> General Science. 2021 July 27;2(7):610-617. doi: 10.37871/jbres1286.
A Cell-Based Assay for Antioxidant Behaviour of Phytochemicals: Influence of Exposure Time and Presence of Serum
Maha J Hashim* and Jeffrey R Fry
- Cellular protection assay
- Serum albumin
- Quercetin
- Epigallocatechin-3-gallate
- Sulforaphane
- Indole-3-carbinol
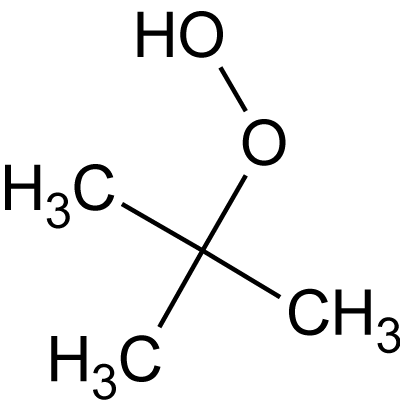
- t-BHP
Abstract
There is considerable interest in the ability of plant-derived antioxidants to protect against oxidative damage associated with disease or exposure to toxic agents. In this study, the cytoprotection effect of the direct antioxidants Quercetin (Q) and Epigallocatechin-3-Gallate (EGCG) and the indirect antioxidants, Sulforaphane (SFN) and Indole-3-Carbinol (I3C) was assessed in a cellular protection assay. This assay involved two cytoprotection patterns: (a) exposure to phytochemical for 20 hours followed by 5-hour exposure to t-Butylhydroperoxide (t-BHP); (b) simultaneous exposure to phytochemicals and t-BHP for 5 hours. HepG2 cells were cultured to a confluent monolayer and exposed to phytochemical +/- t-BHP in serum-free medium or serum-containing medium at high [10%(v/v)] or low [2%(v/v)] levels of foetal bovine serum, after which cell damage mediated by oxidant stress was assessed by uptake of neutral red. Results showed that Q, EGCG and, I3C were effective while SFN was inactive and toxic to the cells by itself at high concentration during long incubation. On the other hand, a short time of incubation with SFN displayed identical results to prolonged exposure. However, I3C was devoid of protection activity. Moreover, results showed that serum has a major impact on antioxidant activity.
Introduction
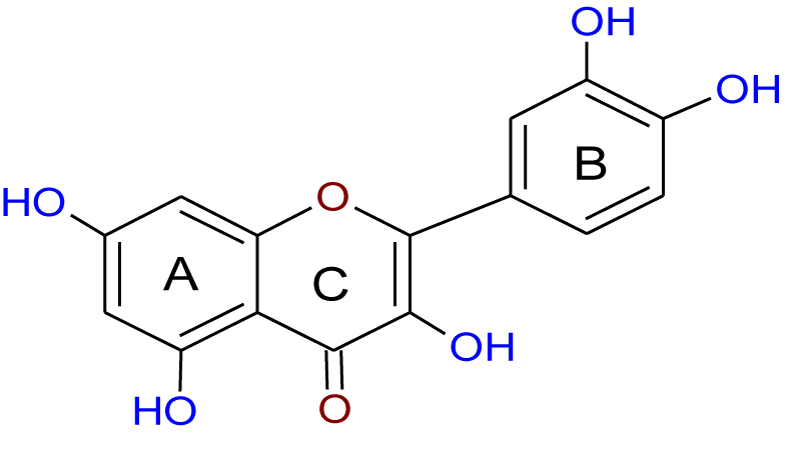
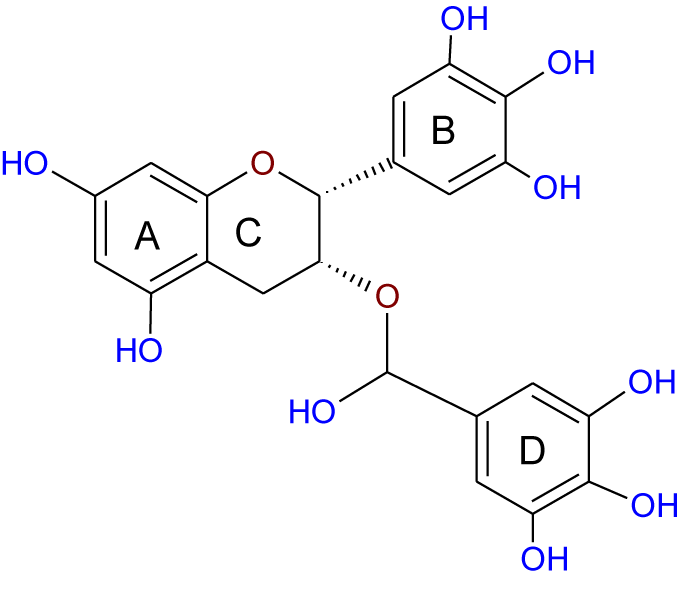
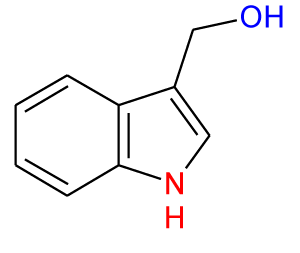
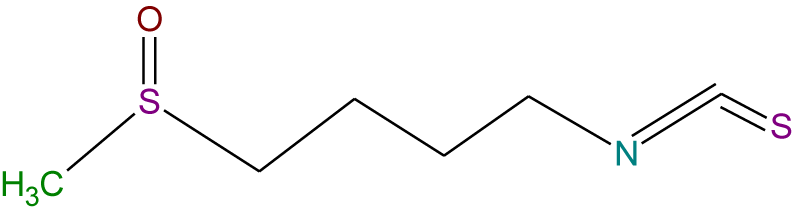
Recently, the importance of plant extracts has emerged as compounds containing antioxidant properties [1], which have contributed greatly as a preventive agent against many diseases [2]. Similarly, several epidemiological studies have shown an inverse relationship between the consumption of vegetables and fruits and the reduction of cancer incidence. These studies attributed this to the effectiveness of the antioxidants in these plants [3-5]. Moreover, these antioxidants and other phytochemicals present in the diet may protect cells from ROS-mediated DNA damage, which otherwise can lead to mutation and subsequent carcinogenesis [3], and so protect from cancer. These protections are either or both of direct (free radical scavenging) or indirect (up-regulation of cytoprotection proteins) actions. Quercetin (Q) (Figure 1) and Epigallocatechin-3-Gallate (EGCG) (Figure 2) are classified as direct antioxidants [6], while Indole-3-Carbinol (I3C) (Figure 3) and Sulforaphane (SFN) (Figure 4) possess an indirect cytoprotection action [6]. High amounts of quercetin are found in garlic, kale, onions, apples [7] as well as in other vegetables, nuts, fruits, tea, seeds and, wine [8], with intakes of between 6 and 31 mg/day [9,10]. Quercetin exhibits a wide range of biological activities [11] including anti-inflammatory [12,13], anti-carcinogenic [14], and antiviral actions [15]. Moreover, it is regarded as one of the most abundant flavonoids and represents an integral part of the human diet. The protective role of quercetin may be attributed to possessing a dual effect as a direct and indirect antioxidant, acting through different mechanisms [10]. Among all green tea constituents, EGCG, which accounts for about 50-60% of total catechin in green tea [16], is the main active ingredient due to its direct and indirect antioxidant effects in tumor growth suppression. These effects are mediated by different pathways including direct induction of apoptosis and arrest of the cell cycle [17,18] and indirect activation of anti-angiogenic and immune functions [19,20]. These effects are achieved selectively by EGCG in cancer cells but not in normal cells [21,22]. Thus, EGCG may exert different toxic and/or beneficial effects in malignant and normal cells respectively through its modulation of signal cascades and regulation of cell cycle which may be diverse in both cell types mentioned. Besides, these differences in EGCG actions may also be attributed to the differences between antioxidant defense mechanisms in normal cells and the mechanism of oxidative stress in cancer cells [23]. There is increasing evidence for the reverse correlation in consumption of a diet rich in cruciferous vegetables such as cauliflower, broccoli, and cabbage and incidence of diverse cancers such as breast, lung, and colon [24]. Decreased cancer risk during cruciferous intake is attributed in part to the high content of glucosinolates which are broken down through hydrolysis catalyzed by the plant enzymemyrosinase released during food preparation, producing isothiocyanates and indole products [25]. Indole-3-carbinol is a naturally occurring isothiocyanate metabolite identified in the cruciferous vegetable family, particularly Brassica, and released upon hydrolysis of glucobrassicin [26]. The first isolation from Brassica oleracea was in 1975 [27] and from that time I3C has emerged as a chemopreventive agent against a wide range of human cancers such as those of the breast, prostate, cervix, and vulva [24]. Moreover, it has been found that I3C can prevent or minimize the risk of carcinogenesis initiation through its involvement in the modulation of drug metabolism. Sulforaphane (1-isothiocyanato-4-(methyl-sulfinyl)butane), is a naturally occurring member of the Isothiocyanate Family (ITC) of chemopreventive agents, has been demonstrated to possess the ability to suppress the growth of cancer cells in different tissues and to delay the appearance of tumors [28]. SFN has emerged as a promising chemopreventive agent in cancer treatment at a low concentration ≤ 50 μM [29] but it has induced apoptosis in normal cells when the concentration has been increased [30]. The molecular mechanism of SFN action on cancer cells has received particular attention due to the effect in prevention and/or delaying the development of preneoplastic cells
Serum albumin is the most abundant protein in the circulatory system [31]; it forms approximately 50% or more of protein interstitial fluid in human aortas [32]. It binds to a large variety of exogenous and endogenous ligands such as drugs, fatty acids, nutrients, and hormones, and transports them to their sites of action. On the other hand, the binding of xenobiotics to albumin can affect their metabolism and biological activity [31]. Therefore, the presence of albumin within experimental constituents such as culture medium may impact the results obtained by assays [8,33].
In this study, we investigated a cellular protection assay in the screening of antioxidant behaviour of model phytochemicals which are known to act as direct and/or indirect antioxidants. The cellular protection assay is based on the protection of HepG2 cells against oxidative stress induced by t-BHP.
Two different cytoprotection patterns were tested in this work: (a) 5-hour exposure to phytochemicals and t-BHP simultaneously where the direct cytoprotection is provided by antioxidant against oxidative stress; (b) exposure to phytochemical for 20 hours followed by 5-hour exposure to t-BHP, the long incubation with antioxidant allows defense against oxidative stress induced by t-BHP indirectly by up-regulation of phase 2 enzymes. Treatment protocol (a) has been used previously by us for the two direct antioxidants, Q and EGCG [33]. Currently, in this study, we are continued the screening of I3C and SFN under the same assay pattern. Treatment protocol (b) was tested for all four compounds Q, EGCG, I3C, and SFN. The effect of the presence or absence of serum on the antioxidant activity in these two treatment scenarios (Figures 1-5).
Materials and Methods
Chemicals
Human hepatoma HepG2 cells were purchased from ECACC, Salisbury, UK. Quercetin, epigallocatechin-3-gallate, sulforaphane, indole-3-carbinol, Hanks ́ balanced salt solution [H9394, without Ca2+ and Mg2+. with phenol red, sterile filtered] Trypsin-EDTA, Eagle’s Minimal Essential medium (MEM) [with Earle’s salts and sodium bicarbonate, without glutamine, sterile filtered], gentamicin [50 mg/ml], glutamine [200 mM], foetal bovine serum (FBS), MEM Non-essential Amino Acid solution neutral red, ethanol solution, tert-butyl hydroperoxide (t-BHP), and glacial acetic acid were purchased from Sigma Chemical Co. Ltd., Poole, Dorset. UK.
HepG2 cells culture
HepG2 cells were cultured in 175 cm2 Nunclon culture flasks in 5% CO2-in-air atmosphere using 50 ml of MEM supplemented with 10% (v/v) foetal bovine serum, 2 μg/ml fungizone, 0.05 mg/ml gentamicin, 1% (v/v) non-essential amino acid solution, 2 mM L-glutamine at 37°C. The culture medium was changed every 72 hours, and cells were confluent after 7 days of sub-culturing. For experiments including treatments, cells were sub-cultured at high density in wells of a 24-well plate in 1 ml medium; under these conditions,the confluence was achieved within 24 hours.
Treatments
Stocks solutions of Q, EGCG, I3C, and SFN were prepared in DMSO and stored at 4°C until use.
Indirect cytoprotection: HepG2 cell cultures were treated with different series of concentrations (0 - 100 μg/ml) of Q, EGCG, I3C, and SFN in a complete culture medium with or without FBS for 20 hours prior to exposure to 0.8 mM t-BHP for 5 hours. A solvent control (culture medium containing 0.5% DMSO) was run concurrently.
Direct cytoprotection: Different series of concentrations (0 - 100 μg/ml) of I3C and SFN and 0.8 mM t-BHP in a complete culture medium with or without FBS was added simultaneously to the cells for 5 hours (i.e., no pre-exposure to the antioxidants).
Effect of serum: The influence of serum on cell survival was assessed by culturing HepG2 cells in a culture medium lacking FBS or containing FBS in the concentration range 0.1 to 10% (v/v) during exposure for 20 hours.
Neutral red assay
Neutral red uptake assay was used to determine the viable cells after treatment and is based on the incorporation and binding of neutral red into the lysosomes of viable cells [34]. Following exposure to the antioxidants, the cells were incubated for 1 hour with a medium containing neutral red (3.3 g neutral red/L of DPBS) after which cells were washed in warm Phosphate-Buffered Saline (PBS). Cold fixative (prepared by mixing 250 ml ethanol, 250 ml distilled water, and 5 ml glacial acetic acid) was used to desorb the stain and the absorbance was read using a spectrophotometer at 540 nm.
Statistical analysis
Results were entered into Graphpad Prism 4, from which mean ± S.D. and TC50 values were determined for SFNand I3C whilst EC50 values were determined for Q, EGCG, I3C, and SFN. Values were derived from at least 3 independent experiments. One-way ANOVA followed by selected comparisons by the Bonferroni method was used to compare the EC50 values obtained under different culture conditions.
Results
Effect of serum
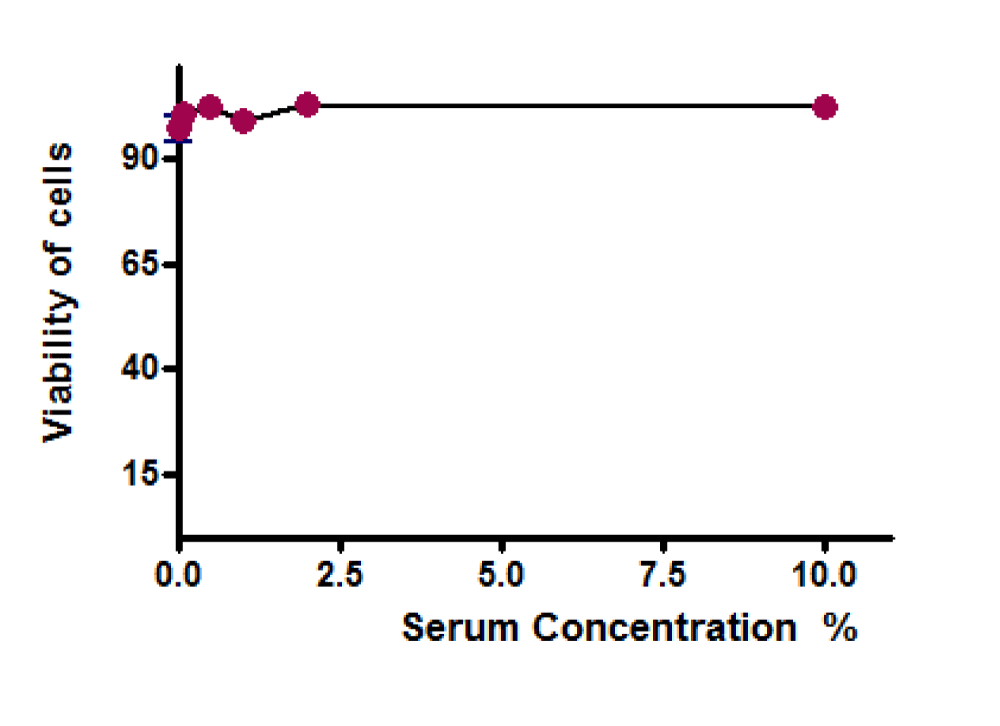
Serum (absence or presence up to 10% v/v) did not affect the viability of the cells when the cells were cultured for 20 hours (Figure 6). This allowed a further study of the cytoprotection provided by the chemicals, under both reduced serum (2% as opposed to 10%) and serum-free conditions.
Indirect cytoprotection: Effect of serum (10% serum)
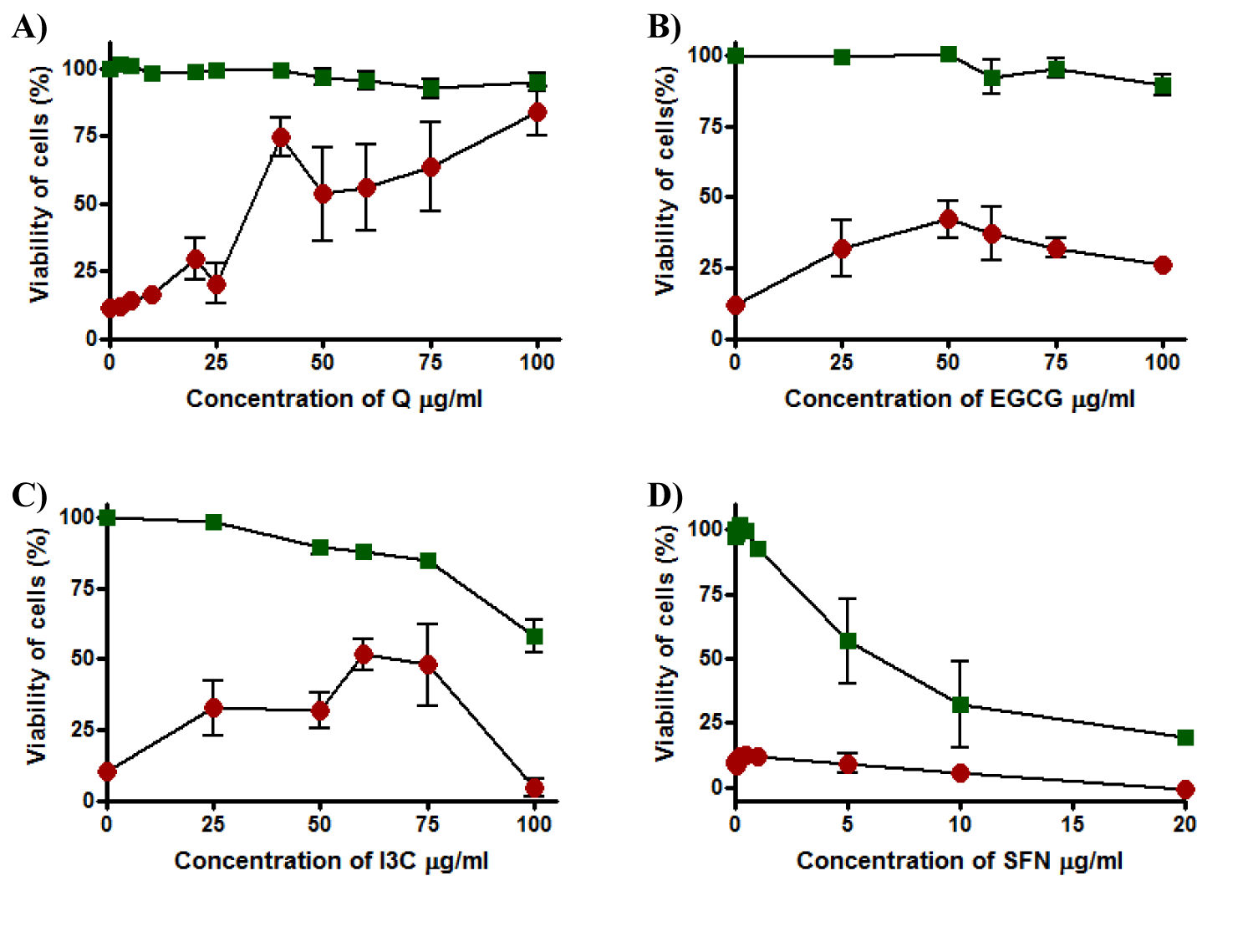
Results showed that Q was not toxic to the cells in the concentration range tested, in that viability was maintained when cells were incubated with Q for 20 hours. Although Q failed to provide full cytoprotection at low concentrations such as 25 µg/ml where viability was 25%, it provided high protection and viability increased to reach 80% at 100 µg/ml and EC50 was 34 ± 5.3 µg/ml (Figure 7a). EGCG showed no cytotoxicity at any concentration in the range tested and maintained cell viability of the cells at 100% to each concentration in the range. On the other hand, cells incubated with EGCG overnight were partially protected from t-BHP toxicity at 50 µg/ml of EGCG to obtain approximately 50% of viability and EC50 was more than 100 µg/ml (Figure 7b). Incubation of HepG2 cells with I3C for 20 hours to detect the toxicity effect of this compound showed an inverse relationship between cell viability and concentration of I3C. At 25 µg/ml, cell viability was recorded 100% and it gradually declined to about 60% at 100 µg/ml of I3C and TC50 was 98.1 ± 0.0. Moreover, when the cells were maintained with I3C for 20 hours and then exposed to t-BHP for 5 hours, there was evidence of cytoprotection against t-BHP and EC50 could not be determined. I3C provided cytoprotection at 60 and 75 µg/ml and the highest cell viability was about 50% at 60µg/ml but when the concentration of I3C was increased, viability dropped to approximately 0% at 100 µg/ml (Figure 7c). SFN was toxic to the cells at a concentration higher than 1 µg/ml, with a marked decline in cell viability from 100% to about 0% when the concentration of SFN increased from 5 to 20 µg/ml where TC50 was 3.9 ± 0.91 µg/ml. Moreover, SFN was inactive and failed to give any small protection to the HepG2 cells at any concentration in the range tested and EC50 could not be determined (Figure7d).
Indirect cytoprotection: Effect of serum (serum-free medium)
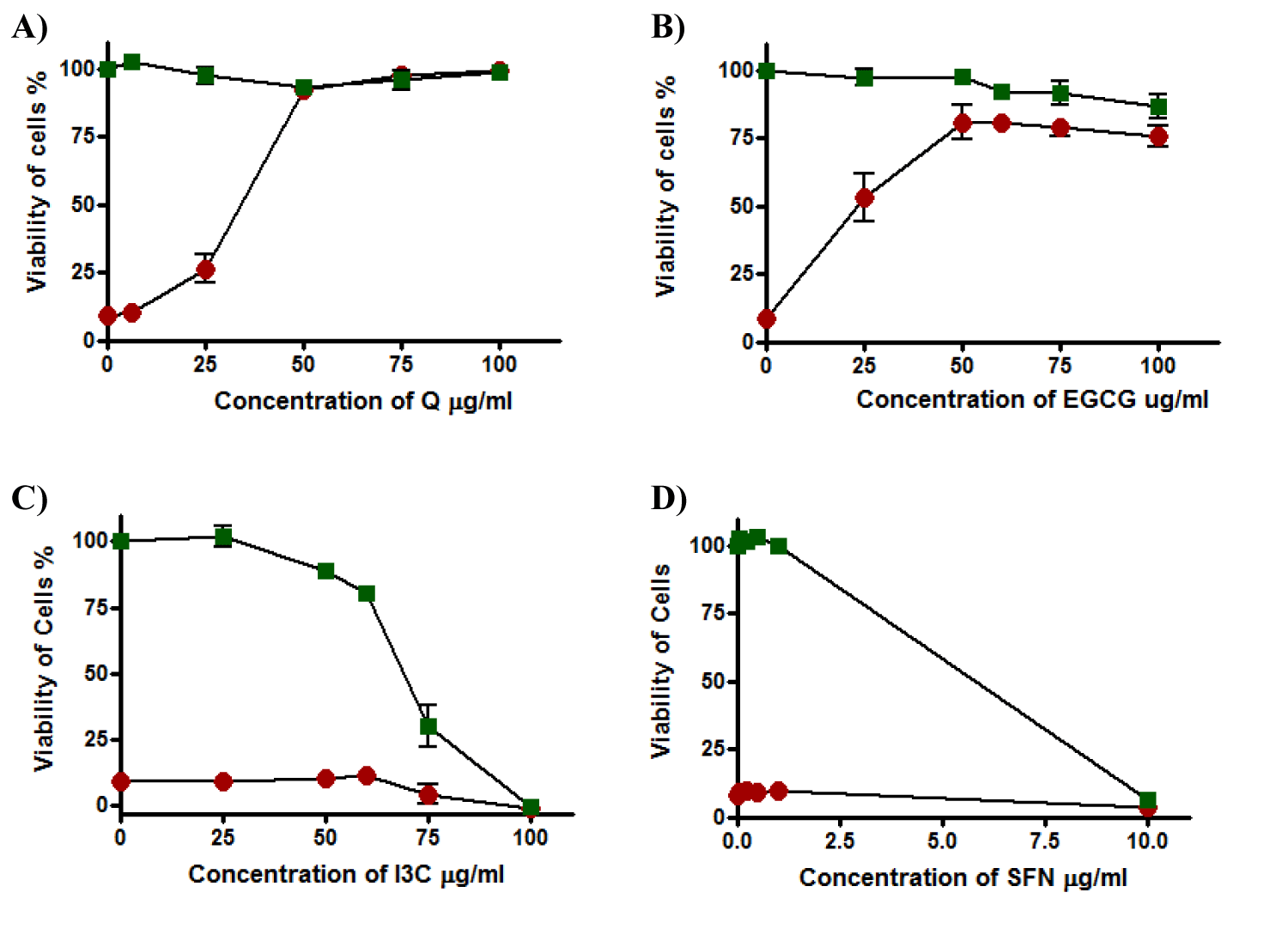
By using the serum-free medium in the experiment, Q was not toxic to the cells at any concentration in the range and cells were still alive by 100%. Furthermore, Q produced concentration-dependent protection against t-BHP with total protection above 50 µg/ml and EC50 was 33.8 ± 4.0 µg/ml (Figure 8a).
A similar pattern of non-toxicity and cytoprotection effect was displayed by EGCG, although complete protection did not occur, cell viability reached about 80% at concentrations 50 µg/ml and above where EC50 was 27.8 ± 9.9 µg/ml (Figure 8b). The viability of cells was markedly affected by I3C which was toxic and caused a decline in viability; it was dropped from about 80% at concentrations 50 µg/ml to about 0% at 100 µg/ml of I3C and TC50 was 72.13 ± 5.9 µg/ml. Moreover, I3C under these conditions failed to protect HepG2 cells against t-BHPtoxicity where EC50 was more than 100 µg/ml (Figure 8c). Results obtained by SFN were identical to the results from cellular assay when HepG2 cells were exposed to SFN for 20 hours using a medium containing 10% serum before exposure to t-BHP (Figure 8d) and EC50 was more than 100 while TC50 was 5.81 ± 0.27 µg/ml.
Direct cytoprotection: Effect of serum (Medium 2% serum)
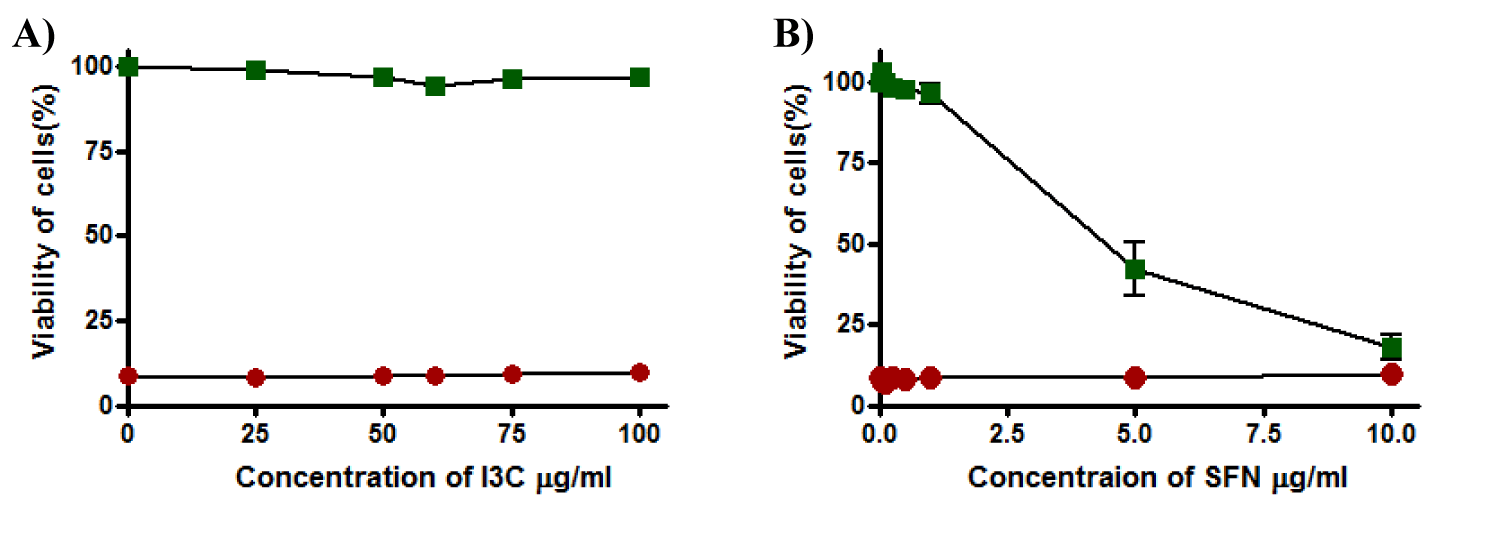
I3C and SFN were inactive due to their inability to protect cells from oxidative stress-induced by t-BHP and keep the viability of the cells near 0% where EC50 for both was more than 100 µg/ml (Figure 9a and 9b). Moreover, a decline in the viability of cells recorded when cells exposed to SFN at a concentration higher than 1µg/ml and reach 10-15% at 10 µg/ml and TC50 was 4.6 ± 0.78 while I3C was not toxic to the cells by itself at any concentration in the range this time and cell viability remained unaffected (Tables 1,2).
Discussion
There is current interest in the involvement of natural antioxidants in cytoprotection against oxidative stress [8]. Our main goal was to detect the influence of the four phytochemicals Q, EGCG, I3C, and SFN on cell viability and to what extent can they provide protection against cellular death stimuli such as t-BHP in the presence and/or absence of serum in the culture medium. According to this, different patterns of cellular assay including direct and indirect cytoprotection were used for this purpose.
At least two aspects can assist to explain the findings of this study, namely the role of albumin and the structure of the antioxidant compounds. Although albumin has antioxidant properties where it can defend against the toxicity effect of, cis-4,7,10,13,16,19-docosahexaenoic acid, a component of fish oil [35], it can interfere in the running of assays and effect on results [36,37] through binding to antioxidant resulting in decrease antioxidant activity [37].
Albumin is a highly soluble protein containing 585 amino acid and has a molecular weight of 66 kDa [38]. It represents approximately 50% of total plasma protein, thus the amount of albumin in plasma is 3.5 g/100ml, and for a medium containing 10% serum as sued in this study, the albumin amount equals 0.35 g/100ml. This amount is not much different from the amount of albumin in Interstitial Fluid (ISF). Thus, albumin content in ISF from human aortas has been estimated to be 0.2 g/100ml [39], the interstitial fluid/plasma ratio for albumin 0.42 [40], and interstitial fluid from rat skin 2.1 g/100ml which represents 63% of plasma albumin concentration, in that the amount of albumin in 10% is 0.21 g/100ml [41]. Accordingly, the use of a medium containing 10% serum approximated exposure of cells to ISF in most tissues. We found that serum does not affect cell viability during incubation for 20 hours (Figure 6) which is identical to our previous study for an incubation period of 5 hours [33] and this indicates that any variations in cytoprotection assessed in further studies may be attributed to the presence of foreign substances such as oxidants and antioxidants.
Concerning indirect cytoprotection, the four compounds displayed different cytoprotection activities. Antioxidant activity of Q was hardly increased (Figures 7a, 8a) and EGCG was enhanced (Figures 7b, 8b) when albumin was omitted. It is obvious that the absence of albumin was responsible for an increase of antioxidant activity considerably, EGCG in particular, where Q and EGCG were being free from binding to albumin. On the other hand, I3C contributed partially to cytoprotection and this incomplete cytoprotection is mainly attributed to the cytotoxicity effect of I3C at higher concentrations where it caused cell death during cellular incubation (Figure 7c). Moreover, the absence of albumin increased the toxicity effect of I3C, possibly caused by an increase in the free (i.e., not bound to albumin) concentration of I3C under this condition [35] and this was clear from decline in TC50 value from 98 to 72 µg/ml.
SFN failed to provide any cytoprotection at any concentrations due to its cytotoxic effects even at low concentrations (Figure 7d). Under this condition, the presence of serum appeared to reduce the toxicity of SFN, and TC50 was increased when the amount of albumin was decreased (Table 2), again possibly due to binding of SFN to serum proteins.
| Table 1: EC50 (the effective concentration of phytochemical providing 50% protection). Values are reported as mean ± S.D; n = number of experiments; *significantly different to 5-hour (10% and 2% serum) (p < 0.001). | |||||
| Antioxidant | EC50 (µg/ml) | ||||
| 20-hours Exposure | 5-hours Exposure | ||||
| Medium Serum 10% |
Medium Serum-free |
Medium Serum 10% |
Medium Serum 2% |
Medium Serum-free |
|
| Q | 34.1 ± 5.3 n = 8 |
33.8 ± 4.0 n = 3 |
39.9 ± 4.5 [34] | 16.1 ± 2.4 [34] | 17.9 ± 1.8 [34] |
| EGCG | > 100 | 27.8 ± 9.9 n = 3 |
84.7 ± 7.4 [34] | 51.2 ± 8.3 [34] | 23.4 ± 7.2 [34] |
| I3C | ~60' n = 2 |
> 100 | ND | > 100 | ND |
| SFN | > 100 | > 100 | ND | > 100 | ND |
| *Significantly different to 5-hour 10% serum (p < 0.001), º significantly different to 5-hour 2% serum (p < 0.001). ND = Not Determined; ' = Response varied between experiments. One-way ANOVA followed by selected comparisons by the Bonferroni method was used to compare the EC50 values obtained under different culture conditions. |
|||||
| Table 2: TC50 values (the concentration of oxidant causing 50% cell death) for I3C and SFN. Values are reported as mean ± S.D of at least three independent experiments. | |||
| Antioxidant | TC50 (µg/ml) | ||
| 20-hour Exposure | 5-hour Exposure | ||
| Medium Serum 10% | Medium Serum-free | Medium Serum 2% | |
| I3C | 98.1 ± 0.0 | 72.13 ± 5.9 | ND |
| SFN | 3.90 ± 0.91 | 5.81 ± 0.27 | 4.60 ± 0.78. |
Under short (5 hours) exposure conditions, I3C and SFN also lacked cytoprotective properties (Figures 9a, 9d respectively). Given that the mechanism of action of direct antioxidant behaviour is based on the principle of scavenging free radicals through a donation of a hydrogen atom from a scavenger, an antioxidant, to the radical and so inactivating it [42,43], this lack of direct cytoprotective response of I3C and SFN can be attributed to the lack of multiple free hydroxyl groups in thteir structure. Ths contrasts with the effective direct cytoprotection preperties of Q and EGCG [33], both of which possess the requisite multiple free hydroxyl groups.
Conclusion
In conclusion, the results of this study demonstrate that the antioxidant activity of Q, EGCG, I3C, and SFN was influenced by both duration of treatment and presence of serum proteins. Q and EGCG were not toxic in any pattern of cellular assay while the toxicity effect of I3C and SFN was influenced by time of incubation and presence of albumin.
Acknowledgment
The authors thank The University of Nottingham and the School of Life Sciences (UK) for all their scientific supports. The author Dr. Maha J Hashim would like to thank her supervisor in Ph.D. (including this research), Dr. Jeffrey R Fry at the School of Life Sciences, The University of Nottingham (UK) for all his guidance and assistance. Also, big thanks from Dr. Maha J Hashim to her father Mr. Jalal Hashim Mohammed Tabana, in Iraq, who has funded her Ph.D. study (including this research) with all living costs in the United Kingdom for five years.
References
- Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004 May;7(3):407-22. doi: 10.1079/phn2003543. PMID: 15153272.
- Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, Chirlaque MD, Dorronsoro M, Jakszyn P, Larrañaga N, Martínez C, Navarro C, Quirós JR, Sánchez MJ, Tormo MJ, González CA. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr. 2007 Jun;85(6):1634-42. doi: 10.1093/ajcn/85.6.1634. Erratum in: Am J Clin Nutr. 2008 Oct;88(4):1181. PMID: 17556703.
- Lopaczynski W, Zeisel SH. Antioxidants, programmed cell death, and cancer. Nutrition Research. 2001;21:295-307. doi: 10.1016/S0271- 5317(00)00288-8.
- Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, de Oliveira Costa Dias G, Morel AF, Nogueira CW, Rocha JB. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006 Aug 30;1107(1):192-8. doi: 10.1016/j.brainres.2006.05.084. Epub 2006 Jul 7. PMID: 16828712.
- Wagner C, Vargas AP, Roos DH, Morel AF, Farina M, Nogueira CW, Aschner M, Rocha JB. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch Toxicol. 2010 Feb;84(2):89-97. doi: 10.1007/s00204-009-0482-3. Epub 2009 Nov 10. PMID: 19902180.
- Maha J Hashim and Jeffrey R Fry. Evaluation of Direct and Indirect Antioxidant Properties of Selected Four Natural Chemical Compounds: Quercetin, Epigallocatechin-3-Gallate, Indole-3-Carbinol and Sulforaphane by DPPH Radical Scavenging Assay. J Biomed Res Environ Sci. 2020 Dec 15;1(8):389-392. doi: 10.37871/jbres1170 Article ID: JBRES1170. https://bit.ly/3zw2Dia
- Hertog MGL, Hollman PCH, Katan MB. Content of Potentially Anticarcinogenic Flavonoids of 28 Vegetables and 9 Fruits Commonly Consumed in the Netherlands. Journal of Agricultural and Food Chemistry. 1992;40(12):2379-2383. https://bit.ly/3kRH87w
- Alía M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol. 2006 Apr 15;212(2):110-8. doi: 10.1016/j.taap.2005.07.014. Epub 2005 Aug 29. PMID: 16126241.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995 Feb 27;155(4):381-6. Erratum in: Arch Intern Med 1995 Jun 12;155(11):1184. PMID: 7848021.
- Kim GN, Jang HD. Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Ann N Y Acad Sci. 2009 Aug;1171:530-7. doi: 10.1111/j.1749-6632.2009.04690.x. PMID: 19723100.
- Hertog MG, Hollman PC. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996 Feb;50(2):63-71. PMID: 8641249.
- Loke WM, Proudfoot JM, Stewart S, McKinley AJ, Needs PW, Kroon PA, Hodgson JM, Croft KD. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem Pharmacol. 2008 Mar 1;75(5):1045-53. doi: 10.1016/j.bcp.2007.11.002. Epub 2007 Nov 12. PMID: 18096136.
- Shimoda K, Kobayashi T, Akagi M, Hamada H, Hamada H. Synthesis of oligosaccharides of genistein and quercetin as potential anti-inflammatory agents. Chemistry Letters. 2008;37:876-877. doi:10.1246/Cl.2008.876. https://bit.ly/3y7VV1t
- Williamson G, Plumb GW, Uda Y, Price KR, Rhodes MJ. Dietary quercetin glycosides: antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis. 1996 Nov;17(11):2385-7. doi: 10.1093/carcin/17.11.2385. PMID: 8968052.
- Fan D, Zhou X, Zhao C, Chen H, Zhao Y, Gong X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia. 2011 Sep;82(6):805-10. doi: 10.1016/j.fitote.2011.04.007. Epub 2011 May 5. PMID: 21570451.
- Hu C, Kitts DD. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol Cell Biochem. 2001 Feb;218(1-2):147-55. doi: 10.1023/a:1007220928446. PMID: 11330830.
- Gupta S, Hussain T, Mukhtar H. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003 Feb 1;410(1):177-85. doi: 10.1016/s0003-9861(02)00668-9. PMID: 12559991.
- Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003 Jul 31;22(31):4851-9. doi: 10.1038/sj.onc.1206708. PMID: 12894226.
- Jung YD, Ellis LM. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int J Exp Pathol. 2001 Dec;82(6):309-16. doi: 10.1046/j.1365-2613.2001.00205.x. PMID: 11846837; PMCID: PMC2517785.
- Zhu M, Gong Y, Yang Z, Ge G, Han C, Chen J. Green tea and its major components ameliorate immune dysfunction in mice bearing Lewis lung carcinoma and treated with the carcinogen NNK. Nutr Cancer. 1999;35(1):64-72. doi: 10.1207/S1532791464-72. PMID: 10624708.
- Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003 Oct;17(13):1913-5. doi: 10.1096/fj.02-0914fje. Epub 2003 Aug 1. PMID: 12897059.
- Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004 Apr 1;23(14):2507-22. doi: 10.1038/sj.onc.1207353. PMID: 14676829.
- Raza H, John A. Green tea polyphenol epigallocatechin-3-gallate differentially modulates oxidative stress in PC12 cell compartments. Toxicol Appl Pharmacol. 2005 Sep 15;207(3):212-20. doi: 10.1016/j.taap.2005.01.004. PMID: 16129114.
- Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, Mayeaux EJ, Tucker A, Turbat-Herrera EA, Mathis JM. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000 Aug;78(2):123-9. doi: 10.1006/gyno.2000.5847. PMID: 10926790.
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997 Feb 28;103(2):79-129. doi: 10.1016/s0009-2797(96)03745-3. PMID: 9055870.
- Bradfield CA, Bjeldanes LF. Effect of dietary indole-3-carbinol on intestinal and hepatic monooxygenase, glutathione S-transferase and epoxide hydrolase activities in the rat. Food Chem Toxicol. 1984 Dec;22(12):977-82. doi: 10.1016/0278-6915(84)90147-9. PMID: 6334634.
- Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975 Apr;54(4):985-8. PMID: 1127728.
- Yanaka A, Zhang S, Tauchi M, Suzuki H, Shibahara T, Matsui H, Nakahara A, Tanaka N, Yamamoto M. Role of the nrf-2 gene in protection and repair of gastric mucosa against oxidative stress. Inflammopharmacology. 2005;13(1-3):83-90. doi: 10.1163/156856005774423863. PMID: 16259730.
- Choi WY, Choi BT, Lee WH, Choi YH. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother. 2008 Nov;62(9):637-44. doi: 10.1016/j.biopha.2008.01.001. Epub 2008 Feb 11. PMID: 18313257.
- Yeh CT, Yen GC. Effect of sulforaphane on metallothionein expression and induction of apoptosis in human hepatoma HepG2 cells. Carcinogenesis. 2005 Dec;26(12):2138-48. doi: 10.1093/carcin/bgi185. Epub 2005 Jul 20. Retraction in: Carcinogenesis. 2014 Jan;35(1):247. PMID: 16033772.
- Trnkova L, Bousova I, Stankova V, Drsata J. Study on the interaction of catechins with human serum albumin using spectroscopic and electrophoretic techniques. Journal of Molecular Structure. 2011;985:243-250. doi: 10.1016/j.molstruc.2010.11.001. https://bit.ly/3iPDgB7
- Smith EB, Staples EM. Plasma protein concentrations in interstitial fluid from human aortas. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):59-75. doi: 10.1098/rspb.1982.0094. PMID: 6187014.
- Maha J Hashim, Jeffrey R Fry. Influence of Extracellular Protein on the Cytoprotective Effects of Two Model Phytochemicals. Mol Biol. 2019;8(1). doi: 10.4172/2168- 9547.1000227.
- Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125-31. doi: 10.1038/nprot.2008.75. PMID: 18600217.
- Kanno S, Kurauchi K, Tomizawa A, Yomogida S, Ishikawa M. Albumin modulates docosahexaenoic acid-induced cytotoxicity in human hepatocellular carcinoma cell lines. Toxicol Lett. 2011 Feb 5;200(3):154-61. doi: 10.1016/j.toxlet.2010.11.009. Epub 2010 Nov 23. PMID: 21108996.
- Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr. 2006 Feb;45(1):19-28. doi: 10.1007/s00394-005-0558-7. Epub 2005 Mar 25. PMID: 15782287.
- Gulden M, Dierickx P, Seibert H. Validation of a prediction model for estimating serum concentrations of chemicals which are equivalent to toxic concentrations in vitro. Toxicology in Vitro. 2006;20(7):1114-1124. doi: 10.1016/j.tiv.2006.02.002
- Rothschild MA, Oratz M, Schreiber SS. Serum-Albumin. Hepatology. 1988;8(2):385-401. doi: 10.1002/hep.1840080234
- Rush GF, Gorski JR, Ripple MG, Sowinski J, Bugelski P, Hewitt WR. Organic Hydroperoxide-Induced Lipid-Peroxidation and Cell-Death in Isolated Hepatocytes. Toxicology and Applied Pharmacology. 1985;78(3):473-483 doi: 10.1016/0041-008x(85)90255-8
- Smith EB, Staples EM. Plasma-Protein Concentrations in Interstitial Fluid from Human Aortas. Proceedings of the Royal Society B-Biological Sciences. 1982; 217(1206):59-75 doi: 10.1098/rspb.1982.0094.
- Rutili G, Arfors KE. Protein Concentration in Interstitial and Lymphatic Fluids from Subcutaneous Tissue. Acta PhysiologicaScandinavica. 1977;99(1):1-8 doi: 10.1111/j.1748-1716.1977.tb10345.x.
- Aukland K, Fadnes H.O. Protein Concentration of Interstitial Fluid Collected from Rat Skin by a Wick Method. Acta Physiologica Scandinavica. 1973. doi:10.1111/j.1748-1716.1973.tb05464.x.
- Chiodo SG, Leopoldini M, Russo N, Toscano M. The inactivation of lipid peroxide radical by quercetin. A theoretical insight. Physical Chemistry Chemical Physics. 2010;12(27):7662-7670 doi:10.1039/B924521a.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.