Biology Group 2025 January 24;6(1):070-081. doi: 10.37871/jbres2057.
Use of Thienopyrimidine Derivatives to Optimize Sorghum Growth and Photosynthesis during the Vegetation Period
Tsygankova VA*, Vasylenko NM, Andrusevich YaV, Kopich VM, Kachaeva MV, Popilnichenko SV, Pilyo SG and Brovarets VS
- Sorghum bicolor L
- IAA
- Methyur
- Kamethur
- Thienopyrimidine derivatives
Abstract
This study is aimed at screening new auxin- and cytokinin-like substances among chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of thienopyrimidine, to improve growth and enhance photosynthesis of an important agricultural crop - grain sorghum (Sorghum bicolor L.) variety Odeske 202 during the vegetation period. A comparative analysis of the morphometric parameters of sorghum plants, as well as the content of photosynthetic pigments in sorghum plants treated with auxin IAA (1H-indol-3-yl)acetic acid or chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) or new compounds, thienopyrimidine derivatives at a concentration of 10-7M was carried out. As a result of the screening, the most physiologically active compounds among thienopyrimidine derivatives were identified, which promote growth and intensification of photosynthesis in sorghum plants, and the relationship between their chemical structure and regulatory effect was analyzed. Based on the obtained results, the practical use of selected compounds, derivatives of thienopyrimidine, as new effective plant growth regulators for improving growth and increasing photosynthesis of sorghum plants during the vegetation period is proposed.
Introduction
Sorghum (Sorghum bicolor L.) Moench) is a strategically important cereal crop after wheat, maize, barley, and rice, used for food, feed, fiber, and fuel production worldwide [1-3]. Currently, plant growth regulators (thiamethoxam, chlormequat chloride, Ethephon (ETH), Uniconazole (S-3307), Paclobutrazol (PBZ), Fluridone, Trinexapac-Ethyl, 2, 3, 5-Triiodobenzoic Acid (TIBA), etc.), phytohormones (Gibberellic Acid (GA3), Jasmonic Acid (JA), Salicylic Acid (SA), Abscisic Acid (ABA), and auxins) and microbial biostimulants are widely used to improve plant growth during the growing season, enhance photosynthesis, increase yield, protect plants from pests, and mitigate sorghum responses to biotic and abiotic stress [4-9].
Nevertheless, a very urgent problem for the agricultural industry is the creation of new effective plant growth regulators to optimize sorghum growth and increase yields against the backdrop of global climate change and declining soil fertility [10,11]. In this regard, the development of new plant growth regulators based on chemical low-molecular-weight nitrogen-containing heterocyclic compounds that exhibit a high plant growth-regulating effect similar to plant hormones is a very promising issue [12,13]. Among these classes of chemical compounds, pyrimidine derivatives are of important practical interest as effective, environmentally friendly plant growth regulators [12-17].
In Ukraine, the most famous representatives of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, which have been studied on various plant species, are derivatives of the sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) [12,18,19]. Numerous studies show that the use of chemical low-molecular-weight nitrogen-containing heterocyclic compounds Methyur and Kamethur as substitutes for plant hormones auxins and cytokinins helps improve plant organogenesis in isolated plant cell cultures, tissues and organs in vitro and postembryonic organogenesis, which is an essential component of the development of plant roots and shoots and their adaptation to environmental conditions [18-24]. Field trials confirm the effectiveness of using chemical low-molecular-weight nitrogen-containing heterocyclic compounds Methyur and Kamethur for pre-sowing seed treatment of wheat, sunflower and sorghum crops to improve cultivation and increase their yields [22-24].
Along with such well-known chemical low-molecular-weight nitrogen-containing heterocyclic compounds, pyrimidine derivatives, as Methyur and Kamethur, studies are being conducted on the plant growth-regulating activity of new chemical low-molecular-weight nitrogen-containing heterocyclic compounds [12,13]. Among these chemical compounds, new representatives of pyrimidine derivatives are very promising substances that regulate plant growth due to their high biological activity, similar to plant hormones auxins and cytokinins [25-38]. An important aspect of increasing crop yields is the intensification of the process of photosynthesis in plant leaves. Studies of various representatives of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, pyrimidine derivatives, indicate their effect on enhancing the biosynthesis of photosynthetic pigments (chlorophylls and carotenoids) in plant cells, which play an important role in ensuring plant productivity [28,29,31,33,35-38]. It is precisely because of these properties that regulate plant growth and photosynthesis that pyrimidine derivatives are of interest for studying the possibility of their practical use in sorghum crops. The environmental safety of the use of pyrimidine derivatives in agriculture lies in their low, non-toxic to the environment and human and animal health concentrations of 10-5M, 10-6M, 10-7M and 10-8M only at the stage of pre-sowing soaking of seeds of agricultural crops [22-38]. The use of such environmentally friendly plant growth regulators will reduce environmental and soil pollution.
The aim of the work is to study the regulatory effect of new chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives, on the growth and photosynthesis of grain sorghum (Sorghum bicolor L.) variety Odeske 202 during the vegetation period.
Materials and Methods
Seed treatment and plant growing conditions
The seeds of grain sorghum (Sorghum bicolor L.) variety Odeske 202 were sterilized with 1% KMnO4 solution for 15-20 min, then treated with 96% ethanol solution for 1 min, after which they were washed three times with sterile distilled water. After this procedure, seeds were placed in the plastic cuvettes (each containing 20-25 seeds) on the perlite moistened with distilled water (control sample) or water solutions of auxin IAA (1H-indol-3-yl)acetic acid or chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur), or thienopyrimidine derivatives (compounds № 1-13) in a concentration of 10-7M (experimental samples). Then seeds were placed in the thermostat for their germination in darkness at the temperature 22°C during 48 h. After the emergence of sprouts, they were placed in a climatic chamber in which sorghum plants were grown for 4 weeks at the 16/8 h light/dark conditions, at the temperature 22-23°C, light intensity of 3000 lux, and air humidity 60-80%. Comparative analysis of morphometric parameters of sorghum plants (average length of shoots and roots (mm)) was carried out at the end of the 4-week period according to the methodological manual [39]. Morphometric parameters determined on experimental plants, in comparison with similar parameters of control plants, were expressed as %.
Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) and thienopyrimidine (compounds № 1-13) were synthesized using methods described in the works [40,41] at the Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine; plant hormone auxin IAA (1H-indol-3-yl)acetic acid) was produced by Sigma-Aldrich, USA, CAS number 87-51-4 (Table 1).
Determination of the content of photosynthetic pigments in sorghum plants
To perform the extraction of photosynthetic pigments (chlorophylls and carotenoids), we homogenized a sample (500 mg) of plant leaves in the porcelain mortar in a cooled at the temperature 10°С 96 % ethanol at the ratio of 1:10 (w:v) with addition of 0.1-0.2 g CaCO3 (to neutralize the plant acids). The 1 mL of obtained homogenate was centrifuged at 8000 g in a refrigerated centrifuge K24D (MLW, Engelsdorf, Germany) during 5 min at the temperature 4°С. The obtained precipitate was washed three times, with 1 mL 96% ethanol and centrifuged at above mentioned conditions. After this procedure, the optical density of chlorophyll a, chlorophyll b and carotenoid in the obtained extract was measured using spectrophotometer Specord M-40 (Carl Zeiss, Germany).
The content of chlorophyll a, chlorophyll b, and carotenoids in plant leaves was calculated in accordance with formula [42]:
Cchl a = 13.36 × A664.2 – 5.19 × A648.6
Cchl b = 27.43 × A648.6 – 8.12A × 664.2
Cchl (a + b) = 5.24 × A664.2 + 22.24 × A648.6
Ccar = (1000 × A470 – 2.13 × Cchl a – 97.64 × Cchlb)/209
Where;
Cchl – Concentration of chlorophylls (µg/ml), Cchl a – concentration of chlorophyll a (µg/ml), Cchl b – concentration of chlorophyll b (µg/ml), Ccar – concentration of carotenoids (µg/ml), А – absorbance value at a proper wavelength in nm.
The chlorophyll and carotenoids content per 1 g of Fresh Weight (FW) of extracted from plant leaves was calculated by the following formula (separately for chlorophyll a, chlorophyll b and carotenoids):
A1 = (C × V)/(1000 × a1).
Where, A1 – content of chlorophyll a, chlorophyll b, or carotenoids (mg/g FW),
C - Concentration of pigments (µg/ml),
V - Volume of extract (ml),
a1 - sample of plant leaves (g).
The content of photosynthetic pigments determined in the leaves of experimental plants in relation to control plants was expressed as %.
Statistical processing of the experimental data was carried out using Student’s t-test with a significance level of p ≤ 0.05; mean values ± standard deviation (± SD). Each experiment was performed three times [43].
Results and Discussion
Comparative analysis of morphometric parameters of sorghum plants. The regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives (compounds № 1-13) on the morphometric parameters of sorghum plants was studied.
The average length of plant shoots and roots (mm) was measured at the end of the 4-week period. The regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives on plant growth was compared with the regulatory effect of auxin IAA (1H-indol-3-yl)acetic acid or chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur), which exhibit phytohormone-like effects on various plant species [18-24,28-38].
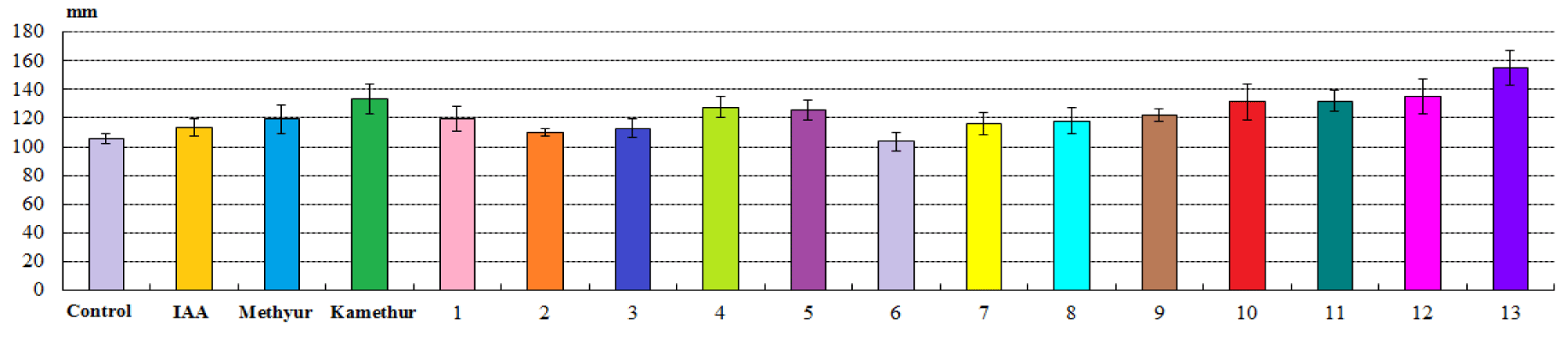
It was shown that the morphometric parameters of sorghum plants treated with chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives (compounds № 1-13) at a concentration of 10-7M were similar to or higher than the morphometric parameters of sorghum plants treated with auxin IAA or derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur), used at a similar concentration of 10-7M (Figure 1).
Comparative analysis of morphometric parameters showed that chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) showed the highest regulatory effect on the growth and development of plant shoots, under the influence of which the average shoot length parameters increased by 12.44 % - under the influence of Methyur and 25.94 % - under the influence of Kamethur, respectively, compared to similar parameters of control sorghum plants (Figure 2).
The highest regulatory effect on the growth and development of plant shoots was also demonstrated by chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 1,4,5, 7-13, under the influence of which the average shoot length parameters increased by 9.28-46.35 %, respectively, compared to similar parameters of control sorghum plants (Figure 2).
Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 2,3,6 showed a lower regulatory effect on the growth and development of shoots, under the influence of which the average shoot length parameters increased by 3.77-6.6 %, respectively, compared to similar parameters of control sorghum plants (Figure 2). Auxin IAA also showed a lower regulatory effect on the growth and development of shoots, under the influence of which the average shoot length parameters increased by 7.31%, respectively, compared to similar parameters of control sorghum plants (Figure 2).
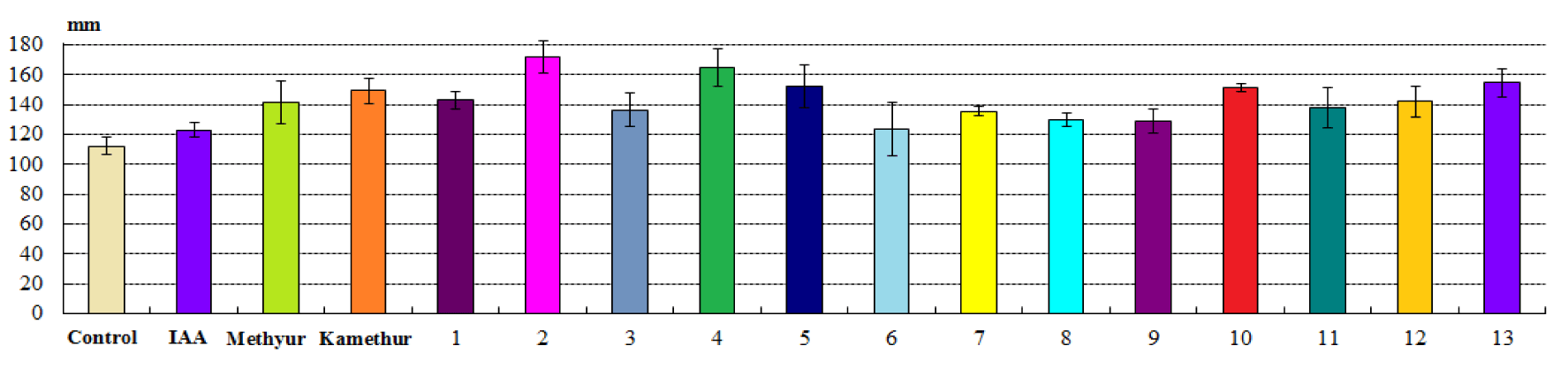
Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) showed the highest regulatory effect on the growth and development of plant roots, under the influence of which the average root length parameters increased by 26.39 % - under the influence of Methyur and 33.9 % - under the influence of Kamethur, respectively, compared to similar parameters of control sorghum plants (Figure 3).
The highest regulatory effect on the growth and development of plant roots was also demonstrated by chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 1,2,4, 5,10-13, under the influence of which the average root length parameters increased by 23-53.61 %, respectively, compared to similar parameters of control sorghum plants (Figure 3).
Auxin IAA showed a lower regulatory effect on the growth and development of roots, under the influence of which the average root length parameters increased by 9.74 %, respectively, compared to similar parameters of control sorghum plants (Figure 3). Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 3,6-9 also showed a lower regulatory effect on the growth and development of roots, under the influence of which the average root length parameters increased by 10.25-21.49 %, respectively, compared to similar parameters of control sorghum plants (Figure 3).
Summarizing the obtained data, it should be noted that the highest regulatory effect in parameters of average shoot and root length (mm) of sorghum plants was demonstrated by chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) and thienopyrimidine derivatives № 1,2,4,5,10-13. The regulatory effect of these chemical compounds applied at a concentration of 10-7M was similar to or exceeded the activity of auxin IAA applied at a similar concentration. Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 3,6-9, showed lower regulatory effect in parameters of average shoot and root length (mm) of sorghum plants.
Thus, the data obtained indicate that the regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) and thienopyrimidine derivatives is based on their auxin-like effect on the main processes of growth and development of roots, shoots, and leaves in postembryonic organogenesis, such as division and expansion of plant cells, as well as cell wall biosynthesis [44-48].
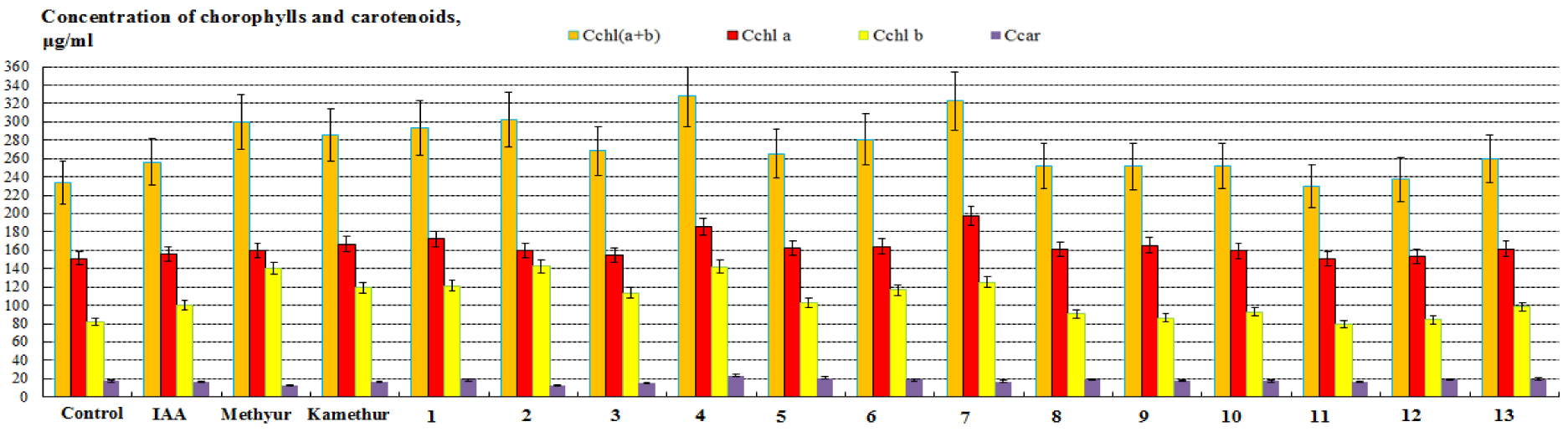
Comparative analysis of the content of photosynthetic pigments in sorghum plants. The regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives (compounds № 1-13) on the content of photosynthetic pigments in sorghum plants was compared with the regulatory effect of auxin IAA (1H-indol-3-yl)acetic acid or chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur). The obtained results are illustrated in Figure 4.
It was shown that the content of photosynthetic pigments in sorghum plants treated with chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives (compounds № 1-13) at a concentration of 10-7 M was similar to or higher than the content of photosynthetic pigments in sorghum plants treated with auxin IAA or derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur), used at a similar concentration (Figure 4).
Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) showed the highest regulatory effect on the content of chlorophylls a and b in sorghum plants. The content of chlorophyll a in sorghum plants increased by 5.59 % - under the influence of Methyur and by 10.2% - under the influence of Kamethur; the content of chlorophyll b in sorghum plants increased by 71.38% - under the influence of Methyur and by 45.46 % - under the influence of Kamethur; the content of chlorophyll a+b in sorghum plants increased by 28.70 % - under the influence of Methyur and by 22.59 % - under the influence of Kamethur, respectively, compared to similar indicators of control sorghum plants (Figure 4).
Studies have shown that chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 1,2,4-7,10 and 13 also had the highest regulatory effect on the content of chlorophylls a and b in sorghum plants, under the influence of which the content of chlorophyll a increased: by 5.31-30.55 %, chlorophyll b increased: by 13-74.24%, chlorophyll a+b increased: by 8,02-40,73 %, respectively, compared to similar indicators of control sorghum plants (Figure 4).
Auxin IAA showed a lower regulatory effect on the content of chlorophylls a and b in sorghum plants, under the influence of which the content of chlorophyll a increased: by 3.16%, chlorophyll b increased: by 22.53%, chlorophyll a, b increased: by 10%, respectively, compared to similar indicators of control sorghum plants (Figure 4). Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 3,8 and 9 also showed a lower regulatory effect on the content of chlorophylls a and b in sorghum plants, under the influence of which the content of chlorophyll a increased: by 2.3-9.27 %, chlorophyll b increased: by 5.6-38.62%, chlorophyll a+b increased by 7.98-15.05 %, respectively, compared to similar parameters of control sorghum plants (Figure 4).
Chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 5 and 11 did not show regulatory effect, the content of chlorophylls a and b in sorghum plants did not statistically significantly differ from similar indicators of control sorghum plants (Figure 4).
Comparative analysis of the content of carotenoids in sorghum plants showed that the highest regulatory effect was demonstrated by chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 1,4-6,8,9,12 and 13, under the influence of which the content of carotenoids increased by 7.99-37.59 %, respectively, compared to similar indicators of control sorghum plants (Figure 4).
Auxin IAA and chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur), and thienopyrimidine derivatives № 2,3,7,10 and 11 did not show regulatory effect; under their influence, the content of carotenoids in sorghum plants did not differ statistically significantly, or was lower than similar indicators of control sorghum plants (Figure 4).
Thus, the conducted studies indicate that chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives, exhibit selectivity of action in the regulation of growth and photosynthesis of sorghum plants. The highest regulatory effect on morphometric parameters and the content of photosynthetic pigments in sorghum plants was demonstrated by chemical nitrogen-containing heterocyclic compounds № 1,2,4,5, 7,10 and 13; compounds № 3,6,8,9,11 and 12 showed a slightly lower regulatory effect.
It can be concluded that the regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium salt and potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) and thienopyrimidine derivatives on increasing the biosynthesis of photosynthetic pigments in sorghum plants (chlorophylls a and b, and carotenoids), is similar to the effect of plant hormones cytokinins. As is known these plant hormones play an important role in regulating leaf development, delay leaf senescence, activate photosynthesis and prevent the degradation of photosynthetic pigments such as chlorophylls and carotenoids in plant leaves [48-51].
Analyzing the obtained results, it can be assumed that the highest regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 1,2,4,5,7,10 and 13 on the growth and photosynthesis of sorghum plants is related to the presence of substituents in their chemical structure (Table 1): compound № 1 contains phenyl group - in position 5 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 2 contains phenyl group in position 5, tetrahydrofuran-2-ylmethyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 4 contains phenyl group in position 5, pyridin-3-ylmethyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 5 contains phenyl group in position 5, 2-(4-methoxyphenyl)ethyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 7 contains a p-tolyl group in position 5, a 3-methoxypropyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 10 contains methyl group in position 5, benzyl group in position 3, carboxyl group in position 6 of the 4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine ring; compound № 13 contains benzyl group in position 3, 4-chlorophenyl group in position 4 of the 3H-thieno[2,3-d]pyrimidin-4-one ring.
| Table 1: Compounds tested. | ||
| Chemical compound | Chemical structure | IUPAC name and relative molecular weight (g/mol) |
| IAA | (1H-indol-3-yl)acetic acid MW = 175.19 | |
| Methyur | sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine MW = 165.17 | |
| Kamethur | potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine MW = 181.28 | |
| 1 | 5-Phenyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 228.274 | |
| 2 | 5-Phenyl-3-(tetrahydrofuran-2-ylmethyl)-3H-thieno[2,3-d]pyrimidin-4-one MW = 312.393 | |
| 3 | 3-Cyclopentyl-5-phenyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 296.394 | |
| 4 | 5-Phenyl-3-pyridin-3-ylmethyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 319.388 | |
| 5 | 3-[2-(4-Methoxyphenyl)-ethyl]-5-phenyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 362.454 | |
| 6 | 3-(2-Methoxyethyl)-5-p-tolyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 300.382 | |
| 7 | 3-(3-Methoxypropyl)-5-p-tolyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 314.409 | |
| 8 | 6-Ethyl-2-mercapto-3-phenyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 288.393 | |
| 9 | (6-Ethyl-4-oxo-3-phenyl-3,4-dihydrothieno[2,3-d]pyrimidin-2-ylsulfanyl)acetic acid MW = 346.43 | |
| 10 | 3-Benzyl-5-methyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine-6-carboxylic acid MW = 300.339 | |
| 11 | 5-Methyl-4-oxo-3-pyridin-4-ylmethyl-3,4-dihydrothieno[2,3-d]pyrimidine-6-carboxylic acid MW = 301.326 | |
| 12 | 5-(4-Chlorophenyl)-3-furan-2-ylmethyl-3H-thieno[2,3-d]pyrimidin-4-one MW = 342.806 | |
| 13 | 3-Benzyl-5-(4-chlorophenyl)-3H-thieno[2,3-d]pyrimidin-4-one MW = 352.845 | |
The decrease of the regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives № 3,6,8,9,11 and 12 on the growth and photosynthesis of sorghum plants can be explained by the presence of substituents in their chemical structures (Table 1): compound № 3 contains a phenyl group in position 5, a cyclopentyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 6 contains p-tolyl group in position 5, 2-methoxyethyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 8 contains ethyl group in position 6, phenyl group in position 3, mercapto group in position 2 of the 3H-thieno[2,3-d]pyrimidin-4-one ring; compound № 9 contains an ethyl group in position 6, a phenyl group in position 3, a sulfonylacetic acid residue in position 2 of the 4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine ring; compound № 11 contains methyl group in position 5, pyridin-4-ylmethyl group in position 3, carboxyl group in position 6 of the 4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine ring; compound № 12 contains 4-chlorophenyl group in position 5, furan-2-ylmethyl group in position 3 of the 3H-thieno[2,3-d]pyrimidin-4-one ring.
It is possible to suggest that the molecular mechanism of action of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, derivatives of sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) and derivatives of thienopyrimidine, by analogy with the molecular mechanism of action of other currently known synthetic auxin-related and cytokinin-related compounds, is carried out through the regulation of auxin and cytokinin signaling in plant cells, or through changes in the level of endogenous plant hormones by modulating the activity of key enzymes of biosynthesis, transport, metabolism, conjugation and oxidation of plant hormones, thereby changing the level of endogenous auxins and cytokinins in plant cells [52-65].
Conclusion
A study was conducted on the regulatory effect of chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives, on the growth and photosynthesis of grain sorghum (Sorghum bicolor L.) variety Odeske 202 during the vegetation period. It has been shown that chemical compounds, derivatives of thienopyrimidine, accelerate the formation and the development of shoots and roots, increase the content of photosynthetic pigments in sorghum plants, similar to auxin IAA (1H-indol-3-yl)acetic or chemical compounds, derivatives of sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur). It was concluded that the selectivity of the regulatory effect of the studied chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives, is explained by the presence of substituents in their chemical structure. The conducted studies provide prospects for the development of new effective sorghum plant growth regulators based on chemical low-molecular-weight nitrogen-containing heterocyclic compounds, thienopyrimidine derivatives.
Statement of Conflict of Interest
The authors are declared that they have no conflict with this research article.
References
- Growth and Production of Sorghum and Millets. Soils, Plant Growth and Crop Production. Verheye, Willy H, editors. EOLSS Publishers; Volume 2. 2010.
- Borrell AK, van Oosterom EJ, Mullet JE, George-Jaeggli B, Jordan DR, Klein PE, Hammer GL. Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol. 2014 Aug;203(3):817-30. doi: 10.1111/nph.12869. Epub 2014 Jun 4. PMID: 24898064.
- Curti MI, Palavecino PM, Savio M, Baroni MV, Ribotta PD. Sorghum (Sorghum bicolor L. Moench) Gluten-Free Bread: The Effect of Milling Conditions on the Technological Properties and In Vitro Bioaccessibility of Polyphenols and Minerals. Foods. 2023 Aug 12;12(16):3030. doi: 10.3390/foods12163030. PMID: 37628029; PMCID: PMC10453239.
- Han LP, Wang X, Guo X, Rao MS, Steinberger Y, Cheng X, Xie GX. Effects of plant growth regulators on growth, yield and lodging of sweet sorghum. Res on Crops. 2011;12(2):372-382.
- Huang J, Shrestha K, Huang Y. Revealing Differential Expression of Phytohormones in Sorghum in Response to Aphid Attack Using the Metabolomics Approach. Int J Mol Sci. 2022 Nov 9;23(22):13782. doi: 10.3390/ijms232213782. PMID: 36430259; PMCID: PMC9699302.
- Lone R, Hassan N, Bashir B, Rohela GK, Malla NA. Role of growth elicitors and microbes in stress management and sustainable production of Sorghum. Plant Stress. 2023;9:100179. doi: 10.1016/j.stress.2023.100179.
- Nimir EAN, Zhou G, Zhu G, Ibrahim ME. Response of some sorghum varieties to GA3 concentrations under different salt compositions. Chilean Journal of Agricultural Research. 2020;80(4):478:486. doi: 10.4067/S0718-58392020000400478.
- Macedo WR, Araújo DK, Santos VM, de Camargo e Castro PR, Fernandes GM. Plant growth regulators on sweet sorghum: physiological and nutritional value analysis. Comunicata Scientiae. 2017;8(1):170-175. doi: 10.14295/CS.v8i1.1315.
- Pravdyva L, Zatserkovna N, Vakhniy S, Khakhula V, Hornovska S. Photosynthetic productivity of sorghum (Sorghum bicolor L. (Moenh) in the conditions of the Right-Bank Forest-Steppe of Ukraine. Scientific Horizons. 2023;26(5):56-64. doi: 10.48077/scihor5.2023.56.
- Msongaleli B, Rwehumbiza F, Tumbo S, Kihupi N. Sorghum yield response to changing climatic conditions in semi-arid central Tanzania: Evaluating crop simulation model applicability. Agricultural Sciences. 2014;5:822-833. doi: 10.4236/as.2014.510087.
- Ondiko JH, Rech CW. Global sorghum production constraints: A review. Annals of Arid Zone. 2022;61(1):1-12.
- Tsygankova VA, Brovarets VS, Yemets AI, Blume YB. Prospects of the development in Ukraine of the newest plant growth regulators based on low molecular heterocyclic compounds of the azole, azine and their condensed derivatives. In Book: Synthesis and bioactivity of functionalized nitrogen-containing heterocycles / Eds. A.I. Vovk. Kyiv: Interservice, 2021. p.246-285.
- Tsygankova VA, Andrusevich Ya V, Shtompel OI, Solomyanny RM, Hurenko AO, Frasinyuk MS, Mrug GP, Shablykin OV, Pilyo SG, Kornienko AM, Brovarets VS. New auxin and cytokinin related compounds based on synthetic low molecular weight heterocycles. Chapter 16, In: Aftab T. Editor. Auxins, cytokinins and gibberellins signaling in plants. Signalling and communication in plants. Springer Nature Switzerland AG. 2022;353-377. doi: 10.1007/978-3-031-05427-3_16.
- Ota C, Kumata S, Kawaguchi S. Novel herbicides, usage thereof, novel thienopyrimidine derivatives, intermediates of the same, and process for production thereof. Patent US20070010402A1. 2007.
- Li JH, Wang Y, Wu YP, Li RH, Liang S, Zhang J, Zhu YG, Xie BJ. Synthesis, herbicidal activity study and molecular docking of novel pyrimidine thiourea. Pestic Biochem Physiol. 2021 Feb;172:104766. doi: 10.1016/j.pestbp.2020.104766. Epub 2020 Dec 25. PMID: 33518053.
- Cansev A, Gülen H, Zengin MK, Ergin S, Cansev M. Use of pyrimidines in stimulation of plant growth and development and enhancement of stress tolerance. WIPO Patent WO 2014/129996A1. 2014.
- Boussemghoune MA,Whittingham WG, Winn CL, Glithro H, Aspinall MB. Pyrimidine derivatives and their use as herbicides. Patent US20120053053 A1. 2012.
- Tsygankova VA, Andrysevich YV, Shtompel OI, Kopich VM, Kluchko SV, Brovaretz VS. Using Pyrimidine Derivatives - Sodium Salt of Methyur and Potassium Salt of Methyur, to Intensify the Growth of Corn. Patent of Ukraine 130921. 2018.
- Tsygankova VA, Andrysevich YV, Shtompel OI, Kopich VM, Kluchko SV, Brovaretz VS. The method of intensifying the growth of corn plants using Methyur potassium salt. Patent of Ukraine 123222. 2021.
- Tsygankova VA, Oliynyk OO, KvaskoO Yu, Pilyo SG, Klyuchko SV, Brovarets VS. Effect of plant growth regulators Ivin, Methyur and Kamethur on the organogenesis of miniature rose (Rosa mini L.) In vitro. Int J Med Biotechnol Genetics. 2022;1-8.
- Pidlisnyuk V, Mamirova A, Newton RA, Stefanovska T, Zhukov O, Tsygankova V, Shapoval P. The role of plant growth regulators in Miscanthus × giganteus utilisation on soils contaminated with trace elements. Agronomy. 2022;12(12):2999. doi: 10.3390/agronomy12122999.
- Tsygankova VA, Voloshchuk IV, Kopich VM, Pilyo SG, Klyuchko SV, Brovarets VS. Studying the effect of plant growth regulators Ivin, Methyur and Kamethur on growth and productivity of sunflower. Journal of Advances in Agriculture. 2023;14:17:24. doi: 10.24297/jaa.v14i.9453.
- Tsygankova VA, Voloshchuk IV, Pilyo SH, Klyuchko SV, Brovarets vs., Enhancing sorghum productivity with Methyur, Kamethur, and Ivin plant growth regulators. Biology and Life Sciences Forum. 2023;27(1):36. doi: 10.3390/IECAG2023-15222.
- Tsygankova VA, Kopich VM, Vasylenko NM, Golovchenko OV, Pilyo SG, Malienko MV, Brovarets VS. Increasing the productivity of wheat using synthetic plant growth regulators Methyur, Kamethur and Ivin. Znanstvena misel journal. 2024;94:22-26. doi: 10.5281/zenodo.13860706.
- Tsygankova VA, Bayer OO, Andrusevich Ya V, Galkin AP, Brovarets VS, Yemets AI, Blume Ya B. Screening of five and six-membered nitrogen-containing heterocyclic compounds as new effective stimulants of Linum usitatissimum L. organogenesis in vitro. Int J Med Biotechnol Genetics. 2016;02(1):1-9. doi: 10.19070/2379-1020-SI02001.
- Tsygankova VA, Andrusevich Ya V, Shtompel OI, Kopich VM, Solomyanny RM, Brovarets VS. Study of regulating activity of synthetic low molecular weight heterocyclic compounds, derivatives of pyrimidine on growth of tomato (Solanum lycopersicum L.) seedlings. International Journal of ChemTech Research. 2019;12(5):26-38. doi: 10.20902/IJCTR.2019.120504.
- Mohilnikova IV, Tsygankova VA, Solomyannyi RM, Brovarets VS, Bilko NМ, Yemets АІ. Screening of growth-stimulating activity of synthetic compounds - pyrimidine derivatives. Reports of the National Academy of Sciences of Ukraine. 2020;10:62-70. doi: 10.15407/dopovidi2020.10.062.
- Tsygankova VA, Kopich VM, Voloshchuk IV, Pilyo SG, Klyuchko SV, Brovarets VS. New growth regulators of barley based on pyrimidine and pyridine derivatives. Sciences of Europe. 2023;124:13-23. doi: 10.5281/zenodo.8327852.
- Tsygankova VA, Andrusevich Ya V, Kopich VM, Voloshchuk IV, Bondarenko OM, Pilyo SG, Klyuchko SV, Brovarets VS. Effect of pyrimidine and pyridine derivatives on the growth and photosynthesis of pea microgreens. Int J Med Biotechnol Genetics. 2023;15-22.
- Tsygankova VA, Andrusevich Ya V, Kopich VM, Voloshchuk IV, Pilyo SG, Klyuchko SV, Brovarets VS. Application of pyrimidine and pyridine derivatives for regulation of chickpea (Cicer arietinum L.) growth. International Journal of Innovative Science and Research Technology (IJISRT). 2023;8(6):19-28. doi: 10.5281/zenodo.8020671.
- Tsygankova VA, Kopich VM, Vasylenko NM, Andrusevich Ya V, Pilyo SG, Brovarets VS. Phytohormone-like effect of pyrimidine derivatives on the vegetative growth of haricot bean (Phaseolus vulgaris L.). Polish Journal of Science. 2024;1(71):6-13. doi: 10.5281/zenodo.10675232.
- Tsygankova VA, Andrusevich Ya V, Vasylenko NM, Pilyo SG, Klyuchko SV, Brovarets VS. Screening of auxin-like substances among synthetic compounds, derivatives of pyridine and pyrimidine. J Plant Sci Phytopathol. 2023;7:151-156. doi: 10.29328/journal.jpsp.1001121.
- Tsygankova VA, Vasylenko NM, Andrusevich Ya V, Kopich VM, Solomyannyi RM, Pilyo SG, Bondarenko OM, Popilnichenko SV, Brovarets VS. New wheat growth regulators based on thioxopyrimidine derivatives. Int J Med Biotechnol Genetics. 2024;23-30.
- Tsygankova VA, Andrusevich Ya V, Vasylenko NM, Kopich VM, Pilyo SG, Solomyannyi RM, Popilnichenko SV, Bondarenko OM, Brovarets VS. The use of thioxopyrimidine derivatives for the regulation of vegetative growth of wheat. Journal of Medicinal Botany. 2024;8:1-7. doi: 10.25081/jmb.2024.v8.8918.
- Tsygankova VA, Andrusevich Ya V, Vasylenko NM, Kopich VM, Solomyannyi RM, Popilnichenko SV, Kozachenko OP, Pilyo SG, Brovarets VS. The use of thioxopyrimidine derivatives as new regulators of growth and photosynthesis of barley. J Plant Sci Phytopathol. 2024;8(2):090-099. doi: 10.29328/journal.jpsp.1001139.
- Tsygankova V, Andrusevich Ya, Kopich V, Vasylenko N, Solomyannyi R, Popilnichenko S, Kachaeva M, Kozachenko O, Pilyo S, Brovarets V. Wheat growth in the vegetative phase under the regulatory effect of furopyrimidine derivatives. The scientific heritage. 2024;140:3-12. doi: 10.5281/zenodo.12720609.
- Tsygankova VA, Andrusevich Ya V, Vasylenko NM, Kopich VM, Popilnichenko SV, Pilyo SG, Brovarets VS. Auxin-like and cytokinin-like effects of new synthetic pyrimidine derivatives on the growth and photosynthesis of wheat. J Plant Sci Phytopathol. 2024;8(1)-15-24. doi: 10.29328/journal.jpsp.1001126
- Tsygankova V, Vasylenko N, Andrusevich Ya, Kopich V, Kachaeva M, Popilnichenko S, Kozachenko O, Pilyo S, Brovarets V. Application of thienopyrimidine derivatives as new eco-friendly wheat growth regulators. Sciences of Europe. 2024;146;8:18. doi: 10.5281/zenodo.13267799.
- Voytsehovska OV, Kapustyan AV, Kosik OI. Plant physiology: Praktykum, Parshikova T.V. Editors. Lutsk: Teren; 2010. p.420.
- Biswas BK, Shin JS, Malpani YR, Hwang D, Jung E, Han SB, Vishakantegowda AG, Jung YS. Enteroviral replication inhibition by N-Alkyl triazolopyrimidinone derivatives through a non-capsid binding mode. Bioorganic & Medicinal Chemistry Letters. 2022;64:128673. doi: 10.1016/j.bmcl.2022.128673.
- Al-Taisan KM, Al-Hazimi HMA, Al-Shihry SS. Synthesis, Characterization and Biological Studies of Some Novel Thieno[2,3-d]pyrimidines. Molecules. 2010;15(6):3932-3957. doi: 10.3390/molecules15063932
- Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Current Protocols in Food Analytical Chemistry (CPFA): John Wiley and Sons, New York; 2001. doi: 10.1002/0471142913.faf0403s01.
- Statistical methods in molecular biology. Bang H, Zhou XK, van Epps HL, Mazumdar M. Editors. Series: Methods in molecular biology, New York: Humana press; 2010;13(620):636.
- Guyomarc'h S, Lucas M, Laplaze L. Postembryonic Organogenesis in Plants: Experimental Induction of New Shoot and Root Organs. Methods Mol Biol. 2022;2395:79-95. doi: 10.1007/978-1-0716-1816-5_5. PMID: 34822150.
- Gallavotti A. The role of auxin in shaping shoot architecture. J Exp Bot. 2013 Jun;64(9):2593-608. doi: 10.1093/jxb/ert141. PMID: 23709672.
- Roychoudhry S, Kepinski S. Auxin in Root Development. Cold Spring Harb Perspect Biol. 2022 May 17;14(4):a039933. doi: 10.1101/cshperspect.a039933. PMID: 34312248; PMCID: PMC9121899.
- Zhang Q, Gong M, Xu X, Li H, Deng W. Roles of Auxin in the Growth, Development, and Stress Tolerance of Horticultural Plants. Cells. 2022 Sep 5;11(17):2761. doi: 10.3390/cells11172761. PMID: 36078168; PMCID: PMC9454831.
- Sosnowski J, Truba M, Vasileva V. The Impact of auxin and cytokinin on the growth and development of selected crops. Agriculture. 2023;13(3):724. doi: 10.3390/agriculture13030724.
- Wu W, Du K, Kang X, Wei H. The diverse roles of cytokinins in regulating leaf development. Hortic Res. 2021;8:118:1-13. doi: 10.1038/s41438-021-00558-3.
- Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int J Mol Sci. 2018 Dec 14;19(12):4045. doi: 10.3390/ijms19124045. PMID: 30558142; PMCID: PMC6321018.
- Zhang YM, Guo P, Xia X, Guo H, Li Z. Multiple Layers of Regulation on Leaf Senescence: New Advances and Perspectives. Front Plant Sci. 2021 Dec 6;12:788996. doi: 10.3389/fpls.2021.788996. PMID: 34938309; PMCID: PMC8685244.
- Calderon-Villalobos LI, Tan X, Zheng N, Estelle M. Auxin perception--structural insights. Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a005546. doi: 10.1101/cshperspect.a005546. Epub 2010 May 26. PMID: 20504967; PMCID: PMC2890193.
- Rigal A, Ma Q, Robert S. Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci. 2014 Jul 30;5:373. doi: 10.3389/fpls.2014.00373. PMID: 25126092; PMCID: PMC4115670.
- Fukui K, Hayashi KI. Manipulation and Sensing of Auxin Metabolism, Transport and Signaling. Plant Cell Physiol. 2018 Aug 1;59(8):1500-1510. doi: 10.1093/pcp/pcy076. PMID: 29668988.
- Hayashi KI. Chemical Biology in Auxin Research. Cold Spring Harb Perspect Biol. 2021 May 3;13(5):a040105. doi: 10.1101/cshperspect.a040105. PMID: 33431581; PMCID: PMC8091948.
- Tsygankova VA, Zayets VN, Galkina LA, Blume Ya B. The phytohormone-mediated action of the synthetic regulators on cell extension growth in higher plants. Biopolymers and Cell. 1999;15(5):432-441. doi: 10.7124/bc.00053B.
- Ricci A, Bertoletti C. Urea derivatives on the move: cytokinin-like activity and adventitious rooting enhancement depend on chemical structure. Plant Biol (Stuttg). 2009 May;11(3):262-72. doi: 10.1111/j.1438-8677.2008.00165.x. Epub 2008 Dec 15. PMID: 19470099.
- Desta B, Amare G. Paclobutrazol as a plant growth regulator. Chem Biol Technol Agric. 2021;8:1. doi: 10.1186/s40538-020-00199-z.
- Mellor N, Band LR, Pěnčík A, Novák O, Rashed A, Holman T, Wilson MH, Voß U, Bishopp A, King JR, Ljung K, Bennett MJ, Owen MR. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc Natl Acad Sci U S A. 2016 Sep 27;113(39):11022-7. doi: 10.1073/pnas.1604458113. Epub 2016 Sep 20. PMID: 27651495; PMCID: PMC5047161.
- Zhang J, Peer WA. Auxin homeostasis: The DAO of catabolism. Journal of Experimental Botany. 2017;68(12):3145-3154. doi: 10.1093/jxb/erx221.
- Hayashi KI, Arai K, Aoi Y, Tanaka Y, Hira H, Guo R, Hu Y, Ge C, Zhao Y, Kasahara H, Fukui K. The main oxidative inactivation pathway of the plant hormone auxin. Nat Commun. 2021 Nov 22;12(1):6752. doi: 10.1038/s41467-021-27020-1. PMID: 34811366; PMCID: PMC8608799.
- Müller K, Dobrev PI, Pěnčík A, Hošek P, Vondráková Z, Filepová R, Malínská K, Brunoni F, Helusová L, Moravec T, Retzer K, Harant K, Novák O, Hoyerová K, Petrášek J. DIOXYGENASE FOR AUXIN OXIDATION 1 catalyzes the oxidation of IAA amino acid conjugates. Plant Physiol. 2021 Sep 4;187(1):103-115. doi: 10.1093/plphys/kiab242. PMID: 34618129; PMCID: PMC8418401.
- Chen L, Zhao J, Song J, Jameson PE. Cytokinin dehydrogenase: a genetic target for yield improvement in wheat. Plant Biotechnol J. 2020 Mar;18(3):614-630. doi: 10.1111/pbi.13305. Epub 2019 Dec 22. Erratum in: Plant Biotechnol J. 2020 Jul;18(7):1634. doi: 10.1111/pbi.13418. PMID: 31782596; PMCID: PMC7004901.
- Khablak SH, Spivak SI, Pastukhova NL, Yemets AI, Blume Ya B. Cytokinin oxidase/dehydrogenase as an important target for increasing plant productivity. Cytology and Genetics. 2024;58(2):115-125. doi: 10.3103/S0095452724020051.
- Tan C, Li S, Song J, Zheng X, Zheng H, Xu W, Wan C, Zhang T, Bian Q, Men S. 3,4-Dichlorophenylacetic acid acts as an auxin analog and induces beneficial effects in various crops. Commun Biol. 2024 Feb 8;7(1):161. doi: 10.1038/s42003-024-05848-9. PMID: 38332111; PMCID: PMC10853179.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.