> Biology Group. 2021 Apr 22;2(4):292-295. doi: 10.37871/jbres1228.
Current Stem Cell Research Status on Hepatic and Pulmonary Sclerosis in COVID-19
Jeongryun Kim1#, Soo Hyun Nam2,3#, Jong Hyun Kim1* and Jiyou Han1#*
2Stem Cell and Regenerative Medicine Institute, Samsung Medical Center, Seoul 06351, Republic of Korea
3Department of Medicine, Sungkyunkwan University School of Medicine, Gyeonggi, Republic of Korea
#These authors contributed equally to this work
Jiyou Han, Ph.D., Department of Biological Sciences, Laboratory of Stem Cell Research and Biotechnology, Hyupsung University, Hwasung-si, 18330, Korea, Tel: +82-10-4572-5573; E-mail:
Jong Hyun Kim, PhD, Department of Biological Sciences, Laboratory of Stem Cell Research and Biotechnology, Hyupsung University, Hwasung-si, 18330, Korea, Tel: +82-10-5025-0446; E-mail:
- Stem cell
- Cell therapy
- Hepatic
- Pulmonary
- Sclerosis
Abstract
Since 2019, Coronavirus Disease (COVID-19) has changed the concept of systemic sclerosis caused by viral infectious diseases. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative organism of COVID-19, has infected more than 1.36 billion people in 188 countries, and nearly 2.29 million have died. Although we have rapidly developed vaccines against COVID-19, the struggle to treat COVID-19 patients who exhibit complicated multiple organ sclerosis has continued ever since. Studies have reported that preexisting liver disease in 3-8% of patients increases metabolic dysfunction and mortality by 4-6-fold in association with the severity of COVID-19. Moreover, both confirmed and cured COVID-19 patients have been reported to develop pulmonary fibrosis, which is often related to poor prognosis of the complications. Therefore, in the present study, we summarize the possible mechanisms underlying the development of hepatic and pulmonary fibrosis caused by SARS-CoV-2 infection, based on recently published data. Furthermore, since stem cell-based treatments have been developed as a novel approach to treat COVID-19 patients with Systemic Sclerosis (SS), we discuss the implementation of stem cell-based treatments as a powerful regenerative tool owing to their notable immunomodulatory and anti-fibrotic effects.
Introduction
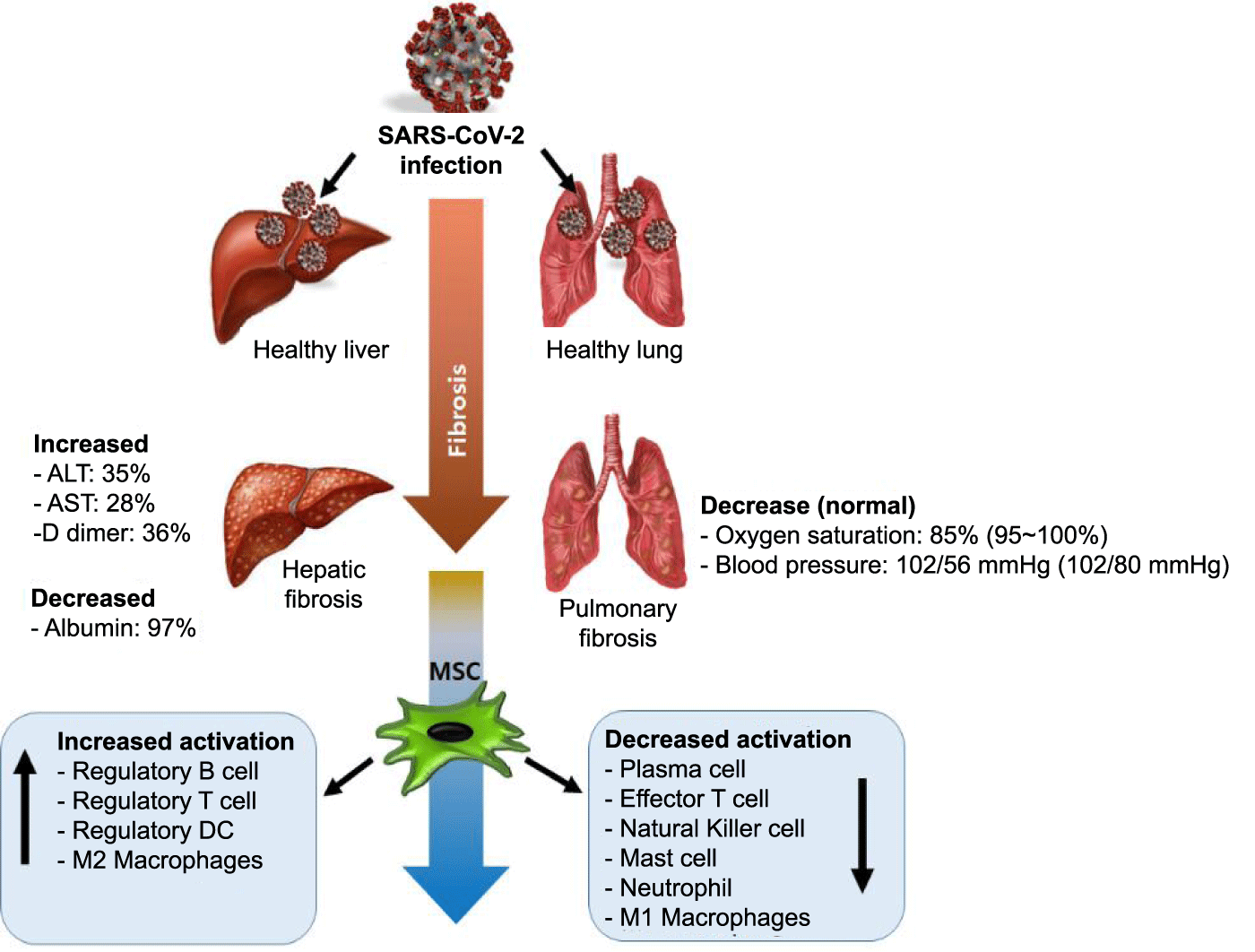
The most severe manifestations of coronavirus disease are atypical pneumonia and sepsis; however, the gastrointestinal tract and liver have recently been reported to be highly affected by SARS-CoV-2. SS generally develops due to fibrosis, which is a pathological process that involves the excessive accumulation of extracellular matrix components, such as collagen, fibronectin, and hyaluronic acid. If this phenomenon persists for a prolonged period, it can lead to a rare chronic autoimmune disease with extensive microvascular injury, endothelial cell damage, activation of immune responses, and progression of tissue fibrosis in various internal organs, especially the liver and lungs.
Various mechanisms underlying the development of hepatic and pulmonary sclerosis in COVID-19 patients have been described, including viral and immune-mediated mechanisms [1]. SARS-CoV-2 is a single-stranded, positive-sense RNA virus belonging to the family Coronaviridae [2]. The sequence homology of SARS-CoV-2 with SARS-CoV and MERS-CoV is 79.5% and 50%, respectively [3]. SARS-CoV-2 binds to the human cells via Angiotensin-Converting Enzyme 2 (ACE2), which is expressed in the liver and lungs [4-6]. Interestingly, ACE2, which serves as the host cell receptor of SARS-CoV-2, plays a protective role against liver and lung damage and is expressed in hepatocytes [7-10].
To date, various therapeutic methods have been employed to manage the symptoms of SS. Studies conducted over several decades have led to the identification of many potential targets to combat fibrotic tissue damage; however, no effective therapies or U.S. Food and Drug Administration (USFDA)-approved antifibrotic agents are currently available [11]. Therefore, the development of antifibrotic therapies to date has relied mainly on a comprehensive understanding of profibrogenic mechanisms in multiple organ systems as well as disease-specific locations [11].
Stem cell-based treatments have been developed as a novel approach to treat several autoimmune diseases. Stem cells, specifically Mesenchymal Stem Cells (MSCs), can be applied as a powerful regenerative tool. They can be advantageous for SS treatment due to their notable immunomodulatory and anti-fibrotic effects. Accordingly, here, we discuss the contemporary status and future perspectives of MSC transplantation for SS treatment [12]. In this review, we reported the current status and highlighted all the implications associated with stem cell therapy for COVID-19.
COVID-19 and hepatic sclerosis
Prior to the emergence of COVID-19 pandemic, cirrhosis and hepatocellular carcinoma accounted for more than 2.5% of all deaths worldwide [13]. Liver cirrhosis leads to immune dysfunction characterized by immunodeficiency and systemic inflammation, which makes patients highly susceptible to SARS-CoV-2 infection. The reported incidence of liver injury associated with COVID-19 ranges from 14.8-53% [14]. Studies reported that SARS-CoV-2 directly binds to ACE2 expressed hepatocytes and cholangiocytes. Importantly, the expression level of ACE2 is much higher in cholangiocytes than hepatocytes (59.7% vs. 2.6%) [15]. Furthermore, SARS-CoV-2 infection impaired the bile acid transportation from cholangiocytes via altering gene involved in canaliculi formed by tight junction [16]. It could lead to liver injury because cholangiocytes has important roles in hepatic regeneration and immune response. A previous study reported that 1 out of 99 COVID-19 patients exhibited a pathological lesion with severe hepatocellular damage and elevated ALT and AST levels, whereas the rest of the patients exhibited moderately elevated ALT and AST levels [17]. The main physiological changes reported to date include increased alanine Aminotransferase (ALT, 13.3–35%), Aspartate aminotransferase (AST, 28-37%), Gamma-Glutamyl Transferase (GGT, 54%), and total bilirubin levels [18]. On the other hand, serum albumin levels was significantly decreased as low as 97% [17]. Furthermore, liver compromise has been associated with the most severe cases of COVID-19.
COVID-19 and pulmonary sclerosis
Pulmonary sclerosis, also known as pulmonary fibrosis, is a condition wherein chronically damaged lung tissue becomes fibrotic and scarred over time. Major symptoms of pulmonary sclerosis include shortness of breath, dry cough, tiredness, weight loss, and nail clubbing. The most common complications associated with pulmonary sclerosis include pulmonary hypertension, respiratory failure, pneumothorax, and lung cancer. Studies have also shown (i) direct evidence of hepatic and pulmonary fibrosis by performing autopsy and pulmonary puncture pathology and (ii) indirect evidence of increased levels of fibrosis-related cytokines [e.g., Transforming Growth Factor (TGF-β), Tumor Necrosis Factor-α (TNF-α), interleukin-6, and interleukin-10] in the peripheral blood of severe patients. To develop a potential treatment against SS, the three major fibrosis-related signaling pathways—TGF-β, Wingless and int (WNT), and YAP/TAZ (known as essential regulators of cell adhesion and cell-cell communication)—in pulmonary fibrosis should be targeted using drugs or stem cell therapy [19,20]. Furthermore, the therapeutic potential of pirfenidone and nintedanib for treating idiopathic as well as COVID-19-induced pulmonary fibrosis should be examined.
Stem cell therapy for sclerosis
Currently, the drugs used to treat COVID-19, such as remdesivir (anti-viral drug), tocilizumab (a drug for rheumatoid arthritis), statins (a drug for cardiovascular disease as HMG-CoA reductase inhibitors), azithromycin (antibiotic for bacterial pneumonia), and chloroquine (a drug for malaria and several inflammatory diseases), exhibit marked hepatotoxic potential, and the patients administered these drugs may develop drug-induced liver injury. Therefore, stem cell therapy may be considered as an alternate treatment option that is currently available for SS associated with COVID-19. MSCs and MSC-like cells exert robust immunomodulatory, regenerative, and anti-fibrotic effects on myofibroblast-mediated fibrotic disease. The recent use of stem cell therapy for critically ill COVID-19 patients in a small group of patients in China as well as the subsequent emergency use authorization of stem cell therapy by the USFDA to the Global Institute of Stem Cell Therapy and Research and Athersys have garnered interest in the medical community for its clinical applications. Consequently, several clinical trials on stem cell-based COVID-19 treatment, which aim to use different cell sources, doses, and diverse targeted patient groups, have been registered [21]. MSC-based therapy for COVID-19 has been suggested to ameliorate cytokine release syndrome and protect alveolar epithelial cells by secreting different types of factors, demonstrating safety and possible efficacy in COVID-19 patients with acute respiratory distress syndrome [22].
Most registered clinical trials have proposed to use MSCs because of their immunomodulatory effects. However, using MSCs to treat immunocompromised COVID-19 patients raises a concern regarding the greater risk of viral and other infections in these patients. Since the immunosuppressive effect of MSCs is non-specific, both alloantigen and viral antigen responses could be repressed, thereby leading to detrimental clinical outcomes. Therefore, recent studies have suggested that exosomes might play an important role in fibrotic diseases and have focused on exosomal cargo dysregulation and their potential diagnostic and therapeutic values in fibrosis [23]. Interestingly, in a recent study, genetically modified hepatocyte growth factor-secreting MSCs effectively decreased the severity of hepatic sclerosis in a corresponding rat model [24]. However, few studies have also reported an association between hepatic sclerosis and COVID-19.
Conclusion
In addition to the shortage of donors for organ transplantation, hepatic and pulmonary sclerosis patients now face a new challenge in the form of COVID-19, which can be life threatening. A major challenge encountered by the scientific community and funding agencies is to develop a successful, highly effective, and sophisticated treatment for major organ sclerosis associated with SARS-CoV-2 infection using stem cell therapy. Since a limited number of clinically approved cell therapies are being pursued as the promising options, there is an urgent need to find more effective candidate therapies while testing the current candidates for sclerosis. In addition to the identification of novel drug treatments and validation of their therapeutic potential, we need to identify novel sclerosis targets as well as cell sources for soft organ sclerosis, which would strengthen our arsenal in the fight against organ sclerosis associated with COVID-19. Understanding stem cell biology in further detail will also broaden our strategies for its control and clinical application worldwide.
Acknowledgement
This work was supported by the Basic Science Research Program (No. 2018R1A2B6002275 to JH and 2018R1C1B6002586 to JHK) and MRC (2016R1A5A2007009 to SHN) from the National Research Foundation of Korea (NRF) funded by the Korea Ministry of Science and ICT (MSIT).
References
- Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, Wu Q, Fang F, Cheng L, Jiao N, Li X, Chen Q. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020 May;92(5):461-463. doi: 10.1002/jmv.25711. Epub 2020 Mar 1. PMID: 32073161; PMCID: PMC7167012.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. PMID: 31978945; PMCID: PMC7092803.
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021 Mar;19(3):155-170. doi: 10.1038/s41579-020-00468-6. Epub 2020 Oct 28. PMID: 33116300; PMCID: PMC7592455.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Hu Y, et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. BioRxiv. 2020. doi:10.1101/2020.01.24.919183.
- Wu HT, Chuang YW, Huang CP, Chang MH. Loss of angiotensin converting enzyme II (ACE2) accelerates the development of liver injury induced by thioacetamide. Exp Anim. 2018 Feb 9;67(1):41-49. doi: 10.1538/expanim.17-0053. Epub 2017 Aug 25. PMID: 28845018; PMCID: PMC5814313.
- Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006 Jun;6(3):271-6. doi: 10.1016/j.coph.2006.03.001. Epub 2006 Apr 3. PMID: 16581295; PMCID: PMC7106490.
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Yang D, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020 Jul 13;24(1):422. doi: 10.1186/s13054-020-03120-0. PMID: 32660650; PMCID: PMC7356137.
- Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020 May 8;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015. Epub 2020 Apr 8. PMID: 32264791; PMCID: PMC7188049.
- Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021 Mar;56(3):218-230. doi: 10.1007/s00535-021-01760-9. Epub 2021 Feb 1. PMID: 33527211; PMCID: PMC7849620.
- Warner FJ, Rajapaksha H, Shackel N, Herath CB. ACE2: from protection of liver disease to propagation of COVID-19. Clin Sci (Lond). 2020 Dec 11;134(23):3137-3158. doi: 10.1042/CS20201268. PMID: 33284956.
- McVicker BL, Bennett RG. Novel Anti-fibrotic Therapies. Front Pharmacol. 2017 May 31;8:318. doi: 10.3389/fphar.2017.00318. PMID: 28620300; PMCID: PMC5449464.
- Haldar D, Henderson NC, Hirschfield G, Newsome PN. Mesenchymal stromal cells and liver fibrosis: a complicated relationship. FASEB J. 2016 Dec;30(12):3905-3928. doi: 10.1096/fj.201600433R. Epub 2016 Sep 6. PMID: 27601441.
- Xing L, Chang X, Shen L, Zhang C, Fan Y, Cho C, Zhang Z, Jiang H. Progress in drug delivery system for fibrosis therapy. Asian J Pharm Sci. 2021 Jan;16(1):47-61. doi: 10.1016/j.ajps.2020.06.005. Epub 2020 Jul 17. PMID: 33613729; PMCID: PMC7878446.
- Ruiz-Velascoa JA, Aldana-Ledesma JM, Ibarra-Estrada MA, Aguirre Díazc SA, Fernández-Ramírez JA, Cárdenas-Lara F, et al. Revista de Gastroenterología de México. 2017;82:301-308. doi: 10.1016/j.rgmxen.2017.08.002.
- Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv Preprint 4 February 2020. doi: 10.1101/2020.02.03.931766.
- Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Liang J, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020 Oct;11(10):771-775. doi: 10.1007/s13238-020-00718-6. PMID: 32303993; PMCID: PMC7164704.
- Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020 May;40(5):998-1004. doi: 10.1111/liv.14435. Epub 2020 Mar 30. PMID: 32170806; PMCID: PMC7228361.
- Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020 Jun;8(5):509-519. doi: 10.1177/2050640620924157. PMID: 32450787; PMCID: PMC7268949.
- Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front Med (Lausanne). 2015 Sep 3;2:59. doi: 10.3389/fmed.2015.00059. PMID: 26389119; PMCID: PMC4558529.
- Mares J, Hartung HP. Multiple sclerosis and COVID-19. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Sep;164(3):217-225. doi: 10.5507/bp.2020.033. Epub 2020 Jul 17. PMID: 32686774.
- Choudhery MS, Harris DT. Stem cell therapy for COVID-19: Possibilities and challenges. Cell Biol Int. 2020 Nov;44(11):2182-2191. doi: 10.1002/cbin.11440. Epub 2020 Aug 22. PMID: 32767687; PMCID: PMC7436138.
- Li Z, Niu S, Guo B, Gao T, Wang L, Wang Y, Wang L, Tan Y, Wu J, Hao J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020 Dec;53(12):e12939. doi: 10.1111/cpr.12939. Epub 2020 Oct 24. PMID: 33098357; PMCID: PMC7645923.
- Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, Kim SJ, An JY, Lee Y, Choi Y, Raj A, Cho SG, Vellingiri B. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep. 2020 Aug;53(8):400-412. doi: 10.5483/BMBRep.2020.53.8.121. PMID: 32731913; PMCID: PMC7473478.
- Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014 Aug 22;46(8):e110. doi: 10.1038/emm.2014.49. PMID: 25145391; PMCID: PMC4150933.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.