Medicine Group. 2024 April 20;5(4):324-344. doi: 10.37871/jbres1900.
Differentiating Compromised Mitochondria of Lung Cancer Cells from Mitochondria in Healthy Cells
Sharma B, Shafaee Z, Twelker K, Bhatia ND, Agriantonis G, Dave J, Mestre J, Ghanta H, Arora S and Whittington JM*
- Mitochondria
- Dysfunctional mitochondria
- Aerobic fermentation
- Mitochondrial metabolism
- Cancer
- Lung cancer
Abstract
Mitochondria are double-walled organelles that generate energy in the form of ATP. ATP is an energy-rich compound and a driver of fundamental cell functions. Mitochondria-oriented studies help design advanced therapies to target lung cancer. Lung cancer is the leading cause of cancer incidences and death globally. Various studies have supported that mitochondrial function is disrupting cancerous cells. Mitochondrial dysfunctions lead to dysregulated ATP synthesis, disturbed respiratory chain, unbalanced mitochondrial fission or fusion, disturbed cellular redox homeostasis, dysregulated apoptosis, and interfered non-smooth intracellular calcium signaling. Dysfunction mitochondria are associated with cancer cell proliferation, metastasis, and death. In this review, we have tried to elaborate on how normal cell mitochondria function differently from the mitochondria of lung cancer cells. It includes various targets such as mitochondrial proteins and related pathways, along with new drug molecules like Militarin analog-1, Dihydromyricetin, and papuamine. As mitochondrial metabolism is associated with the proliferation and metastasis of lung cancer cells, finding interlinks between malfunctioning mitochondria and the process of lung cancer can promote the development of new treatments.
Abbreviations
OXPHOS: Oxidative Phosphorylation; ATP: Adenosine Triphosphate; ETC: Electron Transport Chain; Mtdna: Mitochondrial Deoxyribonucleic Acid; NSCLC: Non-Small Cell Lung Cancer; ROS: Reactive Oxygen Species
Introduction
Mitochondria
Mitochondria are precious organelles that are known to be cellular "powerhouses" [1]. They produce energy from the food we consume [1]. When 10 million billion mitochondria throughout our bodies work together, the bulk of the ATP is generated for our cells [1]. Every day, mitochondria produce nearly fifty kilograms of Adenosine Triphosphate [ATP] [2]. As per Nick Lane, an English biochemist and writer, "Gram per gram, even when sitting comfortably, you are converting 10,000 times more energy than the sun every second" [2]. Specialty and uniqueness lie in the fact that mitochondria contain their DNA, which is very similar to bacterial DNA [3]. This makes them entirely different from other cellular organelles [3]. These organelles are responsible for a remarkable set of cellular functions, for example; generating ATP via oxidative phosphorylation, maintaining cellular redox homeostasis, regulating apoptosis, and intracellular calcium signaling [4-6]. The health of mitochondria is mandatory for the proper functioning of the human body.
Various studies in the past era are based on somatic mutation theory but recent studies are openly challenging this with a perspective that cancer can be a mitochondria-based metabolic disease i.e. supporting mitochondrial metabolic theory [7,8]. A compelling example is the rarity of cancer in chimpanzees or primitive humans. But, cancer is common in humans, why? Diet and lifestyle rather than genetic mutations can be an answer [9,10]. Even in the presence of sufficient oxygen, cancer cells can undergo fermentation to produce lactate and this process is known as aerobic fermentation. It is an indicator of respiratory insufficiency and a characteristic of cancer cells [11-13]. Lactate and succinate production are a key metabolic feature found in a cancer cell [13,14]. Increase in lactate and succinate makes the tumor environment acidic leading to an increase in angiogenesis [15]. Although lactate has its role in cancer progression glucose and glutamine are considered a major source of energy required for cancer growth [16]. Dysfunctional mitochondria can be considered as the fifth cancer hallmark [5]. Hence, we bring forth this literature review by connecting dots to explain how mitochondrial malfunctioning is associated with deadly diseases such as Lung cancer.
Historical facts about mitochondria
Around 1.6 billion years ago, mitochondria were understood to have originated by endocytosis because of their resemblance to α-proteobacteria [17]. It is evident from an evolutionary pattern that there is a close homology of bacterial and mitochondrial respiratory chain complexes [17]. 'Old' bacterial and 'new' eukaryotic-derived proteins together formed mitochondrial proteome [18]. Studies have explicitly shown an evolutionary relationship between mitochondrial translational machinery and bacteria [19]. For example; mutants of Caenorhabditis elegans have contributed to understanding the role of mitochondria in ATP synthesis by tricarboxylic acid cycle and oxidative phosphorylation [20]. Due to the distinct DNA code in their genetic system, they can be differentiated from their bacterial ancestors and their eukaryotic hosts [17]. Recently, mitochondrial similarities and differences from bacterial ribosomes have been determined by Single-Particle Electron Cryomicroscopy [cryo-EM] [21].
In 1865, Swiss physiologist and anatomist Albert von Kolliker observed granular structures in the cells of muscle tissue [22]. In 1870 Eduard Friedrich Wilhelm Pflüger explained that a process known as respiration takes place in cells [23,24]. Later in 1898, based on shape it was named "Mitochondrion" by Carl Benda. This term is the fusion of "mitos", thread, and "chondros", grain [25]. About a century later, the cycle of reactions taking place in mitochondria like the Krebs cycle was discovered by Sir Hans Adolf Krebs, a Nobel prize winner [26]. It helped in the interpretation of the citric acid cycle's role in the intermediate metabolism [27]. In the early 1910s, Kingsbury's postulation revealed that respiration takes place in "mitochondria," [23,24]. David Keilin in 1925 identified pigments and called them cytochromes. Decades back, observations set by Charles MacMunn assisted Keilin with these identifications. Further, It helped various researchers like Warburg, and Hartree to explore and learn more about important concepts like the "respiratory chain," and related enzymes like "dehydrogenases", Warburg's oxygen-reducing respiratory enzyme (The Atmungsferment) [23,27]. Between the late 1920s and the early 1940s, various key aspects of aerobic metabolism came forth, for example, the discovery of ATP by Lohmann in 1929 [28].
In 1931, Warburg demonstrated the nature of the cytochromes and the mode of action of the respiratory enzyme [29]. In 1946, succinoxidase and cytochrome oxidase in an isolated mitochondrion was illustrated by Albert Claude [30]. In the early 1950s, Palade and Sjostrand revealed a detailed structure of mitochondria as a double-membrane structure with curvy twisted cristae through electron micrographs [31]. In 1978, Mitchell received a Nobel Prize for his chemiosmotic theory about the anisotropic arrangement of components which play a role in ATP synthesis within the proton's impermeable inner mitochondrial membrane so electrons transferring and protons expelling across the membrane can be done by complexes. It helped set a base for a meticulous understanding of the processes of OXPHOS [3]. In 1981, the MRC Laboratory of Molecular Biology in Cambridge found the sequence of the human mitochondrial genome [32]. In 1998, the first study on mitochondrial proteomics was published. In 2003, comprehensive research studies explaining mitochondrial proteome by Albert Sickmann, Eric Lander/Matthias Mann, and Steven Taylor were published. In 2016, Khacho and colleagues brought forth the fact that change in mitochondrial shape is a key regulator of neural stem cell fate [33].
In the current scenario, mitochondria-oriented studies help devise new therapies to target lung cancer. A simple explanation of why these discrete organelles are is targeted that they lead a vast array of functions central to cellular life, death, and differentiation U[34,35]. ndoubtedly, mitochondrial function is disrupted in cancerous cells. Even treatment with pharmacological compounds such as chemotherapeutics and antiviral drugs is incompletely safe for the mitochondria of healthy cells [36-38]. Hence, today researchers are making tremendous efforts to learn more about mitochondrial biology so it can be safely targeted to cure various mitochondrial-based diseases especially, lung cancer.
Structure and function of mitochondria in a healthy cell
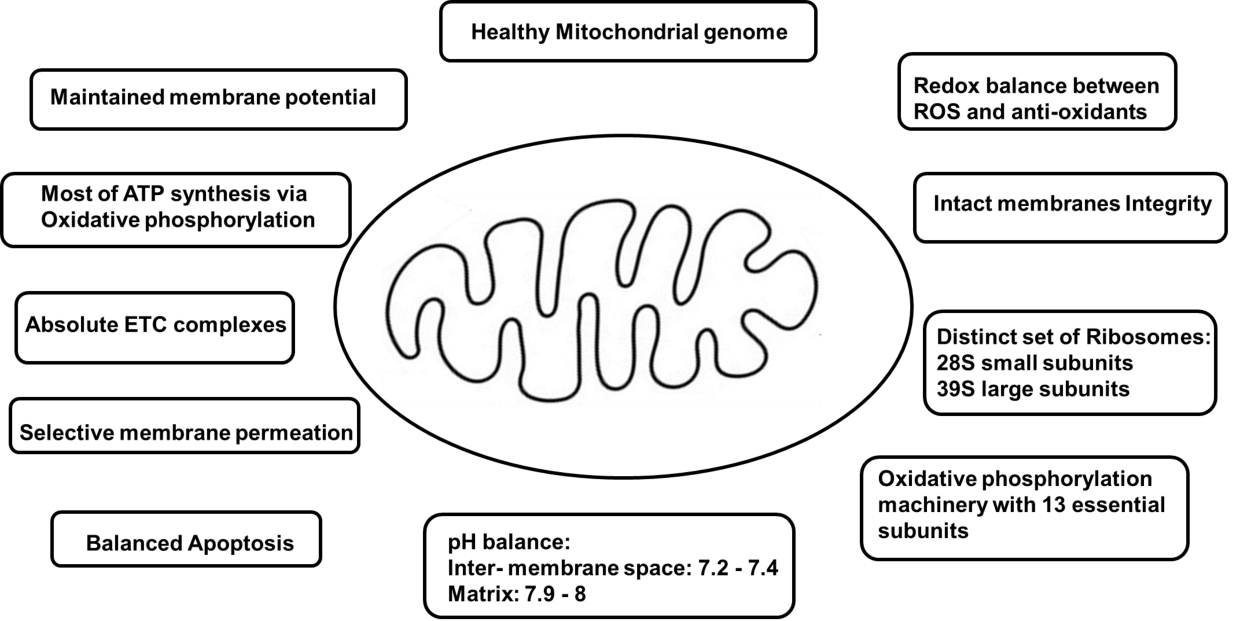
Mitochondria are organelles with double membranes [39]. This double-membrane system separates mitochondria from the cytoplasm [40] and itself is separated by inner membrane space [41]. Various features of mitochondria play a role in fusing, importing proteins, organizing, and maintaining membrane composition along with membrane potential [42,43]. The detailed internal features of mitochondria can be observed by electron microscopy only [44]. The tubular network formed by mitochondria in a cell keeps on dividing and fusing constantly with the help of numerous dynamin-related GTPases [45,46]. When mitochondria are isolated from the cell, fragments are formed after network disintegration which then undergoes spontaneous resealing [47]. Even in isolation, these dynamic organelles [48] are competent for respiration and ATP synthesis [47].
ATP is an energy-rich compound [49] and a driver of fundamental cell functions such as protein folding and degradation, maintaining a tightly regulated interface with other subcellular compartments, building force required for cell division [50,51]. Mitochondria play a role in producing ATP via OXPHOS and are also called cell powerhouses [52]. Different parts of mitochondria include outer and inner membranes, inner membrane space, cristae, and matrix [19,39,51,53,54].
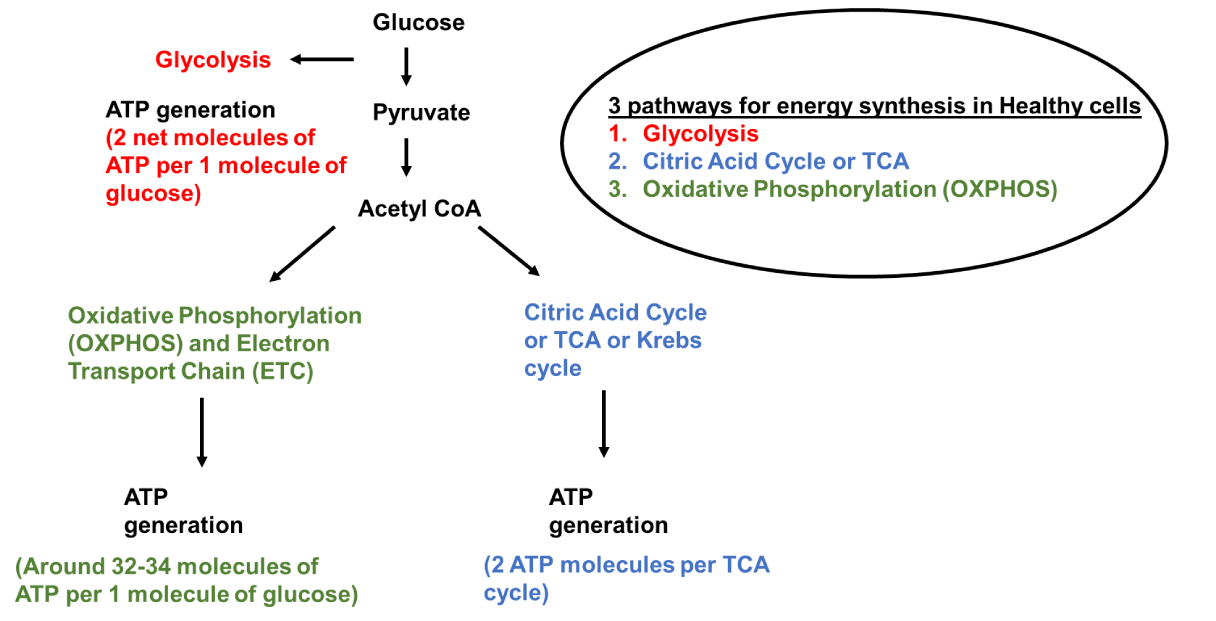
The outer membrane plays a role in defining the overall shape and forms to control molecule permeation along with the separation of mitochondria from cytoplasm [39]. It allows permeation to molecules with a weight less than 6000 Daltons [41] by forming large aqueous channels through lipid bilayer [55] along with the help of porin [55], a pore-forming membrane transport protein like voltage-dependent anion channel VDAC [40]. Inner membrane space empowers ATP synthesis by maintaining a pH gradient by receiving actively transported H+ ions from the mitochondrial matrix [50]. In aerobic cells, the Inner membrane is the site of ATP synthesis employing oxidative phosphorylation and generates around 32-34 ATP per glucose molecule [19,50]. In a healthy cell, 2 molecules of ATP per glucose molecule, are generated by glycolysis, and 2 ATP are generated by the Citric Acid cycle.
The presence of densely packed hydrophobic combinations like double phospholipid cardiolipin makes the inner membrane highly impermeable to most ions and large molecules [50,53]. Upon translation, products of mitochondrial ribosomes like hydrophobic membrane protein subunits are integrated into the inner membrane [56]. It has a high electronegative environment of potential −140 to −180 mV generated by protein complexes of the Electron Transport Chain [ETC] [50]. ETC is located in the inner membrane with proximity to the mitochondrial matrix [57]. It has five complexes: complex I: ubiquinone oxidoreductase, complex II: succinate dehydrogenase, complex III: ubiquinol–cytochrome c oxidoreductase [or cytochrome bc1 complex], complex IV: cytochrome c oxidase, and complex V: ATP synthase [52]. These complexes help in ATP synthesis via oxidative phosphorylation [52]. Electron transporters ubiquinone and cytochrome c also play a crucial role in the mammalian mitochondrial Electron Transport Chain [ETC] [57]. Various pathways involved in energy production in a healthy cell are shown in this paper (Figure 1).
The inner membrane divides into the inner boundary membrane and the cristae [51]. The inner boundary membrane contains carrier proteins that assist in transporting ions, ATP, and ADP between the cytoplasm and the matrix [58]. Cristae are cisternal invaginations [59] of inner membrane disk-like lamellar, tubular, or bag-like extensions of inner boundary membrane [44,59] which were first discovered by electron microscopy of plastic-embedded cells and tissues [31,60]. Several important processes like Mitochondrial energy conversion take place in cristae [51]. Dimerization or multimerization of ATP synthase complexes helps in the stabilization of these mitochondrial features [59]. The most prominent protein complex present in the cristae is mitochondrial F1-Fo ATP synthase [61]. ATP synthase helps in ATP synthesis by rotatory catalysis [61]. Within the cristae membrane, ATP synthase-assisted ATP generation takes place which is driven by a shallow proton gradient between the inter-membrane space [pH 7.2-7.4] and the matrix [pH 7.9-8] [51].
Cristae with numerous folds extend into the interior of mitochondria to form a Mitochondrial matrix [41]. It is surrounded by an inner membrane [54] and has a high protein density of up to 500 mg/ml [62,63]. Proteins have to pass through the outer membrane, intermembrane space, and inner membrane to get into the matrix [62,63]. DNA replication, transcription, protein biosynthesis, and numerous enzymatic reactions take place in this part of mitochondria [64]. The genetic code in animal mitochondria is slightly modified compared to the universal code [65]. Mammalian mitochondria have their genome with 16,000 base pairs of DNAs encoding 2 rRNAs, 22 tRNAs, and 13 polypeptides organized [65]. It contains a distinct set of ribosomes with 28S small subunits and 39S large subunits [66] as well as a small amount of endogenous DNA (mtDNA) [67]. Mitochondrial DNA [mtDNA] can be found in high copy numbers (103-104 copies per cell) in cells [55]. 13 essential subunits of the oxidative phosphorylation machinery are encoded by endogenous DNA, which undergoes transcription and translation [68]. rRNA and tRNA molecules are important parts of translational machinery [68]. In mammalian mitochondria, tRNAs can be differentiated from canonical tRNAs. They are generally shorter than other tRNAs. They don't have conserved or semi-conserved nucleotides. Conserved or semi-conserved nucleotides help in creating an L-shaped tertiary structure of eukaryotic cytoplasmic tRNAs. Characteristics of mitochondria in a healthy cell are explained in this paper (Figure 2).
Mitochondria in lung cancer
Cancer: Cancer is the leading cause of premature death in 57 countries [69]. In 2023, 1,958,310 new cases of cancer and 609,820 death cases due to cancer were projected by the American Cancer Society [70]. As per Global demographic characteristics, in the year 2025, a rise in new cancer cases can exceed more than 20 million [71] and may reach 24 million by 2035 [72]. In the United States, more than 7 million people were infected with COVID-19 by the end of September 2020 [73,74]. The Coronavirus disease 2019 [COVID-19] caused alarming states around the world [75]. As per studies, screenings for breast, colon, prostate, and lung cancers were decreased by 85%, 75%, 74%, and 56%, respectively, especially when COVID-19 was at its peak [76]. Hence, cancer patients experienced huge challenges in accessing care which led to an increase in case numbers [76]. Cancer is a death-causing disease and researchers are investing their tremendous efforts in the search for a cure with minimal adverse effects [77].
Lung cancer: Lung cancer is the leading cause of cancer incidences and mortality globally [78,79]. Based on the origin, lung cancer can be Small-Cell Lung (SCLC) and Non-Small-Cell Lung Cancers (NSCLC) [80]. Neuroendocrine cancers such as Small Cell Carcinoma (SCLC), Squamous Cell Carcinoma (SCC), adenocarcinoma, Large Cell Neuroendocrine Carcinoma (LCNEC), and carcinoid are some most common types of lung cancer [80]. The second most common cancer in males is lung cancer with an estimated 1,369,000 cases [81,82]. The risk of diagnosis in males is 3.8% whereas in females it is 1.77% [81,82]. North America, Northern and Western Europe have the highest incidences in females whereas eastern, central, and western Africa have the lowest cases in both males and females [83]. As per Torre, et al. [84] in the US, for lung cancer diagnosis in both males and females, the average age is 70 years old. Young non-smoker females of age 20-46 years have been diagnosed with NSCLC, more advanced adenocarcinoma [85]. In the US, it is considered as main cause of death in women over 59 years old and in men over 40 years old.
Both genetic and non-genetic factors are responsible for lung cancer [86–89]. Smoking has been stated as the principal cause of lung cancer [72]. It comprises more than 80% of lung cancer cases [81]. In the South African population, male marijuana smokers are found with a 2.4 times increased risk of lung cancer [90]. Exposure to carcinogens like asbestos accounts for 5-10% of global lung cancer cases [91]. Exposure to natural gas like radon or metals like uranium can increase the risk of squamous cell carcinoma of the lungs [92–94]. As per IACR, people working with arsenic are subjected to cancer of the lungs [90,95]. Chronic Obstructive Pulmonary Disease (COPD) [96], tuberculosis [97], HIV [98–100], inflammation and cellular damage [97], and air pollution [101] are causative agents of lung cancer.
According to Genome-Wide Association Studies (GWAS), variants in different chromosomal regions can result in a higher risk of lung cancer [102]. For example: 15q25–26 loci,5p15 locus, and 6p21 locus. 15q25–26 loci can increase nicotine dependence [88], the 5p15 locus includes a gene for the Telomerase Reverse Transcriptase (TERT) [102], and the 6p21 locus regulates G-protein signaling and chances of lung cancer [103]. To get properly treated, prognosis is very important as it is strongly associated with the stage of Lung cancer [104]. According to the U.S. Preventive Services Task Force, low-dose computed tomography [CT] screening is suggested for patients with high risk of Lung cancer [105]. Various new molecules are under laboratory testing with a solo focus on targeting mitochondrial metabolic pathways to combat cancer. Taking current lung cancer statistics into consideration, there is a huge necessity to bring these results to the clinical level. Based on our literature search, there are very few clinical studies targeting mitochondrial metabolism in lung cancer (Table 1).
| Table 1: Clinical studies targeting mitochondria in lung cancer. | |||||
| Treatment | Cancer Type | Stage | Phase | Study Type | ClinicalTrials.gov ID |
| Targeting Mitochondrial Metabolism with Papaverine in Combination With Chemoradiation | Non-Small Cell Lung Cancer | Stage 1 1-11 1 | Phase 1 | Interventional | NCT05136846 |
| Targeting Mitochondrial System using Radiofrequency Ablation | Non-small Cell Lung Cancer | Early stage | Not applicable | Interventional | NCT03840408 |
| Dose Escalation Study of ME-344 in Patients With Refractory Solid Tumors | Non-small Cell Lung Cancer | Locally advanced or metastatic cancer | Phase 1 | Interventional | NCT01544322 |
Dysfunctional mitochondria in lung cancer
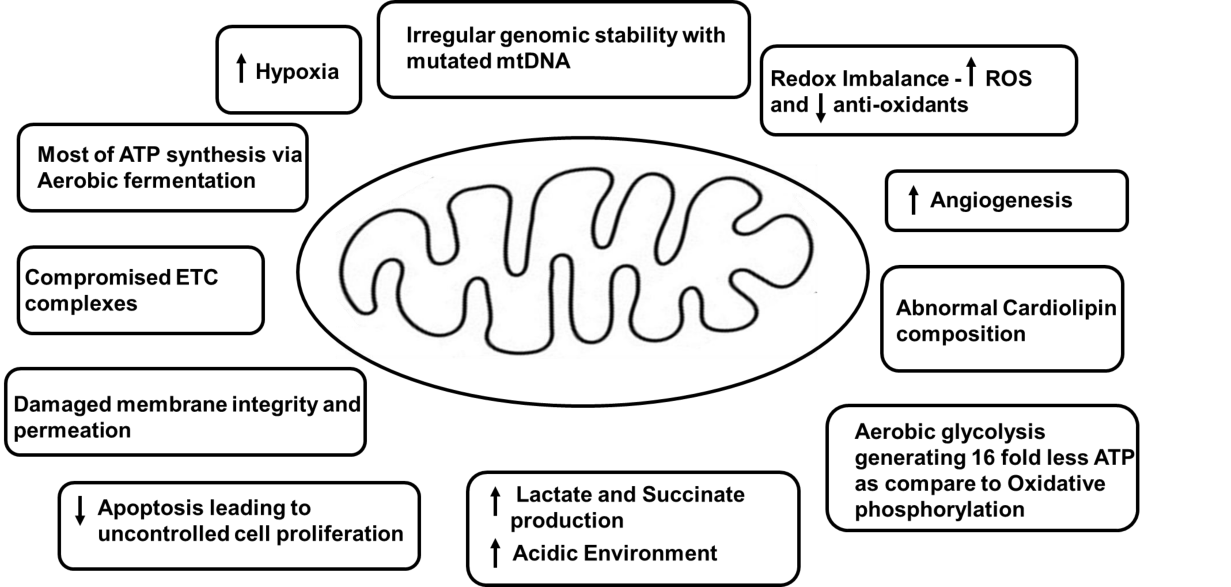
As mentioned in previous sections, mitochondria play a huge role in ATP production at the cellular level in all organs like lungs. According to various studies, whether the energy level is too high or too low, both are associated with detrimental effects on cell viability [106]. In a cell, a low level of ATP can induce necrosis or apoptosis and a high level can disturb membrane pumping with survival inhibition [106]. Irregular changes in ETC, mtDNA, and proton motive gradient of the inner membrane can result in mitochondrial impairment [106,107]. ATP production is affected by mitochondrial dysfunction. Mitochondria in various cancers for instance, lung cancer are structurally and functionally irregular and are not capable of producing normal levels of cellular energy [108–117]. Uncoordinated mitochondrial function can lead to dysregulation in oncogenes and tumor suppressor genes, for example, regulation and expression of p53 [118–120]. Malfunctional mitochondria can amplify cytoplasmic calcium levels, irregular iron-sulfur complexes, and reactive oxygen species and can lead to genome mutability [121-127]. p53 is a tumor suppressor [128]. Its suppression or its disability can downregulate hypoxia-induced apoptosis, ETC dysfunction, and dysregulated oncogenic signaling pathways [129], thus, supporting cancer cell proliferation and survival [129-132]. Any alteration in mitochondrial energy homeostasis, uncontrolled rise in ROS level, radiation, mutagens, and carcinogens can lead to cardiolipin abnormalities [113]. An increase in ROS levels is related to abnormal genome stability, disturbed tumor suppressor gene function, and uncontrolled excess cell proliferation [121,133]. Cardiolipin works closely with proteins of ETC and is the only lipid produced in mitochondria. Abnormal cardiolipin composition and quantity can alter OXPHOS by hindering the uptake of ADP [134-138]. Cancer cells have abnormal cardiolipin content leading to dysregulated ETC [114]. OXPHOS has been shown to have a critical effect on the energy metabolism of lung cancer cells [139,140]. Some mitochondrial characteristics in a cancer cell are shown in this paper (Figure 3).
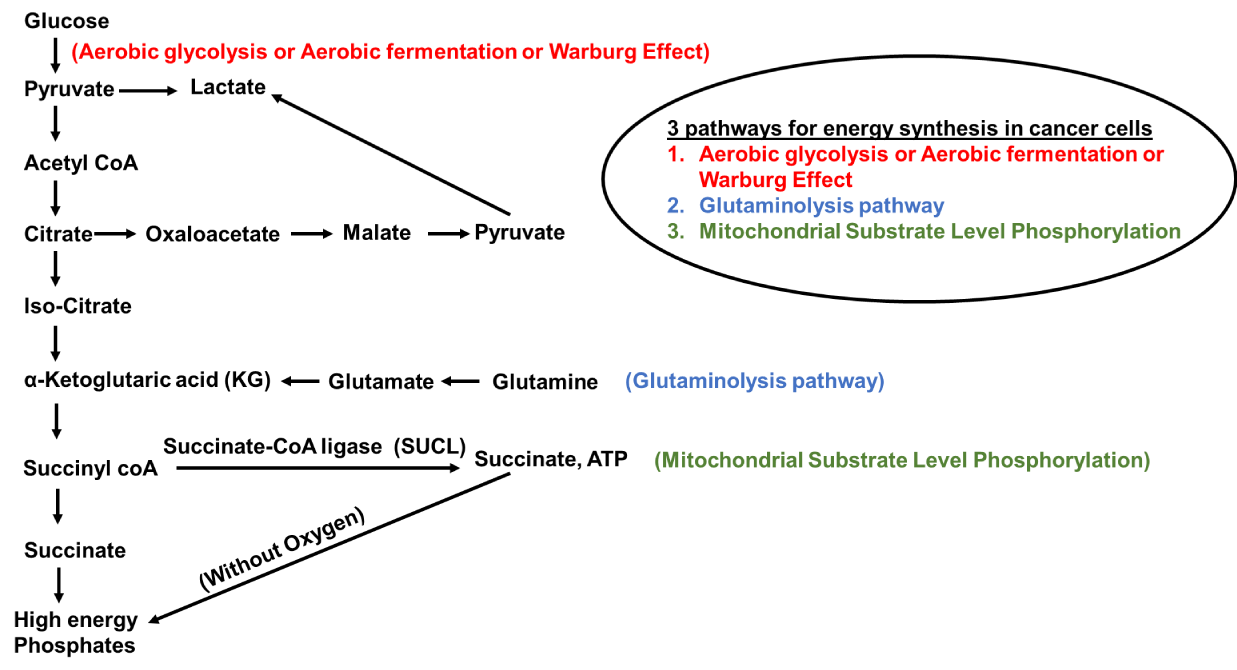
In the 1920s, Otto Warburg observed, that cancer cells produced ATP by undergoing a high level of glycolysis rather than mitochondria-mediated oxidative phosphorylation, and this condition was termed as "Warburg effect." [141]. Even oxygen-rich conditions can't prevent it and hence, this phenomenon was called "aerobic glycolysis" [11,12]. In many types of cancer including lung cancer, an increase in glucose uptake to produce ATP leads to enhanced synthesis of DNA, protein, nucleic acids, and amino acids, an increase in mtDNA mutations resulting in mitochondrial dysfunction and related somatic mutations [142–145]. Aerobic glycolysis or aerobic fermentation or Warburg effect, along with the Glutaminolysis pathway and mitochondrial substrate-level phosphorylation provide supplements for the rapid proliferation of cancer cells as they contribute to the production of biomass precursors, such as nucleotides, amino acids, and lipids [146]. Different pathways involved in energy production in a cancer cell are clearly explained in this paper (Figure 4).
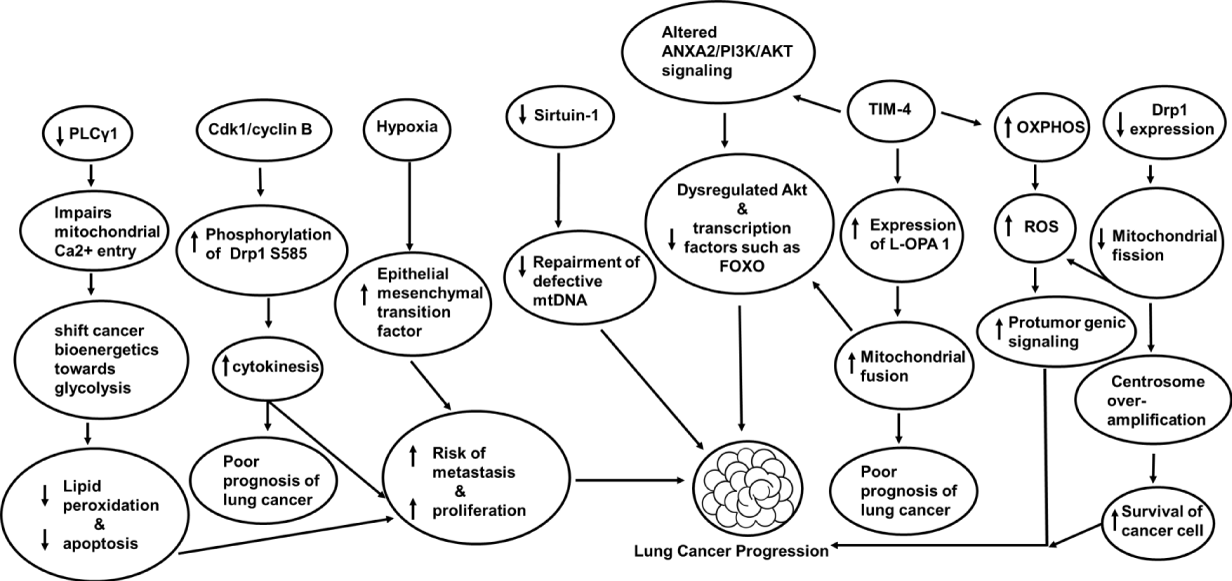
Dysfunctional Mitochondria produce ATP through aerobic glycolysis and produce 16-fold less ATP than OXPHOS [147]. The disbalance of OXPHOS and aerobic glycolysis leads to cytoplasmic accumulation of mitochondrial metabolites with a side effect known as hypoxia [148]. Hypoxia has a proliferative effect on lung cancer because it induces the enlargement of mitochondria and apoptotic resistance [149]. Hypoxia-Inducible Transcription Factor (HIF-1α) helps cells adapt to stress conditions by synthesizing 70 hypoxia-related factors [150]. HIF-1α has been shown to regulate glycolytic conversion leading to low ATP yields in cancer [116,151-153]. Various targets associated with mitochondrial dysfunction in a lung cancer cell are clearly illustrated (Figure 5).
In the study conducted by Yoon, et al. [154], the regulation of c-Jun N-Terminal Kinase (JNK) and p38 Mitogen-Activated Protein Kinase (MAPK) is important in combating lung cancer. They used a Militarin analog-1 to study apoptotic mechanisms against human lung cancer cell lines like A549 lung cancer cells and Bronchial Epithelial Cell Line (BEAS-2B). They found inhibition of ROS generation and JNK/p38 MAPK with enhanced DNA fragmentation, nuclear condensation, mitochondrial membrane permeabilization, cytochrome c release, activation of caspase-9/-3, cleavage of poly (ADP ribose) polymerase, leading to apoptosis in Lung cancer cell lines [154]. Mitochondrial dysfunction in patients with lung-centered disorders like COPD is prone to metastatic lung cancer development [155]. mtDNA repairing mechanism in such patients is defective because of low levels of sirtuin-1 [156]. A hypoxic environment further enhances the potential risk of metastasis due to boosted epithelial-mesenchymal transition factors [96]. Factors like activation of NLRP3 inflammasome [157] and chronic tissue inflammation can damage mitochondria, promoting the growth of lung cancer [158,159]. Cellular mitochondria of the trachea, bronchi, and bronchioles have been implicated in lung cancer [160]. In the study conducted by Kao, et al. [161] Dihydromyricetin, a medicinal flavonoid obtained from Ampelopsis grossedentata has a selective cytotoxic effect against NSCLC cells. It acts by activating caspase-9/-7/-3, increasing intracellular peroxide, inducing poly [ADP-ribose] polymerase (PARP) cleavage in A549 and H1975 cells, activating extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK)1/2. Thus, it induces mitochondrion-derived apoptosis in human NSCLC cells. According to this study, a combination of Dihydromyricetin along with ERK and JNK inhibitors can prove beneficial in preventing NSCLC proliferation [161].
Barrett AG, et al. [162], have shown that targeting mitochondria can be an effective strategy to manage cancer. They used a pentacyclic alkaloidal compound called papuamine which is derived from marine sources like Haliclona sp to target mitochondria in NSCLC. Not only was mitochondrial membrane potential lost but also intracellular ATP production was inhibited. It induced apoptosis and inhibited NSCLC cell viability [163]. TIM-4 is a type I membrane protein and stands for T-cell immunoglobulin and mucin domain-containing molecule [140,164]. It plays an important role in clearing apoptotic cells and prevents autoimmunity. It is highly expressed in human and mouse macrophages and dendritic cells [165,166]. TIM-4 is found to be highly expressed in lung cancer [166]. According to the study conducted by Wang, et al. [167], TIM-4 promotes OXPHOS level and mitochondrial function of lung cancer cells. It upregulates the expression of protein L-OPA1[The unprocessed, N-terminal transmembrane anchored and is the long form of OPA1], and thus regulates mitochondrial morphology and kinetic balance of lung cancer cells. It is involved in the ANXA2/PI3K/AKT signaling pathway [167]. Dysregulation in Akt (acute transforming retrovirus thymoma protein kinase) [168] and inhibition of transcription factors such as forkhead box subfamily O (FOXO) [128] is associated with lung cancer progression [169]. In the study conducted by Saliakoura, et al. [170], PLCγ1 plays a crucial role in lung cancer. On suppression, it impairs Ca2+ entry into the mitochondria and switches cancer bioenergetics towards glycolysis which prevents lipid peroxidation and increases cancer cell proliferation by opposing apoptosis. They found in KRAS-mutant human lung adenocarcinoma cancer cell lines PLCγ1 is poorly expressed during hypoxia with lower patient survival.
When two mitochondria join together, it is termed mitochondrial fusion and when separated into two, is known as mitochondrial fission [171]. Mitofusins (MFN1 and MFN2) and Optic Atrophy 1 (OPA1) are fusion proteins and DRP1, FIS1, MFF, MiD49, and MiD51 are fission proteins [171]. Accumulation of fission proteins on the mitochondrial membrane mediates apoptosis [172]. Silencing Mitofusins 1 and 2 can promote apoptotic cell death [173]. In a study focusing on lung cancer [NSCLC], the expression of a spliced isoform of Mfn1 is found to be upregulated at the mRNA level and is associated with suppressed apoptosis in NSCLC cells [174]. On initiation of apoptosis, Drp1 is recruited to the outer mitochondrial membrane from the cytoplasm [175-178]. Downregulation of Drp1 is shown to lower cytochrome c release, mitochondrial fragmentation, caspase activation, and apoptosis [175,179-180]. On initiation of apoptosis, Drp1 is recruited to the outer mitochondrial membrane from the cytoplasm [175-178]. Prohibitins are inner mitochondrial membrane proteins that play a role in regulating cell cycle and apoptosis [181].
Opa1 works in association with prohibitins (PHB1/2) to regulate cell proliferation [181]. Loss of Opa1 can lead to spontaneous apoptosis [182]. Higher expression of Opa1 has been shown in lung adenocarcinoma cells and is associated with poor prognosis [183]. Based on studies, reduced Drp1 protein expression has been noted in adenocarcinoma alveolar epithelial cells, an NSCLC model, promoting mitochondrial phenotypes elongation, with limited fission and apoptosis [184]. Inhibition of mitochondrial fission can result in mitochondrial dysfunction, loss of mtDNA, elevated ROS, depleted ATP levels [185], cell cycle modification, centrosome overamplification, chromosomal instability, and increased survival of cancer cells [186]. Based on these studies, targeting mitochondrial fission machinery can produce positive outcomes in lung cancer management [55]. Cyclin-dependent kinase 1 [Cdk1]/cyclin B plays an important role in cytokinesis as it allows equal distribution of mitochondria in daughter cells by phosphorylating Drp1 S585 [187], [188]. According to studies cyclin B1 is overly expressed non-small cell lung cancer and is associated with poor prognosis [188]. Inhibiting cyclin-dependent kinases could be used as a therapeutic target in controlling metastatic lung cancer [189,190].
ROS is mentioned in the majority of studies included in this review. Without ROS, studying mitochondrial metabolism in lung cancer can be considered Incomplete. Why? It's because Mitochondria and ROS are very closely associated. Around 90% of intracellular ROS are produced in mitochondria during OXPHOS [191]. The production of ROS can promote tumor growth and progression [192]. In comparison to normal cells, cancer cells generate ROS in larger quantities [193]. ROS can lead to mutations in mtDNA, lower the capacity for DNA "proofreading" [194], increased leakage of electrons leading to dysfunctional ETC, oncogenic transformation, cancer progression, genetic mutations in the mitochondrial genome, and alteration in transport chain signaling pathways [195]. ROS can itself act as a signal to promote protumor genic signaling, facilitate proliferation, adaptation to hypoxia, and survival of cancer cells [196]. That's why ROS are known for their dual role in promoting cancer progression [121]. Mitochondrial complexes I, II, and III subunits generate ROS such as toxic superoxide anion radicals [O2-•], and Hydrogen Peroxide (H2O2). Lack of anti-oxidant action like depleted GSH levels, may create an imbalance between production and neutralization leading to oxidative stress in a cell [191]. Mutation of complex I alters its interaction with complex III generating an incorrect number of electrons resulting in ROS overproduction. That's why, the complex I subunit is of significant target for inhibiting tumor progression and metastasis [197]. As per published scientific literature, complex I appear to be altered frequently in lung cancer, especially in the non-smoker population [198]. Song, et al. [199] have shown that targeting ROS downstream effectors such as JNK and p38MAPK and ROS/MAPK signaling pathway might induce cytotoxicity in cancer cells.
Primary processes such as mitochondrial biogenesis, mitochondrial dynamics, mitophagy, mitochondrial regulation of mtDNA, and mitochondrial unfolded protein response, are involved in the maintenance of mitochondrial quality control [200]. We already discussed mitochondrial dynamics above and here we are elaborating another crucial companion required to maintain mitochondrial quality control. key regulators of mitochondrial biogenesis such as Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 alpha (PGC-1α) are upregulated in lung cancer. PGC-1α promotes metastasis by enhancing oxidative phosphorylation, mitochondrial biogenesis, and oxygen consumption [201]. Deacetylation of PGC-1α by Sirtuin-1 can affect mitochondrial function and biogenesis and is involved in hypoxia-induced chemoresistance in non-small cell lung cancer [202]. Oncogenic pathways like MAPK/ERK and PI3K/AKT/mTOR are upregulated in lung cancer and associated with the regulation of mitochondrial function and biogenesis [203]. Mitophagy is a type of macro autophagy that is essential for removing damaged, excess, or old mitochondria [204]. It has many roles in cancer progression and carcinogenesis. It can be PINK1/PRKN-dependent and PTEN-induced putative kinase 1 (PINK1)/ parkin RBR E3 ubiquitin-protein ligase (PRKN)-independent [205]. Mitophagy targets damaged or dysfunctional mitochondria for degradation to maintain cellular integrity and health. It helps in maintaining the quality and functionality of mitochondria [206]. Mitophagy can act as a tumor promoter or suppressor [207]. Mitophagy mediated by Caveolin-1 (Cav-1)/Parkin can contribute to the resistance of non-small cell lung cancer [208]. In the adaptation to chemotherapy drug treatment, mitophagy acts as a cytoprotectant [209]. Downregulation of Rho-associated coiled-coil-containing protein kinase 1 (ROCK1) can make cav-1-knockdown A549 cells more sensitive toward drugs such as cisplatin [210]. Overexpression of Apurinic Endonuclease 1 (APE1) has been reported to induce Parkin-mediated mitophagy in A549 cells. knockdown of APE1 can promote cell apoptosis and restore the sensitivity of cancer cells toward drugs like cisplatin [209]. Mitochondrial transcription factor A (TFAM], a 25-kDa nDNA-encoded protein [211] is involved in mitochondrial biogenesis [211]. It can translocate into mitochondria and bind to mtDNA, thus important for the maintenance of mtDNA [212]. Mitochondrial quality control proteins such as the dimeric form of DRP1, Sirtuin 3, and BCL2/adenovirus e1B 19 kDa protein-interacting protein 3(BNIP3) determine the integrity level of mitochondria. These could be used to separate patients with lung cancer into high- and low-risk groups [213]
Mitochondria are autonomous [214] and may attain various shapes based on their cellular states such as tubular under normal conditions, round during apoptosis, swollen and distended during necrosis, elongated during autophagy, donut-shaped mediated by mitochondrial calcium [215]. Leucine zipper EF-hand-containing transmembrane protein-1 (LETM1) is an anchoring protein that is involved in mitochondrial morphology, cell viability, and ion homeostasis. Overexpression of LETM1 and mitochondrial fragmentation has been shown in lung cancer cells [216]. Sideroflexin1 (SFXN1) is a potential prognostic biomarker for lung adenocarcinoma. Its high expression is correlated with advanced clinical stage, larger tumor size, expression of multiple immunomodulators, immune cell infiltration [natural killer cells, dendritic cells, activated CD8 + T cells, macrophages], and positive lymph node metastasis [217]. In lung adenocarcinoma, transmembrane protein 70, flavoprotein 2, and NADH dehydrogenase [ubiquinone] act as protective factors. NAD-dependent protein deacetylase sirtuin-5 serves as a protective factor in lung squamous cell carcinoma. NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8 acts as a protective factor in small-cell lung cancer [218]. MitomiRs are regulators of various mitochondrial processes and are an important contributor to the pathogenesis and progression of lung cancer. Expression of mitochondrial proteins along with controlling the functional activity of mitochondria is regulated by a class of microRNA called a MitomiR. MicroRNAs are non-coding RNA molecules. They regulate various mitochondrial activity and mitochondrial genes involved in carcinogenesis processes. These are exported into the cytosol after being encoded in the nuclear genome. These can be derived from mitochondrial DNA and can be located in mitochondria [219]. Levofloxacin has been reported to inhibit mitochondrial respiration and reduce ATP production by inhibiting activities of mitochondrial electron transport chain complexes I and III. It can inhibit proliferation and induce apoptosis in various lung cancer cell lines [220].
Conclusion
Mitochondrial metabolism is closely related to the proliferation and metastasis of lung cancer. Factors like excessive ROS production, dysfunctional mitochondrial metabolism, disbalance in the anti-oxidant system, and alteration in mitochondrial DNA play a crucial role in the progression of lung cancer. Thus, we found different cross-connections between malfunctional mitochondria and the growth of lung cancer. Emerging research facts state cancer is a metabolic disease. To conclude, a research renaissance is required to switch our focus from understanding cancer as a genetic disease to cancer as a mitochondrial metabolic disease with mitochondria as a potential target in the inhibition of lung cancer.
Acknowledgment
Not applicable
Authors contribution
Conceptualization, draft preparation, writing, review, editing, figure and table design is done by Sharma B. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Information for this review was collected from different sources like PubMed, Google Scholar, Scopus, Web of Science, and, clinical trials. gov website.
Institutional review board statement
Not applicable
Informed consent statement
Not applicable
Competing interest
The authors have no competing interest to declare
Funding source
There is no grant support or financial relationship for this manuscript.
References
- Schon EA. Power, sex, suicide Mitochondria and the meaning of life. J Clin Invest. 2006 Jul 3;116(7):1742. doi: 10.1172/JCI29253. PMCID: PMC1483179.
- Nick Lane. Power, Sex, Suicide: Mitochondria and the meaning of life Oxford Landmark Science. 2nd ed. Oxford University Press; 2018. p.1-512.
- Atlante A, Valenti D. Mitochondria Have Made a Long Evolutionary Path from Ancient Bacteria Immigrants within Eukaryotic Cells to Essential Cellular Hosts and Key Players in Human Health and Disease. Curr Issues Mol Biol. 2023 May 19;45(5):4451-4479. doi: 10.3390/cimb45050283. PMID: 37232752; PMCID: PMC10217700.
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012 Sep;13(9):566-78. doi: 10.1038/nrm3412. Epub 2012 Aug 1. PMID: 22850819.
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012 Oct 26;48(2):158-67. doi: 10.1016/j.molcel.2012.09.025. PMID: 23102266; PMCID: PMC3484374.
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010 Sep;11(9):621-32. doi: 10.1038/nrm2952. Epub 2010 Aug 4. PMID: 20683470.
- Seyfried TN, Flores RE, Poff AM, D'Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014 Mar;35(3):515-27. doi: 10.1093/carcin/bgt480. Epub 2013 Dec 16. PMID: 24343361; PMCID: PMC3941741.
- Seyfried TN, Chinopoulos C. Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites. 2021 Aug 25;11(9):572. doi: 10.3390/metabo11090572. PMID: 34564387; PMCID: PMC8467939.
- Puente XS, Velasco G, Gutiérrez-Fernández A, Bertranpetit J, King MC, López-Otín C. Comparative analysis of cancer genes in the human and chimpanzee genomes. BMC Genomics. 2006 Jan 26;7:15. doi: 10.1186/1471-2164-7-15. PMID: 16438707; PMCID: PMC1382208.
- Varki NM, Varki A. On the apparent rarity of epithelial cancers in captive chimpanzees. Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140225. doi: 10.1098/rstb.2014.0225. PMID: 26056369; PMCID: PMC4581030.
- Warburg O. The Metabolism of tumors. In: Smith RR, editor. New York; 1931.
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309-14. doi: 10.1126/science.123.3191.309. PMID: 13298683.
- WEINHOUSE S. On respiratory impairment in cancer cells. Science. 1956 Aug 10;124(3215):267-9. doi: 10.1126/science.124.3215.267. PMID: 13351638.
- Burk D, Woods M, Hunter J. On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst. 1967 Jun;38(6):839-63. PMID: 4381692.
- Seyfried TN. Perspectives on brain tumor formation involving macrophages, glia, and neural stem cells. Perspect Biol Med. 2001 Spring;44(2):263-82. doi: 10.1353/pbm.2001.0035. PMID: 11370160.
- Evangeliou AE, Spilioti MG, Vassilakou D, Goutsaridou F, Seyfried TN. Restricted Ketogenic Diet Therapy for Primary Lung Cancer With Metastasis to the Brain: A Case Report. Cureus. 2022 Aug 2;14(8):e27603. doi: 10.7759/cureus.27603. PMID: 36059366; PMCID: PMC9435310.
- Gray MW, Lang BF, Cedergren R, Golding GB, Lemieux C, Sankoff D, Turmel M, Brossard N, Delage E, Littlejohn TG, Plante I, Rioux P, Saint-Louis D, Zhu Y, Burger G. Genome structure and gene content in protist mitochondrial DNAs. Nucleic Acids Res. 1998 Feb 15;26(4):865-78. doi: 10.1093/nar/26.4.865. PMID: 9461442; PMCID: PMC147373.
- Gabaldón T, Huynen MA. Shaping the mitochondrial proteome. Biochim Biophys Acta. 2004 Dec 6;1659(2-3):212-20. doi: 10.1016/j.bbabio.2004.07.011. PMID: 15576054.
- Christian BE, Spremulli LL. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):1035-54. doi: 10.1016/j.bbagrm.2011.11.009. Epub 2011 Dec 7. PMID: 22172991; PMCID: PMC3314146.
- van der Bliek AM, Sedensky MM, Morgan PG. Cell Biology of the Mitochondrion. Genetics. 2017 Nov;207(3):843-871. doi: 10.1534/genetics.117.300262. Erratum in: Genetics. 2018 Apr;208(4):1673. PMID: 29097398; PMCID: PMC5676242.
- Amunts A, Brown A, Bai XC, Llácer JL, Hussain T, Emsley P, Long F, Murshudov G, Scheres SHW, Ramakrishnan V. Structure of the yeast mitochondrial large ribosomal subunit. Science. 2014 Mar 28;343(6178):1485-1489. doi: 10.1126/science.1249410. PMID: 24675956; PMCID: PMC4046073.
- Kühne W. About the termination of the nerves in the nerve hills of the muscles. Archives for pathological anatomy and physiology and for clinical medicine. 1864;(1–2):187-220. doi: 10.1007/BF02280895.
- Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981 Dec;91(3 Pt 2):227s-255s. doi: 10.1083/jcb.91.3.227s. PMID: 7033239; PMCID: PMC2112799.
- Schon EA. Power, sex, suicide Mitochondria and the meaning of life. J Clin Invest. 2006 Jul 3;116(7):1742. doi: 10.1172/JCI29253. PMCID: PMC1483179.
- Benda C. Mitochondria. Arch F Anat U Physiol Abt. 1898;73:397.
- Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett. 1980 Aug 25;117 Suppl:K1-10. doi: 10.4159/harvard.9780674366701.c143. PMID: 6998725.
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979 Dec 7;206(4423):1148-59. doi: 10.1126/science.388618. PMID: 388618.
- 2About the pyrophosphate fraction in muscle. The Natural Sciences. 1929;17.
- Pagliarini DJ, Rutter J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 2013 Dec 15;27(24):2615-27. doi: 10.1101/gad.229724.113. PMID: 24352419; PMCID: PMC3877752.
- Claude A. FRACTIONATION OF MAMMALIAN LIVER CELLS BY DIFFERENTIAL CENTRIFUGATION : I. PROBLEMS, METHODS, AND PREPARATION OF EXTRACT. J Exp Med. 1946 Jun 30;84(1):51-9. PMID: 19871553; PMCID: PMC2135638.
- PALADE GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953 Jul;1(4):188-211. doi: 10.1177/1.4.188. PMID: 13069686.
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457-65. doi: 10.1038/290457a0. PMID: 7219534.
- Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells. 2019 Jul 16;8(7):728. doi: 10.3390/cells8070728. PMID: 31315173; PMCID: PMC6678812.
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006 Jul 25;16(14):R551-60. doi: 10.1016/j.cub.2006.06.054. PMID: 16860735.
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012 Mar 16;148(6):1145-59. doi: 10.1016/j.cell.2012.02.035. PMID: 22424226; PMCID: PMC5381524.
- Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011 Mar;41(3):661-8. doi: 10.1016/j.nbd.2010.11.017. Epub 2010 Dec 8. PMID: 21145397; PMCID: PMC3031677.
- Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012 Nov;18(11):1639-42. doi: 10.1038/nm.2919. Epub 2012 Oct 28. PMID: 23104132.
- Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003 Oct;2(10):812-22. doi: 10.1038/nrd1201. PMID: 14526384.
- Vogel F, Bornhövd C, Neupert W, Reichert AS. Dynamic subcompartmentalization of the mitochondrial inner membrane. J Cell Biol. 2006 Oct 23;175(2):237-47. doi: 10.1083/jcb.200605138. Epub 2006 Oct 16. PMID: 17043137; PMCID: PMC2064565.
- Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15370-5. doi: 10.1073/pnas.0808115105. Epub 2008 Oct 1. PMID: 18832158; PMCID: PMC2557026.
- Cooper GM, Hausman RE. The cell: a molecular approach. Sinauer Associates. Sunderland, MA. 2000.
- Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004 Sep 17;305(5691):1747-52. doi: 10.1126/science.1100612. Epub 2004 Aug 5. PMID: 15297626.
- Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 2010 Sep;11(9):655-67. doi: 10.1038/nrm2959. PMID: 20729931.
- Perkins GA, Frey TG. Recent structural insight into mitochondria gained by microscopy. Micron. 2000 Jan;31(1):97-111. doi: 10.1016/s0968-4328(99)00065-7. PMID: 10568232.
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007 Nov;8(11):870-9. doi: 10.1038/nrm2275. PMID: 17928812.
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751-80. doi: 10.1146/annurev.biochem.76.071905.090048. PMID: 17362197.
- Alexandre A, Reynafarje B, Lehninger AL. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296-300. doi: 10.1073/pnas.75.11.5296. PMID: 31621; PMCID: PMC392949.
- Lackner LL. Shaping the dynamic mitochondrial network. BMC Biol. 2014 May 27;12:35. doi: 10.1186/1741-7007-12-35. PMID: 24884775; PMCID: PMC4035697.
- Dunn J, Grider MH. Physiology, Adenosine Triphosphate. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2024.
- Smith RA, Hartley RC, Cochemé HM, Murphy MP. Mitochondrial pharmacology. Trends Pharmacol Sci. 2012 Jun;33(6):341-52. doi: 10.1016/j.tips.2012.03.010. Epub 2012 Apr 18. PMID: 22521106.
- Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015 Oct 29;13:89. doi: 10.1186/s12915-015-0201-x. PMID: 26515107; PMCID: PMC4625866.
- Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020 Oct;37:101674. doi: 10.1016/j.redox.2020.101674. Epub 2020 Aug 6. PMID: 32811789; PMCID: PMC7767752.
- Alberts B, Johnson A LJ. Molecular biology of the cell. 4th ed. New York: Garland Science. 2002.
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6803-8. doi: 10.1073/pnas.95.12.6803. PMID: 9618493; PMCID: PMC22642.
- Roberts ER, Thomas KJ. The role of mitochondria in the development and progression of lung cancer. Comput Struct Biotechnol J. 2013 Dec 7;6:e201303019. doi: 10.5936/csbj.201303019. PMID: 24688727; PMCID: PMC3962144.
- Pfeffer S, Woellhaf MW, Herrmann JM, Förster F. Organization of the mitochondrial translation machinery studied in situ by cryoelectron tomography. Nat Commun. 2015 Jan 22;6:6019. doi: 10.1038/ncomms7019. PMID: 25609543.
- Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019 Jul;44(1):3-15. doi: 10.3892/ijmm.2019.4188. Epub 2019 May 8. PMID: 31115493; PMCID: PMC6559295.
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003 Nov 6;426(6962):39-44. doi: 10.1038/nature02056. PMID: 14603310.
- Davies KM, Anselmi C, Wittig I, Faraldo-Gómez JD, Kühlbrandt W. Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13602-7. doi: 10.1073/pnas.1204593109. Epub 2012 Aug 3. PMID: 22864911; PMCID: PMC3427116.
- SJOSTRAND FS. Electron microscopy of mitochondria and cytoplasmic double membranes. Nature. 1953 Jan 3;171(4340):30-2. doi: 10.1038/171030a0. PMID: 13025467.
- von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649-72. doi: 10.1146/annurev.biochem.78.081307.104803. PMID: 19489730.
- Schleyer M, Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339-50. doi: 10.1016/0092-8674(85)90039-x. PMID: 2866845.
- Perkins G, Renken C, Martone ME, Young SJ, Ellisman M, Frey T. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J Struct Biol. 1997 Aug;119(3):260-72. doi: 10.1006/jsbi.1997.3885. PMID: 9245766.
- Kukat C, Wurm CA, Spåhr H, Falkenberg M, Larsson NG, Jakobs S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13534-9. doi: 10.1073/pnas.1109263108. Epub 2011 Aug 1. PMID: 21808029; PMCID: PMC3158146.
- Osawa S, Jukes TH, Watanabe K, Muto A. Recent evidence for evolution of the genetic code. Microbiol Rev. 1992 Mar;56(1):229-64. doi: 10.1128/mr.56.1.229-264.1992. PMID: 1579111; PMCID: PMC372862.
- O'Brien TW. The general occurrence of 55 S ribosomes in mammalian liver mitochondria. J Biol Chem. 1971 May 25;246(10):3409-17. PMID: 4930061.
- Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012 Oct;13(10):659-71. doi: 10.1038/nrm3439. Epub 2012 Sep 20. Erratum in: Nat Rev Mol Cell Biol. 2012 Nov;13(11):726. PMID: 22992591.
- Wisnovsky S, Lei EK, Jean SR, Kelley SO. Mitochondrial Chemical Biology: New Probes Elucidate the Secrets of the Powerhouse of the Cell. Cell Chem Biol. 2016 Aug 18;23(8):917-27. doi: 10.1016/j.chembiol.2016.06.012. Epub 2016 Jul 28. PMID: 27478157.
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021 Aug 15;127(16):3029-3030. doi: 10.1002/cncr.33587. Epub 2021 Jun 4. PMID: 34086348.
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023 Jan;73(1):17-48. doi: 10.3322/caac.21763. PMID: 36633525.
- Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current Challenges in Cancer Treatment. Clin Ther. 2016 Jul;38(7):1551-66. doi: 10.1016/j.clinthera.2016.03.026. Epub 2016 May 2. PMID: 27158009.
- Theodoratou E, Timofeeva M, Li X, Meng X, Ioannidis JPA. Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer. Annu Rev Nutr. 2017 Aug 21;37:293-320. doi: 10.1146/annurev-nutr-071715-051004. PMID: 28826375; PMCID: PMC6143166.
- Garcia M, Lipskiy N, Tyson J, Watkins R, Esser ES, Kinley T. Centers for Disease Control and Prevention 2019 novel coronavirus disease (COVID-19) information management: addressing national health-care and public health needs for standardized data definitions and codified vocabulary for data exchange. J Am Med Inform Assoc. 2020 Jul 1;27(9):1476-1487. doi: 10.1093/jamia/ocaa141. PMID: 32940705; PMCID: PMC7543614.
- Coronavirus disease 2019 (COVID-19) hospital preparedness assessment tool. In: Coronavirus and US Public Health Preparedness. 2020.
- Adam S, Zahra SA, Chor CYT, Khare Y, Harky A. COVID-19 pandemic and its impact on service provision: A cardiology prospect. Acta Cardiol. 2021 Oct;76(8):830-837. doi: 10.1080/00015385.2020.1787636. Epub 2020 Jul 10. PMID: 32646309.
- Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, Markward N, Sullivan M, Peng J, Zhou A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform. 2020 Nov;4:1059-1071. doi: 10.1200/CCI.20.00134. PMID: 33253013; PMCID: PMC7713534.
- Pucci C, Martinelli C, Ciofani G. Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience. 2019;13:961. doi: 10.3332/ecancer.2019.961. PMID: 31537986; PMCID: PMC6753017.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359-86. doi: 10.1002/ijc.29210. Epub 2014 Oct 9. PMID: 25220842.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019 Dec 1;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996. Erratum in: JAMA Oncol. 2020 Mar 1;6(3):444. Erratum in: JAMA Oncol. 2020 May 1;6(5):789. Erratum in: JAMA Oncol. 2021 Mar 1;7(3):466. PMID: 31560378; PMCID: PMC6777271.
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015 Sep;10(9):1243-1260. doi: 10.1097/JTO.0000000000000630. PMID: 26291008.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394-424. doi: 10.3322/caac.21492. Epub 2018 Sep 12. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. PMID: 30207593.
- Lyon F. International agency for research on cancer. Global cancer observatory: Cancer today.2020.
- Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn). 2021;25(1):45-52. doi: 10.5114/wo.2021.103829. Epub 2021 Feb 23. PMID: 33911981; PMCID: PMC8063897.
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1-19. doi: 10.1007/978-3-319-24223-1_1. PMID: 26667336.
- Arnold BN, Thomas DC, Rosen JE, Salazar MC, Blasberg JD, Boffa DJ, Detterbeck FC, Kim AW. Lung Cancer in the Very Young: Treatment and Survival in the National Cancer Data Base. J Thorac Oncol. 2016 Jul;11(7):1121-31. doi: 10.1016/j.jtho.2016.03.023. Epub 2016 Apr 19. PMID: 27103511.
- Patz EF Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, Chiles C, Black WC, Aberle DR; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014 Feb 1;174(2):269-74. doi: 10.1001/jamainternmed.2013.12738. Erratum in: JAMA Intern Med. 2014 May;174(5):828. PMID: 24322569; PMCID: PMC4040004.
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsäter A, Flex A, Aben KKH, de Vegt F, Mulders PFA, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008 Apr 3;452(7187):638-642. doi: 10.1038/nature06846. PMID: 18385739; PMCID: PMC4539558.
- Attanoos RL. Asbestos-related lung disease. Surgical Pathology Clinics. 2010;3(1):109-127. doi: 10.1016/j.path.2010.04.003.
- Zhou Q, Xi S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul Toxicol Pharmacol. 2018 Nov;99:78-88. doi: 10.1016/j.yrtph.2018.09.010. Epub 2018 Sep 15. PMID: 30223072.
- Berthiller J, Straif K, Boniol M, Voirin N, Benhaïm-Luzon V, Ayoub WB, Dari I, Laouamri S, Hamdi-Cherif M, Bartal M, Ayed FB, Sasco AJ. Cannabis smoking and risk of lung cancer in men: a pooled analysis of three studies in Maghreb. J Thorac Oncol. 2008 Dec;3(12):1398-403. doi: 10.1097/JTO.0b013e31818ddcde. PMID: 19057263.
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005 Nov 1;117(2):294-9. doi: 10.1002/ijc.21183. PMID: 15900604.
- Lubin JH, Boice JD Jr, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA, Morrison HI, Radford EP, Samet JM, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995 Jun 7;87(11):817-27. doi: 10.1093/jnci/87.11.817. PMID: 7791231.
- Schubauer-Berigan MK, Daniels RD, Pinkerton LE. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol. 2009 Mar 15;169(6):718-30. doi: 10.1093/aje/kwn406. Epub 2009 Feb 10. PMID: 19208723.
- Roscoe RJ, Deddens JA, Salvan A, Schnorr TM. Mortality among Navajo uranium miners. Am J Public Health. 1995 Apr;85(4):535-40. doi: 10.2105/ajph.85.4.535. PMID: 7702118; PMCID: PMC1615135.
- Marshall G, Ferreccio C, Yuan Y, Bates MN, Steinmaus C, Selvin S, Liaw J, Smith AH. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007 Jun 20;99(12):920-8. doi: 10.1093/jnci/djm004. Epub 2007 Jun 12. PMID: 17565158.
- Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, Barnes PJ, Papi A. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011 Jun;66(6):521-7. doi: 10.1136/thx.2010.156448. Epub 2011 Apr 2. PMID: 21460372.
- Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011 Mar 31;6(3):e17479. doi: 10.1371/journal.pone.0017479. PMID: 21483846; PMCID: PMC3069026.
- Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, Goulet J, Butt AA, Crystal S, Rimland D, Rodriguez-Barradas M, Gibert C, Park LS, Crothers K. HIV as an independent risk factor for incident lung cancer. AIDS. 2012 May 15;26(8):1017-25. doi: 10.1097/QAD.0b013e328352d1ad. PMID: 22382152; PMCID: PMC3580210.
- D'Jaen GA, Pantanowitz L, Bower M, Buskin S, Neil N, Greco EM, Cooley TP, Henry D, Stem J, Dezube BJ, Stebbing J, Aboulafia DM. Human immunodeficiency virus-associated primary lung cancer in the era of highly active antiretroviral therapy: a multi-institutional collaboration. Clin Lung Cancer. 2010 Nov 1;11(6):396-404. doi: 10.3816/CLC.2010.n.051. PMID: 21062730.
- Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D'Souza G, Engels EA, Hessol NA, Brooks JT, Burchell AN, Gill MJ, Goedert JJ, Hogg R, Horberg MA, Kirk GD, Kitahata MM, Korthuis PT, Mathews WC, Mayor A, Modur SP, Napravnik S, Novak RM, Patel P, Rachlis AR, Sterling TR, Willig JH, Justice AC, Moore RD, Dubrow R; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med. 2015 Oct 6;163(7):507-18. doi: 10.7326/M14-2768. PMID: 26436616; PMCID: PMC4711936.
- Cohen AJ, Pope CA 3rd. Lung cancer and air pollution. Environ Health Perspect. 1995 Nov;103 Suppl 8(Suppl 8):219-24. doi: 10.1289/ehp.95103s8219. PMID: 8741787; PMCID: PMC1518961.
- Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager M, Bergen AW, Li Q, Consonni D, Pesatori AC, Wacholder S, Thun M, Diver R, Oken M, Virtamo J, Albanes D, Wang Z, Burdette L, Doheny KF, Pugh EW, Laurie C, Brennan P, Hung R, Gaborieau V, McKay JD, Lathrop M, McLaughlin J, Wang Y, Tsao MS, Spitz MR, Wang Y, Krokan H, Vatten L, Skorpen F, Arnesen E, Benhamou S, Bouchard C, Metspalu A, Metsapalu A, Vooder T, Nelis M, Välk K, Field JK, Chen C, Goodman G, Sulem P, Thorleifsson G, Rafnar T, Eisen T, Sauter W, Rosenberger A, Bickeböller H, Risch A, Chang-Claude J, Wichmann HE, Stefansson K, Houlston R, Amos CI, Fraumeni JF Jr, Savage SA, Bertazzi PA, Tucker MA, Chanock S, Caporaso NE. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009 Nov;85(5):679-91. doi: 10.1016/j.ajhg.2009.09.012. Epub 2009 Oct 15. Erratum in: Am J Hum Genet. 2011 Jun 10;88(6):861. PMID: 19836008; PMCID: PMC2775843.
- Yokota J, Shiraishi K, Kohno T. Genetic basis for susceptibility to lung cancer: Recent progress and future directions. Adv Cancer Res. 2010;109:51-72. doi: 10.1016/B978-0-12-380890-5.00002-8. PMID: 21070914.
- Worley S. Lung cancer research is taking on new challenges: knowledge of tumors' molecular diversity is opening new pathways to treatment. P T. 2014 Oct;39(10):698-714. PMID: 25336866; PMCID: PMC4189696.
- Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014 Mar 4;160(5):330-8. doi: 10.7326/M13-2771. PMID: 24378917.
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012 Oct;12(10):685-98. doi: 10.1038/nrc3365. PMID: 23001348; PMCID: PMC4371788.
- Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159-85. doi: 10.1146/annurev.genet.40.110405.090613. PMID: 16771627.
- John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Med Hypotheses. 2001 Oct;57(4):429-31. doi: 10.1054/mehy.2001.1335. PMID: 11601863.
- Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, Michaud M, Zischka H, Castedo M, Kroemer G. Mitochondrial gateways to cancer. Mol Aspects Med. 2010 Feb;31(1):1-20. doi: 10.1016/j.mam.2009.08.002. Epub 2009 Aug 19. PMID: 19698742.
- Foster CS, Spoerri PE, Glees P, Spoerri O. The mode of mitochondrial degeneration in gliomas. Acta Neurochir (Wien). 1978;43(3-4):229-37. doi: 10.1007/BF01587958. PMID: 707179.
- Rasmussen AK, Chatterjee A, Rasmussen LJ, Singh KK. Mitochondria-mediated nuclear mutator phenotype in Saccharomyces cerevisiae. Nucleic Acids Res. 2003 Jul 15;31(14):3909-17. doi: 10.1093/nar/gkg446. PMID: 12853606; PMCID: PMC165961.
- Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, Zapata JM, Marusawa H, Chamorro M, Reed JC. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002 Nov 15;62(22):6674-81. PMID: 12438266.
- Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res. 2008 Dec;49(12):2545-56. doi: 10.1194/jlr.M800319-JLR200. Epub 2008 Aug 13. PMID: 18703489; PMCID: PMC2582368.
- Arismendi-Morillo GJ, Castellano-Ramirez AV. Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J Electron Microsc (Tokyo). 2008 Jan;57(1):33-9. doi: 10.1093/jmicro/dfm038. PMID: 18230641.
- Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One. 2009 Sep 15;4(9):e7033. doi: 10.1371/journal.pone.0007033. PMID: 19753307; PMCID: PMC2737639.
- Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):5992-7. doi: 10.1073/pnas.0502267102. Epub 2005 Apr 19. PMID: 15840712; PMCID: PMC1087961.
- Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab (Lond). 2005 Oct 21;2:30. doi: 10.1186/1743-7075-2-30. PMID: 16242042; PMCID: PMC1276814.
- Kulawiec M, Safina A, Desouki MM, Still I, Matsui S, Bakin A, Singh KK. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol Ther. 2008 Nov;7(11):1732-43. doi: 10.4161/cbt.7.11.6729. Epub 2008 Nov 4. PMID: 19151587; PMCID: PMC2783327.
- Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009 Nov 1;15(21):6479-83. doi: 10.1158/1078-0432.CCR-09-0889. Epub 2009 Oct 27. PMID: 19861459; PMCID: PMC2783410.
- Kolodner RD, Putnam CD, Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002 Jul 26;297(5581):552-7. doi: 10.1126/science.1075277. PMID: 12142524.
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009 Jul;8(7):579-91. doi: 10.1038/nrd2803. Epub 2009 May 29. PMID: 19478820.
- Traven A, Wong JM, Xu D, Sopta M, Ingles CJ. Interorganellar communication. Altered nuclear gene expression profiles in a yeast mitochondrial dna mutant. J Biol Chem. 2001 Feb 9;276(6):4020-7. doi: 10.1074/jbc.M006807200. Epub 2000 Oct 27. PMID: 11054416.
- Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009 Jun 26;137(7):1247-58. doi: 10.1016/j.cell.2009.04.014. PMID: 19563757; PMCID: PMC2759275.
- Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol Cell. 2004 Apr 9;14(1):1-15. doi: 10.1016/s1097-2765(04)00179-0. PMID: 15068799.
- Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002 Nov 7;21(51):7839-49. doi: 10.1038/sj.onc.1205983. PMID: 12420221.
- Simbula G, Glascott PA Jr, Akita S, Hoek JB, Farber JL. Two mechanisms by which ATP depletion potentiates induction of the mitochondrial permeability transition. Am J Physiol. 1997 Aug;273(2 Pt 1):C479-88. doi: 10.1152/ajpcell.1997.273.2.C479. PMID: 9277345.
- Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, Demazy C, Remacle J, Raes M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002 Jan 15;21(1-2):53-63. doi: 10.1093/emboj/21.1.53. PMID: 11782425; PMCID: PMC125809.
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011 Feb;11(2):85-95. doi: 10.1038/nrc2981. PMID: 21258394.
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006 Jul 14;126(1):107-20. doi: 10.1016/j.cell.2006.05.036. PMID: 16839880.
- Corcoran CA, Huang Y, Sheikh MS. The regulation of energy generating metabolic pathways by p53. Cancer Biol Ther. 2006 Dec;5(12):1610-3. doi: 10.4161/cbt.5.12.3617. Epub 2006 Dec 26. PMID: 17204863.
- Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11848-52. doi: 10.1073/pnas.93.21.11848. PMID: 8876226; PMCID: PMC38147.
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008 Dec 9;14(6):458-70. doi: 10.1016/j.ccr.2008.11.003. PMID: 19061837; PMCID: PMC3038665.
- Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001 Apr 15;61(8):3230-9. PMID: 11309271.
- Kocherginsky N. Acidic lipids, H(+)-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell's Nobel Prize award. Prog Biophys Mol Biol. 2009 Jan;99(1):20-41. doi: 10.1016/j.pbiomolbio.2008.10.013. Epub 2008 Nov 14. PMID: 19049812.
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000 Jul 21;275(29):22387-94. doi: 10.1074/jbc.M909868199. PMID: 10777514.
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008 Sep 8;182(5):937-50. doi: 10.1083/jcb.200801152. Erratum in: J Cell Biol. 2012 Jun 25;197(7):1029. PMID: 18779372; PMCID: PMC2528576.
- Ohtsuka T, Nishijima M, Suzuki K, Akamatsu Y. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J Biol Chem. 1993 Oct 25;268(30):22914-9. PMID: 8226801.
- Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009 Oct;1788(10):2022-31. doi: 10.1016/j.bbamem.2009.05.004. Epub 2009 May 18. PMID: 19450542.
- Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin Cancer Biol. 2022 Nov;86(Pt 2):851-859. doi: 10.1016/j.semcancer.2022.02.002. Epub 2022 Feb 3. PMID: 35122973.
- Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P. Mitochondrial Transfer in Cancer: A Comprehensive Review. Int J Mol Sci. 2021 Mar 23;22(6):3245. doi: 10.3390/ijms22063245. PMID: 33806730; PMCID: PMC8004668.
- Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015 Sep;36(10):7501-13. doi: 10.1007/s13277-015-3473-4. Epub 2015 Apr 25. PMID: 25910707.
- Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer. Mol Cell. 2016 Mar 3;61(5):667-676. doi: 10.1016/j.molcel.2016.02.011. PMID: 26942671; PMCID: PMC4779192.
- Akgul EO, Kurt B, Gulcan Kurt Y, Cayci T. MtDNA depletions and deletions may also be important in pathogenesis of lung cancer. Respir Med. 2013 Nov;107(11):1814. doi: 10.1016/j.rmed.2013.04.024. Epub 2013 Aug 31. PMID: 24035202.
- Wang L, Chen ZJ, Zhang YK, Le HB. The role of mitochondrial tRNA mutations in lung cancer. Int J Clin Exp Med. 2015 Aug 15;8(8):13341-6. PMID: 26550263; PMCID: PMC4612948.
- Liu F, Sanin DE, Wang X. Mitochondrial DNA in Lung Cancer. Adv Exp Med Biol. 2017;1038:9-22. doi: 10.1007/978-981-10-6674-0_2. PMID: 29178066.
- Shen H, Yu M, Tsoli M, Chang C, Joshi S, Liu J, Ryall S, Chornenkyy Y, Siddaway R, Hawkins C, Ziegler DS. Targeting reduced mitochondrial DNA quantity as a therapeutic approach in pediatric high-grade gliomas. Neuro Oncol. 2020 Jan 11;22(1):139-151. doi: 10.1093/neuonc/noz140. PMID: 31398252; PMCID: PMC6954438.
- Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005 Nov 14;24(50):7435-42. doi: 10.1038/sj.onc.1209097. PMID: 16288290.
- Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004 Sep 1;18(17):2095-107. doi: 10.1101/gad.1204904. Epub 2004 Aug 16. PMID: 15314031; PMCID: PMC515288.
- Chiche J, Rouleau M, Gounon P, Brahimi-Horn MC, Pouysségur J, Mazure NM. Hypoxic enlarged mitochondria protect cancer cells from apoptotic stimuli. J Cell Physiol. 2010 Mar;222(3):648-57. doi: 10.1002/jcp.21984. PMID: 19957303.
- de Moura MB, dos Santos LS, Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen. 2010 Jun;51(5):391-405. doi: 10.1002/em.20575. PMID: 20544881.
- Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J. 2004 Aug;18(11):1303-5. doi: 10.1096/fj.03-1001fje. Epub 2004 Jun 4. PMID: 15180958; PMCID: PMC4458073.
- El Mjiyad N, Caro-Maldonado A, Ramírez-Peinado S, Muñoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011 Jan 20;30(3):253-64. doi: 10.1038/onc.2010.466. Epub 2010 Oct 25. PMID: 20972457.
- Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008 Dec;65(24):3981-99. doi: 10.1007/s00018-008-8224-x. PMID: 18766298.
- Yoon DH, Lim MH, Lee YR, Sung GH, Lee TH, Jeon BH, Cho JY, Song WO, Park H, Choi S, Kim TW. A novel synthetic analog of Militarin, MA-1 induces mitochondrial dependent apoptosis by ROS generation in human lung cancer cells. Toxicol Appl Pharmacol. 2013 Dec 15;273(3):659-71. doi: 10.1016/j.taap.2013.10.015. Epub 2013 Oct 22. PMID: 24161344.
- Fetterman JL, Sammy MJ, Ballinger SW. Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology. 2017 Nov 1;391:18-33. doi: 10.1016/j.tox.2017.08.002. Epub 2017 Aug 22. PMID: 28838641; PMCID: PMC5681398.
- Ng Kee Kwong F, Nicholson AG, Harrison CL, Hansbro PM, Adcock IM, Chung KF. Is mitochondrial dysfunction a driving mechanism linking COPD to nonsmall cell lung carcinoma? Eur Respir Rev. 2017 Oct 25;26(146):170040. doi: 10.1183/16000617.0040-2017. PMID: 29070578; PMCID: PMC9488999.
- Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009 Dec;64(12):1082-9. doi: 10.1136/thx.2009.115691. Epub 2009 Sep 23. PMID: 19778933.
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019 Jul-Sep;18(3):121-126. doi: 10.4103/aam.aam_56_18. PMID: 31417011; PMCID: PMC6704802.
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009 Jul;30(7):1073-81. doi: 10.1093/carcin/bgp127. Epub 2009 May 25. PMID: 19468060.
- Cloonan SM, Kim K, Esteves P, Trian T, Barnes PJ. Mitochondrial dysfunction in lung ageing and disease. Eur Respir Rev. 2020 Oct 15;29(157):200165. doi: 10.1183/16000617.0165-2020. PMID: 33060165; PMCID: PMC9488551.
- Kao SJ, Lee WJ, Chang JH, Chow JM, Chung CL, Hung WY, Chien MH. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ Toxicol. 2017 Apr;32(4):1426-1438. doi: 10.1002/tox.22336. Epub 2016 Aug 19. PMID: 27539140.
- Barrett AG, Boys ML, Boehm TL. Total Synthesis of (+)-Papuamine: An Antifungal Pentacyclic Alkaloid from a Marine Sponge, Haliclona sp. J Org Chem. 1996 Jan 26;61(2):685-699. doi: 10.1021/jo951413z. PMID: 11666992.
- Min HY, Jung Y, Park KH, Lee HY. Papuamine Inhibits Viability of Non-small Cell Lung Cancer Cells by Inducing Mitochondrial Dysfunction. Anticancer Res. 2020 Jan;40(1):323-333. doi: 10.21873/anticanres.13956. PMID: 31892583.
- Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010 May;235(1):172-89. doi: 10.1111/j.0105-2896.2010.00903.x. PMID: 20536563; PMCID: PMC2914464.
- Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007 Dec;27(6):927-40. doi: 10.1016/j.immuni.2007.11.011. PMID: 18082433; PMCID: PMC2757006.
- Zhang Q, Wang H, Wu X, Liu B, Liu W, Wang R, Liang X, Ma C, Gao L. TIM-4 promotes the growth of non-small-cell lung cancer in a RGD motif-dependent manner. Br J Cancer. 2015 Nov 17;113(10):1484-92. doi: 10.1038/bjc.2015.323. Epub 2015 Oct 29. PMID: 26512878; PMCID: PMC4815884.
- Wang Y, Wang Y, Liu W, Ding L, Zhang X, Wang B, Tong Z, Yue X, Li C, Xu L, Wu Z, Liang X, Ma C, Gao L. TIM-4 orchestrates mitochondrial homeostasis to promote lung cancer progression via ANXA2/PI3K/AKT/OPA1 axis. Cell Death Dis. 2023;14(2). doi: 10.1038/s41419-023-05678-3.
- Kuo CT, Hsu MJ, Chen BC, Chen CC, Teng CM, Pan SL, Lin CH. Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol Lett. 2008 Feb 28;177(1):48-58. doi: 10.1016/j.toxlet.2007.12.009. Epub 2007 Dec 28. PMID: 18262737.
- Xu CX, Jin H, Shin JY, Kim JE, Cho MH. Roles of protein kinase B/Akt in lung cancer. Front Biosci (Elite Ed). 2010 Jun 1;2(4):1472-84. doi: 10.2741/e206. PMID: 20515818.
- Saliakoura M, Rossi Sebastiano M, Pozzato C, Heidel FH, Schnöder TM, Savic Prince S, Bubendorf L, Pinton P, A Schmid R, Baumgartner J, Freigang S, Berezowska SA, Rimessi A, Konstantinidou G. PLCγ1 suppression promotes the adaptation of KRAS-mutant lung adenocarcinomas to hypoxia. Nat Cell Biol. 2020 Nov;22(11):1382-1395. doi: 10.1038/s41556-020-00592-8. Epub 2020 Oct 19. PMID: 33077911; PMCID: PMC7610419.
- Liu YJ, McIntyre RL, Janssens GE, Houtkooper RH. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech Ageing Dev. 2020 Mar;186:111212. doi: 10.1016/j.mad.2020.111212. Epub 2020 Feb 1. PMID: 32017944.
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004 Sep 27;166(7):1027-39. doi: 10.1083/jcb.200407046. PMID: 15452144; PMCID: PMC2172012.
- Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004 Dec 10;279(50):52726-34. doi: 10.1074/jbc.M408910200. Epub 2004 Sep 30. PMID: 15459195.
- Chung JG, Yeh KT, Wu SL, Hsu NY, Chen GW, Yeh YW, Ho HC. Novel transmembrane GTPase of non-small cell lung cancer identified by mRNA differential display. Cancer Res. 2001 Dec 15;61(24):8873-9. PMID: 11751411.
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005 Oct 21;280(42):35742-50. doi: 10.1074/jbc.M505970200. Epub 2005 Aug 22. PMID: 16115883.
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001 Oct;1(4):515-25. doi: 10.1016/s1534-5807(01)00055-7. PMID: 11703942.
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007 May 7;177(3):439-50. doi: 10.1083/jcb.200610042. Epub 2007 Apr 30. PMID: 17470634; PMCID: PMC2064824.
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006 Aug 23;25(16):3900-11. doi: 10.1038/sj.emboj.7601253. Epub 2006 Jul 27. PMID: 16874299; PMCID: PMC1553198.
- Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005 Apr 20;24(8):1546-56. doi: 10.1038/sj.emboj.7600592. Epub 2005 Mar 24. PMID: 15791210; PMCID: PMC1142564.
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004 Nov;15(11):5001-11. doi: 10.1091/mbc.e04-04-0294. Epub 2004 Sep 8. PMID: 15356267; PMCID: PMC524759.
- Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008 Feb 15;22(4):476-88. doi: 10.1101/gad.460708. PMID: 18281461; PMCID: PMC2238669.
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003 Mar 7;278(10):7743-6. doi: 10.1074/jbc.C200677200. Epub 2002 Dec 31. PMID: 12509422.
- Fang HY, Chen CY, Chiou SH, Wang YT, Lin TY, Chang HW, Chiang IP, Lan KJ, Chow KC. Overexpression of optic atrophy 1 protein increases cisplatin resistance via inactivation of caspase-dependent apoptosis in lung adenocarcinoma cells. Hum Pathol. 2012 Jan;43(1):105-14. doi: 10.1016/j.humpath.2011.04.012. Epub 2011 Jul 27. PMID: 21798574.
- Thomas KJ, Jacobson MR. Defects in mitochondrial fission protein dynamin-related protein 1 are linked to apoptotic resistance and autophagy in a lung cancer model. PLoS One. 2012;7(9):e45319. doi: 10.1371/journal.pone.0045319. Epub 2012 Sep 20. PMID: 23028930; PMCID: PMC3447926.
- Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008 Sep 22;3(9):e3257. doi: 10.1371/journal.pone.0003257. PMID: 18806874; PMCID: PMC2532749.
- Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B. Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci. 2012 Dec 1;125(Pt 23):5745-57. doi: 10.1242/jcs.109769. Epub 2012 Sep 26. PMID: 23015593; PMCID: PMC4074216.
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007 Apr 13;282(15):11521-9. doi: 10.1074/jbc.M607279200. Epub 2007 Feb 14. PMID: 17301055.
- Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000 Aug 1;60(15):4000-4. PMID: 10945597.
- Matthess Y, Raab M, Sanhaji M, Lavrik IN, Strebhardt K. Cdk1/cyclin B1 controls Fas-mediated apoptosis by regulating caspase-8 activity. Mol Cell Biol. 2010 Dec;30(24):5726-40. doi: 10.1128/MCB.00731-10. Epub 2010 Oct 11. PMID: 20937773; PMCID: PMC3004266.
- Feldmann G, Mishra A, Hong SM, Bisht S, Strock CJ, Ball DW, Goggins M, Maitra A, Nelkin BD. Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res. 2010 Jun 1;70(11):4460-9. doi: 10.1158/0008-5472.CAN-09-1107. Epub 2010 May 18. PMID: 20484029; PMCID: PMC3071300.
- Zhang W, Hu X, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat Commun. 2019 Apr 12;10(1):1704. doi: 10.1038/s41467-019-09566-3. Erratum in: Nat Commun. 2019 Jun 10;10(1):2597. Erratum in: Nat Commun. 2019 Oct 4;10(1):4591. PMID: 30979885; PMCID: PMC6461692. .
- Yu M, Nguyen ND, Huang Y, Lin D, Fujimoto TN, Molkentine JM, Deorukhkar A, Kang Y, San Lucas FA, Fernandes CJ, Koay EJ, Gupta S, Ying H, Koong AC, Herman JM, Fleming JB, Maitra A, Taniguchi CM. Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer. JCI Insight. 2019 Jul 23;5(16):e126915. doi: 10.1172/jci.insight.126915. PMID: 31335325; PMCID: PMC6777817.
- Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med. 2017 Mar;104:144-164. doi: 10.1016/j.freeradbiomed.2017.01.004. Epub 2017 Jan 11. PMID: 28088622.
- Księżakowska-Łakoma K, Żyła M, Wilczyński JR. Mitochondrial dysfunction in cancer. Prz Menopauzalny. 2014 May;13(2):136-44. doi: 10.5114/pm.2014.42717. Epub 2014 May 21. PMID: 26327844; PMCID: PMC4520353.
- Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014 Nov;14(11):709-21. doi: 10.1038/nrc3803. PMID: 25342630; PMCID: PMC4657553.
- Idelchik MDPS, Begley U, Begley TJ, Melendez JA. Mitochondrial ROS control of cancer. Semin Cancer Biol. 2017 Dec;47:57-66. doi: 10.1016/j.semcancer.2017.04.005. Epub 2017 Apr 23. PMID: 28445781; PMCID: PMC5653465.
- Solaini G, Sgarbi G, Baracca A. Oxidative phosphorylation in cancer cells. Biochim Biophys Acta. 2011 Jun;1807(6):534-42. doi: 10.1016/j.bbabio.2010.09.003. Epub 2010 Sep 16. PMID: 20849810.
- Dasgupta S, Soudry E, Mukhopadhyay N, Shao C, Yee J, Lam S, Lam W, Zhang W, Gazdar AF, Fisher PB, Sidransky D. Mitochondrial DNA mutations in respiratory complex-I in never-smoker lung cancer patients contribute to lung cancer progression and associated with EGFR gene mutation. J Cell Physiol. 2012 Jun;227(6):2451-60. doi: 10.1002/jcp.22980. PMID: 21830212; PMCID: PMC3256258.
- Song J, Shu L, Zhang Z, Tan X, Sun E, Jin X, Chen Y, Jia X. Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer. Chem Biol Interact. 2012 Jul 30;199(1):9-17. doi: 10.1016/j.cbi.2012.05.005. Epub 2012 Jun 9. PMID: 22687635.
- Kulikov AV, Luchkina EA, Gogvadze V, Zhivotovsky B. Mitophagy: Link to cancer development and therapy. Biochem Biophys Res Commun. 2017 Jan 15;482(3):432-439. doi: 10.1016/j.bbrc.2016.10.088. Epub 2017 Feb 3. PMID: 28212727.
- Kalainayakan SP, FitzGerald KE, Konduri PC, Vidal C, Zhang L. Essential roles of mitochondrial and heme function in lung cancer bioenergetics and tumorigenesis. Cell Biosci. 2018 Nov 2;8:56. doi: 10.1186/s13578-018-0257-8. PMID: 30410721; PMCID: PMC6215344.
- Xu R, Luo X, Ye X, Li H, Liu H, Du Q, Zhai Q. SIRT1/PGC-1α/PPAR-γ Correlate With Hypoxia-Induced Chemoresistance in Non-Small Cell Lung Cancer. Front Oncol. 2021 Jul 26;11:682762. doi: 10.3389/fonc.2021.682762. PMID: 34381712; PMCID: PMC8351465.
- Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018 Mar;28(3):265-280. doi: 10.1038/cr.2017.155. Epub 2017 Dec 8. PMID: 29219147; PMCID: PMC5835768.
- Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31-42. doi:10.1038/cdd.2012.81.
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013 Feb 14;368(7):651-62. doi: 10.1056/NEJMra1205406. PMID: 23406030.
- Kulikov AV, Luchkina EA, Gogvadze V, Zhivotovsky B. Mitophagy: Link to cancer development and therapy. Biochem Biophys Res Commun. 2017;482(3):432-439. doi: 10.1016/j.bbrc.2016.10.088. Epub 2017 Feb 3. PMID: 28212727.
- Liu Y, Fu Y, Hu X, Chen S, Miao J, Wang Y, Zhou Y, Zhang Y. Caveolin-1 knockdown increases the therapeutic sensitivity of lung cancer to cisplatin-induced apoptosis by repressing Parkin-related mitophagy and activating the ROCK1 pathway. J Cell Physiol. 2020 Feb;235(2):1197-1208. doi: 10.1002/jcp.29033. Epub 2019 Jul 4. PMID: 31270811.
- Guan Y, Wang Y, Li B, Shen K, Li Q, Ni Y, Huang L. Mitophagy in carcinogenesis, drug resistance and anticancer therapeutics. Cancer Cell Int. 2021 Jul 5;21(1):350. doi: 10.1186/s12935-021-02065-w. PMID: 34225732; PMCID: PMC8256582.
- Li Z, Wang Y, Wu L, Dong Y, Zhang J, Chen F, Xie W, Huang J, Lu N. Apurinic endonuclease 1 promotes the cisplatin resistance of lung cancer cells by inducing Parkin‑mediated mitophagy. Oncol Rep. 2019 Dec;42(6):2245-2254. doi: 10.3892/or.2019.7345. Epub 2019 Oct 1. PMID: 31578585; PMCID: PMC6826301.
- Gangelhoff TA, Mungalachetty PS, Nix JC, Churchill ME. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 2009 Jun;37(10):3153-64. doi: 10.1093/nar/gkp157. Epub 2009 Mar 20. PMID: 19304746; PMCID: PMC2691818.
- Yu J, Wang Q, Chen N, Sun Y, Wang X, Wu L, Chen S, Yuan H, Xu A, Wang J. Mitochondrial transcription factor A regulated ionizing radiation-induced mitochondrial biogenesis in human lung adenocarcinoma A549 cells. J Radiat Res. 2013 Nov 1;54(6):998-1004. doi: 10.1093/jrr/rrt046. Epub 2013 May 3. PMID: 23645454; PMCID: PMC3823773.
- Kanki T, Ohgaki K, Gaspari M, Gustafsson CM, Fukuoh A, Sasaki N, Hamasaki N, Kang D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004 Nov;24(22):9823-34. doi: 10.1128/MCB.24.22.9823-9834.2004. PMID: 15509786; PMCID: PMC525493.
- Gorbunova AS, Zamaraev AV, Yapryntseva MA, Kovaleva OV, Tchevkina EM, Turkina MV, Zhivotovsky B, Kopeina GS. Prognostic signature based on mitochondria quality control proteins for the prediction of lung adenocarcinoma patients survival. Cell Death Discov. 2023 Sep 25;9(1):352. doi: 10.1038/s41420-023-01649-x. PMID: 37749074; PMCID: PMC10519931.
- Ye C, Shu XO, Pierce L, Wen W, Courtney R, Gao YT, Zheng W, Cai Q. Mutations in the mitochondrial DNA D-loop region and breast cancer risk. Breast Cancer Res Treat. 2010 Jan;119(2):431-6. doi: 10.1007/s10549-009-0397-y. Epub 2009 Apr 21. PMID: 19381801; PMCID: PMC2796283.
- Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013 Jan 17;4(1):e461. doi: 10.1038/cddis.2012.213. PMID: 23328668; PMCID: PMC3564000.
- Tran Q, Lee H, Jung JH, Chang SH, Shrestha R, Kong G, Park J, Kim SH, Park KS, Rhee HW, Yun J, Cho MH, Kim KP, Park J. Emerging role of LETM1/GRP78 axis in lung cancer. Cell Death Dis. 2022 Jun 10;13(6):543. doi: 10.1038/s41419-022-04993-5. PMID: 35680871; PMCID: PMC9184611.
- Liu W, Du Q, Mei T, Wang J, Huang D, Qin T. Comprehensive analysis the prognostic and immune characteristics of mitochondrial transport-related gene SFXN1 in lung adenocarcinoma. BMC Cancer. 2024 Jan 17;24(1):94. doi: 10.1186/s12885-023-11646-z. PMID: 38233752; PMCID: PMC10795352.
- Zhu K, Shi J, Yang R, Zhou C, Liu Z. Evidence based on Mendelian randomization: Causal relationship between mitochondrial biological function and lung cancer and its subtypes. Neoplasia. 2023 Dec;46:100950. doi: 10.1016/j.neo.2023.100950. Epub 2023 Nov 16. PMID: 37976568; PMCID: PMC10685044.
- Kussainova A, Bulgakova O, Aripova A, Khalid Z, Bersimbaev R, Izzotti A. The Role of Mitochondrial miRNAs in the Development of Radon-Induced Lung Cancer. Biomedicines. 2022 Feb 11;10(2):428. doi: 10.3390/biomedicines10020428. PMID: 35203638; PMCID: PMC8962319.
- Song M, Wu H, Wu S, Ge T, Wang G, Zhou Y, Sheng S, Jiang J. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed Pharmacother. 2016 Dec;84:1137-1143. doi: 10.1016/j.biopha.2016.10.034. Epub 2016 Oct 22. PMID: 27780143.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.