> Medicine. 2020 May 27;1(1):003-007. doi: 10.37871/jels1112.
Malignant Cerebral Ischemic Stroke Associated with COVID-19 Infection
Pablo J de la Fuente1*, Iñigo C Pomposo1, Gorka Zabalo1, Javier E Altamirano1, Clara Paternain1, Inigo Sistiaga1, Jone M Iglesias1, Gaizka Bilbao1, Alejandro Carrasco1, Guillermo Carbayo1, Gregorio Catalan1, Edurne Ruiz de Gopegui1, Lara Galbarriatu1, Garazi Bermudez1, and Laura Guio2
2Department of Infectious Diseases, Cruces University Hospital, Barakaldo, Spain
- COVID-19
- Infection
- Stroke
- Ischemia
Introduction: Coronavirus Disease 2019 (COVID-19) originating from Wuhan, China, is spreading around the world. Apart from respiratory, cardiac and vascular complications, acute neurological symptoms and acute cerebrovascular disease have also been observed.
Methods: A 36 year old female with severe cerebral stroke and COVID pneumonia and its clinical characteristics and evolution are described. Results of two retrospective studies about the incidence of Cerebrovascular Disease (CVD) amongst the positive cases for the new coronavirus are shown. An evaluation of the relationship between CVD and previous infections, their stational distribution, and the possible causes of this damage out of the brain is described.
Result: Yanan Li, et al found a 6% of CVD. Ling Mao, et al showed a 5% only amongst the patients defined as severe. Non-severe patients had a 0.8 % incidence. These authors found that in patients with more risk factors that could present more often a severe disease and CVD, some of these factors could in fact be common for a pneumonia or stroke.
We could observe that respiratory infections are described risk factors for CVD, especially for cervico-cerebral Artery Dissections (CAD). The seasonal variation also suggests a possible association.
It is not well known how the new coronavirus spreads through the human body from the lungs. A possible triggering mechanism might be through the interaction with the Angiotensin-Converting Enzyme-2 (ACE-2) or the cytokine cascade that could create blood coagulation disorders.
Conclusion: At this stage of the pandemic, we do not yet know much about the ability of the new coronavirus to produce CVD. This single case report only suggests a possible association between COVID-19 and CVD. More cases with epidemiological data are required to confirm and measure this association, although the role of infections in CVD through a not well-defined mechanism has been described frequently.
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus; COVID-19: Coronavirus Disease 2019; CVD: Cerebrovascular Disease; CAD: Cervico Cerebral Artery Dissection; CT: Computerized Tomography; NIHSS: National institute of Health Stroke Scale; PCR: Polymerase Chain Reaction ; MCA: Middle Cerebral Artery; ACA: Anterior Cerebral Artery; ICA: Internal Carotid Artery; CK: Creatine Kinase; HIV: Human Immunodeficiency Virus; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; ACE-2: Angiotensin Converting Enzyme -2.
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) or Coronavirus Disease 2019 (COVID-19) originating from Wuhan, China, is spreading around the world. The main clinical manifestations of this disease are lung symptoms such as fever, cough and wheezing [1,2]. Apart from the respiratory complications, neurological symptoms and acute Cerebrovascular Disease (CVD) have been observed in patients with COVID-19. As it is shown in retrospective studies, 6% of patients could have CVD, the 83% are ischemic events, amongst the other 17%, the half are haemorrhagic events, and the rest are dural thrombosis or less frequent CVDs [3]. COVID patients with a new onset of CVD are older and more likely to have cardiovascular risk factors. Moreover, these patients used to have a hypercoagulable state and increased inflammatory indicators such as C- reaction protein and D- Dimer. Severe patients more commonly have CVD when compared to non-severe instances [4].
A recent bacterial and viral infection has been described as an individual risk factor for a cerebrovascular ischemia [5]. This association is even higher in the spontaneous cervico-Cerebral Artery Dissection (CAD). The presence of an infection in the month prior to a CVD has been shown twice as common in this group versus other strokes [6,7]. A seasonal variation in spontaneous CVD has been found, showing a higher incidence in autumn and winter, when the frequency of respiratory diseases is at its peak [8].
The incidence of spontaneous CAD is approximately 5 per 100,000 cases per year [9]. It is an important cause of strokes in younger patients, accounting for nearly 20% of strokes in under 45 years patients [10,11]. Hematological diseases could be consider responsible of CAD and other ischemic states in young patients [12].
Aetiopathogenesis of CAD is incompletely understood, although trauma, respiratory infections, and underlying arteriopathy are considered important.
Genetic conditions related with a poor vascular wall are usually thought to be a risk factor for CAD [13]. Environmental factors such as neck manipulation, smoking, oral contraceptives, migraine and hypertension could be part of a badly understood pathogenesis [7].
In this article, we report a case of a patient who presented with a malignant stroke of the left anterior cerebral territory described on the cranial Computerized Tomography (CT) as carotid artery dissection, who was also affected by a COVID-19-related pneumonia.
A 36-year-old female was admitted in the emergency service. She was a nurse assistant in the care of COVID-19 patients and had a history of mixed hyperlipemia, smoking about 10 cigarettes a day, and was under oral contraceptive treatment (Estradio-Nomegestrol).
She was found at home with a language disorder and weakness in right extremities; the last contact with the patient was 36 hours before. She presented with a temperature of 37° C, with all other constants being within normal range. She did not present nausea or vomiting.
On the first neurological examination she had a mixed aphasia with mutism, spontaneous ocular opening with oculocephalic deviation to the left. Right hemiparesis, with right lower extremity plegia, 2/5 strength on the right upper extremity and hemihipoestesia. The right cutaneous plantar reflex was extensor. She got 21 points in the National Institute of Health Stroke Scale (NIHSS).
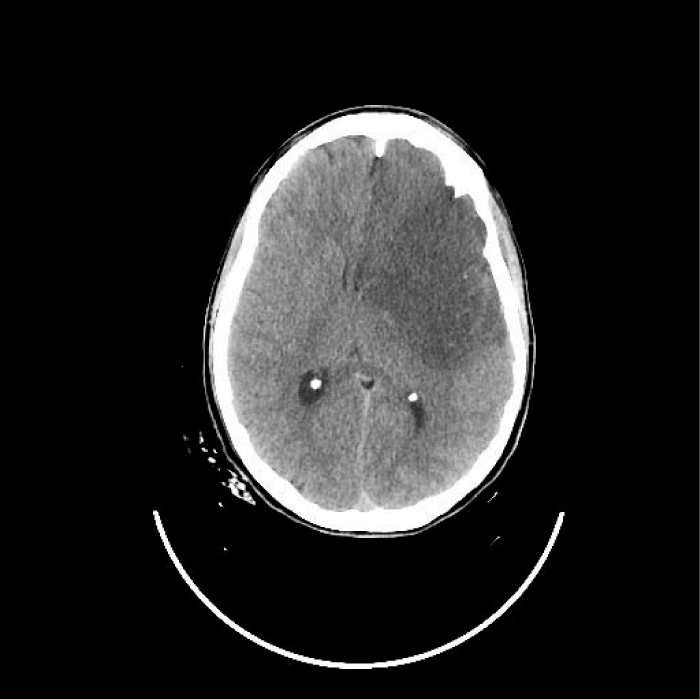
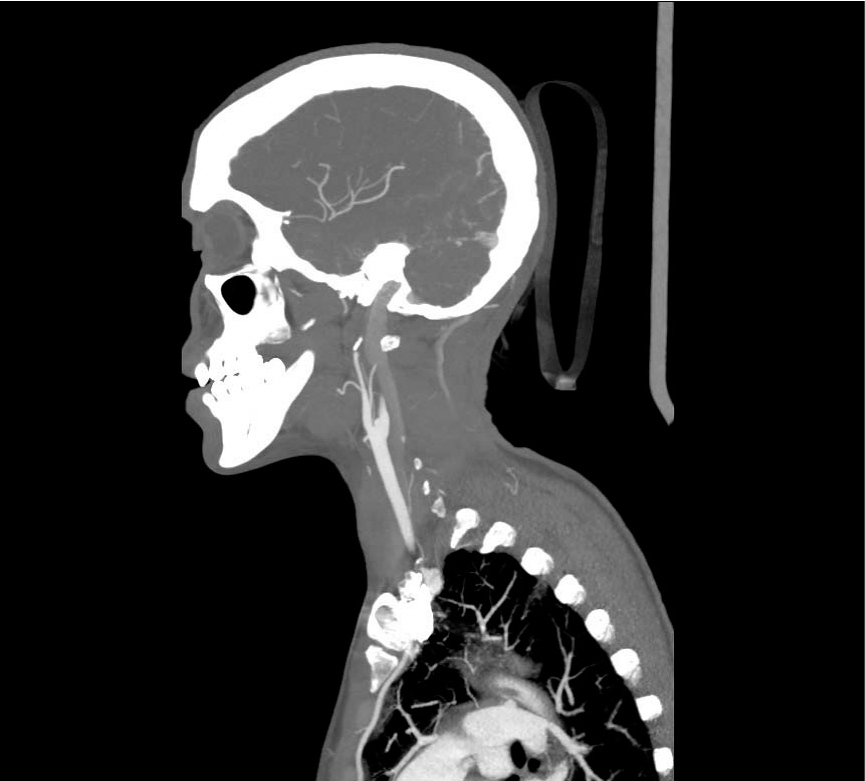
Cranial CT revealed a subacute ischemic lesion of the left Middle Cerebral Artery (MCA) and of Anterior Cerebral Artery (ACA) territory (Figure 1a) associated with a dissection of the left Internal Carotid Artery (ICA) (Figure 1b).
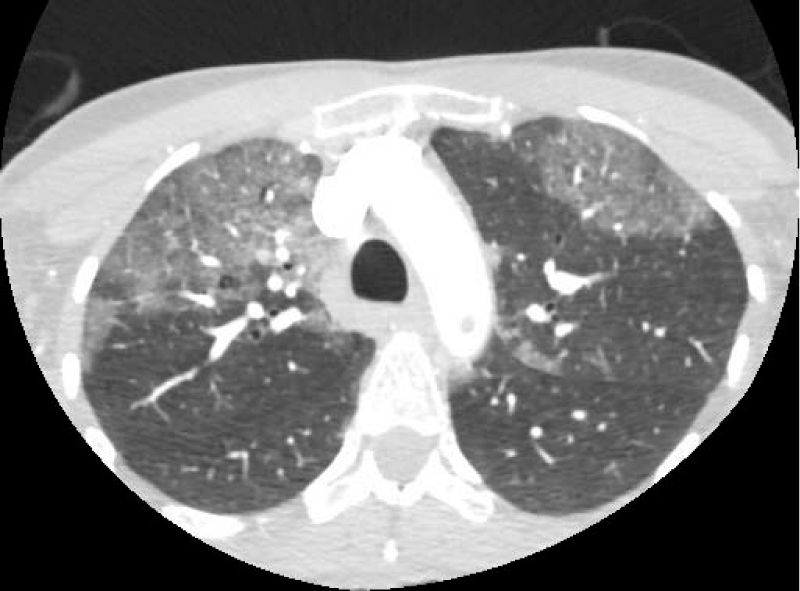
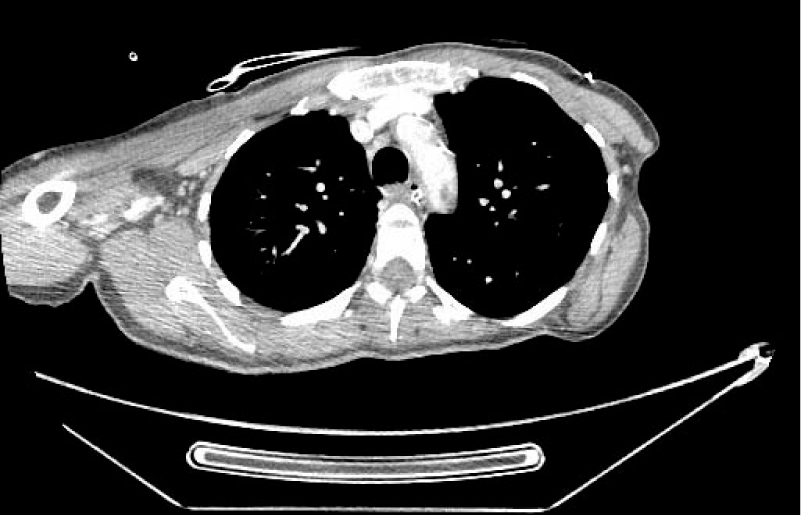
Considering the patient history, a chest CT was also performed where bilateral pulmonary infiltrates compatible with COVID-19 pneumonia were reported (Figure 2). A PCR test for COVID-19 was positive. An echocardiographic study was performed without pathologic discovers.
Blood test performed in the emergency department showed: C-Reactive Protein (PCR) 156 mg/dl, Creatine Kinase (CK) 8,779 U/l, 455,000 platelets/mm3, 23,600 leukocytes/mm3, fibrinogen > 750 mg/dl, LDH 659 U/l and D-Dimer 7,540 ng/ml. Ferritin values were normal (75 ng/ml).
Serological studies were also carried out for HIV, HBV, HCV and Treponema pallidum, all of which were negative, and urine tests for drugs of abuse were positive for cannabis.
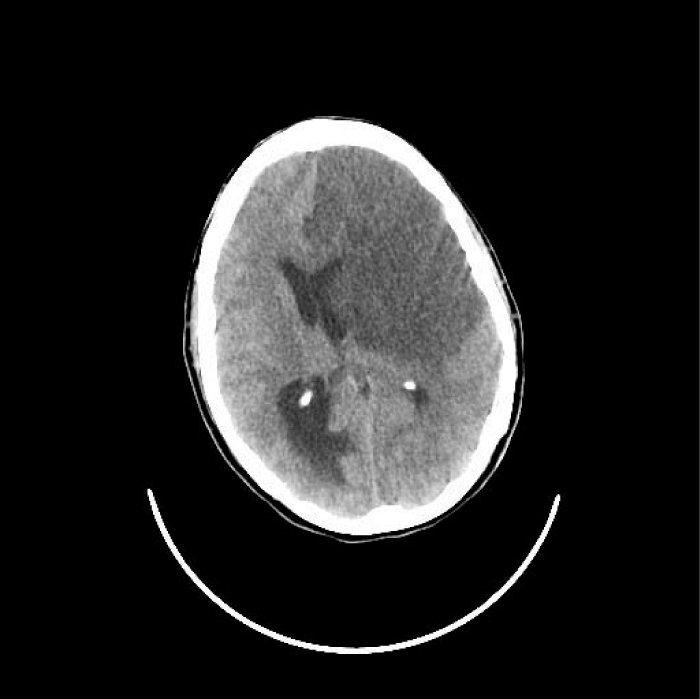
The endovascular treatment was rejected because of the established ischemia. The patient was admitted to the Intensive Care Unit, where antiplatelet therapy was started. 24 hours later, she manifested neurological worsening with associated seizures and later coma with Glasgow Coma Scale (GCS) of 3 and mydriatic middle pupils. A new cranial CT was performed where a malignant infarction of the left MCA and ACA with increased uncal and subfalcine herniation was observed (Figure 1c). A chest CT was also performed where the existence of a thrombus in the left aortic arch and associated thromboembolism of the left upper lobe were confirmed (Figure 3). The patient relatives were informed of the poor prognosis. Surgical approach with decompressive craniectomy was not considered due to the very likely fatal neurological outcome that the patient’s situation presented.
She demonstrated unfavourable clinical evolution, until she died 72h after admission to the emergency room.
Previous studies with coronavirus have already shown cases of damage to the central nervous system. Current evidence suggests that COVID-19 patients commonly have neurological symptoms, more often non-specific and non-severe, such as headache and dizziness [14-16].
We found different incidence of CVD amongst published series. Yanan Li, et al found a 5% of CVD, and it happened in most severe patients, which were generally found to be older and with more risk factors for CVD. Ling Mao, et al showed a 5% only amongst the patients defined as severe. Non-severe patients had a 0.8% incidence. These authors found that in patients with more risk factors that could present more often a severe disease and CVD, some of these factors could in fact be common for a pneumonia or stroke.
It is not well known how the virus spreads through the human body out of the lungs. A possible triggering mechanism might be through the interaction with the Angiotensin-Converting Enzyme-2 (ACE-2) that regulates the blood vessels in all the organs and could increase the ischemia by a general vasoconstriction [17]. Another possible mechanism could be the cytokine cascade that could create blood coagulation disorders [18]. It could be interesting to know if the CVD occurred mostly in the second week of symptomatic infection when the cytokine cascade is thought to take place [19], and whether inhibition of any of these cytokines could be therapeutic option to treat stroke in a proinflammatory context.
Therefore, mild respiratory infections may be related to altered coagulation states that can increase the risk of CVD
More studies need to be performed to determine what role the severity of respiratory infection plays. However, we could observe that, at least in cases of spontaneous CAD it is the most common risk factor or association [7]. The seasonal variation also suggests a possible association [8].
Despite the fact that the patient presented more risk factors for CVD, the low incidence of this pathology in young patients invites us to speculate about the role that COVID-19 may have in this event. Malignant cerebral artery ischemia is a devastating type of ischemic stroke, it happens in the 2.3% of patients with ischemic stroke, and its associated with smoking more than 20 cigarettes a day. Nausea or vomiting could be clinical predictors for this poor evolution [20].
The discovery of a thrombus in the carotid arch could suggest a possible cardioembolic origin for the stroke. It is another factor for a predictable poor prognosis. Cardioembolic stroke has worse outcome comparing with other sybtypes of ischemic stroke [21].
At the time of diagnosis, the patient presented pneumonia with radiological data of complication, although 36 hours earlier she had no serious symptoms. The radiological evolution suggests that the patient could have been in the second week of infection, at which time the cytokine cascade could add one more risk factor.
At this stage of the pandemic, not much is known about the ability – or lack thereof – of the new coronavirus to produce CVD. This single case report only suggests a possible association between the infection from COVID-19 and CVD. More cases with epidemiological data are required to confirm and measure this association, although the role of infections in CVD for a not well-defined mechanism has been described frequently.
In a severe COVID patient with a loss of consciousness level, a CVD should be considered to initiate a treatment if necessary. Another consideration, as shown in this case, is the possibility of a coronavirus infection in a patient with a loss of consciousness, despite the fact that no suggestive or known clinic instance was reported before.
The authors sincerely thank all colleagues of department of Neurosurgery in Cruces university hospital.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. Clinical Characteristics of Coronavirus Disease 2019. N Engl J Med. 2020; 382: 1708-1720.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323: 1061-1069. PubMed: https://pubmed.ncbi.nlm.nih.gov/32031570/
- Li Y, Wang M, Zhou Y, Chang J, Xian Y et al. Acute cerebrovascular disease following COVID-19: A single center. Retrospective, Observational Study. LANCET. 2020.
- Ling Mao, Huijuan Jin, Mengdie Wang, Yu Hu, Shengcai Chen, Quanwei He, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series. JAMA Neurol. 2020; 201127: PubMed: https://pubmed.ncbi.nlm.nih.gov/32275288/
- A J Grau, F Buggle, S Heindl, C Steichen Wiehn, T Banerjee, M Maiwald, et al. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia. Clinical and biochemical studies. Neurology. 1998; 50: 196-203. PubMed: https://pubmed.ncbi.nlm.nih.gov/7886709/
- A J Grau, T Brandt, F Buggle, E Orberk, J Mytilineos, E Werle, et al. Association of cervical artery dissection with recent infection. Arch Neurol. 1999; 56: 851-856. PubMed: https://pubmed.ncbi.nlm.nih.gov/10404987/
- Benoit Guillon, Karine Berthet, Lamia Benslamia, Marion Bertrand, Marie Germaine Bousser, Christophe Tzourio. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. 2003; 34: 79-81. PubMed: https://pubmed.ncbi.nlm.nih.gov/12805497/
- Lucy C Thomas, Lesley Ann Hall, John R Attia, Elizabeth G Holliday, Hugh S Markus, Christopher R Levi. Seasonal variation in spontaneous cervical artery dissection: Comparing between UK and Australian Sites. J Stroke Cerebrovasc Dis. 2017; 26: 177-185. PubMed: https://pubmed.ncbi.nlm.nih.gov/27745777/
- Baumgartner RW. Handbook on cerebral artery dissection. Frontiers of Neurology and Neuroscience. Karge. 2005; 20: 12-15.
- Schievink WI, Mokri B, O'Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994; 330: 393-397. PubMed: https://pubmed.ncbi.nlm.nih.gov/8284004/
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin. 1992; 10: 113-124. PubMed: https://pubmed.ncbi.nlm.nih.gov/1556998/
- Arboix A, Jimenez C, Massons J, Parra O, Besses C. Hematological disordes: A commonly unrecognized cause of acute stroke. Expert review of hematology. 2016; 9: 891-901. PubMed: https://pubmed.ncbi.nlm.nih.gov/27367035/
- Schievink WI, Wijdicks EFM, Michels VV, Vockley J, Godfrey M. Heritable connective tissue disorders in cervical artery dissections: A prospective study. Neurology. 1998; 50: 1166-1169. PubMed: https://pubmed.ncbi.nlm.nih.gov/9566419/
- Sofia Morfopoulou, Julianne R Brown, E Graham Davies, Glenn Anderson, Alex Virasami, Waseem Qasim, et al. Human Coronavirus OC43 Associated with Fatal Encephalitis. N Engl J Med. 2016; 375: 497-498.
- Hussein Algahtani, Ahmad Subahi, Bader Shirah. Neurological complications of middle east respiratory syndrome coronavirus: A report of two cases and review of the Literature. Case Rep Neurol Med. 2016; 3502683; PubMed: https://pubmed.ncbi.nlm.nih.gov/27239356/
- Carod Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020; 70: 311‐322. PubMed: https://pubmed.ncbi.nlm.nih.gov/32329044/
- Wenhui Li, Chengsheng Zhang, Jianhua Sui, Jens H Kuhn, Michael J Moore, Shiwen Luo, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005; 24: 1634-1643. PubMed: https://pubmed.ncbi.nlm.nih.gov/15791205/
- Sajjad Muhammad, Emanuel Haasbach, Maria Kotchourko, Anne Strigli, Antje Krenz, Dirk A Ridder, et al. Influenza virus infection aggravates stroke outcome. Stroke. 2011; 42: 783-791. PubMed: https://pubmed.ncbi.nlm.nih.gov/21293018/
- Zhe Xu, Lei Shi, Yijin Wang, Jiyuan Zhang, Lei Huang, Chao Zhang, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8: 420-422. PubMed: https://pubmed.ncbi.nlm.nih.gov/32085846/
- Arboix A, Garcia Eroles L, Oliveres M, Comes E, Sanchez MJ, Massons J. Malignant middle cerebral artery infarction: A clinical study of 32 patients. Rev Invest Clin. 2015; 67: 64-70. PubMed: https://pubmed.ncbi.nlm.nih.gov/25857586/
- Arboix A, Alio J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. 2011; 9: 367-379. PubMed: https://pubmed.ncbi.nlm.nih.gov/21438816/
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley