Biology Group 2025 January 20;6(1):031-055. doi: 10.37871/jbres2055.
Cell-Free Therapy Based on Adipose Stem Cell-Derived Exosomes to Inhibit Scar Formation Proactively: Mechanisms and Clinical Targeting Applications
Xinhao Cheng1, Haijiang Dong1#, Yu Li1, Shuying Yu2, Guangren Yue1 and Ximei Wang1*
2Zhengzhou Key Laboratory of Tissue Engineering and Plastic Reconstruction, Zhengzhou, Henan Province, China
#These authors are contributed equally
- Exosomes
- Scar
- Cytokine
- Proactive inhibition
- Molecular targeted therapy
- Bioengineering
Abstract
This review provides an in-depth analysis of the potential benefits of cell-free therapy based on adipose-derived exosomes (ADSC-Exos) in inhibiting scar formation. The review highlights the advantages of using ADSC-Exos. It also explores the complex mechanisms by which ADSC-Exos inhibit scar formation, including their role in hemostasis, inflammation, cell proliferation, tissue remodeling, and their regulation of critical molecules (platelets, inflammatory factors, extracellular matrix molecules, collagen molecules) and crucial cells (macrophages, endothelial cells, fibroblasts), as well as their modulation of epithelial-mesenchymal transition. Additionally, the article examines different delivery methods and engineering approaches for optimizing the targeting of adipose stem cell-derived exosomes in traumatic scarring. It points out the potential of using liposomes to construct molecular-targeted exosomes in precision medicine. Finally, the review summarizes current research and provides insights into the future development of exosomes in scar treatment. In conclusion, this article reveals the potential of cell-free therapy based on ADSC-Exos as a promising treatment for inhibiting scar formation.
Introduction
Pathologic scars often cause tremendous psychological pressure on patients. Currently, the main treatment methods for scarring include compression therapy, topical drug therapy, laser therapy, plasma ion beam therapy, intra-scar drug injection therapy, cryotherapy, radiation therapy, surgery, and silicone gel application. These methods have specific efficacy and shortcomings [1]. Therefore, the need for an ideal method for treating hyperplastic scarring has become a worldwide problem. In previous studies, ADSCs have been successfully studied as a means of promoting scarless healing of minor wounds [2], and cell-free therapies based on adipose-derived stem cell exosomes (ADSC-exosomes/exos) have more advantages and potentials in terms of therapeutic efficacy and clinical application and have a bright future for development.
Exosomes
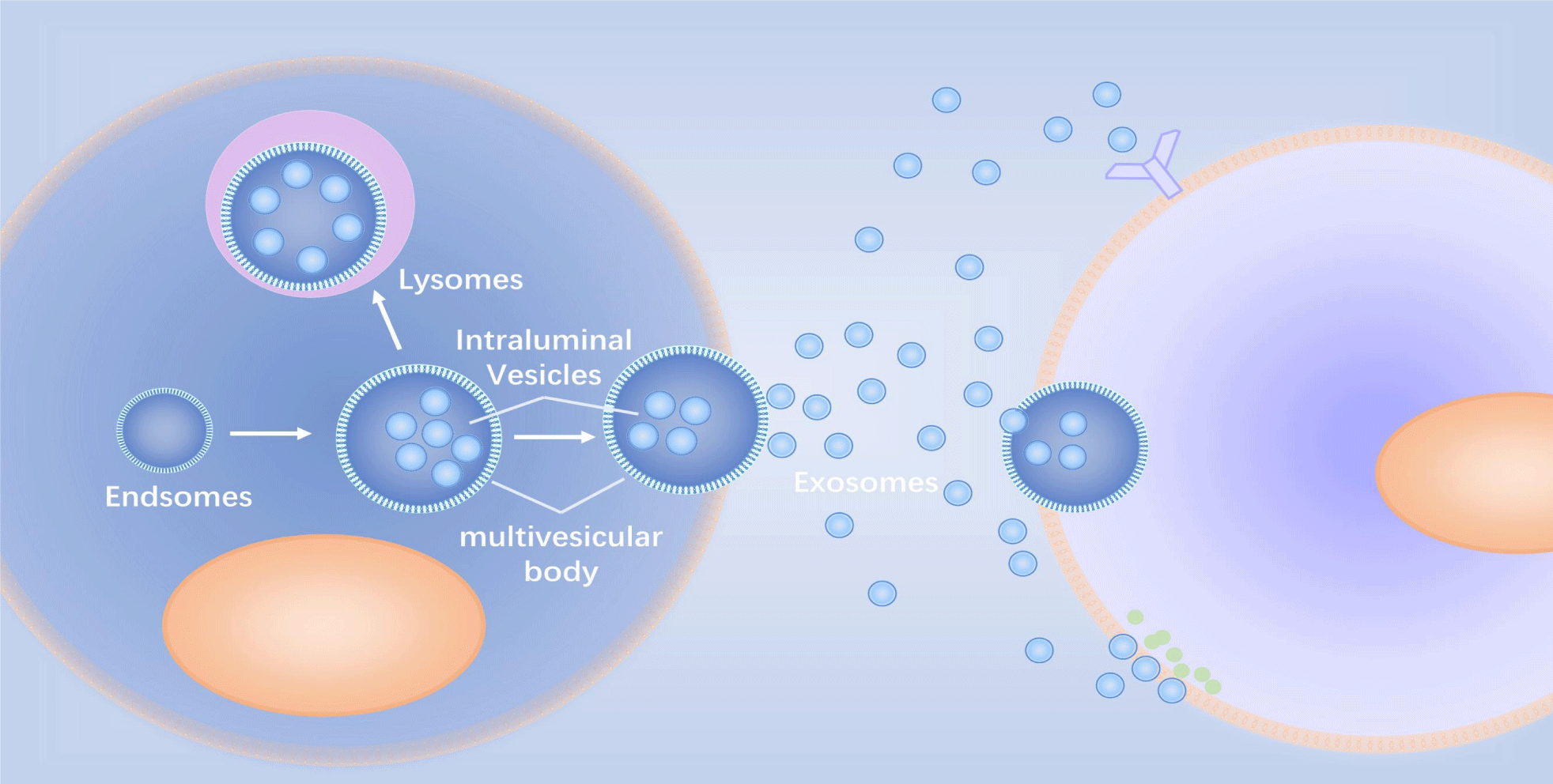
Exosomes are extracellular lipid bilayers released by cells (Figure 1). These microvesicles have diameters ranging from 30 to 200 nanometers, and nanoparticle tracking analyzers have shown that their densities range from 1.09 to 1.18 g/ml [3,4]. Additionally, they appear "disk-like," "cup-like," or "teat-like" under an electron microscope due to their negative staining; “cup-like” can be used to distinguish cell-derived vesicles from similarly sized particles [5,6].
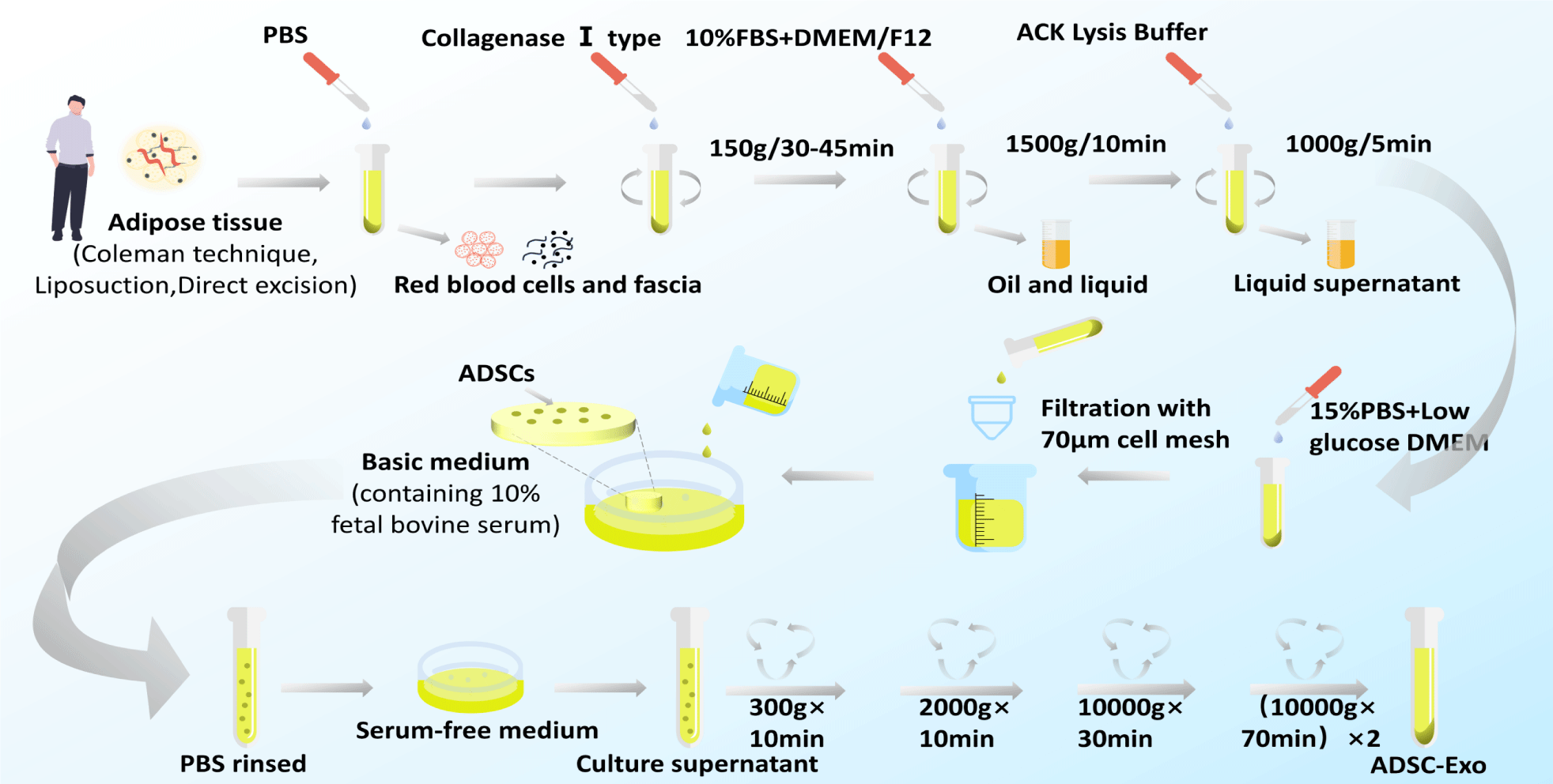
Currently, there is no gold standard for the isolation and purification of exosomes. Methods for exosome isolation include sequential Ultracentrifugation (UC), gradient ultracentrifugation, ultrafiltration, size-exclusion chromatography, polymer precipitation, immunoaffinity capture, and microfluidics-based techniques [7]. UC is the most commonly used (Figure 2). The combination of separation methods is an effective method and a future trend to improve the purity of exosomes.
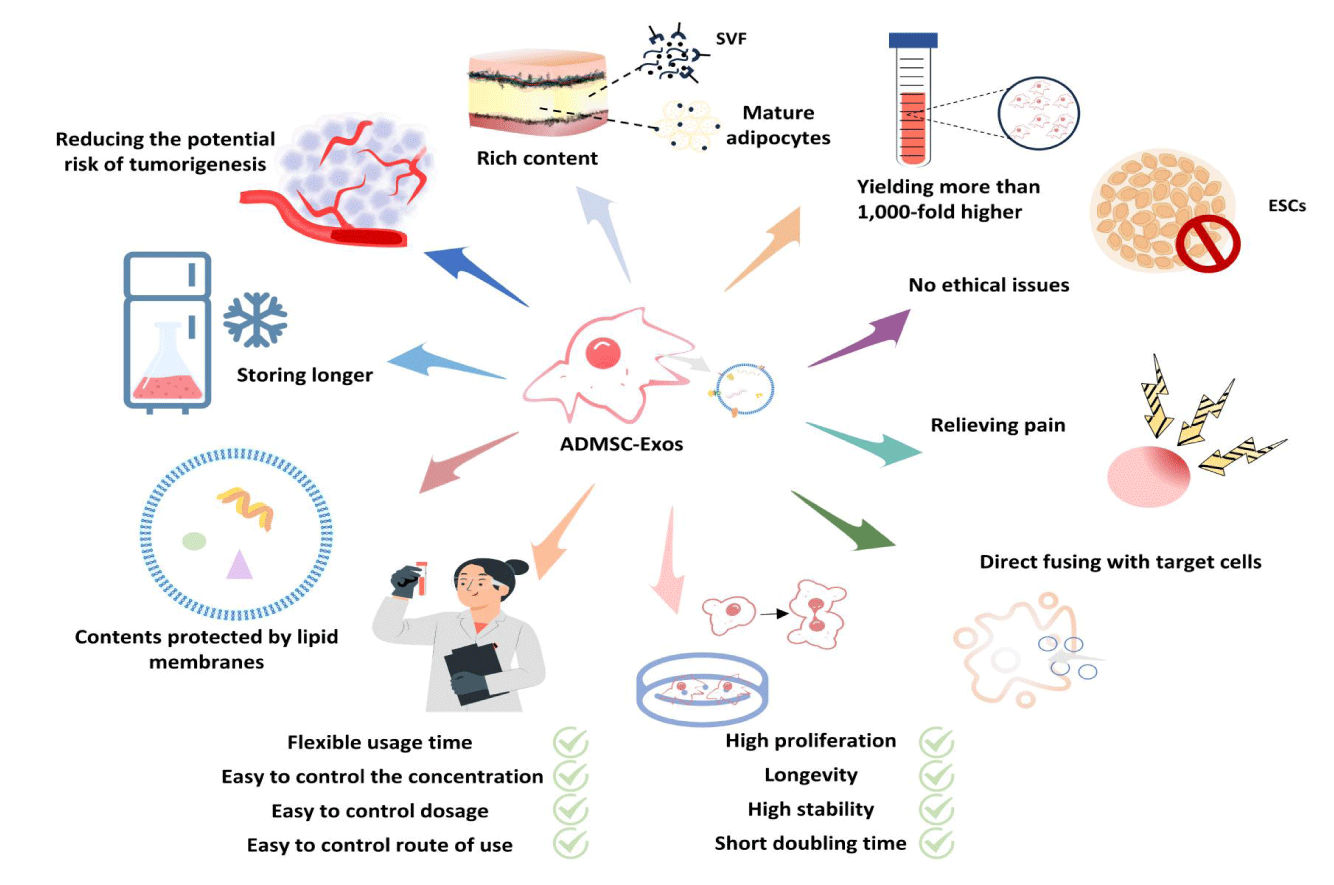
The cells with the most exosome secretion capacity are Mesenchymal Stem Cells (MSCs) [8]. Among them, ADSCs are ideal for obtaining exosomes in scar inhibition. Regarding their characteristics, ADSCs play a crucial role in skin functionality [8]. Firstly, resident ADSCs in the skin are considered key regulators of skin function and play a vital role in tissue repair and regeneration. On one hand, they replace, repair, and regenerate dead or damaged cells [9]. On the other hand, they allow for the continuous recruitment of mature specialized cells from the basal epidermal layer to its outer layers [10,11]. Their interaction with skin cells regulates skin homeostasis and healing [12]. Secondly, ADSCs secrete abundant substances, including exosomes, growth factors, and cytokines. ADSCs and the Dermal Fibroblasts (DF) differentiated from them [13,14] are the primary sources of extracellular matrix proteins that maintain skin structure and function. These molecules can regulate the functions of surrounding cells and participate in skin growth, repair, and regeneration. Additionally, ADSCs possess anti-inflammatory and antioxidant properties by alleviating skin inflammation and protecting the skin from oxidation stress damage achieved by clearing free radicals [15,16]. This is of great significance in improving skin health and delaying the skin aging process. Compared to MSCs from other sources, ADSCs offer a number of advantages [16,17]. Compared to bone marrow stem cells, ADSCs exhibit higher collection rates, lack donor/recipient cell fusion characteristics, are less prone to contamination in culture, have higher proliferation activity during long-term cultivation, are better suited to survive in hypoxic environments and demonstrate outstanding anti-inflammatory, phagocytic, and anti-apoptotic abilities [18-20]. Compared to embryonic stem cells, they have no ethical restrictions and lower immunogenicity. In contrast to induced pluripotent stem cells, ADSCs have higher purity and can be better controlled to prevent generating unwanted heterogeneous cell types. In addition, ADSCs are more effective in producing collagen than other types of stem cells. They can also differentiate into three developmental types of dermal cells, including the endoderm, mesoderm, and ectoderm [21]. These characteristics make them an ideal stem cell source for obtaining exosomes.
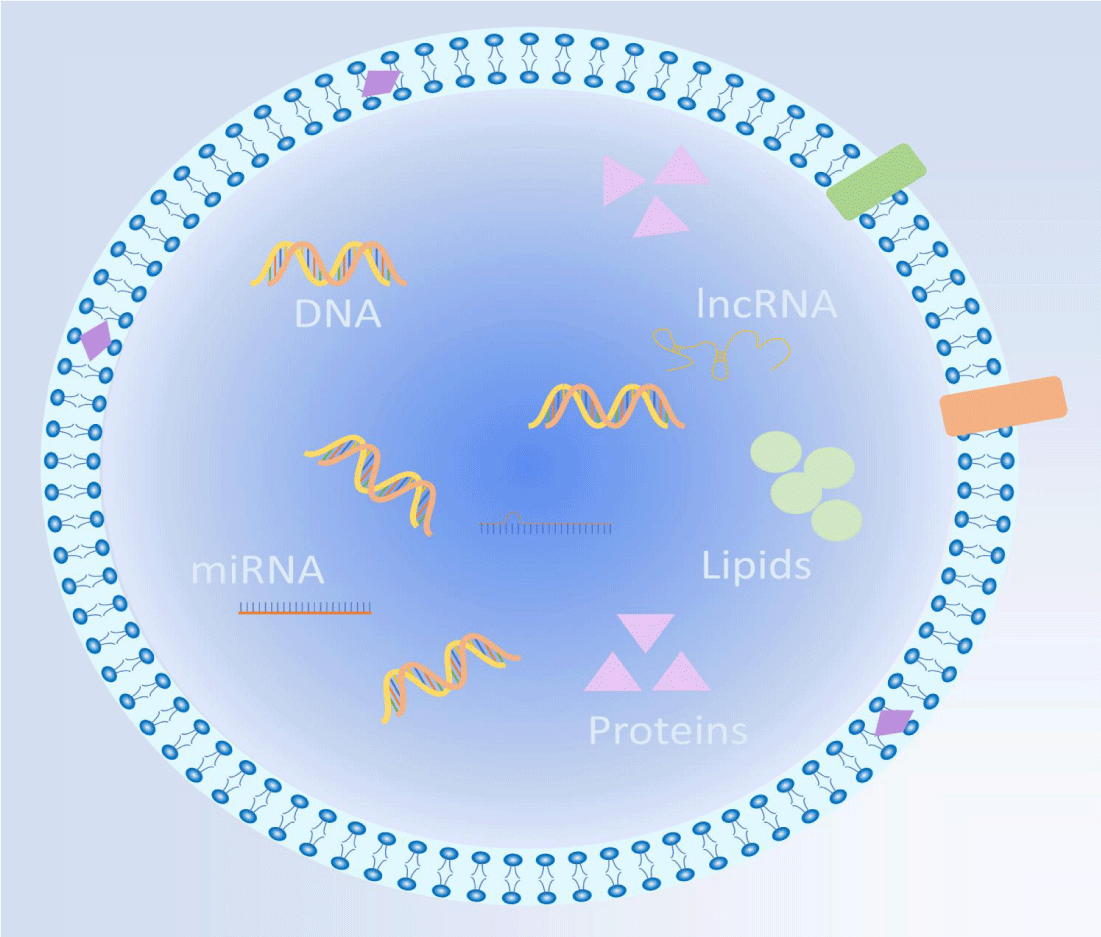
ADSC-Exos carry essential information and macromolecules from their source ADSC [6,22,23] (Figure 3), and their compositions may vary depending on the research method; in general, they encompass a range of essential components that include:1)Proteins: ADSC-Exos contain various proteins such as cytokines, growth factors, and collagen, among others. Notably, they harbor a diverse array of angiogenic factors, including VEGFB, VEGFD, ANG, ANGPTL2, and ANGPTL5, with VEGFD and VEFGB being predominantly expressed within ADSC-Exos [15]. These proteins play pivotal roles in cellular signaling, facilitating cell repair and regeneration. 2) RNA and DNA: ADSC-Exos encompass different types of nucleic acids such as mRNA, miRNA, and lncRNA. For instance, miRNAs present in ADSC-Exos inhibit genes like NPM1, PDCD4, CCL5, and NUP62, promoting dermal fibroblast proliferation. Additionally, ADSC-Exos releases lncRNA MALAT1, which enhances dermal fibroblast migration and accelerates the healing of ischemic wounds [24]. Furthermore, ADSC-Exos releases lncRNA Let-7a-5p, specifically targeting TGF-R1 to modulate the Smad pathway, effectively reducing fibrosis in LSF [25]. Moreover, ADSCP2, a novel peptide derived from ADSC-Exos, exhibits remarkable attenuation of proliferative scar fibrosis both in vitro and in vivo [26]. 3)Lipids: ADSC-Exos contain lipids such as membrane lipids, lipohormones, and lipid degradation products, which contribute to critical cellular functions, including cell membrane repair and cell signaling processes. ADSC-Exos not only substantially contributes to the efficacy of ADSCs but also plays a prominent role in tissue repair and regeneration by acting as paracrine mediators of intercellular signaling [4].
The importance of ADSC-based stem cell therapies in regenerative medicine has been confirmed by tissue engineering and cellular therapy strategies [17,27-29] which have shown positive effects on promoting wound healing and scar prevention. However, exosomes and other active substances have been reported to be the main factors through which ADSCs exert their biological effects [30,31]. Additionally, ADSCs-Exos have more advantages in terms of therapeutic efficacy and clinical applications (Figure 4), so cell-free therapies based on ADSC-Exos have broader prospects for application [32,33].
Inflammatory cytokine and scar formation
Scar formation is intricately linked to wound healing, and inflammatory cytokines play a major role in both processes [34]. The typical progression of wound healing involves four interconnected phases, each governed by growth factors released by different cells involved in tissue repair. Scarring can occur when these cells or growth factors are disrupted or imbalanced during any stage of the healing process [35-38].
Hemostasis
This phase should be initiated as soon as possible after an injury has occurred. Platelets play a critical role by aggregating, followed by degranulating [39-43], releasing and activating a series of favorable growth factors (e.g., TGF-β, EGF, IGF-I, and PDGF.) and producing Fibronectin(FN), the former of which regulates relevant physiological processes including the coagulation cascade, and the latter of which can control the wandering course of inflammatory cells. (e.g., polymorphonuclears, macrophages, mastocytes, epithelial cells, vascular endothelial cells, and fibroblasts) [44,45]. At this stage, increased FN and granulation tissue due to platelet dysfunction can result in wound tissue hyperplasia and the formation of pathological scars.
Inflammatory
After successful hemostasis, the subsequent phase typically lasts 3-5 days, and the essence and core of this phase are the results of growth factor regulation. Vascular and cellular responses, immune reactions, blood clotting, and breakdown of fibrous material characterize this phase. At the end of this phase and during proliferative phases, macrophages mainly secrete growth factors when neutrophils are replaced with macrophages. The recruitment and activation of various cells, including fibroblasts, endothelial cells, and epithelial cells, are stimulated by growth factors. These growth factors play an important role in initiating the healing process. At the wound site, the growth factors draw in and activate fibroblasts, endothelial cells, and epithelial cells, which begin the healing process. Moreover, the inflammatory response is converted to tissue hyperplasia [46-48] from the early stages to pathological scar formation, macrophage polarization results in spatiotemporal diversity of M1 and M2 macrophages, and there is a close relationship between an increase in M2 cells and susceptibility to abnormal scar pathogenesis [49]. At this stage, macrophages inappropriately release cytokines, such as increased local profibrotic cytokines PDGF, TGF-β, and IGF-Ⅰ, which can lead to proliferative scar formation [50-52]. An imbalance between a sustained inflammatory response and a local immune inflammatory response is the leading cause of scar formation [37].
Cell multiplication and formation of connective tissue
During wound healing, cells enter this phase on day three postinjury, when the inflammatory response subsides and tissue-repairing cells gradually proliferate. Two primary events occur during this phase: the formation of granulation tissue, which involves neovascularization, the deposition of ECM, and re-epithelialization [53,54].
Neovascularization:. Neovascularization provides the necessary oxygen supply to stimulate repair [55]. The endothelium is mainly responsible for vascular construction during this period [56]. Growth factors with chemotactic effects and collagenases with degradative effects secreted by inflammatory cells are associated with the initiation of endothelial cell migration, especially growth factors such as aFGF, bFGF, TGF-β, and EGF, which play a vital role in regulating the whole process of neovascularization [57]. Wound hypoxia enhances the inflammatory state and affects various metabolic processes, including fibroblast activity and collagen synthesis, leading to hyperplastic scar formation [58-60].Extracellular matrix: The formation of ECM is the key to this stage [61]. It initiates during the inflammatory response, during which macrophages secrete signaling molecules such as TGF-β, PDGF, IL-1, TNF, and FGF. Additionally, factors such as collagen peptides, FGF-7, C5a, fibronectin peptides, EGF, PDGF, and TGF-β contribute to the migration of fibroblasts. [52,53,62,63]. Fibroblasts exhibit high sensitivity to TGF-1. Fibroblasts express PDGF, TCF-p, and other growth factors that regulate the production of large amounts of the matrix proteins hyaluronate, fibronectin, and types I and III procollagen. [64,65]. Proliferative scars can be formed if the ECM is insufficiently degraded or synthesized too much, or both [52].
Epithelialization: Epithelialization is essential for wound coverage and healing [66]. Keratinocytes at the wound margin are regulated primarily by matrix Metalloproteinases (MMPs), which detach from the dermis and migrate toward the wound defect, continue to proliferate, and migrate laterally across the wound, altering the epidermal layer, i.e., re-epithelialization. Scar hyperplasia is highly likely to occur if the wound completes re-epithelialization more than three weeks before the wound is closed [63,67-69].
Epithelial-mesenchymal transition: Epithelial-Mesenchymal Transition (EMT) refers to the biological process by which different types of epithelial cells are transformed into mesenchymal cells through a series of biological changes under the influence of different factors [70,71]. During EMT, pseudopods appear at the anterior end of cuboid bone keratinocytes, and the cells transform into a spindle shape that promotes cell migration. EMT is necessary for normal re-epithelialization and ECM deposition: persistent and uncontrolled transformation from epithelial cells to fibroblasts and myofibroblasts may lead to pathological scarring; in later stages, unabated inflammation can affect EMT and lead to abnormal scarring [6,72].
Wound contraction and tissue remodeling
This stage is initiated after re-epithelialization is complete and can continue for several weeks or even two years postinjury, during which wound contraction and the conversion of granulation tissue to scar tissue occurs predominantly.
Wound contraction: Fibroblasts are recruited to the wound site by the chemokine CCL-2 and differentiate into myofibroblasts [73]. Myofibroblasts can attach to matrix elements such as collagen and fibronectin through integrin receptors in the extracellular matrix. Furthermore, fibroblasts are stimulated by TGF-β1 to express α-smooth muscle actin, which initiates the contraction of the fiber matrix gel. Prolonged contraction leads to permanent tissue retraction [74].
Tissue remodeling: New ECM molecules (e.g., fibronectin, Col-III, and Col-I) are sequentially deposited. This deposition process leads to a gradual increase in the strength of scar tissue, which eventually plateaus at approximately seven weeks post trauma. Subsequently, various cell types, including keratinocytes, fibroblasts, and macrophages, secrete an array of matrix-degrading enzymes that degrade excess ECM components. For instance, types I, II, III, X, and VIII of collagen are particularly susceptible to Mesenchymal Collagenase (MCC) or MMP-1 degradation; we discuss the breakdown of denatured collagen by gelatinase (MMP-2), which is capable of breaking down all forms of denatured collagen, including types V and X; and we discuss the role of stromelysin (MMP-3) in degrading collagen types IV, V, VI, and IX, as well as adhesive glycoproteins and proteoglycans. As the tissue remodeling process progresses, Col-I replaces Col-III, and dermal fibroblasts produce elastin and fibronectin, contributing to the formation of elastic fibers. Fibroblasts and vascular cells eventually undergo apoptosis [75,76]. The extent of scarring is determined by the amount of ECM synthesized and degraded [77]. Excessive collagen deposition during this stage can lead to the formation of pathological scars [78,79].
Mechanisms of Adipose Stem Cell-Derived Exosomes Proactive Inhibition of Scar Formation
ADSC-Exos is active in regulating the cells and inflammatory factors involved in newly formed wounds and established scars. Active involvement is critical in promoting optimal wound healing and preventing scar formation. The authors emphasized the significant contribution of ADSC-Exos to the active modulation of inflammatory factors throughout this process.
Bidirectional regulation of the whole process of wound healing
Due to the nature of scar formation mechanisms, the process of inhibiting scar formation is multifaceted and intricate. The review categorizes the mechanisms of inhibiting scar formation by ADSC-Exos into three categories and summarizes the specific mechanisms in table 1.
| Table 1: Mechanism of scarring inhibition by adipose stem cell-derived exosomes. | ||||||
| Healing phase | Action level | Functional characteristics | ||||
| Modulation of the immune-inflammatory response | Inflammatory factor level | It contains immunomodulatory proteins such as TNF-a, MCSF, and RBP-4 [80]. | ||||
| Upregulation of MIP1α(CCL3) and MCPIP1(ZC3H12A) expression promotes early inflammation [81]. | ||||||
| Reduces IFN α secretion, exerts immunosuppressive effects, and inhibits T-lymphocyte activation [82].⁑ | ||||||
| Cellular level | Coordinate the role of CD4 T-lymphocytes in the immune system (e.g., coordinate the balance between their various subsets) [82] | |||||
| Promotes differentiation of monocytes into M1 macrophages [80]. | ||||||
| MiR-155 induces adipocyte-derived macrophages in obese mice to differentiate into M1, causing chronic inflammation and an imbalance in the ratio of M1 to M2 macrophages in adipose tissue [83]. | ||||||
| It inhibits T-lymphocyte differentiation, reduces proliferation, and stimulates interferon-γ release in vitro [81].⁑ | ||||||
| M1/M2 polarization | Upon upregulation of TSG-6 expression, ADMSC-Exo (5 μg mL-1) induced polarization of M2 macrophages through the expression of miR-34a-5p, miR-124-3p, and miR-146a-5p, thereby attenuating immune response and inflammation [84]. ⁑ | |||||
| Regulation of macrophage polarization by miRNA shuttling converts macrophages from M1 to M2 phenotype [85].⁑ | ||||||
| Upregulated expression of M2 macrophage markers to regulate macrophage polarization and increased mRNA levels of M2-associated arginase-1 and IL-10 [86,87].⁑ | ||||||
| ADSC-Exos activated arginase-1 and STAT-3, which induced macrophage polarization to M2 and significantly inhibited LPS and IFN-γ-stimulated macrophage inflammatory responses. [87].⁑ | ||||||
| Signaling pathway level | Decreased inflammatory infiltration and increased collagen deposition in wound skin tissue via the lncRNA-XIST↑/miR-96-5p↓/DDR2↑ axis [108]. | |||||
| Promotes fibroblast proliferation and migration and inhibits inflammation by activating the PI3K/AKT pathway [109]. | ||||||
| By regulating inflammatory and oxidative signaling axes, it can significantly protect tissues and organs from ischemia-reperfusion injury(IRI) [110]. | ||||||
| By delivering miR-132 and miR-146a, ADMSC-Exo (5 μg mL-1) exhibited anti-inflammatory effects by targeting the ROCK1/PTEN pathway [88]. ⁑ | ||||||
| Pro-neovascularization | Inflammatory factor level | Promoting flap survival and increasing capillary density with a role in IRI [111]. | ||||
| The combination of microdermabrasion with ADSCs upregulates cytokines such as VEGF, IL-6, HGF, and EGF [112]. | ||||||
| Hypoxia-treated ADSC-Exos had a higher ability to form capillary networks than non-hypoxia-treated ADSC-Exos, and ADSC-Exos pretreated with H2O2 promoted angiogenesis [113]. | ||||||
| ADMSC-Exo containing the mmu_circ_0000250 modification has been shown to promote Sirt 1 expression via miR-128-3p adsorption, increase angiogenesis, and inhibit apoptosis via autophagy activation, as well as promote wound healing in diabetic rats [114]. | ||||||
| Coincubation of HUVEC with ADMSC or ADMSC-Exo, achieved by expression of miR-126-3p, enhanced fibroblast proliferation, migration, angiogenesis, and further enhancement [101]. | ||||||
| ADMSC-Exo-miR-126-3p promotes wound healing, collagen deposition, and neovascularization by downregulating PIK3R2 in alloderm-deficient rats [101]. | ||||||
| MiR-125a was translocated to endothelial cells (100 μg mL-1) by ADMSC-Exo and may promote migration and sprouting of vascular endothelial tip cells by inhibiting the expression of DLL4, thereby promoting angiogenesis in vitro [97]. | ||||||
| ADSC-Exo overexpressing Nrf2 significantly stimulated foot wound healing in diabetic rats by promoting the proliferation and angiogenesis of vascular endothelial progenitor cells and inhibiting the expression of inflammatory proteins and ROS production, which was associated with a reduction of oxidative stress and apoptosis during wound healing induced by Nrf2 [115]. | ||||||
| These miRs significantly increased the expression levels of ANGPT1 and flk1 (KDR) and decreased the expression of VASH1 and THBS1, thereby increasing angiogenesis [88]. | ||||||
| MiR-18a-5p↓/HIF-1↑/VEGF↑ is a novel miRNA regulatory pathway that affects diabetic wound healing in hypoxia-treated ADMSC-circ-Gcap14 [116]. | ||||||
| Promotes angiogenesis by delivering miR378a-3p [102]. | ||||||
| MiR-590-3p impedes angiogenesis in human dermal microvascular endothelial cells by binding and inhibiting VEGFA [103]. ⁑ | ||||||
| ADMSC-Exo-miR-21 promotes endothelial cell angiogenesis by targeting PTEN deletion on chromosome 10, leading to AKT activation and extracellular regulation of the ERK1/2 signaling pathway, which enhances HIF-1α and VEGF expression [90]. | ||||||
| In addition to enhancing HUVEC viability, migration, and angiogenesis, ADMSC-Exo-miR-125a-3p (exo: 25 μg mL-1) inhibited PTEN in mouse wound granulation tissue and activated the PI3K/AKT pathway to promote wound healing and angiogenesis [91]. | ||||||
| High miR-21 expression levels can downregulate TGF-β1 protein levels, thereby reducing wound scar formation [22]. | ||||||
| Induced more nuclear translocation of P-ERK in fibroblasts [92]. | ||||||
| Transporters of functional cytoskeletal proteins, such as vimentin, serve as promoters of fibroblast proliferation, migration, and ECM secretion [117]. | ||||||
| Resulted in increased gene expression of N-calmodulin, cyclin D1, PCNA, Col-1, and Col-3 [118]. | ||||||
| Exos is internalized by fibroblasts and stimulates proliferation, migration, and collagen synthesis in a dose-dependent manner [118]. | ||||||
| Regulation of fibroblast Col-III to Col-I, TGF-3 to TGF-1, and MMP3 to TIMP-1 ratios [92]. | ||||||
| Accelerates skin wound healing by optimizing fibroblast properties. Modulates fibroblast differentiation to influence ECM reconstruction; shifts fibroblasts to an endogenous state and inhibits their differentiation [99]. | ||||||
| ADMSC-Exo-miR-378 protects HaCaT cells from oxidative damage by targeting calpain I, including promoting proliferation and migration and reducing apoptosis [104]. | ||||||
| ADMSC-Exo (10 μgmL-1) contains miRNAs (e.g., has-miR-4484, -619-5p, -6879-5p) expressed by the NPM1, PDCD4, CCL5, and NUP62) genes and promotes regeneration of skin fibroblasts by stimulating dermal fibroblast proliferation [100]. | ||||||
| Accelerates skin cell proliferation | Signaling pathway level | ADMSC-Exo-miR-21 (Exo: 2 mL) increased MMP-9 expression and decreased TIMP-1 expression, and promoted migration and proliferation of HaCaT cells through the PI3K / AKT pathway [93]. | ||||
| In H2O2-injured HaCaT and Human dermal fibroblast, HDF, AMDSC - Exo - lncRNA - MALAT1 binds to miR - 124 and activates the Wnt/β - catenin pathway to promote cell proliferation and migration and inhibit apoptosis [94]. | ||||||
| A key regulatory role of the miR-19b/CCL1↓/TGF-β↑ pathway is to promote wound healing [119]. | ||||||
| ADMSC-Exo (50 μg mL -1) promotes wound healing by inhibiting miR-19b expression via the (lncRNA H19/Mir-19b/SOX9 axis) lncRNA-H19, upregulating SOX9 to activate the Wnt/β-catenin pathway, and promoting human skin fibroblast proliferation, migration, and invasion by acting on the lncRNA-MALAT1/miR-378a↓ / FGF2↑ axis [95,96] | ||||||
| ADSC-Exos may increase MMP3 levels in fibroblasts in an ERK / MAPK signaling pathway manner [92]. | ||||||
| Increased expression of downstream genes in the ERK/MAPK pathway (c-Jun, c-Fos), and the increased expression was almost eliminated by the P-ERK-specific inhibitor U0126 [92]. | ||||||
| Regulates collagen remodeling | Inflammatory factor level | In the early stages, EXO promotes collagen remodeling by synthesizing Col-I. and Col-III [89]. | ||||
| Intravenous injection of ADSC-Exos increased the ratio of TGF-β3 to TGF-β1 in vivo [92]. | ||||||
| Accelerated MMP3 expression in dermal fibroblasts of the skin resulted in a high ratio of MMP3 to tissue inhibitors of TIMP1, which facilitated ECM remodeling [92]. | ||||||
| MiR-495 is a therapeutic target for proliferative scars [105]. | ||||||
| MiR-192-5p was highly expressed in ADMSC-Exo (20 μgmL-1) and reduced proliferative scar formation by downregulating IL-17RA expression, inhibiting Samd2/Smad3 expression, and decreasing the levels of pro-fibrotic proteins, collagen deposition, and fibroblasts' transdifferentiation to myofibroblasts [108]. | ||||||
| Late stages reduce scarring by inhibiting collagen formation [89].⁑ | ||||||
| MiR-449 inhibits protein translation in ADMSC by targeting the 3′-UTR of PLOD1 mRNA [106] ⁑ | ||||||
| Cellular level | Intravenous ADSC-Exos increases the ratio of collagen III to collagen I and regulates fibroblast differentiation and gene expression [97]. | |||||
| Stimulates the rebuilding of the extracellular matrix by regulating fibroblast differentiation and gene expression, promoting wound healing and preventing scarring [45]. | ||||||
| It may prevent fibroblasts from differentiating into myofibroblasts [92].⁑ | ||||||
| Signaling pathway level | ADMSC-Exo overexpression of miR-29a reduces scar formation by inhibiting the TGF-β2/Smad3 signaling pathway [107]. | |||||
| Legends: the part of the table without ⁑ represents that the action of ADSC-Exos promotes the development of the positive process of traumatic healing; conversely, the section of the table with ⁑ indicates that the action of ADSC-Exos encourages the progression of adverse processes in traumatic healing. | ||||||
From table 1, it can be seen that:
- the regulatory mechanism of ADSC-Exos for the overall wound healing process is bidirectional, with an overall forward predominance; at the same time, the regulation of some molecules like collagen by ADSC-Exos will also be bidirectional, with an overall predominance of regulatory mechanisms favoring the inhibition of scarring [80-87].
- It cannot be said that promoting the regulation of reverse development is detrimental to wound healing because sometimes reverse regulation is beneficial for wound healing [84-87]. Similarly, it cannot be said that promoting positive regulation is always beneficial [81-83].
- ADSC-Exos can be actively involved throughout the entire process of wound healing and prevention of scar formation. During the second stage, ADSC-Exos regulates the mechanism of the immunological-inflammatory response. During the third stage, they promote neovascularization and enhance skin cell proliferation. ADSC-Exos also controls collagen remodeling during the fourth stage of wound healing.
- ADSC-Exos do not only directly regulate inflammatory factors but also indirectly regulate them by modulating multiple signaling pathways, including the Wnt/β-catenin, PK 1/2, ERK/MAPK, and PI3K/AKT pathways, thereby facilitating wound healing and scar formation [91-96].
- Research on the regulatory mechanism of ADSC-Exos on cells has focused mainly on fibroblasts [45,92,97-100] and macrophages [83-87].
- The function of ADSC-Exos usually depends on the nucleic acids or proteins they carry and express, and some scholars have focused on the role of a series of Exo-miRs in regulating wound healing via ADSC-Exos [83,84,88,91-98,100-107].
- The nucleic acids or proteins carried by ADSC-Exos are secreted by the cell of origin. Therefore, exosomes usually play the same biological roles as their cell of origin.
- ADSC-Exos can improve their biological ability after pretreatment, such as with a low oxygen environment for operational pretreatment and with H2O2 for pharmacological pretreatment. For example, ADSC-Exos pretreated with hypoxia can improve their biological ability. The ability of hypoxia-pretreated ADSC-Exos to form neovascularization networks was greater than that of nonhypoxia-pretreated ADSC-Exos, and the ability of H2O2-pretreated ADSC-Exos to promote angiogenesis was greater than that of non-H2O2 pretreated ADSC-Exos [113].
Some fundamental mechanisms and molecules in preventing scar formation
ADSC-Exos prevents scar formation through various molecular mechanisms, primarily involving the transfer of specific miRNAs and proteins. Some fundamental mechanisms and molecules involved are as follows:
- Regulation of fibrosis signaling pathways: ADSC-Exos can modulate the activation of several signaling pathways involved in scar formation, such as Wnt/β-catenin, TGF-β2/Smad3, and PI3K/AKT, by carrying or expressing miR-19b [95,96], miR-29a [107], and miR-125a-3p [91], thereby reducing fibrosis and scar formation.
- Anti-inflammatory effects: Delivering miR-155, miR-34a-5p, miR-124-3p, and miR-146a-5p, they can regulate the balance of macrophage differentiation, inhibit the release of pro-inflammatory cytokines like IL-1β and TNF-α, and promote the secretion of anti-inflammatory cytokines like IL-10. This balanced immune response helps limit inflammation and subsequent scar formation [83,84].
- Modulation of fibroblast activity: ADSC-Exos carrying miR-21 and miR-let7b can target and inhibit the expression of destructive genes in fibroblasts, thereby reducing scar formation. In addition to its potential role in promoting fibroblast activity, GDF11 may interact with TGF-β signaling pathways, which are crucial for producing skin cells with youthful characteristics [9,120-123]. Certain inflammatory factors, such as IL-10, can promote the conversion of fibroblasts to mature fibrocytes and inhibit scar tissue formation.
- Induction of angiogenesis: Adequate blood supply is crucial for wound healing and scar prevention. ADSC-Exos contain angiogenic factors such as VEGF and can regulate the promotion of neovascularization through the delivery of miR-125a, miR-31, miR-126-3p, miR378a-3p, and miR-21, thereby improving tissue regeneration and reducing scar formation [90,97,101,102]. ADSCs also interact with endothelial cells of microvessels, secreting increasing levels of IL-6, IL-8, and MCP-1 to regulate skin inflammation [124].
- Promotion of cell migration and proliferation: ADSC-Exos can stimulate the migration and proliferation of various cell types involved in wound healing, such as by delivering Exo-miR-378 [104]. This contributes to normal tissue regeneration and minimizes scar formation. ADSCs regulate and resolve their microenvironmental problems by secreting inflammatory factors such as TGF-β and ECM to optimize the involvement of corneal-forming cells and DFs in the healing process [9,120-122].
- Regulation of collagen remodeling: ADSC-Exos can reduce hypertrophic scar formation by delivering miR-192-5p, which decreases fibrotic proteins and collagen deposition and the differentiation level of fibroblasts into myofibroblasts [125].
It is important to note that inflammatory factors can contribute to scar inhibition through various mechanisms, such as regulating immune responses, inhibiting excessive proliferation, and promoting cell apoptosis, and that the specific miRNAs and proteins present in ADSC-Exos may vary depending on various factors, including the origin and culture conditions of the ADSCs. Different studies have identified multiple miRNAs and proteins associated with the anti-scarring effects of ADSC-Exos, and ongoing research continues to uncover additional molecules and mechanisms involved.
Multi-process intervention for wound healing
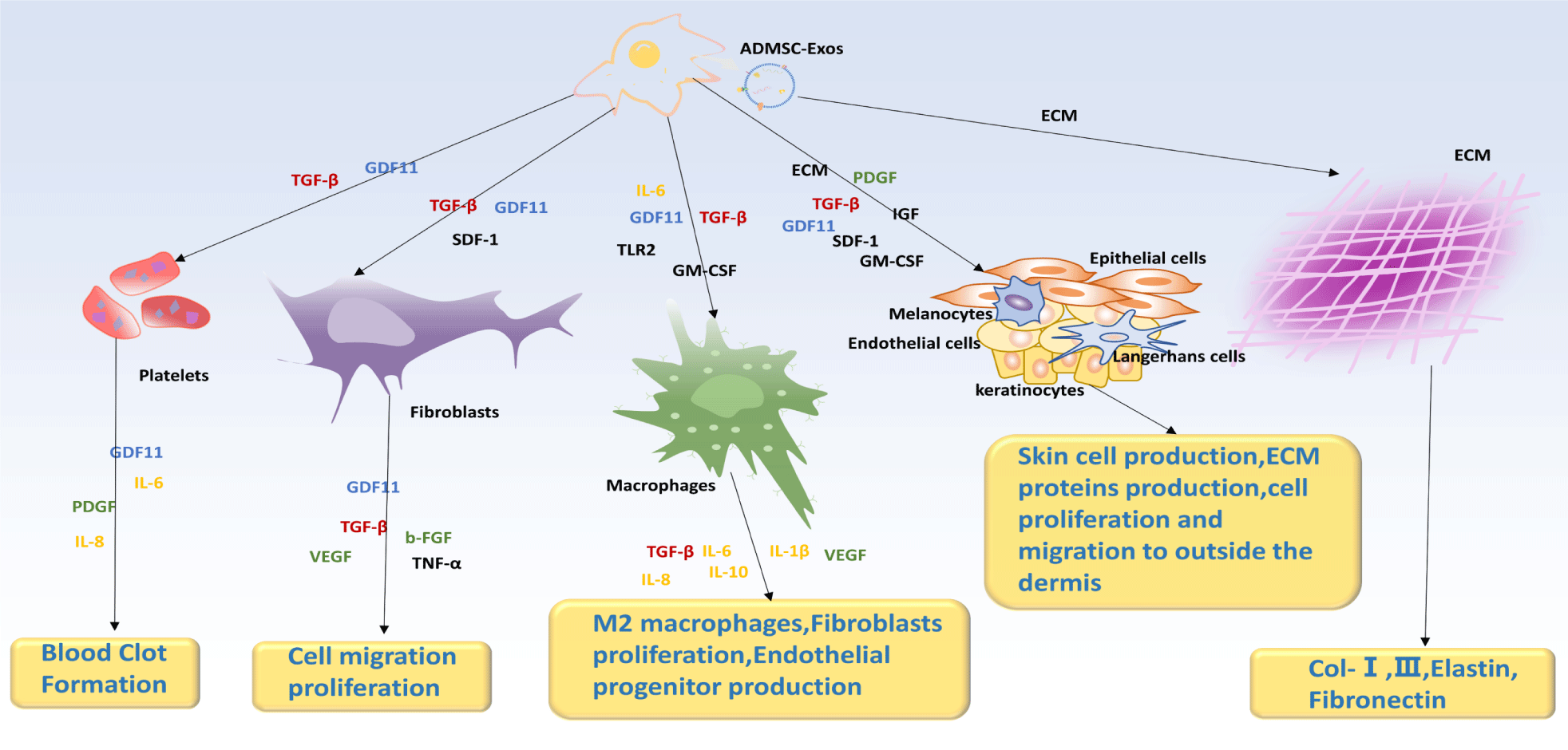
Considering how dynamic and interactive the processes of scar formation and wound healing are, therapeutic strategies in which ADSC-Exos are actively involved in multiple processes for wound healing can lead to better healing outcomes. For example, ADSC-Exos exerts its effect on fibroblasts, macrophages, and skin cells by secretion of inflammatory factors (Figure 5). GDF11 and TGF-β are present at almost all stages, and they promote the proliferation of fibroblasts, macrophages, and ADSCs, leading to immune responses, cell proliferation, and angiogenesis [12]. ADSC-Exo-miR-486-5p (Exo: 200 μg/100 μL) increases the viability and migratory properties of HSF and HMEC by decreasing Sp5/cyclinD2 (CCND2) pathway activity and increasing HMEC neovascularization activity, and it accelerates wound healing throughout the skin, increases epithelial regeneration, reduces scar thickness, and enhances collagen synthesis and angiogenesis [126]; the upregulation of MMP-7 expression has been shown to accelerate wound healing in diabetic rats by promoting the proliferation and migration of keratinocytes, leading to improved inflammation control, re-epithelialization, tissue matrix remodeling, vascular growth, and maturation [127-129]; and when ADSCs are present, skin fibroblasts proliferate more easily during the early phases of wound healing, which promotes collagen production as well as the healing process. However, during the later stages of wound healing, ADSCs have been shown to inhibit fibroblast proliferation and collagen synthesis [13-139].
Clinical Targeting Applications of Adipose Stem Cell-Derived Exosomes in Traumatic Scarring
The targeted application of ADSC-Exos to human wounds can be achieved through various methods, depending on the specific therapeutic goals and applications. These approaches include injection or infusion, local application, and bioengineering techniques, all beneficial in wound healing.
Injection or infusion
ADSC-Exos can be applied to the body by injection or intravenous infusion [89,132]. ADSC-Exos are emerging as promising candidates for delivering therapeutic substances such as drugs, genes, or other functional molecules with remarkable precision and specificity; thus, ADSC-Exos, which can act as ideal carriers for targeted therapy, enable the efficient delivery of therapeutic substances to specific sites of interest [132-135]. For example, ADSC-Exos can be modified to target cellular vesicles and release drugs that promote wound healing in treating large generalized wounds.
Local application
Locally applied ADSC-Exos can be applied directly to specific injury sites in the body by binding to gels, Microneedles (MNs), etc. Exosomes are pivotal in promoting wound healing and regeneration by selectively targeting specific growth factors and cell signaling molecules.
Adipose stem cell-derived exosomes in combination with hydrogels: The building blocks of hydrogels are hydrophilic polymers that undergo physical or chemical cross-linking to form three-dimensional networks [136-138] and are considered ideal alternatives for tissue repair engineering due to their ability to resist infection, absorb wound exudates, maintain water balance and gas exchange, load and deliver bioactive factors, and bioadaptability [139-142]. Gel-based drug delivery systems, as opposed to conventional systems, preserve the form and function of cells or bioactive molecules for an extended amount of time [143]; At present, hydrogel matrix-loaded ADSC-Exos are manifested in three primary forms: (1) physical binding to hydrogels, (2) covalent cross-linking based on the active precursor, and (3) in-situ gelation method. 3D printing technology has rapidly advanced and is widely utilized to enhance the functional aspects of hydrogel scaffolds, which enables precise manipulation of the scaffolds' porosity, pore shape, and overall geometry, leading to improved performance and functionality.
Compared with other hydrogels, DNA hydrogels are 3D polymer networks with DNA as the structural unit. Which combines the matrix structure of hydrogels with DNA's unique biological functions, such as precise and efficient self-assembly ability, excellent structural precision controllability, diversified stimulus responsiveness, good biocompatibility, and biodegradability [141,144,145]. Therefore, DNA hydrogels have certain advantages as carriers for exosome delivery systems, such as biocompatibility, effective binding to drugs, easy triggering of stimuli, and effective active targeting. Additionally, different types of inorganic nanoparticles, small molecules of drugs, and functional biomolecules can be used to dope porous networks with moderate loading efficiencies. In addition, highly programmable DNA hydrogels allow for synergistic loading of drugs with different physicochemical properties, enabling high-performance delivery systems, and release rates are controlled by DNA hydrogels modulating the type of DNA strand segments [146-150].
Currently, a significant number of multifunctional DNA hydrogels capable of delivering ADSC-Exos have been created by researchers, particularly in the areas of tissue repair and drug delivery [151,152], such as a novel multifunctional DNA hydrogel with a collodion structure, MpHt-DNA hydrogel-A/b with a wound patch structure, and E7-P-b-DNA gel for targeted recruitment of stem cells.
Adipose stem cell-derived exosomes in combination with microneedles: Microneedles (MNs) have attracted attention for their unique minimally invasive nature, visualization performance, ability to overcome biological barriers, accurate and sustained drug release performance [153,154]. The combined application of exosome-injected MNs can achieve both advantages to promote each other. In addition to improving the stability and adhesion of microneedles and reducing their resistance when entering the skin, Zhou, et al. [155] designed and 3D printed biomimetic and macro deformable DNA gel microneedles (P-DNAgelMNs) with a shark's barbed groove structure and a flat, angled structure resembling a crab claw. These MNs also have intrinsic adhesive properties that allow them to avoid the laborious dressing application. At the same time, P-DNAgelMNs also have excellent characteristics as follows. P-DNAgelMNs exhibit strong biocompatibility and induce biological behaviors in cells [155]. Biomaterials with particular biochemical properties, growth factors, active substances, and EVs can regulate their polarization, i.e., ADSC-Exos can regulate their polarization [156,157]. Concurrently, P-DNAgelMNs can activate pathways that reduce inflammation and pain; mRNA expression of Trpvl and CCL2, two common inflammatory pain signaling factors, gradually decreases with optimization of the MNs design structure, resulting in less pain. [158,159]. Its needle cap exhibits excellent tensile and elastic deformation ability at concentrations of 10%, and the elastic deformation dressing can better accommodate patients' joints [160-162]. Its dissolution rate decreases as concentration increases, offering a solution to excess tissue fluid in ulcerative wounds. Bacteria can have their cell membranes destroyed by the cationic peptide that MNs inject into the exosome, allowing the contents of the bacterium to seep out and ultimately leading to its death, so the MNs are suitable for the wounds of diabetic patients with low immunity and high risk of infection [160-163].
Engineering of adipose stem cell-derived exosomes
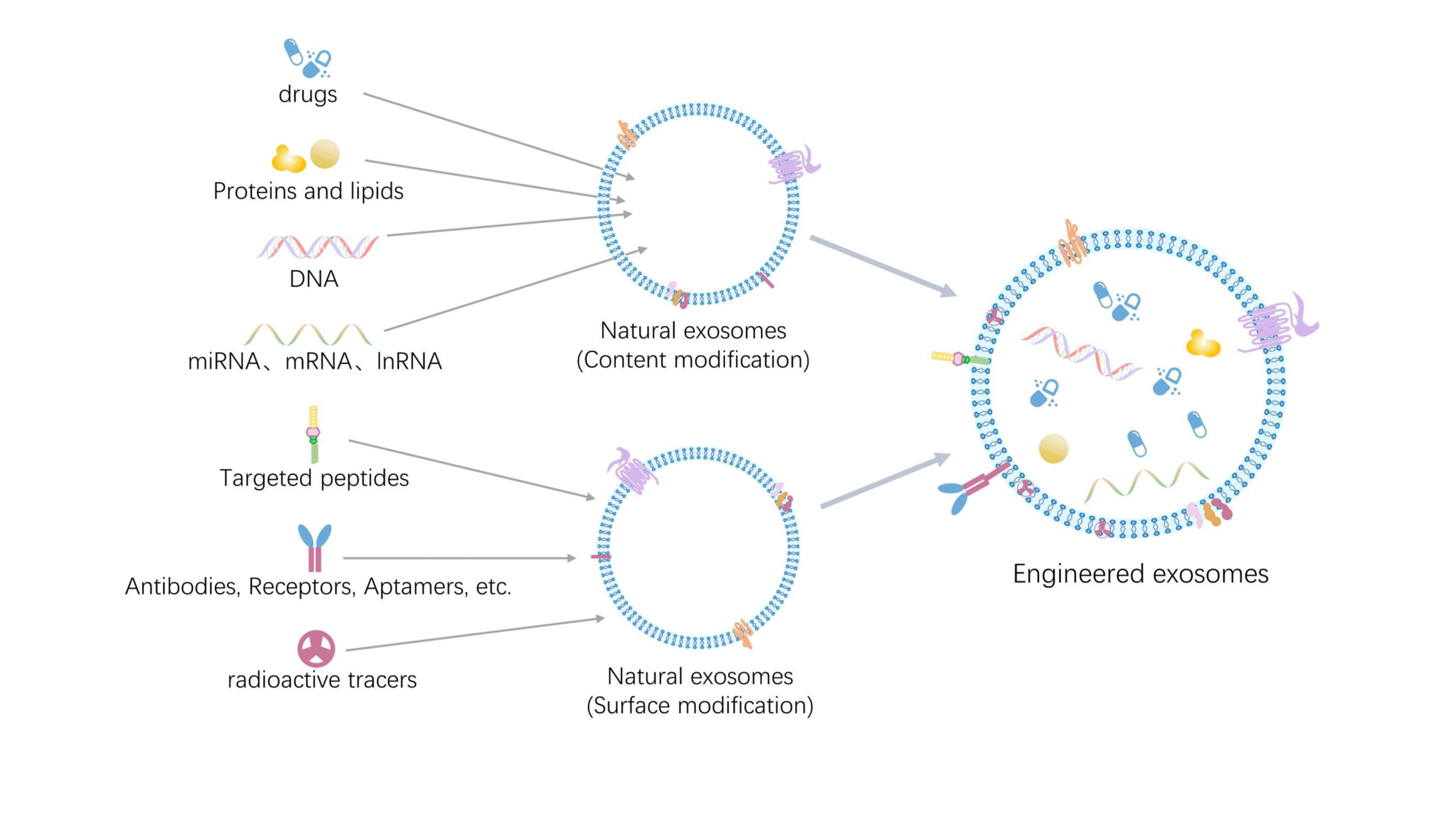
The use of natural ADSC-Exos is limited by limited drug loading capacity, insufficient yield for clinical use, limited biodistribution, targeting dependent on the parent cell, and some off-target effects [166-168]. Engineered approaches can help increase the scalability of ADSC-Exos, improve their targeting properties, and expand the range of biomedical applications of ADSC-Exos beyond their intrinsic capabilities [169]. In this context, constructing engineered ADSC-Exos as alternatives to natural ADSC-Exos has become an effective strategy for addressing the above issues.
Typical methods: Currently, there are two main techniques for preparing engineered ADSC-Exos: surface modification and content modification. Surface modification techniques include covalent modification (using covalent bonds to attach specific molecules to the surface of exosomes, such as spot chemistry), donor cell modification (using gene transfection, changing the donor cell culture environment, and drug-cell incubation method), and noncovalent modification (using electrostatic, receptor-ligands, and other non-covalent bonding to attach particular substances to the exosome membrane binding). While content modification techniques include bioengineering (where the medication binds to the donor cells or exosomes by co-incubation), genetic engineering transfection, and physical methods (such as ultrasound, electroporation, freeze-thaw cycling, extrusion, etc.) [171-175] (Figure 6).
Nanoengineering: Typical approaches for harvesting targeted exosomes typically involve using antibodies and peptides as targeting ligands. Still, several inherent drawbacks hamper their clinical application, including batch-to-batch variability, decreased specificity, immunogenicity, instability, safety concerns, variable transfection results, and mutagenesis. In addition, this bioengineering strategy is very time-consuming, and the identification of the molecule will have to be repeated each time the molecule is targeted for a new entire process.
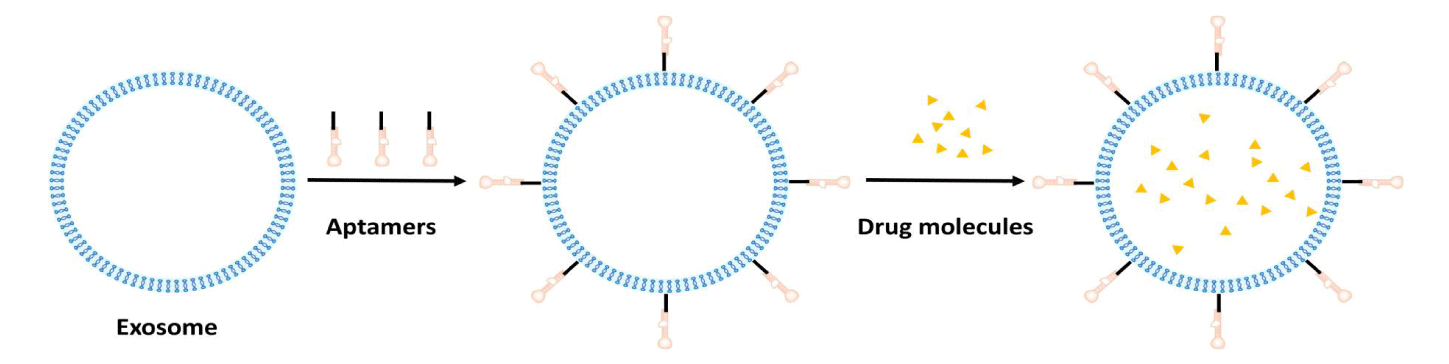
Through the Systematic Evolution of Ligands by Exponential Enrichment (SELEX) screening, single-stranded DNA or RNA molecules known as aptamers or chemical antibodies can be generated. These molecules exhibit high affinity and specificity when binding to their targets through the same mechanism as antibody-antigen binding, forming specific three-dimensional structures [176-179]. Aptamers are superior to antibodies in precision medicine because their molecular mass, immunogenicity, and toxicity are lower, as well as their greater stability, ease of modification, and superior tissue penetration [176,180,181]. From a translational research perspective, the main advantages of aptamers are their scalability and absence of batch-to-batch variation in drug production, as they are synthetic nanocarriers chemically synthesized without the involvement of animals or cultured cells [182].
The synthetic nanocarriers used to construct molecularly targeted exosomes are mainly liposomes, metal nanocarriers, silica carriers, and polymer nanocarriers. Lipid ligands are usually used to utilize the specific biochemical properties of exosomal lipid bilayers for surface nanoengineering [183-188]. Molecularly targeted exosomes can be prepared by three main methods: (1) intracellular preloading; (2) surface modification of exosomes by antibody coupling; and (3) physical methods, which are used to hybridize exosomes with nanocarriers utilizing membrane extrusion, freeze-thaw cycling, ultrasound, electroporation, PEG induction, coincubation, etc [189-196].
Traditional drug delivery vehicles (such as metal particles, nanogels, and liposomes) have been widely used, but their synthesis costs are high, and their stability could be better.
Combining an aptamer-functionalized exosome drug delivery system (Figure 7). with traditional therapeutic means, such as acoustic power therapy, RNA interference technology, and chemical treatment, synergizes active and passive targeting mechanisms, thereby maximizing the effectiveness of the drug and enhancing therapeutic efficiency. Combining exosomes and aptamers to form molecularly targeted exosomes is expected to constitute the next generation of intelligently engineered nanovesicles for precision medicine.
Conclusion
In conclusion, ADSC-Exos are critical for wound healing and reducing the growth of excessive scars. Cell-free therapies offer numerous advantages in mitigating pathological scarring, as they actively regulate relevant cells and their associated inflammatory factors throughout the different phases of wound healing. Furthermore, the multiple regulatory mechanisms employed by ADSC-Exos are complementary. There are various clinical approaches for targeting ADSC-Exos, among which 3D-printed hydrogel drug delivery systems and nanoengineered aptamer-functionalized exosome drug delivery systems have the most potential for research and translational applications. Finally, we can infer that ADSC-Exos exhibits active targeting effects in inhibiting scar formation, suggesting their potential application in overcoming the challenges of scar management in clinical settings. However, there are still challenges to using this treatment in several areas.
Challenges
Long-term efficacy and safety of adipose stem cell-derived exosomes
First, in terms of long-term effectiveness, most of the studies have observed an improvement in the therapeutic efficacy of ADSC-Exos in scar prevention in the short term, but the long-term effect requires longer follow-up and observation; in addition, exosomes may be affected by storage and handling conditions, which also need to be studied further. Secondly, in terms of safety, ADSC-Exos is basically safe because it is derived from autologous tissues without rejecting allografts and has no cytoplasmic nucleus. Still, more evaluations are needed to determine whether other adverse reactions, such as infections and allergies, may occur during treatment.
Impact of recipients-individualized factors on efficacy
Firstly, age is associated with the ability to repair and regenerate tissue. Younger people typically have better self-healing and repair capabilities and, therefore, may be more likely to benefit from ADSC-Exos treatment. Secondly, different skin types have different physiological characteristics and responsiveness. Certain skin types may be more susceptible to scar formation; therefore, individualized treatment regimens may be needed for recipients based on their skin types. Lastly, a recipient's underlying health condition may also impact the efficacy of ADSC-Exos; for example, recipients with chronic inflammatory or immune system disorders and recipients with other chronic diseases or have been on some medications for a long time may require more attention and monitoring. It is important to note that while these specific factors may impact the efficacy of ADSC-Exos in preventing scarring, the current research needs to be more comprehensive to determine the exact relationship between them. Therefore, physicians and researchers must consider their recipients' individual differences and specific circumstances in practice and conduct detailed evaluation and monitoring.
Clinical translation of adipose stem cell-derived exosomes in scar inhibition
There needs to be clear guidelines for determining the optimal dose of exosomes. Secondly, choosing the appropriate route of administration is also an important issue. ADSC-Exos can be administered by several routes, such as injection, intravenous infusion, or local application; however, different administration routes may impact exosome distribution, bioavailability, and therapeutic efficacy.
Scalability and production challenges
The production of ADSC-Exos is a technology-intensive task requiring highly specialized equipment and personnel. Currently, ADSC-Exos production is mainly prepared by culturing ADSCs and extracting the exosomes they secrete. Although this approach is feasible for scaling up ADSC-Exos production for clinical use, it still presents several challenges.
One major challenge is that increasing production requires a large number of ADSCs, which need to be provided with sufficient donors to enable the engineered preparation of ADSC-Exos. Another challenge is that, unlike traditional drug manufacturing, exosome production requires more time and effort to monitor and control the details of the production process, and a standardized production process and quality control system needs to be established to ensure the consistency and quality of the exosome. The current cost of ADSC-Exos production is also very high, mainly due to the need for equipment, which is very expensive, complex cultivation processes, and specialized personnel. Technological improvements and process optimization are needed to reduce costs and increase production efficiency. Lastly, quality control is also an essential issue in ADSC-Exos production. Since ADSC-Exos is a biological product, its quality is affected by many factors, such as cell differentiation status, purity of exosome isolation, etc. A comprehensive quality control system is needed to ensure that the quality of ADSC-Exos meets the clinical requirements.
Regulation and ethics
Regarding regulatory pathways, the approval of ADSC-Exos as therapeutic requires undergoing clinical trials. Regulatory agencies have the authority to impose requirements on the design and execution of these trials, which may include aspects such as sample size, treatment protocols, data collection, and analysis. Additionally, regulatory agencies should mandate pharmaceutical companies to submit comprehensive quality control and production process documentation to ensure the consistency and quality of ADSC-Exos. These measures aim to achieve the intended regulatory objectives.
Regarding ethical considerations, using ADSC-Exos as a therapeutic requires adherence to moral principles and regulations, including patient informed consent, protection of personal privacy, data security, and fair allocation of resources. Biological ethical issues related to ADSC-Exos must also be considered, including cell source selection, donor-informed consent, rights protection, and treatment safety and efficacy evaluation.
Patient education
Patient education and awareness must be considered in preventing and managing scars. Firstly, we can disseminate information about ADSC-Exos to patients through various online and offline channels, organize regular lectures and seminars specifically tailored for patients, and invite experts to share relevant knowledge about ADSC-Exos. Secondly, doctors should communicate thoroughly with patients, jointly develop treatment plans, and provide necessary guidance and support. Additionally, doctors should conduct regular follow-ups with patients undergoing ADSC-Exos treatment to understand their treatment outcomes and responses, emphasizing the importance of scar prevention and management. Thirdly, we should strive to establish patient support groups, enabling patients to share experiences and information. Patients can gain comprehensive information about ADSC-Exos through these measures, empowering them to actively choose and participate in treatment while better controlling and managing their health conditions.
In vivo and Ex vivo model selection
In the research on preventing scars using adipose-derived stem cell exosomes, selecting appropriate in vivo and in vitro models for investigation is crucial. In vivo models can more accurately simulate the complex physiological environment of human tissues and organs, but they also involve issues related to animal ethics and experimental regulations. On the other hand, in vitro models offer better controllability and maneuverability, allowing for precise control of experimental conditions and easier acquisition and processing of samples. Based on four criteria- research objectives, degree of simulation, controllability, maneuverability, and ethical and safety requirements- scientists can choose the model that best suits their research needs. By combining the advantages of different models, a comprehensive evaluation and validation can be conducted.
Prospects
To date, the mechanisms underlying the generation of ADSC-Exos and their role in wound healing have been extensively investigated domestically and internationally. Significant progress has been made in developing diverse and comprehensive techniques for isolating and enriching exosomes. Moreover, research on exosomes as a targeted drug delivery system is also flourishing. However, the safe and sustainable clinical application of targeted scar prevention using extracellular vesicles has not yet been achieved, nor has its engineering production. In addition, the delivery pathways of ADSC-Exos for targeted scar prevention are also limited. Building upon these advancements, future research directions hold promise in exploring the content of ADSC-Exos, nonstem cell-derived ADSC-Exos, laser delivery of exosomes, and the combination of ADSCs-Exos and surgical techniques.
Research on the contents of ADSC-Exos holds the potential to advance cell-free therapy using these vesicles toward further molecularly targeted therapies. This advancement can pave the way for precision medicine in scar prevention, wherein customized treatment plans can be provided based on individual genetic and phenotypic characteristics. This approach aims to enhance the effectiveness of scar prevention and minimize adverse reactions. Exosomes derived from non-stem cell sources can be obtained from animals and plants. They offer the advantages of convenient acquisition and low cost, making them more suitable for the engineered production of exosomes. Additionally, plant-derived exosomes do not carry zoonotic or human pathogens compared to mammalian-derived exosomes. This facilitates engineered exosomes' streamlined extraction and production processes while improving their safety in clinically targeted applications. Laser therapy has been widely used to improve the appearance and texture of scars. Using lasers' focusing and directional properties, exosomes can be precisely delivered to wound areas by adjusting laser parameters, achieving high-precision positioning and non-contact selective delivery. ADSC-Exos can provide growth factors and ECM components, promoting skin healing and repair after laser treatment. For larger or depressed scars, surgical techniques may be necessary. During surgery, ADSC-Exos can facilitate wound healing, reduce inflammation, and improve postoperative scar formation, thereby contributing to better surgical outcomes and reduced complications.
Nomenclature
Resource identification initiative
Not applicable.
Life science identifiers
Not applicable.
Additional Requirements
None.
Conflict of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
XMW: Final review of the manuscript and overall supervision; XHC & HJD: Conceived and designed the study, performed literature sorting, and drafted the manuscript; YL: Revised and edited the manuscript; SYY& GRY: preparation of the initial draft. All authors agree to be accountable for the content of the work.
Funding
Not applicable.
Acknowledgment
Thanks to Komla-Dzah Julius Kodzo for his linguistic support of the manuscript.
Translational Impact Statement
The review highlights adipose stem cell-derived exosomes' potential to revolutionize scar management. Understanding their mechanisms offers a non-invasive, cell-free alternative to traditional therapies. This approach could significantly improve scar treatment outcomes, addressing a critical need in clinical practice. The study's findings have translational and clinical significance, paving the way for innovative scar prevention strategies with broad therapeutic implications.
References
- Zhang T, Wang XF, Wang ZC, Lou D, Fang QQ, Hu YY, Zhao WY, Zhang LY, Wu LH, Tan WQ. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020 Sep;129:110287. doi: 10.1016/j.biopha.2020.110287. Epub 2020 Jun 12. PMID: 32540643.
- Yu J, Wang MY, Tai HC, Cheng NC. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018 Sep 1;77:191-200. doi: 10.1016/j.actbio.2018.07.022. Epub 2018 Jul 12. PMID: 30017923.
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019 Jun 20;88:487-514. doi: 10.1146/annurev-biochem-013118-111902. PMID: 31220978.
- Hade MD, Suire CN, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021 Aug 1;10(8):1959. doi: 10.3390/cells10081959. PMID: 34440728; PMCID: PMC8393426.
- van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012 Jul;64(3):676-705. doi: 10.1124/pr.112.005983. Epub 2012 Jun 21. PMID: 22722893.
- Li C, Wei S, Xu Q, Sun Y, Ning X, Wang Z. Application of ADSCs and their Exosomes in Scar Prevention. Stem Cell Rev Rep. 2022 Mar;18(3):952-967. doi: 10.1007/s12015-021-10252-5. Epub 2021 Sep 12. PMID: 34510359; PMCID: PMC8942892.
- Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020 Feb 19;10(8):3684-3707. doi: 10.7150/thno.41580. PMID: 32206116; PMCID: PMC7069071.
- Balaji S, Keswani SG, Crombleholme TM. The Role of Mesenchymal Stem Cells in the Regenerative Wound Healing Phenotype. Adv Wound Care (New Rochelle). 2012 Aug;1(4):159-165. doi: 10.1089/wound.2012.0361. PMID: 24527298; PMCID: PMC3839028.
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009 Mar;10(3):207-17. doi: 10.1038/nrm2636. Epub 2009 Feb 11. PMID: 19209183; PMCID: PMC2760218.
- Cappuzzello C, Doni A, Dander E, Pasqualini F, Nebuloni M, Bottazzi B, Mantovani A, Biondi A, Garlanda C, D'Amico G. Mesenchymal Stromal Cell-Derived PTX3 Promotes Wound Healing via Fibrin Remodeling. J Invest Dermatol. 2016 Jan;136(1):293-300. doi: 10.1038/JID.2015.346. PMID: 26763449.
- Mazini L, Rochette L, Amal S, Admou B, Malka G. Adipose derived stem cells (ADSCs) Immunomodulation Impact on Skin Tissue Repair. JES. 2020;4(1):1-9. doi: 10.23880/jes-16000136.
- Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020 Feb 14;21(4):1306. doi: 10.3390/ijms21041306. PMID: 32075181; PMCID: PMC7072889.
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008 Feb 15;180(4):2581-7. doi: 10.4049/jimmunol.180.4.2581. PMID: 18250469.
- Cuevas-Diaz Duran R, González-Garza MT, Cardenas-Lopez A, Chavez-Castilla L, Cruz-Vega DE, Moreno-Cuevas JE. Age-related yield of adipose-derived stem cells bearing the low-affinity nerve growth factor receptor. Stem Cells Int. 2013;2013:372164. doi: 10.1155/2013/372164. Epub 2013 Nov 24. PMID: 24376462; PMCID: PMC3859201.
- Lin Z, Shibuya Y, Imai Y, Oshima J, Sasaki M, Sasaki K, Aihara Y, Khanh VC, Sekido M. Therapeutic Potential of Adipose-Derived Stem Cell-Conditioned Medium and Extracellular Vesicles in an In Vitro Radiation-Induced Skin Injury Model. Int J Mol Sci. 2023 Dec 7;24(24):17214. doi: 10.3390/ijms242417214. PMID: 38139042; PMCID: PMC10743562.
- Li Y, Shi G, Liang W, Shang H, Li H, Han Y, Zhao W, Bai L, Qin C. Allogeneic Adipose-Derived Mesenchymal Stem Cell Transplantation Alleviates Atherosclerotic Plaque by Inhibiting Ox-LDL Uptake, Inflammatory Reaction and Endothelial Damage in Rabbits. Cells. 2023 Jul 26;12(15):1936. doi: 10.3390/cells12151936. PMID: 37566014; PMCID: PMC10417209.
- Kocan B, Maziarz A, Tabarkiewicz J, Ochiya T, Banaś-Ząbczyk A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017;2017:1653254. doi: 10.1155/2017/1653254. Epub 2017 Jul 5. PMID: 28757877; PMCID: PMC5516761.
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016 Sep 12;6:32993. doi: 10.1038/srep32993. Erratum in: Sci Rep. 2020 Apr 16;10(1):6693. doi: 10.1038/s41598-020-63068-7. PMID: 27615560; PMCID: PMC5018733.
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. PMID: 22468918.
- Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, Feng G, Cai Y, Xia C, Liu H, Shen W, Hu X, Ouyang H. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am J Sports Med. 2019 Jun;47(7):1722-1733. doi: 10.1177/0363546519848678. Epub 2019 May 17. PMID: 31100005.
- Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020 Dec 10;2020:8810813. doi: 10.1155/2020/8810813. PMID: 33488736; PMCID: PMC7787857.
- Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020 Jul 22;11(1):312. doi: 10.1186/s13287-020-01831-3. PMID: 32698868; PMCID: PMC7374967.
- Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016 Apr 1;126(4):1152-62. doi: 10.1172/JCI81129. Epub 2016 Apr 1. PMID: 27035807; PMCID: PMC4811150.
- Zhang D, Fu Q, Fu H, Zeng J, Jia L, Chen M. 3D-bioprinted human lipoaspirate-derived cell-laden skin constructs for healing of full-thickness skin defects. Int J Bioprint. 2023 Mar 23;9(4):718. doi: 10.18063/ijb.718. PMID: 37323499; PMCID: PMC10261198.
- Wang L, Li T, Ma X, Li Y, Li Z, Li Z, Yu N, Huang J, Han Q, Long X. Exosomes from human adipose-derived mesenchymal stem cells attenuate localized scleroderma fibrosis by the let-7a-5p/TGF-βR1/Smad axis. J Dermatol Sci. 2023 Oct;112(1):31-38. doi: 10.1016/j.jdermsci.2023.09.001. Epub 2023 Sep 6. PMID: 37743142.
- Wang JW, Zhu YZ, Ouyang JY, Nie JY, Wang ZH, Wu S, Yang JM, Yi YY. Adipose-Derived Stem Cell Extracellular Vesicles Improve Wound Closure and Angiogenesis in Diabetic Mice. Plast Reconstr Surg. 2023 Feb 1;151(2):331-342. doi: 10.1097/PRS.0000000000009840. Epub 2022 Nov 8. PMID: 36696316.
- Chaker D, Mouawad C, Azar A, Quilliot D, Achkar I, Fajloun Z, Makdissy N. Inhibition of the RhoGTPase Cdc42 by ML141 enhances hepatocyte differentiation from human adipose-derived mesenchymal stem cells via the Wnt5a/PI3K/miR-122 pathway: impact of the age of the donor. Stem Cell Res Ther. 2018 Jun 19;9(1):167. doi: 10.1186/s13287-018-0910-5. PMID: 29921325; PMCID: PMC6009972.
- García-Contreras M, Vera-Donoso CD, Hernández-Andreu JM, García-Verdugo JM, Oltra E. Therapeutic potential of human adipose-derived stem cells (ADSCs) from cancer patients: a pilot study. PLoS One. 2014 Nov 20;9(11):e113288. doi: 10.1371/journal.pone.0113288. PMID: 25412325; PMCID: PMC4239050.
- Franck CL, Senegaglia AC, Leite LMB, de Moura SAB, Francisco NF, Ribas Filho JM. Influence of Adipose Tissue-Derived Stem Cells on the Burn Wound Healing Process. Stem Cells Int. 2019 Feb 11;2019:2340725. doi: 10.1155/2019/2340725. PMID: 30886634; PMCID: PMC6388323.
- L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019 Apr;46:1-9. doi: 10.1016/j.cytogfr.2019.04.002. Epub 2019 Apr 2. PMID: 30954374.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017 Aug 25;18(9):1852. doi: 10.3390/ijms18091852. PMID: 28841158; PMCID: PMC5618501.
- Aryani A, Denecke B. Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine? Mol Neurobiol. 2016 Mar;53(2):818-834. doi: 10.1007/s12035-014-9054-5. Epub 2014 Dec 15. PMID: 25502465; PMCID: PMC4752585.
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015 Jul 1;6(1):127. doi: 10.1186/s13287-015-0116-z. PMID: 26129847; PMCID: PMC4529699.
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017 Jun 9;356(6342):1026-1030. doi: 10.1126/science.aam7928. Epub 2017 Jun 8. PMID: 28596335.
- Lee HJ, Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018 Mar 2;19(3):711. doi: 10.3390/ijms19030711. PMID: 29498630; PMCID: PMC5877572.
- Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P, Cai JQ, Shen XZ, Tan WQ. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020 Dec 4;11:603187. doi: 10.3389/fimmu.2020.603187. PMID: 33343575; PMCID: PMC7746641.
- Xiaojie W, Banda J, Qi H, Chang AK, Bwalya C, Chao L, Li X. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022 Aug;66:26-37. doi: 10.1016/j.cytogfr.2022.03.001. Epub 2022 Mar 31. Erratum in: Cytokine Growth Factor Rev. 2022 Dec;68:116. doi: 10.1016/j.cytogfr.2022.09.002. PMID: 35690568.
- Ma J, Yong L, Lei P, Li H, Fang Y, Wang L, Haojie Chen, Qi Zhou, Wei Wu, Libo Jin, Da Sun, Xingxing Zhang. Advances in microRNA from adipose-derived mesenchymal stem cell-derived exosome: Focusing on wound healing. J Mater Chem B. 2022;10(46):9565-77. doi: 10.1039/D2TB01987F.
- Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017 Mar 14;38(11):785-791. doi: 10.1093/eurheartj/ehw550. PMID: 28039338; PMCID: PMC11110018.
- Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ Res. 2018 Jan 19;122(2):337-351. doi: 10.1161/CIRCRESAHA.117.310795. PMID: 29348254; PMCID: PMC5777300.
- Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021 Mar;46:100733. doi: 10.1016/j.blre.2020.100733. Epub 2020 Jul 12. PMID: 32682574; PMCID: PMC7354275.
- Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015 May;29(3):153-62. doi: 10.1016/j.blre.2014.10.003. Epub 2014 Oct 31. PMID: 25468720; PMCID: PMC4452143.
- Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017 Jun;36(2):195-198. doi: 10.1007/s10555-017-9677-x. PMID: 28667366; PMCID: PMC5709181.
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997 Apr 4;276(5309):75-81. doi: 10.1126/science.276.5309.75. PMID: 9082989.
- An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, Gu L, Zhang C, Wang B, Wei W, Li D, Wu J. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021 Mar;54(3):e12993. doi: 10.1111/cpr.12993. Epub 2021 Jan 17. PMID: 33458899; PMCID: PMC7941238.
- Lee JY, Oh N, Park KS. Ell3 Modulates the Wound Healing Activity of Conditioned Medium of Adipose-derived Stem Cells. Dev Reprod. 2017 Sep;21(3):335-342. doi: 10.12717/DR.2017.21.3.335. Epub 2017 Sep 30. PMID: 29082349; PMCID: PMC5651700.
- Lee JY, Oh N, Park KS. Ell3 Modulates the Wound Healing Activity of Conditioned Medium of Adipose-derived Stem Cells. Dev Reprod. 2017 Sep;21(3):335-342. doi: 10.12717/DR.2017.21.3.335. Epub 2017 Sep 30. PMID: 29082349; PMCID: PMC5651700.
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015 Aug;173(2):370-8. doi: 10.1111/bjd.13954. Epub 2015 Jul 14. PMID: 26175283; PMCID: PMC4671308.
- Xu X, Gu S, Huang X, Ren J, Gu Y, Wei C, Lian X, Li H, Gao Y, Jin R, Gu B, Zan T, Wang Z. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020 Mar 11;8:tkaa006. doi: 10.1093/burnst/tkaa006. PMID: 32341919; PMCID: PMC7175772.
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010 Apr 1;184(7):3964-77. doi: 10.4049/jimmunol.0903356. Epub 2010 Feb 22. PMID: 20176743.
- Bagabir R, Byers RJ, Chaudhry IH, Müller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol. 2012 Nov;167(5):1053-66. doi: 10.1111/j.1365-2133.2012.11190.x. PMID: 23106354.
- Li N, Bai B, Zhang H, Zhang W, Tang S. Adipose stem cell secretion combined with biomaterials facilitates large-area wound healing. Regen Med. 2020 Nov;15(11):2311-2323. doi: 10.2217/rme-2020-0086. Epub 2020 Dec 15. PMID: 33320721.
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003 Jul;83(3):835-70. doi: 10.1152/physrev.2003.83.3.835. PMID: 12843410.
- Abomaray FM, Al Jumah MA, Alsaad KO, Jawdat D, Al Khaldi A, AlAskar AS, Al Harthy S, Al Subayyil AM, Khatlani T, Alawad AO, Alkushi A, Kalionis B, Abumaree MH. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016;2016:5184601. doi: 10.1155/2016/5184601. Epub 2016 Feb 10. PMID: 27087815; PMCID: PMC4764756.
- Wang S, Amato KR, Song W, Youngblood V, Lee K, Boothby M, Brantley-Sieders DM, Chen J. Regulation of endothelial cell proliferation and vascular assembly through distinct mTORC2 signaling pathways. Mol Cell Biol. 2015 Apr;35(7):1299-313. doi: 10.1128/MCB.00306-14. Epub 2015 Jan 12. PMID: 25582201; PMCID: PMC4355541.
- Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000 Dec;5(1):40-6. doi: 10.1046/j.1087-0024.2000.00014.x. PMID: 11147674.
- Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012 Jul 19;120(3):613-25. doi: 10.1182/blood-2012-01-403386. Epub 2012 May 10. PMID: 22577176.
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010 Aug;163(2):257-68. doi: 10.1111/j.1365-2133.2010.09804.x. Epub 2010 Apr 15. PMID: 20394633.
- Lynam EC, Xie Y, Dawson R, Mcgovern J, Upton Z, Wang X. Severe hypoxia and malnutrition collectively contribute to scar fibroblast inhibition and cell apoptosis. Wound Repair Regen. 2015 Sep;23(5):664-71. doi: 10.1111/wrr.12343. Epub 2015 Sep 22. Erratum in: Wound Repair Regen. 2015 Nov-Dec;23(6):956. doi: 10.1111/wrr.12377. PMID: 26174572.
- Siddiqui A, Galiano RD, Connors D, Gruskin E, Wu L, Mustoe TA. Differential effects of oxygen on human dermal fibroblasts: acute versus chronic hypoxia. Wound Repair Regen. 1996 Apr-Jun;4(2):211-8. doi: 10.1046/j.1524-475X.1996.40207.x. PMID: 17177815.
- Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019 Jan;75-76:12-26. doi: 10.1016/j.matbio.2018.01.002. Epub 2018 Jan 10. PMID: 29330022.
- Atkin L. Chronic wounds: the challenges of appropriate management. Br J Community Nurs. 2019 Sep 1;24(Sup9):S26-S32. doi: 10.12968/bjcn.2019.24.Sup9.S26. PMID: 31479336.
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020 Sep;10(9):200223. doi: 10.1098/rsob.200223. Epub 2020 Sep 30. PMID: 32993416; PMCID: PMC7536089.
- Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in Chronic Wounds. Int J Mol Sci. 2016 Dec 11;17(12):2085. doi: 10.3390/ijms17122085. PMID: 27973441; PMCID: PMC5187885.
- Henriksen JL, Sørensen NB, Fink T, Zachar V, Porsborg SR. Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies. Cells. 2020 Nov 26;9(12):2545. doi: 10.3390/cells9122545. PMID: 33256038; PMCID: PMC7761075.
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015 Jan 5;5(1):a023267. doi: 10.1101/cshperspect.a023267. PMID: 25561722; PMCID: PMC4292081.
- Wilkins RG, Unverdorben M. Wound cleaning and wound healing: a concise review. Adv Skin Wound Care. 2013 Apr;26(4):160-3. doi: 10.1097/01.ASW.0000428861.26671.41. PMID: 23507692.
- Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019 Jun;146:344-365. doi: 10.1016/j.addr.2018.06.019. Epub 2018 Jul 5. PMID: 29981800.
- Wound Healing: A Cellular Perspective. PMC. 2023;Oct 11.
- Harless WW. Cancer treatments transform residual cancer cell phenotype. Cancer Cell Int. 2011 Jan 7;11(1):1. doi: 10.1186/1475-2867-11-1. PMID: 21214935; PMCID: PMC3022788.
- Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009 Jun;119(6):1417-9. doi: 10.1172/JCI39675. PMID: 19487817; PMCID: PMC2689122.
- Yuan FL, Sun ZL, Feng Y, Liu SY, Du Y, Yu S, Yang ML, Lv GZ. Epithelial-mesenchymal transition in the formation of hypertrophic scars and keloids. J Cell Physiol. 2019 Dec;234(12):21662-21669. doi: 10.1002/jcp.28830. Epub 2019 May 20. PMID: 31106425.
- Zhu Z, Hou Q, Li M, Fu X. Molecular mechanism of myofibroblast formation and strategies for clinical drugs treatments in hypertrophic scars. J Cell Physiol. 2020 May;235(5):4109-4119. doi: 10.1002/jcp.29302. Epub 2019 Oct 14. PMID: 31612497.
- Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res. 2016 Apr 26;5:F1000 Faculty Rev-752. doi: 10.12688/f1000research.8190.1. PMID: 27158462; PMCID: PMC4847562.
- Lebonvallet N, Laverdet B, Misery L, Desmoulière A, Girard D. New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation. Exp Dermatol. 2018 Sep;27(9):950-958. doi: 10.1111/exd.13681. Epub 2018 Jun 28. PMID: 29742295.
- Cohen BE, Geronemus RG, McDaniel DH, Brauer JA. The Role of Elastic Fibers in Scar Formation and Treatment. Dermatol Surg. 2017 Jan;43 Suppl 1:S19-S24. doi: 10.1097/DSS.0000000000000840. PMID: 27399940.
- Mir M, Ali MN, Barakullah A, Gulzar A, Arshad M, Fatima S, Asad M. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018 Mar;7(1):1-21. doi: 10.1007/s40204-018-0083-4. Epub 2018 Feb 14. PMID: 29446015; PMCID: PMC5823812.
- Bitto A, Irrera N, Pizzino G, Pallio G, Mannino F, Vaccaro M, Arcoraci V, Aliquò F, Minutoli L, Colonna MR, Galeano MR, Brines M, De Ponte C, Collino M, Squadrito F, Altavilla D. Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. Biochim Biophys Acta Mol Basis Dis. 2018 Feb;1864(2):632-639. doi: 10.1016/j.bbadis.2017.12.006. Epub 2017 Dec 7. PMID: 29223734.
- Van Putte L, De Schrijver S, Moortgat P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: a systematic review. Scars Burn Heal. 2016 Dec 5;2:2059513116676828. doi: 10.1177/2059513116676828. PMID: 29799552; PMCID: PMC5965313.
- Kranendonk ME, Visseren FL, van Balkom BW, Nolte-'t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014 May;22(5):1296-308. doi: 10.1002/oby.20679. Epub 2014 Jan 9. PMID: 24339422.
- Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014 Nov 4;5:556. doi: 10.3389/fimmu.2014.00556. PMID: 25414703; PMCID: PMC4220146.
- Bolandi Z, Mokhberian N, Eftekhary M, Sharifi K, Soudi S, Ghanbarian H, Hashemi SM. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4+ T cell. Life Sci. 2020 Oct 15;259:118218. doi: 10.1016/j.lfs.2020.118218. Epub 2020 Aug 9. PMID: 32784057.
- Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016 Dec;8(6):505-517. doi: 10.1093/jmcb/mjw040. Epub 2016 Sep 25. PMID: 27671445.
- Zhou Y, Wang J, Li H, Liang X, Bae J, Huang X, Li Q. Efficacy and Safety of Cell-Assisted Lipotransfer: A Systematic Review and Meta-Analysis. Plast Reconstr Surg. 2016 Jan;137(1):44e-57e. doi: 10.1097/PRS.0000000000001981. PMID: 26710060.
- Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, Parodi PC, Fabris M, Niazi KR, Soon-Shiong P, Curcio F. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018 Sep 6;8(1):13325. doi: 10.1038/s41598-018-31707-9. PMID: 30190615; PMCID: PMC6127134.
- Heo JS, Choi Y, Kim HO. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019 Nov 5;2019:7921760. doi: 10.1155/2019/7921760. PMID: 31781246; PMCID: PMC6875419.
- Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019 Aug 7;10(1):242. doi: 10.1186/s13287-019-1358-y. PMID: 31391108; PMCID: PMC6686455.
- Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. 2022 Feb 17;12(1):2776. doi: 10.1038/s41598-022-06824-1. PMID: 35177768; PMCID: PMC8854709.
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016 Sep 12;6:32993. doi: 10.1038/srep32993. Erratum in: Sci Rep. 2020 Apr 16;10(1):6693. doi: 10.1038/s41598-020-63068-7. PMID: 27615560; PMCID: PMC5018733.
- An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G, Li D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci Rep. 2019 Sep 6;9(1):12861. doi: 10.1038/s41598-019-49339-y. PMID: 31492946; PMCID: PMC6731308.
- Pi L, Yang L, Fang BR, Meng XX, Qian L. Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol Cell Biochem. 2022 Jan;477(1):115-127. doi: 10.1007/s11010-021-04251-w. Epub 2021 Sep 28. PMID: 34581942.
- Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Hu B, Song J, Chen L. Author Correction: Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2021 Feb 1;11(1):3245. doi: 10.1038/s41598-021-82225-0. Erratum for: Sci Rep. 2017 Oct 17;7(1):13321. doi: 10.1038/s41598-017-12919-x. PMID: 33526797; PMCID: PMC7851382.
- Yang C, Luo L, Bai X, Shen K, Liu K, Wang J, Hu D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020 Mar 15;681:108259. doi: 10.1016/j.abb.2020.108259. Epub 2020 Jan 9. PMID: 31926164.
- Cooper DR, Wang C, Patel R, Trujillo A, Patel NA, Prather J, Gould LJ, Wu MH. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv Wound Care (New Rochelle). 2018 Sep 1;7(9):299-308. doi: 10.1089/wound.2017.0775. Epub 2018 Sep 4. PMID: 30263873; PMCID: PMC6158770.
- Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021 Sep;101(9):1254-1266. doi: 10.1038/s41374-021-00611-8. Epub 2021 May 27. PMID: 34045678.
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016 Sep 12;6:32993. doi: 10.1038/srep32993. Erratum in: Sci Rep. 2020 Apr 16;10(1):6693. doi: 10.1038/s41598-020-63068-7. PMID: 27615560; PMCID: PMC5018733.
- Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016 Jun 1;129(11):2182-9. doi: 10.1242/jcs.170373. PMID: 27252357.
- Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, He T, Shen K, Wang Y, Liu J, Zhang W, Wang H, Zheng Z, Hu D. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021 Mar 31;12(1):221. doi: 10.1186/s13287-021-02290-0. Erratum in: Stem Cell Res Ther. 2021 Sep 3;12(1):490. doi: 10.1186/s13287-021-02568-3. PMID: 33789737; PMCID: PMC8010995.
- Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl Med. 2016 Apr;5(4):440-50. doi: 10.5966/sctm.2015-0177. Epub 2016 Mar 1. PMID: 26933040; PMCID: PMC4798737.
- Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018 Oct;27(10):1170-1172. doi: 10.1111/exd.13451. Epub 2017 Nov 10. PMID: 28940813.
- Ma J, Zhang Z, Wang Y, Shen H. Investigation of miR-126-3p loaded on adipose stem cell-derived exosomes for wound healing of full-thickness skin defects. Exp Dermatol. 2022 Mar;31(3):362-374. doi: 10.1111/exd.14480. Epub 2021 Dec 15. PMID: 34694648.
- Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, Pass HI. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013 Sep;8(9):1156-62. doi: 10.1097/JTO.0b013e318299ac32. PMID: 23945385; PMCID: PMC4123222.
- Sun Y, Xiong X, Wang X. The miR-590-3p/VEGFA axis modulates secretion of VEGFA from adipose-derived stem cells, which acts as a paracrine regulator of human dermal microvascular endothelial cell angiogenesis. Hum Cell. 2020 Jul;33(3):479-489. doi: 10.1007/s13577-019-00315-8. Epub 2020 Apr 10. PMID: 32277427.
- Zheng T, Shao W, Tian J. Exosomes derived from ADSCs containing miR-378 promotes wound healing by targeting caspase-3. J Biochem Mol Toxicol. 2021 Oct;35(10):e22881. doi: 10.1002/jbt.22881. Epub 2021 Aug 15. PMID: 34392575.
- Guo B, Hui Q, Xu Z, Chang P, Tao K. miR-495 inhibits the growth of fibroblasts in hypertrophic scars. Aging (Albany NY). 2019 May 14;11(9):2898-2910. doi: 10.18632/aging.101965. PMID: 31085805; PMCID: PMC6535065.
- Xu M, Fang S, Xie A. Posttranscriptional control of PLOD1 in adipose-derived stem cells regulates scar formation through altering macrophage polarization. Ann Transl Med. 2021 Oct;9(20):1573. doi: 10.21037/atm-21-4978. PMID: 34790779; PMCID: PMC8576667.
- Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021 Nov;24(5):758. doi: 10.3892/mmr.2021.12398. Epub 2021 Sep 3. PMID: 34476508; PMCID: PMC8436211.
- Zhu J, Quan H. Adipose-derived stem cells-derived exosomes facilitate cutaneous wound healing by delivering XIST and restoring discoidin domain receptor 2. Cytokine. 2022 Oct;158:155981. doi: 10.1016/j.cyto.2022.155981. Epub 2022 Aug 8. PMID: 35952595.
- Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, Xiao Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021 Jul 7;19(1):202. doi: 10.1186/s12951-021-00942-0. PMID: 34233694; PMCID: PMC8261989.
- Chang CL, Chen HH, Chen KH, Chiang JY, Li YC, Lin HS, Sung PH, Yip HK. Adipose-derived mesenchymal stem cell-derived exosomes markedly protected the brain against sepsis syndrome induced injury in rat. Am J Transl Res. 2019 Jul 15;11(7):3955-3971. PMID: 31396312; PMCID: PMC6684905.
- Pu CM, Liu CW, Liang CJ, Yen YH, Chen SH, Jiang-Shieh YF, Chien CL, Chen YC, Chen YL. Adipose-Derived Stem Cells Protect Skin Flaps against Ischemia/Reperfusion Injury via IL-6 Expression. J Invest Dermatol. 2017 Jun;137(6):1353-1362. doi: 10.1016/j.jid.2016.12.030. Epub 2017 Feb 3. PMID: 28163069.
- Luo Y, Yi X, Liang T, Jiang S, He R, Hu Y, Bai L, Wang C, Wang K, Zhu L. Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model. Stem Cell Res Ther. 2019 Aug 30;10(1):279. doi: 10.1186/s13287-019-1389-4. PMID: 31470890; PMCID: PMC6717360.
- Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018 Feb 26;497(1):305-312. doi: 10.1016/j.bbrc.2018.02.076. Epub 2018 Feb 8. PMID: 29428734.
- Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, Zhao S, Yuan H, Yang X, Shi J, Zhao H. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020 May 1;318(5):C848-C856. doi: 10.1152/ajpcell.00041.2020. Epub 2020 Mar 11. PMID: 32159361.
- Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018 Apr 13;50(4):1-14. doi: 10.1038/s12276-018-0058-5. PMID: 29651102; PMCID: PMC5938041.
- Wang Z, Feng C, Liu H, Meng T, Huang W, Long X, Wang X. Hypoxic Pretreatment of Adipose-Derived Stem Cells Accelerates Diabetic Wound Healing via circ-Gcap14 and HIF-1α/VEGF Mediated Angiopoiesis. Int J Stem Cells. 2021 Nov 30;14(4):447-454. doi: 10.15283/ijsc21050. PMID: 34456191; PMCID: PMC8611313.
- Parvanian S, Yan F, Su D, Coelho-Rato LS, Venu AP, Yang P, Zou X, Jiu Y, Chen H, Eriksson JE, Cheng F. Exosomal vimentin from adipocyte progenitors accelerates wound healing. Cytoskeleton (Hoboken). 2020 Oct;77(10):399-413. doi: 10.1002/cm.21634. Epub 2020 Oct 17. PMID: 32978896.
- Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, He T, Shen K, Wang Y, Liu J, Zhang W, Wang H, Zheng Z, Hu D. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021 Mar 31;12(1):221. doi: 10.1186/s13287-021-02290-0. Erratum in: Stem Cell Res Ther. 2021 Sep 3;12(1):490. doi: 10.1186/s13287-021-02568-3. PMID: 33789737; PMCID: PMC8010995.
- Cao G, Chen B, Zhang X, Chen H. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomal microRNA-19b Promotes the Healing of Skin Wounds Through Modulation of the CCL1/TGF-β Signaling Axis. Clin Cosmet Investig Dermatol. 2020 Dec 15;13:957-971. doi: 10.2147/CCID.S274370. PMID: 33364805; PMCID: PMC7751444.
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008 Sep-Oct;16(5):585-601. doi: 10.1111/j.1524-475X.2008.00410.x. PMID: 19128254.121. Blanpain C, Fuchs E. Epidermal Stem Cells of the Skin. Annu Rev Cell Dev Biol. 2006 Nov 1;22(1):339–73.
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339-73. doi: 10.1146/annurev.cellbio.22.010305.104357. PMID: 16824012; PMCID: PMC2405915.
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007 Feb 9;128(3):445-58. doi: 10.1016/j.cell.2007.01.014. PMID: 17289566; PMCID: PMC2408375.
- Rochette L, Mazini L, Meloux A, Zeller M, Cottin Y, Vergely C, Malka G. Anti-Aging Effects of GDF11 on Skin. Int J Mol Sci. 2020 Apr 9;21(7):2598. doi: 10.3390/ijms21072598. PMID: 32283613; PMCID: PMC7177281.
- Bachmann S, Jennewein M, Bubel M, Guthörl S, Pohlemann T, Oberringer M. Interacting adipose-derived stem cells and microvascular endothelial cells provide a beneficial milieu for soft tissue healing. Mol Biol Rep. 2020 Jan;47(1):111-122. doi: 10.1007/s11033-019-05112-y. Epub 2019 Oct 3. PMID: 31583562.
- Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, He T, Shen K, Wang Y, Liu J, Zhang W, Wang H, Zheng Z, Hu D. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021 Mar 31;12(1):221. doi: 10.1186/s13287-021-02290-0. Erratum in: Stem Cell Res Ther. 2021 Sep 3;12(1):490. doi: 10.1186/s13287-021-02568-3. PMID: 33789737; PMCID: PMC8010995.
- Lu Y, Wen H, Huang J, Liao P, Liao H, Tu J, Zeng Y. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020 Sep;24(17):9590-9604. doi: 10.1111/jcmm.15387. Epub 2020 Jul 14. PMID: 32666704; PMCID: PMC7520275.
- Song Y, You Y, Xu X, Lu J, Huang X, Zhang J, Zhu L, Hu J, Wu X, Xu X, Tan W, Du Y. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Biopotentiated Extracellular Matrix Hydrogels Accelerate Diabetic Wound Healing and Skin Regeneration. Adv Sci (Weinh). 2023 Oct;10(30):e2304023. doi: 10.1002/advs.202304023. Epub 2023 Sep 15. PMID: 37712174; PMCID: PMC10602544.
- Xie J, Wu W, Zheng L, Lin X, Tai Y, Wang Y, Wang L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front Pharmacol. 2022 Feb 28;13:828627. doi: 10.3389/fphar.2022.828627. PMID: 35295323; PMCID: PMC8919367.
- Pasternak B, Schepull T, Eliasson P, Aspenberg P. Elevation of systemic matrix metalloproteinases 2 and 7 and tissue inhibitor of metalloproteinase 2 in patients with a history of Achilles tendon rupture: pilot study. Br J Sports Med. 2010 Jul;44(9):669-72. doi: 10.1136/bjsm.2008.049411. Epub 2008 Jul 15. PMID: 18628360.130.
- Huayllani MT, Sarabia-Estrada R, Restrepo DJ, Boczar D, Sisti A, Nguyen JH, Rinker BD, Moran SL, Quiñones-Hinojosa A, Forte AJ. Adipose-derived stem cells in wound healing of full-thickness skin defects: a review of the literature. J Plast Surg Hand Surg. 2020 Oct;54(5):263-279. doi: 10.1080/2000656X.2020.1767116. Epub 2020 May 19. PMID: 32427016.
- Qiu H, Liu S, Wu K, Zhao R, Cao L, Wang H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J Cosmet Dermatol. 2020 Mar;19(3):574-581. doi: 10.1111/jocd.13215. Epub 2019 Nov 21. PMID: 31755172.
- Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ, Nawaz M, Szafert S, Mahmoodi M, Jabbari E, Asghari R, Rezaie J. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020 May 15;249:117447. doi: 10.1016/j.lfs.2020.117447. Epub 2020 Feb 19. PMID: 32087234.
- Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, Yamato H, Yotsumoto K, Tanaka U, Taketomi T, Uchiumi T, Le AD, Shi S, Nishimura F. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021 Mar 1;122:306-324. doi: 10.1016/j.actbio.2020.12.046. Epub 2020 Dec 24. Erratum in: Acta Biomater. 2025 Jan 1;191:428-429. doi: 10.1016/j.actbio.2024.11.029. PMID: 33359765; PMCID: PMC7897289.
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011 Apr;29(4):341-5. doi: 10.1038/nbt.1807. Epub 2011 Mar 20. PMID: 21423189.
- Du J, Wan Z, Wang C, Lu F, Wei M, Wang D, Hao Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021 Jul 13;11(17):8185-8196. doi: 10.7150/thno.59121. PMID: 34373736; PMCID: PMC8344009.
- Wang X, Nakamoto T, Dulińska-Molak I, Kawazoe N, Chen G. Enzymatically cross-linked hydrogels based on a linear poly(ethylene glycol) analogue for controlled protein release and 3D cell culture. J Mater Chem B. 2016;4(1):37-45.
- Leijten J, Seo J, Yue K, Santiago GT, Tamayol A, Ruiz-Esparza GU, Shin SR, Sharifi R, Noshadi I, Álvarez MM, Zhang YS, Khademhosseini A. Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater Sci Eng R Rep. 2017 Sep;119:1-35. doi: 10.1016/j.mser.2017.07.001. Epub 2017 Jul 25. PMID: 29200661; PMCID: PMC5708586.
- Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M. Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohydr Polym. 2016 Aug 20;147:379-391. doi: 10.1016/j.carbpol.2016.04.024. Epub 2016 Apr 9. PMID: 27178944.
- Ashworth C. DNA nanotechnology: Building big with DNA bricks. Nat Rev Mater. 2018 Jan 3;3(1):17092.
- Sun W. DNA nanotechnology: DNA robots that sort cargoes. Nat Nanotechnol. 2017 Dec;12(12):1120. doi: 10.1038/nnano.2017.236. Epub 2017 Dec 6. PMID: 29209013.
- Li F, Lyu D, Liu S, Guo W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv Mater. 2020 Jan;32(3):e1806538. doi: 10.1002/adma.201806538. Epub 2019 Aug 5. PMID: 31379017.
- Jung S, Abel JH, Starger JL, Yi H. Porosity-Tuned Chitosan-Polyacrylamide Hydrogel Microspheres for Improved Protein Conjugation. Biomacromolecules. 2016 Jul 11;17(7):2427-36. doi: 10.1021/acs.biomac.6b00582. Epub 2016 Jun 28. PMID: 27351270.
- Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013 Jan;2(1):57-71. doi: 10.1002/adhm.201200197. Epub 2012 Sep 3. PMID: 23184739.
- Li F, Tang J, Geng J, Luo D, Yang D. Polymeric DNA hydrogel: Design, synthesis and applications. Progress in Polymer Science. 2019;98:101163. doi: 10.1016/j.progpolymsci.2019.101163.
- Wang Z, Li W, Gou L, Zhou Y, Peng G, Zhang J, Liu J, Li R, Ni H, Zhang W, Cao T, Cao Q, Su H, Han YP, Tong N, Fu X, Ilegems E, Lu Y, Berggren PO, Zheng X, Wang C. Biodegradable and Antioxidant DNA Hydrogel as a Cytokine Delivery System for Diabetic Wound Healing. Adv Healthc Mater. 2022 Nov;11(21):e2200782. doi: 10.1002/adhm.202200782. Epub 2022 Sep 27. PMID: 36101484.
- Mao X, Chen G, Wang Z, Zhang Y, Zhu X, Li G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem Sci. 2017 Nov 22;9(4):811-818. doi: 10.1039/c7sc03716c. PMID: 29629148; PMCID: PMC5873223.
- Wang H, Wang X, Lai K, Yan J. Stimulus-Responsive DNA Hydrogel Biosensors for Food Safety Detection. Biosensors (Basel). 2023 Feb 24;13(3):320. doi: 10.3390/bios13030320. PMID: 36979532; PMCID: PMC10046603.
- Li C, Faulkner-Jones A, Dun AR, Jin J, Chen P, Xing Y, Yang Z, Li Z, Shu W, Liu D, Duncan RR. Rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew Chem Int Ed Engl. 2015 Mar 23;54(13):3957-61. doi: 10.1002/anie.201411383. Epub 2015 Feb 5. PMID: 25656851.
- Liu S, Liu Y, Zhou L, Li C, Zhang M, Zhang F, Zhentao Ding, Yongqiang Wen, Peixun Zhang. XT-type DNA hydrogels loaded with VEGF and NGF promote peripheral nerve regeneration via a biphasic release profile. Biomater Sci. 2021 Dec 7;9(24):8221–34. 10.1039/D1BM01377G.
- Mo F, Jiang K, Zhao D, Wang Y, Song J, Tan W. DNA hydrogel-based gene editing and drug delivery systems. Adv Drug Deliv Rev. 2021 Jan;168:79-98. doi: 10.1016/j.addr.2020.07.018. Epub 2020 Jul 23. PMID: 32712197.
- Lu S, Wang S, Zhao J, Sun J, Yang X. A pH-controlled bidirectionally pure DNA hydrogel: reversible self-assembly and fluorescence monitoring. Chem Commun. 2018;54(36):4621-4. doi: 10.1039/C8CC01603H.
- Huang X, Li J, Luo J, Gao Q, Mao A, Li J. Research progress on double-network hydrogels. Materials Today Communications. 2021 Dec;29:102757. doi:10.1016/j.mtcomm.2021.102757.
- Sun Y, Liu J, Wang H, Li S, Pan X, Xu B, Hailong Yang, Qingyuan Wu, Wenxuan Li, Xin Su, Zhijun Huang, Xindong Guo, Huiyu Liu. NIR laser‐triggered microneedle‐based liquid band‐aid for wound care. Adv Funct Materials. 2021;(29):2100218. doi: 10.1002/adfm.202100218.
- Zhang Q, Shi L, He H, Liu X, Huang Y, Xu D, Yao M, Zhang N, Guo Y, Lu Y, Li H, Zhou J, Tan J, Xing M, Luo G. Down-Regulating Scar Formation by Microneedles Directly via a Mechanical Communication Pathway. ACS Nano. 2022 Jul 26;16(7):10163-10178. doi: 10.1021/acsnano.1c11016. Epub 2022 May 26. Erratum in: ACS Nano. 2023 Jun 13;17(11):11070-11071. doi: 10.1021/acsnano.3c03389. PMID: 35617518; PMCID: PMC9331171.
- Yu Y, Li P, Zhu C, Ning N, Zhang S, Vancso GJ. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv Funct Materials.;29(35):1904402. doi: 10.1002/adfm.201904402.
- Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018 Oct 28;435:80-91. doi: 10.1016/j.canlet.2018.08.001. Epub 2018 Aug 8. Erratum in: Cancer Lett. 2023 Aug 1;568:216292. doi: 10.1016/j.canlet.2023.216292. PMID: 30098399.
- Liu A, Wang Q, Zhao Z, Wu R, Wang M, Li J, Sun K, Sun Z, Lv Z, Xu J, Jiang H, Wan M, Shi D, Mao C. Nitric Oxide Nanomotor Driving Exosomes-Loaded Microneedles for Achilles Tendinopathy Healing. ACS Nano. 2021 Aug 24;15(8):13339-13350. doi: 10.1021/acsnano.1c03177. Epub 2021 Jul 29. PMID: 34324304.
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005 Sep;115(9):2393-401. doi: 10.1172/JCI25437. Epub 2005 Aug 18. Erratum in: J Clin Invest. 2010 Jan;120(1):394. PMID: 16110328; PMCID: PMC1187934.
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010 Apr;126(1):56-68. doi: 10.1016/j.pharmthera.2010.01.002. Epub 2010 Feb 1. PMID: 20117131; PMCID: PMC2839017.
- Grennan D. Diabetic Foot Ulcers. JAMA. 2019 Jan 1;321(1):114. doi: 10.1001/jama.2018.18323. PMID: 30620372.
- Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, Xu T, Zhang X, Lin C, Gao W, Guo Y, Lei B. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano. 2019 Sep 24;13(9):10279-10293. doi: 10.1021/acsnano.9b03656. Epub 2019 Sep 9. PMID: 31483606.
- Zhou L, Pi W, Cheng S, Gu Z, Zhang K, Min T, et al. Multifunctional DNA Hydrogels with Hydrocolloid‐Cotton Structure for Regeneration of Diabetic Infectious Wounds. Adv Funct Materials. 2021 Nov;31(48):2106167. dOI: 10.1002/adfm.202106167.
- Zhao P, Feng Y, Zhou Y, Tan C, Liu M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact Mater. 2022 Jun 14;20:355-367. doi: 10.1016/j.bioactmat.2022.05.035. Erratum in: Bioact Mater. 2024 Jun 14;40:275-279. doi: 10.1016/j.bioactmat.2024.06.006. PMID: 35784635; PMCID: PMC9207301.
- Yang X, Ding C, Wu M, Xu X, Ke X, Xu H, et al. Biomineral interface with superior cell adhesive and antibacterial properties based on enzyme-triggered digestion of saliva acquired pellicle-inspired polypeptide coatings. Chemical Engineering Journal. 2021 Jul;415:128955. doi: 10.1016/j.cej.2021.128955.
- Cheng L, Cai Z, Ye T, Yu X, Chen Z, Yan Y, Yufei Yan, Jin Qi, Lei Wang, Zhihong Liu, Wenguo Cui, Lianfu Deng. Injectable polypeptide‐protein hydrogels for promoting infected wound healing. Adv Funct Materials. 2020;30(25):2001196. doi: 10.1002/adfm.202001196.
- Gao J, Dong X, Wang Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods. 2020 May 1;177:114-125. doi: 10.1016/j.ymeth.2019.11.012. Epub 2019 Nov 30. PMID: 31790730; PMCID: PMC7198327.
- Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020 Dec;27(1):585-598. doi: 10.1080/10717544.2020.1748758. PMID: 32264719; PMCID: PMC7178886.
- Liu Y, Wang Y, Lv Q, Li X. Exosomes: From garbage bins to translational medicine. Int J Pharm. 2020 Jun 15;583:119333. doi: 10.1016/j.ijpharm.2020.119333. Epub 2020 Apr 26. PMID: 32348800.
- Man K, Brunet MY, Jones MC, Cox SC. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials (Basel). 2020 Sep 15;10(9):1838. doi: 10.3390/nano10091838. PMID: 32942556; PMCID: PMC7558114.
- Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015 Jun 10;207:18-30. doi: 10.1016/j.jconrel.2015.03.033. Epub 2015 Mar 31. Erratum in: J Control Release. 2021 Nov 10;339:232-234. doi: 10.1016/j.jconrel.2021.09.027. PMID: 25836593; PMCID: PMC4430381.
- Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020 Sep 22;15:6917-6934. doi: 10.2147/IJN.S264498. PMID: 33061359; PMCID: PMC7519827.
- Hajipour H, Farzadi L, Roshangar L, Latifi Z, Kahroba H, Shahnazi V, Hamdi K, Ghasemzadeh A, Fattahi A, Nouri M. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 2021 Jun 15;275:119351. doi: 10.1016/j.lfs.2021.119351. Epub 2021 Mar 15. PMID: 33737084.
- Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021 Jan 10;329:894-906. doi: 10.1016/j.jconrel.2020.10.020. Epub 2020 Oct 12. PMID: 33058934.
- Guo P, Busatto S, Huang J, Morad G, Moses MA. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv Funct Mater. 2021 Oct 26;31(44):2008326. doi: 10.1002/adfm.202008326. Epub 2021 Jan 20. PMID: 34924915; PMCID: PMC8680268.
- Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015 May 10;205:35-44. doi: 10.1016/j.jconrel.2014.11.029. Epub 2014 Dec 4. PMID: 25483424.
- Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017 Mar;16(3):181-202. doi: 10.1038/nrd.2016.199. Epub 2016 Nov 3. Erratum in: Nat Rev Drug Discov. 2017 Jun;16(6):440. doi: 10.1038/nrd.2017.86. PMID: 27807347; PMCID: PMC5700751.
- Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjić N. 2'-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998 Aug 7;273(32):20556-67. doi: 10.1074/jbc.273.32.20556. PMID: 9685413.
- Wang T, Yin W, AlShamaileh H, Zhang Y, Tran PH, Nguyen TN, Li Y, Chen K, Sun M, Hou Y, Zhang W, Zhao Q, Chen C, Zhang PZ, Duan W. A Detailed Protein-SELEX Protocol Allowing Visual Assessments of Individual Steps for a High Success Rate. Hum Gene Ther Methods. 2019 Feb;30(1):1-16. doi: 10.1089/hgtb.2018.237. PMID: 30700146.
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990 Aug 3;249(4968):505-10. doi: 10.1126/science.2200121. PMID: 2200121.
- Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018 Oct 16;16(1):81. doi: 10.1186/s12951-018-0403-9. PMID: 30326899; PMCID: PMC6190562.
- Gefen T, Castro I, Muharemagic D, Puplampu-Dove Y, Patel S, Gilboa E. A TIM-3 Oligonucleotide Aptamer Enhances T Cell Functions and Potentiates Tumor Immunity in Mice. Mol Ther. 2017 Oct 4;25(10):2280-2288. doi: 10.1016/j.ymthe.2017.06.023. Epub 2017 Aug 8. PMID: 28800954; PMCID: PMC5628791.
- Rahimizadeh K, AlShamaileh H, Fratini M, Chakravarthy M, Stephen M, Shigdar S, Veedu RN. Development of Cell-Specific Aptamers: Recent Advances and Insight into the Selection Procedures. Molecules. 2017 Nov 27;22(12):2070. doi: 10.3390/molecules22122070. PMID: 29186905; PMCID: PMC6149766.
- Zhang D, Wu T, Qin X, Qiao Q, Shang L, Song Q, Yang C, Zhang Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019 Sep 11;19(9):6635-6646. doi: 10.1021/acs.nanolett.9b02903. Epub 2019 Aug 12. PMID: 31393134.
- Alhasan AH, Patel PC, Choi CH, Mirkin CA. Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small. 2014 Jan 15;10(1):186-92. doi: 10.1002/smll.201302143. Epub 2013 Sep 17. PMID: 24106176; PMCID: PMC3947239.
- Sancho-Albero M, Encabo-Berzosa M del M, Beltrán-Visiedo M, Fernández-Messina L, Sebastián V, Sánchez-Madrid F, Manuel Arruebo, Jesús Santamaría, Pilar Martín-Duque. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11(40):18825-36. doi: 10.1039/C9NR06183E.
- Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019 Aug 23;10(1):3838. doi: 10.1038/s41467-019-11718-4. PMID: 31444335; PMCID: PMC6707218.
- Han J, Zhang L, Cui M, Su Y, He Y. Rapid and Accurate Detection of Lymph Node Metastases Enabled through Fluorescent Silicon Nanoparticles-Based Exosome Probes. Anal Chem. 2021 Jul 27;93(29):10122-10131. doi: 10.1021/acs.analchem.1c01010. Epub 2021 Jul 13. PMID: 34255475.
- Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine: Nanotechnology, Biology and Medicine. 2018 Oct 1;14(7):1973–85.
- Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, Hu C, Zhang L, Guo H, Gao S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018 Oct;14(7):1973-1985. doi: 10.1016/j.nano.2018.05.020. Epub 2018 Jun 20. PMID: 29935333.
- Van Deun J, Roux Q, Deville S, Van Acker T, Rappu P, Miinalainen I, Heino J, Vanhaecke F, De Geest BG, De Wever O, Hendrix A. Feasibility of Mechanical Extrusion to Coat Nanoparticles with Extracellular Vesicle Membranes. Cells. 2020 Jul 29;9(8):1797. doi: 10.3390/cells9081797. PMID: 32751082; PMCID: PMC7464356.
- Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, Hyeon T, Han IB, Kim BS. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018 Aug 8;18(8):4965-4975. doi: 10.1021/acs.nanolett.8b01816. Epub 2018 Jul 13. PMID: 29995418.
- Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019 Aug;94:482-494. doi: 10.1016/j.actbio.2019.05.054. Epub 2019 May 24. PMID: 31129363.
- Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, Shiku H, Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016 Feb 25;6:21933. doi: 10.1038/srep21933. PMID: 26911358; PMCID: PMC4766490.
- Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014 Mar 1;448:41-9. doi: 10.1016/j.ab.2013.12.001. Epub 2013 Dec 9. PMID: 24333249; PMCID: PMC3954633.
- Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano. 2018 Jul 24;12(7):6830-6842. doi: 10.1021/acsnano.8b02053. Epub 2018 Jul 10. PMID: 29975503.
- Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv Sci (Weinh). 2018 Jan 30;5(4):1700611. doi: 10.1002/advs.201700611. PMID: 29721412; PMCID: PMC5908366.a
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.