Biology Group 2025 June 16;6(6):652-660. doi: 10.37871/jbres2119.
Serological Identification of Partial-D Variants in a Sub-Saharan African Population: A Cross-Sectional Study From Yaoundé, Cameroon
Jeanne Manga Messina-Mbeti1,6*, Jobert Richie Nansseu2,3, Francoise Ngo Sack4,5, Leopold Mbous Nguimbous6, Annie Rachel Epote1 and Suzanne Belinga1
2Department of Public Health, Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I, Yaoundé, Cameroon
3Department for the Control of Disease, Epidemics and Pandemics, Ministry of Public Health, Yaoundé, Cameroon
4Blood Bank Unit of the Yaoundé Central Hospital, Yaoundé, Cameroon
5Faculty of Medicine and Pharmaceutical Sciences of the University of Douala, Douala, Cameroon
6School of Health Sciences, Catholic University of Central Africa, Yaoundé, Cameroon
- D blood grouping
- Partial D variants
- Cameroon
- Sub-Saharan Africa
Abstract
Objective: This study aimed to identify partail-D variants in African black populations living in Africa.
Methods: A cross-sectional study was carried out at the CPC Hematology Laboratory from April 2016 to June 2017. A 5 ml volume of blood was collected in an EDTA tube, from consent patients for the ABO/D blood grouping. Three Bio-Rad antisera reagents characterized by monoclonal anti-D antibody were used for D antigen testing: IgG (MS-26)/IgM (TH-28) , anti-DVI- [LHM59/20 (LDM3) +175-2] and anti-DVI+ [ESD-1M+175-2]. An extended partial typing panel of 12 anti-D (LHM76/58; LHM76/59; LHM174/102; LHM50/2B; LHM169/81; ESD1; LHM76/55; LHM77/64; LHM70/45; LHM59/19; LHM169/80; LHM57/17) was used to identify partial D on discrepant results.
Results: Of the 3 439 patients sampled, 1.6% with a median age of 24 years showed discordant results. Men were 2.7 times (1.3-5.5) more likely to have concordant results (p = 0.005) than women. Variant characterization revealed 7.3% weak D and 87.3% partial D including 36.4% DFR, 32.7% DV, 14.6% DIII, 1.8% DAR-E, and 1.8% DHK/DAU-4.
Conclusion: The use of anti-D reagents from different cell lines is essential for the detection of D variants. The identification of these variants by larger panels of antisera is highly needed and their confirmation by molecular approaches is becoming paramount.
Abbreviations
AIHA: Autoimmune Hemolytic Anemia; CI: Confidence Interval; CPC: Centre Pasteur of Cameroon; HDFN: Hemolytic Disease of the Fetus and Newborn; IgG: Immunoglobulin G; IgM: Immunoglobulin M; IQR: Interquartile Range
Introduction
The RH system is known as the most polymorphic of blood systems. It includes approximately sixty antigens. The most immunogenic of all, the D antigen responsible for the RH1 phenotype, is considered to be a mosaic of epitopes that are present in RH1 positive subjects and all absent in RH1 negative individuals [1]. It is also the most important in the clinical context because of its antibody stimulated by intake of the antigen in a subject lacking it, which is responsible for immune hemolytic transfusion accidents, Hemolytic Disease of the Fetus and Newborn (HDFN), and Autoimmune Hemolytic Anemia (AIHA) [2].
However, some RH1 subjects may produce an anti-D alloantibody directed against one or more epitopes absent from their red blood cells, thus defining the "partial D" phenotype or weakly expressed for "weak D" phenotype [2,3]. This high variability of the D antigen explains the discrepancies noted between two serological determinations, thus putting biologists in a decision-making impasse when rendering the results [2]. Thus, false-negative results in blood donors are clinically problematic because they can induce alloimmunization in recipients, and false-positive results are critical for recipients because they favour exposure to antigens that they do not truly have and consequently the production of alloantibodies [4]. The frequency of this alloimmunization due to discordant results has been estimated at 36% in Brazil [5].
Knowledge of the D variants is then important to prevent alloimmunization and HDFN [6]. Through numerous studies, it has been found that their distribution is mainly ethnic and racial. In Africa, 7-8% are partial variants are found, in Europe 93% are weak D, 0.027% are Del, and in Asia 30% are Del [7,8]. However, in Cameroon, to the best of our knowledge, there are no current data on circulating D variants because of the use of low-sensitivity serological methods in analytical laboratories alongside the difficulty of setting-up means of characterizing these variants. Hence, the objective of this study was to search for these variants in our context through the reactive specificities of antisera reagents and to identify them by using a specific serological panel.
Methods
Study design, setting and population
A cross-sectional study was conducted at the Hematology laboratory of the Centre Pasteur of Cameroon (CPC) from April 2016 to June 2017. This is a reference laboratory situated in the capital city of Cameroon, Yaoundé, a cosmopolitan metropolis with a very heteregeneous population made up of five ethnic groups and a considerable number of foreigners from neighboring nations and the Sub-Saharan African region. The CPC receives biological samples coming from all over the country. It sees approximately 150 000 patient records per year and provides services in training, public health, research, environment analysis, microbiology, biochemistry and hematology medical analysis. This study was approved by the Ethics Committee of the School of Health Sciences of the Catholic University of Central Africa. Participants were consenting patients living in Yaoundé and its surroundings who applied for ABO/D blood grouping at the CPC during the study period.
Data collection and laboratory investigations
Socio-demographic data were collected from patients’ digital records. In addition, a volume of 5 mL of blood was collected from all patients in EDTA vacutainer tubes for D antigen screening.
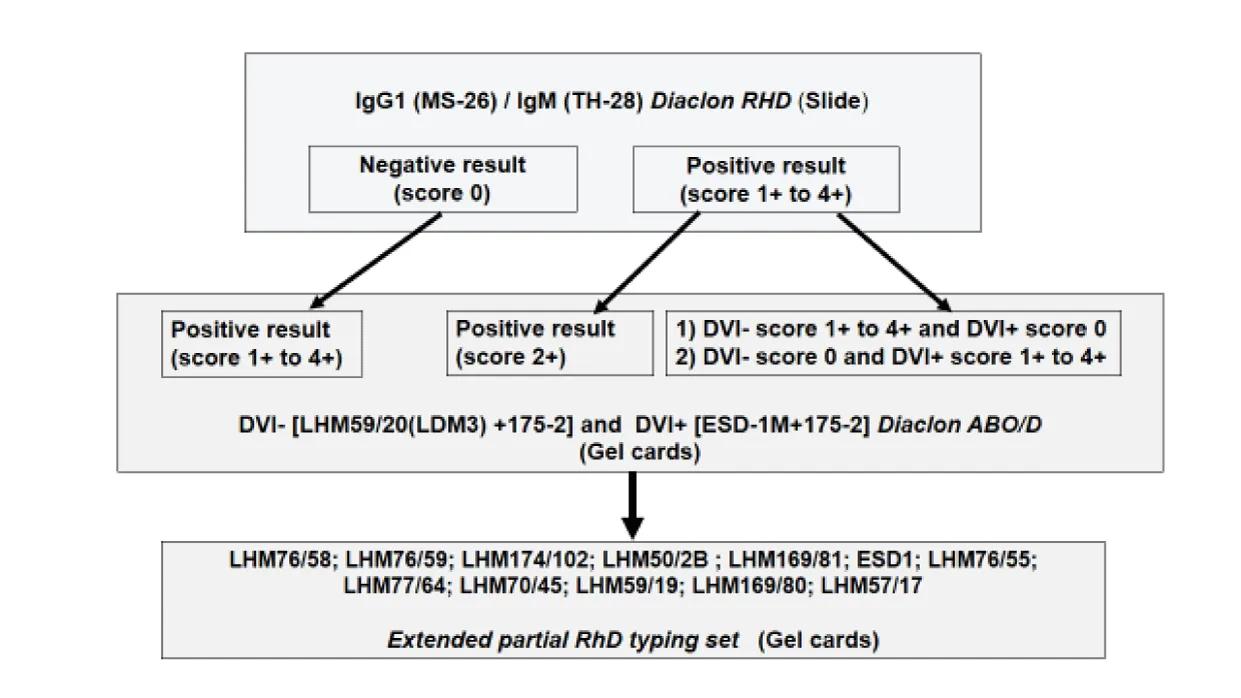
Our laboratory routinely receives a wide range of patients, including those undergoing pretransfusion or preoperative testing, antenatal screening, routine health assessments, and blood group confirmation. To address these diverse diagnostic needs and ensure accurate RhD typing, three monoclonal anti-D reagents with complementary reactivity profiles are used in routine practice. The first reagent, combining MS-26 (IgG) and TH-28 (IgM), is used for initial D antigen detection by slide direct agglutination test. The second, anti-DVI– [LHM59/20 (LDM3) + 175-2] on gel card, does not detect the DVI phenotype and is suitable for identifying patients at risk of alloimmunization. The third, anti-DVI+ [ESD-1M + 175-2] on gel card also, is capable of detecting the DVI phenotype and is essential for donor screening. Together, these reagents allow for comprehensive and clinically appropriate RhD typing, including the detection of partial D variants.
Samples exhibiting qualitative discrepancies or atypical reactivity scores, as defined by a flowchart with three profiles, were further analyzed using an extended partial RhD typing panel of 12 monoclonal anti-D reagents (LHM76/58, LHM76/59, LHM174/102, LHM50/2B, LHM169/81, ESD1, LHM76/55, LHM77/64, LHM70/45, LHM59/19, LHM169/80, LHM57/17) to identify partial D variants including DII/DNU, DIII, DIV, DV, DCS, DVI, DOL, DFR, DAR-E, DHK/DAU-4, DBT, and weak D (Figure 1).
Statistical Analysis
Data were coded and registered in Microsoft Excel Office 360, and subsequently analysed with SPSS version 28.0 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY, USA). Qualitative variables are presented using frequencies and percentages. Quantitative variables are summarised with their median and Interquartile Range [(IQR): 25th-75th percentiles]. The Chi-2 test was used to compare qualitative variables and the Mann-Whitney U test served for the comparison of quantitative variables. The odds ratio and its 95% Confidence Interval (CI) were used to assess the risk of discordant results. The level of agreement between D screening techniques was assessed by the kappa coefficient (k). The statistically significant threshold was set at p < 0.05.
Results
The study included 3439 patients. Women (n = 2 264; 65.8%) were more represented than men with a male/female sex ratio of 0.52. The median age was 24 years with an IQR between 7-31 years.
Antisera reactivity
The anti-D MS-26/TH28 had 11.9% score 0 and 0.1% score 1+. The scores 2+ and 3+ were recorded only with antisera LHM59/LDM3 and ESD-1M (Table 1). A total of 3384 (98.4%) concordant results were obtained, leaving 55 (1.6%) discordant results of which 52 (1.5%) were reported from the score 0 of MS-26/TH28 antibody (Table 2).
| Table 1: Antisera reactivity. | ||||||
| DiaClon Anti-D MS-26/TH-28 | Anti-DVI- LHM59/20 (LHM3) + 175-2 | Anti-DVI+ ESD-1M+175-2 | ||||
| n | Percentage (%) | n | Percentage (%) | n | Percentage (%) | |
| Negative (score 0) | 410 | 11.9 | 362 | 10.5 | 358 | 10.4 |
| Positive | ||||||
| Score 1+ | 2 | 0.1 | 1 | 0.0 | 1 | 0.0 |
| Score 2+ | 0 | 0.0 | 23 | 0.7 | 20 | 0.6 |
| Score 3+ | 0 | 0.0 | 6 | 0.2 | 23 | 0.7 |
| Score 4+ | 3 027 | 88.0 | 3 047 | 88.6 | 3 037 | 88.3 |
| Total | 3 439 | 100.0 | 3 439 | 100.0 | 3 439 | 100.0 |
| Table 2: Distribution of concordant results, D phenotypes and D varaints. | ||
| Number of patients | Percentage (%) | |
| Concordant results | ||
| Yes | 3 384 | 98.4 |
| No | ||
| Profile 1a | 52 | 1.5 |
| Profile 2b | 1 | 0.0 |
| Profile 3c | 2 | 0.1 |
| Total | 3 439 | 100.0 |
| D phenotypes | ||
| D | 3 026 | 88.0 |
| d | 358 | 10.4 |
| Partial | 48 | 1.4 |
| Weak | 4 | 0.1 |
| Not identified | 3 | 0.1 |
| Total | 3 439 | 100.0 |
| D variants | ||
| DFR | 20 | 36.4 |
| DV | 18 | 32.7 |
| DIII | 8 | 14.5 |
| DAR | 1 | 1.8 |
| DHK | 1 | 1.8 |
| Weak | 4 | 7.3 |
| Not identified | 3 | 5.5 |
| Total | 55 | 100.0 |
| aScore 0 MS-26; bScore 1+to4+ MS-26 and score ≤ 2+ LHDM/ESD; cScore 1+to4+ MS-26 and discordance betwenn LHMD and ESD | ||
Frequency of phenotype D
The RHD positive phenotype represented 3026 (88.0%) results, the RHD negative phenotype represented 358 (10.4%) results and 55 (1.6%) were discordant results (Table 2). The identification of variants from the discordant results showed 48 (87.3%) of partial D phenotypes. DFR and DV were the most represented with respective frequencies of 36.4% and 32.7% (Table 2).
Association between gender, age and concordance of results
The median age of patients with both concordant and discordant results was 24 years with no difference in distribution (p = 0.513). In the D variant group, females represented 83.6% (46/55) of patients. Men were 2.7 times (1.3-5.5) more likely to have concordant results than women (p = 0.005; Table 3).
| Table 3: Distribution of concordant results by age and gender. | |||||
| Concordance | OR (95%CI) | p-value | |||
| Yes | No | ||||
| Age | Median (IQR), years | 24.0 (7-31) | 24.0 (18-32) | 0.513 | |
| Minimum, years | 0 | 0 | |||
| Maximum, years | 87 | 64 | |||
| Gender | Male, n (%) | 1 161 (34.3) | 9 (16.4) | 1.0 | 0.005* |
| Female, n (%) | 2 223 (65.7) | 46 (83.6) | 2.7 (1.3-5.5) | ||
| *p < 0.05; CI: cofidence interval, IQR: interquartile range; OR: odds ratio | |||||
Reactivity of antisera and D variants
Based on the variant selection flowchart, the monoclonal IgM MS-26/TH 28 gave weakened reaction (Score 0 to 1+) for 54 (98.2%) of the 55 results. All variants had a positive reaction with the ESD-1M+175-2 monoclonal anti-D reagent. The weak D and partial DFR, DV, DIII presented many different profiles of reaction with the three antisera reagents (Table 4).
| Table 4: Distribution of D variants by antisera reactivity. | ||||
| Level of agglutination | ||||
| Variants | MS-26 | LHM | ESD | n (%) |
| Weak | 0 | 0 | 4+ | 1 (1.8) |
| 0 | 2+ | 2+ | 1 (1.8) | |
| 0 | 3+ | 3+ | 1 (1.8) | |
| 0 | 4+ | 3+ | 1 (1.8) | |
| DFR | 0 | 0 | 2+ | 1 (1.8) |
|
0 | 1+ | 2+ | 1 (1.8) |
| 0 | 2+ | 2+ | 6 (10.9) | |
| 0 | 2+ | 3+ | 6 (10.9) | |
| 0 | 3+ | 1+ | 1 (1.8) | |
| 0 | 4+ | 2+ | 2 (3.6) | |
| 0 | 4+ | 3+ | 2 (3.6) | |
| 0 | 4+ | 4+ | 1 (1.8) | |
| DV | 0 | 2+ | 2+ | 3 (5.5) |
| 0 | 2+ | 3+ | 4 (7.3) | |
| 0 | 3+ | 3+ | 2 (3.6) | |
| 0 | 3+ | 4+ | 1 (1.8) | |
| 0 | 4+ | 2+ | 1 (1.8) | |
| 0 | 4+ | 3+ | 2 (3.6) | |
| 0 | 4+ | 4+ | 3 (5.5) | |
| 1+ | 2+ | 2+ | 1 (1.8) | |
| 1+ | 4+ | 3+ | 1(1.8) | |
| DIII | 0 | 2+ | 2+ | 1 (1.8) |
| 0 | 3+ | 3+ | 1 (1.8) | |
| 0 | 4+ | 2+ | 2 (3.6) | |
| 0 | 4+ | 3+ | 1 (1.8) | |
| 0 | 4+ | 4+ | 3 (5.5) | |
| DHK | 4+ | 0 | 4+ | 1 (1.8) |
| DAR/E | 0 | 0 | 4+ | 1 (1.8) |
| Unknown | 0 | 2+ | 3+ | 1 (1.8) |
| 0 | 4+ | 2+ | 1 (1.8) | |
| 0 | 4+ | 3+ | 1 (1.8) | |
| Total | 55 (100.0) | |||
Comparison of methods
The comparability of results between MS-26/TH28 and LHM59/LDM3, MS-26/TH28 and ESD-1M, LHM59/LDM3 and ESD-1M gave the respective concordance rates of 98.50% (88.0% positive, 10.5% negative), 98.5% (88.1% positive, 10.4% negative) and 99.9% (89.5% positive, 10.4% negative). Their agreement measure yielded a k coefficient ≥ 0.92 with a significant correlation (p < 0.001; Tables S1-S3). The three antisera reagents demonstrated sensitivities greater than 98.0%. Their specificities ranged from 99.7% to 100%, 88.1% to 100%, and 87.3% to 98.9% for MS-26/TH 28, LHM59/LDM3, and ESD-1M, respectively.
Discussion
In this study involving 3439 patients, RhD typing was performed using two complementary agglutination based serological techniques: slide and gel card, with three distinct anti-D reagents. The cohort was predominantly female (65.8%), with a median age of 24 years, which reflects the high frequency of blood group testing in women of childbearing age for obstetric care and prevention of foeto-maternal alloimmunization [9].
The phenotypic distribution revealed 88% RhD-positive and 10.4% RhD-negative individuals, frequencies similar to those observed in North African and Eastern European populations, where approximately 90% are RhD-positive and 10% are RhD-negative [10-14]. These frequencies are lower than those reported in Asia (≥ 93% D and d≤ 7%), [1,15-17] in Latin America (D ≥ 95% and D ≤ 5%),[18,19] and in several Sub-Saharan African studies where the RhD-positive frequency reaches 97–99% and RhD-negative 1-3% [19,20]. This variation highlights the influence of ethnic diversity on RHD expression. The relatively higher frequency of RhD-negative individuals in our study may also be due to the sensitivity of the serological techniques used in our study, including the use of three different monoclonal reagents and a confirmatory technique based on gel filtration.
Analytical performance of the three reagents demonstrated high sensitivity (>98%) and specificity ranging from 87.3% to 100%, depending on the reagent and technique used. The concordance between reagents and techniques was excellent. However, discrepancies between methods were observed in 1.6% of cases, illustrating the molecular complexity of the RH system and the limitations of standard serology in detecting all RhD variants. The frequency of RhD variants found in our study (1.6%) is higher than those reported in most published studies : 1.43% in Brazil [21], 0.14% in Albania [14], 0.8% in Pakistan [17], 0.05-0.52% in Morocco [12,13], 0.02% in Korea [22], 0.2-1% in Caucasians [23], and in Uganda, a Sub-Saharan African country, where this frequency was 0.7% [24]. It apeared low compared to 4.5% in Egypt [25]. These differences may be explained by the diversity of antisera used, the ethnic diversity of studied populations, and the sensitivity of the methods employed.
Extended serological analysis revealed that the majority of variants identified in our study were partial D variants (87.3%), followed by weak D (7.3%) and uncharacterized variants (5.4%). This distribution differs from those observed in European and North African populations, where weak D variants are more frequent: 90% in Denmark (partial D 10%) [26], 90.1% in Belgium (partial D 9.9%) [27], and 81.8% in Albania [14]. In Morocco, partial D variants are predominant (40.7-65.2%), followed by weak D (25-26.1%) and uncharacterized variants (8.7-34.37%) [12-13]. In Latin America, the predominance of partial variants was also reported in Brazil (75%) compared to weak variants (25%), [5] though another Brazilian study reported a nearly equal distribution: 48.5% partial D and 50.5% weak D. [28]. In India, the frequency of partial D variants reached 81%, compared to 12% for weak D and 7% uncharacterized [29]. The predominance of partial D variants in our population supports the hypothesis that these variants are more frequent in individuals of African origin, with frequencies reaching up to 31% in some studies [30,31].
Subtype analysis of partial D variants showed that the most frequent were DFR (36.4%) and DV (32.7%), followed by DIII (14.5%), DAR/E and DHK/DAU-4 (1.8% each). In India, DFR (37%) and DOL (23%) were the most frequent, followed by DAR, DV, DMH, DCS, and DVI, accounting for approximately 21% of variants [29]. In Korea, DVa was the predominant subtype [22]. In Brazil, the most common subtypes were DIVa (18.2%), DIV type 4 (12.7%), DIVb (5.5%), DAR (30.9%), DVI (29.1%), and DFR (3.6%) [5]. In North Africa, variant distribution is more heterogeneous: in Morocco, DVI (9.37%), DV (6.25%), DIII (6.25%), and DVII (18.75%) were reported and in Egypt, DIII (34.6%) and DV (15.4%) were predominant [13,32,33]. These observations highlight the need to study populations with diverse ethnic backgrounds to better understand the distribution of RhD variants and their implications in transfusion medicine, in the follow-up of women of reproductive age and in the prevention of feto-maternal alloimmunization.
Serological analysis showed that weak D variants often present profiles similar to partial or uncharacterized variants. This reinforces the difficulty of serological discrimination between different variant types and the need for molecular characterization. These overlapping serological reactivity patterns may be due to shared epitope expression, reagent sensitivity, and the specificity of different anti-D clones [1,22,34,35]. Thus, serological tests alone are insufficient, and combining serological and molecular by analysis is essential for reliable RhD typing.
In our cohort, variant carriers were predominantly female (83.6%) with a median age similar to the overall population. This is contrary to some studies, such as one conducted in China, which showed a male predominance (59.4%) [36]. The high frequency of RhD variants in women of childbearing age highlights the importance of accurate RhD typing in this population to prevent alloimmunization and hemolytic disease of the fetus and newborn. In transfusion practice, most carriers of RhD variants are classified as RhD-positive when donating blood, but are managed as RhD-negative when receiving transfusions, due to the risk of alloimmunization upon exposure to normal D antigen. Consequently, anti-D immunoglobulin prophylaxis is recommended in clinical situations at risk of sensitisation. However, weak D types 1, 2, and 3 have been shown not to induce alloimmunization and are therefore managed as RhD-positive, without the need for prophylaxis [3,16,17,37].
Our findings underscore the molecular complexity and genetic diversity of the RHD in our population. The differences observed in RHD variant frequency and distribution compared to other regions support the need for molecular epidemiological studies adapted to local contexts. Combining molecular and serological methods is crucial to improve transfusion safety and reduce alloimmunization risk.
The main limitations of our study include its monocentric design and the use of convenience sampling, which may limit generalizability to the entire Cameroonian population. However, the Centre Pasteur of Cameroon, as a national reference center, receives samples from a broad geographic and ethnic base, particularly in Yaoundé and its surroundings, making our population relatively representative. The large sample size, the use of multiple serological and molecular techniques, and the methodological rigor contribute to the robustness of our findings. To our knowledge, this is the first study in Cameroon and one of the few in Central and Sub-Saharan Africa to estimate the prevalence and distribution of RhD variants in the general population. These findings provide a solid basis for improving transfusion practices and call for further research into the sociodemographic, genetic, and environmental determinants of RhD variant expression.
Conclusion
D type differentiation and identification are important for blood products selection and the prevention of post-erythrocyte alloimmunization and HDFN. This study identified the presence of some clinically relevant D variants in the Cameroonian population through a set of antisera panels. The findings of this study revealed that the serological D typing reagents should be provided, taking into account variant D antigen within the ethnic group in the context of Sub-Saharan Africa. It appears important to use a combination of monoclonal reagents from different cell lines in routine practice, in order to detect D variants and confirm all discordant or weakly positive reactions. In addition, the MS-26/TH28 antibody may be helpful for D antigen typing in patients and pregnant women, but not in blood donors, in our case. However, the contribution of molecular biology is essential for the confirmation of these variants and the search for possible mutations contributing to their appearance.
Funding Statement
There was no specific funding source to be mentioned.
Acknowledgments
The authors thank the staff of the Hematology Department of the Centre Pasteur of Cameroon for their support in data collection.
Author Contributions
JMMM designed the research, interpreted the data, and wrote the first draft of the manuscript. JRN performed statistical analysis, interpreted the data, and critically reviewed and revised the manuscript. FNS collected the data and critically reviewed the manuscript. LMN collected the data and wrote the first draft of the manuscript. ARE interpreted the data and critically reviewed the manuscript. SB designed the research, interpreted the data, and rewiewed the manuscript. All authors agreed on the final version of the manuscript.
Novelty statements
What is the NEW aspect of your work?
This paper emphasizes the importance of differential monoclonal antibody reactivity in the screening of D-variants present in Sub-Saharan African populations.
What is the CENTRAL finding of your work?
Many negative D-type serologic responses obtained with some antisera hide certain D-variant phenotypes specific to Sub-Saharan African populations.
What is (or could be) the SPECIFIC clinical relevance of your work?
Practitioners should take into account the low reactivity and discrepancy results between antisera to highlight the presence of partial variants in D blood grouping because of the importance of their screening in the selection of blood products to be transfused and the management of the mother after delivery of a carrier child. Hence, they should ask for D variant screening when requesting the ABO/Rh group typing, especially before blood transfusion. Lab technicians should consider undertaking at least two different monoclonal antibody methods to detect partial-D antigens.
Data Availability Statement
The authors declare that data supporting the findings of this study are included in the article (and its supplementary files), and that additional information is available from the corresponding author upon reasonable request. No data from other sources was used in this manuscript.
Prior Publication
Abstract and presentation of part of this data were presented as follows
- Societé Française de Transfusion Sanguine 2021 congress, November 2021
- Transfusion Clinique et Biologique 28 (2021) S27-S47 (OR5-4).
Ethics approval statement
The study was approved by the Ethics Committee of the School of Health Sciences of the Catholic University of Central Africa.
Patient Consent Statement
All patients provided written informed consent.
Permission to Reproduce Material
This article may be distributed and reproduced if the original work is properly cited.
Clinical Trial Registration
Not applicable.
Conflicts of Interest Statement
The authors declare that they have no conflicts of interest.
References
- S S, Shastry S, B PB. Variable reactivity of Rh D antigen and its serological characterization. Acta Clin Belg. 2021 Oct;76(5):346-350. doi: 10.1080/17843286.2020.1735115. Epub 2020 Feb 28. PMID: 32108563.
- Ouchari M, Jemni Yacoub S, Houissa B, Abdelkefi S, Chakroun T, Bouslama M, Jerray I, Belhedi S, Hmida S. Système RH : dépistage de D partiels avec RHD/RHCE gène hybride [System RH: screening of partials D with RHD/RHCE hybrid gene]. Transfus Clin Biol. 2013 Mar;20(1):35-9. French. doi: 10.1016/j.tracli.2012.11.002. Epub 2013 Mar 21. PMID: 23523094.
- Daniels G. Variants of RhD--current testing and clinical consequences. Br J Haematol. 2013 May;161(4):461-70. doi: 10.1111/bjh.12275. Epub 2013 Feb 25. PMID: 23432139.
- Menegati SFP, Santos TD, Macedo MD, Castilho L. Discrepancies between red cell phenotyping and genotyping in daily immunohematology laboratory practice. Transfus Apher Sci. 2020 Feb;59(1):102585. doi: 10.1016/j.transci.2019.06.020. Epub 2019 Jul 9. PMID: 31303508.
- Campos FC, Mota MA, Aravechia MG, Torres KB, Bub CB, Kutner JM, Castilho L. Variant RHD Types in Brazilians With Discrepancies in RhD Typing. J Clin Lab Anal. 2016 Nov;30(6):845-848. doi: 10.1002/jcla.21946. Epub 2016 Apr 13. PMID: 27076392; PMCID: PMC6806703.
- Credidio DC, Pellegrino J, Castilho L. Serologic and molecular characterization of D variants in Brazilians: impact for typing and transfusion strategy. Immunohematology. 2011;27(1):6-11. PMID: 22356480.
- Geoff D. Rh and RHAG blood group systems. In: Human blood groups. Wiley-Blackwell; 2013:182-258. doi:10.1002/9781118493595.ch5.
- Kacem N. Determination of RHD gene zygosity in the Tunisian population: Impact of "Rh Box" polymorphisms on the relevance of molecular analyses. Aix-Marseille University and Monastir University; 2013.
- Belinga S, Ngo Sack F, Bilong C, Manga J, Mengue MA, Tchendjou P. High prevalence of anti-D antibodies among women of childbearing age at Centre Pasteur of Cameroon. Afr J Reprod Health. 2009 Sep;13(3):47-52. PMID: 20690261.
- Ajhoun I. Prevalence of ABO and RH-Kell blood group phenotypes among blood donors at the hmimv blood transfusion center in Rabat. Mohammed V University. Rabat. 2022.
- El Ghali B, Mohammed O, Hicham Y, Mustapha AA. Les fréquences phénotypiques et génotypiques des systèmes ABO et Rh dans la population marocaine : Expérience du Service de Transfusion de l´Hôpital Militaire Avicenne. Marrakech. PAMJ-CM. 2020;2. doi: 10.11604/pamj-cm.2020.2.140.20679.
- El Housse H, El Wafi M, Ouabdelmoumene Z, Zarati F, Alid R, Nourichafi N, Bouisk K, Benajiba M, Férec C, Fichou Y, Habti N. Comprehensive phenotypic and molecular investigation of RhD and RhCE variants in Moroccan blood donors. Blood Transfus. 2019 Mar;17(2):151-156. doi: 10.2450/2018.0153-18. Epub 2018 Oct 24. PMID: 30418133; PMCID: PMC6476734.
- Kabiri Z, Benajiba M, Hajjout K, Dakka N, Bellaoui H. Testing for Partial RhD with a D-Screen Diagast Kit in Moroccan Blood Donors with Weak D Expression. J Blood Transfus. 2014;2014:204301. doi: 10.1155/2014/204301. Epub 2014 Oct 28. PMID: 25530908; PMCID: PMC4228700.
- Xhetani M, Seferi I, Férec C, Zoraqi G, Fichou Y. Distribution of Rhesus blood group antigens and weak D alleles in the population of Albania. Blood Transfus. 2014 Oct;12(4):565-9. doi: 10.2450/2014.0240-13. Epub 2014 Jun 12. PMID: 24960662; PMCID: PMC4212038.
- Subramaniyan R. Prevalence of D variants in the Indian donor population. Hematol Transfus Cell Ther. 2019 Apr-Jun;41(2):190-193. doi: 10.1016/j.htct.2018.09.004. Epub 2018 Dec 31. PMID: 31084770; PMCID: PMC6517790.
- Pahuja S, Pujani M, Sethi N, Kushwaha S, Jain M, Kumari R. Frequency of variant D in Delhi, India. Asian J Transfus Sci. 2014 Jul;8(2):142-3. doi: 10.4103/0973-6247.137459. PMID: 25161360; PMCID: PMC4140062.
- Usman M, Rizwan M, Iqbal N, Shaikh NU, Mehmood HO. Frequency of RH-D negative and weak D in Pakistani population. 2020;23(1):5.
- Canizalez-Román A, Campos-Romero A, Castro-Sánchez JA, López-Martínez MA, Andrade-Muñoz FJ, Cruz-Zamudio CK, Ortíz-Espinoza TG, León-Sicairos N, Gaudrón Llanos AM, Velázquez-Román J, Flores-Villaseñor H, Muro-Amador S, Martínez-García JJ, Alcántar-Fernández J. Blood Groups Distribution and Gene Diversity of the ABO and Rh (D) Loci in the Mexican Population. Biomed Res Int. 2018 Apr 23;2018:1925619. doi: 10.1155/2018/1925619. PMID: 29850485; PMCID: PMC5937518.
- Blood type frequencies by country including the Rh factor - Rhesus negative. 2022.
- Kabemba BH, Kabobo KI, Mukena TS, Ngiele MD, Kabingie NG, Kasolva TC, Pungwe KJ, Kasendue EP. Frequency of erythrocyte phenotypes in blood group systems ABO and rhesus at moba, province of Tanganyika, democratic Republic of Congo. OALib. 2017;04(03):1-12. doi: 10.4236/oalib.1103421.
- Schmidt LC, Castilho L, Vieira OV, Sippert E, Gaspardi AC, Martins ML, da Silva Malta MC. Impact of a confirmatory RhD test on the correct serologic typing of blood donors. Rev Bras Hematol Hemoter. 2015 Sep-Oct;37(5):302-5. doi: 10.1016/j.bjhh.2015.06.001. Epub 2015 Jul 9. PMID: 26408363; PMCID: PMC4685092.
- Jeong D, Oh S, Song EY, Hong YJ, Park KU. Molecular Characteristics of the Serological Weak D Phenotype in Koreans. Diagnostics (Basel). 2021 May 21;11(6):920. doi: 10.3390/diagnostics11060920. PMID: 34063775; PMCID: PMC8223775.
- Sandler SG, Queenan JT. A Guide to Terminology for Rh Immunoprophylaxis. Obstet Gynecol. 2017 Sep;130(3):633-635. doi: 10.1097/AOG.0000000000002190. PMID: 28796682.
- Ojok P, Oyet C, Webbo F, Mwambi B, Taremwa IM. Prevalence of RhD variants among blood donors at Gulu Regional Blood Bank, Gulu, Northern Uganda. J Blood Med. 2017 Sep 15;8:151-154. doi: 10.2147/JBM.S145550. PMID: 28979173; PMCID: PMC5608233.
- Hussein E, Teruya J. Weak D types in the Egyptian population. Am J Clin Pathol. 2013 Jun;139(6):806-11. doi: 10.1309/AJCP1T9FGZBHIQET. PMID: 23690125.
- Christiansen M, Samuelsen B, Christiansen L, Morbjerg T, Bredahl C, Grunnet N. Correlation between serology and genetics of weak D types in Denmark. Transfusion. 2008 Jan;48(1):187-93. doi: 10.1111/j.1537-2995.2007.01504.x. Epub 2007 Sep 27. PMID: 17900277.
- Van Sandt VS, Gassner C, Emonds MP, Legler TJ, Mahieu S, Körmöczi GF. RHD variants in Flanders, Belgium. Transfusion. 2015 Jun;55(6 Pt 2):1411-7. doi: 10.1111/trf.12947. Epub 2014 Nov 21. PMID: 25413499.
- Person RDM, Arnoni CP, Muniz JG, Vendrame TAP, Latini FRM, Cortez AJP, Pellegrino J Jr, Castilho LM. Serologic strategy in detecting RHD altered alleles in Brazilian blood donors. Hematol Transfus Cell Ther. 2020 Oct-Dec;42(4):365-372. doi: 10.1016/j.htct.2019.08.004. Epub 2019 Oct 13. PMID: 31780389; PMCID: PMC7599269.
- Kulkarni S, Kasiviswanathan V, Ghosh K. A simple diagnostic strategy for RhD typing in discrepant cases in the Indian population. Blood Transfus. 2013 Jan;11(1):37-42. doi: 10.2450/2012.0006-12. Epub 2012 Jul 12. PMID: 22871818; PMCID: PMC3557475.
- Sandler SG, Flegel WA, Westhoff CM, Denomme GA, Delaney M, Keller MA, Johnson ST, Katz L, Queenan JT, Vassallo RR, Simon CD; College of American Pathologists Transfusion Medicine Resource Committee Work Group. It's time to phase in RHD genotyping for patients with a serologic weak D phenotype. College of American Pathologists Transfusion Medicine Resource Committee Work Group. Transfusion. 2015 Mar;55(3):680-9. doi: 10.1111/trf.12941. Epub 2014 Dec 1. PMID: 25438646; PMCID: PMC4357540.
- Wagner FF, Moulds JM, Tounkara A, Kouriba B, Flegel WA. RHD allele distribution in Africans of Mali. BMC Genet. 2003 Sep 24;4:14. doi: 10.1186/1471-2156-4-14. PMID: 14505497; PMCID: PMC222912.
- Hussein E, Teruya J. Serologic findings of RhD alleles in Egyptians and their clinical implications. Transfus Apher Sci. 2014 Oct;51(2):184-7. doi: 10.1016/j.transci.2014.08.014. Epub 2014 Aug 27. PMID: 25219636.
- Abdelrazik AM, Elshafie SM, Ezzat Ahmed GM, Abdelaziz HM. Combining serology and molecular typing of weak D role in improving D typing strategy in Egypt. Transfusion. 2013 Nov;53(11 Suppl 2):2940-4. doi: 10.1111/trf.12100. Epub 2013 Jan 30. PMID: 23362929.
- Miranda MR, Dos Santos TD, Castilho L. Systematic RHD genotyping in Brazilians reveals a high frequency of partial D in transfused patients serologically typed as weak D. Transfus Apher Sci. 2021 Dec;60(6):103235. doi: 10.1016/j.transci.2021.103235. Epub 2021 Aug 8. PMID: 34389204.
- Bub CB, Aravechia MG, Costa TH, Kutner JM, Castilho L. RHD alleles among pregnant women with serologic discrepant weak D phenotypes from a multiethnic population and risk of alloimmunization. J Clin Lab Anal. 2018 Jan;32(1):e22221. doi: 10.1002/jcla.22221. Epub 2017 Apr 4. PMID: 28374955; PMCID: PMC6816983.
- Yan L, Wu J, Zhu F, Hong X, Xu X. Molecular basis of D variants in Chinese persons. Transfusion. 2007 Mar;47(3):471-7. doi: 10.1111/j.1537-2995.2006.01138.x. PMID: 17319828.
- British Committee for Standards in Haematology; Milkins C, Berryman J, Cantwell C, Elliott C, Haggas R, Jones J, Rowley M, Williams M, Win N. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. British Committee for Standards in Haematology. Transfus Med. 2013 Feb;23(1):3-35. doi: 10.1111/j.1365-3148.2012.01199.x. Epub 2012 Dec 6. Erratum in: Transfus Med. 2022 Feb;32(1):91. doi: 10.1111/tme.12842. PMID: 23216974.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.