Medicine Group. 2024 February 28;5(2):214-220. doi: 10.37871/jbres1884.
Induction Therapy with Bortezomib Cyclophosphamide Dexamethasone and Conditioning with Lenalidomide for Al Renal Amyloidosis: Long-Term Follow Up
Greco Rosita1*, Agata Mollica1, Botta Cirino2, Gentile Massimo3, Enrica Martino4 and Papalia Teresa1
2Department of Health Promotion, Mother and Child Care, Internal Medicine, University of Palermo, Italy
3Hematology Unit, Annunziata Hospital, Cosenza, Italy
4Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, Italy
- Renal amyloidosis
- Lenalidomide
- Partial remission
- Proteinuria
Abstract
Objectives: In our study we evaluated the long term efficacy of protocol with bortezomib in induction and lenalidomide in maintenance for AL renal Amyloidosis transplant-ineligible patients. Methods. A prospective analysis of 16 transplant-ineligible patients with histological diagnosis of renal amyloidosis. All patients had overt nephrotic syndrome. Bortezomib-based (CyBorD) regimen was used for 9 cycles. The patients with PRR were treated with Lenalidomide and dexamethasone.
Results: The mean age was 63±7aa and 7/16 were males. The amyloid deposits were FLC k in 6/16 and lambda in 10/16. At onset the mean proteinuria was 13.1±3.6gr/24h with average MDRD of 37.2ml/min. At onset 3 patients had Myeloma. 3/16 patients died for cardiac arrhythmia. CyBorD regimen was completed in 13/16. After 9 CyBorD cycles: 3/13 showed a Complete Renal Response (CRR) and Hematological Response (CHR); 10/13 showed a Partial Renal Response (PRR) and Partial Hemathological Response (PHR), but one died for Sepsi. The 9 patients in PRR were treated with Lenalidomide. After median 6 cycles 7/9 showed a CRR and CHR. Haemodialysis was started in 2/9. All the patients with CRR have been in remission for 30 months on average. Conclusion. Maintenance therapy with Lenalidomide is effective for complete and sustained renal remission.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a rare life-threatening disease caused by light chains that are toxic to vital organs such as the heart, kidneys, liver and peripheral nervous system, and that misfold and assemble as amyloid fibrils and deposit both in affected organs and systemically in the vasculature and other tissues [1-3]. This process causes progressive organ dysfunction, leading to organ failure and death if it is not arrested by effective therapy. Renal involvement is common in light-chain (AL) amyloidosis. The kidney is involved in 70% of patients and renal failure limits the therapeutic options. Typical clinical manifestations of kidney damage are proteinuria, nephrotic syndrome (i.e., concomitant proteinuria, hypoalbuminemia, and peripheral edema), renal insufficiency, and End-Stage Renal Disease (ESRD) in need of hemodialysis [4-7]. Pathological heart involvement is present in up to 90% of patients, and approximately 50% present with diastolic heart failure at the time of diagnosis [8]. Amyloid deposition within the myocardium results in thickening of ventricular and atrial walls leading to restrictive cardiopathy responsible for progressively increasing asthenia, dyspnoea and lower limb edema. Peripheral nerve involvement, present in 20% of patients with AL amyloidosis, is characterized by painful, slowly progressing sensorimotor peripheral polyneuropathy, similar to diabetic neuropathy. Autonomic neuropathyis often responsible for severe postural hypotension.
Monoclonal Gammopathy of Undetermined Significance (MGUS) is a known precursor of both MM and AL, and progresses to AL in 1.0% of cases with a relative risk of 8.8 as compared to the general population [10-13]. New therapeutic strategies with bortezomib and lenalidomide have improved the prognosis for high-risk patients who are transplant-ineligible. The aim of our study was to evaluate the long term efficacy of therapeutic protocol utilizing bortezomib in induction and lenalidomide in conditioning for PRR (partial renal response) in AL renal Amyloidosis transplant-ineligible patients.
Methods
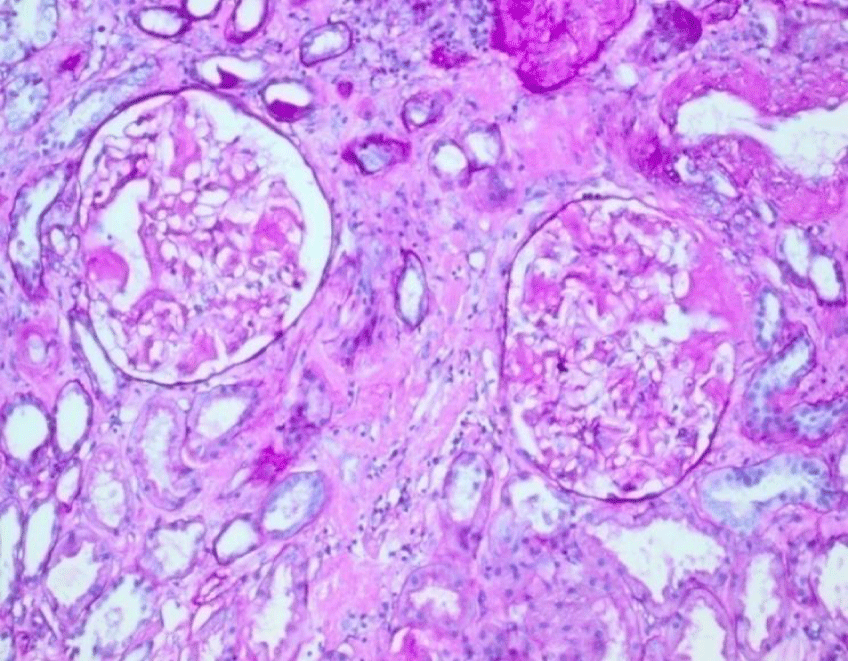
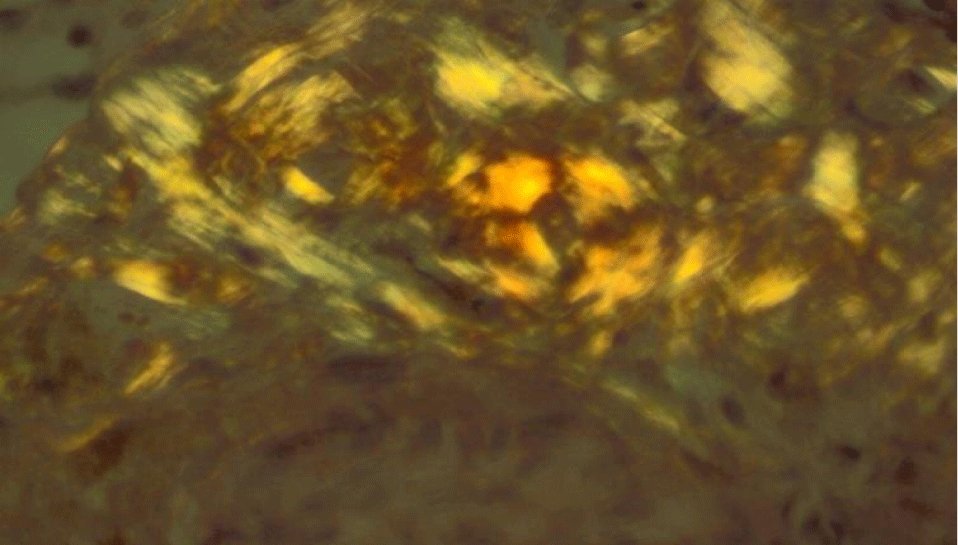
This is a prospective analysis of 16 transplant-ineligible patients (7 males and 9 females) with histological diagnosis of renal amyloidosis admitted to our Nephrology Department from 1/01/2010 to 01/10/2020. All patients have overt nephrotic syndrome at the diagnosis. Two/thirds of them have renal failure. A lower proportion of patients have involvement of the heart. The diagnosis of amyloid is based on the kidney biopsy finding, by light microscopic examination, of amorphous extracellular Congo red positive deposits (Figure 1), which display characteristic dichroism and apple green birefringence under polarised light (Figure 2). Amyloid typing relies primarily on immunohistochemistry on paraffin-embedded tissue sections, and immunofluorescence microscopy on frozen sections showing staining of deposits with a conjugate specific for kappa or lambda Light Chains (LC). Nephelometric quantification of Free Light Chains (FLC) in serum is useful in establishing the presence of a monoclonal protein in all patients. In addition a bone marrow biopsy is used in assessing plasma cell clonality and determining the plasma cell burden. Abdominal fat aspirate was performed in all patients.
All individuals whose data has been used in the clinical research studies described in this thesis gave explicit informed consent by signing a consent form whilst visiting the centre.
Bortezomib-based (CyBorD) regimen is used (Bortezomib 1.3 mg/m2on days 1-4-8-11in the first three cycles and after on days 1-8-15-22 subcutaneously; Cyclophosphamide 200 mg/m2p.o. and Dexamethasone 40 mg p.o. on days 1-8-15-22 of the 21-day cycle for 9 cycles). Infection prophylactic therapy for Pneumocystis and Herpes Zoster was recommended, the doses adjusted to renal function. Antibiotic prophylaxis would have been started if neutropenia appeared. Hematological and organ response were evaluated according to the novel criteria of the International Society of Amyloidosis (CHR: Negative serum and urine IFE, normal dFLC; PHR: dFLC decrease ≥ 50%). The patients with partial renal response (PRR: decreased of 24 hour urine protein and serum creatinine > 50% over baseline) were treated with cycles of Lenalidomide (dose adjustments for renal function, orally on days 1 trough 21 of each 28-days cycle) in combination with dexamethasone (20 mg on days 1-4, 9-12, 17-20 in the first 4 cycles and after 10 mg on days 1-4) and prophylactic anti-thrombotic treatment (aspirin 100 mg).
Results
The mean age was 63±7 aa and 7/16 (43.7%) were males. By immunohistochemistry the protein composition of the amyloid deposits was FLC k in 6/16 and FLC lambda in 10/16. Periombelical fat aspirate was positive in 5 patients (31.2%). At onset the mean proteinuria was 13.1 ± 3.6 gr/24h with average MDRD of 37.2 ml/min (III stage CKD), average pro-BNP 420.1 and SIV 12.5 mm. At onset three patients (18.7%) had confirmed multiple myeloma (Clone of plasma cells proliferation > 30% in the bone marrow) and severe cardiac failure. 8/16 (50%) patients showed the pheripheral nerve involvement, characterized by painful and sensorimotor peripheral polyneuropaty at electromyography and postural hypotension. The genetic variant of transthyretin (TTR) and serum amyloid A component did not occur in anybody.
3/16 patients died for cardiac arrhythmia at 3 and 4 months after CyBorD cycles initiation (Table 1). Therefore CyBorD regimen was completed in 13/16 patients.
| Table 1: After BD regimen 3/13 (23%) patients showed a CRR, after Lenalidomide regimen 7/13 (53.8%) patients showed a CRR. Total number of patients in CRR are 76.8%. | ||||
| Symptoms | N° patients/tot (16) At onset |
CR after 9 CyBorD (Tot 13 patients) |
PR after CyBorD (Tot 13 patients) |
CR after Lenalidomide (Tot 13 patients) |
| Cardiac failure | 3 (18.7%) | All died | / | |
| Arrhytmia | ||||
| MM | ||||
| Postural Hypotension Peripheral Neuropathy |
8 (50%) | 100% | / | |
| LVH | 13 (81.2%) | 3 (23%) | 10 (77%) | 100% |
| Nephrosic Syndrome | 16 (100%) | 3 (23%) | 10 (77%) | 7 (53.8%) |
| Renal Failure | 8 (50%) | |||
| LVH: Left Ventricular Hypertrophy; MM: Myeloma Multiple; CR: Complete Remission; PR: Partial Remission; CRR: Complete Renal Remission; Cybord: Cyclophosphamide/Bortezomib/Desamethasone. | ||||
After 9 CyBorD cycles: 3/13 patients showed a complete renal response (CRR: MDRD > 90 ml/min, proteinuria/24h< 300 mg) and CHR (normal serum FLC ratio); 10/13 (76.9%) showed aPRR and partial hemathological response (PHR, dFLC decrease >50% compared to baseline) but one of them with colostomy for previous diverticulitis died of sepsis (Table 1). All patients with CRR or PRR showed a complete cardiological response (normal BNP and SIV). Adverse events during therapy: HZV nevralgy in 3.
The 8 patients with Peripheral Polyneuropathy and Autonomic Neuropathy showed complete remission of hortostatic hypotension, but partial remission of peripheral neuropathy, already after the first CyBorD cycles.
The 9 patients in PRR were treated with Lenalidomide. After median 6 treatment cycles 7/9 (77.7%) patients showed a CRR and CHR (Table 1). We decided to discontinue the therapy for adverse events after an average of 6 cycles in 3 patients: breast cancer in one, transient troponin I increase and angina in another patient, pneumonia events in one. The pneumoniae infectious and anginal episodes regressed with drug discontinuation. In the others four patients the treatment was discontinued after one year. Haemodialysis was started in 2/9 (22.2%) patients.
One patients died for breast cancer, but in CRR. All the others patients with CRR, including the two who suspended Lenalidomide for its toxic effects (angina and pneumonia events), are still now in remission after an average of 48 months.
Discussion
All organs can be affected in systemic AL amyloidosis. Amyloid heart disease is a major prognosis factor as it account for approximately 75% of deaths, due to heart failure or arrhythmia [13-14]. In fact even our patients 3/16 with the cardiac involvement and the significant cardiovascular symptoms (severe cardiac failure and arrhytmia) had a bad progression. In the others 13 patients the cardiac involvement (increase of proBNP and SIV) has been only an incidental findings without any functional consequences. After CyBorD treatment 3/13 (23%) showed a complete cardiological response, the other 10 improved instead during conditioning with Lenalidomide. Our work shows that the severe cardiac involvement at onset worsened the outcome of patient survival [15].
Various types of amyloidoses are characterized by the development of peripheral neuropathy [16]. Immunoglobulin light chains aggregate and form amyloid deposits. Amyloid deposits or amyloid protein aggregates have been found at different locations within peripheral nerves [17]. Since the nerves are most commonly affected in ATTR amyloidosis [18], but TTR genetic testing was performed in all our patients and was found to be negative. Peripheral nerve involvement, present in 20% of patients with AL amyloidosis, is characterized by painful, slowly progressing sensorimotor peripheral polyneuropathy, similar to diabetic neuropathy [5]. Carpal tunnel syndrome is also common [5]. In our population the 50% of patients manifested severe postural hypotension and peripheral neuropathy. After 9 CyBorD cycles all patients had already showed complete remission of hortostatic hypotension, while during conditioning with Lenalidomide they showed painful peripheral neuropathy improvement.
The AL Amyloidosisis caused by the deposition of monoclonal light chains produced by an underlying clonal plasma cell proliferative disorder such as Multiple Myeloma (MM). AL amyloidosis accounts for more than three-fourths of patients and is caused by plasma cell clone that in approximately 50% of cases infiltrates the bone marrow by less than 10%. Most patients present organ damage caused by the monoclonal protein produced by small dangerous plasma cell clones [9,11]. In our population at onset three patients (18.7%) had confirmed multiple myeloma (Clone of plasma cells proliferation > 30% in the bone marrow). In all of them the coexistence of Multiple Myeloma correlated with worse renal survival (hemodialysis startup) and death of the patients.
Kidney involvement is the most frequent symptom, found in two thirds of patients at the time of diagnosis.
Characteristic presentation is with heavy proteinuria (composed mainly of albumin, with detectable urine monoclonal Ig LC in most cases), with nephrotic syndrome and decreased glomerular filtration rate in 20 to 45% of cases [5]. The diagnosis of renal amyloidosis relies on the pathological demonstration of renal amyloid and/or, when a kidney biopsy is not available, on histological evidence from another tissue with proteinuria ≥0.5 g/day predominantly composed of albumin [19,20]. All our patients had overt nephrosic syndrome and 8/16 (50%) had renal failure (III stage CKD, DOQI guidelines) at diagnosis. All our patients underwent echo-guided renal biopsy and all patients showed the histological characteristic features of Amyloidosis. The diagnosis of Amyloidosis is based on the finding, by light microscopic examination, of Congo red-positive glomerular deposits in the mesangium, capillary walls and Bowman’s capsule (Figure 1), with typical apple-green birefringence under polarized light (Figure 2). By immunohistochemistry the protein composition of the amyloid deposits was FLC k in 6/16 and FLC lambda in 10/16 patients.
The treatment of AL amyloidosis has been only based on anti-plasma cell chemotherapy for many years. By suppresing the plasma clone, chemotherapy reduces the concentration of toxic light chains, with necessary to improve organ dysfuction and long survival. High-dose melphalan with autologous stem cell transplantation (HDM/SCT) can fully eradicate production of amyloidogenic light chains, improve function of affected organs, and decrease mortality. However, this aggressive approach is not effective for all patients and has significant toxicities that limit its use. Melphalan administered orally with dexamethasone in repeated cycles, bortezomib, and lenalidomide are newer treatments that are being used increasingly for AL amyloidosis generally with less toxicity than high-dose melphalan with autologous stem cell transplantation (HDM/SCT). Lenalidomide has been shown to be an immunomodulator, affecting both cellular and humoral limbs of the immune system. It has also been shown to have anti-angiogenic properties. The angiogenesis plays an important pathogenic role in multiple myeloma as reflected by increased bone marrow microvascular density and VEGF (vascular endothelial growth factor) levels. Therefore this immunomodulatory agent was initially developed as a treatment for multiple myeloma. Early studies suggested to be effectived for AL amyloidosis, but a worsening of kidney function had observed in Lenalidomide induction therapy [21, 23,24,27]. To date, no randomized clinical trials of modern treatment approaches have been published, and there is little evidence in support of a standard treatment strategy in AL amyloidosis. In the largest study of frontline treatment with CyBorD of patients with AL amyloidosis, the overall hematologic response rate was 60%, with CHR in 23% of cases and Renal Response (RR) in 25% [22]. Prospective trials and large retrospective series proved the efficacy and tolerability of bortezomib in Al Amyloidosis [25,26].
In our study all patients (n.16) were treated in induction with CyBorD and 13 patients completed the regimen for 9 cycles. The three patients who died after only 3 cycles for cardiac arrhythmia, were those who had Multiple Myeloma and worse cardiac involvement at onset. After BD regimen 3/13 (23%) patients showed a CRR, 10/13 (76.9%) showed a PRR but one patient died for Sepsis in a patient with colonstomy for previous perforated diverticulitis. The treatment with Bortezomib has been well tolerated by our population. During the therapy only 3 patients slipped a VZV infection, despite prophylaxis with Acyclovir. No patient presented with myelosuppression, no patient showed gastroenterological complications.
In our previous experience we have observed an early recurrence of renal amyloid disease (relapse of nephrosic proteinuria after about 6 months) in PRR treated with CyBorD. Therefore we decided to use a conditioning therapy with Lenalidomide in AL renal Amyloidosis transplant-ineligible population with PRR.
Therefore the 9 patients in PRR were treated with Lenalidomide, dose adjustments for renal function, and 7/9 showed a CRR and CHR. We decided to discontinue the therapy for adverse events after an average of 6 cycles in 3 patients: breast cancer in one, transient troponin I increase and angina in another patient, pneumonia events in one. The pneumoniae infectious and anginal episodes regressed with drug discontinuation. 2/9 patients started hemodialysis for ESRD.
Lenalidomide should be used with caution in patients with relevant proteinuria or low eGFR [27]. It’s an immunomodulatory agent that was initially developed as a treatment for multiple myeloma [28]. Lenalidomide has been shown to induce apoptosis of MM cells and inhibit angiogenesis and blocks the binding of MM cells to the bone marrow stromal cells [29]. In our patients in PPR with Lenalidomide-base therapy the patients’ proteinuria disappeared. Morever the treatment with dose-adjusted lenalidomide for the renal dysfunction didn’t cause worsening of serum creatinine. Lenalidomide was well tolerated in our population.
Conclusion
Even today the diagnosis of AL amyloidosis is often late although combinations such as heart failure and nephrotic syndrome or “left ventricular hypertrophy” on echocardiographic evidence should raise suspicion of this disesase, In case of a strong clinical suspicious, as nephrotic syndrome, biopsies should be obtained at additional sites (es. renal biopsy) in patients with negative abdominal fat aspirate.
In our study we treated patients with AL Amyloidosis with a very intensive induction therapy (9 CyBorD cycles). The patients in PRR are treated with dose-adjusted lenalidomide for the renal dysfunction. Currently 10 patients (10/16, 62.5%) maintain complete renal remission.
We conclude the following Lenalidomide therapy is very effective at increasing the number of complete renal remissions. In addition, the two treatment regimens (induction therapy with CyBorD and conditioning with Lenalidomide) kept patients in remission for more than 30 months on average.
Further studies on larger samples are needed to validate the data.
Conclusion
Even today the diagnosis of AL amyloidosis is often late although combinations such as heart failure and nephrotic syndrome or “left ventricular hypertrophy” on echocardiographic evidence should raise suspicion of this disesase, In case of a strong clinical suspicious, as nephrotic syndrome, biopsies should be obtained at additional sites (es. renal biopsy) in patients with negative abdominal fat aspirate.
In our study we treated patients with AL Amyloidosis with a very intensive induction therapy (9 CyBorD cycles). The patients in PRR are treated with dose-adjusted lenalidomide for the renal dysfunction. Currently 10 patients (10/16, 62.5%) maintain complete renal remission.
We conclude the following Lenalidomide therapy is very effective at increasing the number of complete renal remissions. In addition, the two treatment regimens (induction therapy with CyBorD and conditioning with Lenalidomide) kept patients in remission for more than 30 months on average.
Further studies on larger samples are needed to validate the data.
Contributions
All authors R.Greco, C. Botta, M. Gentile and T. Papalia contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rosita Greco, Teresa Papalia. The first draft of the manuscript was written by Rosita Greco and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
All authors don’t declare interests that are directly or indirectly related to the work submitted for publication. In addition, all authors have not financial interests that could impart bias on the work submitted for publication. The authors have no financial or proprietary interests in any material discussed in this article.
Consent to Publish Statement
Written informed consent for publication their medical case (including publication of images) was obtained from all subjects. State whether written informed consent was obtained from participants (or their parents/legal guardians/next-of-kin) for publication of the details of their medical case and any accompanying images.
Funding Sources
None
Statement of Ethics
Published research complies with the guidelines for human studies and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent to Publish Statement
Written informed consent for publication their medical case (including publication of images) was obtained from all subjects. State whether written informed consent was obtained from participants (or their parents/legal guardians/next-of-kin) for publication of the details of their medical case and any accompanying images.
References
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003 Aug 7;349(6):583-96. doi: 10.1056/NEJMra023144. PMID: 12904524.
- Comenzo RD. Current treatment options in oncology. Amyloidsis. 2006;7:225-236. doi: 10.1007/s11864-006-0015-8.
- Gurung R, Li T. Renal Amyloidosis: Presentation, Diagnosis, and Management. Am J Med. 2022 Apr;135 Suppl 1:S38-S43. doi: 10.1016/j.amjmed.2022.01.003. Epub 2022 Jan 24. PMID: 35085515.
- Milani P, Merlini G, Palladini G. Novel Therapies in Light Chain Amyloidosis. Kidney Int Rep. 2017 Nov 28;3(3):530-541. doi: 10.1016/j.ekir.2017.11.017. PMID: 29854961; PMCID: PMC5976806.
- Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005 Nov 10;1753(1):11-22. doi: 10.1016/j.bbapap.2005.08.014. Epub 2005 Sep 2. PMID: 16198646.
- Nishi S, Alchi B, Imai N, Gejyo F. New advances in renal amyloidosis. Clin Exp Nephrol. 2008 Apr;12(2):93-101. doi: 10.1007/s10157-007-0008-3. Epub 2008 Jan 5. PMID: 18175051.
- Bergesio F, Ciciani AM, Manganaro M, Palladini G, Santostefano M, Brugnano R, Di Palma AM, Gallo M, Rosati A, Tosi PL, Salvadori M; Immunopathology Group of the Italian Society of Nephrology. Renal involvement in systemic amyloidosis: an Italian collaborative study on survival and renal outcome. Nephrol Dial Transplant. 2008 Mar;23(3):941-51. doi: 10.1093/ndt/gfm684. Epub 2007 Oct 19. PMID: 17951308.
- Ekelund L. Radiologic findings in renal amyloidosis. AJR Am J Roentgenol. 1977 Nov;129(5):851-3. doi: 10.2214/ajr.129.5.851. PMID: 410249.
- Müller AM, Geibel A, Neumann HP, Kühnemund A, Schmitt-Gräff A, Böhm J, Engelhardt M. Primary (AL) amyloidosis in plasma cell disorders. Oncologist. 2006 Jul-Aug;11(7):824-30. doi: 10.1634/theoncologist.11-7-824. PMID: 16880241.
- Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2018 Jan 18;378(3):241-249. doi: 10.1056/NEJMoa1709974. PMID: 29342381; PMCID: PMC5852672.
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006 Oct 15;108(8):2520-30. doi: 10.1182/blood-2006-03-001164. Epub 2006 Jun 22. PMID: 16794250.
- Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012 Nov 22;120(22):4292-5. doi: 10.1182/blood-2012-07-445304. Epub 2012 Oct 9. PMID: 23047823.
- Leung N. My patient with monoclonal gammopathy of undetermined significance has a kidney problem. Journal of Onco-Nephrology. 2017;1:18-23. doi: 10.5301/jo-n.5000005.
- Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007 Nov 27;50(22):2101-10. doi: 10.1016/j.jacc.2007.08.028. Epub 2007 Nov 13. Erratum in: J Am Coll Cardiol. 2011 Mar 29;57(13):1501. PMID: 18036445.
- Kapoor P, Thenappan T, Singh E, Kumar S, Greipp PR. Cardiac amyloidosis: a practical approach to diagnosis and management. Am J Med. 2011 Nov;124(11):1006-15. doi: 10.1016/j.amjmed.2011.04.013. PMID: 22017778.
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016 Jun 25;387(10038):2641-2654. doi: 10.1016/S0140-6736(15)01274-X. Epub 2015 Dec 21. PMID: 26719234.
- Schönland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, Lohse P, Röcken C. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012 Jan 12;119(2):488-93. doi: 10.1182/blood-2011-06-358507. Epub 2011 Nov 21. PMID: 22106346.
- Benson MD, Dasgupta NR, Rao R. Diagnosis and Screening of Patients with Hereditary Transthyretin Amyloidosis (hATTR): Current Strategies and Guidelines. Ther Clin Risk Manag. 2020 Aug 14;16:749-758. doi: 10.2147/TCRM.S185677. PMID: 32884276; PMCID: PMC7434568.
- Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005 Aug;79(4):319-28. doi: 10.1002/ajh.20381. PMID: 16044444.
- Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005 Aug;79(4):319-28. doi: 10.1002/ajh.20381. PMID: 16044444.
- Weiss BM, Wong SW, Comenzo RL. Beyond the plasma cell: emerging therapies for immunoglobulin light chain amyloidosis. Blood. 2016 May 12;127(19):2275-80. doi: 10.1182/blood-2015-11-681650. Epub 2016 Feb 23. PMID: 26907632.
- Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, Basset M, Hawkins P, Merlini G, Wechalekar AD. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015 Jul 30;126(5):612-5. doi: 10.1182/blood-2015-01-620302. Epub 2015 May 18. PMID: 25987656.
- Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016 Jul 14;128(2):159-68. doi: 10.1182/blood-2016-01-629790. Epub 2016 Apr 6. PMID: 27053535.
- Milani P, Merlini G, Palladini G. Novel Therapies in Light Chain Amyloidosis. Kidney Int Rep. 2017 Nov 28;3(3):530-541. doi: 10.1016/j.ekir.2017.11.017. PMID: 29854961; PMCID: PMC5976806.
- Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Bladé J, Fermand JP, Hassoun H, Heffner L, Kukreti V, Vescio RA, Pei L, Enny C, Esseltine DL, van de Velde H, Cakana A, Comenzo RL. Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis. Blood. 2014 Oct 16;124(16):2498-506. doi: 10.1182/blood-2014-04-568329. Epub 2014 Sep 8. PMID: 25202139; PMCID: PMC4199951.
- Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V, Gillmore JD, Palladini G. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010 Feb 20;28(6):1031-7. doi: 10.1200/JCO.2009.23.8220. Epub 2010 Jan 19. PMID: 20085941.
- Specter R, Sanchorawala V, Seldin DC, Shelton A, Fennessey S, Finn KT, Zeldis JB, Dember LM. Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol Dial Transplant. 2011 Mar;26(3):881-6. doi: 10.1093/ndt/gfq482. Epub 2010 Aug 5. PMID: 20693160; PMCID: PMC3108346.
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD; Multiple Myeloma (009) Study Investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007 Nov 22;357(21):2133-42. doi: 10.1056/NEJMoa070596. PMID: 18032763.
- Furukawa M, Ohkawara H, Ogawa K, Ikeda K, Ueda K, Shichishima-Nakamura A, Ito E, Imai JI, Yanagisawa Y, Honma R, Watanabe S, Waguri S, Ikezoe T, Takeishi Y. Autocrine and Paracrine Interactions between Multiple Myeloma Cells and Bone Marrow Stromal Cells by Growth Arrest-specific Gene 6 Cross-talk with Interleukin-6. J Biol Chem. 2017 Mar 10;292(10):4280-4292. doi: 10.1074/jbc.M116.733030. Epub 2017 Jan 31. PMID: 28154173;
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.