Medicine Group. 2024 July 10;5(7):743-756. doi: 10.37871/jbres1950.
Blast Lung Injury in Rats: Consequences on Alveolar Macrophage Function
Yalda Hadizamani1,2#, Ueli Moehrlen1,3#, Hanno Huwer4#, Uz Stammberger1,5, Ulrich C Liener6, Florian Gebhard7, Lia Bally8, Albrecht Wendel9, Rudolf Lucas10-12 and Jürg Hamacher1,2,9,13,14*
2Pneumology, Clinic for General Internal Medicine, Lindenhofspital Bern, 3012 Bern, Switzerland
3Pediatric Surgery, University Children’s Hospital Zurich, 8032 Zurich, Switzerland
4Department of Cardiothoracic Surgery, Völklingen Heart Centre, Völklingen, Germany
5STM ClinMedRes Consulting, 4056 Basel, Switzerland
6Department of Trauma, Hand, Plastic and Reconstructive Surgery, University of Ulm-Surgical Centre, Steinhövelstraße 9, 89075 Ulm, Germany
7Department of Traumatology, Hand-, Plastic-, and Reconstructive Surgery, Centre of Surgery, Univer-sity of Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany
8Department of Diabetes, Endocrinology, Clinical Nutrition and Metabolism Inselspital, Bern University Hospital and University of Bern, 3010 Bern, Switzerland
9Biochemical Pharmacology, Department of Biology, University of Konstanz, D-78457 Konstanz, Ger-many
10Vascular Biology Center, Augusta University, Augusta, GA, United States
11Department of Pharmacology and Toxicology, Augusta University, Augusta, GA, United States
12Department of Medicine, Medical College of Georgia, Augusta University, Augusta, GA, United States
13Medical Clinic V - Pneumology, Allergology, Intensive Care Medicine, and Environmental Medicine, Faculty of Medicine, Saarland University, University Medical Centre of the Saarland, D-66421 Hom-burg, Germany
14Institute for Clinical & Experimental Surgery, Faculty of Medicine, Saarland University, D-66421 Homburg, Germany
#Authors are contributed equally
- Alveolar Macrophages (AMs)
- Primary blast lung injury
- Acute Lung Injury (ALI)
- Phagocytosis
- Respiratory burst
- Inflammation
- Tissue repair
Abstract
Background: Despite research into the mechanisms of injury in blast-induced thoracic trauma using animal or clinical models, some questions regarding the exact nature of such inju-ries have not been fully addressed. The present study aims to investigate the temporal altera-tions in the quantity and functions of Alveolar Macrophages (AMs) associated with intrapulmo-nary haemorrhage in a prospective rodent model of primary blast lung injury.
Methods: Female Wister rats were randomly divided into two experimental groups: 1. the blast group, which re-ceived a single standardised blast wave; 2. the control group, which received no trauma but un-derwent the same procedure as the experimental group. AMs were harvested, cultured, and stimulated. The number of AMs was determined, and their capacity for phagocytosis, respiratory burst, and release of cytokines was determined, with or without the contribution of blood com-ponents.
Results: The number of AMs exhibited a time-dependent pattern of changes. Phagocy-tosis of dead Escherichia coli (E. coli) and superoxide production by AMs decreased 10 min and 24 h after trauma, but both parameters increased significantly 96 h after the insult. These results sug-gest a transiently impaired response of AMs to a bacterial stimulus in the initial period after thoracic trauma. However, the inclusion of blood components in the experiment further reduced the phagocytic capacity and respiratory burst of AMs.
Conclusions: Blunt thoracic trauma in the present form and intensity impaired two important antibacterial functions of innate immunity, including the phagocytosis and superoxide generation capacity of AM and their ability to pro-duce TNF. These changes are followed by a time-dependent recovery. Alveolar haemorrhage has been shown to be at least partially involved in the impairment of the phagocytosis capacity of AMs.
Introduction
Blast injuries caused by high-intensity blast waves can lead to organ dysfunction in multiple systems and can be fatal [1]. These injuries are often associated with the rifting and rupturing of biological tissues in thin-walled organs and organs with different inter-nal densities [2]. After the eardrum, the lung is the organ most sensitive to blast waves due to its air-filled nature [3]. Indeed, injuries to the lungs occur mainly as a result of the torso and lung exposure to blast waves, irrespective of injury to other systems [2], and are the main cause of mortality and severe injury due to blast waves in Syria, Afghanistan, Jemen, Irak, Nigeria, during the Russian war against the population and military forces in Ukraine, or in accidents, mainly at work.
Following a primary blast lung injury, radiological and clinical evidence of Acute Lung Injury (ALI) appears within some minutes to 12 h after the insult [1]. Blast waves can cause lung contusions, parenchymal injury [1], intrapulmonary haemorrhage [4], and in-flammatory responses in resident cells, ultimately promoting the formation of pulmonary oedema, which in turn impairs pulmonary gas exchange and induces hypoxia [4]. This situation can worsen over time [4]. Of further prognostic importance appears to be further mediastinal injury, including to the heart and the thoracic aorta, as recently analysed in a UK military trauma registry.
AMs represent the most abundant innate immune cells in the distal lung parenchy-ma, where they are located on the luminal surface of the alveolar space. Recent research has shown that they comprise a heterogeneous population that typically consists of two subclasses during inflammation: resident airspace macrophages that arise from the yolk sac during embryogenesis and likely self-renew throughout life without being replaced by circulating monocytes [5]; and another population that is recruited from monocytes. AMs overall have a dynamic, complex multi-functional effector anti- or pro-inflammatory role in the alveolar space [5,6]. During nocturnal micro-aspiration, which is thought to be present in a considerable proportion of humans [7], they might phagocytose bacteria or gastric or pharyngolaryngeal content and have an anti-inflammatory effect. In contrast, their role may be different in bacterial pneumonia or inflammation that occurs in life-threatening lung injuries like ALI or acute respiratory distress syndrome (ARDS) [8]. Depending on the composition of the intra-alveolar milieu, AMs can have a pro-inflammatory M1 or an anti-inflammatory M2 phenotype, the latter of which elimi-nates apoptotic cells and participates in wound healing and fibrosis [9].
Thus, under physiological conditions, AMs perform protective and rather an-ti-inflammatory roles. They can internalise inhaled particles and pathogens, resolve in-flammation, and foster pulmonary tissue repair. In addition, AMs can employ alternative strategies to limit pulmonary infection [10], including immobilisation and killing of mi-croorganisms by releasing Macrophage Extracellular Traps (METs), which are fibres composed of DNA and cellular proteins [11]. Moreover, activated AMs can produce and release reactive oxygen species (ROS), proteases and anti-proteases, bioactive lipids, cyto-kines like interleukin (IL)-1 and -6, Tumour Necrosis Factor (TNF) and anti-inflammatory cytokines like IL-10, chemokines like monocyte chemotactic protein 1 and macrophage in-flammatory protein 2, complement factors, acid metabolites, and growth factors, which each can perform role(s) in pro- and/or anti-inflammatory status [12-15]. All in all, ROS are generated via “respiratory burst” and are involved with a multitude of intracellular proteases in microbial killing.
As previously mentioned, after an infection or trauma, AMs can take on an inflam-matory or anti-inflammatory role either immediately or with some delay .Various re-searchs has suggested conflicting results regarding the performance of AMs in wave blast-induced trauma. On the one hand, following blunt chest trauma, AMs were shown to promote Alveolar Epithelial Type 2 (AT2) cell apoptosis [16], perhaps through the gener-ation of apoptosis-inducing cytokines such as TNF and/or the excessive generation of ROS [17]. On the other hand, early after trauma AMs have been shown to alleviate post-traumatic local inflammation by silencing chemokine release from AT2 cells.
The current study, along with the two other papers with the same setting constitutes a trilogy of research within the same project focusing on rat blast thorax trauma [18,19]. This particular study uniquely evaluates the impact of blast on Alveolar Macrophages (AMs) to understand their role after blast-induced lung injury, emphasising the im-portance of delineating the timeline of AM involvement as either deleterious or beneficial [20]. As such, the main aim of the current study was to investigate whether blunt chest trauma may change the number and function of AMs (phagocytosis, oxidative burst ca-pacity, and TNF release) and with what timeline, using AMs isolated by Bronchoalveolar Lavage (BAL) of rats undergone blast-induced lung injury in an ex vivo setting.
Materials and Methods
Experimental set-up
Blunt lung injury was induced upon applying a single blast wave focused on the thorax. The characteristics and dimensions of the blast wave generator and the detailed experimental conditions are described and illustrated in our previous works [18,19]. Fe-male Wistar rats (230 ± 20 g) were obtained from Harlan Winkelman GmbH (Borchen, Germany) and were kept under controlled conditions (24°C, 55% humidity, 12 hours day/night rhythm) on standard laboratory chow and water ad libitum. Animals were anaesthetised in a bell jar flooded with a Halothane®/O2 gas mixture through a Halo-thane-evaporator (flow: 2 L/min oxygen, 4% Halothane) and divided into two groups: a control group and a blast group. The blast group was then subjected to a blast pressure wave (~3.2 ± 0.41 bar, during~629 ± 29µs) generated by a laboratory shock tube device placed at 3.5 cm in the upper part of the rat’s thorax. The control group underwent the same procedure without receiving the blast. Animals in both groups were sacrificed at 10 min, 24 h, and 96 h after the blast.
Preparation of AMs
AMs were harvested by BAL. For that purpose, rats were tracheotomised, and the al-veolar space was rinsed five times with 10 ml of cold NaCl 0.9%. The last three times, the thoracic cage was softly massaged to increase the number of isolated BAL cells. The re-covery of fluid was always > 75%. The lavage fluids were pooled and centrifuged (4°C) for 10 min at 270 x g, and the pellet was resuspended in 20-50 ml of cold RPMI 1640 and centrifuged for another 5 min (190 x g, 4°C). The pellet was again resuspended in RPMI 1640 with phenol red, 10% heat-inactivated fetal calf serum (FCS), and 1% penicil-lin/streptomycin.
AMs number
The BAL fluid was mixed with a Trypan blue solution (1:2) and incubated for 1 mi-nute at RT. Cell numbers were assayed using a Neubauer counting chamber. Cytospin preparations were used to determine the BAL cell differential. Depending on the total cell counts, the BAL samples were first diluted (2-4 folds) in an isolation medium before pipet-ting 200 μl into the cytospin inserts. The samples were centrifuged at 90 x g in a Minifuge RF for 7 min at RT. The cells were fixed and stained with a May-Grünwald/Giemsa solu-tion. For every experiment, two cytospins were made, and a digital photograph was taken (Nikon Coolpix 995 through a special ocular with a Zeiss Axiovert 50). The number of AMs was assessed using standard morphological criteria for differentiation.
Culturing of AMs
BAL cells were cultured at an initial density of 5 x 105/ml (except stimulation with endotoxin and cytokines determinations) in 96-well plates in 200 µl/well RPMI 1640 (phenol red, 10% FCS, 1% penicillin/streptomycin) for two hours at 37°C. Non-adherent BAL cells were removed by washing the cells three times with a fresh medium. The re-maining adherent AMs were cultured in 200 µl/well RPMI 1640 (without phenol red, 10% FCS, 1% penicillin/streptomycin) and were either not stimulated or treated with endotox-in, lipoteichoic acid, or whole, dead bacteria. Regular initial control microscopies were performed to ascertain purity and quality of the procedures. All experiments were run, at least in duplicate. Supernatants were harvested for TNF measurement or subjected to a phagocytosis and superoxide production assay, respectively. In some experiments, either whole blood, plasma, washed erythrocytes, hemolyzed erythrocytes, or hemolysate were added together with the bacterial stimulus.
Measurement of phagocytosis capacity
Stimulation with dead, fluorescent-labelled bacteria: AMs were incubated with either a dead, fluorescent-labelled G+ Staphylococcus aureus (S. aureus) wood strain without protein A, tetramethylrhodamine conjugate, or G- (E. coli, K-12 strain), tetramethylrhodamine conjugate (both from Molecular Probes, Eugene, OR, USA) in a final concentration of 250 µg/ml. Both reagents were stored as concentrated stocks at 4°C and diluted with the medium after sonicating immediately before use. After three hour of incubation at 37°C, cells, supernatants were either harvested and analysed or subjected to different assays as described below.
Stimulation with endotoxin: AMs were cultured at an initial density of 106 cells/ml (100 µl/well) or 5 x 105 cells/ml (200 µl/well) in the presence of various concentrations of Lipopolysaccharide (LPS) from Salmonella abortus equi (0, 10, 100, 1,000, 10,000, 100,000 ng/ml) or in-house purified Lipo-Teichoic Acid (LTA) from S. aureus (0, 2, 10, 50, 100 µg/ml). Both reagents were stored as concentrated stocks at -20°C and diluted with medium immediately before use. Restored endotoxin was sonicated for 2 min before use. After three or four hours of incubation at 37°C, the supernatant was either harvested and analyzed or subjected to another assay as described below.
Fluorescent-labelled E. coli/S. aureus phagocytosis assay: This assay measures the ability of phagocytes to engulf fluorescently-labelled E. coli/S. aureus particles. Prepared BAL AMs were cultured in 96-well plates (5 x 105 cells/ml, 200 µl/well), allowed to adhere for 2h and stimulated with dead, labelled E. coli/S. aureus (final concentration: 250 µg/ml) as described. After 3h of incubation, phagocytosis was stopped by washing the cells three times with Phosphate-Buffered Saline solution (PBS) to eliminate non-phagocytosed bacteria. The cells were lysed by the addition of 100 µl/well PBS and 0.1% Triton X-100. Fluorescence was determined at 530 nm excitation and 590 nm emission wavelengths using a fluorescence microplate reader. Cells without bacteria were used to determine the background fluorescence. Viability assays such as Trypan blue and Sytox/Hoechst staining at various steps during the assay procedure were used as an index for physiological culture conditions. Furthermore, complete inhibition of ingestion at 0°C was used as a negative control for this quantitative phagocytic assay [21].
Assessment of superoxide generation
Generation of superoxide after stimulation with either whole dead bacteria or bacte-rial toxins was performed by assaying the superoxide-sensitive reduction of the wa-ter-soluble and cell-impermeable tetrazolium salt WST-1. Isolated BAL cells were seeded in 96-well plates after 2h of adhesion, as described before 500 µM WST-1 in Hanks Bal-anced Saline Solution (HBSS) was added to each well. In the experiments with whole, dead bacteria, either 250 µg/ml E. coli or S. aureus was added to half of the wells. If bacterial toxin was used for stimulation, 0.1 µg/ml of LPS and 100 µg/ml LTA were selected as concentrations. Therefore, three diverse groups (non-stimulated, stimulated with bacterial toxin and stimulated with whole bacteria) have been defined. To one well of each of these groups, 1000 U/ml of the superoxide neutralising enzyme Superoxide Dismutase (SOD) was added. Subsequently, plates were incubated at 37°C for 3h. After 3h, the extinction of the single wells was read by a plate reader at a wavelength of 450 nm, and the concentra-tion of superoxide, as well as the rate of its production, was calculated.
Measurement of TNF release
For TNF measurements, BAL samples or cell culture supernatants were stored at -70°C and quantified by sandwich-Enzyme-Linked Immunosorbent assay technique (ELI-SA) using antibody pairs of polyclonal rabbit anti-rat/mouse TNF and recombinant TNF serving as standard. ELISA plates were coated overnight at 4°C with 50 µl/well with 0.5% rabbit anti-rat TNF serum (in-house prepared), diluted 1:200 in coating buffer. After blocking with 200 µl/well PBS/3% Bovine Serum Albumin (BSA) at pH 7.0 for 2 hours at room temperature, the plates were washed twice with PBS/0.05% Tween 20. Samples and standard (50 µl/well) were added and incubated for 3 hours. After four wash cycles, 100 µl/well 0.5 mg/ml tracer antibody (biotin-labelled polyclonal anti-mouse/rat TNF) in PBS/3% BSA were added and incubated for 45 minutes. After another six wash cycles, plates were incubated for 30 minutes with 100 µl/well 50 ng/ml streptavidin-peroxidase in PBS/3% BSA. Following eight wash cycles, 100 µl/well TMB liquid substrate solution was added and incubated for 5-15 minutes. After the addition of 50 µl/well stop solution (1 M H2SO4), absorption was measured at 450 nm using a reference wavelength of 690 nm in an ELISA reader. The detection limit of the assay was 10 pg/ml.
Preparation of washed and haemolyzed erythrocytes and hemolysate
Peripheral venous blood was drawn from rats. To prevent coagulation, heparin (Liquemin®) in NaCl, 1:10 was added. The blood was diluted 10-fold in ice-cold NaCl and centrifuged for 5 min at 270 x g and 4°C. Supernatant was carefully removed and the washing procedure was repeated twice. Then, a 10-fold volume of phenol red-free RPMI 1640 (Gibco) was added, and the erythrocyte cell count was determined using the Neubauer chamber. After another 5 minutes of centrifugation at 270 x g and 4°C, cell pellet was diluted in RPMI 1640 (without phenol red, PAA, Pasching, Austria) to a final concen-tration of 8 x 109 erythrocytes/ml, which corresponds approximately to whole blood erythrocyte counts. The experiments were performed with 1.25 % washed erythrocytes. To determine contamination with leukocytes, we also assessed leukocyte counts. To generate hemolysed erythrocytes, the washed erythrocytes were frozen in liquid nitrogen and then thawed at room temperature. This procedure was repeated three times. The hemolysate was obtained after centrifugation for 10 min at 10,600 x g and 4°C.
Lysis of erythrocytes
BAL fluid was centrifuged for 10 minute at 270 x g, and the pellet was resuspended in medium (1:20) containing 0.17 M ammonium chloride to lyse the erythrocytes and in-cubated for 5 min at room temperature. To stop the lysis, a five-fold excess of fresh medi-um was added and the pellet was resuspended and centrifuged again for 5 min at 190 x g.
Statistics
Data in the figures are given as mean ±SEM, unless otherwise stated, and data in the tables as mean ±SD. Data from the entire curves from the two experiments were compared by two-way ANOVA. Values of p < 0.05 were considered statistically significant. Data from the end or other time points were analysed by one-way ANOVA. In case of differ-ences between groups, post-tests were performed as indicated in the legends (Dunnet’s Multiple Comparison Test, Tukey’s Multiple Comparison Test, or Bonferroni's Multiple Comparison Test); statistics were performed with the Graph Pad Prism 9.0.0 software (Graph Pad Software, San Diego, CA, USA).
Results
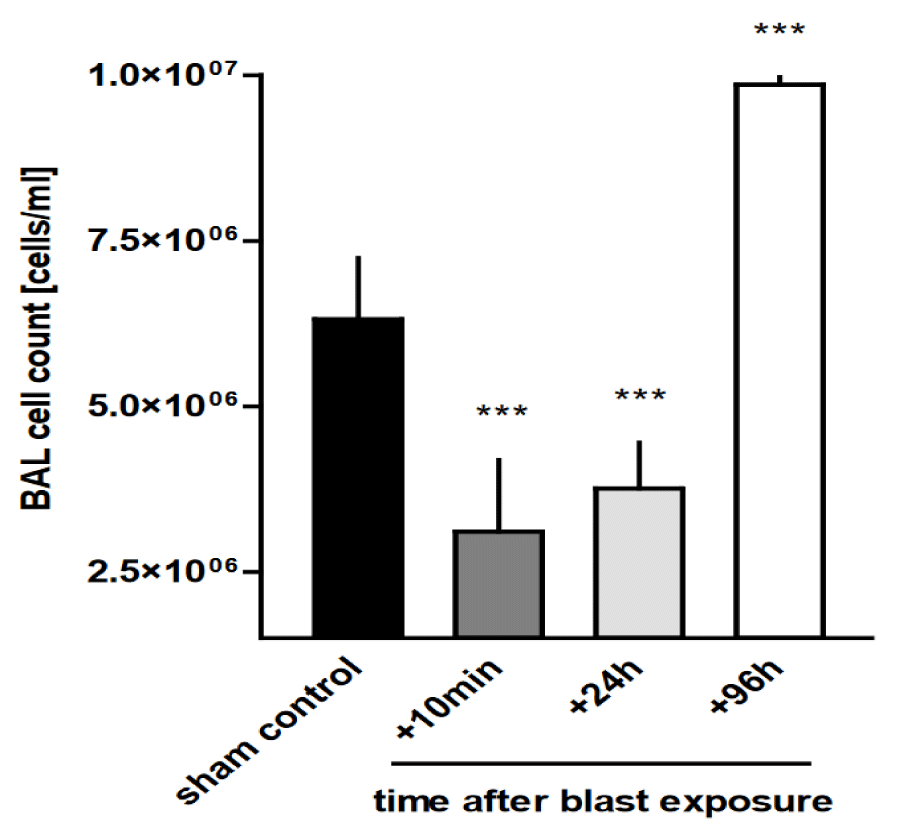
Blast trauma alters AMs count in BAL fluid: As shown in figure 1, the number of macrophages in BAL samples collected from the alveolar space of rats after thoracic trauma depended strongly on the time after blast ex-posure, ranging from (3.11 ± 1.08) x 106 cells/ml at 10 min after trauma to (9.86 ± 0.34) x 106 cells/ml at 96h after trauma and was significantly different from non-injured controls (6.32 ± 0.92 x 106 cells/ml) (p < 0.001).
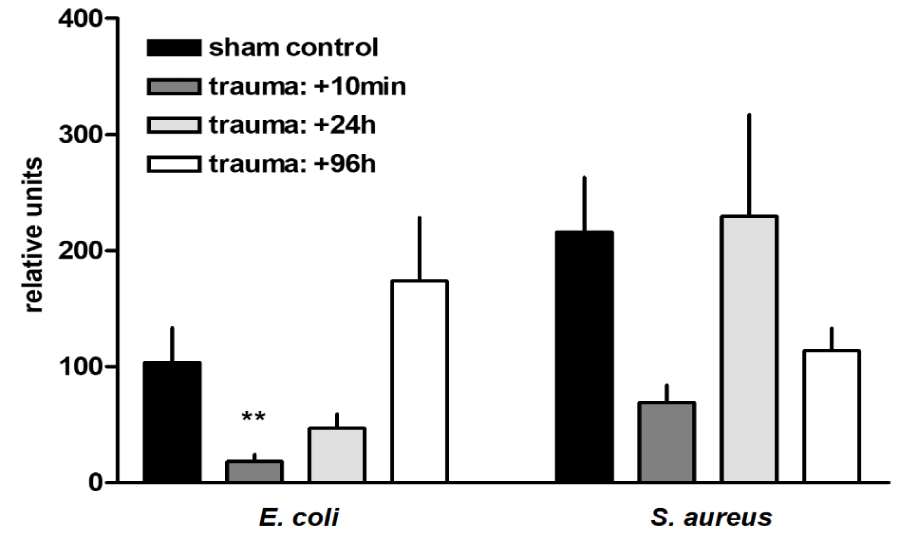
Trauma-related modulation of AMs phagocytic capacity: Initially, after trauma, the AMs phagocytic capacity for dead, fluorescently labelled G- E. coli was significantly reduced by 82% (p < 0.001) in comparison to sham controls. By contrast, the phagocytic capacity of dead G+ S. aureus bacteria was not significantly im-paired after trauma (Figure 2).
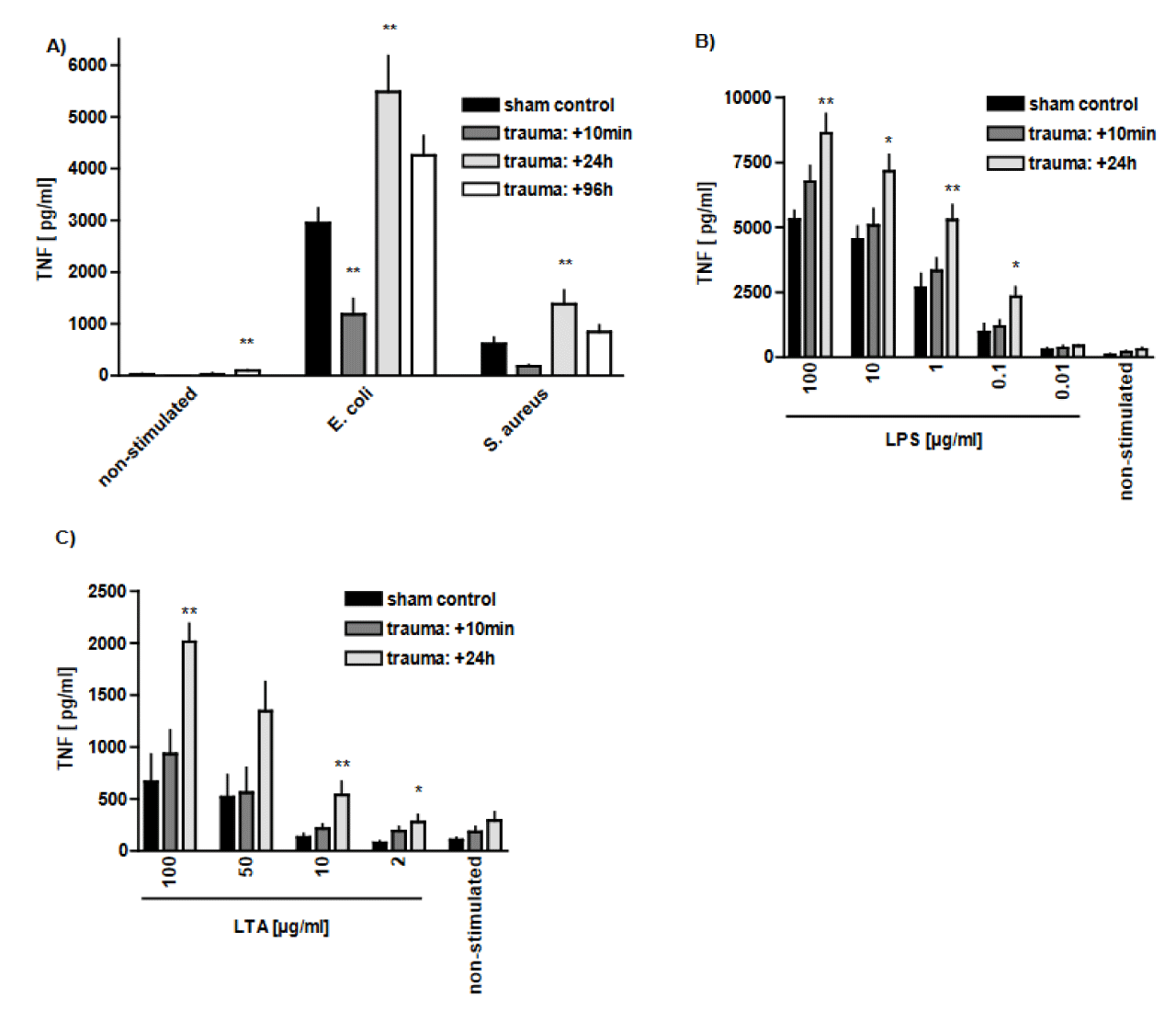
Trauma-related modulation of TNF release after Gram-positive and Gram-negative stimulation: Generation of TNF by AMs is investigated in either trauma or control group, in the absence or presence of additional treatment with G+ and G-stimulation. In the non-stimulated group (in the absence of G+ bacteria), release of TNF by AMs increased up to about 3-fold 96 h after blast injury in comparison to sham controls (p < 0.01). It indicates that the macrophages are primed to release TNF following thoracic trauma. Similarly, stimulation of the AMs with whole, dead E. coli or S. aureus after the blast injury further increased TNF release, as compared to control group at 24 h after trauma (p < 0.01). E. coli stimulation could generally induce higher levels of TNF release comparing to S. aureus (Figure 3A). However, shortly after trauma, i.e., after 10 min, stimulation with bacteria failed to increase TNF release, as compared to sham controls (Figure 3A). In addition, in case of stimulation with E. coli, TNF level was even decreased at this time point (10 min after trauma) (p < 0.01) (Figure 3A). Subsequently, there was more than 1.5-fold and 3-fold increase in TNF release in response to LPS and LTA stimulation (100 µg/ml), respectively, 24 h after trauma, as compared to sham controls (p < 0.01) (Figures 3(B,C)). It shows the ability of AMs to increase TNF in response to LPS stimulation to higher levels than LTA stimulation. Note that the measurements are TNF structure measurements and not measurements of bioactivity.
The impact of trauma-induced alveolar haemorrhage on AMs' function
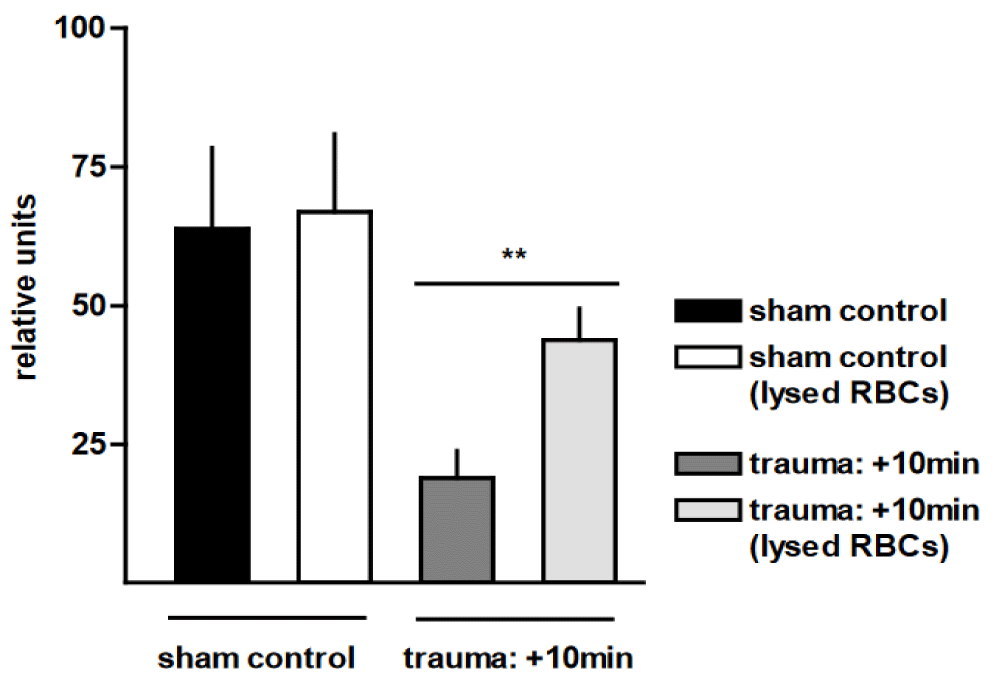
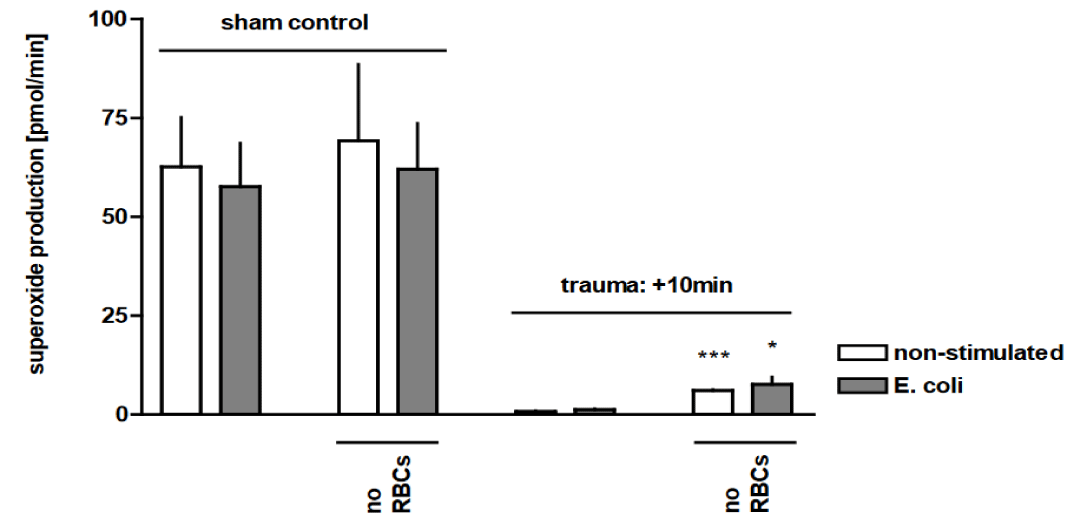
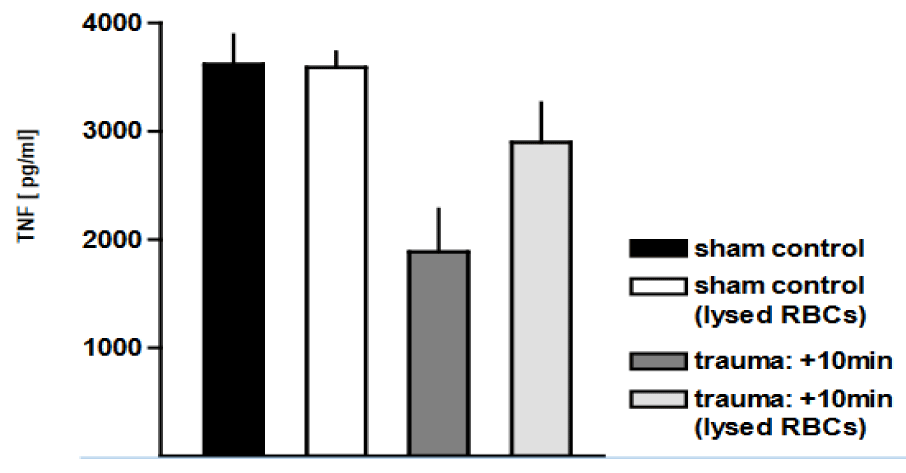
The most frequent and obvious injury detected in our rat blast model was bilateral pulmonary haemorrhage, due to disruption of the alveolar parenchyma and extravasation of blood into the interstitial and alveolar spaces. Therefore, we tested whether the observed macrophage dysregulation after trauma could at least partially be attributed to the ac-companying alveolar haemorrhage. Removal of Red Blood Cells (RBCs) resulted in a more than 2-fold increase in phagocytic capacity of AMs from traumatised rat lungs for labelled E. coli (p <0.01) (Figure 4). We demonstrated that thoracic trauma yielded a marked decrease in the production of superoxide by macrophages, as compared to non-injured sham con-trols. Lysis of the RBCs resulted in an up to 6-fold (p < 0.05) and 8-fold (p < 0.0001) increase in superoxide production in traumatised rat lungs with and without E. coli stimulation, respectively, in comparison with injured animals with haemorrhagic BAL (Figure 5). We subsequently investigated whether the trauma-associated alveolar haemorrhage affects the TNF release from AMs in response to E. coli particles. Figure 6 shows that in the pres-ence of RBCs, TNF release was reduced by 48 ± 21% as a consequence of pressure wave exposure. Removal of RBCs did not influence TNF release of AMs after trauma. In contrast to the phagocytosis capacity and the oxidative burst capacity, TNF expression is not in-fluenced by alveolar haemorrhage in trauma-related alterations of the function of AMs.
The effect of different blood components on AMs' function in control animals
Since at least a part of the trauma-induced macrophage dysregulation was shown to be due to the associated alveolar heamorrhage, we tested whether and how different blood components could mediate the observed effects. For this purpose, we prepared washed and hemolyzed erythrocytes, haemolysate of erythrocytes, and plasma from peripheral venous blood. We then investigated how these different blood components affected mac-rophage function. To simulate the in vivo conditions after thorax trauma, whole blood and prepared components were used at a final concentration of 1.25%, i.e. in an 80 fold dilu-tion. This roughly corresponds to the red blood cell count found in bronchoalveolar lavage fluids of traumatised rats.
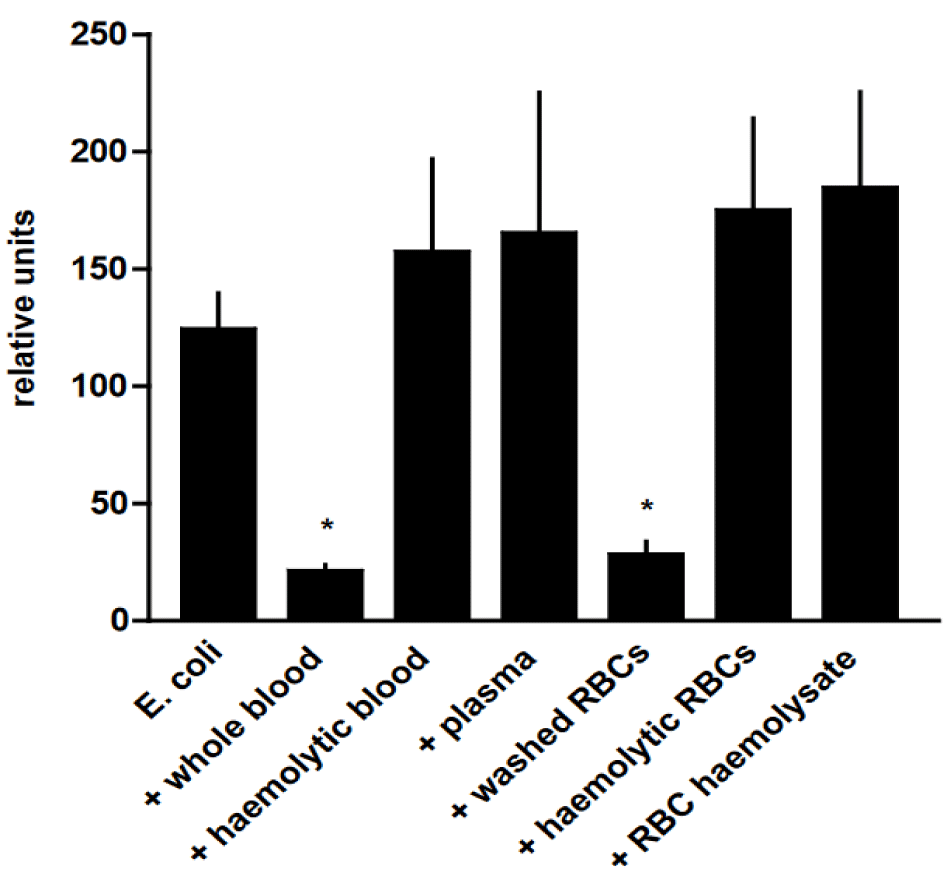
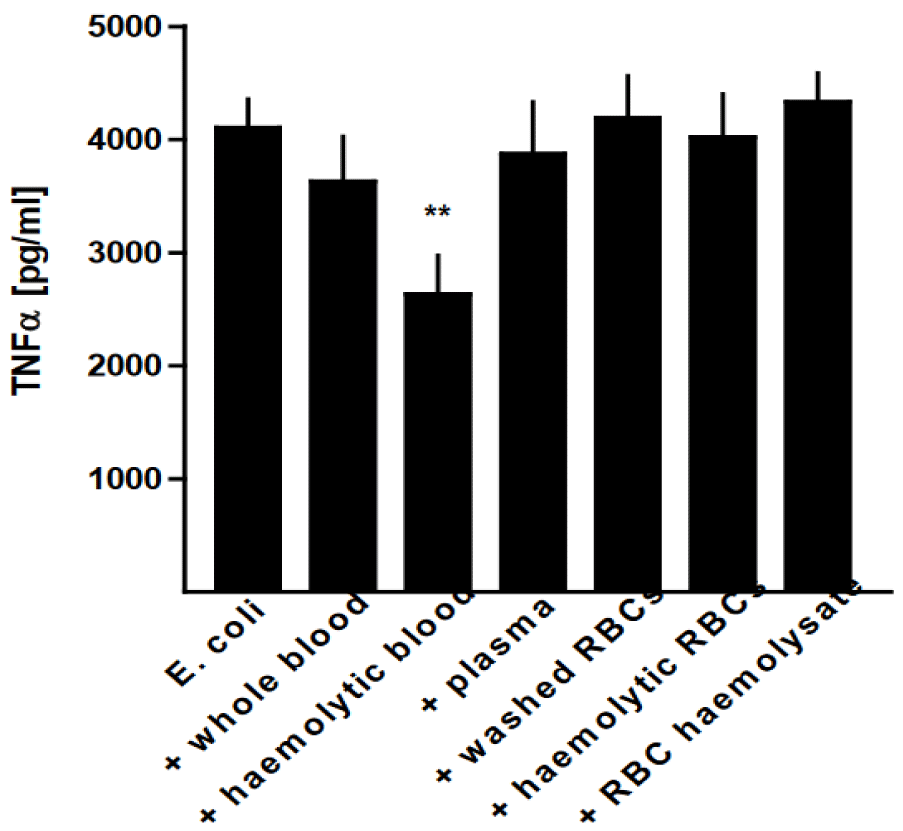
The phagocytic capacity of AMs was significantly diminished in the presence of whole blood and washed RBCs (p < 0.05) in comparison with the phagocytic activity of pure AMs, whereas the presence of hemolytic blood, plasma, hemolytic erythrocytes, and red blood cell hemolysate had no effect (Figure 7).
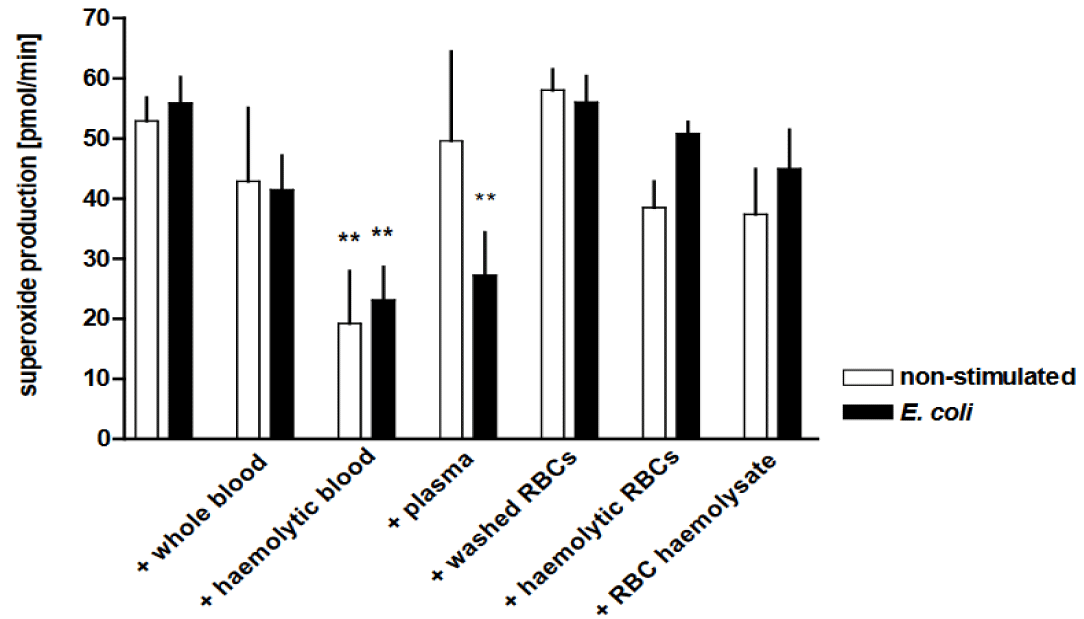
The oxidative burst capacity was significantly reduced by the addition of haemolytic blood to the AMs, both with or without stimulation with E. coli (p < 0.01) (Figure 8). Further-more, the addition of plasma yielded a decrease in superoxide generation (p < 0.01), alt-hough to a lesser extent than with E. coli and afflicted by variations between the individual experiments.
In line with the reduced AMs’ oxidative burst capacity in the presence of haemolytic blood, E. coli-induced TNF release was also significantly reduced (p < 0.01), whereas the addition of the other blood components was without effect (Figure 9). The experiments de-scribed above were also assessed with whole blood and prepared blood components at a final concentration of 5%. Compared to 1.25%, there were no statistically significant dif-ferences detectable in the phagocytotic and oxidative burst activity or the TNF release. Whole intact blood cells as well as cellular debris seeded together with harvested macro-phages interfered with the macrophage adhesion. The results of the different in vitro ex-periments are summarised in tables 1,2.
| Table 1: Thorax trauma-related changes in macrophage functions. | |||||
| time after blast exposure | |||||
| stimulus | read out | sham control | 10min | 24h | 96h |
| non-stimulated | phagocytosis | ♦ | ♦ | ♦ | ♦ |
| oxidative burst | ♦ | ↓↓ | ↓ | ♦ | |
| TNF release | ♦ | ♦ | ♦ | ↑↑ | |
| E. coli/S. aureus | phagocytosis | ↑/↑ | ↓↓/♦ | ♦/♦ | ♦/♦ |
| oxidative burst | ♦/♦ | ↓↓/↓↓ | ↓↓/↓ | ♦/♦ | |
| TNF release | ♦/♦ | ↓↓/♦ | ↑↑/↑↑ | ♦/♦ | |
| LPS/ LTA | oxidative burst | ♦/♦ | ↓↓/↓↓ | ♦/♦ | ↑↑/↑↑ |
| TNF release | ♦/♦ | ♦/♦ | ↑↑/↑↑ | / | |
| ♦ No change, ↑ upregulation, ↓ downregulation (compared to sham control. | |||||
| Table 2: Influence of different blood components on macrophage functions. | ||||||
| non-traumatised controls | ||||||
| non-stimulated | E. coli | |||||
| addition of: | phagocytosis | oxidative burst | TNF release | phagocytosis | oxidative burst | TNF release |
| cell culture medium | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| whole blood | ♦ | ♦ | ♦ | ↓ | ♦ | ♦ |
| haemolytic blood | ♦ | ↓↓ | ♦ | ♦ | ↓↓ | ↓↓ |
| plasma | ♦ | ♦ | ♦ | ♦ | ↓↓ | ♦ |
| washed RBCs | ♦ | ♦ | ♦ | ↓ | ♦ | ♦ |
| haemolytic RBCs | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| RBC haemolysate | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| ♦ No change, ↓ downregulation (compared to sham control). | ||||||
Discussion
The aim of this study is to investigate the response of AMs in terms of alterations in AMs count and function using an ex vivo setting. Accordingly, AMs were isolated from in vivo settings of rats undergoing blast-induced lung injury. The model represents a pul-monary injury with a major shortest-time injury imposed by a blast wave, after which all biological responses are ultimately “on a way to repair”, in contrast to e.g. archetypic lip-opolysaccharide instillation models where injury extends over a long period of hours or even days; while injury and repair are in a dominant and complex interrelationship.
Our results demonstrate that the number of AMs in BAL fluid decreased within the first 24 h, but then increased by 1.5-fold after 96 h. The initial decrease in AMs number in BAL fluid may be the consequence of increased cell death due to exposure to the blast or increased trauma-related adhesion of the AMs to resident lung epithelial and/or endothe-lial or interstitial cells or debris, including post-haemorrhagic thrombocoagulative clots mixed with alveolar space liquid and cell contents, including erythrocytes. However, this hypothesis can only be evidenced by means of quantitative stereometry over time. The in-creased BAL AMs count in the preceding post-traumatic phases is characteristic of an acute or chronic inflammatory state, as has been similarly reported in previous studies. The contribution of recruited circulating monocytes turning to AMs versus the increase of the resident airspace macrophages pool by self-expansion in the blast-injured and thus inflamed alveolar space [5,22] has not been tested in our study.
In the current experiment, Ams’ phagocytotic capacity for dead, fluorescently labelled E. coli was reduced, whereas the phagocytotic capacity for S. aureus particles, was not sig-nificantly impaired. Since the E. coli particles were suspended homogeneously, the S. au-reus particles despite sonication remained rather aggregates and this might affect particle counts. On the other hand, the assay discriminated within groups concerning superoxide production. Furthermore, surfactant proteins might play a role, some of which are highly linked to the complement system. They are capable of precoat bacteria and foreign parti-cles in order to facilitate AMs phagocytosis and bactericidal activities [23]. Similarly, to the post-trauma E. coli reduction results, reduced phagocytosis 1 h after surgical interventions in the mice model occurred, which is an indication of the contribution of peritoneal mac-rophages to the altered immune function [24].
Basal TNF release by AMs from traumatised rat lungs was increased 96h after the trauma, and this was further augmented by an additional bacterial stimulus. With regard to TNF release, AMs from both control and trauma groups were more susceptible to LPS stimulation than to LTA and more to E. coli than to S. aureus stimulation. LTA has been described as a weak inducer of cytokine release in human blood, as compared to LPS [25]. We further identified an increased TNF response of AMs to LPS/LTA and whole bacteria stimulation 24h after blast exposure. This is in line with previous findings [26], which have detected increased TNF, IL-6 and H2O2 secretion of splenic macrophages in response to endotoxin or Phorbol Myristate Acetate (PMA) stimulation up to 7 days after trauma. It has been moreover demonstrated that increased inflammatory mediators secretion does not necessarily translate into improved immunologic protection, as demonstrated by the functional impairment of macrophage antigen presentation [26].
The reason behind the decline in AMs phagocytosis as well as superoxide production appears to be multifactorial. A key mechanism of the initial suppression of macrophage function might be the release of trauma-related early mediators like eicosanoids, such as prostaglandin E2. Blast wave lung injury may among others also cause a stress response, which in turn may cause immune system dysfunction that leads to prolonged infection susceptibility. As physical or even psychological stresses trigger the hypothalamic-pituitary-adrenal axis, a marked rise in the serum level of glucocorticosteroids may occur. Glucocorticoid receptors are expressed in macrophages [27] and have the potential to downregulate macrophage functions [28,29]. Furthermore, stress-induced pro-inflammatory cytokines, such as IL-1, IL-6 and TNF besides being key proinflamma-tory mediators, cause the release of glucocorticoids via the release of corticotropin [30,31]. Thus, an unknown part of the impaired AMs’ function following blast wave lung injury may be glucocorticoid-mediated.
In addition, many mediators perform complex roles. For instance, TNF has pro- as well as anti-inflammatory features that are often neglected [32]. The impaired phagocytic and microbicidal capacity after thoracic trauma observed in this setting could be linked to a depressed antigen presentation or other features of the specific immune response. Ac-cordingly, preliminary findings gave evidence that Keyhole Limped Hemocyanin (KLH)-sensitized rats developed cutaneous energy about four days after severe trauma, as e.g. shown in mouse laparotomy in contrast to mouse laparoscopy with CO2 insufflation [24], which underlines that the intensity of the induced trauma has systemic repercussions over a period of time and that this includes aspects of immunosuppression and may parallel AMs M1 versus M2 reactivity.
Another important factor contributing to the initial suppression of phagocytosis and oxidative burst capacity in AMs is trauma-related alveolar bleeding. Of importance is that most probably a very huge part of the alveolar-capillary disruption leading to bleeding is the blast itself with its mechanical rupture of those structures, most probably an injury occurring within milliseconds. This “first hit” situation of probably pure alveolar capil-lary bleeding with its “mechanical” breakdown of the alveolar-capillary membrane is pathogenically probably highly different from an ALI or ARDS due to pulmonary or peritoneal bacterial inflammation or respiratory COVID-19 infection, and therefore the alveolar content seems at the initial post-blast situation that of pure trauma-related bleeding and therefore of highly different contents in terms of inflammatory mediators like eicosanoids, cytokines and growth factors in contrast to pulmonary, peritoneal or COVID-19 related acute lung injury or ARDS where barrier breakdown is a complex inflammatory process .
Removal of the RBCs resulted in a marked increase in the phagocytosis of E. coli particles and superoxide anion production (with or without E. coli stimulation) by AMs from traumatised rat lungs. Whereas the phagocytosis capacity of control AMs was reduced in the presence of intact blood cells (whole blood and washed RBCs), the oxidative burst and the TNF release remained unaffected. By contrast, hemolytic blood decreased superoxide production and TNF release but had no effect on the phagocytotic capacity of E. coli particles. These results suggest an anti-oxidative blood cell component that may quench the superoxide anion production.
As only hemolytic blood and neither RBCs hemolysate nor plasma altered the oxidative burst (at least without E. coli) nor TNF release, one hypothesis would be that an intra-cellular component and a plasma component may act together, like e.g. glutathione and glutathione peroxidase while quenching the superoxide. Because both respiratory burst and TNF release were unaffected by either whole blood or RBCs, a steric interference be-tween E. coli and AMs as the cause for the diminished phagocytosis seems unlikely. Thus, there are two possible explanations: 1. Intact blood cells bind to the macrophage surface, thereby downregulating the phagocytosis of E. coli particles and/or 2. A preferential phagocytic engulfment of RBCs occurs, as compared to E. coli. The latter hypothesis is supported by the finding that after haemorrhage large quantities of hemoglobin, hemo-siderin and ingested RBCs can be detected in AMs [33,34]. However, further stud-ies are required to differentiate these possibilities.
This study has many important limitations. We did not differentiate the M1 versus M2 phenotypes of alveolar macrophages by our approach. In addition, halothane impacts mediator release during lung inflammation [35,36]; as such, anaesthesia may influence the AMs function . In addition, vagus nerve stimulation can suppress inflammation. In a prospectively randomized, controlled study using rats, blast pressure wave caused an immediate vagally mediated reflex-like pulmonary defensive reflex [37]. Similarly, the rats studied in this setting had a low breathing frequency immediately after the blast pursued by fast shallow breathing. This triad of apnoea, bradycardia, and hypotension [38,39] is merely seen in animals in which just the thorax is exposed to the blast, but not in animals undergoing abdominal blast exposure [39]. These changes have been attributed to air em-bolism and direct cardiac injury [40] and may probably further be mediated by vagal re-flexes [37]. Experimental animal studies [41] and clinical observations following blunt chest trauma reveal a variety of EKG disturbances, from ventricular extrasystoles to ven-tricular fibrillation,that has usually been temporarily observed but might account for fa-talities following blast injury and might exacerbate the triad of apnoea, bradycardia and hypotension.
BAL fluid in healthy, nonsmoking adults contains 80-90% AMs, 5-15% lymphocytes, 1-3% polymorphonuclear neutrophils, 1% eosinophils, and <1% mast cells [42]. Previous studies have shown that blast lung injury results in severe immunodysfunction of lymphocytes and splenic macrophages in mouse model [43] and likewise pulmonary contusion impairs macrophage and lymphocyte immune functions and increases mortality associated with a subsequent septic challenge [44]. However, in the current study, we have not investigated the performance of T cell subsets after blast lung injury. There is definitely a huge interaction between these cells, which have not been the focus of the current study. Clinically, the huge alveolar surface of about 140 m2 in humans is only protectable by an excellent immune system [45], impressively evident in immunodeficient humans including those with cancer therapy or haematological cancers, and especially during neutropenia or even agranulocytosis, and in patients with immunosuppression due to a variety of inflammatory diseases. Due to both interspecies and interpersonal differences, specific immune deficiencies and specific occurrence of infective complications may occur. Ac-accordingly, bacteria, their toxins, viruses, fungi or other microorganisms may play roles in stimulating AMs reactions.
Conclusion
Following blast wave-induced lung injury in rats, in vitro examinations show a phasic and time-dependent response of AMs to endotoxin and whole bacteria stimulation in terms of initially suppressed phagocytic capacity and superoxide production, as well as a delayed increase in TNF release. Part of the impaired AMs’ function was shown to be due to the RBCs upon exposition to alveolar haemorrhage. Blast lung injury-induced AM dysfunction in the current intensity and setting appears to be associated with time-dependent recovery, which is corroborated by parameters like AM abundance and antimicrobial functions including superoxide production, phagocytic function, and TNF release.
Acknowledgement
We acknowledge the work of Dr Katja Eichert, her experimental work and her resulting Ph. D. thesis constitute the basis for this paper. We further acknowledge the intellectual and technical inputs from Ulrich Liener, Markus Knöferl, Florian Gebhard, Lothar Kinzl (University of Ulm), Bruno Erne (Technical Dept., Univ. of Konstanz), Clemens Braun, Anne Hildebrandt (University of Konstanz), Sandra Unger, Manfred Kind (Pathology, Konstanz Hos-pital).
Competing Interests Statement
The work of JH was supported by a grant from the Deutsche Forschungsgemeinschaft (FOR 321/2-1; research group “Endogenous tissue injury: Mechanisms of autodestruction”) and by the Herrmann Josef Schieffer Prize of the “Freunde des Universi-tätsklinikums Homburg e.V.”. The work of YH, UM, RL and JH have been funded by the Lung-en- und Atmungsstiftung Bern, Switzerland, which provided non-restricted financial support toward research on lung, respiratory and sleep-related respiratory diseases as well as related rehabilitation and lifestyle change issues such as exercise, smoking cessation, etc. JH is the chair of Lungen- und Atmungsstiftung Bern. His position did not influence the design of the study, the collection of the data, the analysis or interpretation of the data, the decision to submit the manuscript.
References
- Scott TE, Kirkman E, Haque M, Gibb IE, Mahoney P, Hardman JG. Primary blast lung injury - a review. Br J Anaesth. 2017 Mar 1;118(3):311-316. doi: 10.1093/bja/aew385. PMID: 28203741.
- Herrmann J, Tawhai MH, Kaczka DW. Computational Modeling of Primary Blast Lung Injury: Implications for Ventilator Management. Mil Med. 2019 Mar 1;184(Suppl 1):273-281. doi: 10.1093/milmed/usy305. PMID: 30901433; PMCID: PMC6515895.
- Lemonick DM. Bombings and blast injuries: A primer for physicians. Am J Clin. 2011;8(3):134-140.
- Kirkman E, Watts S. Characterization of the response to primary blast injury. Philos Trans R Soc Lond B Biol Sci. 2011 Jan 27;366(1562):286-90. doi: 10.1098/rstb.2010.0249. PMID: 21149364; PMCID: PMC3013437.
- Mould KJ, Jackson ND, Henson PM, Seibold M, Janssen WJ. Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI Insight. 2019 Mar 7;4(5):e126556. doi: 10.1172/jci.insight.126556. PMID: 30721157; PMCID: PMC6483508.
- Woo YD, Jeong D, Chung DH. Development and Functions of Alveolar Macrophages. Mol Cells. 2021 May 31;44(5):292-300. doi: 10.14348/molcells.2021.0058. PMID: 33972474; PMCID: PMC8175155.
- Lee AS, Ryu JH. Aspiration Pneumonia and Related Syndromes. Mayo Clin Proc. 2018 Jun;93(6):752-762. doi: 10.1016/j.mayocp.2018.03.011. Epub 2018 May 2. PMID: 29730088.
- Niesler U, Palmer A, Radermacher P, Huber-Lang MS. Role of alveolar macrophages in the inflammatory response after trauma. Shock. 2014 Jul;42(1):3-10. doi: 10.1097/SHK.0000000000000167. PMID: 24667621.
- Huang X, Xiu H, Zhang S, Zhang G. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediators Inflamm. 2018 May 13;2018:1264913. doi: 10.1155/2018/1264913. PMID: 29950923; PMCID: PMC5989173.
- Allard B, Panariti A, Martin JG. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front Immunol. 2018 Jul 31;9:1777. doi: 10.3389/fimmu.2018.01777. PMID: 30108592; PMCID: PMC6079255.
- Doster RS, Rogers LM, Gaddy JA, Aronoff DM. Macrophage Extracellular Traps: A Scoping Review. J Innate Immun. 2018;10(1):3-13. doi: 10.1159/000480373. Epub 2017 Oct 7. PMID: 28988241; PMCID: PMC6757166.
- Prakash A, Mesa KR, Wilhelmsen K, Xu F, Dodd-o JM, Hellman J. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology. 2012 Oct;117(4):822-35. doi: 10.1097/ALN.0b013e31826a4ae3. PMID: 22890118; PMCID: PMC3477877.
- Seitz DH, Niesler U, Palmer A, Sulger M, Braumüller ST, Perl M, Gebhard F, Knöferl MW. Blunt chest trauma induces mediator-dependent monocyte migration to the lung. Crit Care Med. 2010 Sep;38(9):1852-9. doi: 10.1097/CCM.0b013e3181e8ad10. PMID: 20543668.
- Seitz DH, Palmer A, Niesler U, Fröba JS, Heidemann V, Rittlinger A, Braumüller ST, Zhou S, Gebhard F, Knöferl MW. Alveolar macrophage phagocytosis is enhanced after blunt chest trauma and alters the posttraumatic mediator release. Shock. 2011 Dec;36(6):621-7. doi: 10.1097/SHK.0b013e318234f8a0. PMID: 21921831.
- Chavko M, Adeeb S, Ahlers ST, McCarron RM. Attenuation of pulmonary inflammation after exposure to blast overpressure by N-acetylcysteine amide. Shock. 2009 Sep;32(3):325-31. doi: 10.1097/SHK.0b013e31819c38f1. PMID: 19174737.
- Seitz DH, Perl M, Mangold S, Neddermann A, Braumüller ST, Zhou S, Bachem MG, Huber-Lang MS, Knöferl MW. Pulmonary contusion induces alveolar type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock. 2008 Nov;30(5):537-44. doi: 10.1097/SHK.0b013e31816a394b. PMID: 18317405.
- Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem. 1997 Aug 15;272(33):20646-9. doi: 10.1074/jbc.272.33.20646. PMID: 9252381.
- Hamacher J, Hadizamani Y, Huwer H, Moehrlen U, Bally L, Stammberger U, Wendel A, Lucas R. Characteristics of inflammatory response and repair after experimental blast lung injury in rats. PLoS One. 2023 Mar 16;18(3):e0281446. doi: 10.1371/journal.pone.0281446. PMID: 36928833; PMCID: PMC10019677.
- Huwer H, Hadizamani Y, Moehrlen U, Stammberger U, Gebhard F, Bally L, Wendel A, Liener UC, Lucas R, Hamacher J. Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role. Biomedicines. 2022 Nov 15;10(11):2930. doi: 10.3390/biomedicines10112930. PMID: 36428498; PMCID: PMC9687465.
- Smith JE, Garner J. Pathophysiology of primary blast injury. J R Army Med Corps. 2019 Feb;165(1):57-62. doi: 10.1136/jramc-2018-001058. Epub 2018 Oct 12. PMID: 30317218.
- Stossel TP. Phagocytosis. Prog Clin Biol Res. 1977;13:87-102. PMID: 400763.
- Mould KJ, Moore CM, McManus SA, McCubbrey AL, McClendon JD, Griesmer CL, Henson PM, Janssen WJ. Airspace Macrophages and Monocytes Exist in Transcriptionally Distinct Subsets in Healthy Adults. Am J Respir Crit Care Med. 2021 Apr 15;203(8):946-956. doi: 10.1164/rccm.202005-1989OC. PMID: 33079572; PMCID: PMC8048748.
- Tino MJ, Wright JR. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996 Apr;270(4 Pt 1):L677-88. doi: 10.1152/ajplung.1996.270.4.L677. PMID: 8928829.
- Moehrlen U, Schwoebel F, Reichmann E, Stauffer U, Gitzelmann CA, Hamacher J. Early peritoneal macrophage function after laparoscopic surgery compared with laparotomy in a mouse mode. Surg Endosc. 2005 Jul;19(7):958-63. doi: 10.1007/s00464-004-2118-2. Epub 2005 May 12. Erratum in: Surg Endosc. 2005 Nov;19(11):1516. Schwöbel, F [corrected to Schwoebel, F]. PMID: 15920692.
- Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001 Feb 5;193(3):393-7. doi: 10.1084/jem.193.3.393. PMID: 11157059; PMCID: PMC2195914.
- McCarter MD, Mack VE, Daly JM, Naama HA, Calvano SE. Trauma-induced alterations in macrophage function. Surgery. 1998 Jan;123(1):96-101. PMID: 9457229.
- Werb Z, Foley R, Munck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J Exp Med. 1978 Jun 1;147(6):1684-94. doi: 10.1084/jem.147.6.1684. PMID: 681878; PMCID: PMC2184310.
- Cech AC, Shou J, Gallagher H, Daly JM. Glucocorticoid receptor blockade reverses postinjury macrophage suppression. Arch Surg. 1994 Dec;129(12):1227-32. doi: 10.1001/archsurg.1994.01420360013001. PMID: 7986150.
- Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007 May 15;109(10):4313-9. doi: 10.1182/blood-2006-10-048215. Epub 2007 Jan 25. PMID: 17255352; PMCID: PMC1885507.
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009 Oct;1179:86-105. doi: 10.1111/j.1749-6632.2009.04984.x. PMID: 19906234; PMCID: PMC3399249.
- Woiciechowsky C, Schöning B, Lanksch WR, Volk HD, Döcke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med (Berl). 1999 Nov;77(11):769-80. doi: 10.1007/s001099900051. PMID: 10619437.
- Lucas R, Hadizamani Y, Enkhbaatar P, Csanyi G, Caldwell RW, Hundsberger H, Sridhar S, Lever AA, Hudel M, Ash D, Ushio-Fukai M, Fukai T, Chakraborty T, Verin A, Eaton DC, Romero M, Hamacher J. Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance. Front Physiol. 2022 Feb 21;12:793251. doi: 10.3389/fphys.2021.793251. PMID: 35264975; PMCID: PMC8899333.
- Finley TN, Aronow A, Cosentino AM, Golde DW. Occult pulmonary hemorrhage in anticoagulated patients. Am Rev Respir Dis. 1975 Jul;112(1):23-9. doi: 10.1164/arrd.1975.112.1.23. PMID: 1147381.
- Grissom CK, Albertine KH, Elstad MR. Alveolar haemorrhage in a case of high altitude pulmonary oedema. Thorax. 2000 Feb;55(2):167-9. doi: 10.1136/thorax.55.2.167. PMID: 10639537; PMCID: PMC1745671.
- Giraud O, Seince PF, Rolland C, Leçon-Malas V, Desmonts JM, Aubier M, Dehoux M. Halothane reduces the early lipopolysaccharide-induced lung inflammation in mechanically ventilated rats. Am J Respir Crit Care Med. 2000 Dec;162(6):2278-86. doi: 10.1164/ajrccm.162.6.9807113. PMID: 11112152.
- Itoh T, Hirota K, Hisano T, Namba T, Fukuda K. The volatile anesthetics halothane and isoflurane differentially modulate proinflammatory cytokine-induced p38 mitogen-activated protein kinase activation. J Anesth. 2004;18(3):203-9. doi: 10.1007/s00540-004-0237-5. PMID: 15290420.
- Irwin RJ, Lerner MR, Bealer JF, Mantor PC, Brackett DJ, Tuggle DW. Shock after blast wave injury is caused by a vagally mediated reflex. J Trauma. 1999 Jul;47(1):105-10. doi: 10.1097/00005373-199907000-00023. PMID: 10421195.
- Irwin RJ, Lerner MR, Bealer JF, Brackett DJ, Tuggle DW. Cardiopulmonary physiology of primary blast injury. J Trauma. 1997 Oct;43(4):650-5. doi: 10.1097/00005373-199710000-00015. PMID: 9356063.
- Guy RJ, Kirkman E, Watkins PE, Cooper GJ. Physiologic responses to primary blast. J Trauma. 1998 Dec;45(6):983-7. doi: 10.1097/00005373-199812000-00001. PMID: 9867037.
- CLEMEDSON CJ, HULTMAN HI. Air embolism and the cause of death in blast injury. Mil Surg. 1954 Jun;114(6):424-37. PMID: 13165075.
- Guy RJ, Watkins PE, Edmondstone WM. Electrocardiographic changes following primary blast injury to the thorax. J R Nav Med Serv. 2000;86(3):125-33. PMID: 11346922.
- Stanzel F. Bronchoalveolar Lavage. Principles and Practice of Interventional Pulmonology. 2012 May 22:165–76. doi: 10.1007/978-1-4614-4292-9_16. PMCID: PMC7176194.
- Knöferl MW, Liener UC, Perl M, Brückner UB, Kinzl L, Gebhard F. Blunt chest trauma induces delayed splenic immunosuppression. Shock. 2004 Jul;22(1):51-6. doi: 10.1097/01.shk.0000127684.64611.5c. PMID: 15201702.
- Perl M, Gebhard F, Brückner UB, Ayala A, Braumüller S, Büttner C, Kinzl L, Knöferl MW. Pulmonary contusion causes impairment of macrophage and lymphocyte immune functions and increases mortality associated with a subsequent septic challenge. Crit Care Med. 2005 Jun;33(6):1351-8. doi: 10.1097/01.ccm.0000166352.28018.a9. PMID: 15942355.
- Gehr P, Bachofen M, Weibel ER. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol. 1978 Feb;32(2):121-40. doi: 10.1016/0034-5687(78)90104-4. PMID: 644146.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.