General Science Group. 2024 July 10;5(7):737-742. doi: 10.37871/jbres1949.
The Effect of Humidity on the Mass and Unit Cell Volume of Montmorillonite near Room Temperature
Haiyan Sun1, I-Ming Chou1*, Dejun Huang2 and Pingjing Li1
2Malvern Panalytical (China), Shanghai 200233, China
- Montmorillonite
- SWy-2
- X-ray powder diffraction
- Humidity buffer
Abstract
Phase equilibria between montmorillonite (Swy-2 from Wyoming, USA) and H2O vapor phase were established by using the humidity buffer technique at 0.1 MPa and room temperature (23 ± 0.5°C). The Relative Humidity (RH; equivalent to the activity of H2O) levels of the system were defined between 6.5% and 97.4% by using six different salt-saturated aqueous solutions. There was no direct physical contact between Swy-2 sample and the buffer solution to prevent any metal ion exchanges between them. The reaction time at each humidity buffer was 10 days, after which the mass of the sample was determined and X-ray powder diffraction spectrum was collected. The experiments were carried out in two directions: one is adsorption (i.e., from low to high RH), and the other is desorption (i.e., from high to low RH), and the equilibrium of the system was demonstrated by the adsorption-desorption reversal experiments in terms of both mass change and crystal parameters (d-001 spacing and unit cell volume). When compared our data with those from previous study for Na-saturated montmorillonite, either synthetic or natural, the Na replacement in the crystal structure favored the retention capacity of water, but only under RH higher than about 62%.
Introduction
Montmorillonite, the main component in bentonite, is often called a "universal clay" due to its special physical and chemical properties. Bentonite has been widely used in many fields, including environmental protection, agriculture and animal husbandry, and medical, food, construction and petroleum industries [1-6]. It is also commonly used as an engineering barrier for high-level radioactive waste or underground waste treatment [1] due to its low permeability under compact condition, as well as its high expansion capacity with capability of retaining pollutants. The multiple utilizations of bentonite are mostly resulted from the characteristic hydration, expansion, cation exchange, and surface complexation properties of montmorillonite [7,8].
Because montmorillonite has good swelling ability, hydrophilicity, and strong adsorption performance, investigations on the relationship between its swelling ability and interlayer distance of montmorillonite crystal structure have been continuously carried out. In previous studies, the durations of humidity-controlled experiments were relatively short, generally not exceeding 12 hours [3], and the experimental system may not necessarily reach equilibrium. In other studies, in order to control experimental humidity (or activity of H2O), montmorillonite was immersed in an aqueous solution containing salt (e.g., NaCl) during experiment before the subsequent treatments of the sample were carried out [9-16]; in these studies, the range of controlled humidity levels is rather limited, and the direct contact of montmorillonite with an aqueous solution tends to change the composition of the montmorillonite sample through metal-ion exchange. In this study, the humidity buffer technique [17, and references therein; will be described later] was used to control the humidity of the montmorillonite sample, such that the humidity of the sample at a fixed experimental pressure and temperature condition was defined by a salt-saturated solution in a closed system; there was no direct physical contact between the sample montmorillonite and the buffer solution, and the isopiestic equilibrium between the sample and buffer solution was achieved by the transfer of H2O through the vapor phase in the closed system.
Materials and Methods
Materials
Wyoming montmorillonite (SWy-2) was selected as our starting material. Six humidity buffers were used in this study, which were saturated aqueous solutions containing the following salts: LiBr, MgCl2•6H2O, NaBr, NaCl, KCl and K2SO4. The specific information of the starting materials is listed in table 1.
| Table 1: Information of reagents used in the experiments. | |||
| Reagent | Manufacturer | Batch number | Purity |
| LiBr | Shandong Xixia Reagent Co., Ltd | P0709 | AR (Analytical Reagent) |
| MgCl2•6H2O | Xilong Scientific Co., Ltd | 1909241 | AR |
| NaBr | Shandong Xixia Reagent Co., Ltd | Q3670 | AR |
| NaCl | Guangzhou Chemical Reagent Factory | GB/T1266 | AR |
| KCl | Shandong Xixia Reagent Co., Ltd | X63512 | AR |
| K2SO4 | Shandong Xixia Reagent Co., Ltd | D35699 | AR |
| SWy-2 H2O | Clay Minerals Society Source Clays Repository, USA Merck KGaA, Darmstadt, Germany | 1302-78-9 Milli-Q IQ7000 | Content: 90-100% Ultrapure Water |
Experimental set up
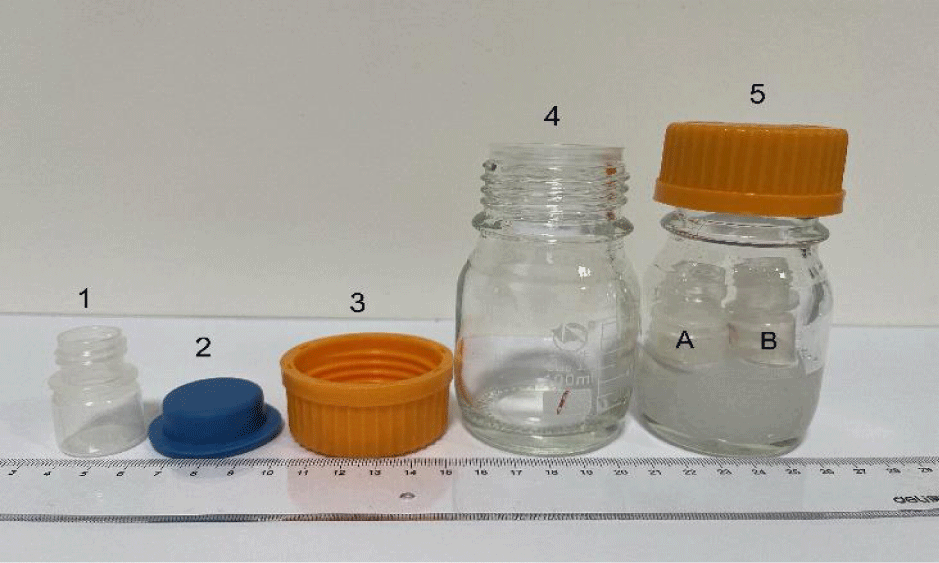
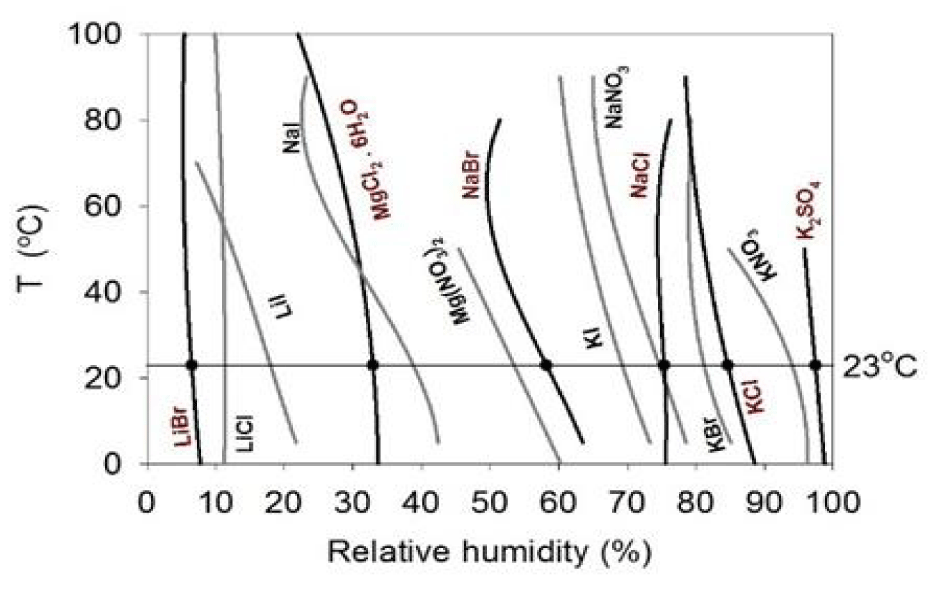
The humidity buffer set up was used to control humidity (or H2O activity) of an enclosed experimental system, as shown in figure 1, in which the montmorillonite sample (SWy-2) was exposed to the vapor phase of the humidity buffer, which is a salt-saturated aqueous solution. The methodology of this technique as well as its development history and applications were summarized in [17]. Six humidity buffers from 28 binary saturated aqueous solutions reported by Greenspan [18] were selected in this study, and the buffered humidity levels at our experimental temperature of 23 ± 0.5°C (controlled by air conditioning) are listed in table 2, such that the Relative Humidity (RH) levels of the samples were controlled between 6.5 and 97.4% under 0.1 MPa of atmospheric pressure (Figure 2).
| Table 2: Relative humidities (% RH) of the six humidity buffers used in this study at 0.1 MPa and 23 ± 0.5°C based on Greenspan [18]. | |
| Humidity Buffer | % RH |
| LiBr | 6.47 ± 0.023 |
| MgCl2. 6H2O | 32.91 ± 0.029 |
| NaBr | 58.20 ± 0.157 |
| NaCl | 75.37 ± 0.018 |
| KCl | 84.65 ± 0.076 |
| K2SO4 | 97.42 ± 0.029 |
Experimental procedures
The following procedures were followed in this study:
Weighed two small plastics bottles A and B, and weighed them again after adding the SWy-2 sample powder. Mettler Toledo (ME204TE/02) was used for weighing (accurate to 0.1 mg).
- Immersed the lower parts of the two sample-loaded small plastics bottles, without caps, in an NaCl-saturated humidity buffer solution in a large glass bottle, which was then sealed by adding a stopper and cap, as shown in item 5 of figure 1.
- Kept the large glass bottle with sample and buffer solution at a temperature of 23 ± 0.5°C for 10 days for the system to reach isopiestic equilibrium.
- Removed the two small plastics bottles, wiped and dried their outer parts, and then weighed bottle A and took a small portion of the sample powder from bottle B for X-ray powder diffraction analysis, and the powder sample was returned to bottle B after non-destructive scanning analysis. The XRD test was performed using an Analytical Empyrean powder diffractometer. The anode material is Cu, with K-Alpha1, K-Alpha1, and K-Beta values of 1.54060, 1.54443, and 1.39225 Å, respectively. The current and voltage during the test are 40 mA and 45 kV, respectively. The range of 2θ is 3-80°, the step size is 0.013°, and the test time is for 10 min each.
- Repeated the above procedures by using other humidity buffer solution listed in table 2, and the experiments were carried out from Exp. No. 1 (NaCl-saturated solution) to No. 10 (NaBr-saturated solution) listed in table 3.
| Table 3: Mass values of SWy-2 sample under different humidity conditions and relative mass changes (Δ mass) using the mass of the sample equilibrated with NaCl-saturated humidity buffer solution as a reference. The equilibrated unit cell volumes of SWy-2 montmorillonite crystals are also listed. | ||||||
| Experimental number | Humidity buffer | % RH | Sample mass (g) | ∆ Mass (mg) | ∆ Mass (%) | Unit cell volume (Å3) |
| 1 | NaCl | 75.37 | 1.0677 | 0 | 0 | 533.70 |
| 2 | KCl | 84.65 | 1.0886 | 20.9 | 1.96 | 557.97 |
| 3 | K2SO4 | 97.42 | 1.1641 | 96.4 | 9.03 | 559.06 |
| 4 | KCl | 84.65 | 1.1063 | 38.6 | 3.62 | 544.30 |
| 5 | NaCl | 75.37 | 1.0905 | 22.8 | 2.14 | 527.47 |
| 6 | NaBr | 58.20 | 1.0515 | -16.2 | -1.52 | 519.20 |
| 7 | MgCl2.6H2O | 32.91 | 1.0124 | -55.3 | -5.18 | 505.49 |
| 8 | LiBr | 6.47 | 0.9472 | -120.5 | -11.29 | 489.63 |
| 9 | MgCl2.6H2O | 32.91 | 0.9815 | -86.2 | -8.07 | 498.46 |
| 10 | NaBr | 58.20 | 1.0251 | -42.6 | -3.99 | 519.40 |
Results
Mass changes of montmorillonite during adsorption and desorption
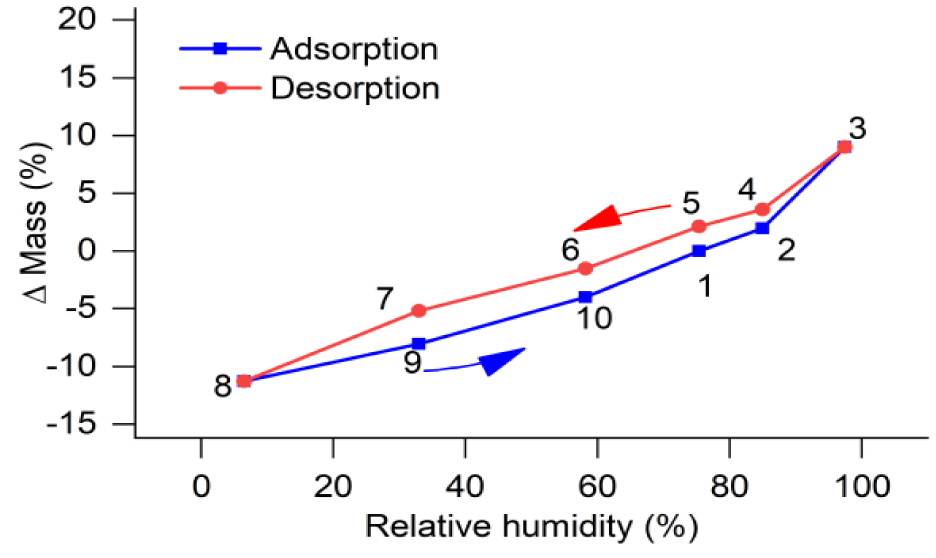
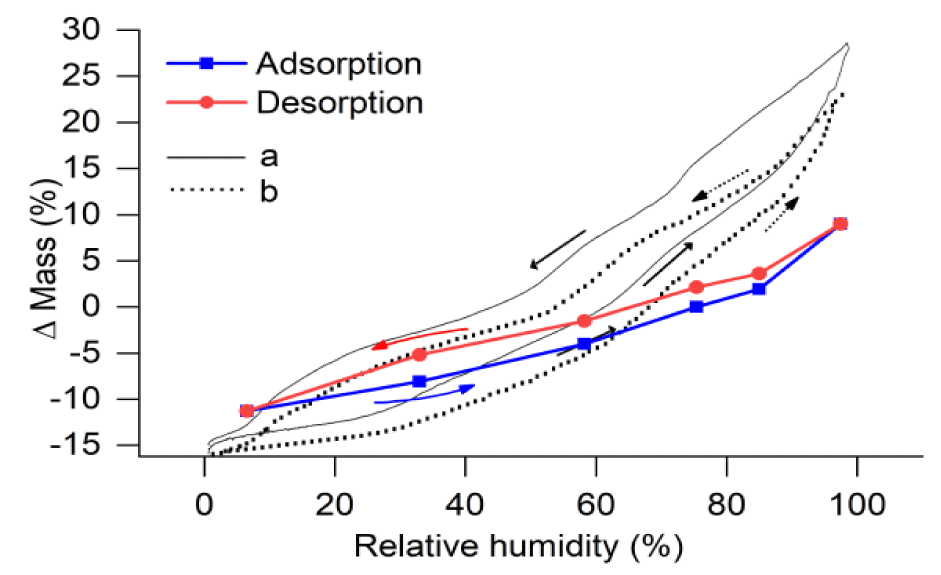
The mass values of SWy-2 sample, after equilibrated with salt-saturated humidity buffer solutions at 23 ± 0.5°C for 10 days, are listed in table 3. During data processing, the mass of SWy-2 sample equilibrated with the NaCl-saturated solution was used as a reference, with a mass change of 0 at 75.37 % RH, and the values of relative mass change (Δ mass) at various % RH are listed in table 3, together with the percentage values of mass change. The relation between Δ mass (%) and % RH during adsorption and desorption processes are shown in figure 3.
Variation of unit cell volumes of montmorillonite crystals with humidity
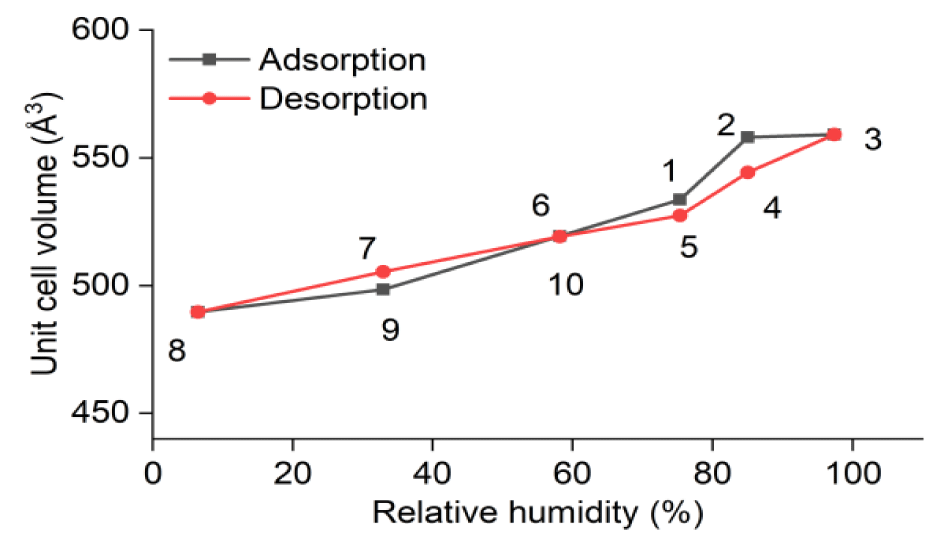
The obtained X-ray powder diffraction (XRD) data of SWy-2 montmorillonite were subjected to XRD phase identification and full spectrum fitting analysis (Rietveld analysis method) using High Score Plus software to obtain the unit cell volume of the sample crystals under different humidity conditions. The specific unit cell volumes are listed in table 3, and the trends of their changes with humidity are shown in figure 4.
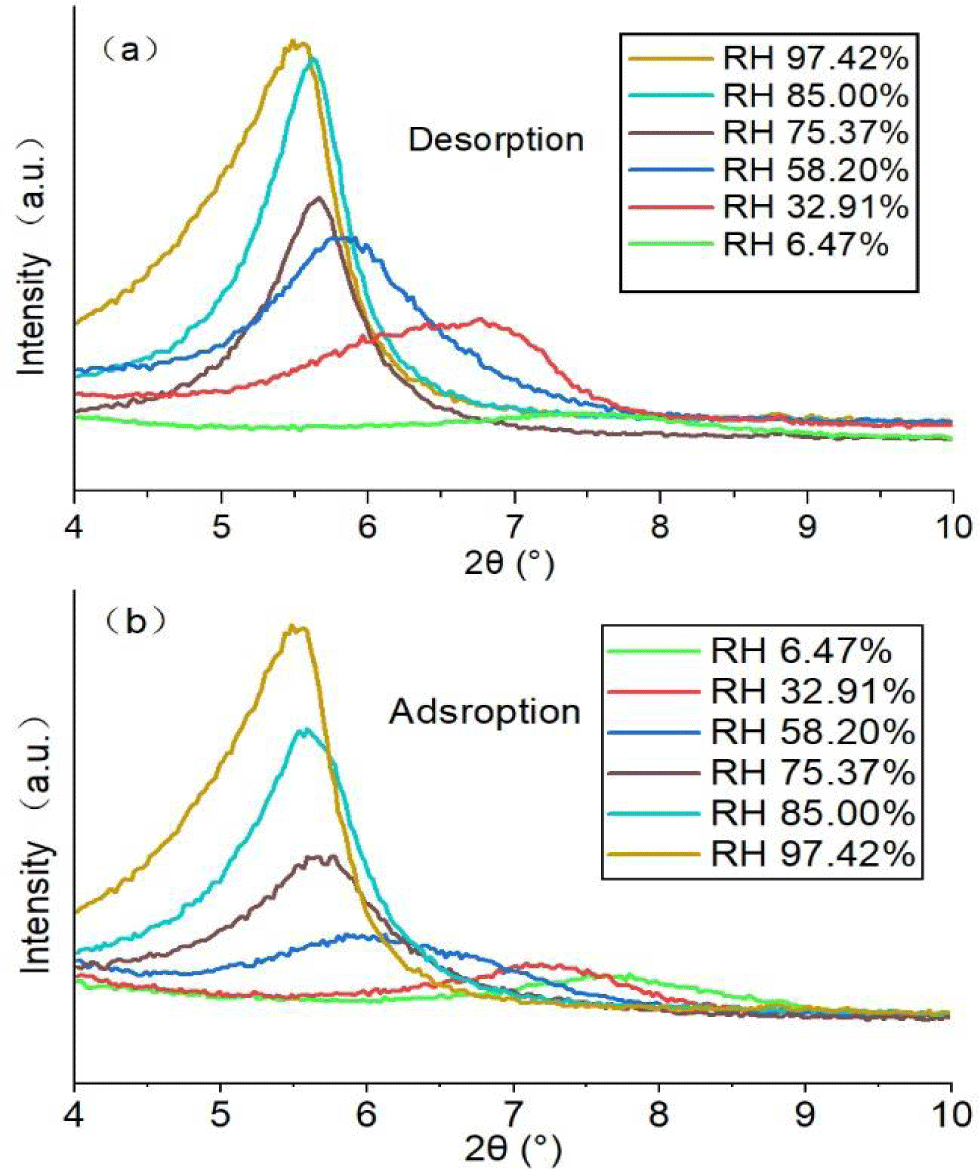
Variations of XRD patterns and d (001) of SWy-2 crystals with humidity
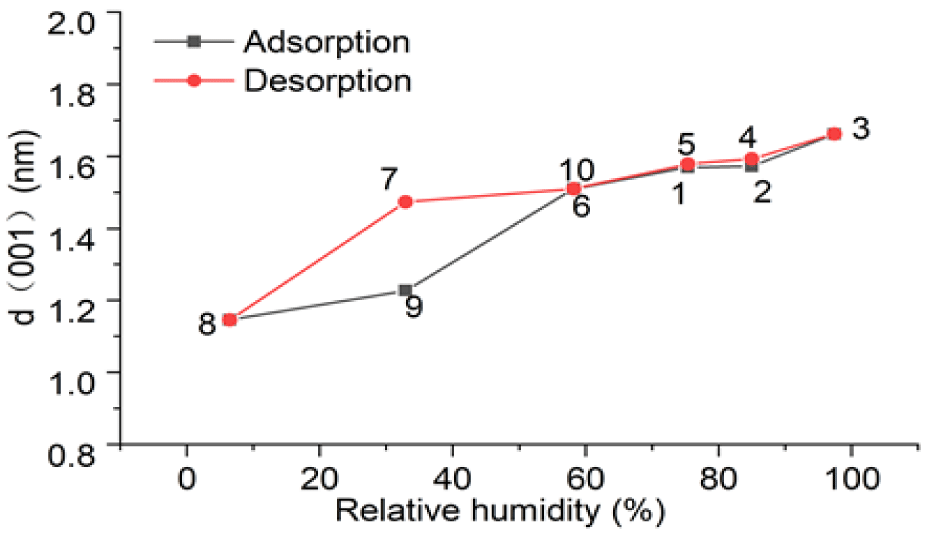
The X-ray diffraction patterns of SWy-2 montmorillonite crystals collected at various humidity conditions are shown in figure 5, and their equilibrium d (001) spacing values are shown in figure 6.
During desorption of SWy-2 montmorillonite crystals (Figure 5a), the characteristic peak for (001) spacing shifted toward a higher diffraction angle, resulting in a decrease in the inter-planar spacing (d-001), as shown in figure 6.
Discussion
In this study, two plastics sample bottles containing weighed amounts of natural SWy-2 montmorillonite (A and B shown in item 5 of figure 1) were equilibrated with the H2O vapor in an enclosed system (item 5 in figure 1), buffered by six salt-saturated aqueous solutions at 23 ± 0.5°C and 0.1 MPa. During experiments, the material loaded in sample bottle A was not disturbed, except the gain or loss of H2O through isopiestic equilibrium with the buffer solutions, while that in bottle B was changed when some of which was taken out for X-Ray analysis, and it was not possible to return all of the sample powder back to bottle B. This is the reason why we used two sample bottles, and the mass changes during adsorption and desorption processes were determined for bottle A and listed in table 3. The mass changes of natural SWy-2 montmorillonite sample equilibrated under different humidity conditions at 23 ± 0.5°C, shown in figure 3, indicate that the mass of the sample at a fix humidity after desorption process (red dot) is higher than that after adsorption process (blue dot), such that the desorption trend (red line) is above the adsorption trend (blue line). In figure 7, these trends are compared with those of a previous study at 303 K [16] for: (a) synthetic Na-MMT (synthetic sodium montmorillonite) shown by the solid black lines; and (b) natural Na-SWy-2 (sodium-saturated SWy-2 montmorillonite) shown by the dotted lines. Their general trends in both desorption and adsorption processes are similar to ours, except their “Δ mass” values are higher than ours at RH levels higher than about 65%, indicating the favorable effect of Na replacement in the structure of their crystals for the retention capacity of water at relatively high humidity conditions. In other words, to increase the retention capacity of water in the applications of montmorillonite, pre-saturation of montmorillonite with Na is a good choice only when the environments are high in humidity.
Figure 6 shows that the variations of d(001) inter-layer spacing are not affected by the desorption or adsorption processes, except at 32.91% RH buffered by the MgCl2.6H2O-saturated solution. At this RH, a tight reversal in terms of d(001) spacing could not be reached for unknown reason(s); further studies are needed for clarification.
Conclusion
The effects of humidity on the physical properties of natural SWy-2 montmorillonite, including its mass, unit cell volume and d (001) spacing, were examined at 23°C and 0.1 MPa by using the humidity buffer technique described by Chou IM, et al. [17].
The comparison of our data for the relationship between the mass of SWy-2 montmorillonite and humidity with previous data [16] for Na-saturated montmorillonite, either synthetic of natural, indicates that the addition of Na in the structure of montmorillonite favors the retention capacity of water either in the adsorption or desorption processes, in the environments with relatively high humidity with RH greater than 65%. Therefore, in lower humidity environments, the incorporation of Na in montmorillonite will not always increase its capacity of water retention.
During our experiments, montmorillonite sample and humidity buffer solution were physically separated, and the isopiestic equilibria were achieved through the connected vapor phase, such that the sample was not contaminated by the buffer.
The long duration of experiments (10 days) ensured the system reached equilibrium. However, the tight reversal of d (001) spacing of SWy-2 montmorillonite crystal could not be reached between adsorption and desorption processes at RH of 32.9% (Figure 6), and further study is needed to clarify this.
Acknowledgment
We would like to thank the support of the National Natural Science Foundation of China (Grant No. 42130109) the Hainan Provincial Natural Science Foundation (No. 422RC745, and No. 221RC1086), and the Deep-sea Infrastructure and Support Platform Improvement Program of DSTIC (No. DSTIC-SSPT-2022005, and No. DSTIC-SSPT-2022008).
References
- Bergaya F, Theng BKG, Lagaly G. Clay science. Developments in Clay Science. 2013;5:819-855. doi: 10.1016/B978-0-08-098258-8.00028-6.
- Chen G, Yan CJ, Shou JF, Han XS, Chen LS, Mei J. A Study on the factors influencing the quantitative analysis method of montmorillonite by X-ray diffraction. Non-Metallic Mines. 2011;34(1):3. doi:10.3969/j.issn.1000-8098.2011.01.018.
- Dai SY, Wang N, Ma YX, Wang B, Li G. A Study on the factors influencing the qualitative and quantitative analysis of montmorillonite (Powder) by X-ray powder diffraction. Journal of Nanjing Normal University (Natural Science Edition). 2019;42(2):7. doi:10.3969/j.issn. 1001-4616.2019.02.019.
- Lei SM, Hao Q, Xiong BH, Zeng XB. The characteristics and development prospects of montmorillonite minerals. Resources, Environment and Engineering. 2006;20(5):5. doi: 10.3969/j.issn.1671-1211.2006.05.019.
- Shi HP, Wu RF, Yang W. Development and application of montmorillonite. Inner Mongolia Petrochemical. 2004;30(2):3. doi: CNKI:SUN:NMSH.0.2004-02-010.
- Uddin F. montmorillonite: An introduction to properties and utilization conflict of interest author details. 2019. doi: 10.5772/intechopen.77987.
- Murray HH. Applied clay mineralogy today and tomorrow. Clay Minerals. 1999;34(1):39-39. doi: 10.1180/000985599546055.
- Caglar B, Afsin B, Tabak A, Eren E. Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chemical Engineering Journal. 2009;149(1-3):242-248. doi: 10.1016/j.cej.2008.10.028.
- Chantel C, Tester, Shaul A, Benjamin G, Jillian F. Short- and long-range attractive forces that influence the structure of montmorillonite osmotic hydrates. Langmuir. 2016;32(46):12039-12046. doi:10.1021/acs.langmuir.6b03265.
- Svensson PD, Hansen S. Freezing and thawing of montmorillonite: A time-resolved synchrotron X-ray diffraction study. Applied Clay Science. 2010;49(3):127-134. doi: 10.1016/j.clay.2010.04.015.
- Borralleras P, Segura I, Aranda AGM, Aguado A. Influence of experimental procedure on d-spacing measurement by XRD of montmorillonite clay pastes containing PCE-based superplasticizer. Cement and Concrete Research. 2019;116: 266. doi: 10.1016/j.cemconres.2018.11.015.
- Zeng MF, Zheng X, Wang YD, Shu GQ, Zhao J, Xu MD, Qi CZ, Cao XZ, Wang BY. Investigation of organophilic montmorillonites supported palladium catalytic composites by combined positron annihilation lifetime spectroscopy and X-ray diffraction. Radiation Physics and Chemistry. 2019;165:108343. doi: 10.1016/j.radphyschem.2019.108343.
- Yoshimoto S, Ohashi F, Kameyama. X-ray diffraction studies of intercalation compounds prepared from aniline salts and montmorillonite by a mechanochemical processing. Solid State Communications. 2005;136(5):251-256. doi: 10.1016/j.ssc.2005.08.017.
- Amorim C, Lopes RT, Barroso RC, Queiroz JC, Alves DB, Perez CA, Schelin HR. Effect of clay–water interactions on clay swelling by X-ray diffraction. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2007;580(1):768-770. doi: 10.1016/j.nima.2007.05.103.
- Zhai YG, Ci XM, Zou X, Guo LL. Mineral composition and chemical composition analysis of medicinal montmorillonite clay. Chinese Herbal Medicine. 2002;33(4):3. doi:10.7501/ j.issn.0253-2670.2002.4.134.
- Lydie Le Forestier, Fabrice Muller, Frédéric Villiéras, Manuel Pelletier. Textural and hydration properties of a synthetic montmorillonite compared with a natural Na-exchanged clay analogue. Applied Clay Science, Elsevier, 2010, 48, pp.18-25. 10.1016/j.clay.2009.11.038.insu-00433524.
- Chou IM, Seal RR, Robert R, Wang A. The stability of sulfate and hydrated sulfate minerals near ambient conditions and their significance in environmental and planetary sciences. Journal of Asian Earth Sciences. 2013;62(1):734-758. doi: 10.1016/ j.jseaes.2012.11.027.
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J. Res. National Bur. Stnd. 1977;81(1):81-89. doi:10.6028/jres.081A.011.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.