Medicine Group 2025 January 13;6(1):025-030. doi: 10.37871/jbres2054.
Hemodynamic Optimization with Acumen Assisted Fluid Management (AFM): The Importance of Flow
Martinez Lydia, Ana Perez Carbonell*, Garcia-Romero J, Munoz JL and Monserrat J
Background
Anesthesiology has been one of the disciplines that has most successfully integrated AI-based technologies, allowing for personalization and precision in patient management, especially, during surgical procedures.
The ability to analyze large volumes of data in real time, together with the automation of clinical decisions has optimized monitoring and identification of pathological states.
Within this context, intraoperative fluid therapy is a critical area in our field, where AI has proven its usefulness.
As we already know, the objective of intraoperative fluid therapy is to provide fluids, to increase the patient's Stroke Volume (SV) and, therefore, Cardiac Output (CO). However, its administration must be careful, since a positive fluid balance due to excessive administration, is associated with increased morbidity and mortality. In the same way, hypovolemia or preload-dependency poses a risk of hypoperfusion. Therefore, predicting how our patient will respond to volume administration is crucial to adequately perform this resuscitation and hemodynamic management with fluids.
The Assisted Fluid Management (AFM) software includes an AI and machine learning algorithm that integrates patient hemodynamic variables and individually assesses preload-dependency continuously during the procedure. This algorithm learns from the patient and predicts the response to fluids based on the previous response and the current hemodynamic situation, supporting and facilitating decision-making.
Given the frequent and continuous changes in cardiac function during an intervention, this software allows for an “individualized hemodynamic management.”
We present now the next case report, in which the AFM algorithm was used together with a fluid meter, for the administration of goal-directed fluid therapy during a surgical procedure.
Case Report
This is a 55-year-old, 90kg male with no relevant medical history, diagnosed with a sigmoid cancer, who underwent laparoscopic sigmoidectomy using robotic surgery (Hugo). We did an intravenous anesthetic induction and a TAP block as regional analgesia. Advanced hemodynamic monitoring with minimally invasive cardiac output monitoring was used, supported by the HPI (Hypotension Prediction Index) and AFM systems.
After performing the pneumoperitoneum, the patient presented an increase in Blood Pressure (BP) together with an increase in Systemic Vascular Resistance (SVR) that required adjustment of the patient's analgesia with intravenous remifentanil infusion and occasional urapidil boluses to control it.
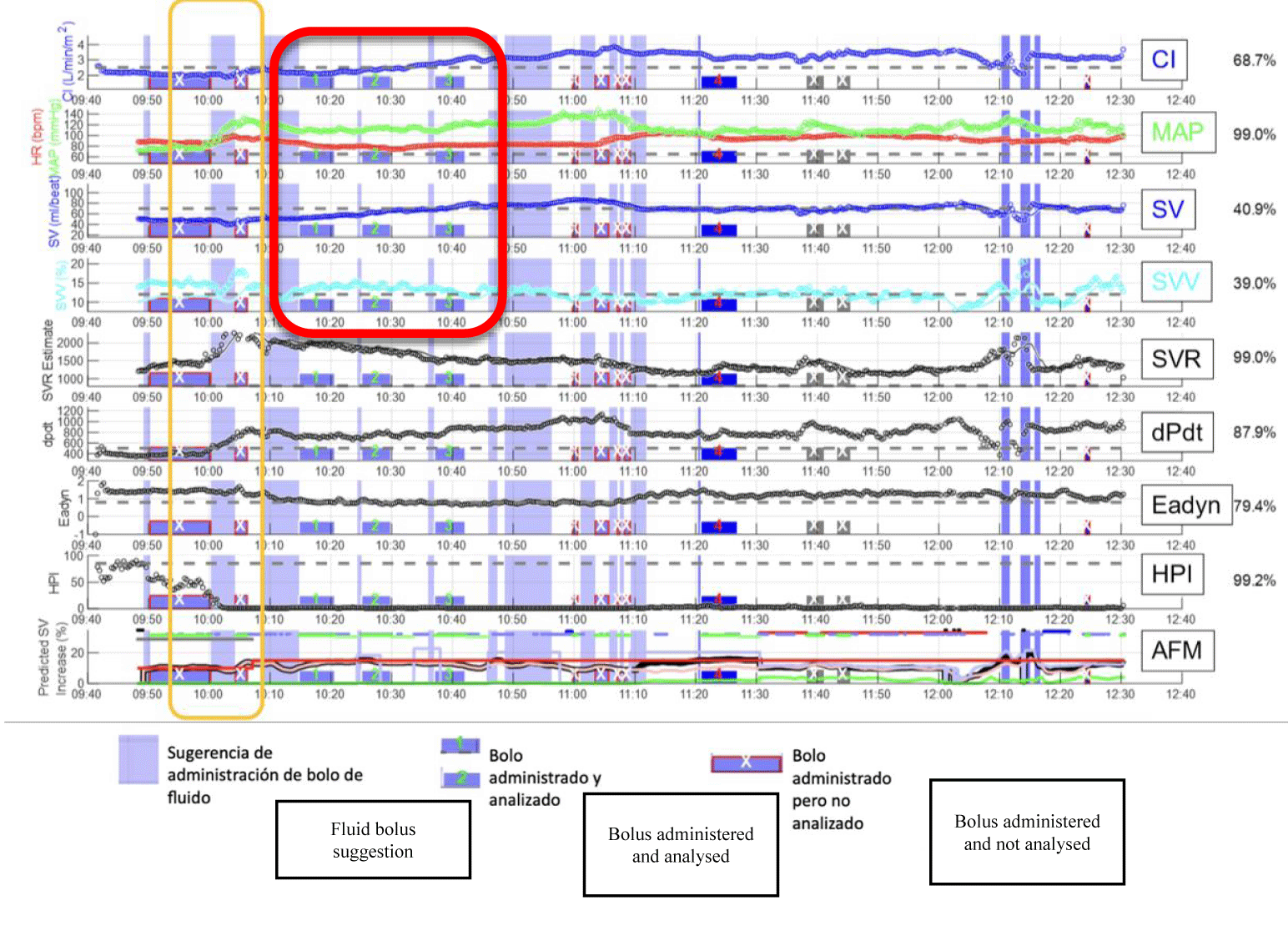
Although the patient maintains normotension during all the procedure (MAP > 65 mmHg 99.0%), AFM recommends administering fluids bolus. The bolus was accepted and turns out positive for the patient, with an increase in SV > 15% and Cardiac Index (CI) from 2.1 to 3.2 l/min/m2 after the administration of 3 fluid boluses (Figure 1).
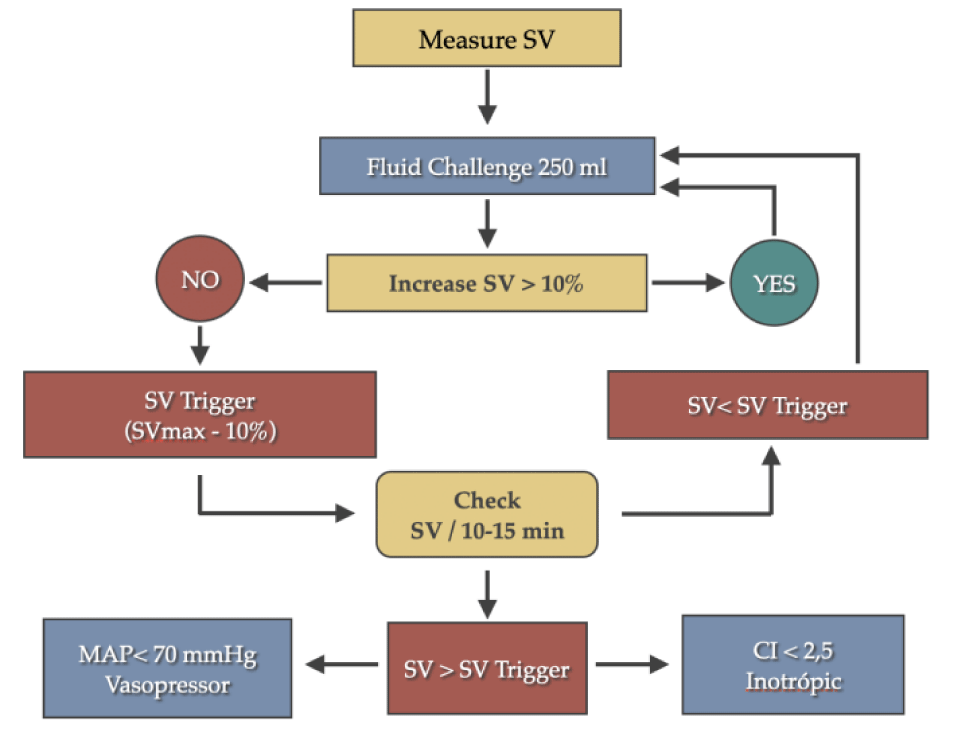
During the rest of the surgery, we kept hemodynamic optimization of the patient, achieving all the targets of the main hemodynamic parameters, as included in our protocol of Goal Directed Fluid Therapy (GDFT) [1] (Figure 2): MAP > 70 mmHg, CI (> 2.5 l/min/m2), maintaining a SVV < 12% (preload-independence zone).
The surgery lasted 2 hours and 50 minutes, where a total of 1200 milliliters of crystalloids were administered.
Results
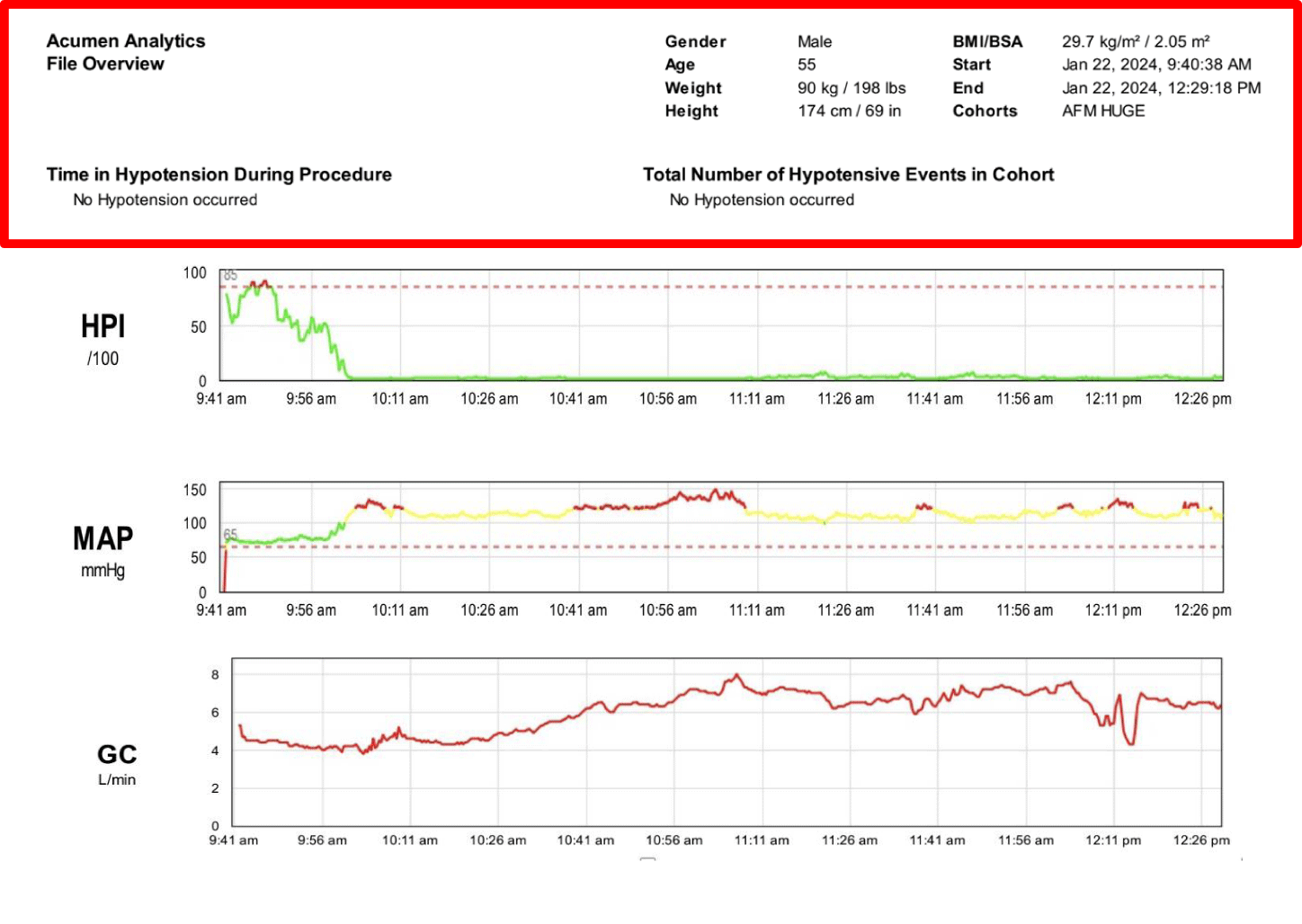
After analyzing the case data using the Acumen Analytics system we observed that the patient had a total of 0 minutes of hypotension, and the administration of fluids following the AFM algorithm also allowed to optimize CI values after fluid administration (Figure 3).
Discussion
The impact of hypotension in surgical patients has been studied for years. The development of intraoperative hypotension is detrimental to the patient as it is associated with adverse outcomes that we know, such as, acute kidney injury, myocardial injury, stroke, and even increased mortality. The chances of an adverse outcome increase with the duration and/or magnitude of hypotension and even short periods of hypotension appear to be associated with adverse outcomes. These outcomes occur due to the phenomenon of hypoperfusion.
Therefore, if our patient has normal blood pressure, does that mean that are managing the patient's hemodynamics the best as possible and can ensure that hypoperfusion is not occurring?
Although many cohort studies have suggested an association between perioperative hypotension and poor outcome [3]. Randomized Controlled Trials (RCTs) have failed to demonstrate improved outcomes with higher blood pressure maintenance.
A specific value of arterial pressure is the result of CO (ventricular function) and the arterial system regulation (arterial load/SVR). We can observe that combinations of different CO and SVR changes represent different physiological states underlying a blood pressure value, but also, pathological states. Thus, with a normal BP value, this may be due to an increase in arterial load, to compensate for a drop in CO; this decrease in flow, together with the increase in SVR, may compromise tissue perfusion and therefore cause organic damage [4].
Avoiding intraoperative hypotension is crucial to reduce its possible adverse effects, but hypotension does not always cause hypoperfusion, we must also understand that a normotensive patient may not have adequate CO and perfusion, as occurs in certain states of shock. CO is not only a determinant of BP but also associates with organ perfusion, viewing organ perfusion as a percentage share of CO [5]. Thus we highlight the importance of monitoring and optimizing CO.
Preload-dependence identifies a patient who can improve their CO (flow) with fluid administration if they have an adequate vascular tone. If a patient is preload-dependent, will benefit from fluid administration if it is beneficial for the patient to increase CO. In our case, the CO was low (CI 2.1), and fluids increase SV > 10% and CO > 2.5 l/min/m2 will lead the arterial system to regulate itself, normalizing the SVR values and thus optimizing organic perfusion.
There is evidence about the importance of maintaining CO in mayor abdominal surgery [6] and AFM helps clinicians to optimize the amount and timing of fluid administration and in addition the recommendations made by the software obtain better responses [7] (greater absolute SV increase) therefore AFM is a very useful tool that allows for personalized fluid administration avoiding fluid boluses with little or no response allowing the patient to be kept in the preload-independence zone with less fluid administration
Joosten's study [8] is a prospective randomized controlled trial that compares AI-assisted or manual hemodynamic management, and it is shown that individualized AI-assisted management reduces intraoperative hypotension, compared to a manually directed approach, as well as, maintaining the patient in the preload-independence zone for a significant longer time [9] thus being superior to current goal-guided fluid therapy protocols.
Maheshwari showed the greater effectiveness of this AFM software in fluid administration, 66% of the fluid administered by boluses recommended by the software, resulted in the desired increases in vs., (95% CI, 62 to 70%), compared to the reference rate of 30%, when the AFM software is not used7. However, such a high percentage reported by Joosten of time in the preload-independence zone was not achieved. This was due in most cases to a lack of adherence by physicians to AFM indications and the main reason for this lack of adherence was that the patient presented normal BP values when AFM recommended administering a fluid bolus, when AFM is an algorithm to optimize CO and flow, not BP.
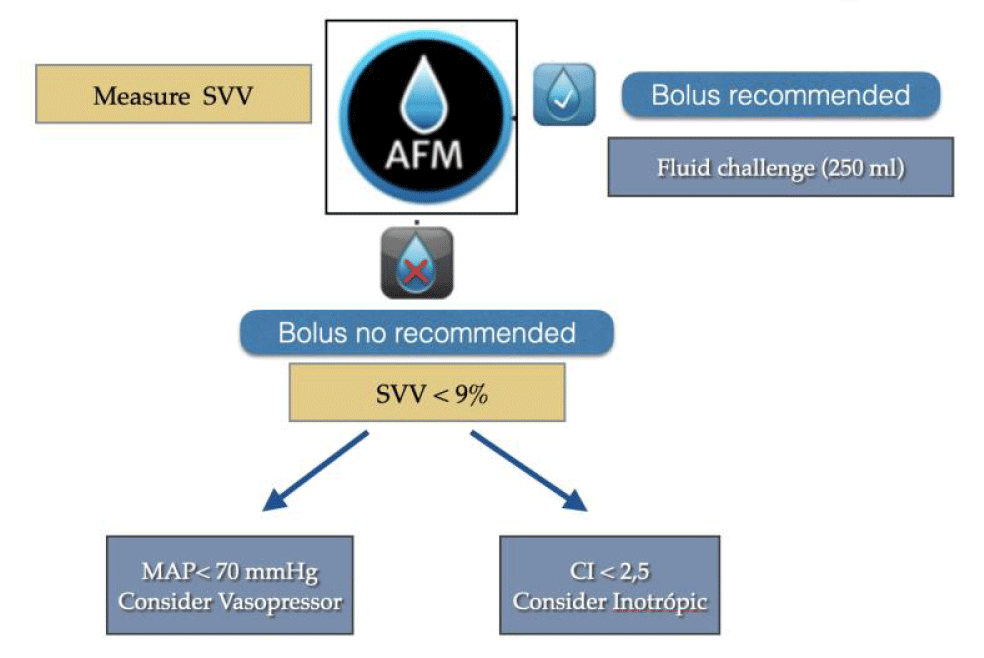
Therefore, in a hemodynamic protocol, AFM is placed in the optimization of preload and, as can be seen in figure 4. In our algorithm, manual DGFT is replaced by AFM.
Some of the limitations of this software, we mainly highlight those that limit the use of SVV, such as atrial fibrillation, spontaneous breathing or procedures with the open thorax. Others would also be the use of this type of systems in patients with severe valvular injuries.
Learning Points
The AFM software, which combines the identification of the preload-dependence state with the patient's hemodynamic response to fluid administration, helps to discriminate between hemodynamic situations in which the patient responds to fluids and hemodynamic situations in which does not respond.
AFM is a preload (SV) optimization algorithm and assesses current response to fluids and make a prediction of the patient`s response to a bolus and should be considered a tool to optimize flow (CO) and oxygen delivery.
References
- Resalt-Pereira M, Muñoz JL, Miranda E, Cuquerella V, Pérez A. Goal-directed fluid therapy on laparoscopic colorectal surgery within enhanced recovery after surgery program. Rev Esp Anestesiol Reanim (Engl Ed). 2019 May;66(5):259-266. English, Spanish. doi: 10.1016/j.redar.2019.01.007. Epub 2019 Mar 10. PMID: 30862401.
- Wijnberge M, Schenk J, Bulle E, Vlaar AP, Maheshwari K, Hollmann MW, Binnekade JM, Geerts BF, Veelo DP. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open. 2021 Jan 8;5(1):zraa018. doi: 10.1093/bjsopen/zraa018. PMID: 33609377; PMCID: PMC7893468.
- Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology. 2017 Jan;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PMID: 27792044.
- Meng L. Heterogeneous impact of hypotension on organ perfusion and outcomes: a narrative review. Br J Anaesth. 2021 Dec;127(6):845-861. doi: 10.1016/j.bja.2021.06.048. Epub 2021 Aug 12. PMID: 34392972; PMCID: PMC8978210.
- Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015 Nov;123(5):1198-208. doi: 10.1097/ALN.0000000000000872. PMID: 26402848.
- Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, Hinds C, Rowan K; OPTIMISE Study Group. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014 Jun 4;311(21):2181-90. doi: 10.1001/jama.2014.5305. Erratum in: JAMA. 2014 Oct 8;312(14):1473. PMID: 24842135.
- Maheshwari K, Malhotra G, Bao X, Lahsaei P, Hand WR, Fleming NW, Ramsingh D, Treggiari MM, Sessler DI, Miller TE; Assisted Fluid Management Study Team. Assisted Fluid Management Software Guidance for Intraoperative Fluid Administration. Anesthesiology. 2021 Aug 1;135(2):273-283. doi: 10.1097/ALN.0000000000003790. PMID: 33901281.
- Joosten A, Rinehart J, Van der Linden P, Alexander B, Penna C, De Montblanc J, Cannesson M, Vincent JL, Vicaut E, Duranteau J. Computer-assisted Individualized Hemodynamic Management Reduces Intraoperative Hypotension in Intermediate- and High-risk Surgery: A Randomized Controlled Trial. Anesthesiology. 2021 Aug 1;135(2):258-272. doi: 10.1097/ALN.0000000000003807. PMID: 33951140; PMCID: PMC8277754.
- Joosten A, Hafiane R, Pustetto M, Van Obbergh L, Quackels T, Buggenhout A, Vincent JL, Ickx B, Rinehart J. Practical impact of a decision support for goal-directed fluid therapy on protocol adherence: a clinical implementation study in patients undergoing major abdominal surgery. J Clin Monit Comput. 2019 Feb;33(1):15-24. doi: 10.1007/s10877-018-0156-x. Epub 2018 May 19. PMID: 29779129.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.