severe CT finding. Although the results were statistically insignificant, they support the accuracy of our hypothesis, while severe positivity rate in thoracic CT was found to be lower in patients with the development of anosmia-ageusia. The greatest limitation in this part of our study was the fact that the anosmia-ageusia symptom onset time and thoracic CT evaluation periods were not standard in all patients and that CT findings were evaluated retrospectively. In fact, some patients received CT at the onset of symptoms, while some others received CT in the following days, depending on the clinical course and a standard assessment thus could not be made. Moreover, not every patient was evaluated by CT.
In our study, the association of the development of anosmia-ageusia with the date of COVID-19 diagnosis was found to be statistically significant (p < 0.01). These patients were diagnosed in September 2020 (29.8%) at most and in July 2020 (1.0%) at the least. The COVID-19 diagnosis of patients who did not develop anosmia-ageusia was made in September (36.9%) at most and in December (2.3%) at the least. This may be due to common virus at that time, neurosensitive type trait that was a variant mutant.
The association of the development of anosmia-ageusia with perceptual, concentration and attention deficit was found to be statistically significant (p < 0.01). While 24.6% of these patients suffered from perceptual, concentration and attention deficit, only 8.4% of those who did not develop anosmia-ageusia experienced these symptoms. This result suggests that the virus mutant may be neutrophilic. That is, the virus mutant that causes the loss of smell and taste is not pulmono-philic or has a lower pulmonophilicity. The opposite is also among our hypotheses; that is, the pulmonary-sensitive virus variant has lower neuro-sensitivity. Or, different virus mutants create viral loads in the patient and symptoms develop depending on the majority of the virus mutants. We consider these results to be significant in terms of clinical predictive value for long-term follow-up of the patients and predictions of possible complications.
In our study, anosmia-ageusia was observed in 69.5% of patients who used favipiravir (p < 0.01). There was no such as correlation between the use of chloroquine and anticoagulants (p > 0.05). This may be due to the fact that only chloroquine was administered in the treatment in the early stages of the disease, while favipiravir was not used widely. In the first periods when chloroquine was used more widely, anosmia-ageusia was observed less in the presentation of disease, while it was observed at a higher rate when favipiravir was added to the treatment. This may be due to the fact that the neutrophilic type, which is a variant mutant virus, did not spread in the early stages.
Another remarkable result of our study was that the association of the development of anosmia-ageusia with smoking was statistically significant (p < 0.05). While 18.5% of these patients were smokers, the rate of smokers in patients who did not develop anosmia-ageusia was 13.9%, which is a predictable finding; because smoking alone causes the development of epithelial damage and thus a decrease in the sense of taste and smell.
Conclusion
Especially patients presenting with a sudden development of anosmia-ageusia should be considered as positive patients and must be isolated, mask wearing-social distance rules must be followed. Quarantine and isolation periods can be extended in these patients, keeping in mind the prolonged RT-PCR positivity as well. Or, if the control RT-PCR is to be checked, this should be examined after a longer time. The incidence of having headache, absence of perception and concentration is high in these patients, so we recommend close follow-up in this respect. Besides, these patients are more likely to develop central nervous system complications. Quitting smoking in patients who smoke and develop anosmia-ageusia may reduce symptom severity, duration of anosmia-ageusia and prolonged RT-PCR positivity.
Hospitalization rates of patients presenting with anosmia-ageusia were lower and their disease progression were milder. We suggest that there are mainly neuro- or pulmono-philic variants of the virus. The determination of these characteristics is of great importance for the long-term follow-up of these patients and predictions of complications.
Acknowledgment
We thank Murat Sungun and Ozkan Görgülü for contributions. We thank all the study participants for their participation and contribution. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. DZ, AB, LH, YŞ. Acquisition of data: all authors. DZ, AB, LH, YŞ. Drafting of the manuscript: YB, Critical revision of the manuscript: DZ, LH.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Becker RC. COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020 Oct;50(3):499-511. doi: 10.1007/s11239-020-02230-4. PMID: 32700024; PMCID: PMC7373848.
- Mondal R, Lahiri D, Deb S, Bandyopadhyay D, Shome G, Sarkar S, Paria SR, Thakurta TG, Singla P, Biswas SC. COVID-19: Are we dealing with a multisystem vasculopathy in disguise of a viral infection? J Thromb Thrombolysis. 2020 Oct;50(3):567-579. doi: 10.1007/s11239-020-02210-8. PMID: 32627126; PMCID: PMC7335630.
- Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: A review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol. 2020 Jun;38(2):69-77. doi: 10.12932/AP-030520-0826. PMID: 32563234.
- Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, Klimek L, Wang DY, Liu Z. The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr Allergy Asthma Rep. 2020 Aug 3;20(10):61. doi: 10.1007/s11882-020-00961-1. PMID: 32748211; PMCID: PMC7397453.
- Wong DKC, Gendeh HS, Thong HK, Lum SG, Gendeh BS, Saim A, Salina H. A review of smell and taste dysfunction in COVID-19 patients. Med J Malaysia. 2020 Sep;75(5):574-581. PMID: 32918429.
- Cetinkaya EA. Coincidence of COVID-19 Infection and Smell-Taste Perception Disorders. J Craniofac Surg. 2020 Sep;31(6):e625-e626. doi: 10.1097/SCS.0000000000006601. PMID: 32398625; PMCID: PMC7282409.
- Daruich A, Martin D, Bremond-Gignac D. Ocular manifestation as first sign of Coronavirus Disease 2019 (COVID-19): Interest of telemedicine during the pandemic context. J Fr Ophtalmol. 2020 May;43(5):389-391. doi: 10.1016/j.jfo.2020.04.002. Epub 2020 Apr 17. PMID: 32334847; PMCID: PMC7164841.
- Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. 2020 Aug;55(4):e125-e129. doi: 10.1016/j.jcjo.2020.03.003. Epub 2020 Apr 2. PMID: 32284146; PMCID: PMC7124283.
- Casalino G, Monaco G, Di Sarro PP, David A, Scialdone A. Coronavirus disease 2019 presenting with conjunctivitis as the first symptom. Eye (Lond). 2020 Jul;34(7):1235-1236. doi: 10.1038/s41433-020-0909-x. Epub 2020 Apr 28. PMID: 32346108; PMCID: PMC7186940.
- Wege H, Watanabe R, ter Meulen V. Relapsing subacute demyelinating encephalomyelitis in rats during the course of coronavirus JHM infection. J Neuroimmunol. 1984 Aug;6(5):325-36. doi: 10.1016/0165-5728(84)90022-5. PMID: 6086712; PMCID: PMC7119698.
- Alves de Sousa F, Pinto Costa R, Xará S, Nóbrega Pinto A, Almeida E Sousa C. SARS-CoV-2 and hearing: An audiometric analysis of COVID-19 hospitalized patients. J Otol. 2021 Jul;16(3):158-164. doi: 10.1016/j.joto.2021.01.005. Epub 2021 Feb 3. PMID: 33558808; PMCID: PMC7857034.
- Sriwijitalai W, Wiwanitkit V. Hearing loss and COVID-19: A note. Am J Otolaryngol. 2020 May-Jun;41(3):102473. doi: 10.1016/j.amjoto.2020.102473. Epub 2020 Apr 2. PMID: 32276732; PMCID: PMC7132500.
- Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, Müller-Wieland D, Hartmann B, Dreher M, Müller T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020 Nov-Dec;174:106197. doi: 10.1016/j.rmed.2020.106197. Epub 2020 Oct 20. PMID: 33120193; PMCID: PMC7573668.
- Foster KJ, Jauregui E, Tajudeen B, Bishehsari F, Mahdavinia M. Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020 Oct;125(4):481-483. doi: 10.1016/j.anai.2020.07.023. Epub 2020 Jul 24. PMID: 32717301; PMCID: PMC7380219.
- Kakodkar P, Kaka N, Baig MN. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19). Cureus. 2020 Apr 6;12(4):e7560. doi: 10.7759/cureus.7560. PMID: 32269893; PMCID: PMC7138423.
- Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, Bonten MMJ, Dahly DL, Damen JAA, Debray TPA, de Jong VMT, De Vos M, Dhiman P, Haller MC, Harhay MO, Henckaerts L, Heus P, Kammer M, Kreuzberger N, Lohmann A, Luijken K, Ma J, Martin GP, McLernon DJ, Andaur Navarro CL, Reitsma JB, Sergeant JC, Shi C, Skoetz N, Smits LJM, Snell KIE, Sperrin M, Spijker R, Steyerberg EW, Takada T, Tzoulaki I, van Kuijk SMJ, van Bussel B, van der Horst ICC, van Royen FS, Verbakel JY, Wallisch C, Wilkinson J, Wolff R, Hooft L, Moons KGM, van Smeden M. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020 Apr 7;369:m1328. doi: 10.1136/bmj.m1328. Update in: BMJ. 2021 Feb 3;372:n236. Erratum in: BMJ. 2020 Jun 3;369:m2204. PMID: 32265220; PMCID: PMC7222643.
- Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J, Gómez-López A, Monreal E, Parra-Díaz P, Cortés-Cuevas JL, Galán JC, Fragola-Arnau C, Porta-Etessam J, Masjuan J, Alonso-Cánovas A. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020 Sep;27(9):1738-1741. doi: 10.1111/ene.14273. Epub 2020 May 16. PMID: 32320508; PMCID: PMC7264557.
- Haehner A, Draf J, Dräger S, de With K, Hummel T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82(4):175-180. doi: 10.1159/000509143. Epub 2020 Jun 11. PMID: 32526759; PMCID: PMC7360503.
- Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020 Oct;26(10):1426-1427. doi: 10.1016/j.cmi.2020.05.017. Epub 2020 May 22. PMID: 32447049; PMCID: PMC7242197.
- Boscolo-Rizzo P, Borsetto D, Hopkins C, Polesel J. Challenges in interpreting the diagnostic performance of symptoms to predict COVID-19 status: The case of anosmia. Int Forum Allergy Rhinol. 2020 Sep;10(9):1113-1115. doi: 10.1002/alr.22650. Epub 2020 Jul 17. PMID: 32588537; PMCID: PMC7361777.
- Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020 Jul;10(7):821-831. doi: 10.1002/alr.22592. Epub 2020 Jun 7. PMID: 32329222; PMCID: PMC7264572.
- Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 Jul;10(7):806-813. doi: 10.1002/alr.22579. Epub 2020 Jun 1. PMID: 32279441; PMCID: PMC7262089.
- Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C. Sudden and Complete Olfactory Loss of Function as a Possible Symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 Jul 1;146(7):674-675. doi: 10.1001/jamaoto.2020.0832. PMID: 32267483.
- Mutiawati E, Fahriani M, Mamada SS, Fajar JK, Frediansyah A, Maliga HA, Ilmawan M, Emran TB, Ophinni Y, Ichsan I, Musadir N, Rabaan AA, Dhama K, Syahrul S, Nainu F, Harapan H. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms - a systematic review and meta-analysis. F1000Res. 2021 Jan 21;10:40. doi: 10.12688/f1000research.28393.1. PMID: 33824716; PMCID: PMC7993408.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.