Medicine Group 2025 June 16;6(6):661-665. doi: 10.37871/jbres2120.
Modulation of Inflammation in Cardiac Function after Myocardial Infarction
Behrooz G Sharifi* and Prediman K Shah
Introduction
Acute Myocardial Infarction (MI) remains a leading cause of death worldwide. Although reperfusion therapies have improved survival following the initial ischemic event, many patients are still at elevated risk of developing heart failure due to a process known as ventricular remodeling [1]. Consequently, there is a pressing need for new therapies that can limit infarct size and prevent maladaptive remodeling and heart failure.
Following MI, the heart undergoes a robust inflammatory response, initially marked by the infiltration of neutrophils, which are rapidly cleared, followed by the sustained presence of pro-inflammatory monocytes and macrophages [2,3]. Over time, these monocytes can transition into reparative phenotypes [2,4]. The prevailing hypothesis is that uncontrolled inflammation drives disease progression, and targeting pro-inflammatory pathways may protect against adverse remodeling after MI reviewed in [5,6].
However, despite this rationale, numerous anti-inflammatory therapies have failed to demonstrate clinical benefit. Corticosteroids and other agents have shown limited or no efficacy in fibrotic diseases such as idiopathic pulmonary fibrosis, where inflammation does not correlate well with disease stage or prognosis, and anti-inflammatory treatment has not improved outcomes [7]. Similarly, clinical trials aimed at reducing cardiac inflammation have yielded disappointing results [8-10]. For instance, early studies suggested TNFα inhibition with etanercept might enhance cardiac function [10], but larger trials were terminated for lack of efficacy [9]. Another trial using infliximab, a chimeric monoclonal anti-TNFα antibody, was also discontinued [9].
In a meta-analysis of patients with acute myocarditis, corticosteroid treatment was associated with improved left ventricular ejection fraction but did not affect survival [11]. Overall, interventions targeting inflammation-including those controlling the broader immune response [12-13] - have not translated into improved clinical outcomes in heart failure. As a result, current clinical guidelines advise against the use of steroids and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in the acute and early post-ST-Segment Elevation Myocardial Infarction (STEMI) phase, due to concerns about potential harm [14].
While the pathogenesis of MI is primarily vascular, the immune system, especially the innate immune arm, plays a critical role in orchestrating the response to myocardial injury. This response is multifaceted, involving the immediate recognition of tissue damage, the recruitment of immune cells, and the regulation of healing and scar formation. However, dysregulation of these processes can result in adverse cardiac remodeling and the progression to heart failure.
Innate Immune Cell Recruitment and Function
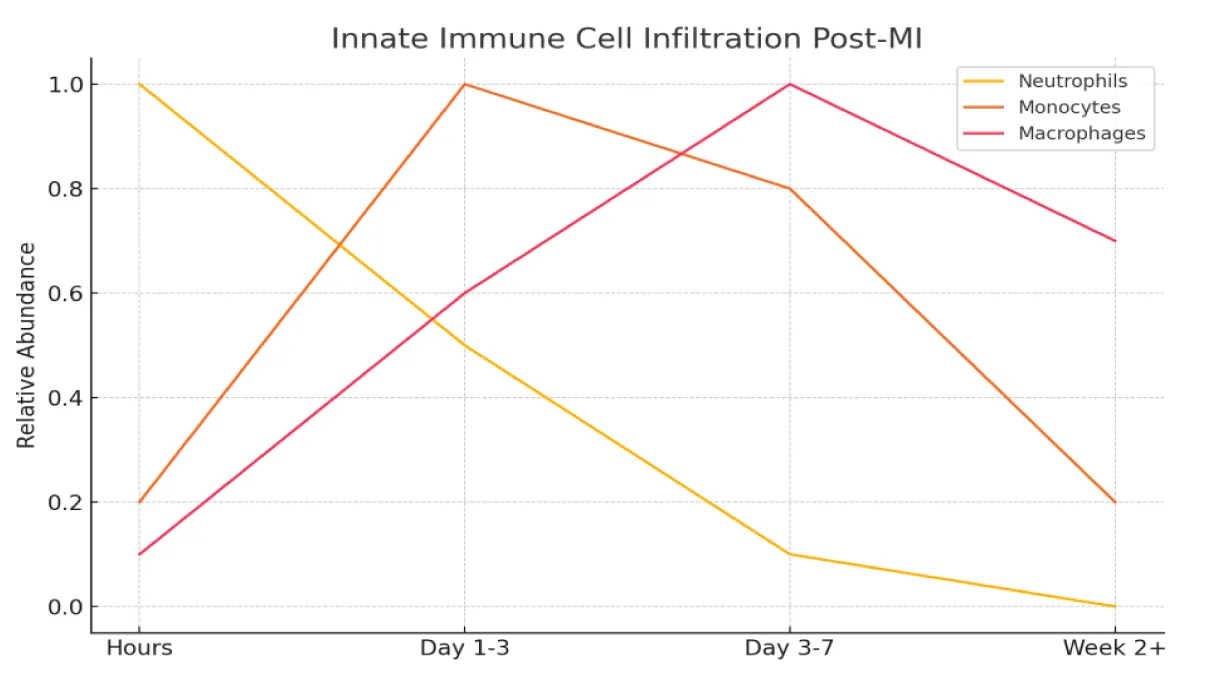
The initial immune response following MI is marked by a tightly regulated infiltration of innate immune cells. Neutrophils are the first to arrive, infiltrating the infarcted myocardium within hours of injury. Their primary functions include phagocytosis of necrotic debris, generation of Reactive Oxygen Species (ROS), and secretion of proteolytic enzymes. Neutrophils can also form Neutrophil Extracellular Traps (NETs) to help contain local inflammation [15,16]. Beyond their direct antimicrobial actions, neutrophils play a critical role in orchestrating the subsequent immune response by modulating the activity of both innate and adaptive immune cells, including macrophages and lymphocytes [15].
Neutrophils exhibit functional heterogeneity and can be classified into two major phenotypes: pro-inflammatory N1 (Ly6G⁺ CD206⁻) and anti-inflammatory N2 (Ly6G⁺ CD206⁺) subtypes. N1 neutrophils express high levels of inflammatory mediators such as IL-1β, IL-12a, and TNF-α, and are characterized by a CD49d^high CD11b^low surface expression pattern. In contrast, N2 neutrophils express anti-inflammatory markers like IL-10 and display a CD49d^low CD11b^high phenotype. In mouse models of MI, N1 (CD206⁻) neutrophils dominate during the first 7 days post-infarction, while N2 (CD206⁺) neutrophils gradually increase between days 5 to 7 [17].
Following neutrophil infiltration, monocyte are recruited to the injured myocardium in response to chemokines such as CCL2 [18]. Fate mapping analyses and lineage tracing experiments have indicated that tissue macrophages in many organs are of early embryonic origin [19-21]. Monocytes are a heterogeneous population of myeloid cells that originate from progenitors in the bone marrow, and traffic via the bloodstream to peripheral tissues. Monocytes are bone marrow-derived circulating cells that localize to injured and inflamed tissues and differentiate locally into diverse myeloid cell populations [22]. Early after MI, pro-inflammatory (M1-like) macrophages predominate, contributing to further debris clearance and cytokine production. As the healing phase progresses, a shift toward reparative (M2-like) macrophages supports tissue repair and scar formation (Figure 1).
Recent studies also suggest that monocytes and macrophages possess the ability to develop innate immune memory, a concept traditionally reserved for adaptive immune cells such as T and B lymphocytes. This form of "trained immunity" enables innate cells to respond more robustly upon re-exposure to the same or similar stimuli. This enhanced responsiveness is mediated by epigenetic and transcriptional reprogramming triggered by the initial inflammatory encounter [23,24]. These findings underscore the complexity and adaptability of the innate immune system in the context of myocardial injury and repair.
Dendritic cells contribute to the post-MI immune response by processing and presenting antigens, thereby serving as a critical link between the innate and adaptive immune systems. In parallel, the complement system plays a significant role in mediating inflammation following acute MI. When dysregulated, complement activation exacerbates tissue damage, contributing to larger infarct sizes and worse clinical outcomes [25]. After MI, the complement cascade is activated, promoting opsonization of necrotic cells, recruitment of immune cells, and the production of pro-inflammatory cytokines.
Initiation of the Innate Immune Response
The innate immune response to MI is initiated almost immediately following ischemic injury. As cardiomyocytes undergo necrosis, they release Damage-Associated Molecular Patterns (DAMPs), including High-Mobility Group Box 1 (HMGB1), ATP, mitochondrial DNA, and heat shock proteins. These DAMPs are recognized by Pattern Recognition Receptors (PRRs), such as Toll-Like Receptors (TLRs) and NOD-Like Receptors (NLRs), which are expressed on resident and circulating immune cells, including macrophages, dendritic cells, and neutrophils.
Among the PRRs, Toll-Like Receptor 4 (TLR4) plays a pivotal role in post-MI inflammation by activating downstream signaling pathways, notably NF-κB, leading to the production of pro-inflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α), Interleukin-1β (IL-1β), and interleukin-6 (IL-6). In parallel, activation of the NLRP3 inflammasome facilitates the maturation and secretion of IL-1β, further amplifying the inflammatory cascade and contributing to adverse cardiac outcomes [17,26].
Resolution of Inflammation and Tissue Repair
Timely resolution of inflammation is critical to preventing chronic inflammation and adverse cardiac remodeling after myocardial infarction. Key processes involved in this resolution phase include:
- Efferocytosis – the clearance of apoptotic neutrophils by macrophages;
- Macrophage phenotypic switching – the transition from a pro-inflammatory to a reparative (anti-inflammatory) phenotype; and
- Secretion of reparative growth factors – such as Vascular Endothelial Growth Factor (VEGF) and Transforming Growth Factor-β (TGF-β), which promote angiogenesis and tissue repair.
Disruptions in any of these mechanisms can impair healing and contribute to pathological outcomes, including ventricular dilation, fibrosis, and the progression to heart failure.
Therapeutic Implications
The inflammatory response is essential for initiating tissue repair following MI. However, as previously discussed, clinical efforts to broadly suppress inflammation in MI patients have generally yielded disappointing results. In some cases, extensive anti-inflammatory interventions have even produced harmful effects. Therefore, rather than completely inhibiting inflammation, therapeutic strategies should aim to restore the balance between pro-inflammatory and reparative immune cell populations.
This approach involves either enhancing the body's intrinsic anti-inflammatory mechanisms or selectively inhibiting key pro-inflammatory pathways. The goal is to minimize the harmful effects of excessive inflammation while preserving its beneficial roles in tissue repair and remodeling [27,28].
A better understanding of innate immune mechanisms in MI has paved the way for more targeted therapies. For example, IL-1β inhibitors such as canakinumab have shown promise in reducing recurrent MI and cardiovascular mortality [28]. In addition, TLR antagonists and NLRP3 inflammasome inhibitors are being investigated as strategies to limit excessive inflammatory signaling. Modulating monocyte and macrophage recruitment, particularly through CCR2 antagonism, is another promising approach to fine-tune post-MI inflammation and promote optimal healing.
Conclusion
Innate immunity plays a pivotal role in the response to myocardial infarction, driving both the initial inflammatory reaction and subsequent tissue repair. However, dysregulation of this system can exacerbate cardiac injury and promote maladaptive remodeling. Ongoing research and the development of precisely targeted therapies hold promise for leveraging the beneficial aspects of innate immunity while minimizing its detrimental effects.
References
- Eapen ZJ, Tang WH, Felker GM, Hernandez AF, Mahaffey KW, Lincoff AM, Roe MT. Defining heart failure end points in ST-segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ Cardiovasc Qual Outcomes. 2012 Jul 1;5(4):594-600. doi: 10.1161/CIRCOUTCOMES.112.966150. PMID: 22811505.
- Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013 Jun 7;112(12):1624-33. doi: 10.1161/CIRCRESAHA.113.300890. PMID: 23743228; PMCID: PMC3753681.
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016 Jan;17(1):34-40. doi: 10.1038/ni.3324. PMID: 26681460.
- Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013 Sep;62:24-35. doi: 10.1016/j.yjmcc.2013.04.023. Epub 2013 May 2. PMID: 23644221.
- Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014 May;11(5):255-65. doi: 10.1038/nrcardio.2014.28. Epub 2014 Mar 25. PMID: 24663091; PMCID: PMC4407144.
- Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016 Apr;93:149-55. doi: 10.1016/j.yjmcc.2015.11.015. Epub 2015 Nov 21. PMID: 26593722; PMCID: PMC4846552.
- Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001 Jan 16;134(2):136-51. doi: 10.7326/0003-4819-134-2-200101160-00015. PMID: 11177318.
- Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschöpe C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009 Feb;11(2):119-29. doi: 10.1093/eurjhf/hfn043. PMID: 19168509; PMCID: PMC2639409.
- Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res. 2016 Jun 24;119(1):159-76. doi: 10.1161/CIRCRESAHA.116.308030. PMID: 27340274.
- Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res. 2016 Jun 24;119(1):159-76. doi: 10.1161/CIRCRESAHA.116.308030. PMID: 27340274.
- Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013 Oct 18;2013(10):CD004471. doi: 10.1002/14651858.CD004471.pub3. PMID: 24136037; PMCID: PMC8094275.
- Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE; VISTA-16 Investigators. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014 Jan 15;311(3):252-62. doi: 10.1001/jama.2013.282836. PMID: 24247616.
- Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017 Mar 14;38(11):774-784. doi: 10.1093/eurheartj/ehw224. PMID: 27354052.
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Jan 29;61(4):485-510. doi: 10.1016/j.jacc.2012.11.018. Epub 2012 Dec 17. Erratum in: J Am Coll Cardiol. 2013 Sep 10;62(11):1039. PMID: 23256913.
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011 Jul 25;11(8):519-31. doi: 10.1038/nri3024. PMID: 21785456.
- Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair. 2013 Jun 3;6(1):11. doi: 10.1186/1755-1536-6-11. PMID: 23731794; PMCID: PMC3681584.
- Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016 Jun 24;119(1):91-112. doi: 10.1161/CIRCRESAHA.116.303577. PMID: 27340270; PMCID: PMC4922528.
- Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med. 2018 Jun;24(6):711-720. doi: 10.1038/s41591-018-0064-0. Epub 2018 Jun 4. PMID: 29867229; PMCID: PMC7301893.
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012 Apr 6;336(6077):86-90. doi: 10.1126/science.1219179. Epub 2012 Mar 22. PMID: 22442384.
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013 Jan 24;38(1):79-91. doi: 10.1016/j.immuni.2012.12.001. Epub 2012 Dec 27. Erratum in: Immunity. 2013 May 23;38(5):1073-9. PMID: 23273845; PMCID: PMC3908543.
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013 Apr 18;38(4):792-804. doi: 10.1016/j.immuni.2013.04.004. PMID: 23601688; PMCID: PMC3853406.
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010 Feb 5;327(5966):656-61. doi: 10.1126/science.1178331. Erratum in: Science. 2010 Dec 3;330(6009):1319. PMID: 20133564; PMCID: PMC2887389.
- Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, Riksen NP. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016 Nov;254:228-236. doi: 10.1016/j.atherosclerosis.2016.10.019. Epub 2016 Oct 12. PMID: 27764724.
- Chakraborty S, Singh A, Wang L, Wang X, Sanborn MA, Ye Z, Maienschein-Cline M, Mukhopadhyay A, Ganesh BB, Malik AB, Rehman J. Trained immunity of alveolar macrophages enhances injury resolution via KLF4-MERTK-mediated efferocytosis. J Exp Med. 2023 Nov 6;220(11):e20221388. doi: 10.1084/jem.20221388. Epub 2023 Aug 24. PMID: 37615937; PMCID: PMC10450795.
- Ghosh M, Rana S. The anaphylatoxin C5a: Structure, function, signaling, physiology, disease, and therapeutics. Int Immunopharmacol. 2023 May;118:110081. doi: 10.1016/j.intimp.2023.110081. Epub 2023 Mar 28. Erratum in: Int Immunopharmacol. 2023 Dec;125(Pt A):111089. doi: 10.1016/j.intimp.2023.111089. PMID: 36989901.
- Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018 Apr;15(4):203-214. doi: 10.1038/nrcardio.2017.161. Epub 2017 Nov 16. PMID: 29143812.
- Liu Y, Xu J, Wu M, Kang L, Xu B. The effector cells and cellular mediators of immune system involved in cardiac inflammation and fibrosis after myocardial infarction. J Cell Physiol. 2020 Dec;235(12):8996-9004. doi: 10.1002/jcp.29732. Epub 2020 Apr 30. PMID: 32352172.
- Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013 Sep;43(9):986-95. doi: 10.1111/eci.12118. Epub 2013 Jun 17. PMID: 23772948; PMCID: PMC3745791.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.