> Biology. 2020 October 19;1(6):219-232. doi: 10.37871/jbres1147.
-
Subject area(s):
- Antivirology
- Biology
- Infectious Diseases
- Antiretrovirology
Challenges and Opportunities to Develop Diagnostics and Therapeutic Interventions for Severe Acute Respiratory Syndrome- Corona Virus 2 (SARS-COV-2)
Jaison Jeevanandam1,3, Subhamoy Banerjee2 and Rajkumar Paul1*
2School of Life Sciences, B.S. Abdur Rahman Crescent Institute of Science & Technology, Chennai - 600 048, India
3CQM - Centro de Química da Madeira, MMRG, Universidade da Madeira, Campus da Penteada, 9020-105 Funchal, Portugal
- SARS-CoV-2
- Covid 19
- Diagnostics
- Therapeutic interventions
- Real time PCR
- Vaccine
Severe Acute Respiratory Syndrome-Corona Virus 2 (SARS-CoV-2) or Corona Virus Disease 19 (COVID-19) is playing havoc all over the world since December 2019. Despite being a family member of coronaviridae, which has previously affected mankind twice in last one decade, the novel corona virus, as it is named left medical practitioners and scientists defenseless. The major challenge is twofold identification and therapeutic intervention. Several approaches, including real-time PCR have already been taken for quick identification of Covid19. Due to very fast evolving rate, accurate identification is still a challenge for most of the detection methods developed in last three months. Several proposals for therapeutic intervention have also put forth by scientists, ranging from vaccine to RNA therapy. In this article, a comprehensive review is made from the scattered scientific literatures and is fine-tuned further with possible diagnostic and therapeutic interventions.
The novel corona virus or SARS-CoV-2 is alleged to be originated from the city of Wuhan, China, has been currently turned into a pandemic and affected almost every country (213 countries) throughout the world (https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/). Corona virus is a unique class of virus, that exists under the large viral of coronaviridae, which is a single stranded positive RNA sense virus. The size of virus particle ranges from 70-90 nm [1]. Due to presence of spikes on the outer surface, which resembles a crown under an electron microscope, these viruses are called as corona virus, as per Latin word 'corona' which means 'crown' [2]. Further, the corona viruses are sub-classified into alpha, beta, gamma and delta subtypes. These viruses are endemic in non-human vertebrates, such as bats, civet cat, camel as well as Pangolin and few of them are able to infect human [3]. According to Center for Disease Control and Prevention (CDC), USA, till date, seven different types of corona virus have been found to infect humans. Alpha and beta corona virus have been found till date to infect humans. There are two alpha subtypes namely 229E, NL63, and five beta subtypes namely OC43, HKU1, SARS-CoV (severe acute respiratory syndrome), MERS-CoV (Middle East respiratory syndrome) and COVID-19 (corona virus disease 2019) (https://www.cdc.gov/coronavirus/types.html). SARS-CoV was first identified in the year 2002, which has infected the low respiratory tract of several individuals in Foshan, Chine, and has resulted in Acute Respiratory Distress Syndrome (ARDS), multi-organ failure and septic shock with high Case Fatality Ratio (CFR). Later, it has been transported to Hong Kong from where it has been spread globally, infected about 8098 people, led to the mortality of 774 individuals, and was controlled in the year 2003. However, a higher virulent strain of SARS causing CoV named MERS has been first identified in Jordon in the year 2012 and has spread rapidly, infected around 2494 individuals with 34.4% of fatality rate in Middle East countries. In 2019, the same virus has been evolved into novel coronavirus with capability to spread rapidly and cause respiratory as well as immunity related complication in individuals, especially among older age group people [4]. According to Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses, virus is officially named as Severe Acute Respiratory Syndrome-Corona Virus 2 (SARS-CoV-2) which was intermittently called 2019 novel corona virus (2019-nCoV) [5]. In the current paper, we shall mention the disease as COVID-19 and viruses as SARS-CoV-2.
SARS-CoV-2 is airborne, swiftly evolving and highly contagious in nature, which is evident by the infection numbers, which is escalating at an exponential rate in past 5 months. Till now, the mortality rate is calculated between 2%-3%, however, there are numerous factors, including age, comorbidity of the patient and health facility, that influence the mortality rate [6]. As a result, the reported mortality rate is found to have less than 1% in some countries whereas higher than 10% in some other countries [7]. There are various studied have been conducted on the virus incubation period in human which in turn helps the policy makers to quarantine and observe suspected individual [7]. The results suggested the incubation time may vary greatly, but 14 days of isolation and observation may be sufficient to determine the infection [8]. There are different theories of mechanism of SARS-CoV-2 infection has come forward and different treatment approaches have been proposed. Out of multiple approaches to contain the infection and treat the infected patients, one model has been adapted globally, which is early detection of the infected individual and treat them in a quarantine facility. Hence, it is evident that we need a sensitive and a rapid diagnostic system to identify the infected, but asymptomatic patients. The second part is, of course, finding suitable and target specific therapeutic interventions. Vaccine is considered to be hopeful therapeutic agent against Covid-19 to combat them and control its transmission. As of now total 115 various types of vaccine candidates have been identified out of which 78 are confirmed based on publicly available data and 5 of which has been approved for clinical trial [9]. For developing any successful and precise therapeutic and diagnostic measures, it is important to know the genetics of SARS-CoV-2 and its exclusive structure.
Corona virus has the largest genome among all the single stranded RNA viruses known till date with 26-32 kb of size. It possesses a 5' cap and a 3' poly-A tail. There are 14 Open Reading Frame (ORF) in SARS-CoV-2, which encodes for 27 unique proteins [10]. In the 5' end, there are two ORFs, namely ORF1a and1b that encodes for two polypeptides, such as pp1a and pp1ab. The frame shift of one nucleotide towards 5' end (-1 frame-shift) between ORF1a and ORF1b produce two polypeptides. Polypeptide1a (molecular weight 440-510 kDa) gets cleaved into 11 Nonstructural proteins (Nnsps) and polypeptide1ab (molecular weight 740-810 kDa) gets sliced into 15 Nsps [11]. In the 3' end, other ORFs generates four Exemptional Structural Proteins, Namely Spike (S), Envelope (E), Nucleoprotein (N), Membrane (M) and eight distinct accessory type of proteins, namely 3a, 3b, p6, 7a, 7b, 8b, 9b and ORF14 [10]. Moreover, it is worthy to note that the proteolytic polypeptide cleavage is facilitated via Nsp3 and Nsp5 [11].
Among structural type of proteins, Spike protein (S) (~150 kDa) is a glycoprotein, which has a significant role in the attachment of the virus to cells. It is glycosylated and it has three domains-N terminal domain consists of S1 and S2subdomain and protrude outwards, a domain of transmembrane and a cytoplasmic structure at the C terminal end. It intermingles with the Angiotensin Converting Enzyme 2 in human (hACE2), after being cleaved by Transmembrane Serine Protease 2 (TMPRSS2) at the S1 and S2 junction [12]. Membrane (M) protein is comparatively smaller (~25-30 kDa) but more abundant. It has three transmembrane domains and plays a significant role in membrane dynamics like shaping the virion and membrane curvature and nucleocapsid binding [13]. The Envelope (E) protein is also a small, yet extremely Diverse Protein (~8-12 kDa). Although E protein is not fully characterized, yet it is speculated that E protein may be a transmembrane protein ion channel activity. Also, E protein enables the viral assembly and release and certain studies indicated that the E protein is essential in pathogenicity, especially viral lethality [14]. Nucleocapsid (N) protein is a heavily phosphorylated protein, which plays a variety of functions. It has an affinity towards both host and viral RNA, but phosphorylation of the N protein changes the affinity towards viral RNA over host RNA [15]. Also, N protein found to play role in viral genome packaging, acts as a structural subunit of replicase complex and mediates the tethering of the viral genome to replication transcription complex [16]. Also, it was found to influence host cell response by modulating chaperone activity and cell cycle regulation [17].
Non-Structural Proteins (Nsp) of Betacoronaviridae family members have a diverse function ranging from blocking the host immune response to RNA polymerization. Non-structural proteins are categorized as Nsp1 to Nsp 16. Inhibition of the immune system is caused by different Nsp is different ways. Nsp1 inhibits interferon signaling [18], Nsp3 blocks host innate immune response and are essential in cytokine storm by promoting cytokine expression [19], Nsp5 acts like Protease (3CLpro) and cleaves pp1a [20] and inhibits interferon signaling [21]. Nsp6 restricts autophagosome expansion [22], Nsp12 acts like RNA dependent RNA polymerase by its C terminal domain [23], Nsp14 and 15 has an exo- and endo-ribonuclease activities [24] and Nsp15 helps the viral machinery to evade double stranded RNA sensors [25]. Nsp16 along with Nsp10 mediates ribose 2'-O methylation [26]. So, it is evident that beta coronaviridae family has an extremely sophisticated genome organization and understanding its function at the molecular level will help to design effective therapeutic measures.
The virus particle attaches the human cell via spike protein and hACE2 binding. Upon binding, spike protein is cleaved by TMPRSS2 and helps the processed S protein to fuse with host membrane [15]. In SARS-CoV-2, the cell entry is preactivated by proprotein convertase furin, hence, the dependence on host protease is reduced. Cell mediated endocytosis helps the virus to internalize into the human (host) cell [27]. After entering into the host cell, lysosomal proteases like cathepsin cleaves the viral structure and helps to release the genomic RNA inside the cell. Later, the virus develops a 'slippery' sequence (5'-UUUAAAC-3') and a pseudoknot RNA structure to translate two polypeptides by frame-shifting mechanism. The polypeptides are cleaved into Nsps and some of the Nsps take part in Replication-Transcription Complex (RTC) formation to provide an amiable environment for viral replication [15].
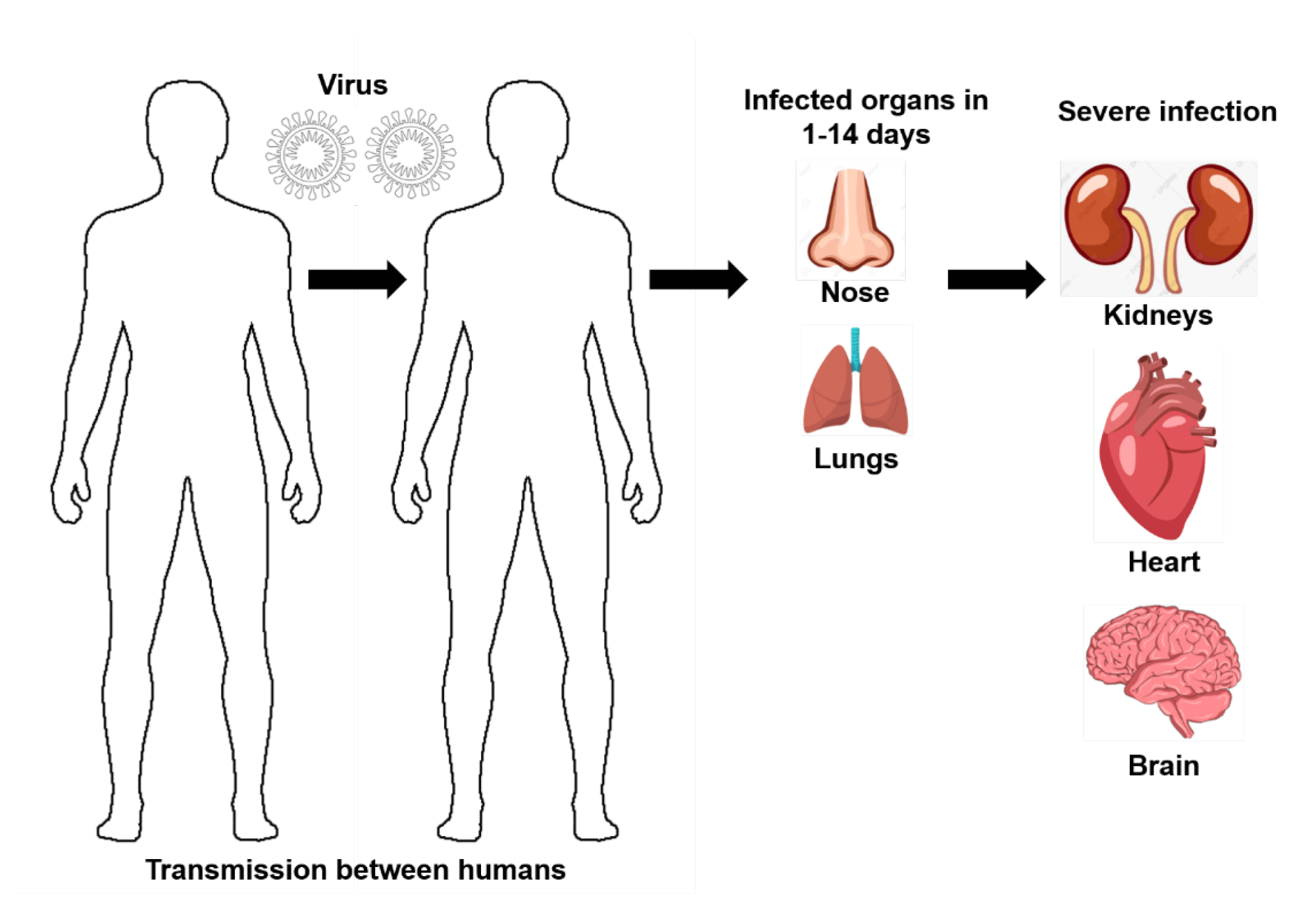
The pathophysiology of any pathogen-mediated infections are required to be understood elaborately for the effective designing of treatment modalities and drugs to combat them [28]. SARS-CoV-2, which is a new virus pathogen that has ability to cause Severe Acute Respiratory Syndrome (SARS) and has infected 13 million people from December 2019 to May 2020 [29]. The pathophysiology of SARS-CoV-2 is in high demand to be revealed in recent times as it is a new virus (novel Coronavirus or 2019-nCoV) [4,30], no proper treatment methods until now, governments have implemented lockdown measures to reduce the crowd for mitigating the spread of this virus, which eventually declines the economy of a country [31] and understanding their pathophysiological pathways can help to combat this viral infection effectively in the upcoming days [32]. The SARS-CoV-2 virus was first recognized in certain respiratory illness patients from December 2019, in the city of Wuhan, China [33], and received this name as its 80% of the genome sequence is similar to SARS-CoV (coronavirus family, that caused SARS), which has spread rapidly in the year 2002 [34]. These types of viruses possess Ribonucleic Acid (RNA) as their genetic materials, which provides them enhanced potential to mutate rapidly and can generate sub-species [35]. The viral genome is protected by a protein capsid [36] and a Spike (S) protein, which binds with the host cell [37]. The protein capsid in SARS-CoV-2 is made up of three unique protein groups, such as E (capsid protein), M (membrane protein) and N (nucleoprotein) [38]. Even though, other proteins in the capsid are useful in the maintenance of genome structural integrity, the spike proteins are the one, that can bind with receptors in humans to initiate its replication [39]. The SARS-CoV-2 infection and its transmission from the person to another starts via microdroplets, that are airway generated by the infected person and expelled, while sneezing, coughing or other similar activities [40] as displayed in figure 1. The microdroplets with the virus particles will reach another person and can enter them via epithelial conjunctiva and the upper respiratory tract cells [41]. However, the aerosol transmission of viral particles is still under investigation and it has been estimated that these particles can reside in an unventilated space for several hours [42]. Recently, several researchers suggested that the aerosol can stay longer in the air with the viral load, which may transmit the virus effectively in a closed or air conditioned environment [43,44].
The virus with the double domain surface glycoproteins (spike protein), that are inhaled will bind with the epithelial cell in the nasal cavity via Angiotensin Converting Enzyme Type 2 (ACE2) receptor and initiates its replication by the stages, such as entry, uncoating, replication, transcription, translation, virion assembly and release [45,46]. It has been proven in the in vitro data that the coronaviruses family possesses enhanced ability to bind with the ciliated cells in the respiratory tract as the primary target in the conducting airways [47]. The virus will replicate in the epithelial cells and spreads to other parts of the respiratory tract in the initial 1-2 days of infection, which is called as an asymptomatic state [48]. In this stage, a person infected with the virus can be identified by analyzing their nose and throat swab samples, which will contain high viral load [49]. However, recent studies also proved that the existence of virus (low viral load) can be detected in saliva, sputum, tracheal and pharyngeal swabs, pleural effusion fluid, broncho-alveolar lavage and in certain cases, urine and semen [50]. The samples from the patients will be subjected to Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) analysis to detect the single RNA sequence of SARS-CoV-2 to label them as positive or negative cases [51].
In the next phase, the virus spread more in the respiratory tract cells and the viral load will increase along the conducting airways, which eventually activates an innate immune response to show certain mild to severe symptoms, such as uncomfortable to breathe and fever [52]. The immune response will vary with the age, gender and various other factors in covid-19 positive patients and the cytokine-based innate immune response will be triggered to protect the unaffected cells as well as inhibit the replicating virus [53]. Above 80% of the SARS-CoV-2 infected patients are either asymptomatic or with mild symptoms such as fever, if their immune response is better without any comorbid conditions [54]. However, these patients can spread the virus to others through air transmission as mentioned earlier [55]. It is worthy to note that the level of CXCL 10, which is an interferon responsive gene, which is expressed in viral infected epithelial cells along with other beta and lambda interferons. This CXCL 10 gene possesses superior ratio of signal to noise in the response towards type 2 alveolar cells and are recommended to be beneficial as a potential disease marker for SARS diagnosis [56-58].
The rest of 20% population affected by SARS-CoV-2 will progress to the next stage, which will show moderate symptoms, such as pneumonia with fever and cough without hypoxemia, severe symptoms, including pneumonia with hypoxemia, which leads to critical conditions in patients such as Acute Respiratory Distress Syndrome (ARDS), along with other conditions namely encephalopathy, shock, heart failure, acute kidney injury, myocardial injury and coagulation dysfunction [59]. Out of this 20% severely infected patients, 2% of fatality rate can be observed, mostly for patients with comorbid conditions and age [60]. In this stage, the viral load will increase in the lungs'gas exchange units and critically contaminates the sub pleural and peripheral type 2 alveolar cells [61,62]. Later, the lung cells will undergo apoptosis, due to high viral load and starts to gradually spread towards other organs [57]. The result from the pathological studies revealed that the SARS-CoV-2 possess ability to diffuse damage in the alveolar region with a few giant multinucleate cells and hyaline membranes rich in fibrin [63]. Thus, vigorous response of innate and acquired immunity as well as epithelial cell regeneration are required to treat severe symptom exhibiting patients and recover them from the infection [64]. The 2% mortality rate in infected patients is mostly due to reduced immune response in old-age and comorbid populations with reduced ability to repair the damaged epithelial cells. In addition, these groups of infected patients will have reduced mucociliary clearance, which eventually facilitates the escalated virus spread in the gas exchange units of the lungs and leads to death [65].
Effect of SARS-CoV-2 in various organs
Apart from general pathophysiology, which is focused in the lungs, SARS-CoV-2 have a specific mechanism of action to infiltrate damages in other organs. It has been reported that the kidney, heart and brain are the most affected organs, due to corona-type virus infection, especially SARS-CoV-2, other than the lungs.
Lungs: Lungs are the most affected organ due to any nasal pathogenic viral infections and SARS-CoV-2 also severely affect these organs. It can be noted that the lung serves as a replication spot for these novel coronaviruses, cause pulmonary damage and spread to other organs after severely affecting the lungs and nasal pathway [11]. Ziehr, et al. [66,67]. stated the respiratory pathophysiology of mechanically ventilated, severely infected SARS-CoV-2 patients. The study was performed in Massachusetts General Hospital and Beth Israel Deaconess Medical Center among 66 patients with the severe infection of SARS-CoV-2 and are managed with invasive mechanical ventilation to support patients with respiratory failure. Out of 66 patients, 12% of patients already had pre-existing pulmonary disease and 34% of patients are former or current smokers with a median age of 58 years. In this study, 85% of patients are identified to have trifling to moderate Acute Respiratory Distress Syndrome (ARDS) and are confirmed by Berlin criteria to admit them in the Intensive Care Unit (ICU). The mechanical ventilation for 16 was proven to successfully improve the respiratory failure condition in severely infected, 62.1% of SARS-CoV-2 patients, a tracheostomy was performed in 21.2% of patients, 75.8% of patients were discharged from ICU and 16.7% of patients were dead. This study revealed that the respiratory failure in SARS-CoV-2 patients was exhibited in a similar gas exchange and mechanics of the respiratory system, and the effect of mechanical ventilators is based on the pre-existing co-morbid conditions and age factor of patients. Further, the pathophysiology revealed that the pre-existing therapies for ARDS along with the mechanical ventilation facility will be beneficial in recovering the patient from the respiratory viral infection [68].
Kidney: Recently, several reports demonstrated that the SARS-CoV-2 viruses target and damage kidney cells, next to the lungs. Martinez-Rojas, et al. [69] reported that SARS-CoV-2 viral infection can lead to multi-organ failure. The study emphasized that the attachment of viral particles with ACE2 promotes them to infect distinct host cells by disrupting the homeostasis of renin-angiotensin-aldosterone system. These disruptions largely lead to abnormalities in renal parts, such as hematuria, proteinuria and Acute Injuries in Kidney (AKI). Further, these coronavirus types possess ability to infect tubular epithelial cells and podocytes, that can eventually lead to renal abnormalities. Likewise, Diao, et al. [70] showed via retrospective glomerular filtration rate analysis, among 85 infected SARS-CoV-2 patients, that the viral infection affects kidneys, next to the lungs. The results revealed that the 27.06% of SARS-CoV-2 infected patients developed acute renal failure condition, while 65.22% of elderly patients with comorbid conditions, such as hypertension and heart failure are easier to develop renal failure and lead to mortality. The postmortem report of kidney tissues stained with hematoxylin and Eosin stains revealed severe acute tubular necrosis and lymphocyte infiltration. Further, the study established the viral nucleoprotein antigen accumulation n the kidney tubules via immunohistochemistry analysis and the existence of virus-like particles in kidneys, via electron micrographs. The study concluded that the infection of SARS-CoV-2 induces, CD68+ macrophages to infiltrate tubulointerstitium and enhances the deposition of complement C5b-9 on tubules. Likewise, Zhang, et al. [71] demonstrated the genetic roadmap of kidney participation in the SARS-CoV-2 infected patients. In this study, the protein and gene expression of ACE2 level is determined in kidney with its spatial characterization via The Human Protein Atlas [72]. The ACE2 receptor is expressed in several organs, including kidney, and the corona virus binds and replicates in humans via ACE2 receptor to cause acute respiratory illness [73]. However, the viral RNA has also been noticed in the urine samples, which has concluded the involvement of kidney in Covid-19 infection. The study concluded that the existence of ACE2 receptor in the kidney tubules will cause injury, which eventually leads to the consequences of covid-19 infection in the kidney [74]. Further, Zhang and Liang also mentioned the latent risk of the kidney as a defenseless organ to the infection caused by novel coronavirus 2019 [75]. In the study, they have reported the prevalence of non-respiratory symptoms, such as myalgia, diarrhea and fatigue in moderate cases and acute kidney injury in severe cases, affected with SARS-CoV-2. Further, pathophysiological studies exhibited that the acute kidney injury can be a multifactorial mechanism, that are triggered via 2019-nCoV direct infection, which elevates responses of inflammation and immunity and leads to toxic reactions, due to respiratory failure [75]. Contrarily, Wang, et al. [76] reported by analyzing 116 patients infected by covid-19 and admitted in a hospital in Wuhan, China, that the infection caused by SARS-CoV-2 does not lead to an acute and direct injury in the renal structures. This study showed that the severe injury in the lungs due to viral load has led to multiple organ damage, including kidney.
Cardiovascular system: Apart from the lungs and kidneys, certain patients have also exhibited damages in the cardiovascular system. Zheng, et al. [77] reported that the infection of SARS-CoV-2 virus in humans can lead to acute myocardial injury and chronic damages to the cardiovascular system. In addition, covid-19 patients with preexisting cardiovascular diseases are under the high risk category, and the viral infection can lead to death. Likewise, Groß, et al. [78] revealed the implications of ACE2 receptor-dependent SARS-CoV-2 interaction on the cardiovascular system of the host. In this study, the authors provided a molecular insight on the reason behind the increased mortality rate among elderly, covid-19 infected people with immunocompromised condition and cardiovascular disease, due to cardiopulmonary failure. The study showed that the binding of corona type virus with ACE2 can degrade angiotensin II, which is a master Renin-Angiotensin-Aldosterone System (RAAS) regulator and converts them into vasodilatory molecules with cardio-protective effects. This mechanism potentially leads to cardiovascular system failure in elder patients and cause death. Additionally, Chen, et al. [79] revealed that the novel coronavirus 2019 possess ability to cause fulminant myocarditis, which causes inflammation in cardiac cells and patients with this syndrome have high mortality of about 40-70%. This study showed that the several infected patients of covid-19 will have increased levels of cardiac troponin I and new-onset arrhythmias causing cardiopulmonary failure.
Brain: It has been reported recently that the brain and central nervous system is also affected by the viral infection caused by 2019-nCoV. Natoli, et al. [80] demonstrated the neurological impact of SARS-CoV-2 via neurological sequelae from animal models of MERS and SARS. In this study, the affinity of the covid-19 viral spike protein with ACE2 receptor has been identified in neurons, which revealed their neuro-invasive potential, similar to SARS and MERS. Thus, the translational lessons from the animal models infected with SARS and MERS provided ample evidence that SARS-CoV-2 can enter and damage the brain. Further, Moriguchi, et al. [81] reported that the SARS-coronovirus-2 can lead to meningitis or encephalitis in severely affected patients. In a particular case reported by the authors, the patient had fever and fatigue in day 1 and prescribed with Laninamivir and antipyretic agents for 2-5 days. Later, the patient developed severe symptoms and became unconscious after 9th day. The nasopharyngeal swab analysis of the patient did not detect the presence of any viral load, however, the viral load was detected in cerebrospinal fluid. Furthermore, Zanin, et al. [82] mentioned that novel coronavirus can induce spine and brain demyelinating lesions in severely infected patients. The study suggested that the virus can cause Systemic Inflammatory Response Syndrome (SIRS)-like immune disorder and can trigger hypoxic neurotoxicity and injuries in central nervous system. Moreover, Paniz-Mondolfi, et al. [83] reported the existence of 2019-nCoV in the capillary and neural endothelial cells in the frontal lobe tissue of the severely infected patient via postmortem examination. The study emphasized that the virus present in the neural tissue can worsen neurological symptoms and damage central nervous system.
Other organs: In children, mostly the virus does not show any symptoms as they possess a less quantity of ACE2 in their upper respiratory tract. However, there can be milder symptoms such as fever and cough [84]. In addition, Verdoni, et al. [85] reported that the children affected with SARS-CoV-2 in the Italian epicenters are developing a severe Kawasaki-like disease, which is an acute self-limiting vasculitis of the medium caliber vessels. In certain cases, the binding nature of corona virus with ACE2 has led to a potential damage in the gastrointestinal tract. Interestingly, Grassia, et al. [86] reported that the patients with preexisting inflammatory bowel diseases are not developing severe symptoms against SARS-CoV-2 and several investigations are under research to identify, which drug or factor reduces the symptom. Kumar, et al. [87] revealed the enhanced ACE2 expression in 2019-nCoV infected patients, especially in the tissues of gastro-intestine with the digestive pathogenesis symptoms and also emphasized mortality related to diabetes and recurrence of diseases in the patients of covid-19. Moreover, Carvalho, et al. [88] showed that the gastrointestinal infection of SARS-CoV-2 can lead to hemorrhagic colitis, which can lead to detection of virus in fecal samples and symptoms, such as vomiting, diarrhea, nausea and pain in the abdomen. However, extensive research in the future will reveal several unanswered questions in the pathophysiology of this viral infection, which affects multiple organs.
Rapid diagnosis of infectious agents is a key step to fight against an outbreak. When a new virus infects human, diagnosis becomes a challenging task. The diagnosis is divided into 3 major parts- specimen collection, virus isolation and identification. It is worthy to note that the PCR method is highly beneficial in the detection of the virus, while it exists in a person. Another technique applied with limited success is immunodiagnostic method. Here, the detail procedure shall be discussed to shed light on diagnostic procedure.
Specimen collection
Being an airborne pathogen, in the early stage of infection, corona virus targets upper respiratory tract. When a patient with mild pneumonia and history of coming in contact with suspected individual reaches the lab or hospital for testing, the Nasopharyngeal (NP) or Pharyngeal (OP) swab is collected. NP swab collection generally elicits tears and OP swab collection create a 'gag' response. Some reports suggest NP swab has a much higher identification rate compared to OP swab. As SARS-CoV 2 is airborne and highly contagious, hence, utmost care should be taken by the medical personnel who is collecting the samples. Personal Protective Equipment (PPE) should be used while collecting the Covid19 samples [89]. In crisis situation like scarcity of PPEs, non-availability of health care personnel due to high patient number, saliva or nasal wash samples that are collected by the clinicians can be utilized as specimens for the patients who are showing pneumonia like symptoms. Sputum from lower respiratory tract can be used as a specimen. Serum samples should be collected in two separate occasions, acute which is in the first week of illness and convalescent which is over 2-3 weeks.
Specimen transport
Collected specimen should be kept in viral transport medium and transported in refrigerated condition. As per WHO and CDC guidelines (https://www.cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medium.pdf), Viral Transport Medium (VTM) is constituted as follows: Hanks Balanced Salt Solution (HBSS) mixed with calcium and magnesium ions, but a pH indicator like phenol red should not be present. This medium should be supplemented with sterile and heat inactivated Fetal Bovine Serum (FBS) and antibiotics e.g. gentamicin and amphotericin B. According to Indian Council of Medical Research (ICMR) guidelines (https://www.mohfw.gov.in/pdf/5Sample%20collection_packaging%20%202019-nCoV.pdf), specimen samples must be kept in VTM and transported to the lab in refrigerated condition (≤4°C). The testing of the sample should be done when kept at ≤4°C within 5 days. Longer storage is possible at -70°C. Bronchoalveolar lavage storage time is much less (≤48h) compared to NP and OP swab. Biopsy or autopsy tissue samples should be transported in sterile normal saline and should be tested ≤24 h. serum samples should be collected in a serum separator tube and transported in 4°C. It can be stored around 5 days before testing at 4°C.
Covid-19 diagnosis
Diagnosis is an important part of fighting a pandemic. In a large population, identifying the potential candidates is a challenging task. In addition to this, diagnosis of a relatively unknown virus is always challenging. Multiple approaches have been proposed (Table 1). Some of the key approaches are documented in the current review.
| Table 1: Different diagnostic techniques with their advantages and disadvantages. | ||
| Diagnostic technique | Advantages | Disadvantages |
| Real time PCR or qRT PCR | High sensitivity Confirmatory test |
False positive may arise if the primers are incorrect (Toms, et al. 2020) Minor contamination during sample collection affects the result |
| Loop mediated isothermal amplification | Cheap compared to qRT PCR Less complex instrument Can be used at the point of care |
Primer designing is complex and may produce confusing result |
| Imaging technique | Direct observation of the condition of the internal organs | Expensive instrumentation, often available in specialized hospitals Highly trained manpower Not a confirmatory test |
| Serological test | Easy to conduct with basic diagnostic lab facilities Less expensive compared to qRT PCR |
Early detection not possible Indirect test High level of variability Patients with other infection may give false positive results Virus specific identification requires high viral titre |
Real time PCR
It is also named as reverse transcription quantitative PCR. It is regarded as the gold standard to detect SARS-CoV-2 all across the globe. Currently, several COVID-19 testing are conducted with the genetic material of virus that is collected from throat and nose swabs, using a workhorse molecular biology tool known as reverse transcription polymerase chain reaction RT-PCR. The principle of this test is to amplify a specific sequence of viral genes, where short sequences of complementary genes named primers can help to initiate the copying process. As it is a highly specific and sensitive technique, scientist and medical practitioners depend on this technique. Dozens of labs and research consortia around the world worked round the clock to develop testing kits to fulfill the demand. Labs, throughout the world, have modified their PCR for SARS-CoV-2tests, via distinct primers that can target specific sequence of the viral genes. Examples are the US FDA's emergency use authorized kits by Roche Diagnostics Cobas and Logix Smart COVID-19 test kit. Other kits like Nova cyst by French Clinical Diagnostics, Bioeasy kit by Shenzhen Bioeasy Biotechnology Co Ltd, China are being used by European countries. Already, certain reports from Spain have mentioned that the Bioeasy kit is recognizing only 30 of the positive cases [90-92].
It is a Nucleic Acid Amplification Test (NAAT), where rapid analysis can be obtained. It is widely applied to various types of pathogen detection like virus, bacteria and protozoa (especially malaria). The simplicity of the technique enables it to be converted into mobile unit and can be used at the point of care like railway station, airport, and rural areas. Reverse transcription loop mediated isothermal amplification process or RT-LAMP is a cheaper and faster process compared to qRT PCR. It depends on one step amplification technique. Unlike PCR, which needs thermal cycling, RT LAMP is carried out at a temperature between 60-65°C and use DNA polymerase. This technique employs a set of four primers [93] to recognize six unique sequences in target DNA sequence. There are forward as well as backward inner primers with dual sequences of primer. The first set is for initial stage priming and the second is for later stage self-priming process. Among four primers, both primers are utilized at the early stage, but later the stand displacement is done by inner primers. In the first report, the reaction is carried out at 65°C for 1 hour [93]. Later it was observed that the inclusion of two additional primers can shorten the reaction time by half [94]. The current LAMP method uses total six primers which are specific against eight unique sites. Besides inner and outer primers, which are able to identify six distinct sites, the loop of two primers elevates the speed and the efficiency of amplification [95]. Previously, the efficacy of LAMP was shown to detect SARS [96] and RT-LAMP assay for MERS detection [97] corona virus with high sensitivity. In LAMP, reverse transcription reaction is done in the same tube prior to amplification; hence it makes the identification process faster. In one Covid 19 detection kit, researchers have designed an RT-LAMP system, where viral detection limit is 80 copies of viral RNA molecule/mL of sample [98] and the reaction can be completed in 20 min at 65°C. The designed primers are specific to ORF1ab, S, N1 and N15 genes. In another work, researchers have identified Covid 19 in a one or two stage process. In the two-stage isothermal amplification termed as Penn-RAMP by the authors, the first stage conducted at 38°C termed as recombinase polymerase amplification and second stage LAMP at 63°C. The amplicon was detected by leuco crystal violet stain and colorimetric identification was possible [99]. In another study, researchers have targeted N gene. They have shown the limit of detection 118 copies and with the help of fluorescent dye, it can be identified with 30 min and if the initial template number is 200 copies, then it can be identified in visual detection at 40 min ([100]. In another study, researchers have targeted ORF1ab region and the reaction was conducted at 65°C. The visible color change was observed within 20 min when the template RNA copy number was 1000. The detection capability was further extended by using SYBR green dye [101].
Imaging techniques
Imaging techniques are highly beneficial in any disease diagnosis. As Covid 19 is an acute respiratory syndrome, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography-Computed Tomography (PET-CT), lung imaging by ultrasound and application of artificial intelligence is analysis and decision outcome plays crucial role in treatment assessment and modalities [102]. Although RT-PCR based screening is considered as gold standard, yet it has detection limit and a patient with respiratory illness cannot be opted out of Covid-19, even if the RT-PCR result is negative. Chest CT acts as a complementary examination for RT-PCR. The characteristic images of infected lungs, such as ground glass opacity and pulmonary fibrosis are used to consolidate the diagnosis [103]. 18F-Flurodeoxygluucose (FDG)-PET CT is also employed to measure the effect of SARS-CoV-2 on the lungs. The axial image shows ground glass opacity and increased 18F-FDG uptake. The increased uptake takes place because of highly activated neutrophils swarm in the infected area. Supported by sample collection site (e.g: nasopharengeal swab eliminates influenza) and RT-PCR data, 18F-FDG-PET CT may give crucial information about the patient [104].
Serological Test
Many reports have emerged that young adults and children, who have not been exposed to any of the coronaviral family members may show very minimal or no symptoms and simply behave as carriers to transmit the infection to others. Here is the necessity for a test that could show the extent of viral spread in a community and provide useful public-health information. A serological test-based detection would perfectly fulfill this requirement. A Singapore based group from Duke-NUS Medical School used serological tests to aid in contact tracing, but at the time the test had not been broadly validated for clinical use. Another group from New York City hospital used serological tests to better understand how quickly COVID-19 patients start to develop antibodies to the virus. It could also help to identify recovered patients who could then donate their SARS-CoV-2 antibody-rich serum to help treat critically ill patients. Another key application would be to identify people who have developed likely immunity to the virus. They might be able to treat patients safely or take on other front-line jobs during the pandemic [91,105].
Vaccine development
The most alarming situation is that there is no proper medical prescription for patients to mitigate the effects of this viral disease. In addition, there is no vaccine available to control the spread which will lead an escalation in the positive cases, in the upcoming days. The SARS-CoV-2 has S protein on the surface of the virus, guiding its entry into the host cell. More interestingly the Receptor Binding Domain (RBD) of S protein has the potential role in this matter. Therefore, the target of this study is the RBD domain to stop the viral infection. COVID-19 recovered patient has antibody against SARS-CoV-2 [106]. The RBD domain is the most exposed part of SARS-CoV-2, however, there is no recommended treatment for COVID-19 as there exists no proper suggestion from randomized controlled trials [107]. Interestingly, US based Vir Biotechnology, Inc. have developed VIR-7831 and VIR-7832, with high affinity towards the S protein of SARS-CoV-2. Therefore, S protein, particularly the RBD domain of S1 and S2 subunit would be a critical vaccine contender for long run COVID-19treatment [108]. Likewise, a ChAdOx1 nCoV-19 vaccine prepared by Oxford university, mRNA-1273 prepared by Moderna, Inc. and BNT162 by Pfizer pharmaceutical company shows promising results to develop immunity in hosts against SARS-CoV-2 viruses by targeting their spike proteins [109-111]. Similarly, INO-4800 prepared by Inovio pharmaceuticals (USA) is a DNA-based synthetic vaccine, that targets antigen in the surface of S protein and protein-based vaccine was synthesized by targeting ectodomain soluble spike protein immunogen in emulsion to combat against the replication of SARS-CoV-2 virus in the host [55,112]. Further, PicoVacc vaccine targeting neutralizing antibody of coronavirus and CoroFlu fabricated by Bharat Biotech International Ltd. as a nasal flu vaccine to produce neutralizing antibody against viruses were also recently recommended as a probable vaccine to produce immunity against SARS-CoV-2 in human hosts [113,114]. Furthermore, Shenzhen Geno-Immune Medical Institute of China prepared LV-SMENP-DC vaccine by using lentiviral vector with the synthetic minigene to produce antigen-specific cytotoxic T-lymphocytes and pathogen-specific artificial antigen-presenting cell to inhibit viral protein as an effective vaccine candidate against 2019-nCoV [53]. However, all these vaccines are in Phase I clinical trials and it will take a long time to reach the commercial pharmaceutical market.
Antiviral drugs
Several preexisting antiviral drugs that are used in the treatment of previously common infections caused by viruses, such as SARS, MERS, Ebola and Nipah are currently utilized for SARS-CoV-2treatment. Remdesivir is a capable antiviral drug that are used for Ebola treatment and are prescribed for 2019-nCoV treatment as it is a nucleotide-based antiviral drug [115]. Similarly, Ribavirin is also a nucleotide-based antiviral drug, that are used for the treatment of certain viral hemorrhagic fevers, respiratory syncytial virus and hepatitis C, and are prescribed for SARS-CoV-2 treatment [116]. Likewise, Galidesivir and BCX4430 are the other antiviral nucleotide analogues with the ability to effectively treat Ebola, Hepatitis C and Marburg virus, and are recommended for the treatment of severely infected patients with covid-19 [114-117]. Moreover, Baricitinib, that targets Janus kinase and are already in usage as a curative agent for rheumatoid arthritis without any inflammatory response and Lopinavir, which targets viral 3CLpro proteases and are permitted for HIV treatment in combination with ritonavir are also recommended for covid-19 treatment in some countries [115,118]. Furthermore, Arbidol is a potential drug antiviral efficacy that isbeneficial in preventing the entry of influenza type coronaviruses by inhibiting ACE2 binding spike protein of the virus and Darunavir, that are used to reduce the infection of HIV, are also widely suggested against SARS-CoV-2 for effective treatment [119,120]. Besides, Nitazoxanide, that can target and inhibit the protein expression of the virus, which are already in use for the treatment of diarrhea caused by viral, protozoan and helminthic infection, were also under extensive research to be beneficial for SARS-CoV-2 infection treatment [13]. However, extensive trials in preclinical as well as clinical arenas are vital to confirm the effectiveness of these drugs against Covid-19 and can only be used in combinations, instead of a single drug.
Repurposed drugs
The SARS-CoV-2 virus has been transmitted to almost 200 countries within 3 months and there is no specific drug to counteract the infection or its associated complications. A new drug, that are specific to counteract the covid-19 infection, will take a long time (several months or even years) to reach the commercial market as it should cross the barrier of preclinical and clinical trials. Thus, already existing drugs that are utilized for other infection treatments are repurposed to help SARS-CoV-2affected patients. The main purpose of designating these already existing drugs is that they are approved and certified to be safe of human for the treatment of other infections. BCG vaccine, which is commonly used against tuberculosis, is repurposed and are under extensive research to be used as a vaccine candidate for the current covid-19 infection to improve immunity in patients [121]. However, in vitro, in vivo and clinical analysis are compulsory to confirm their efficacy in improving immunity of the host against SARS-CoV-2. Later, Chloroquine phosphate used as an effective antimalarial drug are repurposed for severely infected covid-19 patient treatment and early studies showed that they are effective in reducing upper respiratory issues in SARS-CoV-2 infected patients. However, elderly patients may develop cardiovascular problems, when high doses of these drugs are prescribed, which a major limitation of this drug is [122]. Similarly, Hydroxychloroquine in combination with antibacterial agent named azithromycin were prescribed to covid-19 patients for combating pneumonia-like complications. Even though, these drugs are already in use for the treatment of malaria and bacterial infections, it may lead to cardiovascular issues in elderly patients and cannot be prescribed for patients of all age groups [123-126]. Further, convalescent plasma from the processed blood of covid-19 disease recovered patients were used to treat severely infected patients as it will contain antibodies to improve immunity against SARS-CoV-2. Furthermore, initial studies in Italian and Chinese epicenters showed promising results among high risk patients. However, the extraction of plasma is a costly and time-consuming process, which cannot be affordable in developing and underdeveloped countries [16,17,127]. All these reports presented the extensive researches which will help in upcoming days to understand the exact pathophysiology of this viral infection is required to design novel or repurpose preexisting drugs to combat and eradicate this infection in the near future.
Rehabilitation programs
In addition to diagnostic, vaccine development, antiviral drugs and drug repurposing approaches, rehabilitation programs were also considered to be a significant method for the recovery of SARS-CoV-2 infected patients. Zhu, et al. [128] reported the early possible pulmonary rehabilitation for SARS-CoV-2 pneumonia with the experience from an Intensive Care Unit (ICU) in Shenyang, China. In this study, a 41-year-old man with SARS-CoV-2 infection was successfully treated with 11 days of mechanical ventilation and 9 days of oxygenation via extracorporeal membrane along with conventional supportive care. Further, an individualized and meticulous ICU rehabilitation program was provided to the patient after weaning process. The ICU rehabilitation program includes four components, such as postural change and prone position, respiratory training to restore respiratory muscle strength and lung volume, early mobilization and physical exercises, and psychological intervention as well as sleep promotion. Later, Aytur, et al. [129] released a guideline for the acute and subacute rehabilitation based on the pulmonary rehabilitation principles in SARS-CoV-2 infection. In this study, they have provided several guidelines of pulmonary rehabilitation required for mild disease, mild and severe pneumonia as well as acute ARDS stage. Further, they also drafted the regulations for rehabilitation approaches after discharging the patient infected by COVID-19 from hospitals. In both these studies, it is noteworthy that physiotherapy plays a key role in the rehabilitation process to regain muscle strength of lungs and to improve mobilization of the patient with certain psychological impact. Jangra and Saxena (2020) also mentioned that physiotherapy can play a crucial role in the respiratory rehabilitation and management of patients with SARS-CoV-2 infection. Dyspnea is a condition in SARS-CoV-2 infected patients, where the lung muscle is tightened and inspiratory muscle training as well as breathing exercises can be beneficial to improve dyspnea. Moreover, physiotherapy was reported to be useful for assisting the positioning of COVID-19 patients to relax them from respiratory distress and to prevent secondary complications. Furthermore, early rehabilitation via physiotherapy can be recommended to recovered patients for limiting or preventing ICU-acquired weakness [130]. Likewise, Dehesh (2020) stated that stress may lead to negative effects on COVID-19 patients with back pain or musculoskeletal disorders, which can be reduced with firm physiotherapy guidelines [131]. Similarly, Diwate (2020) agreed to the fact that physiotherapy is useful in the physical rehabilitation and treatment of COVID-19 patients and recommended 'expert consensus and recommendation for physiotherapy management for COVID-19 in Indian set up' guidelines, which is approved by Maharashtra State Council for Occupational Therapy and Physiotherapy, Mumbai (India) for physiotherapists to decide and plan treatment for SARS-CoV-2 infected patients [132]. All these studies emphasized that physiotherapy can be a beneficial approach for the proper rehabilitation, during and after ICU-based treatments for COVID-19 patients.
SARS-CoV-2 viruses are spreading rapidly in the last few months and the recent studies showed that their strain virulence is reducing rapidly. However, the elderly people and patients with comorbidity are under high risk and are prone to mortality, due to this viral infection. Thus, social distancing, lockdown measures, rapid diagnosis in large scale and preexisting drugs are the only available strategies to combat the pandemic of this viral infection, until the discovery of a potential vaccine. Scientist are working hard to identify a potential drug and vaccine candidate, where are few are successful and are under in phase I clinical trials, which gives promising status that a vaccine will be available within next year. Even before the vaccine, rapid diagnostic tools and drugs to reduce or treat the health complications instigated by the infection of virus, will be available in markets as mentioned in this review. In the near future, more critical knowledge on the pathophysiology of SARS-CoV-2 viruses will be available, which will eventually lead to the discovery of highly efficient and target specific drug or vaccine to eradicate this viral infection and save lives of several millions of people.
We acknowledge the Academy of Competitive Examination and Research Training for providing the platform for studies and idea development. The author (Dr. Jaison Jeevanandam) acknowledge the support of FCT-Fundação para a Ciência e a Tecnologia (Base Fund UIDB/00674/2020 and Programmatic Fund UIDP/00674/2020, Portuguese Government Funds), ARDITI-Agência Regional para o Desenvolvimento da InvestigaçãoTecnologia e Inovação through the project M1420-01-0145-FEDER-000005-CQM+ (Madeira 14-20 Program).
- Kim JM, Chung YS, Jo HJ, Lee NJ, Kim MS, Woo SH, Park S, Kim JW, Kim HM, Han MG. Identification of Coronavirus Isolated from a Patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020 Feb;11(1):3-7. doi: 10.24171/j.phrp.2020.11.1.02. PMID: 32149036; PMCID: PMC7045880.
- Poutanen SM. Human Coronaviruses. Principles and Practice of Pediatric Infectious Diseases. 2012:1117-1120.e4. doi: 10.1016/B978-1-4377-2702-9.00224-5. Epub 2013 Feb 10. PMCID: PMC7152163.
- Daga MK, Kumar N, Aarthi J, Govind Mawari. From SARS-CoV to Coronavirus Disease 2019 (COVID-19)-A Brief Review. Journal of Advanced Research in Medicine. 2019;(4):1-9. doi: 10.24321/2349.7181.201917
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020 Apr 14;52(4):583-589. doi: 10.1016/j.immuni.2020.03.007. Epub 2020 Apr 6. PMID: 32259480; PMCID: PMC7136867.
- Gorbalenya AE, Baker SC, Baric RS. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. doi: 10.1038/s41564-020-0695-z.
- Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J. 2020 May 7;55(5):2000524. doi: 10.1183/13993003.00524-2020. Erratum in: Eur Respir J. 2020 Sep 24;56(3): PMID: 32269088; PMCID: PMC7144257.
- Roussel Y, Giraud-Gatineau A, Jimeno MT, Rolain JM, Zandotti C, Colson P, Raoult D. SARS-CoV-2: Fear versus data. Int J Antimicrob Agents. 2020 May;55(5):105947. doi: 10.1016/j.ijantimicag.2020.105947. Epub 2020 Mar 19. PMID: 32201354; PMCID: PMC7102597.
- Jiang X, Rayner S, Luo MH. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. 2020 May;92(5):476-478. doi: 10.1002/jmv.25708. Epub 2020 Feb 24. PMID: 32056235; PMCID: PMC7166592.
- Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020 May;19(5):305-306. doi: 10.1038/d41573-020-00073-5. PMID: 32273591.
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J, Quan L, Xia Z, Tan W, Cheng G, Jiang T. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020 Mar 11;27(3):325-328. doi: 10.1016/j.chom.2020.02.001. Epub 2020 Feb 7. PMID: 32035028; PMCID: PMC7154514.
- Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Kwe Yinda C, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv [Preprint]. 2020 Apr 22:2020.04.15.043166. doi: 10.1101/2020.04.15.043166. Update in: Nature. 2020 Jun 9. PMID: 32511319; PMCID: PMC7239049.
- Walls AC, Park YJ, Tortorici MA, Abigail Wall, Andrew TM Guire, David Veesler. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-292e6. https://tinyurl.com/rca7ukk
- Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020 Apr;92(4):418-423. doi: 10.1002/jmv.25681. Epub 2020 Feb 7. Erratum in: J Med Virol. 2020 Aug 2. PMID: 31967327; PMCID: PMC7167049.
- Pervushin K, Tan E, Parthasarathy K, Lin X, Jiang FL, Yu D, Vararattanavech A, Soong TW, Liu DX, Torres J. Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 2009 Jul;5(7):e1000511. doi: 10.1371/journal.ppat.1000511. Epub 2009 Jul 10. PMID: 19593379; PMCID: PMC2702000.
- Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. doi: 10.1007/978-1-4939-2438-7_1. PMID: 25720466; PMCID: PMC4369385.
- Kang S, Yang M, Hong Z, Zhang L, Huang Z, Chen X, He S, Zhou Z, Zhou Z, Chen Q, Yan Y, Zhang C, Shan H, Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm Sin B. 2020 Jul;10(7):1228-1238. doi: 10.1016/j.apsb.2020.04.009. Epub 2020 Apr 20. PMID: 32363136; PMCID: PMC7194921.
- McBride R, van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014 Aug 7;6(8):2991-3018. doi: 10.3390/v6082991. PMID: 25105276; PMCID: PMC4147684.
- Tanaka T, Kamitani W, DeDiego ML, Enjuanes L, Matsuura Y. Severe acute respiratory syndrome coronavirus nsp1 facilitates efficient propagation in cells through a specific translational shutoff of host mRNA. J Virol. 2012 Oct;86(20):11128-37. doi: 10.1128/JVI.01700-12. Epub 2012 Aug 1. PMID: 22855488; PMCID: PMC3457165.
- Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. 2018 Jan;149:58-74. doi: 10.1016/j.antiviral.2017.11.001. Epub 2017 Nov 8. PMID: 29128390; PMCID: PMC7113668.
- Stobart CC, Sexton NR, Munjal H, Lu X, Molland KL, Tomar S, Mesecar AD, Denison MR. Chimeric exchange of coronavirus nsp5 proteases (3CLpro) identifies common and divergent regulatory determinants of protease activity. J Virol. 2013 Dec;87(23):12611-8. doi: 10.1128/JVI.02050-13. Epub 2013 Sep 11. PMID: 24027335; PMCID: PMC3838113.
- Zhu X, Fang L, Wang D, Dang Wang, Yuting Yang, Jiyao Chen, Xu Ye, Mohamed Frahat Foda, Shaobo Xiao. Porcine deltacoronavirus nsp5 inhibits interferon-beta production through the cleavage of NEMO. Virology. 2017;502: 33-38. https://tinyurl.com/yyrs7wct
- Cottam EM, Whelband MC, Wileman T. Coronavirus NSP6 restricts autophagosome expansion. Autophagy. 2014 Aug;10(8):1426-41. doi: 10.4161/auto.29309. Epub 2014 Jun 11. PMID: 24991833; PMCID: PMC4203519.
- te Velthuis AJ, Arnold JJ, Cameron CE, van den Worm SH, Snijder EJ. The RNA polymerase activity of SARS-coronavirus nsp12 is primer dependent. Nucleic Acids Res. 2010 Jan;38(1):203-14. doi: 10.1093/nar/gkp904. Epub 2009 Oct 29. Erratum in: Nucleic Acids Res. 2011 Nov;39(21):9458. PMID: 19875418; PMCID: PMC2800238.
- Minskaia E, Hertzig T, Gorbalenya AE, Campanacci V, Cambillau C, Canard B, Ziebuhr J. Discovery of an RNA virus 3'->5' exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):5108-13. doi: 10.1073/pnas.0508200103. Epub 2006 Mar 20. PMID: 16549795; PMCID: PMC1458802.
- Deng X, Hackbart M, Mettelman RC, O'Brien A, Mielech AM, Yi G, Kao CC, Baker SC. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc Natl Acad Sci U S A. 2017 May 23;114(21):E4251-E4260. doi: 10.1073/pnas.1618310114. Epub 2017 May 8. PMID: 28484023; PMCID: PMC5448190.
- Chen Y, Su C, Ke M, Jin X, Xu L, Zhang Z, Wu A, Sun Y, Yang Z, Tien P, Ahola T, Liang Y, Liu X, Guo D. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2'-O-methylation by nsp16/nsp10 protein complex. PLoS Pathog. 2011 Oct;7(10):e1002294. doi: 10.1371/journal.ppat.1002294. Epub 2011 Oct 13. PMID: 22022266; PMCID: PMC3192843.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11727-11734. doi: 10.1073/pnas.2003138117. Epub 2020 May 6. PMID: 32376634; PMCID: PMC7260975.
- Pirofski LA, Casadevall A. The Damage-Response Framework as a Tool for the Physician-Scientist to Understand the Pathogenesis of Infectious Diseases. J Infect Dis. 2018 Aug 14;218(suppl_1):S7-S11. doi: 10.1093/infdis/jiy083. PMID: 30124977; PMCID: PMC6093430.
- Marco Cascella, Michael Rajnik, Arturo Cuomo, Scott CD, Raffaela DN. Features, evaluation and treatment coronavirus (COVID-19), in Statpearls. 2020; StatPearls Publishing.
- Gengler I, Wang JC, Speth MM, Sedaghat AR. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: A systematic review of the current evidence. Laryngoscope Investig Otolaryngol. 2020 Apr 16;5(3):354-359. doi: 10.1002/lio2.384. PMID: 32587887; PMCID: PMC7262250.
- Barnett ML, Mehrotra A, Landon BE. Covid-19 and the Upcoming Financial Crisis in Health Care. NEJM Catalyst Innovations in Care Delivery. 2020;1(2). doi: 10.1056/CAT.20.0153
- Ripoll S, Gercama I, Jones T. Social Science in Epidemics: Ebola Virus Disease Lessons Learned. 2018. https://tinyurl.com/y6fo3kx4
- Yang X, Yu Y, Xu J, Huaqing Shu, Jia'an Xia, Hong Liu, Yongran Wu, Lu Zhang, Zhui Yu, Minghao Fang, Ting Yu, Yaxin Wang, Shangwen Pan, Xiaojing Zou, Shiying Yuan, You Shang. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020 May;8(5): 475-481. doi: 10.1016/S2213-2600(20)30079-5
- Xu J, Zhao S, Teng T, Abdalla AE, Zhu W, Xie L, Wang Y, Guo X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020 Feb 22;12(2):244. doi: 10.3390/v12020244. PMID: 32098422; PMCID: PMC7077191.
- Wang M, Li M, Ren R, Li L, Chen EQ, Li W, Ying B. International Expansion of a Novel SARS-CoV-2 Mutant. J Virol. 2020 Jun 1;94(12):e00567-20. doi: 10.1128/JVI.00567-20. PMID: 32269121; PMCID: PMC7307084.
- Khedkar PH, Patzak A. SARS-CoV-2: What do we know so far? Acta Physiol (Oxf). 2020 Jun;229(2):e13470. doi: 10.1111/apha.13470. Epub 2020 Apr 11. PMID: 32220035; PMCID: PMC7228362.
- Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol Cell. 2020 May 21;78(4):779-784.e5. doi: 10.1016/j.molcel.2020.04.022. Epub 2020 May 1. PMID: 32362314; PMCID: PMC7194065.
- Chatterjee S. Understanding the Nature of Variations in Structural Sequences coding for Coronavirus Spike, Envelope, Membrane and Nucleocapsid Proteins of SARS-CoV-2. Envelope, Membrane and Nucleocapsid Proteins of SARS-CoV-2. 2020 March; 1-18. https://tinyurl.com/yxe2fqgv
- Luan J, Lu Y, Jin X, Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020 May 21;526(1):165-169. doi: 10.1016/j.bbrc.2020.03.047. Epub 2020 Mar 19. PMID: 32201080; PMCID: PMC7102515.
- Wilson NM, Norton A, Young FP, Collins DW. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020 Aug;75(8):1086-1095. doi: 10.1111/anae.15093. Epub 2020 May 8. PMID: 32311771; PMCID: PMC7264768.
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL; HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 May;26(5):681-687. doi: 10.1038/s41591-020-0868-6. Epub 2020 Apr 23. PMID: 32327758.
- Kowalik MM, Trzonkowski P, Łasińska-Kowara M, Mital A, Smiatacz T, Jaguszewski M. COVID-19 - Toward a comprehensive understanding of the disease. Cardiol J. 2020;27(2):99-114. doi: 10.5603/CJ.a2020.0065. Epub 2020 May 7. PMID: 32378729.
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-1567. doi: 10.1056/NEJMc2004973. Epub 2020 Mar 17. PMID: 32182409; PMCID: PMC7121658.
- Meselson M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N Engl J Med. 2020 May 21;382(21):2063. doi: 10.1056/NEJMc2009324. Epub 2020 Apr 15. PMID: 32294374; PMCID: PMC7179963.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020 Mar 17;94(7):e00127-20. doi: 10.1128/JVI.00127-20. PMID: 31996437; PMCID: PMC7081895.
- Ruggiero E, Richter SN. G-quadruplexes and G-quadruplex ligands: targets and tools in antiviral therapy. Nucleic Acids Res. 2018 Apr 20;46(7):3270-3283. doi: 10.1093/nar/gky187. PMID: 29554280; PMCID: PMC5909458.
- Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005 Dec;79(24):15511-24. doi: 10.1128/JVI.79.24.15511-15524.2005. PMID: 16306622; PMCID: PMC1316022.
- Wickramaratchi MM, Pieris A, Fernando AJS. Review on Identification of Major Infectious Site and Disease Progression Pathway for Early Detection of Novel Corona Virus Covid-19. 2020. doi: 10.31222/osf.io/fu9p8
- Pan Y, Zhang D, Yang P, Leo LMP, Quanyi Wang. Viral load of SARS-CoV-2 in clinical samples. The Lancet infectious diseases. 2020;20(4): 411-412. doi: 10.1016/S1473-3099(20)30113-4
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 May 12;323(18):1843-1844. doi: 10.1001/jama.2020.3786. PMID: 32159775; PMCID: PMC7066521.
- Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020 Apr 21;323(15):1502-1503. doi: 10.1001/jama.2020.2783. PMID: 32105304; PMCID: PMC7047852.
- Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020 Apr 16;55(4):2000607. doi: 10.1183/13993003.00607-2020. PMID: 32269085; PMCID: PMC7144260.
- Ong EZ, ZiyingOng, Yvonne FZC, Wan YL, Natalie MYL, Shirin K, Salahudeen MHM, Kian S, Chan, Anthony TT, Antonio Bertoletti, Eng E, Jenny GH. A dynamic immune response shapes COVID-19 progression. Cell Host & Microbe. 2020 June;27(6): 879-882e2. doi: 10.1016/j.chom.2020.03.021
- Hageman JR. The Coronavirus Disease 2019 (COVID-19). Pediatr Ann. 2020 Mar 1;49(3):e99-e100. doi: 10.3928/19382359-20200219-01. PMID: 32155273.
- Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, Zhou L, Liu J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-411. doi: 10.1016/S1473-3099(20)30114-6. Epub 2020 Feb 19. PMID: 32087116; PMCID: PMC7158985.
- Hancock AS, Stairiker CJ, Boesteanu AC, Monzón-Casanova E, Lukasiak S, Mueller YM, Stubbs AP, García-Sastre A, Turner M, Katsikis PD. Transcriptome Analysis of Infected and Bystander Type 2 Alveolar Epithelial Cells during Influenza A Virus Infection Reveals In Vivo Wnt Pathway Downregulation. J Virol. 2018 Oct 12;92(21):e01325-18. doi: 10.1128/JVI.01325-18. PMID: 30111569; PMCID: PMC6189488.
- Qian Z, Travanty EA, Oko L, Edeen K, Berglund A, Wang J, Ito Y, Holmes KV, Mason RJ. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013 Jun;48(6):742-8. doi: 10.1165/rcmb.2012-0339OC. PMID: 23418343; PMCID: PMC3727876.
- Tang NL, Chan PK, Wong CK, To KF, Wu AK, Sung YM, Hui DS, Sung JJ, Lam CW. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005 Dec;51(12):2333-40. doi: 10.1373/clinchem.2005.054460. Epub 2005 Sep 29. PMID: 16195357; PMCID: PMC7108146.
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020 Jun;215:108427. doi: 10.1016/j.clim.2020.108427. Epub 2020 Apr 20. PMID: 32325252; PMCID: PMC7169933.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. doi: 10.1001/jama.2020.2648. PMID: 32091533.
- Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008 Mar 1;372(1):127-35. doi: 10.1016/j.virol.2007.09.045. Epub 2007 Nov 26. PMID: 18022664; PMCID: PMC2312501.
- Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, Huang H, Li C. Chest CT Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features. Invest Radiol. 2020 May;55(5):257-261. doi: 10.1097/RLI.0000000000000670. PMID: 32091414; PMCID: PMC7147284.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25;: PMID: 32085846; PMCID: PMC7164771.
- Nikolaos MN, John GN, Lori BP, Jason CG, Yasuaki U, Huixing W, Atsushi S, Kara EL, Huan L, Mitchell RW, Kevan LH, Francis XMC. Mitogenic stimulation accelerates influenza-induced mortality by increasing susceptibility of alveolar type II cells to infection. 2017;114(32). E6613-E6622. doi: 10.1073/pnas.1621172114
- Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Sun J, Leung R, Tsang KW. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001 Mar;163(4):983-8. doi: 10.1164/ajrccm.163.4.9909121. PMID: 11282777.
- Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018 Feb 20;319(7):698-710. doi: 10.1001/jama.2017.21907. PMID: 29466596.
- Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Reply to Epelbaum: Standards and Stereotypes in COVID-19. Am J Respir Crit Care Med. 2020 Aug 1;202(3):470-471. doi: 10.1164/rccm.202005-1944LE. PMID: 32510977; PMCID: PMC7397799.
- Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020 Jun 15;201(12):1560-1564. doi: 10.1164/rccm.202004-1163LE. PMID: 32348678; PMCID: PMC7301734.
- Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020 Jun 1;318(6):F1454-F1462. doi: 10.1152/ajprenal.00160.2020. Epub 2020 May 15. PMID: 32412303; PMCID: PMC7303722.
- Diao B, Wang C, Wang R. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020. doi: 10.1101/2020.03.04.20031120.
- Zhang YM, Zhang H. Genetic Roadmap for Kidney Involvement of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin J Am Soc Nephrol. 2020 Jul 1;15(7):1044-1046. doi: 10.2215/CJN.04370420. Epub 2020 Apr 23. PMID: 32327413; PMCID: PMC7341780.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419. doi: 10.1126/science.1260419. PMID: 25613900.
- Molina R, Oliva B, Fernandez FN. A collection of designed peptides to target SARS-Cov-2–ACE2 interaction: PepI-Covid19 database. bioRxiv. 2020. doi: 10.1101/2020.04.28.051789
- Zhang YM, Zhang H. Genetic Roadmap for Kidney Involvement of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin J Am Soc Nephrol. 2020 Jul 1;15(7):1044-1046. doi: 10.2215/CJN.04370420. Epub 2020 Apr 23. PMID: 32327413; PMCID: PMC7341780.
- Zhang F, Liang Y. Potential risk of the kidney vulnerable to novel coronavirus 2019 infection. Am J Physiol Renal Physiol. 2020 May 1;318(5):F1136-F1137. doi: 10.1152/ajprenal.00085.2020. Epub 2020 Mar 30. PMID: 32223555; PMCID: PMC7191387.
- Lunwen W, Xun L, Hui C, Shaonan Y, Yan L, Dong L, Zuojiong G. SARS-CoV-2 infection does not significantly cause acute renal injury: an analysis of 116 hospitalized patients with COVID-19 in a single hospital, Wuhan, China. 2020. doi: 10.1101/2020.02.19.20025288
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-260. doi: 10.1038/s41569-020-0360-5. PMID: 32139904; PMCID: PMC7095524.
- Groß S, Jahn C, Cushman S, ChristianBär, ThomasThum. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. Journal of Molecular and Cellular Cardiology. 2020;144: 47-53. doi: 10.1016/j.yjmcc.2020.04.031
- Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020 May;45(3):230-232. doi: 10.1007/s00059-020-04909-z. PMID: 32140732; PMCID: PMC7080076.
- Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 Apr 25:10.1111/ene.14277. doi: 10.1111/ene.14277. Epub ahead of print. PMID: 32333487; PMCID: PMC7267377.
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020 May;94:55-58. doi: 10.1016/j.ijid.2020.03.062. Epub 2020 Apr 3. PMID: 32251791; PMCID: PMC7195378.
- Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, Fontanella MM. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). 2020 Jul;162(7):1491-1494. doi: 10.1007/s00701-020-04374-x. Epub 2020 May 4. PMID: 32367205; PMCID: PMC7197630.
- Paniz-Mondolfi A, Bryce C, Grimes Z. Central Nervous System Involvement by Severe Acute Respiratory Syndrome Coronavirus -2 (SARS-CoV-2). Journal of Medical Virology. 2020.
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 Infection in Children. N Engl J Med. 2020 Apr 23;382(17):1663-1665. doi: 10.1056/NEJMc2005073. Epub 2020 Mar 18. PMID: 32187458; PMCID: PMC7121177.
- Lucio V, Angelo M, Annalisa G, Laura M, Maurizio R, Matteo C, Ezio B, Lorenzo DA. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet. 2020 June;395(10239): 1771-1778. doi: 10.1016/S0140-6736(20)31103-X
- Grassia R, Soro S, Conti CB. Inflammatory Bowel Diseases and Biological Treatment in SARS-CoV-2 Era. Why Not? Inflamm Bowel Dis. 2020 Jun 18;26(7):e71. doi: 10.1093/ibd/izaa110. PMID: 32386056; PMCID: PMC7239122.
- Kumar A, Faiq MA, Pareek V, Raza K, Narayan RK, Prasoon P, Kumar P, Kulandhasamy M, Kumari C, Kant K, Singh HN, Qadri R, Pandey SN, Kumar S. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med Hypotheses. 2020 Nov;144:110271. doi: 10.1016/j.mehy.2020.110271. Epub 2020 Sep 13. PMCID: PMC7487155.
- Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, DeBenedet AT. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am J Gastroenterol. 2020 Jun;115(6):942-946. doi: 10.14309/ajg.0000000000000667. PMID: 32496741; PMCID: PMC7172485.
- Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol. 2020 May 26;58(6):e00512-20. doi: 10.1128/JCM.00512-20. PMID: 32245835; PMCID: PMC7269383.
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, Fung AY, Ng AC, Zou Z, Tsoi HW, Choi GK, Tam AR, Cheng VC, Chan KH, Tsang OT, Yuen KY. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020 Apr 23;58(5):e00310-20. doi: 10.1128/JCM.00310-20. PMID: 32132196; PMCID: PMC7180250.
- Kontou PI, Braliou GG, Dimou NL, Nikolopoulos G, Bagos PG. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics (Basel). 2020 May 19;10(5):319. doi: 10.3390/diagnostics10050319. PMID: 32438677; PMCID: PMC7278002.
- Liang G, Yihui H, Mengqi T, Shipei W, Sichao C, Wei L, Wei Z, Danyang C, Lin Z, Min W, Meng W, Qi H, Haibo X, Wen Z, Zeming L. Confusion and Thinking on the Diagnosis and Treatment of Patients with Negative RT-PCR Results for SARS-CoV-2. 2020; 1-28. https://tinyurl.com/yxu2cmhl
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000 Jun 15;28(12):E63. doi: 10.1093/nar/28.12.e63. PMID: 10871386; PMCID: PMC102748.
- Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002 Jun;16(3):223-9. doi: 10.1006/mcpr.2002.0415. PMID: 12144774.
- Kashir J, Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med Hypotheses. 2020 Aug;141:109786. doi: 10.1016/j.mehy.2020.109786. Epub 2020 Apr 25. PMID: 32361529; PMCID: PMC7182526.
- Poon LL, Leung CS, Tashiro M, Chan KH, Wong BW, Yuen KY, Guan Y, Peiris JS. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin Chem. 2004 Jun;50(6):1050-2. doi: 10.1373/clinchem.2004.032011. Epub 2004 Mar 30. PMID: 15054079; PMCID: PMC7108160.
- Shirato K, Yano T, Senba S, Akachi S, Kobayashi T, Nishinaka T, Notomi T, Matsuyama S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virol J. 2014 Aug 8;11:139. doi: 10.1186/1743-422X-11-139. PMID: 25103205; PMCID: PMC4132226.
- Wei EH, Boon L, Chia CH, Dan X, Wei W, Yejiong Y, Huidong J, Yun W, Yida Z, Mengmeng J, Hong C, Xiuming Z, Hui w, Zhanfeng C. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020; doi: 10.1111/1751-7915.13586
- El-Tholoth M, Bau HH, Song J. A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. ChemRxiv [Preprint]. 2020 Feb 19. doi: 10.26434/chemrxiv.11860137. PMID: 32511284; PMCID: PMC7251958.
- Lu R, Wu X, Wan Z, Li Y, Jin X, Zhang C. A Novel Reverse Transcription Loop-Mediated Isothermal Amplification Method for Rapid Detection of SARS-CoV-2. Int J Mol Sci. 2020 Apr 18;21(8):2826. doi: 10.3390/ijms21082826. PMID: 32325642; PMCID: PMC7216271.
- Yu L, Wu S, Hao X, Dong X, Mao L, Pelechano V, Chen WH, Yin X. Rapid Detection of COVID-19 Coronavirus Using a Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) Diagnostic Platform. Clin Chem. 2020 Jul 1;66(7):975-977. doi: 10.1093/clinchem/hvaa102. PMID: 32315390; PMCID: PMC7188121.
- Dong D, Tang Z, Wang S, Hui H, Gong L, Lu Y, Xue Z, Liao H, Chen F, Yang F, Jin R, Wang K, Liu Z, Wei J, Mu W, Zhang H, Jiang J, Tian J, Li H. The role of imaging in the detection and management of COVID-19: a review. IEEE Rev Biomed Eng. 2020 Apr 27;PP. doi: 10.1109/RBME.2020.2990959. Epub ahead of print. PMID: 32356760.
- Adam B, Xueyan M, Mingqian H, Yang Y, Zahi A, Ning Z, Kaiyue D, Bin L, Xiqi Z, Kunwei L, Shaolin L, Hong S, Adam J, Michael C. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3): 200463. doi: 10.1148/radiol.2020200463
- Deng Y, Lei L, Chen Y, Zhang W. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020 Jul;47(7):1634-1635. doi: 10.1007/s00259-020-04767-1. Epub 2020 Mar 21. PMID: 32198615; PMCID: PMC7087529.
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):e38. doi: 10.1056/NEJMc2007575. Epub 2020 Apr 8. PMID: 32268022; PMCID: PMC7161262.
- Ballout RA, Sviridov D, Bukrinsky MI, Remaley AT. The lysosome: A potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. FASEB J. 2020 Jun;34(6):7253-7264. doi: 10.1096/fj.202000654R. Epub 2020 May 5. PMID: 32367579; PMCID: PMC7383733.
- Chen WH, Hotez PJ, Bottazzi ME. Potential for developing a SARS-CoV Receptor-Binding Domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum Vaccin Immunother. 2020 Jun 2;16(6):1239-1242. doi: 10.1080/21645515.2020.1740560. Epub 2020 Apr 16. PMID: 32298218; PMCID: PMC7482854.
- Shi F, Xie Y, Shi L, Xu W. Viral RNA polymerase: a promising antiviral target for influenza A virus. Curr Med Chem. 2013;20(31):3923-34. doi: 10.2174/09298673113209990208. PMID: 23931274.
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato V, Bushmaker T, Flaxman A, Ulaszewska M, Feldmann F, Allen ER, Sharpe H, Schulz J, Holbrook M, Okumura A, Meade-White K, Pérez-Pérez L, Bissett C, Gilbride C, Williamson BN, Rosenke R, Long D, Ishwarbhai A, Kailath R, Rose L, Morris S, Powers C, Lovaglio J, Hanley PW, Scott D, Saturday G, de Wit E, Gilbert SC, Munster VJ. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv [Preprint]. 2020 May 13:2020.05.13.093195. doi: 10.1101/2020.05.13.093195. Update in: Nature. 2020 Jul 30; PMID: 32511340; PMCID: PMC7241103.
- Shi Y, Wang N, Zou QM. [Progress and challenge of vaccine development against 2019-novel coronavirus (2019-nCoV)]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020 Jun 6;54(6):614-619. Chinese. doi: 10.3760/cma.j.cn112150-20200317-00366. PMID: 32234130.
- Singh T, Heston SM, Langel SN, Blasi M, Hurst JH, Fouda GG, Kelly MS, Permar SR. Lessons from COVID-19 in children: Key hypotheses to guide preventative and therapeutic strategies. Clin Infect Dis. 2020 May 8:ciaa547. doi: 10.1093/cid/ciaa547. Epub ahead of print. PMID: 32382748; PMCID: PMC7239258.
- Smith TRF, Patel A, Ramos S. Rapid development of a synthetic DNA vaccine for COVID-19. 2020, Research Square. https://tinyurl.com/y4juygdl
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H, Ge X, Kan B, Hu Y, Liu J, Cai F, Jiang D, Yin Y, Qin C, Li J, Gong X, Lou X, Shi W, Wu D, Zhang H, Zhu L, Deng W, Li Y, Lu J, Li C, Wang X, Yin W, Zhang Y, Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 Jul 3;369(6499):77-81. doi: 10.1126/science.abc1932. Epub 2020 May 6. PMID: 32376603; PMCID: PMC7202686.
- Ella KM, Mohan VK. Coronavirus Vaccine: Light at the End of the Tunnel. Indian Pediatr. 2020 May 15;57(5):407-410. doi: 10.1007/s13312-020-1812-z. Epub 2020 Apr 15. PMID: 32291382; PMCID: PMC7240229.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020 Jan 10;11(1):222. doi: 10.1038/s41467-019-13940-6. PMID: 31924756; PMCID: PMC6954302.
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O'Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, García-Sastre A, Shokat KM, Shoichet BK, Krogan NJ. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 Jul;583(7816):459-468. doi: 10.1038/s41586-020-2286-9. Epub 2020 Apr 30. PMID: 32353859; PMCID: PMC7431030.
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014 Apr 17;508(7496):402-5. doi: 10.1038/nature13027. Epub 2014 Mar 2. PMID: 24590073; PMCID: PMC7095208.
- Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020 Feb 15;395(10223):e30-e31. doi: 10.1016/S0140-6736(20)30304-4. Epub 2020 Feb 4. Erratum in: Lancet. 2020 Jun 20;395(10241):1906. PMID: 32032529; PMCID: PMC7137985.
- Dierynck I, De Wit M, Gustin E, Keuleers I, Vandersmissen J, Hallenberger S, Hertogs K. Binding kinetics of darunavir to human immunodeficiency virus type 1 protease explain the potent antiviral activity and high genetic barrier. J Virol. 2007 Dec;81(24):13845-51. doi: 10.1128/JVI.01184-07. Epub 2007 Oct 10. PMID: 17928344; PMCID: PMC2168871.
- Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020 Mar 16;14(1):64-68. doi: 10.5582/bst.2020.01030. Epub 2020 Feb 9. Erratum in: Biosci Trends. 2020;14(1):E1. PMID: 32037389.
- Fukui M, Kawaguchi K, Matsuura H. Does TB vaccination reduce COVID-19 infection? No evidence from a regression discontinuity analysis. No Evidence from a Regression Discontinuity Analysis. 2020 April. medRxiv. doi: 10.1101/2020.04.13.20064287
- Roujian L, Xiang Z, Juan L, Peihua N, Bo Y, Honglong W, Wenling W, Hao S, Baoying H, Na Z, Yuhai B, Xuejun M, Faxian Z, Liang W, Tao H, Hong Z, Zhenhong H, Weimin Z, Li Z, Jing C, Yao M, Ji W, Yang L, Jianying Y, Zhihao X, Jinmin M, William JL, Dayan W, Wenbo X, Edward CH, George FG, Guizhen W, Weijun C, Weifeng S, Wenjie T. 2020 January. doi: 10.1016/S0140-6736(20)30251-8
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Epub 2020 Mar 20. PMID: 32205204; PMCID: PMC7102549.
- Sandeep S, McGregor K. Energetics Based Modeling of Hydroxychloroquine and Azithromycin Binding to the SARS-CoV-2 Spike (S) Protein-ACE2 Complex. 2020. https://tinyurl.com/y4oozfb8
- Srinivasa A, Tosounidou S, Gordon C. Increased Incidence of Gastrointestinal Side Effects in Patients Taking Hydroxychloroquine: A Brand-related Issue? J Rheumatol. 2017 Mar;44(3):398. doi: 10.3899/jrheum.161063. PMID: 28250164.
- Waetzig V, Riffert J, Cordt J, Reinecke K, Haeusgen W, Boehm R, Cascorbi I, Herdegen T. Neurodegenerative effects of azithromycin in differentiated PC12 cells. Eur J Pharmacol. 2017 Aug 15;809:1-12. doi: 10.1016/j.ejphar.2017.05.002. Epub 2017 May 4. PMID: 28479141.
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9490-9496. doi: 10.1073/pnas.2004168117. Epub 2020 Apr 6. PMID: 32253318; PMCID: PMC7196837.
- Zhu C, Wu Y, Liu H, Ban Y, Ma X, Zhang Z. Early pulmonary rehabilitation for SARS-CoV-2 pneumonia: Experience from an intensive care unit outside of the Hubei province in China. Heart Lung. 2020 Sep-Oct;49(5):449-450. doi: 10.1016/j.hrtlng.2020.04.007. Epub 2020 Apr 16. PMID: 32312554; PMCID: PMC7161510.
- Kurtaiş Aytür Y, Köseoğlu BF, Özyemişçi Taşkıran Ö, Ordu-Gökkaya NK, Ünsal Delialioğlu S, Sonel Tur B, Sarıkaya S, Şirzai H, Tekdemir Tiftik T, Alemdaroğlu E, Ayhan FF, Duyur Çakıt BD, Genç A, Gündoğdu İ, Güzel R, Demirbağ Karayel D, Bilir Kaya B, Öken Ö, Özdemir H, Soyupek F, Tıkız C. Pulmonary rehabilitation principles in SARS-COV-2 infection (COVID-19): A guideline for the acute and subacute rehabilitation. Turk J Phys Med Rehabil. 2020 May 12;66(2):104-120. doi: 10.5606/tftrd.2020.6444. PMID: 32760887; PMCID: PMC7401689.
- Jangra MK, Saxena A. Significance of physiotherapy in “SARS-CoV-2/COVID-19: An Epidemic”. Ann Thorac Med. 2020 Jul-Sep;15(3):179-180. doi: 10.4103/atm.ATM_169_20. Epub 2020 Jun 18. PMID: 32831942; PMCID: PMC7423205.
- Dehesh P. Risk Management of COVID-19 Infection in Physiotherapy: Recommendations. Journal of Biology and Today's World. 2020;9(7): 1-2. https://tinyurl.com/y42lz2xp
- Diwate AD. Role of Physiotherapy in Covid-19 Patients. Vims. Journal of Physical Therapy. 2020;2(1): 1-2.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.