Medicine Group. 2024 July 11;5(7):757-762. doi: 10.37871/jbres1951.
Surgical Approach to Retroperitoneal Sarcomas with Involvement of the Permeable Inferior Vena Cava: Use of the Femorojugular Bypass
Cambeiro Cabre L*, Homs Samso R, Rosello Diez E, Solans Solerdelcoll M, Gine Serven M, Lorenzo Vaquerizo L, Sanchez Cabus S, Moral Duarte A and Gonzalez Lopez JA
- Retroperitoneal sarcoma
- Inferior vena cava
- Femorojugular bypass
- Surgical resection
- Hemodynamic complications
- Overall survival
Abstract
Introduction: Retroperitoneal sarcomas with Inferior Vena Cava (IVC) involvement are rare cases and complex to manage. Radical surgical resection is the only potentially curative treatment and sometimes clamping of the IVC is necessary, which can cause hemodynamic alterations. In this study, we describe the use of femorojugular bypass with perfusion pump as a technique to optimize the hemodynamic situation during the resection of retroperitoneal sarcomas with IVC involvement.
Methods: We collected data from patients presenting with retroperitoneal sarcomas with IVC involvement operated at the Hospital de la Santa Creu i Sant Pau (Barcelona), a sarcoma referral center, between March 2021 and May 2023. The technical details of the surgical procedures were analyzed, including the IVC involvement, surgical approach to treatment, and the use of femorojugular bypass. Postoperative complications and oncologic outcomes were also recorded.
Results: A total of seven patients diagnosed with retroperitoneal sarcoma with IVC involvement were operated on. In two of the cases, a femorojugular bypass was performed prior to clamping the IVC. In five of the cases, radical resection of the tumor was achieved without leaving macroscopic disease. The mean estimated blood loss was 2.21 L and there was no postoperative mortality. Overall survival was 75% at 12 months with a mean follow-up of 11.86 months.
Conclusion: The use of femorojugular bypass during the resection of retroperitoneal sarcomas with IVC involvement may improve the hemodynamic situation of the patient and allow for complete tumor resection. This multidisciplinary surgical approach in referral centers may be effective in the treatment of this rare disease. However, further studies are needed to confirm these results and to establish standardized management guidelines.
Introduction
Retroperitoneal Sarcomas (RPS) originating as a primary tumor in the Inferior Vena Cava (IVC) or with secondary involvement of the IVC are very rare cases; to give perspective, IVC leiomyosarcomas account for only 2% of all leiomyosarcomas.
Primary and secondary vena cava tumors are rare and often malignant. They can originate in the vein wall, invade the walls secondarily, or grow into the vena cava forming a tumor thrombus [1].They can also cause extrinsic compression of the vein lumen. Most patients have local, regional, or distant metastatic disease at the time of diagnosis [2].
The most frequent primary tumor of the vena cava (both inferior and superior) is leiomyosarcoma, affecting the IVC in 60% of patients [1,3].
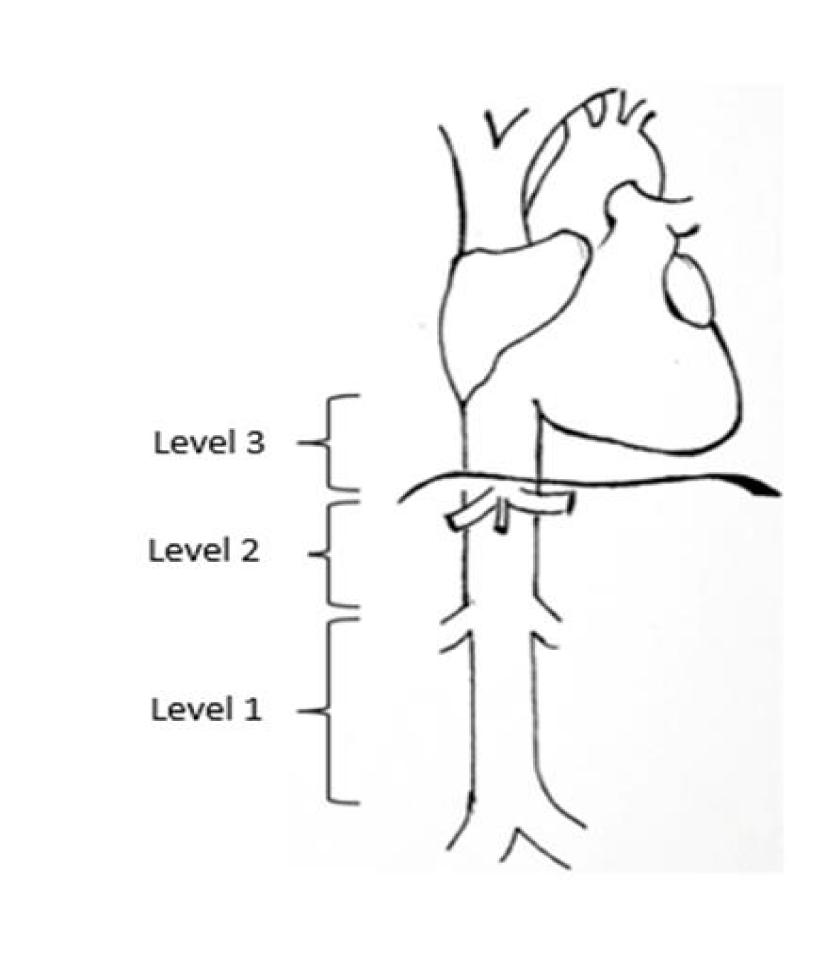
The most commonly used classification system [1,3], is according to location (Figure 1), where the IVC is divided into three segments:
- Segment I (low): below the renal veins; present in 34% of the cases.
- Segment II (middle): between the hepatic veins and the renal veins. These account for about 42% of the cases and have a better prognosis.
- Segment III (superior): from the right atrium to the hepatic veins. This type is present in 24.3% of the cases.
Secondary tumors affect the vena cava more frequently than primary tumors and have different etiologies (lymphoma, teratoma, angiosarcoma, liposarcoma or retroperitoneal leiomyosarcoma, hepatic/pancreatic tumors, and renal tumors, amongst others).
Similar to other intra-abdominal locations of these types of tumors, with the exception of lymphoma, radical surgery is the only potentially curative treatment. Radical surgery involves removal of both the primary tumor and the adjacent organs affected or included in the compartment in which the tumor is located. The lack of proven efficacy in adjuvant therapies further emphasizes the importance of surgical treatment.
One of the main factors in the prognosis of RPS is an R0 resection without leaving macroscopic disease, as residual malignant tissue increases the risk of local recurrence of the tumor. In cases of RPS with IVC involvement, this objective is a challenge at the surgical level, with radical resection sometimes requiring resection and repair of the IVC. This requires the intervention of surgeons with extensive experience and the collaboration of different specialties.
There are three generally accepted surgical approaches: resection and ligation of the cava, resection and primary suture, or resection accompanied by reconstruction with prosthesis. The approach used in most series is resection with associated reconstruction (using the patient's own biological materials, from bovine pericardium/ cadaver grafts, or synthetic prostheses) [4].
During this type of surgery, it may be necessary to clamp the IVC, which causes a decrease in venous return and, consequently, in cardiac preload. As a result, in most cases, especially if the patient is hypovolemic, there is a decrease in cardiac output. This decrease in cardiac output can produce alterations in the hemodynamic state of the patient such as hypotension, poor peripheral perfusion, metabolic acidosis, and even cardiac arrest [5].
Clamping of the inferior vena cava may not have hemodynamic repercussions if it is performed in patients who present chronic obstruction due to tumor invasion, due to patients developing compensation mechanisms by connecting the gonadal vessels and lumbar plexus with the azygos system.
If the patient does not hemodynamically tolerate a permeable inferior vena cava clamp, i.e., if he/she presents hypotension refractory to high-dose perfusion of vasoconstrictor drugs (mean arterial pressure < 65 mmHg) and with a consequent reduction in cardiac output that could lead to poor organ perfusion (lactacidemia in arterial blood gas analysis), it would be necessary to employ an extracorporeal circulation method to optimize venous return, and thus, optimize hemodynamic status and perfusion to target organs. One alternative could be a jugulofemoral venous bypass with a pump.
The venovenous bypass [5]. was first used in the 1960s for orthotopic liver transplantation, but its use is mainly described during IVC resection in renal tumors. There is very little literature on the use of this type of bypass in retroperitoneal sarcoma resections.
Percutaneous cannulation is usually performed using the ultrasound-guided Seldinger technique, or semi-Seldinger cannulation, with one cannula in the femoral vein and another in the right jugular vein. The femoral cannula drains blood from the patient to the extracorporeal circulation pump (inflow cannula), and the jugular cannula returns blood from the pump to the patient (outflow cannula). Arterial cannulas are used for both locations, usually 18 Fr for the jugular and 20-22 Fr for the femoral cannula. The position of the jugular cannula can be guided by transesophageal echocardiography or by direct palpation of the right atrium if the thorax is open. The tip of the femoral cannula is at the level of the iliac vein. A centrifugal pump is usually used as an extracorporeal circulation pump, without a reservoir for the blood, and is a closed circuit that avoids the entry of air. The flow rate can vary between 0.5-2.5 L/min depending on the patient's blood volume, degree of bleeding, etc. A heat exchanger is associated with the circuit to maintain normothermia. During venous-venous bypass it is important to heparinize at full doses, with a Target Clotting Time (ACT) greater than 250 seconds, to avoid thrombosis formation within the circuit.
In addition, the cannulas and tubulures are made of biocompatible material to reduce the risk of thrombosis. Venous-venous bypass allows better control of the patient's hemodynamics, by returning blood from the lower hemibody to the right atrium and adapting the return flow with the pump controller according to the patient's hemodynamic status.
This technique improves the perioperative hemodynamic situation and operating conditions during IVC clamping, facilitating safe resection of advanced PRRS.
The aim of this study is to review the series of cases of RPS with IVC involvement in a sarcoma referral center, focusing on the description of the surgical approach to treatment (and use of femorojugular bypass). The associated morbidity and oncologic outcomes are also analyzed.
Material and Methods
Study design
A retrospective observational study was designed. Data was collected from patients presenting with retroperitoneal sarcoma with Inferior Vena Cava (IVC) involvement, operated on between March 2021 and May 2023 at the Hospital de la Santa Creu i Sant Pau (Barcelona), a sarcoma referral center. Sex, age, and preoperative tumor characteristics were collected. Intraoperative variables included technical details of the surgeries performed, the IVC involvement, and the use of the femorojugular bypass. Postoperative complications and oncologic outcomes were also recorded.
Surgical particularities for femorojugular bypass
The material used to perform the femorojugular bypass was an 18Fr arterial perfusion cannula (Edwards Lifescience OptiSite, Irvine, CA, USA) for cannulation of the femoral vein and internal jugular vein.
Cannula placement in the femoral vein and internal jugular vein was performed with ultrasound guidance to ensure accurate placement.
During the femorojugular bypass, blood flow between 0.5 to 2.3 liters per minute was established. For anticoagulation during the bypass, intravenous heparin was administered and the Activated Clotting Time (ACT) was maintained at around 250 seconds. At the end of clamping, the anticoagulation administered during the femorojugular bypass was reversed using protamine (an agent used to neutralize the anticoagulant effects of heparin and restore normal coagulation) in a 1:1 ratio.
Statistical analysis
Data were analyzed with Excel and Statistical Package for Social Sciences (SPSS 21) software. Continuous variables are expressed as means, medians, and interquartile ranges. Cumulative survival was analyzed with the Kaplan-Meier survival curve.
Results
This retrospective series included a total of 7 patients affected by PRRS with IVC involvement who underwent surgery at the Hospital de la Santa Creu i Sant Pau (Barcelona) during the period described. Patient demographics and tumor histology are listed in table 1.
| Table 1: Demographic data. | |
| Demographic and Disease Data | |
| Average / median age (years) | 59.86 (49-76)/55 |
| Gender Male Woman | 6 1 |
| Tumor size (cm), mean / median | 13.71 (3-26)/11 |
| Histological subtype Leiomyosarcoma Fibromyxoid sarcoma Liposarcoma | 3 1 3 |
| Histological grade Low grade High grade | 1 6 |
| Tumor status Primary Recurrence | 4 3 |
| IVC involvement Primary tumor IVC Infiltration of IVC Contact with IVC (no infiltration) | 2 1 4 |
| Distant disease Yes No | 1 6 |
Four patients had primary tumors with IVC involvement, only two of them being a leiomyosarcoma that originated as a primary tumor in the IVC itself. Three patients presented with a recurrence of a previously operated retroperitoneal liposarcoma. Only one of the patients showed distant disease in the form of a single bone metastasis in the right iliac crest.
In all seven cases, surgery was performed with the possibility of performing a vein-vein bypass prior to clamping the IVC, although this was only necessary in three of the cases. However, in one of them, an injury to the common femoro-iliac vein occurred during cannulation that required immediate repair and extracorporeal circulation was ruled out.
In the first case where veno-venous bypass was used, vena cava clamping was performed for 7 minutes which produced a decrease in central venous pressure from 10 to 8 mmHg and a decrease in blood pressure figures (down to 60/30 mmHg), necessitating the start of vasoactive drugs such as noradrenaline (requirements up to 45 mL/h). Consequently, a femorojugular bypass was used for 12 minutes, and an increase in blood pressure and a decrease in noradrenaline requirements down to 25mL/h were observed.
In the second case in which the bypass was used, when the vena cava was clamped, there was also a decrease in blood pressure from 110/50 mmHg to 90/40 mmHg and the need to increase the dose of noradrenaline up to 20 mL/h. Once the bypass was started (total duration of 55 minutes), an improvement in hemodynamic status was also observed (increase in blood pressure up to 100/50 mmHg), which led to a decrease in noradrenaline supply to 10 mL/h.
Regarding the technical details of the surgeries (Table 2), one of the patients required resection of an IVC segment, preserving the insertion of the left renal vein, and the subsequent reconstruction was with a 10-cm tabularized bovine pericardium patch. Two other patients required opening of the IVC (one due to direct infiltration of the tumor and the other due to partial tumor thrombosis from the right renal vein), which was repaired in both cases with simple suture. In the remaining four patients, IVC infiltration was ruled out intraoperatively and neither resection nor reconstruction was required.
| Table 2: Data related to surgery. | |
| Surgery | |
| Resection R0/R1 R2 | 5 2 |
| Number of organs resected (including IVC) 1 2 3 > 3 | 3 2 1 1 |
| IVC reconstruction procedures Yes Simple suture Tabularized bovine pericardium patch No | 3 - 2 - 1 4 |
| Vein-vein bypass Yes No | 2 5 |
| IVC clamping Yes No | 5 2 |
| Mean blood loss (L) | 2.214 |
| Intraoperative blood transfusions (medium/median) | 3.14 / 2 |
| Clavien-Dindo 0 I/II IIIa IIIb IVa/IVb/V | 1 5 0 1 0 |
| Specific complications Case 1: No complications Case 2: Acute renal failure, paralytic ileus, bilateral pleural effusion, seroma, biloma. Case 3: Edema in the legs Case 4: Acute kidney failure (AKI), Atrial Fibrillation (AF) debut, paralytic ileus, and groin wound infection, surgical site fluid collection, neuropathic pain in the right arm. Case 5: Jejunal anastomosis failure (reoperation), intra-abdominal fluid collections. Case 6: Paralytic ileus Case 7: Perihepatic hematomas, inguinocrural hematoma. | |
In five of the cases a radical resection of the tumor was performed, leaving no macroscopic disease. In one other patient it was not possible to perform bloc resection of the tumor, leaving macroscopic disease. In the last patient, it was detected intraoperatively that the tumor was in intimate contact with the superior mesenteric artery and the celiac trunk without a dissection plane, so it was considered unresectable and the surgery was not continued.
The mean estimated blood loss for the procedures was 2.21 L. On average, patients required 3.14 red blood cell transfusions intraoperatively, with a median of 2.
Only one of the patients in the series presented major postoperative complications, in the form of a jejunojejunal anastomosis failure that required an urgent second surgery for reestablishment of the jejunojejunal anastomosis. There was no postoperative mortality in any of the cases.
Minor complications (Clavien I or II) were observed in five cases. Two of the patients experienced paralytic ileus that required parenteral nutrition support. Two patients developed acute renal failure, which resolved without the need for renal replacement therapy. One patient developed a biloma in the resection bed (after requiring resection of the hepatic segment VI during the operation), which was resolved conservatively with antibiotic therapy. One patient experienced hematoma at the perihepatic level and at the level of the groin wound, which were managed conservatively with transfusion support and without the need for intervention.
All complications are related to the type of surgery and do not seem to be related to the use of by-pass or IVC clamping.
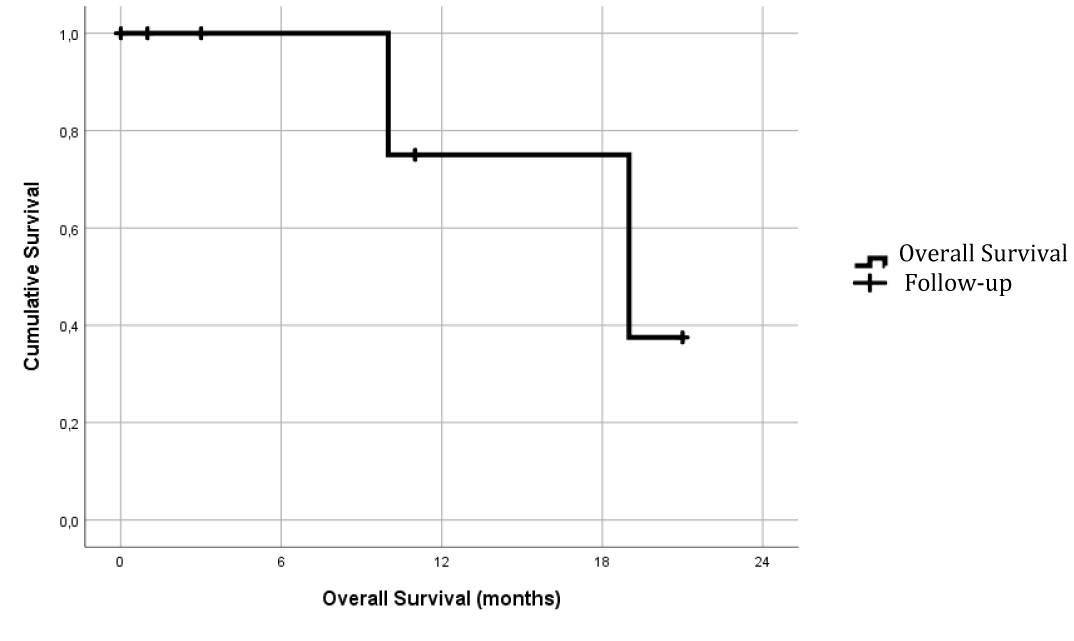
At a median follow-up of 11.86 months (median 10 months and with an interquartile range of 4-20 months), overall survival (Figure 2) at 12 months was 75%.
At the date of publication, of the five patients in whom radical resection of the disease was achieved, only one of them had presented local and pulmonary recurrence 8 months after surgery. The other four are alive and free of recurrence.
Of the two patients in whom macroscopic resection of the tumor could not be completed, both showed disease progression during follow-up. One of them was the only exitus of the series 11 months after surgery.
Conclusion
Cava leiomyosarcoma is an uncommon disease, which makes it difficult to standardize both its diagnosis and treatment, and for this reason multidisciplinary management in referral centers is recommended.
The main (and potentially curative) treatment is surgical resection, where resection of the vena cava and subsequent reconstruction are necessary.
Performing a femorojugular bypass can be useful in those patients who do not hemodynamically tolerate the clamping of the inferior vena cava, that is, who present hypotension (mean arterial pressure < 65 mmHg) refractory to vasoconstrictor drugs at high doses, and poor organ perfusion with lactacidemia. With this strategy, an improvement in the hemodynamic status of the patient is achieved, which allows for complete tumor resection.
A femorojugular bypass is a technique that should be considered during the surgical excision of retroperitoneal tumors that present infiltration of, or suspicion of possible lesions to, the vena cava, especially in patients with a patent inferior vena cava, to avoid the complications of an unexpected clamping.
A limitation of this review is that it has a low number of cases (given the low incidence of the pathology), so it would be interesting to conduct future research with a larger number of cases (conducting multicenter studies) and include a control group to better evaluate the effectiveness of the procedure.
References
- Stanley JC, Veith F, Wakefield TW. Current therapy in vascular and endovascular surgery. 5th ed. Elsevier - Health Sciences Division; 2014.
- Gloviczki P, Dalsing MC, Eklöf B, Lurie F, Wakefield TW, Gloviczki ML. Handbook of venous and lymphatic disorders. 4th ed. CRC Press; 2017. doi: 10.1201/9781315382449. 2017.
- Galland B. Rutherford's Vascular Surgery, 7th edn. Ann R Coll Surg Engl. 2011 Mar;93(2):176. doi: 10.1308/003588411X561062. PMCID: PMC3293326.
- Pérez-de-Villar JM, Arjona-Sanchez A, Rufián-Andujar B, Valenzuela-Molina F, Sánchez-Hidalgo JM, Rodriguez-Ortiz L, Casado-Adam A, Viyuela-García C, Rufián-Peña S, Caro-Cuenca T, Moreno-Vega A, Briceño-Delgado J. Surgical treatment of vena cava leiomyosarcomas: Series of cases in a referral center and literature review. Cir Esp (Engl Ed). 2022 Aug;100(8):481-487. doi: 10.1016/j.cireng.2022.05.020. Epub 2022 May 18. PMID: 35597419.
- Wong D, Hockley J, Parys S, Hodder R, Jansen S, Newman M. Current Technology Venous-Venous Bypass Improves the Safety of Resection of Sarcoma and Benign Retroperitoneal Tumours Involving the Inferior Vena Cava. Eur J Vasc Endovasc Surg. 2022 Nov;64(5):575-576. doi: 10.1016/j.ejvs.2022.07.049. Epub 2022 Aug 12. PMID: 35964890.
- Kulaylat MN, Karakousis CP, Doerr RJ, Karamanoukian HL, O'Brien J, Peer R. Leiomyosarcoma of the inferior vena cava: a clinicopathologic review and report of three cases. J Surg Oncol. 1997 Jul;65(3):205-17. doi: 10.1002/(sici)1096-9098(199707)65:3<205::aid-jso11>3.0.co;2-2. PMID: 9236931.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.