Medicine Group . 2023 March 12;4(2):387-393. doi: 10.37871/jbres1687.
The Possible Therapeutic Application of CO on COVID-19
Zhang Rui-Gang*
Summary
An outbreak of pneumonia caused by a novel Coronavirus (2019-nCoV) is ongoing in China [1]. The disease caused by 2019-nCoV was recently named as COVID-19 by WHO. Although the case-fatality rate of COVID-19 (about 2.3% up to now) is lower than SARS, they share many similarities [2,3]. Early studies have shown that increased pro-inflammatory cytokines were associated with pulmonary inflammation and extensive lung damage in SARS patients [4], while the latest report on COVID-19 showed that 2019-nCoV infection lead to high amounts of both Th1 and Th2 cytokines [5]. Moreover, ICU patients had higher levels of GCSF, IP10, TNFα, MCP1, IL2, IL7, IL10, MIP1A, suggesting the cytokine storm was associated with disease severity [5]. Corticosteroid therapy was frequently gave as a combined regimen for possible benefit by reducing inflammatory-induced lung injury. However, the drug is immunosuppressive and may delay viral clearance if given before viral replication is controlled [6], side-effects of corticosteroid also occurred in other cases [7]. Therefore, novel anti-inflammatory molecules could be considered in the treatment of COVID-19.
Accumulating evidence suggests a protective role of Carbon monoxide (CO), which is produced from the catabolism of heme via Heme oxygenase (HO), in the lungs and many other organ systems [8]. The anti-inflammatory properties of HO-1/CO has been demonstrated during pulmonary inflammation and lung injury through inhibiting Th17 cell-mediated immune response [9], suppressing NLRP3 inflammasome activation [10], decreasing the release of segmented neutrophils from the bone marrow [11], affecting PMN migration and improving microvascular permeability [12], while the inducers or stimulators varies from OVA, sepsis, LPS and oxidative stress [9-13]. CO was defined as novel Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [14] that accelerates resolution of inflammation [15]. In this review, we will briefly summarize the anti-virus effects of CO, with an emphasis on its interaction with purinergic signaling.
Purinergic Signaling in Virus Infection
Increased levels of extracellular nucleotides were detected during virus infection such as Respiratory Syncytial Virus (RSV), parainfluenza virus and HIV [16-19]. By activating P2Y receptor-mediated signaling pathways, ATP or UTP contributes to the accumulation of ions/fluid in the respiratory tract and reduction of Alveolar Fluid Clearance (AFC) [19,20]. The impaired AFC was due to suppressed Na+ absorption and enhanced Cl- secretion mediated by ATP or UTP during virus infection [19,21]. Interestingly, SARS-CoV spike protein and envelop protein transfected human airway epithelial cells (H441) cells showed decreased amiloride-sensitive Na+ currents as well as ENaC protein level, indicating that lung edema in SARS infection may be partially due decreased levels and activity of ENaC at the apical surfaces of lung epithelial cells [22].
Apart from ion transport, purinergic signal also participates in host inflammatory responses during virus infection. Calven J, et al. [23] reported that Rhinovirus (RV) infected Bronchial Smooth Muscle Cell (BSMC) supernatants exhibited elevated ATP, Blocking of purinergic signaling with suramin inhibited BSMC expression of IL-33. Taken together, nucleotides participates in airway virus infection, therefore, purinergic signaling appears to be a new pharmacological target against virus [24].
Anti-purinergic Effects of CO
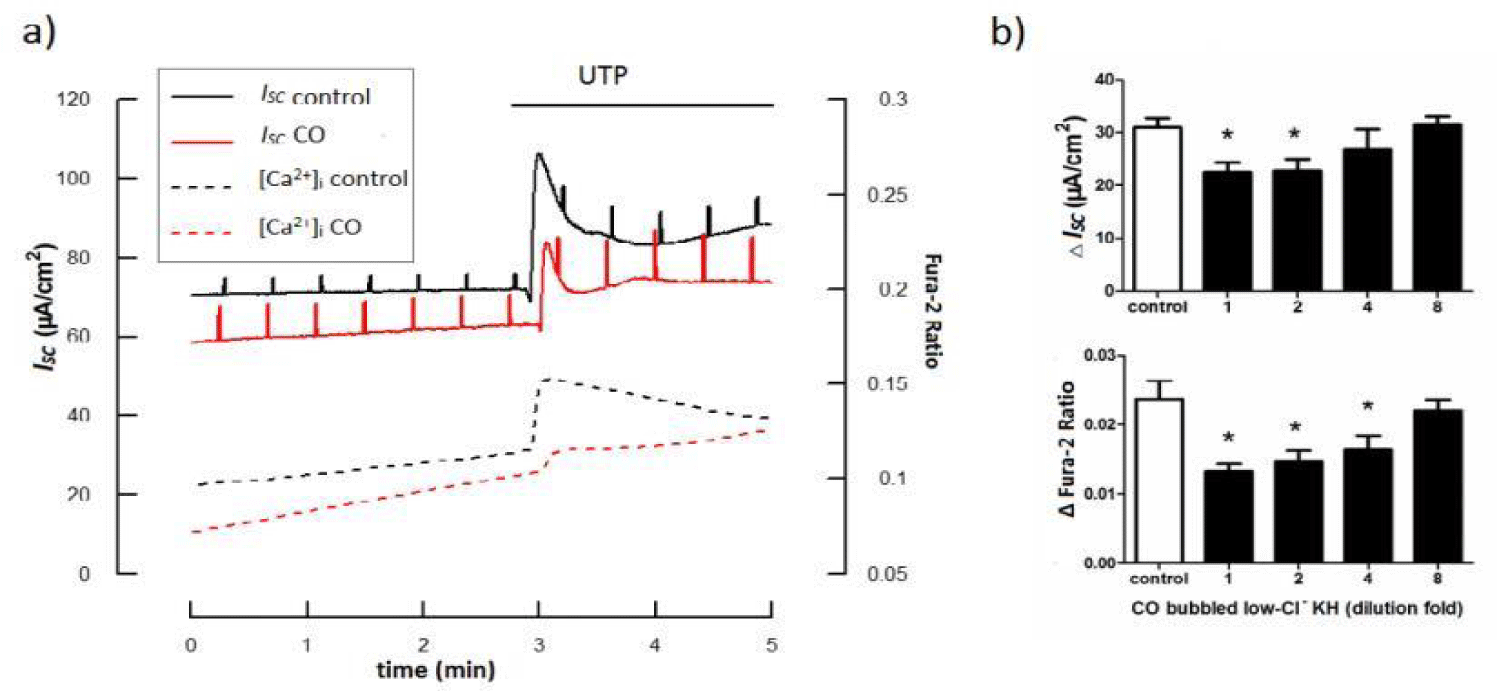
Apart from its well-defined anti-inflammatory effects, CO is also an emerging regulator of ion channels, modulating several classes of ion channels, including examples from calcium-activated K+ (BK(Ca)), voltage-activated K+ (K(v)) and Ca2+ channel (L-type) families, ligand-gated P2X receptors (P2X2 and P2X4), tandem P domain K+ channels (TREK1) and the epithelial Na+ channel (ENaC) [25]. Though there’s no evidence demonstrating the direct effects of CO on Cl- channels, it can regulate Cl- transport in other ways. Extracelluar nucleotides are known to activate Cl- secretion through either [Ca2+]i or cAMP dependent pathway, contributing to the maintainess of Airway Surface Liquid (ASL) [26-30]. In lung diseases characterized by impaired oxygen and CO2 transport, an increase in the ASL height, which is often observed during lung inflammation, might further aggravate the symptoms [31]. We’ve previously reported a inhibitory role of CO on P2Y receptor-mediated [Ca2+]i increase and IP3 formation [32]. Recently, by utilizing a simultaneous measurement combing electrophysiology and fluorescent, we further demonstrated that CO significantly suppressed UTP-evoked [Ca2+]i increase and Cl- secretion (Figure 1). We also tested the effect of CO on another important Cl- secretion pathway by detecting intracellular cAMP and Cl- secretion simultaneously [33]. Data revealed a strong inhibitory effect of CO on either [Ca2+]i or cAMP dependent Cl- secretion. Given that CO also directly suppress ENaC [34], we hypothesize that CO is a functional inhibitor against nucleotides-induced ion transport, and therefore can be used to alleviate edema.
Apart from modulating purinergic signaling mediated ion transport, HO-1/CO also reduced nucleotide-induced pro-inflammatory pathway activation, such as ERK1/2 MAPK as well as NF-κB, resulting in reduced IL-6 and IL-8 secretion [33,35].
Other Anti-virus Properties of CO
CO exerts its protective effect partially through modulating ROS production derived from either NADPH oxidase or respiratory chain [36,37]. It was reported CO plays a protective role in acute lung injury [38]. In an influenza virus infected mouse model, transfection of HO-1 resulted in suppression of both pathological changes and intrapulmonary hemorrhage; enhanced survival of animals; and a decrease of inflammatory cells in the lung [39]. Though CO could be produced by HO-1 in vivo, external CO can also induce the expression of HO-1, further strengthen the effect of CO [40,41]. We also found an increased expression of HO-1 induced by CO releasing molecule 3 (data not published). HO-1 was found to suppress hepatitis C virus and dengue virus replication in biliverdin dependent manner [42,43]. A latest study showed that pre-treatment of A549 alveolar cell and primary cultures of Human Tracheal Epithelial (HTE) cells with relative low dose of CO (100 ppm) resulted in reduced RV14 titers in the supernatants and RV RNA levels in A549 and HTE cells, CO exposure also increased the expression levels of Interferon (IFN)-gamma mRNA and protein [44].
Coronavirus are enveloped virus that fuse with a host cell membrane in order to deliver their genome into the host cell. Specific cues in the endosomal microenvironment induce conformational changes in the viral fusion proteins leading to viral and host membrane fusion, acidification of the endosomal microenvironment is required for successful fusion and release of the viral genome into the cytoplasm, such as SARS-CoV [45], NL63 [46] and MERS-CoV [47]. In dendritic cells, HO-1 derived CO reduced cargo transport, endosome-to-lysosome fusion, and antigen processing, dampening the production of peptide-MHC complexes on the surface [48]. Tardif V, et al. [49] also demonstrated that CO significantly reduced the efficiency of fusion between late endosomes and lysosomes, therefore blocked antigen trafficking at the level of late endosome-lysosome fusion in dendritic cells. Whether HO-1/CO has similar effect in the airway epithelial cells during virus infection remains unknown.
Additionally, CO is an important gaseous smooth muscle dilator [8] through activating PKG and/or BK(Ca) [50,51]. As previously described, COVID-19 patients showed higher inflammatory cytokine level [5], while many cytokines could facilitate bronchial smooth muscle contractility, including IL-17 [52], IL-4 [53], IL-13 [54], TNF-α [55]. Furthermore, some virus are capable of directly increase smooth muscle contraction. It was recently reported that RV infection lead to Airway Hyper Responsiveness (AHR) by increasing [Ca2+]i mobilization [56]. Therefore, CO therapy not only provides anti-inflammation and anti-hypersecretion effect, but also alleviates airway narrowing. Together with its potential role in anti-virus infection, CO application may provide a comprehensive protective support to patients with COVID-19.
Safety and Clinical Trials of CO
The successful demonstration of CO-dependent protection in numerous animal models of disease has evoked the intriguing proposition that CO may be applicable as a molecular medicine in corresponding human disease states [57]. Exogenous administration of low concentration of CO by inhalation has been tested or currently in clinical trial to evaluate it’s potential to reduce inflammation (NCT00094406), (NCT00122694), (NCT00531856). In a phase II clinical trial aimed to test the effect of inhaled CO on COPD, patients inhaled 2 hours of 100-125 ppm CO for 4 consecutive days showed reduced sputum eosinophils and improved responsiveness to methacholine. The median COHb reached after the fourth inhalation session of 100 ppm CO was 2.6%, with a highest individual value of 3.5%. After 125 ppm inhalation the median COHb was 3.1%, with the highest individual value reaching 4.5% [58], below the levels of COHb “achieved” with smoking of 20 cigarettes•day-1 where the 24-h average COHb levels reach 5.3% on average, with peaks >6% [59].
Remarks
CO is a potential candidate for therapeutic application during virus infected lung diseases. The unique advantage of CO is that it is an electroneutral gaseous molecule, which can diffuse easily across any membranes to exert its multiple function without interacting to unnecessary reactions like NO did [8,57,60,61]. Cytokine storm evoked by over-activated immune responses is a lethal characteristic during virus infection [62-64], CO could be a substitution of corticosteroid to control immune reaction and inflammation. CO can also alleviate ASL and alveolar fluid overproduction through either directly modulating ion channels or interacting with purinergic signaling pathways. Furthermore, CO and HO-1 showed potential anti-virus replication and inhibits endosome fusion to prevent virus release, which is worthwhile further studies. Additionally, CO also protects host from oxidative stress as well as smooth muscle hypercontractility. The comprehensive effects of CO makes it a possible therapeutic support for virus infection-induced lung disease including the prevalent COVID-19.
Acknowledgment
The work was supported by the National Natural Science Foundation of China (Grant No.: 82000008) and GuangDong Basic and Applied Basic Research Foundation (Grant No.: 2019A1515110126).
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. PMID: 31978945; PMCID: PMC7092803.
- Perlman S. Another Decade, Another Coronavirus. N Engl J Med. 2020 Feb 20;382(8):760-762. doi: 10.1056/NEJMe2001126. Epub 2020 Jan 24. PMID: 31978944; PMCID: PMC7121143.
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020 Feb 20;382(8):692-694. doi: 10.1056/NEJMp2000929. Epub 2020 Jan 24. PMID: 31978293.
- Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004 Apr;136(1):95-103. doi: 10.1111/j.1365-2249.2004.02415.x. PMID: 15030519; PMCID: PMC1808997.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299.
- Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004 Dec;31(4):304-9. doi: 10.1016/j.jcv.2004.07.006. PMID: 15494274; PMCID: PMC7108318.
- Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006 Sep;3(9):e343. doi: 10.1371/journal.pmed.0030343. PMID: 16968120; PMCID: PMC1564166.
- Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005 Dec;57(4):585-630. doi: 10.1124/pr.57.4.3. PMID: 16382109.
- Zhang Y, Zhang L, Wu J, Di C, Xia Z. Heme oxygenase-1 exerts a protective role in ovalbumin-induced neutrophilic airway inflammation by inhibiting Th17 cell-mediated immune response. J Biol Chem. 2013 Nov 29;288(48):34612-26. doi: 10.1074/jbc.M113.494369. Epub 2013 Oct 4. PMID: 24097973; PMCID: PMC3843074.
- Luo YP, Jiang L, Kang K, Fei DS, Meng XL, Nan CC, Pan SH, Zhao MR, Zhao MY. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int Immunopharmacol. 2014 May;20(1):24-32. doi: 10.1016/j.intimp.2014.02.017. Epub 2014 Feb 26. PMID: 24583148.
- Konrad FM, Braun S, Ngamsri KC, Vollmer I, Reutershan J. Heme oxygenase-1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow. Am J Physiol Lung Cell Mol Physiol. 2014 Nov 1;307(9):L707-17. doi: 10.1152/ajplung.00145.2014. Epub 2014 Aug 29. PMID: 25172914.
- Konrad FM, Knausberg U, Höne R, Ngamsri KC, Reutershan J. Tissue heme oxygenase-1 exerts anti-inflammatory effects on LPS-induced pulmonary inflammation. Mucosal Immunol. 2016 Jan;9(1):98-111. doi: 10.1038/mi.2015.39. Epub 2015 May 6. PMID: 25943274.
- Wei J, Fan G, Zhao H, Li J. Heme oxygenase-1 attenuates inflammation and oxidative damage in a rat model of smoke-induced emphysema. Int J Mol Med. 2015 Nov;36(5):1384-92. doi: 10.3892/ijmm.2015.2353. Epub 2015 Sep 22. PMID: 26397736.
- Sulaieva O, Wallace JL. Gaseous mediator-based anti-inflammatory drugs. Curr Opin Pharmacol. 2015 Dec;25:1-6. doi: 10.1016/j.coph.2015.08.005. Epub 2015 Aug 28. PMID: 26319186.
- Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013 Jun 15;190(12):6378-88. doi: 10.4049/jimmunol.1202969. Epub 2013 May 6. PMID: 23650615; PMCID: PMC3679316.
- Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med. 2006 Mar 15;173(6):673-82. doi: 10.1164/rccm.200508-1200OC. Epub 2005 Dec 30. PMID: 16387801; PMCID: PMC2662951.
- Swartz TH, Dubyak GR, Chen BK. Purinergic Receptors: Key Mediators of HIV-1 Infection and Inflammation. Front Immunol. 2015 Nov 26;6:585. doi: 10.3389/fimmu.2015.00585. PMID: 26635799; PMCID: PMC4659914.
- Zhou F, Liu X, Gao L, Zhou X, Cao Q, Niu L, Wang J, Zuo D, Li X, Yang Y, Hu M, Yu Y, Tang R, Lee BH, Choi BW, Wang Y, Izumiya Y, Xue M, Zheng K, Gao D. HIV-1 Tat enhances purinergic P2Y4 receptor signaling to mediate inflammatory cytokine production and neuronal damage via PI3K/Akt and ERK MAPK pathways. J Neuroinflammation. 2019 Apr 4;16(1):71. doi: 10.1186/s12974-019-1466-8. PMID: 30947729; PMCID: PMC6449963.
- Kunzelmann K, König J, Sun J, Markovich D, King NJ, Karupiah G, Young JA, Cook DI. Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem. 2004 Nov 19;279(47):48760-6. doi: 10.1074/jbc.M409747200. Epub 2004 Sep 10. PMID: 15364905.
- Chen S, Shenk T, Nogalski MT. P2Y2 purinergic receptor modulates virus yield, calcium homeostasis, and cell motility in human cytomegalovirus-infected cells. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18971-18982. doi: 10.1073/pnas.1907562116. Epub 2019 Sep 3. PMID: 31481624; PMCID: PMC6754545.
- Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2004 Jan;286(1):L112-20. doi: 10.1152/ajplung.00218.2003. Epub 2003 Aug 29. PMID: 12948936.
- Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol. 2009 Mar;296(3):L372-83. doi: 10.1152/ajplung.90437.2008. Epub 2008 Dec 26. PMID: 19112100; PMCID: PMC2660211.
- Calvén J, Akbarshahi H, Menzel M, Ayata CK, Idzko M, Bjermer L, Uller L. Rhinoviral stimuli, epithelial factors and ATP signalling contribute to bronchial smooth muscle production of IL-33. J Transl Med. 2015 Aug 29;13:281. doi: 10.1186/s12967-015-0645-3. PMID: 26318341; PMCID: PMC4552418.
- Ferrari D, Idzko M, Müller T, Manservigi R, Marconi P. Purinergic Signaling: A New Pharmacological Target Against Viruses? Trends Pharmacol Sci. 2018 Nov;39(11):926-936. doi: 10.1016/j.tips.2018.09.004. Epub 2018 Oct 3. PMID: 30292585.
- Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011 Jul 1;589(Pt 13):3055-62. doi: 10.1113/jphysiol.2011.206706. Epub 2011 Apr 26. PMID: 21521759; PMCID: PMC3145923.
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994 Jan;10(1):38-47. doi: 10.1165/ajrcmb.10.1.7507342. PMID: 7507342.
- Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016 Mar;71(3):284-7. doi: 10.1136/thoraxjnl-2015-207588. Epub 2015 Dec 30. PMID: 26719229.
- Wong CH, Ko WH. Stimulation of Cl- secretion via membrane-restricted Ca2+ signaling mediated by P2Y receptors in polarized epithelia. J Biol Chem. 2002 Mar 15;277(11):9016-21. doi: 10.1074/jbc.M111917200. Epub 2002 Jan 4. PMID: 11779875.
- Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010 Aug 1;21(15):2639-48. doi: 10.1091/mbc.E09-12-1004. Epub 2010 Jun 16. PMID: 20554763; PMCID: PMC2912350.
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004 Aug 27;279(35):36855-64. doi: 10.1074/jbc.M405367200. Epub 2004 Jun 21. PMID: 15210701; PMCID: PMC2943374.
- Hug MJ. CO2 - friend or foe? J Physiol. 2016 Mar 15;594(6):1521. doi: 10.1113/JP271882. PMID: 26995259; PMCID: PMC4799978.
- Zhang RG, Yip CY, Ko WH. Regulation of Intracellular Calcium by Carbon Monoxide in Human Bronchial Epithelial Cells. Cell Physiol Biochem. 2017;42(6):2377-2390. doi: 10.1159/000480029. Epub 2017 Aug 18. PMID: 28957808.
- Zhang RG, Yip CY, Ko WH. Carbon Monoxide Inhibits Cytokine and Chloride Secretion in Human Bronchial Epithelia. Cell Physiol Biochem. 2018;49(2):626-637. doi: 10.1159/000493026. Epub 2018 Aug 30. PMID: 30165347.
- Althaus M, Fronius M, Buchäckert Y, Vadász I, Clauss WG, Seeger W, Motterlini R, Morty RE. Carbon monoxide rapidly impairs alveolar fluid clearance by inhibiting epithelial sodium channels. Am J Respir Cell Mol Biol. 2009 Dec;41(6):639-50. doi: 10.1165/rcmb.2008-0458OC. Epub 2009 Feb 27. PMID: 19251942.
- Zhang RG, Pan K, Hao Y, Yip CY, Ko WH. Anti-inflammatory action of HO-1/CO in human bronchial epithelium in response to cationic polypeptide challenge. Mol Immunol. 2019 Jan;105:205-212. doi: 10.1016/j.molimm.2018.12.002. Epub 2018 Dec 13. PMID: 30553057.
- Taillé C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005 Jul 8;280(27):25350-60. doi: 10.1074/jbc.M503512200. Epub 2005 Apr 29. PMID: 15863496.
- Ryter SW, Choi AM. Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp. 2007;280:165-75; discussion 175-81. PMID: 17380794.
- Faller S, Hoetzel A. Carbon monoxide in acute lung injury. Curr Pharm Biotechnol. 2012 May;13(6):777-86. doi: 10.2174/138920112800399185. PMID: 22201607.
- Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001 Oct;8(19):1499-507. doi: 10.1038/sj.gt.3301540. PMID: 11593363.
- Yeh PY, Li CY, Hsieh CW, Yang YC, Yang PM, Wung BS. CO-releasing molecules and increased heme oxygenase-1 induce protein S-glutathionylation to modulate NF-κB activity in endothelial cells. Free Radic Biol Med. 2014 May;70:1-13. doi: 10.1016/j.freeradbiomed.2014.01.042. Epub 2014 Feb 7. PMID: 24512908.
- Chi PL, Liu CJ, Lee IT, Chen YW, Hsiao LD, Yang CM. HO-1 induction by CO-RM2 attenuates TNF-α-induced cytosolic phospholipase A2 expression via inhibition of PKCα-dependent NADPH oxidase/ROS and NF-κB. Mediators Inflamm. 2014;2014:279171. doi: 10.1155/2014/279171. Epub 2014 Jan 29. PMID: 24616552; PMCID: PMC3927740.
- Tseng CK, Lin CK, Wu YH, Chen YH, Chen WC, Young KC, Lee JC. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci Rep. 2016 Aug 24;6:32176. doi: 10.1038/srep32176. PMID: 27553177; PMCID: PMC4995454.
- Zhu Z, Wilson AT, Luxon BA, Brown KE, Mathahs MM, Bandyopadhyay S, McCaffrey AP, Schmidt WN. Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: mechanism for the antiviral effects of heme oxygenase? Hepatology. 2010 Dec;52(6):1897-905. doi: 10.1002/hep.23921. PMID: 21105106; PMCID: PMC3058505.
- Deng X, Yasuda H, Sasaki T, Yamaya M. Low-Dose Carbon Monoxide Inhibits Rhinovirus Replication in Human Alveolar and Airway Epithelial Cells. Tohoku J Exp Med. 2019 Apr;247(4):215-222. doi: 10.1620/tjem.247.215. PMID: 30971638.
- Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018 Apr;517:3-8. doi: 10.1016/j.virol.2017.12.015. Epub 2017 Dec 21. PMID: 29275820; PMCID: PMC7112017.
- Milewska A, Nowak P, Owczarek K, Szczepanski A, Zarebski M, Hoang A, Berniak K, Wojarski J, Zeglen S, Baster Z, Rajfur Z, Pyrc K. Entry of Human Coronavirus NL63 into the Cell. J Virol. 2018 Jan 17;92(3):e01933-17. doi: 10.1128/JVI.01933-17. PMID: 29142129; PMCID: PMC5774871.
- Burkard C, Verheije MH, Wicht O, van Kasteren SI, van Kuppeveld FJ, Haagmans BL, Pelkmans L, Rottier PJ, Bosch BJ, de Haan CA. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014 Nov 6;10(11):e1004502. doi: 10.1371/journal.ppat.1004502. Erratum in: PLoS Pathog. 2015 Feb;11(2):e1004709. PMID: 25375324; PMCID: PMC4223067.
- Riquelme SA, Pogu J, Anegon I, Bueno SM, Kalergis AM. Carbon monoxide impairs mitochondria-dependent endosomal maturation and antigen presentation in dendritic cells. Eur J Immunol. 2015 Dec;45(12):3269-88. doi: 10.1002/eji.201545671. Epub 2015 Oct 23. PMID: 26461179.
- Tardif V, Riquelme SA, Remy S, Carreño LJ, Cortés CM, Simon T, Hill M, Louvet C, Riedel CA, Blancou P, Bach JM, Chauveau C, Bueno SM, Anegon I, Kalergis AM. Carbon monoxide decreases endosome-lysosome fusion and inhibits soluble antigen presentation by dendritic cells to T cells. Eur J Immunol. 2013 Nov;43(11):2832-44. doi: 10.1002/eji.201343600. Epub 2013 Aug 21. PMID: 23852701.
- Kinhult J, Uddman R, Cardell LO. The induction of carbon monoxide-mediated airway relaxation by PACAP 38 in isolated guinea pig airways. Lung. 2001;179(1):1-8. doi: 10.1007/s004080000043. PMID: 11479689.
- Ameredes BT, Otterbein LE, Kohut LK, Gligonic AL, Calhoun WJ, Choi AM. Low-dose carbon monoxide reduces airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol. 2003 Dec;285(6):L1270-6. doi: 10.1152/ajplung.00145.2003. Epub 2003 Aug 1. PMID: 12896878.
- Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012 Mar 4;18(4):547-54. doi: 10.1038/nm.2684. PMID: 22388091; PMCID: PMC3321096.
- Martin G, O'Connell RJ, Pietrzykowski AZ, Treistman SN, Ethier MF, Madison JM. Interleukin-4 activates large-conductance, calcium-activated potassium (BKCa) channels in human airway smooth muscle cells. Exp Physiol. 2008 Jul;93(7):908-18. doi: 10.1113/expphysiol.2008.042432. Epub 2008 Apr 10. PMID: 18403443; PMCID: PMC4115791.
- Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, Yang J. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther. 2010 Jun;23(3):182-9. doi: 10.1016/j.pupt.2009.12.005. Epub 2010 Jan 4. PMID: 20045483.
- Chen H, Tliba O, Van Besien CR, Panettieri RA Jr, Amrani Y. TNF-[alpha] modulates murine tracheal rings responsiveness to G-protein-coupled receptor agonists and KCl. J Appl Physiol (1985). 2003 Aug;95(2):864-72; discussion 863. doi: 10.1152/japplphysiol.00140.2003. Epub 2003 May 2. PMID: 12730147.
- Parikh V, Scala J, Patel R, Corbi C, Lo D, Bochkov YA, Kennedy JL, Kurten RC, Liggett SB, Gern JE, Koziol-White CJ. Rhinovirus C15 Induces Airway Hyperresponsiveness via Calcium Mobilization in Airway Smooth Muscle. Am J Respir Cell Mol Biol. 2020 Mar;62(3):310-318. doi: 10.1165/rcmb.2019-0004OC. PMID: 31533004; PMCID: PMC7055698.
- Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009 Sep;41(3):251-60. doi: 10.1165/rcmb.2009-0170TR. Epub 2009 Jul 17. PMID: 19617398; PMCID: PMC2742746.
- Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007 Dec;30(6):1131-7. doi: 10.1183/09031936.00163206. Epub 2007 Aug 22. PMID: 17715164.
- Zevin S, Saunders S, Gourlay SG, Jacob P, Benowitz NL. Cardiovascular effects of carbon monoxide and cigarette smoking. J Am Coll Cardiol. 2001 Nov 15;38(6):1633-8. doi: 10.1016/s0735-1097(01)01616-3. PMID: 11704374.
- Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411-49. doi: 10.1146/annurev.pharmtox.46.120604.141053. PMID: 16402911.
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006 Apr;86(2):583-650. doi: 10.1152/physrev.00011.2005. PMID: 16601269.
- Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008 Sep;43(3):336-41. doi: 10.1016/j.cyto.2008.07.009. Epub 2008 Aug 9. PMID: 18694646.
- Yoo JK, Kim TS, Hufford MM, Braciale TJ. Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol. 2013 Dec;132(6):1263-76; quiz 1277. doi: 10.1016/j.jaci.2013.06.006. Epub 2013 Aug 1. PMID: 23915713; PMCID: PMC3844062.
- Vázquez Y, González L, Noguera L, González PA, Riedel CA, Bertrand P, Bueno SM. Cytokines in the Respiratory Airway as Biomarkers of Severity and Prognosis for Respiratory Syncytial Virus Infection: An Update. Front Immunol. 2019 Jun 4;10:1154. doi: 10.3389/fimmu.2019.01154. PMID: 31214165; PMCID: PMC6557983.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.