Medicine Group 2025 January 24;6(1):056-069. doi: 10.37871/jbres2056.
Comparative Study of Biomarkers Dependent on Biological, Molecular Factors and Their Clinical Significance in Breast Cancer Treatment
Alina Oana Rusu-Moldovan1,2, Daniela Luminita Zob3, Adrian Vasile Comanici4,5*, Maria Mihaela Comanici6,7 and Dan Mihu2
2Iuliu Hatieganu University of Medicine and Pharmacy, Second Clinic of Obstetrics and Gynecology, 8 Victor Babes Str., Cluj-Napoca, Romania
3Institute of Oncology Busharest, Department of Oncology II, 252 Fundeni Road, Bucharest, Romania
4Department of Endocrinology "Titu Maiorescu" University of Medicine and Pharmacy, 031593 Bucharest, Romania
5Department of Endocrinology, C.F.2 Clinical Hospital. 011464 Bucharest, Romania
6Department of Imunology "Titu Mairescu" University of Medicine and Pharmacy. 031483 Bucharest, Romania
7Department of Imunology, C.F.2 Clinical Hospital. 011464 Bucharest, Romania
- Breast cancer
- Her2
- Hormone receptors
- Ki-67
- Cerb-2
- Prediction
- Prognosis
Abstract
Breast cancer clinically represents a heterogeneous disease. Over decades, the integration of prognostic and predictive markers in treatment decisions has led to a more individualized and optimized therapy. Prognosis describes the risk of disease recurrence and disease-related death after diagnosis without the influence of therapy and prediction illustrates the probability of efficacy or response of a specific therapeutic measure.
The present study evaluated the clinical significance of Ki-67 index, ER, PGR, cerb-2 and Her2 receptors as prognostic markers and predictors of recurrence in different molecular subtypes of breast cancer. We analyzed the relationship of these receptors with different clinicopathological factors.
We have processed samples from 130 patients hospitalized in the Surgery Department III of the Bucharest Institute of Oncology. Biological samples have been taken by breast biopsy punctures or by excision of the tumors and analyzed them histopathologically and immunohistochemically.
Improved understanding of breast cancer biology and genetics together with the utilization of classical biomarkers and the identification of new markers or profiles is increasingly defining the clinical decisions are to be made to minimize overtreatment, undertreatment, and incorrect treatment.
Introduction
Clinically, breast cancer is a heterogeneous disease. In the last decades, the integration of predictive and prognostic markers into the therapeutic decision has led to the emergence of individualized treatments. While prognosis describes the risk of disease recurrence and the survival rate without treatment, prediction identifies the probability of response rate to the applied treatment [1].
Clinical decisions are important to minimize the over- or under-assessment of therapeutic conduct, but especially in the application of correct treatment.
Malignant progression requires heterotypic processes regulating epithelialmesenchymal transition, hypoxia, desmoplasia and angiogenesis [2,3]. Cancer progression involves the following mechanisms: degradation of proliferative signaling and growth inhibitory factors, activation of oncogenes and loss of tumor suppressor genes resulting in suppression of apoptosis and senescence [3]. The understanding of the pathophysiological mechanisms of breast cancer is driven by the advent of implementation of new molecular techniques, risk assessment, implementation of targeted therapies, individualized treatment application [4,5]. The prognostic signatures of genes that may help to characterize tumors may allow more tailored therapies for individual patients [6,7].
Uncontrolled proliferation is a hallmark of malignancy and can be assessed by various methods, including counting mitotic indices in stained tissue sections, incorporation of labeled nucleotides into DNA, and cytometric assessment of cell fraction flux in the S-phase of cell division [7].
The development of specific systemic treatment options for early-detected breast cancer has led to a substantial decrease in mortality in recent years [8]. The progress observed was based on the identification of subgroups of patients who required treatment and by identifying markers that allowed the prediction of the efficacy of certain treatment measures. The best prognostic markers for breast cancer include tumor size, lymph node status, metastases, tumor histologic type, tumor grading, age and peritumoral lymphovascular invasion [1].
Many molecular markers that have been studied have both prognostic and predictive values. Prognostic markers are indicators of aggressiveness, invasiveness, and the degree of metastasis. These markers correlate with survival over time independent of systemic therapy and can be used to select patients at risk. Predictive markers are intended to allow clinicians to track favorable therapeutic outcomes and decide future treatment plans. Mainly, prognostic values of classical factors (Ki67, ER, PGR, Her2) and novel molecular factors (p53, p14ARF, cyclin D1, cyclin E, TBX2/3, BRCA1/2 and VEGF) are specific for breast cancer. The molecular markers are involved in the regulation of the p53 membrane antigen tumor suppressor pathway, which elicits a response to DNA damage. They play a role in the process of angiogenesis and metastasis, leading to the development of breast cancer [9].
Tumor size is a strong prognostic factor independent of neoplasia, showing a positive correlation with axillary lymph node status [10]. The efficacy of chemotherapy is independent of tumor size. For early breast cancer, axillary lymph node status is still the most important prognostic factor [11,12]. According to international and national guidelines, patients with invaded lymph nodes should be treated with adjuvant and neoadjuvant chemotherapy regardless of the immunohistochemical status of the primary tumor. In lymph node-negative patients, additional prognostic and predictive markers should be taken into account to make an appropriate adjuvant treatment decision.
The prognostic impact of histologic subtype is limited. The degree of cell differentiation is another value-dependent prognostic factor.
Determination of hormone receptor expression is a widely accepted standard procedure for breast pathology. It is relevant that Estrogen Receptor (ER) and Progesterone Receptor (PGR) have prognostic and predictive value, even if the predictive power is much stronger and consequently more frequently used. The predictive value of the presence of hormone receptors is aimed at tracking the benefit of hormone treatment over time [13,14].
The impact of Her2 on breast cancer biology confirms the clear involvement of a target molecule and leads to the development of a highly effective therapeutic option in the application of the monoclonal antibody Trastuzumab. The discovery of this molecule has opened up the field of individualized treatment previously associated with endocrine therapy. Overexpression of the Her2 gene is strongly correlated with an aggressive tumor, the presence of hormone receptors in low percentage, a high cell proliferation index, and a consequent decrease in overall survival [15].
BRCA1 and BRCA2 genetic mutations located in chromosome 17q21 and 13q13, respectively, are involved in breast carcinogenesis. The presence of the mutation is a predictor of neoplastic disease.
Despite screening programs or opportunistic screening, advances in therapeutic approaches and the understanding of the molecular biology of the neoplastic cell, breast cancer remains the leading cause of cancer death in women over 50.
Because breast cancer is a molecularly heterogeneous disease, its successful management involves a multidisciplinary approach, requiring both local disease control and systemic therapy tailored to the histopathologic, genetic and molecular status.
Working Hypothesis
In this study the interrelationship between prognostic factors associated with predictive factors according to the clinico-biological characteristics of the tumor (histopathologic type, molecular subtype) was highlighted. It also highlighted the importance of identifying these factors as essential in the under- or over-assessment of cytostatic treatment.
Inclusion criteria
- Patients aged 35-87 years
- Patients whose diagnosis was made by biopsy puncture or surgical excision of the tumor formation
- Patients who have no history of breast neoplasm or other neoplasia
- Patients who agreed to be involved in the study
Exclusion criteria
- Patients already diagnosed and undergoing systemic therapy
- Patients who have been irradiated in the chest cavity
- Patients with breast lesions other than breast carcinoma
- Patients who refused to participate in the study
Material and Methods
Between 01.01.2017 - 30.09.2019 samples were collected and processed from 130 patients hospitalized in the Surgical Section III of the Bucharest Oncological Institute. The biological samples were taken by breast biopsy puncture or by excision of tumor formation and analyzed histopathologically and immunohistochemically. From the group of 130 patients only 30 patients had blood and salivary samples taken in order to identify the presence of BRCA1 and BRCA2 mutations.
Tissue samples from tumors were obtained by processing in the pathological anatomy laboratory of BIO according to the working protocol of art.1, annex 1 of OG no. 1217/2010 on the indicated working techniques for processing and staining of cytopathological and histopathological preparations. Immunohistochemistry (IHC) was performed by highlighting antigens using antibodies that recognize the antigenic site. The antigen-antibody reaction was visualized using chromatographic detection.
Statistical analysis
Descriptive statistics were calculated and data were presented using indicators of centrality, location and distribution.
The Shapiro-Wilk test was used to test the normal distribution and the variance was tested with the F-test.
The t-test (Student) was used for data with normal distribution and the non-parametric
Mann-Whitney (U) test was used for values with non-uniform distribution or ranks. The χ2 test was also used for statistical processing of the data in some cases. RR (relative risk or risk ratio), RE (risk in the exposed) and RN (risk in the unexposed) were calculated.
The Pearson correlation coefficient (r) was used to detect the correlation between two continuous quantitative variables with a normal (uniform) distribution. For variables with a non-uniform distribution, the Spearman rank correlation coefficient (ρ) was used. The analysis of correlation coefficients was performed using Colton's rule.
The significance threshold for the tests used was α = 0.05 (5%), 0.01 (1%) or 0.001.
StatsDirect v.2.7.2 was used for the statistical processing of the data. The graphical representation of the results was done with Excel (Microsoft Office 2010).
Ethics
The approval of the studies initiated at BIO was granted after prior evaluation of the Scientific Council according to the minutes of 14.05.2016 and updated by the Ethics Commission of the Bucharest Oncology Institute with no.15920 of 12.11.2018.
Results
The 130 patients were divided into the following age groups:
- 35-44 years (n = 14)
- 45-54 years (n = 26)
- 55-64 years (n = 39)
- 65-74 years (n = 32)
- ≥ 75 years (n = 19 )
Following IHC examination, patients were categorized according to specific receptors (ER, PGR, Ki67, Her2) into molecular subgroups as follows: 57 patients belong to the LUMINAL A subgroup, 38 patients to the LUMINAL B subgroup, 5 patients to the Her2 subtype (enriched) and 30 patients to the Triple Negative subgroup.
The average age of the studied group was 60.92 years with limits between 35 and 87 years.
In the statistical analysis of age values, highly statistically significant differences (p < 0.01) were observed between the ages of patients in the Luminal A - Triple Negative sublots (Table 1).
| Table 1: Comparative analysis for age values in the studied group and sublots and statistical significance. | ||||||||
| Lots | Media | ES | Mediana | DS | Min | Max | Semnificaţia statistică | (p) |
| Whole lot | 60.92 | 1.042 | 62 | 11.884 | 34 | 87 | ||
| HER2 - | 61.21 | 1.200 | 63 | 11.878 | 34 | 86 | HER2- vs., HER2+ | 0.5112 |
| HER2 + | 60 | 2.130 | 61.5 | 12.048 | 35 | 87 | cerB2- vs., cerB2+ | 0.2024 |
| cerB 2- | 62.11 | 1.354 | 63 | 11.490 | 35 | 86 | Luminal A vs., Luminal B | 0.2557 |
| cerB 2+ | 59.43 | 1.614 | 62 | 12.295 | 34 | 87 | Luminal A vs., HER 2 enriched | 0.766 |
| Luminal A | 63.37 | 1.516 | 64 | 11.447 | 35 | 80 | Luminal A vs., Triple Negative | 0.0038 |
| Luminal B | 61.29 | 1.926 | 61.5 | 11.873 | 39 | 87 | Luminal B vs., HER 2 enriched | 0.8013 |
| HER 2 enriched | 62.4 | 3.776 | 62 | 8.444 | 49 | 71 | Luminal B vs., Triple Negative | 0.0521 |
| Triple negative | 55.53 | 2.185 | 51.5 | 11.968 | 34 | 79 | HER 2 enriched vs., Triple Negative | 0.229 |
Immunohistochemical receptor values were statistically analyzed according to the age of the patients.
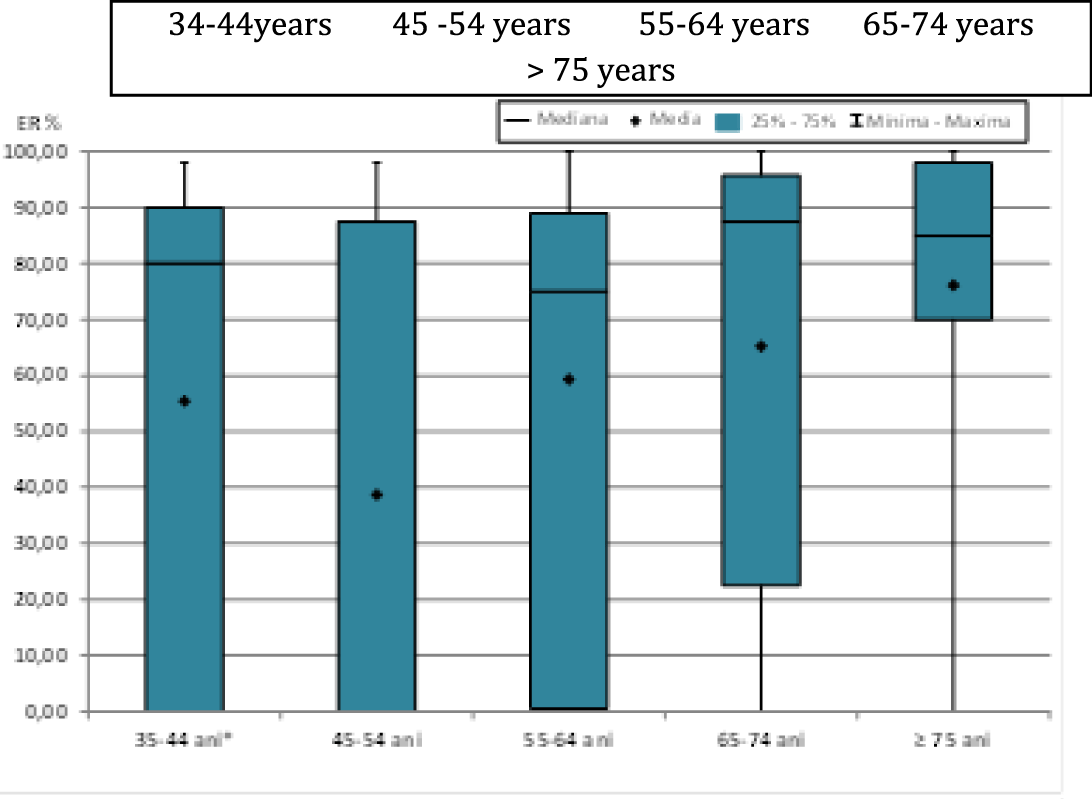
When analyzing estrogen receptor values (ER%) according to the age groups studied (Figure 1), highly statistically significant differences (p < 0.01) were observed between age groups 45-54 years compared to 65-74 years or ≥ 75 years and statistically significant differences (p < 0.05) between age groups 55-64 years compared to ≥75 years (Table 2).
| Table 2: Comparative analysis for ER% values by age and statistical significance. | ||||||||||
| Lots | Media | ES | Median | DS | Min | Max | Statistic semnification | (p) | ||
| 35-44 years | 55.43 | 11.625 | 80 | 43.497 | 0 | 98 | 35-44 vs., 45-54 | 0.281 | 45-54 vs., 65-74 | 0.009 |
| 45-54 years | 38.69 | 8.524 | 0 | 43.467 | 0 | 98 | 35-44 vs., 55-64 | 0.9782 | 45-54 vs., ≥75 | 0.0027 |
| 55-64 years | 59.31 | 6.221 | 75 | 38.853 | 0 | 100 | 35-44 vs., 65-74 | 0.2259 | 55-64 vs., 65-74 | 0.1367 |
| 65-74 years | 65.22 | 7.252 | 87.5 | 41.026 | 0 | 100 | 35-44 vs., ≥75 | 0.1079 | 55-64 vs., ≥75 | 0.0452 |
| ≥75 years | 76.11 | 7.226 | 85 | 31.499 | 0 | 100 | 45-54 vs., 55-64 | 0.0976 | 65-74 vs., ≥75 | 0.4676 |
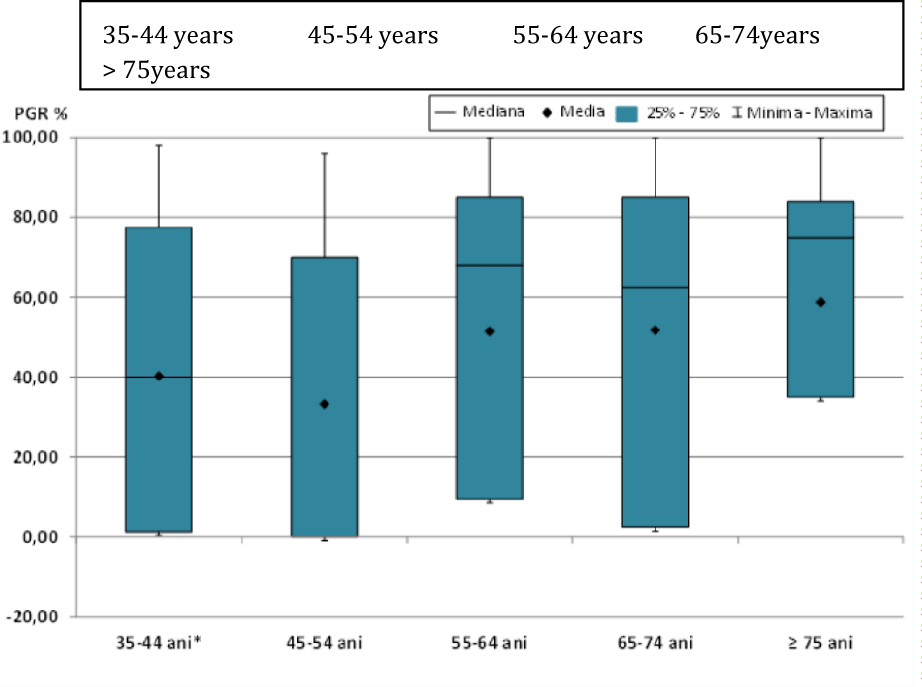
In the statistical analysis of progesterone receptor values (PGR%) according to the age groups studied (Figure 2), statistically significant differences (p < 0.05) were observed between the age groups 45-54 years compared to 55-64 years, 65-74 years or ≥ 75 years (Table 3).
| Table 3: Comparative analysis for PGR % values by age and statistical significance. | ||||||||||
| Lots | Media | ES | Median | DS | Min | Max | Statistic semnification | (p) | ||
| 35-44 years | 40.21 | 10.545 | 40 | 39.456 | 0 | 98 | 35-44 vs., 45-54 | 0.3259 | 45-54 vs., 65-74 | 0.0367 |
| 45-54 years | 33.23 | 7.607 | 0 | 38.786 | 0 | 96 | 35-44 vs., 55-64 | 0.4415 | 45-54 vs., ≥75 | 0.023 |
| 55-64 years | 51.49 | 5.940 | 68 | 37.097 | 0 | 100 | 35-44 vs., 65-74 | 0.428 | 55-64 vs., 65-74 | 0.9074 |
| 65-74 years | 51.78 | 6.783 | 62.5 | 38.369 | 0 | 100 | 35-44 vs., ≥75 | 0.2628 | 55-64 vs., ≥75 | 0.6055 |
| ≥75 years | 58.74 | 7.836 | 75 | 34.155 | 0 | 100 | 45-54 vs., 55-64 | 0.0306 | 65-74 vs., ≥75 | 0.7374 |
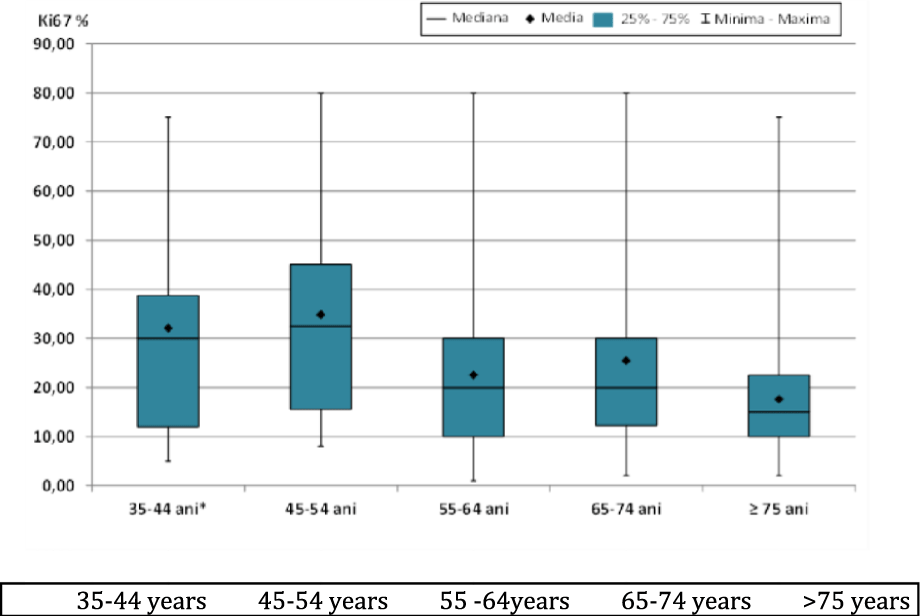
The concordance of the Ki67 antigen values (Ki67 %) according to the age groups studied (Figure 3), showed highly statistically significant differences (p < 0.01) between the age groups 45-54 years compared to 55-64 years or ≥75 years and statistically significant differences (p < 0.05) between the age groups 45-54 years compared to 65-74 years (Table 4).
| Table 4: Comparative analysis for Ki67% values by age and statistical significance. | ||||||||||
| Lots | Media | ES | Median | DS | Min | Max | Statistic semnification | (p) | ||
| 35-44 years | 32.07 | 6.102 | 30 | 22.832 | 5 | 75 | 35-44 vs., 45-54 | 0.6971 | 45-54 vs., 65-74 | 0.0412 |
| 45-54 years | 34.81 | 3.933 | 32.5 | 20.052 | 8 | 80 | 35-44 vs., 55-64 | 0.1723 | 45-54 vs., ≥ 75 | 0.001 |
| 55-64 years | 22.49 | 2.775 | 20 | 17.328 | 1 | 80 | 35-44 vs., 65-74 | 0.2931 | 55-64 vs., 65-74 | 0.6097 |
| 65-74 years | 25.44 | 3.606 | 20 | 20.398 | 2 | 80 | 35-44 vs., ≥ 75 | 0.0504 | 55-64 vs., ≥ 75 | 0.2446 |
| ≥ 75 years | 17.58 | 3.737 | 15 | 16.290 | 2 | 75 | 45-54 vs., 55-64 | 0.0081 | 65-74 vs., ≥ 75 | 0.0971 |
The evaluation of predictive and prognostic markers according to the molecular subtypes analyzed, age and tumor stage was performed and revealed statistically significant elements. Thus, in the statistical analysis of estrogen receptor (ER %) and progesterone receptor (PGR %) values, statistically intensely significant differences (p < 0.001) were observed between Luminal A - Triple negative and Luminal B - Triple negative (Tables 5,6).
| Table 5: Comparative analysis for ER% values in the studied group and sublots and statistical significance. | ||||||||
| Lots | Media | ES | Mediana | DS | Min | Max | Semnificaţia statistică | (p) |
| Whole lot | 58.68 | 3.596 | 80 | 41.006 | 0 | 100 | ||
| HER2 - | 57.45 | 4.217 | 80 | 41.750 | 0 | 100 | HER2- vs., HER2+ | 0.7857 |
| HER2 + | 62.44 | 6.901 | 80 | 39.036 | 0 | 100 | cerB2- vs., cerB2+ | 0.2124 |
| cerB 2- | 62.31 | 4.771 | 80 | 40.480 | 0 | 100 | Luminal A vs., Luminal B | 0.7425 |
| cerB 2+ | 54.17 | 5.457 | 77.5 | 41.559 | 0 | 100 | Luminal A vs., HER 2 enriched | - |
| Luminal A | 82.98 | 2.348 | 88 | 17.729 | 0 | 100 | Luminal A vs., Triple Negative | < 0.0001 |
| Luminal B | 76.24 | 4.884 | 90 | 30.107 | 0 | 100 | Luminal B vs., HER 2 + | - |
| HER 2 enriched | 0 | 0.000 | 0 | 0.000 | 0 | 0 | Luminal B vs., Triple Negative | < 0.0001 |
| Triple negative | 0.03 | 0.033 | 0 | 0.183 | 0 | 1 | HER 2 + vs., Triple Negative | - |
| Table 6: Comparative analysis for PGR% values in the studied lot and sublots and statistical significance. | ||||||||
| Lots | Media | ES | Mediana | DS | Min | Max | Semnificaţia statistică (p) | |
| Whole lot | 47.75 | 3.334 | 55 | 38.014 | 0 | 100 | ||
| HER2 - | 44.63 | 3.952 | 40 | 39.120 | 0 | 100 | HER2- vs., HER2+ | 0.1707 |
| HER2 + | 57.31 | 5.862 | 72.5 | 33.160 | 0 | 98 | cerB2- vs., cerB2+ | 0.656 |
| cerB 2- | 48.74 | 4.492 | 60 | 38.113 | 0 | 100 | Luminal A vs., Luminal B | 0.0525 |
| cerB 2+ | 46.53 | 5.014 | 49 | 38.187 | 0 | 100 | Luminal A vs., HER 2 + | - |
| Luminal A | 70.09 | 3.593 | 80 | 27.130 | 0 | 100 | Luminal A vs., Triple Negative | < 0.0001 |
| Luminal B | 57.84 | 4.947 | 62.5 | 30.493 | 0 | 100 | Luminal B vs., HER 2 + | - |
| HER 2 + | 0 | 0.000 | 0 | 0.000 | 0 | 0 | Luminal B vs., Triple Negative | < 0.0001 |
| Triple | ||||||||
| negative | 0.50 | 0.338 | 0 | 1.852 | 0 | 9 | HER 2 + vs., Triple Negative | - |
The association of the Ki67 antigen value (Ki67 %) within Luminal A - Luminal B, Luminal A - enriched Her2, Luminal A - triple negative and Luminal B - triple negative subgroups showed statistically highly significant differences (p < 0.001) and statistically highly significant differences (p < 0.01) between enriched Her2 - triple negative (Table 7).
| Table 7: Comparative analysis for Ki67% values in the studied group and sublots and statistical significance. | ||||||||
| Lots | Medi a | ES | Median a | DS | Mi n | Ma x | Semnificaţia statistică | (p) |
| Whole lot | 25.99 | 1.73 2 | 20 | 19.74 4 | 1 | 80 | ||
| HER2 - | 27.26 | 2.18 6 | 20 | 21.63 8 | 2 | 80 | HER2- vs., HER2+ | 0.7863 |
| HER2 + | 22.13 | 2.06 6 | 20 | 11.68 9 | 1 | 40 | cerB2- vs., cerB2+ | 0.9382 |
| cerB 2- | 26.47 | 2.45 1 | 20 | 20.79 9 | 2 | 80 | Luminal A vs., Luminal B | < 0.0001 |
| cerB 2+ | 25.27 | 2.39 2 | 20 | 18.37 6 | 1 | 70 | Luminal A vs., HER 2 enriched | 0.0003 |
| Luminal A | 10.75 | 0.64 7 | 10 | 4.885 | 2 | 25 | Luminal A vs., Triple Negative | < 0.0001 |
| Luminal B | 28.37 | 1 . 43 3 | 30 | 8.836 | 1 | 45 | Luminal B vs., HER 2 enriched | 0.4552 |
| HER 2 enriched | 26 | 3.67 4 | 25 | 8.216 | 20 | 40 | Luminal B vs., Triple Negative | < 0.0001 |
| Triple negative | 51.93 | 3.73 7 | 55 | 20.47 0 | 8 | 80 | HER 2 enriched vs., Triple Negative | 0.0038 |
For statistical analysis of tumor staging, patients were grouped as follows: Tis+T1, T2 and T3+T4. Note that in the Her2 enriched sublot there were only patients in Tis+T1 and T2 stages.
In the comparative analyses of tumor stages between Her2- vs Her2+ sublots, tumor stages between cerB 2- vs cerB 2+ sublots, tumor stages between Luminal A vs., enriched Her2 sublots, no statistically significant association of any tumor stage with any of the 6 sublots was observed (p > 0.05).
In the comparative analysis of tumor stages between Luminal A vs Luminal B sublots, a statistically semi-significant association of Luminal A sublot with stage Tis+T1 compared to stage T2 was observed (p < 0.05) (Table 8). In the comparative analysis of tumor stages between Luminal A vs Triple-negative sublots, a statistically semi-significant association of Luminal A sublot with Tis+T1 stage was observed in comparison with T2 stage (p < 0.05) (Table 9).
| Table 8: Comparative analysis for Tumour stages (T) between Luminal A-Luminal B sublots and statistical significance. | ||||||
| Lots(n) | T3+T4 | Tis+T1 | p | RR | RE (%) | RN (%) |
| Luminal A | 3 | 39 | 0.8469 | 1.357 | 7.14 | 5.26 |
| Luminal B | 1 | 18 | ||||
| Lots (n) | T3+T4 | T2 | p | RR | RE (%) | RN (%) |
| Luminal A | 3 | 15 | 0.3040 | 3.333 | 16.67 | 5.00 |
| Luminal B | 1 | 19 | ||||
| Lots (n) | T2 | Tis+T1 | p | RR | RE (%) | RN (%) |
| Luminal A | 15 | 39 | 0.0391 | 0.5409 | 27.78 | 51.35 |
| Luminal B | 19 | 18 | ||||
| Table 9: Comparative analysis for Tumour stages (T) between Luminal A-Triple negative sublots and statistical significance. | ||||||
| Lots (n) | T3+T4 | Tis+T1 | p | RR | RE (%) | RN (%) |
| Luminal A | 3 | 39 | 0.4606 | 0.5 | 7.14 | 14.29 |
| Triple negative | 2 | 12 | ||||
| Lots(n) | T3+T4 | T2 | p | RR | RE (%) | RN (%) |
| Luminal A | 3 | 15 | 0.6688 | 1.5 | 16.67 | 11.11 |
| Triple negative | 2 | 16 | ||||
| Lots (n) | T2 | Tis+T1 | p | RR | RE (%) | RN (%) |
| Luminal A | 15 | 39 | 0.0183 | 0.4861 | 27.78 | 57.14 |
There was no statistically semi-significant association of any tumor stage with any of the Luminal B vs.,Her2 enriched, Luminal B vs Triple negative or Her2 enriched vs Triple negative sublots (p > 0.05).
For statistical analysis of the degree of aggressiveness, patients were grouped as follows: G1, G2+G2/G3 and G3. It should be noted that in the enriched Her2 sublot there were only patients with G2+G2/G3 and G3 aggression grades.
Between Her2- vs Her2+ sublots and cerB 2- vs cerB 2+ sublots, no statistically significant association of any degree of aggressiveness with any of the 4 sublots was observed (p > 0.05).
Within the Luminal A vs Luminal B sublots, a statistically semi-significant association of the Luminal A sublot with grades G1 and G2+G2/G3 was observed in comparison with grade G3 (p < 0.01) (Table 10).
| Table 10: Comparative analysis for degree of aggressiveness (G) between Luminal A-Luminal B sublots and statistical significance. | ||||||
| Loturi (n) | G1 | G3 | p | RR | RE (%) | RN (%) |
| Luminal A | 11 | 4 | 0.0097 | 3.3 | 73.33 | 22.22 |
| Luminal B | 4 | 14 | ||||
| Loturi (n) | G2 + G2/G3 | G3 | p | RR | RE (%) | RN (%) |
| Luminal A | 42 | 4 | 0.0015 | 1.552 | 91.30 | 58.82 |
| Luminal B | 20 | 14 | ||||
| Loturi (n) | G2 + G2/G3 | G1 | p | RR | RE (%) | RN (%) |
| Luminal A | 42 | 11 | 0.7036 | 0.9509 | 79.25 | 83.33 |
| Luminal B | 20 | 4 | ||||
Between the Luminal A vs Triple Negative sublots, a statistically significant association of the Luminal A sublot with grades G1 and G2+G2/G3 compared to G3 (p < 0.01) (Table 11), and a statistically significant association of the Luminal B sublot with grade
| Table 11: Comparative analysis for the degree of aggressiveness (G) between Luminal A- Triple negative sublots and statistical significance. | ||||||
| Lotsi (n) | G1 | G3 | p | RR | RE (%) | RN (%) |
| Luminal A | 11 | 4 | 0.0003 | 6.111 | 73.33 | 12.00 |
| Triple negative | 3 | 22 | ||||
| Lots(n) | G2 + G2/G3 | G3 | p | RR | RE (%) | RN (%) |
| Luminal A | 42 | 4 | < 0.0001 | 4.93 | 91.30 | 18.52 |
| Triple negative | 5 | 22 | ||||
| Lots (n) | G2 + G2/G3 | G1 | p | RR | RE (%) | RN (%) |
| Luminal A | 42 | 11 | 0.3352 | 1.268 | 79.25 | 62.50 |
| Triple negative | 5 | 3 | ||||
G2+G2/G3 compared to G3 (p < 0.01) was also observed in comparison with the Triple Negative subgroup (Table 12).
| Table 12: Comparative analysis for the degree of aggressiveness (G) between the sublots of Luminal B-Triple negative and statistical significance. | ||||||
| Lots (n) | G3 | G1 | p | RR | RE (%) | RN (%) |
| Luminal B | 14 | 4 | 0.4087 | 0.8838 | 77.78 | 88.00 |
| Triple negative | 22 | 3 | ||||
| Lots (n) | G3 | G2 + G2/G3 | p | RR | RE (%) | RN (%) |
| Luminal B | 14 | 20 | 0.0035 | 0.5053 | 41.18 | 81.48 |
| Triple negative | 22 | 5 | ||||
| Lots (n) | G2 + G2/G3 | G1 | p | RR | RE (%) | RN (%) |
| Luminal B | 20 | 4 | 0.2706 | 1.333 | 83.33 | 62.50 |
| Triple negative | 5 | 3 | ||||
In the comparative analysis of the degree of aggressiveness between the age groups studied, no statistically semi-significant association (p > 0.05) of any degree of aggressiveness was observed in the comparison of 35-44 years vs., 45-54 years, 55-64 years, ≥ 75 years, 45-54 years vs 55-64 years, 65-74 years, ≥ 75 years, 55-64 years vs., 65-74 years, ≥
75 years and 65-74 years vs., ≥ 75 years. Only when comparing the age groups 35-44 years* vs 65-74 years, a statistically significant (p < 0.05) association of the 65-74 years age group with grade G2+G2/G3 compared to G1 was observed.
Statistical correlation analysis between the values of the studied indicators showed for:
- sublot Her2-
- good positive correlation between G-Ki67%, ER%-PGR% (p < 0.001)
- good negative correlation between ER%-Ki67%, PGR%-Ki67% (p < 0.001)
- an acceptable positive correlation between V-ER% (p < 0.001), V-PGR%, T-G, T-Ki67% (p < 0.01)
- an acceptable negative correlation between G-ER%, G-PGR% (p < 0.001), VKi67% (p < 0.01), T-PGR% (p < 0.05)
- Her2+ sublot
- a good positive correlation between T-G (p < 0.01)
- an acceptable positive correlation between G-Ki67% (p < 0.01), T-Ki67%, ER%-PGR% (p < 0.05), V-T.
• Triple Negative:
- a good positive correlation between ER%-PGR% (p < 0.001)
- an acceptable positive correlation between T-Ki67% (p < 0.05)
- an acceptable negative correlation between T-PGR%, G-ER% (p < 0.05), ER%Ki67%.
Of the 130 patients, 57 patients belong to the Luminal A subtype.
Following the histopathologic and immunohistochemical examination, 54.39% (31 patients) underwent hormone therapy.
The decision criteria for choosing this type of treatment for this batch were :
- low and medium aggressiveness
- genetic determination of BRCA1 mutation in 4 patients with G2 which was undetectable
- a Ki67 proliferation index below 15%
3.51% (2 patients) benefited from hormone therapy and surgery in the gynecological sphere following BRCA2 mutation detection; 7.02% (4 patients) from hormone therapy and Herceptin administration.
11 patients (19.30%) were given chemotherapy treatment associated with hormone therapy.
The recommendation criteria were :
- medium and high aggressiveness
- Ki67 proliferation index above 15%
The remaining 9 patients benefited from the following therapeutic regimens:
- 1 patient (1.75%) received chemotherapy combined with hormone therapy, Herceptin and gynecologic surgery (G3, Her2 positive, BRCA2 present).
- 5 patients (8.77%) were administered chemotherapy, hormone therapy and Herceptin (BRCA1, G2, Ki67 mutation over 15%)
- 2 patients (3.51%) benefited from chemotherapy, hormone therapy and surgery in the gynecological sphere (G3)
- 1 patient (1.75%) received chemotherapy combined with Herceptin and
underwent hormone suppression (BRCA1 and 2 present, Her₂ positive)
The molecular subtype Luminal B includes 38 patients who, according to immunohistochemical, histologic and molecular genetic elements, benefited from the following therapeutic regimens:
- 1 patient (2.63%) - chemotherapy (G3, Ki67 over 15%)
- 2 patients (5.26%) - chemotherapy + gynecologic surgery (BRCA 2 present, G2, Ki67 over 15%)
- 4 patients (10.53%) - chemotherapy associated with hormone therapy (G2, G3, BRCA 1 present, Ki67 between 15-25%)
- 8 patients (21.05%) - chemotherapy, hormone therapy and Herceptin (G2, G3, Her2 positive, Ki67 between 20-25%)
- 19 patients (50%) - chemotherapy associated with hormone therapy (BRCA1 and BRCA2 mutation present in 4 of the patients, G2, G3, Ki67 over 15%)
- 4 patients (10.53%) - chemotherapy associated with hormone therapy and surgical hormone suppression (BRCA2 present, G3, KI67 20%).
In the Triple Negative subgroup there are 30 patients who have benefited from the following therapeutic conduit:
- 29 patients (96.67%) - chemotherapy (BRCA1 mutation present in 5 patients, G2/G3 in 7 patients, G3 in 22 patients and Ki67 over 55%)
- 1 patient (3.33%) - follow-up (BRCA 1 and 2 mutation undetectable, G1, Ki67 30%).
In the Her₂-enriched subtype there are 5 patients of which 2 patients are receiving chemotherapy and Herceptin therapy (Her 2 present, G2) and 3 patients are receiving chemotherapy combined with monoclonal therapy with Herceptin and Perjeta (Her2 present, G3, Ki67 over 45%).
Discussion
The predictive rate of patients' long-term survival and response to treatment of breast neoplasm has been estimated using classical markers such as Ki67, ER, PGR, Her2. Although numerous genetic and phenotypic alterations have been reported in breast cancer, only a fraction of them have been fully identified and reported in clinical trials [16].
Breast tumor growth is often influenced by the presence of female sex hormones. Determination of cellular concentrations of ER and PGR in the tumor is currently used to predict the prognosis of patients as well as for hormone therapy decision [17].
In the normal epithelium of the female mammary gland ER has been detected in 7-17% of cells. It is estimated that about 70-80% of female breast tumors express ER. Tumors showing ER are characterized by slower growth, differentiation and better prognosis determined by an appropriate treatment regimen, which correlates with survival time after surgical removal of the primary lesion [18].
Some clinical studies have shown that estrogen receptor expression was identified in 78% of cases. In postmenopausal women, a positive nuclear ER was observed in 73% of patients. Women in whom ER-positivity occurs in more than 10% of tumor cells are classified as suitable for hormone therapy, as this type of treatment is essential [19,20].
Retrospective clinical studies have shown that only 70% of Progesterone Receptor (PGR)-positive and 25-30% of PGR-negative but ER-positive tumors respond to hormonal therapy [19]. ER and PGR receptors at the time of surgery are used as prognostic biomarkers [20]. ER positivity is strongly associated with age at diagnosis, being more common among postmenopausal women [21].
In the present study, the mean age of the study group was 60.92 years with limits between 35 and 87 years. From the group of 130 patients, they were divided into age groups as follows: 35-44 years (n = 14), 45-54 years (n = 26), 55-64 years (n = 39), 65-74 years (n = 32) and ≥ 75 years (n = 19).
Rosen, et al. [22] and Fernandopulle SM, et al. [23], reported an increased incidence of poorly differentiated tumors (53%) and ER-negative cancer
Singhai R, et al. [24], identified in their study about 60% of tumors as poorly differentiated. Out of our group of patients only 42 (32.30%) had poorly differentiated tumors.
When analyzing estrogen receptor values (ER%) according to the age groups studied, highly statistically significant differences (p < 0.01) were observed between age groups 45-54 years compared to 65-74 years and statistically significant differences (p < 0.05) between age groups 55-64 years compared to ≥ 75 years. Also, in the statistical analysis of age values, highly statistically significant differences (p < 0.01) were observed between the ages of patients in the Luminal A - Triple Negative sublots. These aspects have also been reported by Badowska-Kozakiewicz AM, et al. [25,26], who demonstrated that the presence of estrogen receptors are in close correlation with young age and recurrence period
In statistical analysis of progesterone receptor values (PGR%) according to the age groups studied, statistically significant differences (p < 0.05) were observed between the age groups 45-54 years compared to 55-64 years.
The concordance of Ki67 antigen values (Ki67%) according to the age groups studied revealed highly statistically significant differences (p < 0.01) between the 45-54 age groups compared with 55-64 years and statistically significant differences (p < 0.05) between the 45-54 age groups compared with 65-74 years.
In the comparative analysis we highlighted the following aspects:
- In the Luminal A sublot, tumors between 0-2cm, with G1/G2 aggressiveness grade predominate.
- In the Luminal B sublot, tumors between 2-5 cm are predominant, with G2/G3 aggressiveness grade
- In the age group 35-44 years, tumors with G2/G3 aggressiveness predominate, in contrast to patients aged 65-74 years, who have tumors with G1 aggressiveness.
These aspects were also reported in the study by Badowska-Kozakiewicz AM, et al. [25]. Similar results were reported in Kollias J, et al. [27], but they also reported that young women present aggressive forms of the disease.
Witters L, et al. [28], demonstrated that premenopausal women with ER-positive tumors and the presence of Her2 have little benefit from treatment with anti-Her2 monoclonal antibodies alone.
Elledge RM, et al. [30], observed the relationship between the percentage of cells displaying estrogen receptors on the membrane surface and the response to tamoxifen and survival rate among women with metastatic breast cancer [29].
Bardou VJ, et al. [30], showed a low risk of death in patients with an increased percentage of membrane receptors undergoing adjuvant hormonal therapy.
In the study group, we performed genetic testing for BRCA1 and 2 mutations in 30 patients. The presence of the mutation has a preventive role in the development of neoplastic disease and a predictive role in active disease.
There is no single management in breast cancer risk reduction for BRCA mutation carriers, and this was recently reviewed by Bougie O, et al. [31].
Although surgical procedures are curative and low-risk for BRCA mutation carriers, individualized therapies for hereditary breast cancer are still desirable [31]. DNA defects, which are responsible for tumorigenesis, also provide an appropriate therapeutic strategy when cells turn malignant. Because the BRCA1 mutation is responsible for the dysregulation of DNA repair pathways, BRCA1-deficient tumor cells are more vulnerable to DNAdamaging agents such as platinum-based chemotherapeutics such as Cisplatin and its derivative Carboplatin [32,33]. Poly ADP-ribose polymerase (PARP) inhibitors, are a novel therapeutic option for the treatment of BRCA-defective breast and ovarian cancers [34,36]. PARP inhibitors are enzymes with an essential role in the repair of single-stranded DNA defects [34].
Conclusions
The use of classical markers such as Ki67, ER, PR, and HER2 for the prediction of patients’ survival and treatment response of breast cancer has been well established, and thus, they will be continued to be used as useful laboratory tests. Although numerous genetic and phenotypic alterations have been reported in breast cancer, only a handful have been fully identified and brought to clinical studies.
The amount of receptors present on the cell surface correlates closely with the age and hormonal status of the woman (menopause).
The percentage ratio of ER and PGR is not constant and varies with disease progression. Hormone receptors have been shown to have both prognostic and predictive value, being an indicator of long-term survival in mildly aggressive disease.
The discovery of the Her2 antigen has changed the therapeutic perspective of breast cancer. The use of BRCA1/2 testing is important in personalizing treatment. Because of the high cost of testing, screening should be limited to high-risk women, such as young women with a family history of breast cancer. The personalized therapy cannot be initiated without the detection of essential biomarkers: ER, PGR, Ki67, Her2.
A example of personalized therapeutic management began with the successful introduction of anti-Her2 monoclonal antibody therapy.
Understanding the molecular biology of breast cancer in conjunction with the use of classical biomarkers and the identification of novel ones are indispensable elements in the management of personalized therapy.
Biomarkers can provide information about the presence, progression, and treatment response of breast cancer.
References
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr; American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007 Nov 20;25(33):5287-312. doi: 10.1200/JCO.2007.14.2364. Epub 2007 Oct 22. PMID: 17954709.
- Dickson RB, Lippman ME. Autocrine and paracrine growth factors in the normal and neoplastic breast. In: Harris JR, Lippman ME, Morrow M. eds. Philadelphia; Lippincott Williams &Wilkins. 2000. 303.
- Pakkiri P, Lakhani SR, Smart CE. Current and future approach to the pathologist's assessment for targeted therapy in breast cancer. Pathology. 2009 Jan;41(1):89-99. doi: 10.1080/00313020802563551. PMID: 19089744.
- Masood S. Prognostic/predictive factors in breast cancer. Clin Lab Med. 2005 Dec;25(4):809-25, viii. doi: 10.1016/j.cll.2005.08.012. PMID: 16308094.
- Debies MT, Welch DR. Genetic basis of human breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2001 Oct;6(4):441-51. doi: 10.1023/a:1014739131690. PMID: 12013533.
- de Snoo F, Bender R, Glas A, Rutgers E. Gene expression profiling: decoding breast cancer. Surg Oncol. 2009 Dec;18(4):366-78. doi: 10.1016/j.suronc.2009.07.005. PMID: 19879448.
- Kuderer NM, Lyman GH. Gene expression profile assays as predictors of distant recurrence-free survival in early-stage breast cancer. Cancer Invest. 2009 Nov;27(9):885-90. doi: 10.3109/07357900903275142. PMID: 19832034; PMCID: PMC3523732.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14-20;365(9472):1687-717. doi: 10.1016/S0140-6736(05)66544-0. PMID: 15894097.
- Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, Inoue K. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin Med Insights Oncol. 2010 Apr 20;4:15-34. doi: 10.4137/cmo.s4773. PMID: 20567632; PMCID: PMC2883240.
- Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018 Oct;52(Pt 1):56-73. doi: 10.1016/j.semcancer.2017.08.010. Epub 2017 Sep 4. PMID: 28882552.
- Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R; Austrian Breast and Colorectal Cancer Study Group. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004 Aug;240(2):306-12. doi: 10.1097/01.sla.0000133355.48672.22. PMID: 15273556; PMCID: PMC1356408.
- Ferguson NL, Bell J, Heidel R, Lee S, Vanmeter S, Duncan L, Munsey B, Panella T, Orucevic A. Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J. 2013 Jan-Feb;19(1):22-30. doi: 10.1111/tbj.12059. Epub 2012 Dec 13. PMID: 23240985.
- von Minckwitz G, Untch M, Nüesch E, Loibl S, Kaufmann M, Kümmel S, Fasching PA, Eiermann W, Blohmer JU, Costa SD, Mehta K, Hilfrich J, Jackisch C, Gerber B, du Bois A, Huober J, Hanusch C, Konecny G, Fett W, Stickeler E, Harbeck N, Müller V, Jüni P. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011 Jan;125(1):145-56. doi: 10.1007/s10549-010-1228-x. Epub 2010 Nov 3. PMID: 21042932.
- EBCTCG Esobo: Highlights from the early breast cancer trialist collaborative group (EBCTCG) 2005-2006 worldwide overview. Breast Cancer Res Treat. 2009.
- Rakha EA, Chmielik E, Schmitt FC, Tan PH, Quinn CM, Gallagy G. Assessment of Predictive Biomarkers in Breast Cancer: Challenges and Updates. Pathobiology. 2022;89(5):263-277. doi: 10.1159/000525092. Epub 2022 Jun 21. PMID: 35728576.
- Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008 Dec 15;14(24):8019-26. doi: 10.1158/1078-0432.CCR-08-0974. PMID: 19088018.
- Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018 Oct;52(Pt 1):56-73. doi: 10.1016/j.semcancer.2017.08.010. Epub 2017 Sep 4. PMID: 28882552.
- Wu JR, Zhao Y, Zhou XP, Qin X. Estrogen receptor 1 and progesterone receptor are distinct biomarkers and prognostic factors in estrogen receptor-positive breast cancer: Evidence from a bioinformatic analysis. Biomed Pharmacother. 2020 Jan;121:109647. doi: 10.1016/j.biopha.2019.109647. Epub 2019 Nov 13. PMID: 31733575.
- Li CJ, Chen HM, Lai JC. Diagnostic, Prognostic, and Predictive Biomarkers in Breast Cancer. J Oncol. 2020 Mar 7;2020:1835691. doi: 10.1155/2020/1835691. PMID: 32256579; PMCID: PMC7091542.
- Tarighati E, Keivan H, Mahani H. A review of prognostic and predictive biomarkers in breast cancer. Clin Exp Med. 2023 Feb;23(1):1-16. doi: 10.1007/s10238-021-00781-1. Epub 2022 Jan 15. PMID: 35031885.
- Middleton LP, Chen V, Perkins GH, Pinn V, Page D. Histopathology of breast cancer among African-American women. Cancer. 2003 Jan 1;97(1 Suppl):253-7. doi: 10.1002/cncr.11021. PMID: 12491489.
- Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016 Dec;13(4):496-504. doi: 10.20892/j.issn.2095-3941.2016.0066. PMID: 28154782; PMCID: PMC5250608.
- Fernandopulle SM, Cher-Siangang P, Tan PH. Breast carcinoma in women 35 years and younger: a pathological study. Pathology. 2006 Jun;38(3):219-22. doi: 10.1080/00313020600699268. PMID: 16753742.
- Singhai R, Patil V, Patil A. Status of HER-2/neu receptors and Ki-67 in breast cancer of Indian women. Int J Appl Basic Med Res. 2011 Jan;1(1):15-9. doi: 10.4103/2229-516X.81974. PMID: 23776766; PMCID: PMC3657947.
- Badowska-Kozakiewicz AM, Patera J, Sobol M, Przybylski J. The role of oestrogen and progesterone receptors in breast cancer - immunohistochemical evaluation of oestrogen and progesterone receptor expression in invasive breast cancer in women. Contemp Oncol (Pozn). 2015;19(3):220-5. doi: 10.5114/wo.2015.51826. Epub 2015 May 28. PMID: 26557763; PMCID: PMC4631285.
- Potemski P, Pluciennik E, Bednarek AK, Kusinska R, Kubiak R, Kordek R. Evaluation of oestrogen receptor expression in breast cancer by quantification of mRNA. Histopathology. 2007 Dec;51(6):829-36. doi: 10.1111/j.1365-2559.2007.02886.x. PMID: 18042072.
- Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer--histopathological and prognostic considerations. Br J Cancer. 1997;75(9):1318-23. doi: 10.1038/bjc.1997.223. PMID: 9155052; PMCID: PMC2228226.
- Witters L, Engle L, Lipton A. Restoration of estrogen responsiveness by blocking the HER-2/neu pathway. Oncol Rep. 2002 Nov-Dec;9(6):1163-6. PMID: 12375012.
- Liu G, Wang L, Ji L, He D, Zeng L, Zhuo G, Zhang Q, Wang D, Pan Y. Identifying prognostic markers in spatially heterogeneous breast cancer microenvironment. J Transl Med. 2023 Aug 29;21(1):580. doi: 10.1186/s12967-023-04395-x. PMID: 37644433; PMCID: PMC10463390.
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003 May 15;21(10):1973-9. doi: 10.1200/JCO.2003.09.099. PMID: 12743151.
- Bougie O, Weberpals JI. Clinical Considerations of BRCA1- and BRCA2-Mutation Carriers: A Review. Int J Surg Oncol. 2011;2011:374012. doi: 10.1155/2011/374012. Epub 2011 Aug 8. PMID: 22312502; PMCID: PMC3263675.
- Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, Son BH, Yoo KY, Kang D; KOHBRA Research Group; Korean Breast Cancer Society. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010 Jul;122(1):11-25. doi: 10.1007/s10549-010-0859-2. Epub 2010 Apr 8. PMID: 20376556.
- Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Dent R, Lubinski J, Narod S. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010 Jan 20;28(3):375-9. doi: 10.1200/JCO.2008.20.7019. Epub 2009 Dec 14. PMID: 20008645.
- Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, Inoue K. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin Med Insights Oncol. 2010 Apr 20;4:15-34. doi: 10.4137/cmo.s4773. PMID: 20567632; PMCID: PMC2883240.
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009 Jul 9;361(2):123-34. doi: 10.1056/NEJMoa0900212. Epub 2009 Jun 24. PMID: 19553641.
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010 Jul 24;376(9737):235-44. doi: 10.1016/S0140-6736(10)60892-6. Epub 2010 Jul 6. PMID: 20609467.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.