> Biology Group. 2021 May 27;2(5):408-411. doi: 10.37871/jbres1250.
Cell, Time and Knowledge: Some Conjectures
Diosey Ramon Lugo-Morin*
- Knowledge
- Time
- Cell
- DNA
- COVID-19
Introductıon
This review is a reflective analysis that aims to explore the potential of cells as time capsules, capable of providing humanity with useful knowledge to face the challenges of today’s world.
Based on cell-theory [1-5], today we know that life is generated from pre-existing life, i.e. the basic unit of life that is a cell originates from another cell [6], this process is called the cell cycle and consists of several phases [7,8] (i) DNA synthesis and replication or data transfer; (ii) cell division, here the already duplicated DNA molecules separate to original nuclei, the nuclei have identical genetic content to the original cell and (iii) finally, cytokinesis where reorganisation of the cell membrane occurs in the new nuclei forming the new cells. These phases are more complex and give rise to multiple additional processes that we will not mention here because this analysis is not about molecular biology.
Based on the principles of cell-theory we can point out that all living organisms are composed of cells in differentiated quantities [9]. Due to the cell cycle we can conclude that there is persistence, resilience and transformation of life [10]. The continuous cellular interaction has generated fascinating evolutionary processes that in the last two decades have been unveiled (e.g., perception, memory, learning, emotions), information that is inscribed in our genetic code [11-13].
The case of memory in cells is a matter of debate, memory in cells is defined as the prolonged response to a transient stimulus (e.g., differentiation in which a precursor cell makes a permanent, heritable cell fate decision in response to transient signals) [14].
The ability to understand memory in cells has made important advances, the study by Ajo-Franklin, et al. [14] proposed to develop a cellular memory device which would allow to address a positive transcriptional feedback loop aimed at conferring “memory” of a stimulus to a yeast cell and its progeny. Another interesting study showing memory in cells was described by Sible [15] showing through differentiation how a cell “remembers”.
The usefulness of cellular memory can be seen in vaccine development, in this logic the study by Macallan, et al. [16] argues the importance of this cellular function against future pathogenic challenges, which will open new opportunities for modulation and modification of vaccine responses. Moreover, the use of nanotechnologies could accelerate their utility in vaccine development; the use of nanobots controlled by nanocomputers with today’s science is a reality. The application of nanobots in medicine offers a new range of tools for various uses, e.g., nanomaterials, in situ diagnosis, treatment of damaged cells [17-20].
Previous approaches, leads us to the first conjecture, that today’s cells are derived from ancestral cells. According to Weismann [21], the principle of immortality of cells derives from the fact that the first cells, due to the cell cycle, have been dividing up to the present day to form today’s cells. Weismann’s approach is part of an ongoing debate that may lead to support for these claims [22].
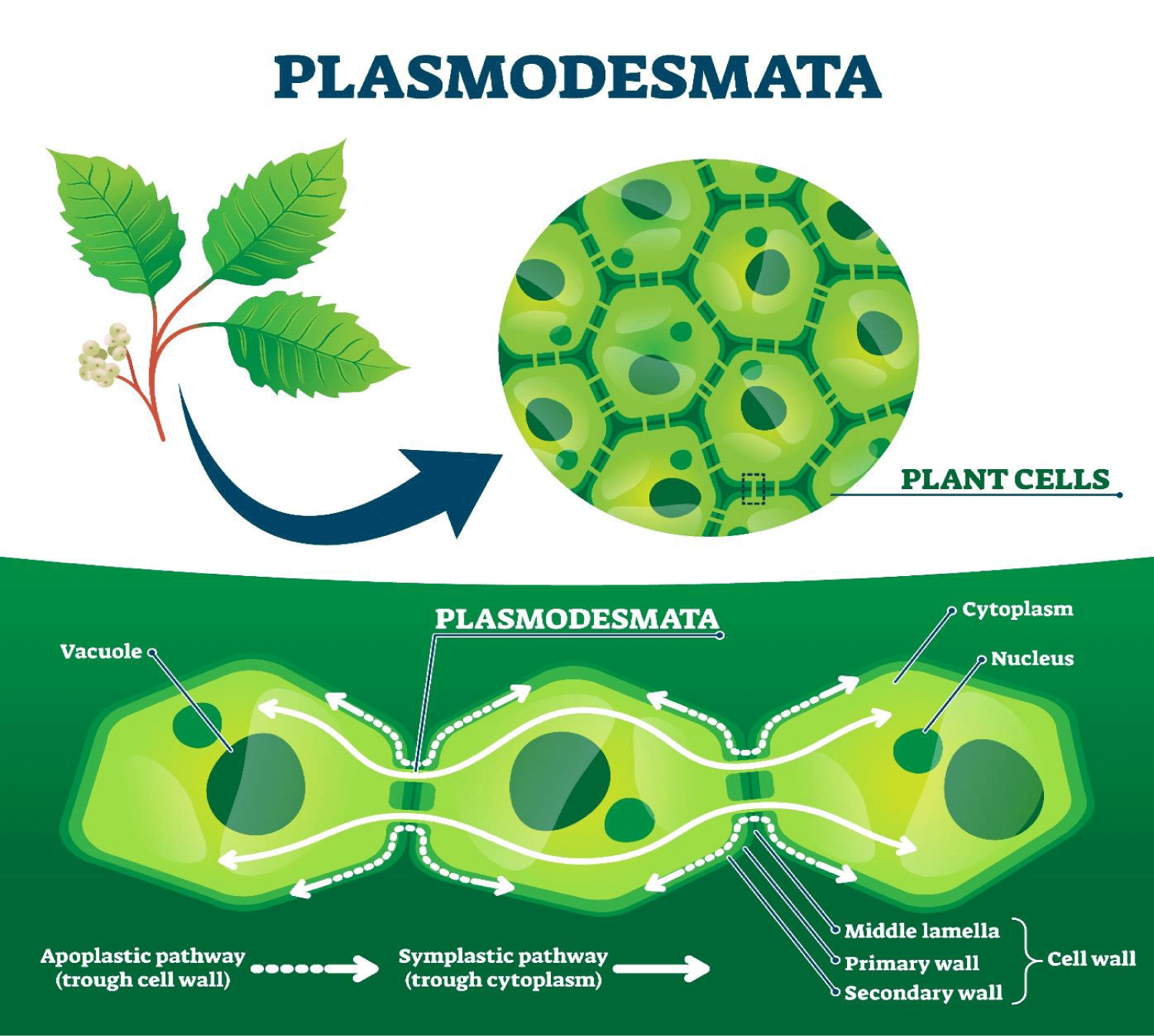
The first conjecture leads us to the second conjecture: if today’s cells originated from ancient cells, it is logical to think that in the process of DNA duplication the information contained in these cells has been transferred for millions of years, if we recognise that the first hominids already existed in the Pliocene, the acquired knowledge inscribed in DNA is enormous [23]. The cellular communication strategy is based on two mechanisms: plasmodesmata and specialised molecules associated with the cell wall (see Figure 1) [24,25].
Based on the previous approach, we have a third conjecture: if we know how cells communicate with each other, we can then have access to the information contained in each cell. The information (e.g., perceptions, memory, learning, emotions) accumulated over millions of years in cells has given humans evolutionary advantages, and human capacities over time have been shaped by the environmental context, one example being their capacity for resilience [26].
The three conjectures that are proposed lead us to a single path, to unveil the knowledge that cells have been able to accumulate over time. Even if it seems impossible in practice, the theory shows us that the knowledge exists to reach the frontiers of DNA.
Access to Knowledge through Time: Fantasy or Reality?
The analysis carried out shows us a world of possibilities that a century ago would not have been possible to reach, the advance of science has been able to unveil highly specialised knowledge of cells to an unimaginable limit. Access to the contents of a cell emulates access to time capsules with information accumulated via perception, memory, emotions that human beings have inscribed in their DNA during their existence.
With current scientific knowledge we know that cells are time capsules containing knowledge about the ancestors of humans, accessing the worldview of the ancestors can give us clues to possible solutions to current challenges (e.g., climate change, pandemics, food insecurity).
Discoveries about the functionality of the plasmodesmata now provide detailed insights into cell-to-cell communication. The study by Suoqin, et al. [27] using a tool (CellChat: http://www.cellchat.org) was able to quantitatively infer and analyse intercellular communication networks from single cell RNA sequencing data. With the CellChat tool it was possible to gain insight into the signalling dynamics for cells and how those cells and signals coordinate for functions using network analysis and pattern recognition methods. The refinement of this tool allows us to expand its uses and generate unconventional visualisations, first in the field of medicine and then in the exploration of other fields of science.
In this logic, we have the advances in nanotechnologies applied to medicine, which currently projects the repair of the human body [28]. The field of nanofabrication is also notable [29]. Other studies address the application of nanotechnology for cell repair [30]. Knowledge about nanobots and their use at the cellular level has made impressive progress and may constitute a tool that facilitates the transfer of information from a cell.
According to Mitra [30] a programmed nanobot constructed from carbon nanotubes can kill cancer cells using nanolasers, clean infected cells (with nanochemistry) and repair damaged tissue.
Access to the information contained in cells is possible; the studies mentioned above show that it is possible. Access to thousands of years of accumulated knowledge can make the difference for a more sustainable world, prevention and control of pandemics [31], advances in food design to mitigate food insecurity. For example, the world has historically suffered from many pandemics, all of which are contained in cells, and accessing this information could give us clues to prevent and control future pandemics.
By Way of Conclusion: Thinking about the COVID-19 Pandemic
The unexpected presence of the COVID-19 pandemic has surprised the world, knowledge has been overwhelmed by the characteristics of this pandemic, however, science has been able to understand the virus in detail and create different vaccines to control the pandemic, but as a response to the virus it is changing, calling into question the effectiveness of the vaccines developed in the face of the appearance of these new variants.
This situation requires a major effort to understand the complexities associated with this type of health emergency. For more than two decades, science has been deepening its understanding of cellular knowledge, and understanding the potential of this knowledge could be the key to finding solutions to future public health challenges.
The knowledge accumulated in a cell has been proven, the development of techniques for accessing this knowledge is not new, but attempts at cell reprogramming, development of cell-to-cell communication protocols and the expected use of nanotechnologies promise an unprecedented breakthrough in uncovering the ancestral knowledge that cells have accumulated to date.
Author Contributions
This manuscript was conceived, designed and written by DRLM and the final version submitted is approved by DRLM.
References
- Leeuwenhoek AV, Observationes D. Anthonii Lewenhoeck, de natis’e semine genitali animalculis. Philosophical Transactions of the Royal Society of London. 1677;12(142):1040-1046.
- Schleiden MJ. Beitrage zur Phytogenesis. Archives of Anatomy, Physiology and Scientific Medicine. 1838;2:137-176.
- Virchow RLK. The position of pathology among biological studies. Proceedings of the Royal Society of London. 1893;53(321-325):114-129. doi: 10.1098/rspl.1893.0015
- BAKER JR. The cell-theory; a restatement, history and critique. Q J Microsc Sci. 1948 Mar;89(Pt 1):103-25. PMID: 18860297.
- Baluska F, Volkmann D, Barlow PW. Eukaryotic cells and their cell bodies: Cell Theory revised. Ann Bot. 2004 Jul;94(1):9-32. doi: 10.1093/aob/mch109. Epub 2004 May 20. PMID: 15155376; PMCID: PMC4242365.
- Padilla-Mejia NE, Makarov AA, Barlow LD, Butterfield ER, Field MC. Evolution and diversification of the nuclear envelope. Nucleus. 2021 Dec;12(1):21-41. doi: 10.1080/19491034.2021.1874135. PMID: 33435791; PMCID: PMC7889174.
- Wolpert L. Evolution of the cell theory. Philos Trans R Soc Lond B Biol Sci. 1995 Sep 29;349(1329):227-33. doi: 10.1098/rstb.1995.0106. PMID: 8577831.
- Ekundayo B, Bleichert F. Origins of DNA replication. PLoS Genet. 2019 Sep 12;15(9):e1008320. doi: 10.1371/journal.pgen.1008320. Erratum in: PLoS Genet. 2019 Dec 19;15(12):e1008556. PMID: 31513569; PMCID: PMC6742236.
- Mora Van Cauwelaert E, Arias Del Angel JA, Benítez M, Azpeitia EM. Development of cell differentiation in the transition to multicellularity: a dynamical modeling approach. Front Microbiol. 2015 Jun 23;6:603. doi: 10.3389/fmicb.2015.00603. PMID: 26157427; PMCID: PMC4477168.
- Wan KY, Jékely G. Origins of eukaryotic excitability. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 15;376(1820):20190758. doi: 10.1098/rstb.2019.0758. Epub 2021 Jan 25. PMID: 33487111; PMCID: PMC7935092.
- Lyon P, Keijzer F, Arendt D, Levin M. Reframing cognition: getting down to biological basics. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 15;376(1820):20190750. doi: 10.1098/rstb.2019.0750. Epub 2021 Jan 25. PMID: 33487107; PMCID: PMC7935032.
- Bechtel W, Bich L. Grounding cognition: heterarchical control mechanisms in biology. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 15;376(1820):20190751. doi: 10.1098/rstb.2019.0751. Epub 2021 Jan 25. PMID: 33487110; PMCID: PMC7934967.
- Lyon P, Kuchling F. Valuing what happens: a biogenic approach to valence and (potentially) affect. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 15;376(1820):20190752. doi: 10.1098/rstb.2019.0752. Epub 2021 Jan 25. PMID: 33487109; PMCID: PMC7935054.
- Ajo-Franklin CM, Drubin DA, Eskin JA, Gee EP, Landgraf D, Phillips I, Silver PA. Rational design of memory in eukaryotic cells. Genes Dev. 2007 Sep 15;21(18):2271-6. doi: 10.1101/gad.1586107. PMID: 17875664; PMCID: PMC1973140.
- Sible J. Thanks for the memory. Nature. 2003; 426: 392-393. doi: 10.1038/426392a
- Macallan DC, Borghans JA, Asquith B. Human T Cell Memory: A Dynamic View. Vaccines (Basel). 2017 Feb 4;5(1):5. doi: 10.3390/vaccines5010005. PMID: 28165397; PMCID: PMC5371741.
- Apoorva M, Vijay K. The promising future in medicine: Nanobots. Biomedical Science and Engineering. 2014; 2(2): 42-47. doi: 10.12691/bse-2-2-3
- Chidchob P, Sleiman HF. Recent advances in DNA nanotechnology. Curr Opin Chem Biol. 2018 Oct;46:63-70. doi: 10.1016/j.cbpa.2018.04.012. Epub 2018 May 9. PMID: 29751162.
- Thiruchelvi R, Sikdar E, Das A, Rajakumari K. Nanobots in today´s world. Research Journal of Pharmacy and Technology. 2020; 13(4): 2033-2039. DOI: 10.5958/0974-360X.2020.00366.2
- Galdopórpora JM, Ibar A, Tuttolomondo MV, Desimone MF. Dual-effect core-shell polyphenol coated silver nanoparticles for tissue engineering. Nano-Structures & Nano-Objects 26: 100716. doi: 10.1016/j.nanoso.2021.100716
- Weismann A. Prof. Weismann’s Theory of Heredity. Nature. 1890;41:317–323. https://tinyurl.com/yp5pp5x2
- Ginsburg S, Jablonka E. Evolutionary transitions in learning and cognition. Philos Trans R Soc Lond B Biol Sci. 2021 Mar 29;376(1821):20190766. doi: 10.1098/rstb.2019.0766. Epub 2021 Feb 8. PMID: 33550955; PMCID: PMC7935133.
- Lovejoy CO. The origin of man. Science. 1981 Jan 23;211(4480):341-50. doi: 10.1126/science.211.4480.341. PMID: 17748254.
- Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, Kloareg B, Stengel DB. Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol. 2011;62:567-90. doi: 10.1146/annurev-arplant-042110-103809. PMID: 21351878.
- Petit JD, Li ZP, Nicolas WJ, Grison MS, Bayer EM. Dare to change, the dynamics behind plasmodesmata-mediated cell-to-cell communication. Curr Opin Plant Biol. 2020 Feb;53:80-89. doi: 10.1016/j.pbi.2019.10.009. Epub 2019 Dec 2. PMID: 31805513.
- Grine FE and Fleagle JG. The First Humans: A Summary Perspective on the Origin and Early Evolution of the Genus Homo. In: Grine FE, Fleagle JG, Leakey RE editors. The First Humans-Origin and Early Evolution of the Genus Homo. Vertebrate Paleobiology and Paleoanthropology.
- Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, Myung P, Plikus MV, Nie Q. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021 Feb 17;12(1):1088. doi: 10.1038/s41467-021-21246-9. PMID: 33597522; PMCID: PMC7889871.
- Perán M, García MA, Lopez-Ruiz E, Jiménez G, Marchal JA. How Can Nanotechnology Help to Repair the Body? Advances in Cardiac, Skin, Bone, Cartilage and Nerve Tissue Regeneration. Materials (Basel). 2013 Mar 28;6(4):1333-1359. doi: 10.3390/ma6041333. PMID: 28809213; PMCID: PMC5452318.
- Mallapragada SK, Brenza TM, McMillan JM, Narasimhan B, Sakaguchi DS, Sharma AD, Zbarska S, Gendelman HE. Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines. Nanomedicine. 2015 Apr;11(3):715-29. doi: 10.1016/j.nano.2014.12.013. Epub 2015 Jan 31. PMID: 25652894; PMCID: PMC4628726.
- Mitra M. Medical nanobot for cell and tissue repair. International Robotics & Automation Journal. 2017; 2(6): 218-222. doi: 10.15406/iratj.2017.02.00038
- Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell. 2021 Apr 1;184(7):1671-1692. doi: 10.1016/j.cell.2021.02.029. Epub 2021 Feb 16. PMID: 33743212; PMCID: PMC7885626.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.