> Medicine. 2020 September 30;1(5):186-189. doi: 10.37871/jbres1141.
-
Subject area(s):
- Oncology
- Cancer
- Cellular Biology
The Negative RT-PCR Test is Enough to Rule Out Covıd-19 in Cancer Patients or Not? Covıd-19 or Not?
Selin Aktürk Esen1*, Öznur Bal1, Efnan Algın1, Yusuf Açıkgöz1, Merve Dirikoç1, Gökhan Uçar1, Lale Damgacı2, Yakup Ergun1, İrfan Esen3, Hürrem Bodur4 and Doğan Uncu1
2Health Sciences University Ankara City Hospital, Department of Radiology, Turkey
3Yıldırım Beyazıt University Yenimahalle Training and Research Hospital, Turkey
4Health Sciences University Ankara City Hospital, Department of Infectious Diseases, Turkey
- COVID-19
- Coronavirus
- Polymerase chain reaction
- Chemotherapy
- Cancer
At the end of 2019, a new coronavirus pneumonia turned into an epidemic in China and then spread to other countries around the world. This disease was identified as the COVID-19 (SARS-CoV-2) disease. SARS-CoV-2 RNA is detected by Reverse-Transcription Polymerase Chain Reaction (RT-PCR). A positive test for SARS-CoV-2 may confirm the diagnosis of COVID-19, but the results of false negativity and false positivity can be confusing. This article describes the negative COVID-19 RNA RT-PCR test results of four cancer patients with symptoms/clinical findings suggestive of COVID-19 and also computed tomography findings consistent with viral pneumonia.
At the end of 2019, a new coronavirus pneumonia was identified in Wuhan, China. The disease was identified by the World Health Organization (WHO) 2020 as the COVID-19 (SARS-CoV-2) disease in February [1]. In Turkey, the first COVID-19 case was reported on March 10, 2020.
This article describes the negative SARS-CoV-2 RNA Reverse-Transcription Polymerase Chain Reaction (RT-PCR) test results of four cancer patients with symptoms/clinical findings suggestive of COVID-19 and also Computed Tomography (CT) findings consistent with viral pneumonia, between 10 March-14 May 2020.
Patient 1
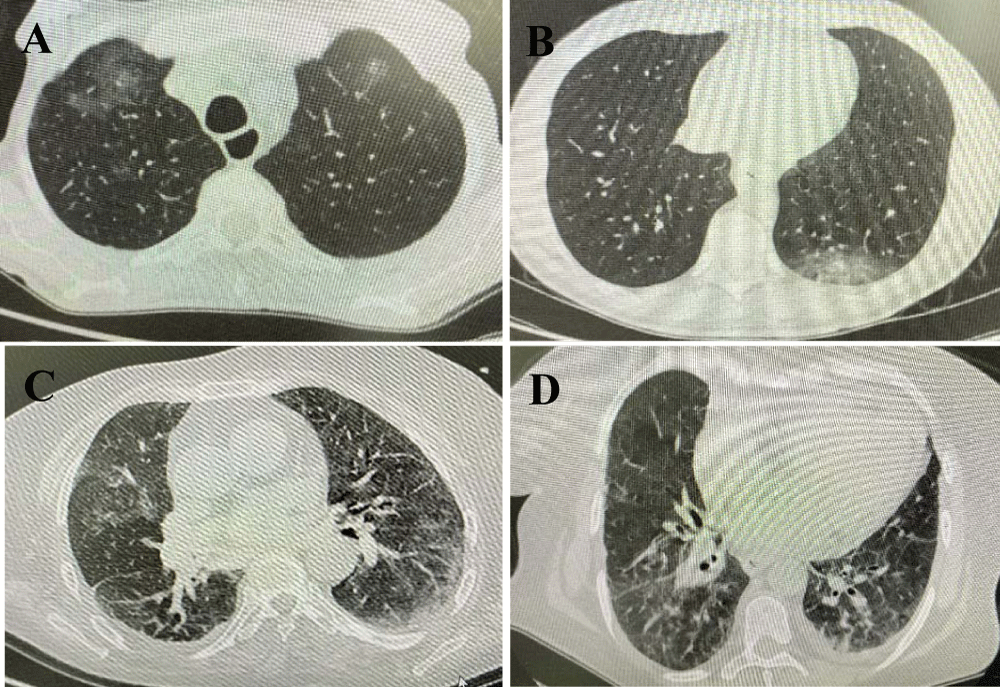
64-year-old male patient who applied with dry cough for 8 days, shortness of breath and subfebrile fever for the last 2 days. He was followed up for 3 years with the diagnosis of gastric adenocarcinoma (Stage 4B). He was receiving folinic acid, fluorouracil and irinotecan chemotherapy regimen as the third line chemotherapy (last date of chemotherapy was 8 days before the onset of symptoms). The Eastern Cooperative Oncology Group (ECOG) performance score was 4. The patient's Respiratory Rate (RR) was 19/minute, arterial Oxygen Saturation (SO2) was 94% without supplementation of oxygen. Renal and liver functions were normal. Other laboratory parameters are shown in table 1. Peripheral multi-focal ground glass opacities were detected in both lungs in thorax Computed Tomography (CT) (Figure 1A). Influenza and other viral respiratory tests were negative. There was no microorganism growth in cultures. Oropharyngeal and nasopharyngeal swabs (OS, NS) for COVID-19 RT-PCR was repeated 5 times in total, one day apart. All were negative. Initially 2x400 mg and at the continuation 2x200 mg hydroxychloroquine, prophylactic enoxaparin was applied for 5 days. Hydroxychloroquine was stopped on the 5th day and the clinical findings of the patient regressed on the 8th day.
| Table 1: Laboratory findings of patients. | |||||
| First laboratory values | Normal range | Patient - 1 | Patient - 2 | Patient - 3 | Patient - 4 |
| Leukocyte x109/L | 3.90 - 10.20 | 8.19 | 0.65 | 7.08 | 8.86 |
| Lymphocyte x109/L | 1.10 - 4.50 | 0.36 | 0.43 | 0.79 | 1.07 |
| Neutrophil x109/L | 1.50 - 7.70 | 7.40 | 0.10 | 5.41 | 7.48 |
| CRP g/L | 0 - 0.005 | 0.117 | 0.068 | 0.339 | 0.480 |
| CRP g/L (on the 5th day of the treatment) |
0.083 | 0.140 | 0.190 | 0.369 | |
| Procalcitonin mcg/L | <0.16 | 0.10 | 0.15 | 7.24 | 0.36 |
| Procalcitonin mcg/L (on the 5th day of the treatment) |
0.05 | 0.06 | 1.80 | 0.32 | |
| AST U/L | <35 | 11 | 19 | 20 | 32 |
| ALT U/L | <50 | 9 | 21 | 39 | 48 |
| LDH U/L | 120-246 | 283 | 605 | 422 | 765 |
| D-dimer mg/dl | <0.55 | 4.96 | 2.00 | 7.56 | 6.52 |
| Ferritin mcg/L | 22-322 | 2003 | 756 | 7103 | 3258 |
| CRP: C-Reactive Protein; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LDH: Lactate Dehydrogenase | |||||
Patient 2
28-year-old male patient who applied with 11 weight loss in 10 days, sore throat, dry cough for 4 days and 39.6 degrees of fever for the last two days. He was followed up for 4 months with the diagnosis of testicular mix germ cell tumor (stage 2A). He was receiving bleomycin, etoposide, cisplatin chemotherapy regimen as the first line chemotherapy (last date of chemotherapy was 10 days before the onset of symptoms). The ECOG performance score was 2. The patient's RR was 26/minute, SO2 was 95% with oxygen support of 4 liters/minute. Renal and liver functions were normal. Other laboratory parameters are shown in table1. He was febrile neutropenic. A consolidation area containing ground-glass opacity was detected around the posterobasal segment of left lower lobe (Figure 1B). Influenza and other viral respiratory tests were negative. There was no microorganism growth in cultures. OPS and NPS for COVID-19 RT-PCR was repeated 5 times in total, one day apart. All were negative. Empirically piperacillin tazobactam, filgrastim (treatment protocol of febrile neutropenia) and hydroxychloroquine (initially 2x400 mg and at the continuation 2x200 mg), prophylactic enoxaparin was started. Hydroxychloroquine treatment was stopped on the 5th day When the patient's clinical, laboratory and CT findings regressed, antibiotherapy was stopped on the 10th day.
Patient 3
58-year-old female patient who applied with dry cough for 6 days and 38.6 degrees of fever for the last one day. She was followed up for 7 years with the diagnosis of ovarian serous carcinoma (Stage 4B). She was receiving carboplatin chemotherapy regimen as the fifth line of chemotherapy (last date of chemotherapy was 7 days before the onset of symptoms). ECOG performance score was 2. The patient's RR was 24/minute. SO2 was 92% with oxygen support of 4 liters/minute. Other laboratory parameters are shown in table 1. Peripheral multi-focal ground glass opacities were detected in thorax CT in both lungs (Figure 1C). Influenza and other viral respiratory tests were negative. There was no microorganism growth in cultures. OPS and NPS for COVID-19 RT-PCR was repeated 5 times in total, one day apart. All were negative. Empirically piperacillin tazobactam and hydroxychloroquine (initially 2x400 mg and at the continuation 2x200 mg), prophylactic enoxaparin was started. Hydroxychloroquine treatment was stopped on the 5th day. When the patient's clinical, laboratory and CT findings regressed, antibiotherapy was stopped on the 10th day.
Patient 4
74-year-old female patient who applied with fatigue, loss of appetite for 10 days, dry cough for 5 days and 39.8 degrees of fever for the last two days. She was followed up for 3 months with the diagnosis of lung adenocarcinoma (Stage 4B). She was receiving paclitaxel carboplatin chemotherapy regimen as the first line of chemotherapy (last date of chemotherapy was 12 days before the onset of symptoms). The ECOG performance score was 3. Liver functions was normal and creatinine was 1.39 mg/dl. The creatinine value was normal 15 days ago. Other laboratory parameters are shown in table 1. Peripheral multi-focal ground glass opacities, pleural effusion on the left side were detected in thorax CT in both lungs (Figure 1D). Influenza and other viral respiratory tests were negative. There was no microorganism growth in cultures. Empirically meropenem and hydroxychloroquine (initially 2x400 mg and at the continuation 2x200 mg), prophylactic enoxaparin was started. Prophylactic enoxaparin was added. OPS and NPS RT-PCR was repeated 3 times in total, one day apart. All were negative. Teicoplanin was added on the 3rd day of treatment since the fever continued. The patient was intubated on the 6th day of treatment due to the suddenly developing of hypoxemia and hypercarbia; and one hour later the patient died. Therefore, RT-PCR test could not be performed in tracheal aspirate.
Signs and symptoms of SARS-CoV-2 can be summarized as fever and/or new cough, shortness of breath, sore throat, muscle aches, rhinorrhea/nasal congestion, and anosmia [2]. Chest CT in patients with COVID-19 commonly demonstrates ground-glass opacities with bilateral, peripheral, and lower lung zone distributions and with a peak in severity at 10 to 12 days after symptom onset [3]. In a retrospective study with 610 patients who were considered COVID-19 pneumonia with clinical and CT findings, the first COVID-19 RT-PCR positivity (PS) rate was found 27.5% [2]. When the second test was performed on these patients, 12.5% positivity was detected [2]. Positivity was observed in the third test in 7 patients, in the fourth test in 4 patients and in the fifth test in 1 patient. Test results of patients who were positive were found to be negative within a few days [2]. Pharyngeal viral load insufficiency, sampling errors, problems during laboratory and transportation may cause this situation [4]. Upper respiratory tract samples may have lower viral loads and lower respiratory tract samples may be more likely to detect viral positivity. In a study of patients with COVID-19, the highest rates of positive viral RNA tests were reported from bronchoalveolar lavage (95%) and sputum (72%), compared with OPS (32%) [3]. In these cases, WHO recommends testing lower respiratory tract samples, if it is possible [6]. Since the number of patients intubated due to pneumonia is lower than those not intubated [7], in non-intubated patients, the lack of bronchoalveolar lavage due to its impracticalness may make it difficult to detect COVID-19. There may be false negativity in PCR due to loss of DNA or RNA in relation to degradation [8]. In a study examining the relationship between chemotherapy and RNA degradation in cancer patients, fragmented RNA was detected due to RNA degradation 8-18 weeks after cytotoxic chemotherapy [9]. The fact that our patients were receiving chemotherapy due to cancer treatment may have caused RNA degradation and caused PCR false negativity.
COVID-19 has become a pandemic outbreak in the world and concerns all segments of the societies. So new studies on this subject are needed to reduce COVID-19 uncertainty and illuminate confusing questions.
- Organization WH. Director-General's remarks at the media briefing on 2019-nCoV on 2020. 2020 February 11.
- Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung TW, Lee EYP, Wan EYF, Hung IFN, Lam TPW, Kuo MD, Ng MY. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020 Aug;296(2):E72-E78. doi: 10.1148/radiol.2020201160. Epub 2020 Mar 27. PMID: 32216717; PMCID: PMC7233401.
- Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, Yang C. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020 Jul;92(7):903-908. doi: 10.1002/jmv.25786. Epub 2020 Apr 5. PMID: 32219885; PMCID: PMC7228231.
- Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, Gao G, Wang S, Ma C, Xie R, Wang F, Tan C, Zhu L, Guo Y, Zhang F. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020 Jul 28;71(15):793-798. doi: 10.1093/cid/ciaa345. PMID: 32221523; PMCID: PMC7184442.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 May 12;323(18):1843-1844. doi: 10.1001/jama.2020.3786. PMID: 32159775; PMCID: PMC7066521.
- Organization WH. Coronavirus disease (COVID-19) technical guidance: Surveillance and case definitions. 2020 February 28.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 February 28;8:475-481. doi: 10.1016/S2213-2600(20)30079-5.
- Sefers S PZ, Tang YW. False positives and false negatives encountered in diagnostic molecular microbiology. Reviews in Medical Microbiology. 2005; 16:59-67.
- Parissenti AM, Guo B, Pritzker LB, Pritzker KP, Wang X, Zhu M, Shepherd LE, Trudeau ME. Tumor RNA disruption predicts survival benefit from breast cancer chemotherapy.
- Breast Cancer Res Treat. 2015 Aug;153(1):135-44. doi: 10.1007/s10549-015-3498-9. Epub 2015 Jul 25. PMID: 26208483.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.