Biology Group . 2023 January 24;4(1):050-063. doi: 10.37871/jbres1648.
Do COVID-19 RNA-Injections Affect Male Fertility? Latest Facts and Perspective
Werner Bergholz1 and Klaus Steger2*
2Molecular Andrology of the Department of Urology, Pediatric Urology and Andrology located at the Biomedical Research Center of the Justus-Liebig University, Giessen, Germany
- COVID-19
- Erectile dysfunction
- Lipid nanoparticles
- Male fertility
- Testicular tumor
- RNA vaccines
- Spermatogenesis
- Spike protein
Abstract
Background: Based on available data on male fertility adverse effects after COVID-19 injections, we draw the reader´s attention to open questions and undeniable risks of the new RNA-based vaccine technology.
Methods: Review and reanalysis of published data on pre- and post-injection semen analyses. Evaluation of UK Yellow Card and US VAERS databases for male fertility adverse effects and of the German deStatis database for monthly live birth rates after start of the governmental vaccination program.
Results: The deStatis database demonstrates a time shift of exactly nine months between the start of the governmental vaccination campaign (April 2021) and an abrupt decline in live births (January 2022). Frequency of male fertility adverse effects is approx. 100 times lower than that of female fertility. Remarkably, report numbers per one million doses are similar between AstraZeneca and BionTech/Pfizer, but significantly increased for Moderna despite overall numbers of administered doses are smaller than that of the other two manufacturers. Both increase in and correlation between erectile dysfunction and heart failure could be demonstrated. Review and reanalysis of published data on pre- and post-injection semen analyses identified a number of limitations of the currently available studies.
Conclusion: There remain still far more questions than answers. Due to the principle “primum non nocere,” any new medical therapy must be banned until harmlessness beyond doubt has been proven. Most importantly, it must be realized that the active ingredient of RNA-based vaccines is not simply mRNA promoting the synthesis of a nota bene viral specific protein, but modRNA specifically designed for longevity and encapsulated in LNPs to bypass biological barriers and get access to all cells, possibly also germ cells. As mRNA is involved in regulation of gene expression, cells have mechanisms at hand to silence mRNA species not required, however, theses protective mechanisms will not work with modRNA.
Introduction
COVID-19 (Coronavirus Disease-2019) caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome-CoronaVirus type-2) was reported to affect the respiratory tract and more particularly the pulmonary structures, however, an additional impact on male and female reproductive organs was demonstrated [1]. Special attention was directed at the membrane receptor ACE-2 (Angiotensin Converting Enzyme type-2) known to mediate viral cell entry and to be extensively expressed in testes [2] and ovaries [3]. With the onset of the governmental vaccination campaign, fear about fertility-related adverse effects has been reported as one of the major causes of vaccine hesitancy [4]. This concern is comprehensible, as germ cells are highly specialized and particularly vulnerable cell types and, therefore, any disruption of DNA integrity will either stop germ cell/embryo development or may be passed on to the next generation with a negative impact on the reproductive and/or the general health situation. Awareness of potential risks and possible consequences associated with COVID-19 injections requires insight into fundamentals of germ cell development (Box 1).
Box 1: Germ cell development at a glance.
During gestational weeks 4-6, primordial germ cells migrate from the yolk sac to the undifferentiated gonads and are then referred to as gonocytes. Around birth, male gonocytes attach to the basal membrane of the seminiferous tubules and differentiate into spermatogonia, which remain silent until onset of spermatogenesis at puberty [5,6]. Spermatogonia then pass three developmental steps [5,7]: (1) Mitotic proliferation and differentiation into primary spermatocytes; (2) Meiotic cleavage into secondary spermatocytes (1st division) and haploid spermatids (2nd division); (3) Differentiation of round spermatids into elongated spermatids and testicular spermatozoa. The latter step, called spermiogenesis, involves complete re-organization of nuclear chromatin by replacing DNA-binding histones by protamines finally resulting in chromatin condensation and stop of transcription [8,9]. In men, a spermatogenic cycle, that is the differentiation of a spermatogonium into testicular spermatozoa, takes 74 days [10]. Testicular spermatozoa are then released into the lumen of the seminiferous tubules and forwarded to the epididymis for final differentiation into mature (epididymal) sperm taking an additional two weeks.
Contrary to men experiencing a continual renewal of their germ cells, women bear only a fixed reservoir of oocytes, of which only around 500 will complete maturation during a woman´s life [6]. As meiosis is arrested both at the diplotene stage in primary oocytes (until finishing 1st meiotic division upon monthly release starting with puberty) and at the metaphase stage in secondary oocytes (until finishing 2nd meiotic division upon fertilization), female germ cells will be even more susceptible for DNA damage related to normal ageing processes and harmful environmental influences.
To improve our understanding of potential risks and damage mechanisms that may negatively affect male fertility, it is highly desirable to accumulate all information that emerged during the COVID-19 pandemic and in conjunction with the worldwide vaccination campaign. Although it is too early to present valid data on possible long-term adverse effects associated with COVID-19 injections, we reviewed publicly available databases and male fertility-related publications to encourage the currently missing scientific dialogue by drawing attention to open questions and undeniable risks of the new RNA-based vaccine technology for normal germ cell differentiation and male fertility.
Methods
The main investigative tools included: (1) Re-analysis of literature data with standard statistical tools available in worksheets, such as Microsoft Excel; (2) Inspection of UK Yellow Card and US VAERS databases for any data with potential relevance to the research question of this project whether there is evidence for a negative impact on male fertility; (3) Inspection of the German national statistics database (deStatis) for the development of the live births rate for a surmised correlation with the start of the COVID-19 vaccination program for the age cohorts contributing to reproduction. For these data, Statistical Process Control (SPC) has been used [11] to analyze the data. Specific details are provided in figure legends and relevant subsections of the results chapter. Details about the analyzed time periods vary from case to case depending on data availability and are given in connection with the presentation of the data and the analysis results.
Results
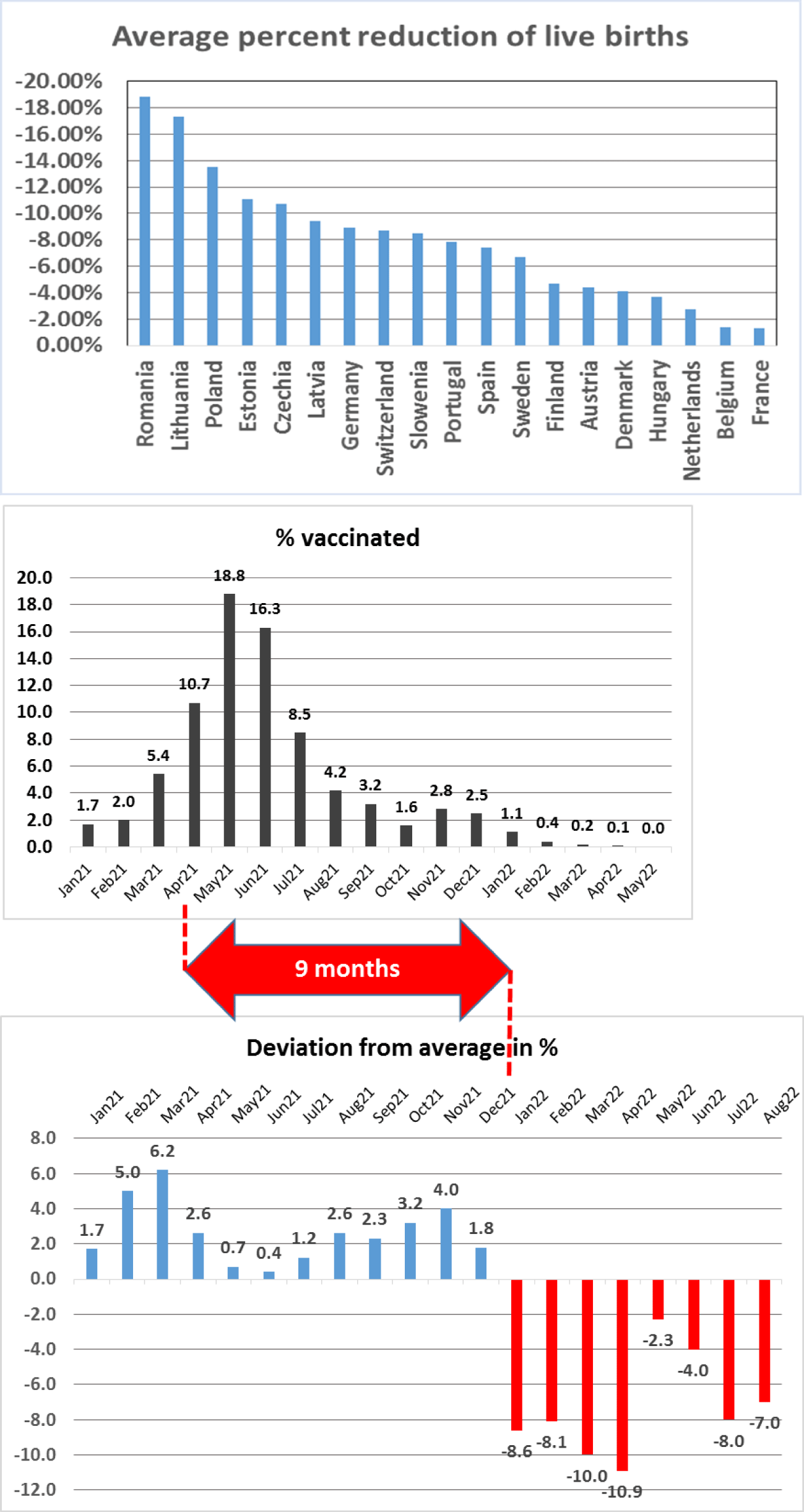
Starting in 2022, countries in Europe reported, to a variable extent, an abrupt and remarkable drop of live births when compared with the corresponding averages 2018-2021 (Figure 1A). Monthly statistics, exemplarily shown for Germany, exhibit a time shift of exactly nine months between the start of the governmental vaccination program upon the reproduction-relevant age cohorts and the observed decline in live births (Figure 1B).
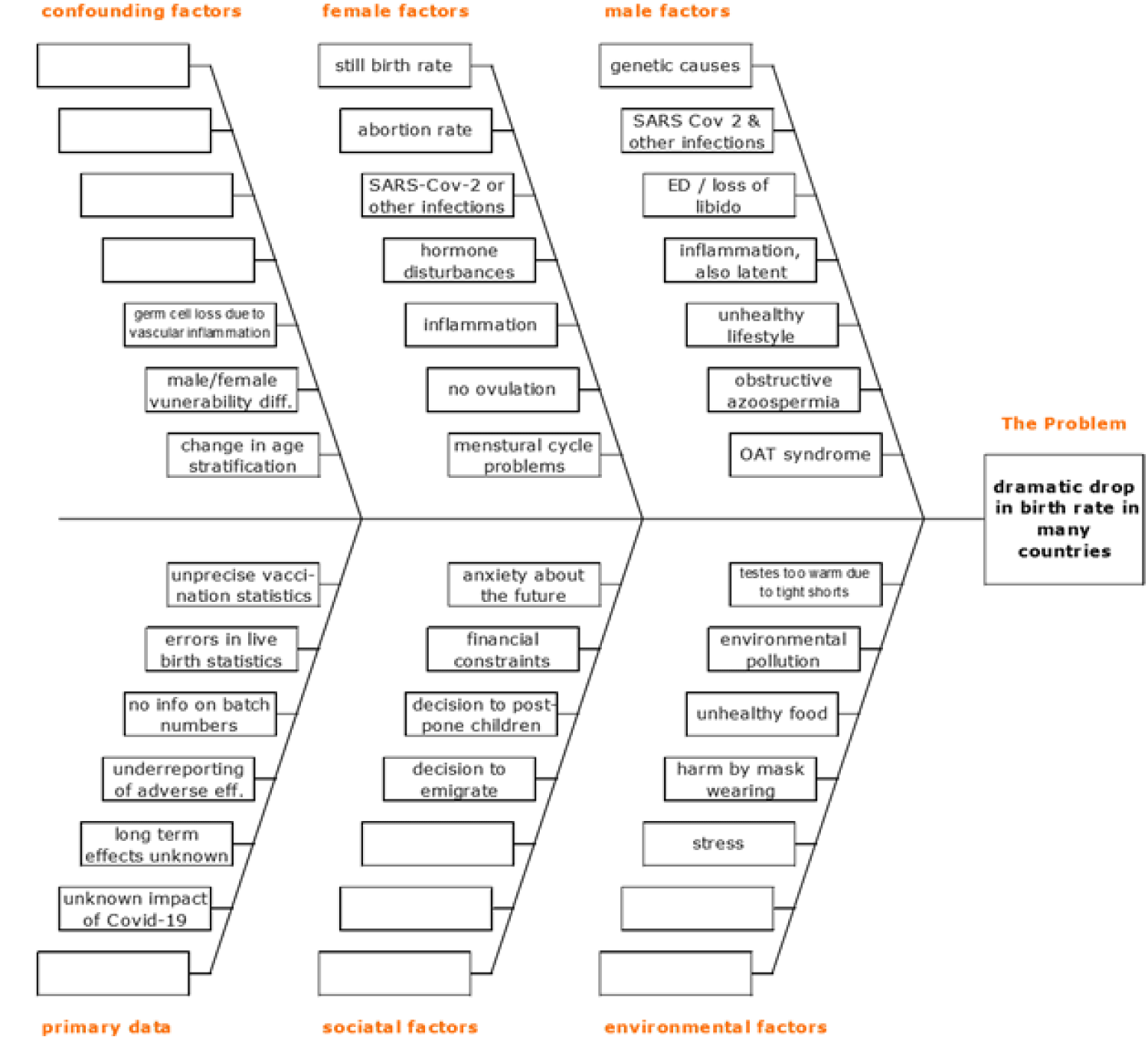
Figure 2 collects conceivable reasons with possible influence on the live births rate. Although incomplete, the presented cause-effect diagram gives an impression of the complexity of the problem. It should be mentioned that public databases provide imprecise primary data. A well-known and generally accepted source of error is underreporting of vaccine-related adverse effects, where estimates vary between 1% and 10% of the real numbers. However, vaccination and birth statistics are considered to be accurate within at least the 10% error margins. Therefore, the presented coincidence between the vaccine uptake and the nine months-lagged abrupt drop of the live births rate can be considered statistically sound. To date, it is still too early to determine whether reduced live birth rates represent only a temporal or a long-term effect. Further, it is not possible to make any claim that the vaccines are the root cause for the abrupt decline of live births, as there may be additional, yet unknown causes not captured in figure 2. Finally, it is a fact that databases lack relevant information on social and psychological burdens caused by family, employment or government´s intervention, such as lockdowns, mask wearing, COVID-19 tests, social distancing and compulsory vaccination. Although each of the listed factors, to a variable extent, may contribute to family planning and natural conception statistics, none of them will per se be able to explain the abrupt, remarkable and unique drop of live births starting in January 2022 in all European countries analysed.
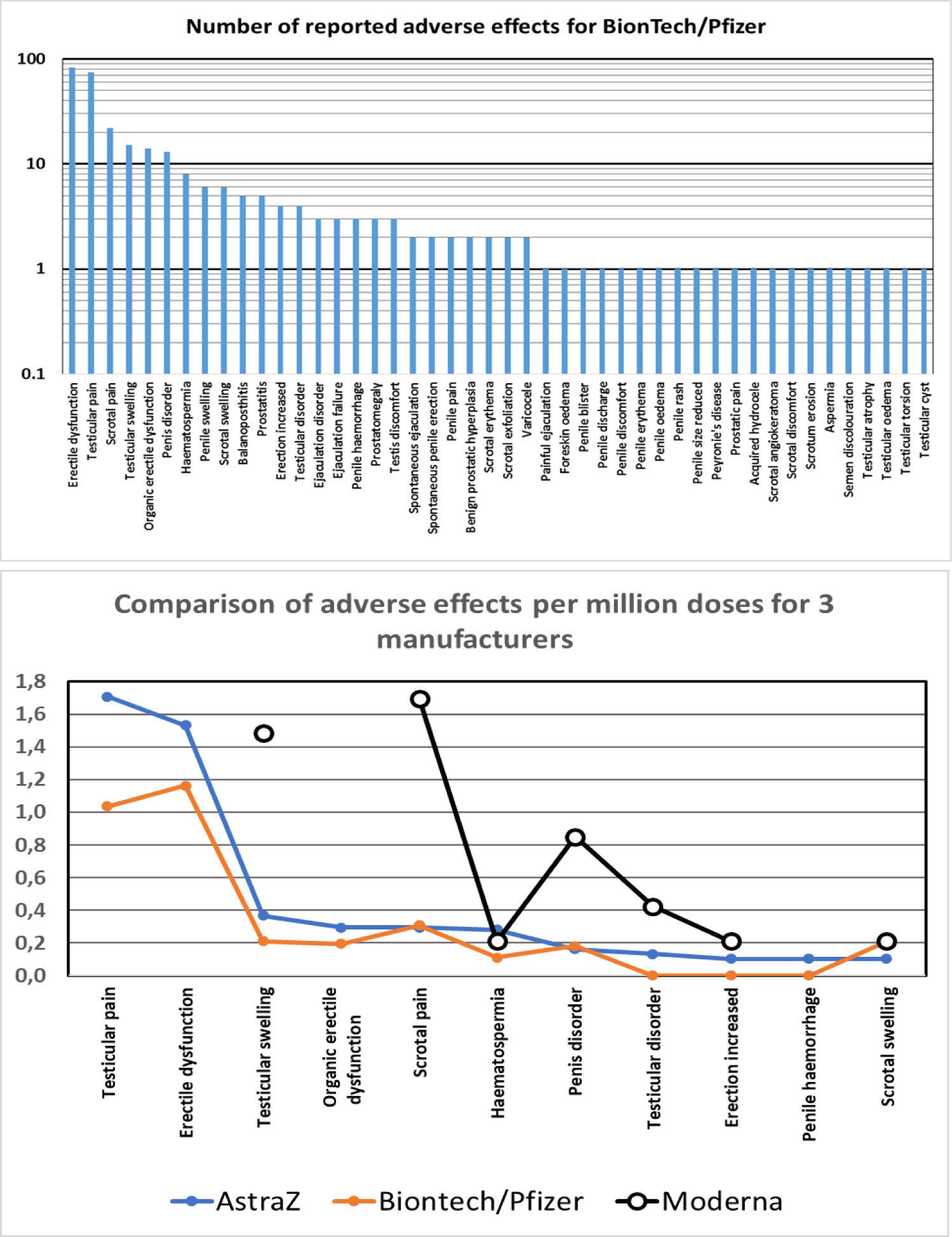
Regarding any changes in live birth rates, the female partner without doubt plays a predominant role, however, involvement of the male partner must not be ignored. Recently, a comprehensive meta-analysis, mainly based on data from soldiers and students, reported a worldwide decrease of the mean sperm concentration (-51.6%) and the total sperm count (-62.3%) between 1973 and 2018 [12]. Again, this general trend cannot be held responsible for the abrupt decline of live births observed from January 2022 onwards all over Europe. In order to gain at least some indirect evidence for and against any significant role for male fertility, different official databases have been examined with regard to adverse effects in connection with male reproductive organs. Figure 3A lists adverse effects reported in the UK Yellow Card scheme as of September 28th 2022. Of note, the frequency of reports for male reproductive organs is lower than for female reproductive organs by a factor of >100 (not shown). Nevertheless, most of the reported adverse effects regarding male reproductive organs can be expected to have also a significant negative impact on a couple´s fertility. Figure 3B compares the three main vaccine manufacturers for the most prominent adverse effects. Remarkably, the number of reports per one million doses is comparable for AstraZeneca and BionTech/Pfizer despite different underlying vaccine technologies, namely DNA in adeno virus vectors and RNA in lipid nanoparticles, respectively. Surprisingly, the frequency of male fertility-related adverse effects reported per one million doses is significantly higher for the Moderna vaccine, although the overall number of administered doses is much smaller than for the other two manufacturers.
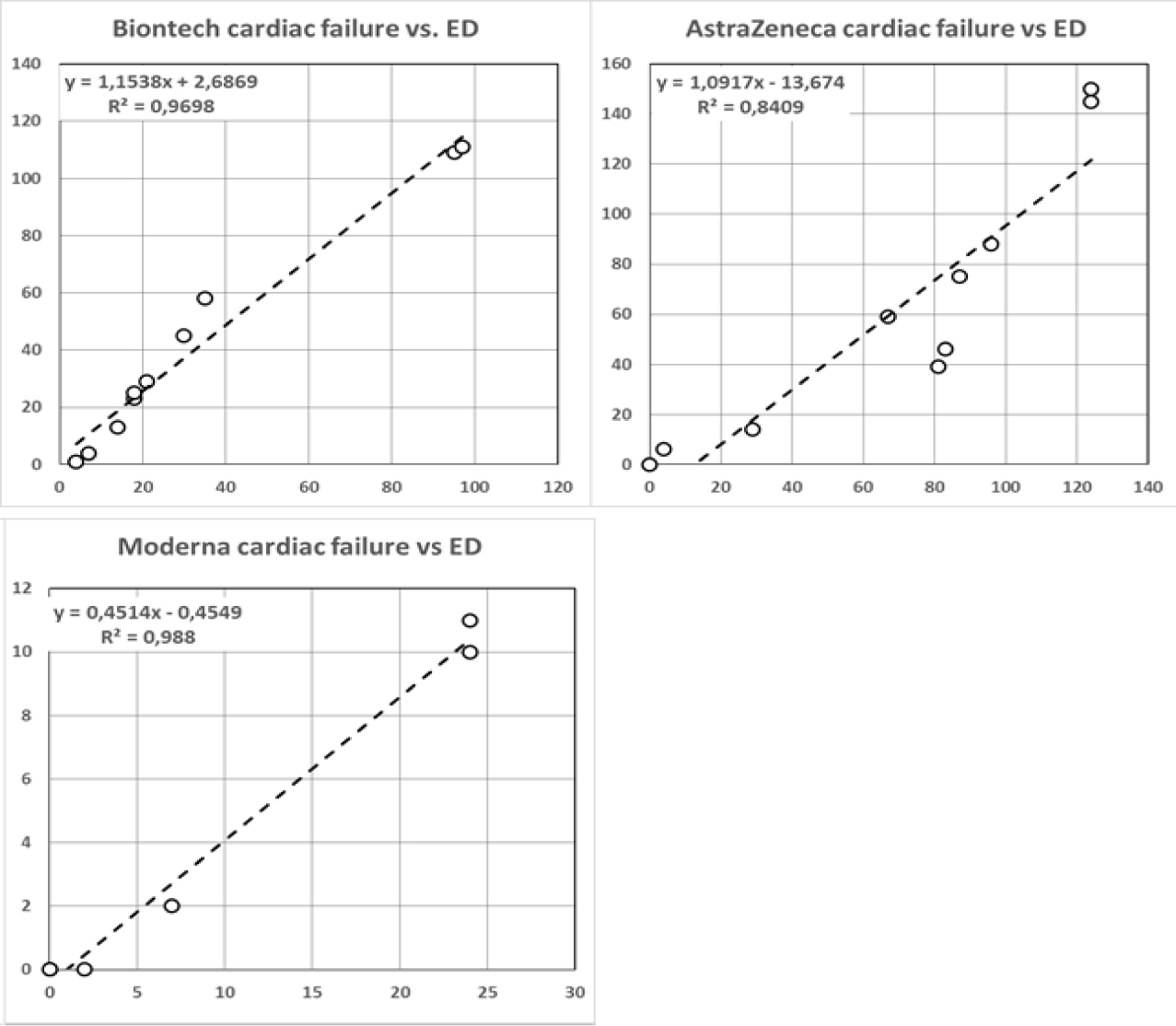
In addition to factors exerting a direct influence on germ cell development followed by a prompt decrease of semen quality, male factor subfertility may originate as a result of a long-term secondary health condition. Exemplarily, we draw the reader´s attention to ED (Erectile Dysfunction), which shares a common pathological basis with cardio-vascular disease, namely endothelial dysfunction of small capillaries in association with blood clots. Based on different diameters between the A. profunda penis (1-2 mm) and the Aa. coronariae (3-4 mm), ED normally precedes clinical manifestation of cardio-vascular diseases, i.e., heat failure, with three to five years [13]. Analysing the UK Yellow Card database (Figure 4), we found a strong correlation between the frequency of ED and heart failure for all of the three administered vaccines. For AstraZeneca and BionTech/Pfizer (BNT162b2, 30 µg RNA/dosis), despite using a different underlying vaccine technology, the proportionality factor is approx. 2/5 that of Moderna. Interestingly, for the Moderna vaccine (mRNA-1273, 100 µg RNA/dosis), ED is more frequently than heart failure suggesting that ED is more likely to happen with increasing RNA content of the vaccine. Of note, composition of lipids in LNPs is different between BionTech/Pfizer and Moderna.
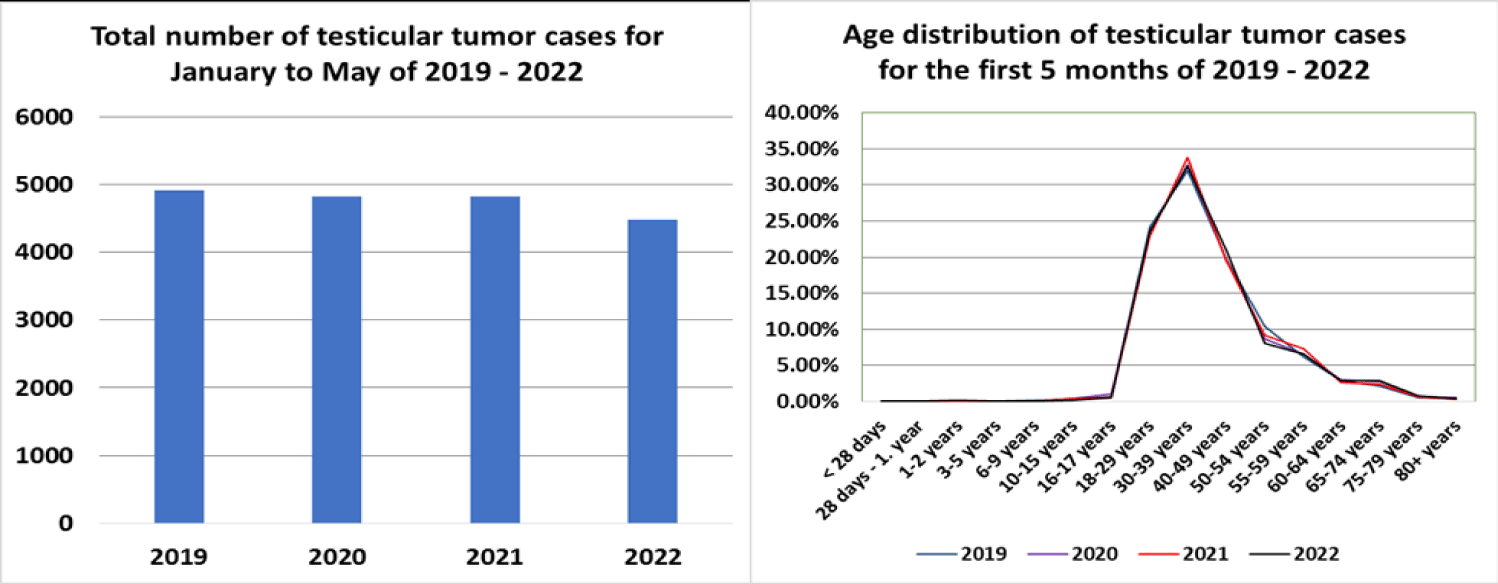
In addition to the well-known adverse effects myocardial infarction and stroke, a commonly reported observation is that both frequency and growth rate of tumors increased since the start of the COVID-19 vaccine program. Even German mainstream media reported that four players of the German first division football league (“1. Bundesliga”) suffered from testicular cancer [14]. We therefore investigated whether there is an increase in the rate of testicular tumors in the general population. To this end, publicly available data for hospital statistics have been examined for this diagnosis. However, as shown in figure 5, no statistically significant increase in the absolute number of the diagnosis testicular tumor in all males could be found. Also, there is no evidence for an age shift when compared with previous years.
Discussion
Our results demonstrating a time shift of exactly nine months between the start of the German government COVID-19 vaccine program in April 2021 and an abrupt decline of live births starting in January 2022 (Figure 1) are in compliance with a study of the German Federal Institute for Population Research [15]. Authors observed no significant changes in fertility rates in late 2020 and early 2021, but reported a prompt and strong decline of approx. -14% in early 2022. The vaccination campaign starts in late 2020, but initially aimed at vulnerable groups, i.e., older people and individuals with underlying health conditions. Vaccine mass enrolment peaked between April and June 2021 (1st dose) and between May and August 2021 (2nd dose) [16]. Implementation of the vaccine program coincides exactly with a nine months-lagged abrupt decline of fertility rates, which remain at a reduced level until the end of the analysed period (September 2022). Fairly soon after the start of the pandemic, it was known that SARS-CoV-2 affects in particular older people [17]. Therefore, it can be assumed that the health crisis had no substantial direct effect on the childbearing behaviour of the young population. However, the authors of the German Federal Institute for Population Research [15] suggest uncertainty and anxiety about the future economic development as the most likely reason for the parents´ decision to postpone their desire to have children, but did not provide any evidence to support their claim. By contrast, a more family-oriented life situation during periods of lockdown and home-office, known as cocooning effect, may have created even a positive influence on the parents´ decision to become children. However, there was indeed a reduced support in patient fertility care for birth clinics in general and for IVF (In-Vitro Fertilization) centers in particular [18]. A variety of additional factors, which may be involved in the decreased live birth rates, are summarized in figure 2, but will not be discussed further in this paper.
Against this background, it is completely incomprehensible that preclinical animal studies only scratched the surface regarding a valid analysis of the possibility of fertility-related adverse effects. While we were unable to find any original data from Moderna´s animal studies, Pfizer/Biontech reported that 30 μg mRNA of the BNT162b2 vaccine were administered to 44 female rats 21 and 14 days prior to mating with unvaccinated males and on gestation days 9 and 20 [19]. No negative effects were observed regarding female fertility and offspring survival. As 22 rats were subjected to Cesarean section at the end of gestation and only 22 rats were monitored to the end of lactation, the results presented in this study do not allow any reliable conclusion to be made about long-term adverse events. This is confirmed by 466 pages from Pfizer´s original documents, which were intended to be kept secret for 75 years, but were released to the Freedom of Information request no. 2021-4389.2022 [20]. It is noteworthy that the biodistribution data provided by Pfizer to EMA (European Medicines Agency) were based on injections with radiolabelled LNPs (Lipid NanoParticles) and luciferase-modified RNA, but did not include the actual vaccine substance or the later administered vaccine BNT162b2. Already 25 minutes following injection, radioactive signals were detected in a variety of tissues and, after 8 to 48 hours, measurements revealed a maximum concentration in the liver. Somewhat lower concentrations were detected in ovaries and testes [21] constituting indirect evidence of potential harmful effects on reproductive organs.
The aforementioned approach is insofar incomprehensible, as it appears pretty easy to study direct detrimental effects on male fertility by performing routine semen analyses.
A meta-analysis [22] on male and female fertility-related adverse effects associated with COVID-19 vaccination, above all, sends a powerful signal on the overall poor quality of the studies published within the last three years. Out of 1,406 screened studies, only 20 matched the inclusion criteria for the meta-analysis with 34.5%, 58.6% and 6.9% exhibiting poor, moderate and good quality, respectively. Regarding data on sperm progressive motility, only five studies were included. Authors criticized the consistently low sample size and the short follow-up time of the analysed studies. In addition, we draw the reader´s attention to the fact that from the five studies included in the meta-analysis, three studies were performed in Russia including solely men injected with the Gam-COVID-Vac(SputnikV) vaccine and one study was performed in China including solely men injected with an “inactivated COVID-19 vaccine” that was not further specified. This means in effect that only one study [23] was analysed that includes men injected with a RNA-based vaccine. This justifies by no means the conclusion drawn by the authors that “based on the studies published so far, there is no scientific proof of any association between COVID-19 vaccines and fertility impairment in men…”
Following, we discuss ten studies performing semen analyses in men pre- and post-injection with a RNA-based vaccine. Six studies [24-29] were performed in sperm banks and four studies [23,30-32] in IVF centers. Only one study [23] was included in the aforementioned meta-analysis [22] on sperm progressive motility representing the most powerful parameter for male fertility. Another three studies [24,29,30] were included regarding sperm concentration. Two studies [25,27] were excluded from the meta-analysis due to poor quality and five studies discussed below were not considered, most likely due to the study deadline of June 8th 2022.
Regarding semen analyses conducted at sperm banks, only one study [26] reported significant differences of semen parameters before versus after COVID-19 vaccination (see below). It must be noted that the remaining five studies exhibit major limitations, that is, a sample size too low and a follow-up time too short to allow any final assessment. Sample size was 33 [25], 45 [24], 47 [28], 72 [29] and 75 [27]. The authors of the latter study [27] even missed to set up a control group and solely compared semen parameters with reference ranges, as defined by the WHO (World Health Organization). Further, authors performed semen analyses on average 37 days after the second COVID-19 injection. This time interval, however, is too short to expect any changes, as one spermatogenic cycle takes 74 days [10]. Although the remaining four studies performed semen analyses approx. 70 days after the second COVID-19 injection, a time interval that matches more or less with the duration of a spermatogenic cycle, it is highly likely that authors predominantly analysed mature (i.e., old) sperm stored in the epididymis. However, due to chromatin condensation, lack of division and poor metabolism, these cells represent probably the most protected cells in a man´s body. To study a possible impact of any environmental parameter on semen quality, a follow-up time of three months, better six months, would have been appropriate to guarantee that “old” epididymal sperm have been replaced. Unexpectedly, one study [24] reported even an elevated sperm concentration and TMSC (Total Motile Sperm Count) after the second COVID-19 injection. Of note, the magnitude of change was within the normal individual variation and, as suggested by the authors, may be due to an increased abstinence time before the second injection. An alternative interpretation of the authors, however, is without any foundation, namely: “Because the vaccines contain mRNA and not the live virus, it is unlikely that the vaccine would affect sperm parameters.” It must be noted that the relevant ingredient of RNA-based vaccines is not mRNA of the virus, but modRNA delivered by lipid nanoparticles (Box 2).
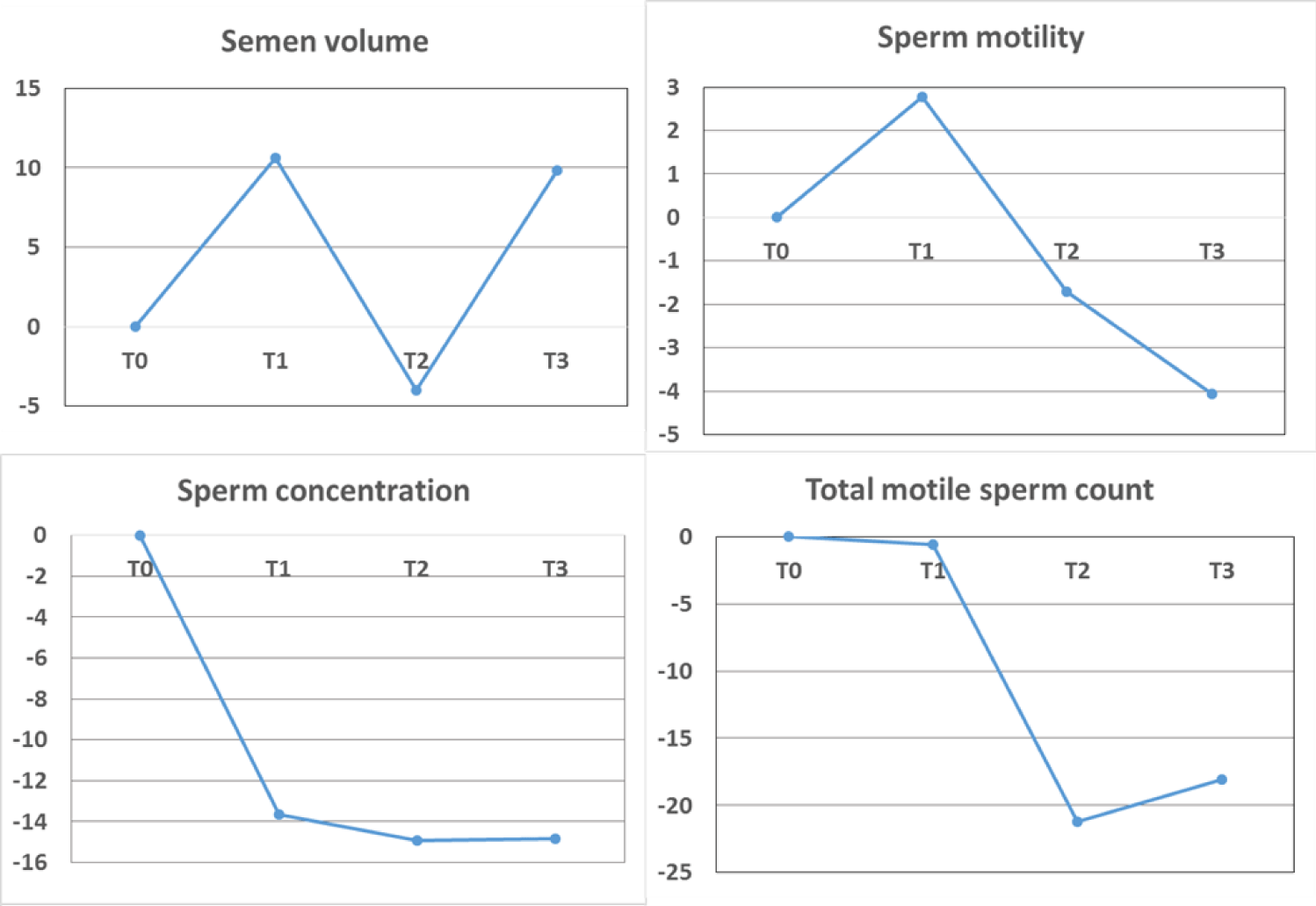
Currently, the most valid data are provided by Gat I, et al. [26]. The authors analysed 216 semen samples from 37 sperm donors and included a pre-vaccination baseline control (T0), as well as three additional evaluations at 15-45 days (T1), 75-125 days (T2) and > 145 days (T3) after completion of vaccination starting seven days after the second injection. The authors reported a decrease of sperm concentration of -15.4% at T2 when compared with T0 resulting in a reduction of the TMSC of -22.1%. Similarly, analysis of first semen sample only and samples’ mean per donor resulted in a reduction of both sperm concentration and TMSC on T2 when compared with T0. Namely, the median decline of 12.0 million/ml and 31.2 million motile spermatozoa on first sample evaluation, respectively, and the median decline of 9.5 million/ml and 27.3 million motile spermatozoa on samples´ mean examination, respectively. Due to “overall recovery” at T3, authors suggested an injection-based systemic immune response (i.e., fever) as the most reasonable explanation for the “temporary decrease” of the sperm concentration. While fever is well-known to negatively affect proper sperm production, the provided explanation is insofar incomprehensible, as diagrams prepared from the published data [26], at T3, still exhibit significantly decreased values for sperm concentration, sperm motility and TMSC (Figure 6).
Despite the valid study design and the high sample number (n = 216), the number of sperm donors (n = 37) is too low to allow for a generalization of the published data. Unfortunately, “samples produced after third (booster) vaccination dose were excluded from the study.” The authors did not provide an explanation for their decision within their publication, but upon request argued that booster represents an additional intervention, which may interfere with the final outcome and, therefore, has been excluded in order to have a clean methodology (personal communication).
The authors mentioned the focus on sperm donors rather than the general population of patients with subfertility as an important limitation of their study. This adoption is based on a study [33], which analysed 2,043 semen samples from 65 sperm donors and 479 semen samples from 74 subfertile men and reported high within-subject and between-subject variations with the sperm count exhibiting the greatest variability and the sperm motility the lowest. As expected, sperm donors revealed an overall lower variability of these parameters than subfertile men.
So far, only four studies performed semen analyses in male partners from women attending IVF centers. Abd ZH, et al. [31] compared semen parameters from 60 men before and > 90 days after COVID-19 injection excluding men reporting infection or post-vaccination fever. The authors demonstrated statistically significant differences for total motility and progressive motility, thus corroborating data reported by Gat I, et al. [26]. The remaining three studies reported no significant differences. The study by Orvieto R, et al. [30] included only 36 men. Of note, the time interval between the second COVID-19 injection and the post-vaccination semen analysis was highly variable with a mean of 33.3 ± 14.9 days. The retrospective study by Reschini M, et al. [23] analysed 106 men. It is reported that the median time interval between the first vaccine dose and the second ART (Assisted Reproductive Technology) attempt was 75 days, with a range between 39 days and 112 days. Besides the fact that men received different vaccine types (DNA- and RNA-based), complete vaccination requires two doses with a time interval of at least two weeks. Consequently, the observation period for most of the analysed samples is again less than one spermatogenic cycle. The study by Karavani G, et al. [32] included 58 men, with 42 displaying normal sperm parameters and 16 exhibiting male factor infertility. All men received at least two BNT162b2 injections. 13 men were analysed 6-9 months after the second dose, whereas 45 men were analysed 9-14 months after the second dose. No significant differences in semen volume, concentration, motility, normal morphology and total motile sperm count were observed. This applied equally to a subgroup of 47 men receiving a third (booster) injection.
Besides a direct effect of COVID-19 injections on male germ cell development and semen quality, indirect effects are also conceivable.
In figure 4, we demonstrate a strong correlation between the frequency of adverse effects for heart failure and ED, both of which share endothelial dysfunction as a common pathological basis. Of note, the vascular endothelium seems to be particularly affected by the spike protein resulting in inflammation and functional impairment. There is evidence that this applies not only for severe SARS-CoV-2 infections [34], but also for the spike protein produced after COVID-19 injection [35,36]. Therefore, it is highly likely that testicular capillaries may also be affected with a delay of several months or even years. Reduced blood supply will then be followed by oxygen and nutrient deficiencies within a defined area and finally will result in germ cell depletion and reduced semen quality.
In addition, long-term cellular stress may promote the development of tumor cells. High levels of LINE1 have been demonstrated not only in sperm [37], but also in association with various tumor tissues [38]. In the latter, it is thought to be caused by inactivation of tumor suppressor genes. As depicted in figure 5, the anecdotal evidence of an increased case number of testicular tumor in four players of the German first division football league (“1. Bundesliga”) after COVID-19 injection [14] is, so far, not expressed by hard statistical data. However, there remains an undeniable risk, when in future, young boys will be vaccinated, as GCNIS (Germ Cell Neoplasia In-Situ) is based on abnormal differentiation of primordial germ cells and, therefore, the time before puberty represents a critical period for the manifestation of testicular tumor.
Perspective
In light of the reported situation of concern, it is about time to open an overdue scientific dialogue on possible long-term adverse effects of COVID-19 RNA-injections on male fertility. An overview on the operating principle of RNA-based COVID-19 vaccine technology is provided in box 2.
Box 2: RNA-based vaccine technology at a glance.
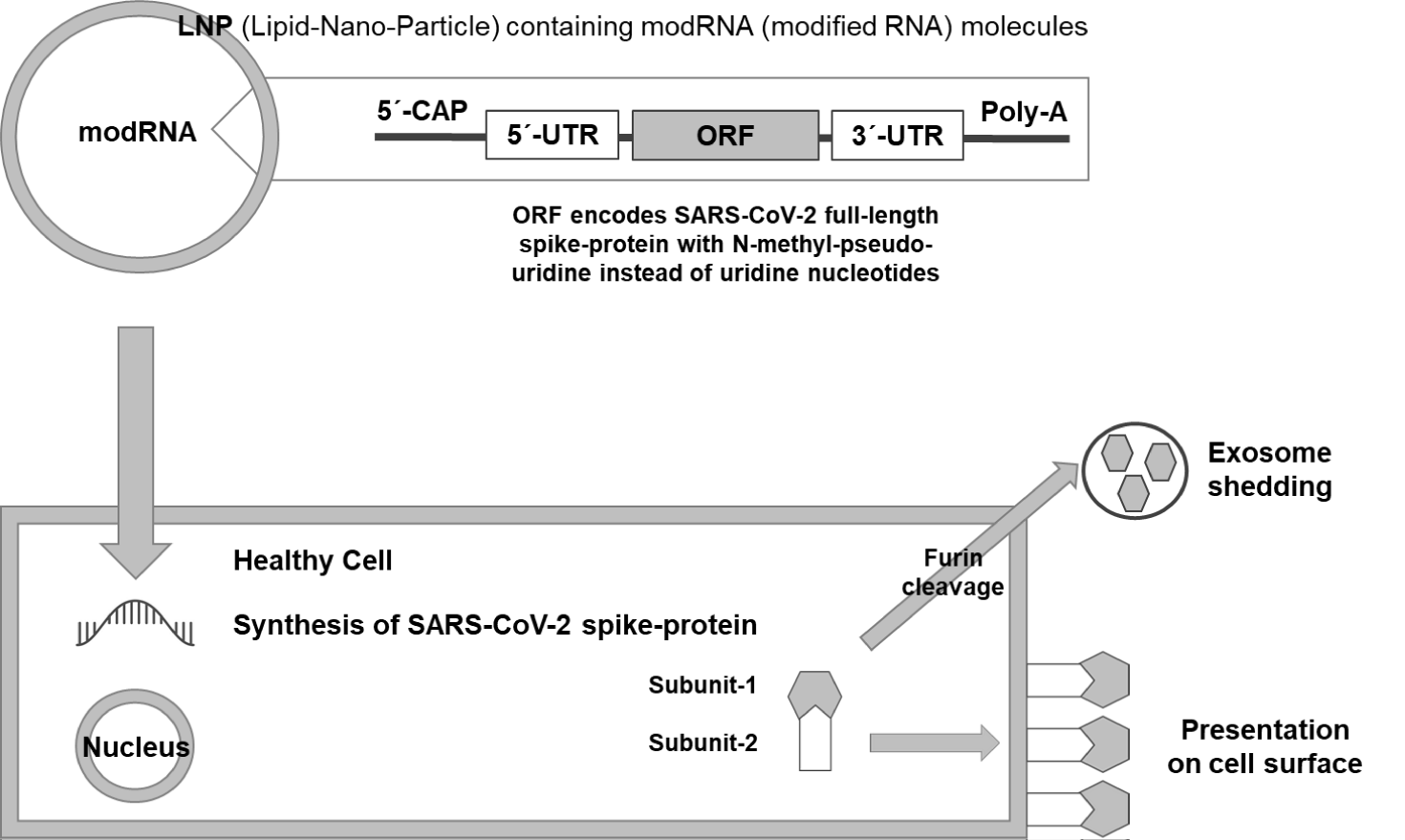
Contrary to conventional vaccines and for the first time in medical history, RNA-based vaccines do not deliver a viral protein, but force healthy cells to produce a viral protein, here the spike protein of SARS-CoV-2. Therefore, RNA-based COVID-19 injections represent gene therapy medicinal products rather than vaccines [39]. To protect the administered RNA against degradation by RNases, it is enclosed in LNPs (Lipid NanoParticles), which due to their small size (< 100 nm) are able to pass biological barriers. As LNPs resemble exosomes, they can also take advantage of the natural endocytosis process. While incorporated phospholipids facilitate cellular uptake, polyethylene glycol is a well known allergen [40]. Although the underlying mechanisms are not yet fully understood, there is evidence that LNPs trigger ROS (Reactive Oxygen Species) production, which is followed by an increase in oxidative stress promoting DNA damage. Accumulation of LNPs in reproductive organs disrupts the hormonal balance and causes inflammation and, therefore, may per se negatively affect reproductive health. For further details regarding LNP technology, refer to reviews by Lan Z, et al. [41] and Wang R, et al. [42]. The administered RNA sequence represents an ORF (Open Reading Frame) encoding for the full length SARS-CoV-2 spike protein, the intended target for the production of neutralizing antibodies. Due to a lack of experience, it can only be speculated how long and to what extent this process will be active. Of note, the inoculated RNA did not mimic viral RNA, but instead is “humanized” in order to promote ribosomal translation by the human cell equipment. This modRNA (modified RNA) comprises a 7-methylguanosine CAP and an UTR (UnTranslated Region) at the 5´end, as well as an UTR and a poly-A tail at the 3´ end, the latter of which is also known to play a vital role in LINE1 mediated retroposition [43]. Within the ORF, uridine nucleotides are replaced by N-methyl-pseudo-uridine nucleotides (preventing recognition by innate immune sensors, i.e., TLRs (Toll Like Receptors)) and adenine/uracil nucleotides at the third position within the codons were replaced by guanine/cytosine nucleotides in order to increase both stability and translational efficiency. As reported on the Biontech homepage [44]: “We demonstrated that the presence of a variety of modified nucleosides in the manufactured mRNA suppresses its intrinsic immune activation, while leading to superior protein production for long duration.“ Due to the presence of a furin cleavage site, subunit-1 of the spike protein can split off and leave the cell via exocytosis. Subsequently, it can be distributed throughout the body by the circulatory system. Likewise alarming is the fact that the full length spike protein is presented at the cell surface of the producing cell, hence tagging it with a “foreign label” that converts the former healthy cell into an apparent target to be attacked by the immune system. For further details regarding RNA technology, refer to reviews by Sahin U, et al. [45] and Seneff S [46]. While cell entry of SARS-CoV-2 via binding of the spike protein subunit-1 to the ACE2 receptor followed by endocytosis is well known, recently, an alternative metalloproteinase pathway has been reported [47]. Here, cell entry occurs via cell cell fusion regulated by the furin cleavage site located within the spike protein subunit-2.
It is suggested that this pathway may explain the emergence of COVID-19 symptoms in fully vaccinated individuals. For further details, refer to review by Sfera A, et al. [47].
As RNA-based vaccines lack reverse transcriptase, politicians and mass media worldwide have denied, and still deny, that administered RNA can be reverse transcribed and integrated into the human genome. However, Zhang L, et al. [48] demonstrated that SARS-CoV-2 RNA can reverse transcribe without reverse transcriptase and, in addition, DNA copies can integrate into the genome of infected human HEK293T cells. Authors reported target site duplications flanking the viral sequences and consensus LINE1 (Long Interspersed Nuclear Element-1) endonuclease recognition sequences at the integration sites suggesting a LINE1-mediated retroposition mechanism. It is well-known that the autonomous retrotransposon LINE1 comprising approx. 17% of the human genome [49] can act as an endogenous reverse transcriptase [50] and exhibit increased expression upon viral infection [51] including SARS-CoV-2 [52]. Zhang L, et al. [48] provided also evidence that, in some patient samples, the majority of viral transcripts appeared to be derived from integrated viral sequences. In addition, Alden M, et al. [53] demonstrated that COVID-19 mRNA vaccine BNT162b2 is able to enter human Huh7 liver cells and will be reverse transcribed into DNA as fast as six hours following exposure. Immunohistochemistry with an antibody against LINE1 ORFp1 (Open Reading Frame RNA-binding protein-1) revealed increased LINE1 nuclear distribution and PCR on genomic DNA amplified the DNA sequence unique to BNT162b2.
While our current knowledge is based on results obtained in cell cultures, it is important to prove whether vaccine-derived RNA will indeed integrate into the genome of a vaccinated individual and, even more important, into the germ cells genome. In the probably rare case of genome integration, the question arises whether these sequences do indeed express viral antigens? In the case when a cell with an integrated and expressed SARS-CoV-2 sequence survives and presents a viral antigen after infection has already been cleared by the immune system, this might induce permanent stimulation of the immune system and, as a consequence, may trigger a condition comparable to that in autoimmunity [54]. Another conceivable scenario might be that the integrated SARS-CoV-2 sequence remains silent, but may be activated upon environmental stimuli comparable to the Herpes zoster “strategy” [55].
Whether viral genetic information can integrate into human genomic DNA is not a question, but a fact, as up to 8% of the human genome does not derive from our ancestors, but from retroviruses, i.e., HERVs (Human Endogenous RetroViruses) [56]. Although SARS-CoV-2 is not a retrovirus, but a single-stranded RNA virus, RNA-integration into human DNA is feasible in various ways, i.e., via the aforementioned LINE1 reverse transcription or the human reverse transcriptase polymerase-theta. For further details, refer to reviews by Domazet-Loso T [57] and Kyriakopoulos AM, et al. [58].
Inheritance of viral-derived genome sequences occurs when viral DNA, reverse transcribed from viral RNA, integrates into DNA of a reproductive cell and, subsequently, is passed on from father or mother to the offspring. Well-known examples with an important role for human evolution are HERV-W and HERV-FRD, expression of which results in the synthesis of syncytin, which is involved in the generation of the syncytiotrophoblast cell layer during placentogenesis [59]. If it should turn out that vaccine-derived RNA, reverse transcribed into DNA, can indeed integrate into a germ cell´s DNA, there is also a high likelihood of inheritance and spike protein production in the offspring. High LINE1 levels have been reported in sperm, which can reverse transcribe exogenous RNA into DNA and deliver plasmids packaging up this DNA to the oocyte upon fertilization. Subsequently, plasmids will propagate themselves within the embryo [37].
Due to massive chromatin condensation, sperm is a transcriptionally inactive cell and, therefore, sperm RNAs have for a long time been considered irrelevant remnants of spermatogenesis. However, sperm RNAs have been demonstrated to play an active role in sperm function, fertility and even conception [60]. While the quantity of RNA in sperm appears to be rather small when compared with the amount in oocytes, it has been reported to be sufficient to have an impact on transgenerational inheritance [61]. For further details regarding sperm RNA, refer to review by Godia M, et al. [62]. It should be noted that the oocyte upon fertilization is exposed not only to the sperm´s haploid genome, but also to potential hosts, i.e., retroviruses and/or retrotransposons, both of which bear the potential to alter the genome of the zygote and to affect the development of the embryo [63]. This potential may also apply to inoculated RNA sequences, which manage to take the path into sperm. It is known that retroviruses and/or retrotransposons can insert into the genome and affect gene expression by acting as an enhancer altering splicing or polyadenylation [64]. While transcription of LINE1 affects chromatin accessibility in mouse embryos, no such genes implicated in embryonic genome activation have so far been identified in LINE1 of sperm [65]. By contrast, disruption of LINE1-encoded reverse transcriptase has been demonstrated in early mouse embryos [66]. In addition, some RNA-types present in both sperm and oocytes, i.e., miRNAs and piRNAs [62,67], have been suggested to degrade parasite-derived RNAs, hence ensuring genome integrity between generations. However, nucleic acids encapsulated in exosomes have been reported to distribute throughout the circulation system, pass the blood-testis-barrier and enter sperm [66,68]. As LNPs containing the modRNA resemble exosomes, it is highly likely that this will also apply to the administered RNA-based vaccines. In a series of experiments in mice xenografted with human tumor cells, soma-to-germline transmission of RNAs has been reported suggesting a LINE1-based reverse transcription and an exosome-based mechanism of transport [69,70].
The bottom line is that there are several conceivable pathways how spike proteins in reproductive organs may affect germ cell development and semen quality. To date, nobody knows whether the reported decrease of semen quality after COVID-19 injections represents only a temporary or a long-lasting effect. The fact that men experience a permanent renewal of their germ cells may act to “wash-out” negative environmental exposures and “reset” the original genetic program. However, this strategy will fail, if vaccine-derived RNA, reverse transcribed into DNA, will manage to integrate into a spermatogonial stem cell´s DNA. In this probably rare case, sperm will forward the genetic information for SARS-CoV-2 spike protein to the offspring. As mechanisms regulating sperm-mediated gene transfer upon fertilization and the significance of sperm LINE1 endogenous reverse transcriptase for potential generation of new genetic information that may be forwarded to the offspring are still far from being understood, new techniques for medical therapies must be excluded until harmlessness beyond doubt has been proven by scientifically valid, long-term clinical studies. In addition to factors with a direct impact on male germ cell development followed by a prompt decrease of sperm quality, male subfertility on a long-term perspective may be an indirect effect caused by general co-morbidities, i.e., testicular tumor, as well as (micro) thromboses in capillaries of the testis (resulting in oxygen and nutrient deficiencies and germ cell loss) and/or the penis (resulting in erectile dysfunction).
Initially, we aimed, inter alia, at re-initiating the currently missing scientific dialogue, however, based on the poor quality of the available studies (additional proof has been provided by a recent meta-analysis [22]), we realized that medical science needs a reality check and must return to reason and evidence in order to restore its damaged credibility. Besides higher sample numbers and longer follow-up observation, future studies should contain information on both vaccine-types and batch numbers in conjunction with obtained results, as there is evidence for a large batch-to-batch variability with a minority of batches causing the majority of severe adverse effects [71]. Interdisciplinary discussion on possible secondary health conditions must not only be allowed, but promoted. Finally, science and medicine must liberate itself from political narratives. In this specific situation, scientists and medical doctors must realize that the active ingredient of “mRNA-based vaccines” is not simply a mRNA molecule carrying the information for the synthesis of a specific protein, nota bene an exogenous viral protein, but modRNA specifically designed for translational efficacy and longevity encapsulated in LNPs to bypass biological barriers and get access to all cells including heart and brain [36]. What is the underlying rationale when an originally healthy cell within heart or brain starts to synthesize a viral protein transforming this cell into a target to be attacked by our immune system? Finally, it has to be kept in mind that mRNA is also involved in the regulation of gene expression [72], which is why cells have mechanisms “at hand” to silence mRNA species not required. Theses protective mechanisms, however, will not work with modRNA [73].
Conflict of Interest
The authors declare no conflict of interest, in particular no financial relationships with organizations that might have an interest in the submitted work and no activities that could appear to have influenced the submitted work. There was no funding of this project.
References
- Rajak P, Roy S, Dutta M, Podder S, Sarkar S, Ganguly A, Mandi M, Khatun S. Understanding the cross-talk between mediators of infertility and COVID-19. Reprod Biol. 2021 Dec;21(4):100559. doi: 10.1016/j.repbio.2021.100559. Epub 2021 Sep 1. PMID: 34547545; PMCID: PMC8407955.
- Wang Z, Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020 Apr 9;9(4):920. doi: 10.3390/cells9040920. PMID: 32283711; PMCID: PMC7226809.
- Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011 Jan;95(1):176-81. doi: 10.1016/j.fertnstert.2010.06.060. Epub 2010 Aug 1. PMID: 20674894.
- Diaz P, Zizzo J, Balaji NC, Reddy R, Khodamoradi K, Ory J, Ramasamy R. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia. 2022 May;54(4):e14361. doi: 10.1111/and.14361. Epub 2021 Dec 30. PMID: 34970749.
- Stukenborg JB, Colón E, Söder O. Ontogenesis of testis development and function in humans. Sex Dev. 2010 Sep;4(4-5):199-212. doi: 10.1159/000317090. Epub 2010 Jul 27. PMID: 20664245.
- Larose H, Shami AN, Abbott H, Manske G, Lei L, Hammoud SS. Gametogenesis: A journey from inception to conception. Curr Top Dev Biol. 2019;132:257-310. doi: 10.1016/bs.ctdb.2018.12.006. Epub 2019 Jan 8. PMID: 30797511; PMCID: PMC7133493.
- Sharma R, Agarwal A. Spermatogenesis: An Overview. Agarwal A, editor. New York: Springer; 2011.
- Schagdarsurengin U, Paradowska A, Steger K. Analysing the sperm epigenome: roles in early embryogenesis and assisted reproduction. Nat Rev Urol. 2012 Nov;9(11):609-19. doi: 10.1038/nrurol.2012.183. Epub 2012 Oct 9. PMID: 23045264.
- Steger K, Balhorn R. Sperm nuclear protamines: A checkpoint to control sperm chromatin quality. Anat Histol Embryol. 2018 Aug;47(4):273-279. doi: 10.1111/ahe.12361. Epub 2018 May 23. PMID: 29797354.
- Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008 Sep-Oct;29(5):469-87. doi: 10.2164/jandrol.107.004655. Epub 2008 May 22. PMID: 18497337.
- ISO 11462-1:2001(en). Guidelines for implementation of Statistical Process Control (SPC). Part 1: Elements of SPC. 1st ed.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. 2022 Nov 15:dmac035. doi: 10.1093/humupd/dmac035. Epub ahead of print. PMID: 36377604.
- Diaconu CC, Manea M, Marcu DR, Socea B, Spinu AD, Bratu OG. The erectile dysfunction as a marker of cardiovascular disease: a review. Acta Cardiol. 2020 Aug;75(4):286-292. doi: 10.1080/00015385.2019.1590498. Epub 2019 Apr 6. PMID: 30955454.
- Blick. Available online: https://tinyurl.com/2q34ajlr (only German).
- Bujard M, Andersson G. Fertility declines near the end of the COVID-19 pandemic: Evidence of the 2022 birth declines in Germany and Sweden. Martin B, Gunnar A. Sweden: BiB Working Paper 6/2022; 2022.
- Antonini M, Eid MA, Falkenbach M, Rosenbluth ST, Prieto PA, Brammli-Greenberg S, McMeekin P, Paolucci F. An analysis of the COVID-19 vaccination campaigns in France, Israel, Italy and Spain and their impact on health and economic outcomes. Health Policy Technol. 2022 Jun;11(2):100594. doi: 10.1016/j.hlpt.2021.100594. Epub 2021 Dec 24. PMID: 34976711; PMCID: PMC8702636.
- Bonanad C, García-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-González V, Fácila L, Ariza A, Núñez J, Cordero A. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J Am Med Dir Assoc. 2020 Jul;21(7):915-918. doi: 10.1016/j.jamda.2020.05.045. Epub 2020 May 25. PMID: 32674819; PMCID: PMC7247470.
- DSouza KN, Orellana M, Ainsworth AJ, Cummings G, Riggan KA, Shenoy CC, Allyse MA. Impact of the COVID-19 Pandemic on Patient Fertility Care. J Patient Exp. 2022 May 6;9:23743735221098255. doi: 10.1177/23743735221098255. PMID: 35548663; PMCID: PMC9083039.
- Bowman CJ, Bouressam M, Campion SN, Cappon GD, Catlin NR, Cutler MW, Diekmann J, Rohde CM, Sellers RS, Lindemann C. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol. 2021 Aug;103:28-35. doi: 10.1016/j.reprotox.2021.05.007. Epub 2021 May 28. PMID: 34058573; PMCID: PMC8163337.
- Freedom of Information request no. 2021-4389. 2022.
- European Medicine Agency (EMA).
- Zaçe D, La Gatta E, Petrella L, Di Pietro ML. The impact of COVID-19 vaccines on fertility-A systematic review and meta-analysis. Vaccine. 2022 Oct 6;40(42):6023-6034. doi: 10.1016/j.vaccine.2022.09.019. Epub 2022 Sep 12. PMID: 36137903; PMCID: PMC9464596.
- Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, Fornelli G, Dolci C, Cermisoni GC, Viganò P, Somigliana E, Coccia ME, Papaleo E. COVID-19 Vaccination Does Not Affect Reproductive Health Parameters in Men. Front Public Health. 2022 Feb 2;10:839967. doi: 10.3389/fpubh.2022.839967. PMID: 35186854; PMCID: PMC8847439.
- Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, Ramasamy R. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA. 2021 Jul 20;326(3):273-274. doi: 10.1001/jama.2021.9976. PMID: 34137808; PMCID: PMC8293015.
- Barda S, Laskov I, Grisaru D, Lehavi O, Kleiman S, Wenkert A, Azem F, Hauser R, Michaan N. The impact of COVID-19 vaccine on sperm quality. Int J Gynaecol Obstet. 2022 Jul;158(1):116-120. doi: 10.1002/ijgo.14135. Epub 2022 Feb 26. PMID: 35128663; PMCID: PMC9087610.
- Gat I, Kedem A, Dviri M, Umanski A, Levi M, Hourvitz A, Baum M. Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology. 2022 Sep;10(6):1016-1022. doi: 10.1111/andr.13209. Epub 2022 Jun 27. PMID: 35713410; PMCID: PMC9350322.
- Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online. 2022 Jan;44(1):145-149. doi: 10.1016/j.rbmo.2021.09.021. Epub 2021 Oct 4. PMID: 34815157; PMCID: PMC8489287.
- Olana S, Mazzilli R, Salerno G, Zamponi V, Tarsitano MG, Simmaco M, Paoli D, Faggiano A. 4BNT162b2 mRNA COVID-19 vaccine and semen: What do we know? Andrology. 2022 Sep;10(6):1023-1029. doi: 10.1111/andr.13199. Epub 2022 Jun 8. PMID: 35647664; PMCID: PMC9348225.
- Safrai M, Herzberg S, Imbar T, Reubinoff B, Dior U, Ben-Meir A. The BNT162b2 mRNA Covid-19 vaccine does not impair sperm parameters. Reprod Biomed Online. 2022 Apr;44(4):685-688. doi: 10.1016/j.rbmo.2022.01.008. Epub 2022 Jan 31. PMID: 35279377; PMCID: PMC8801893.
- Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol. 2021 May 13;19(1):69. doi: 10.1186/s12958-021-00757-6. PMID: 33985514; PMCID: PMC8116639.
- Abd ZH, Muter SA, Saeed RAM, Ammar O. Effects of Covid-19 vaccination on different semen parameters. Basic Clin Androl. 2022 Aug 2;32(1):13. doi: 10.1186/s12610-022-00163-x. PMID: 35915409; PMCID: PMC9343088.
- Karavani G, Chill HH, Meirman C, Gutman-Ido E, Herzberg S, Tzipora T, Imbar T, Ben-Meir A. Sperm quality is not affected by the BNT162b2 mRNA SARS-CoV-2 vaccine: results of a 6-14 months follow-up. J Assist Reprod Genet. 2022 Oct;39(10):2249-2254. doi: 10.1007/s10815-022-02621-x. Epub 2022 Sep 17. PMID: 36114906; PMCID: PMC9483282.
- Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril. 2006 Jan;85(1):128-34. doi: 10.1016/j.fertnstert.2005.06.048. PMID: 16412742.
- Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020 Jun 16;24(1):353. doi: 10.1186/s13054-020-03062-7. PMID: 32546188; PMCID: PMC7296907.
- Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Zhang J, Yuan JX, Malhotra A, Manor U, Wang S, Yuan ZY, Shyy JY. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ Res. 2021 Apr 30;128(9):1323-1326. doi: 10.1161/CIRCRESAHA.121.318902. Epub 2021 Mar 31. PMID: 33784827; PMCID: PMC8091897.
- Mörz M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines (Basel). 2022 Oct 1;10(10):1651. doi: 10.3390/vaccines10101651. PMID: 36298516; PMCID: PMC9611676.
- Pittoggi C, Beraldi R, Sciamanna I, Barberi L, Giordano R, Magnano AR, Torosantucci L, Pescarmona E, Spadafora C. Generation of biologically active retro-genes upon interaction of mouse spermatozoa with exogenous DNA. Mol Reprod Dev. 2006 Oct;73(10):1239-46. doi: 10.1002/mrd.20550. PMID: 16850445.
- Zhang X, Zhang R, Yu J. New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation. Front Cell Dev Biol. 2020 Aug 7;8:657. doi: 10.3389/fcell.2020.00657. PMID: 32850797; PMCID: PMC7426637.
- Cosentino M, Marino F. Understanding the Pharmacology of COVID-19 mRNA Vaccines: Playing Dice with the Spike? Int J Mol Sci. 2022 Sep 17;23(18):10881. doi: 10.3390/ijms231810881. PMID: 36142792; PMCID: PMC9502275.
- Hong L, Wang Z, Wei X, Shi J, Li C. Antibodies against polyethylene glycol in human blood: A literature review. J Pharmacol Toxicol Methods. 2020 Mar-Apr;102:106678. doi: 10.1016/j.vascn.2020.106678. Epub 2020 Jan 23. PMID: 31981619.
- Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine (Lond). 2012 Apr;7(4):579-96. doi: 10.2217/nnm.12.20. PMID: 22471721.
- Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018 Dec 11;13:8487-8506. doi: 10.2147/IJN.S170723. PMID: 30587973; PMCID: PMC6294055.
- Doucet AJ, Wilusz JE, Miyoshi T, Liu Y, Moran JV. A 3' Poly(A) Tract Is Required for LINE-1 Retrotransposition. Mol Cell. 2015 Dec 3;60(5):728-741. doi: 10.1016/j.molcel.2015.10.012. Epub 2015 Nov 12. PMID: 26585388; PMCID: PMC4671821.
- Biontech Homepage.
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014 Oct;13(10):759-80. doi: 10.1038/nrd4278. Epub 2014 Sep 19. PMID: 25233993.
- Seneff S, Nigh G. Worse than the Disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. Int J Vacc Theory Pract Res. 2021;2:38-79. doi: 10.56098/ijvtpr.v2i1.23.
- Sfera A, Thomas KG, Sfera DO, Anton JJ, Andronescu CV, Jafri N, Sasannia S, Kozlakidis Z. Do messenger RNA vaccines induce pathological syncytia? Int J Pathol Clin Res. 2022;8:137. doi: 10.23937/2469-5807/1510137.
- Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2105968118. doi: 10.1073/pnas.2105968118. PMID: 33958444; PMCID: PMC8166107.
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, Szustakowki J; International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001 Feb 15;409(6822):860-921. doi: 10.1038/35057062. Erratum in: Nature 2001 Aug 2;412(6846):565. Erratum in: Nature 2001 Jun 7;411(6838):720. Szustakowki, J [corrected to Szustakowski, J]. PMID: 11237011.
- Coffin JM, Fan H. The Discovery of Reverse Transcriptase. Annu Rev Virol. 2016 Sep 29;3(1):29-51. doi: 10.1146/annurev-virology-110615-035556. Epub 2016 Jul 22. PMID: 27482900.
- Jones RB, Song H, Xu Y, Garrison KE, Buzdin AA, Anwar N, Hunter DV, Mujib S, Mihajlovic V, Martin E, Lee E, Kuciak M, Raposo RA, Bozorgzad A, Meiklejohn DA, Ndhlovu LC, Nixon DF, Ostrowski MA. LINE-1 retrotransposable element DNA accumulates in HIV-1-infected cells. J Virol. 2013 Dec;87(24):13307-20. doi: 10.1128/JVI.02257-13. Epub 2013 Oct 2. PMID: 24089548; PMCID: PMC3838212.
- Yin Y, Liu XZ, He X, Zhou LQ. Exogenous Coronavirus Interacts With Endogenous Retrotransposon in Human Cells. Front Cell Infect Microbiol. 2021 Feb 25;11:609160. doi: 10.3389/fcimb.2021.609160. PMID: 33732659; PMCID: PMC7959850.
- Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol. 2022 Feb 25;44(3):1115-1126. doi: 10.3390/cimb44030073. PMID: 35723296; PMCID: PMC8946961.
- Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 9;7(5):e781. doi: 10.1212/NXI.0000000000000781. PMID: 32518172; PMCID: PMC7309518.
- Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021 Oct 9;60(SI):SI90-SI95. doi: 10.1093/rheumatology/keab345. PMID: 33848321; PMCID: PMC8083327.
- Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci U S A. 2004 Apr 6;101(14):4894-9. doi: 10.1073/pnas.0307800101. Epub 2004 Mar 25. PMID: 15044706; PMCID: PMC387345.
- Domazet-Lošo T. mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes (Basel). 2022 Apr 20;13(5):719. doi: 10.3390/genes13050719. PMID: 35627104; PMCID: PMC9141755.
- Kyriakopoulos AM, McCullough PA, Nigh G, Seneff S. Potential mechanisms for human genome integration of genetic code from SARS-CoV-2 mRNA. Vaccination. PrePrint. 2022. doi:10.22541/au.166203678.82079667/v1.
- Morozov VA, Dao Thi VL, Denner J. The transmembrane protein of the human endogenous retrovirus--K (HERV-K) modulates cytokine release and gene expression. PLoS One. 2013 Aug 7;8(8):e70399. doi: 10.1371/journal.pone.0070399. PMID: 23950929; PMCID: PMC3737193.
- Zhao Y, Li Q, Yao C, Wang Z, Zhou Y, Wang Y, Liu L, Wang Y, Wang L, Qiao Z. Characterization and quantification of mRNA transcripts in ejaculated spermatozoa of fertile men by serial analysis of gene expression. Hum Reprod. 2006 Jun;21(6):1583-90. doi: 10.1093/humrep/del027. Epub 2006 Feb 24. PMID: 16501037.
- Rando OJ. Intergenerational Transfer of Epigenetic Information in Sperm. Cold Spring Harb Perspect Med. 2016 May 2;6(5):a022988. doi: 10.1101/cshperspect.a022988. PMID: 26801897; PMCID: PMC4852801.
- Gòdia M, Swanson G, Krawetz SA. A history of why fathers' RNA matters. Biol Reprod. 2018 Jul 1;99(1):147-159. doi: 10.1093/biolre/ioy007. PMID: 29514212.
- Miller D. Confrontation, Consolidation, and Recognition: The Oocyte's Perspective on the Incoming Sperm. Cold Spring Harb Perspect Med. 2015 May 8;5(8):a023408. doi: 10.1101/cshperspect.a023408. PMID: 25957313; PMCID: PMC4526728.
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007 Apr;8(4):272-85. doi: 10.1038/nrg2072. PMID: 17363976.
- Jachowicz JW, Bing X, Pontabry J, Bošković A, Rando OJ, Torres-Padilla ME. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nat Genet. 2017 Oct;49(10):1502-1510. doi: 10.1038/ng.3945. Epub 2017 Aug 28. PMID: 28846101.
- Spadafora C. Sperm-mediated 'reverse' gene transfer: a role of reverse transcriptase in the generation of new genetic information. Hum Reprod. 2008 Apr;23(4):735-40. doi: 10.1093/humrep/dem425. Epub 2008 Feb 11. PMID: 18270183.
- Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA; Reproductive Medicine Network. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013 Nov-Dec;19(6):604-24. doi: 10.1093/humupd/dmt031. Epub 2013 Jul 14. PMID: 23856356; PMCID: PMC3796946.
- Wykes SM, Visscher DW, Krawetz SA. Haploid transcripts persist in mature human spermatozoa. Mol Hum Reprod. 1997 Jan;3(1):15-9. doi: 10.1093/molehr/3.1.15. PMID: 9239704.
- Cossetti C, Lugini L, Astrologo L, Saggio I, Fais S, Spadafora C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS One. 2014 Jul 3;9(7):e101629. doi: 10.1371/journal.pone.0101629. PMID: 24992257; PMCID: PMC4081593.
- Spadafora C. Soma to germline inheritance of extrachromosomal genetic information via a LINE-1 reverse transcriptase-based mechanism. Bioessays. 2016 Aug;38(8):726-33. doi: 10.1002/bies.201500197. Epub 2016 Jun 17. PMID: 27315018.
- Batch codes and associated deaths, disabilities and illnesses for COVID-19 vaccines.
- Hoernes TP, Hüttenhofer A, Erlacher MD. mRNA modifications: Dynamic regulators of gene expression? RNA Biol. 2016 Sep;13(9):760-5. doi: 10.1080/15476286.2016.1203504. Epub 2016 Jun 28. PMID: 27351916; PMCID: PMC5014007.
- Li J, Boix E. Host Defence RNases as Antiviral Agents against Enveloped Single Stranded RNA Viruses. Virulence. 2021 Dec;12(1):444-469. doi: 10.1080/21505594.2021.1871823. PMID: 33660566; PMCID: PMC7939569.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.