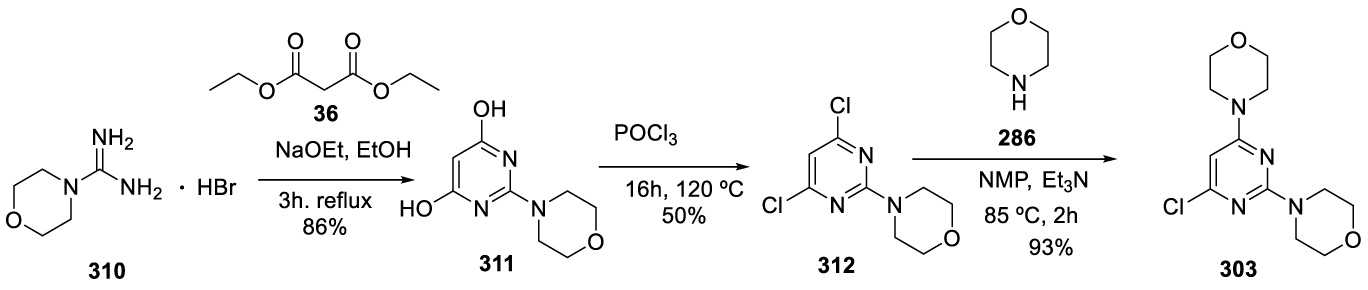
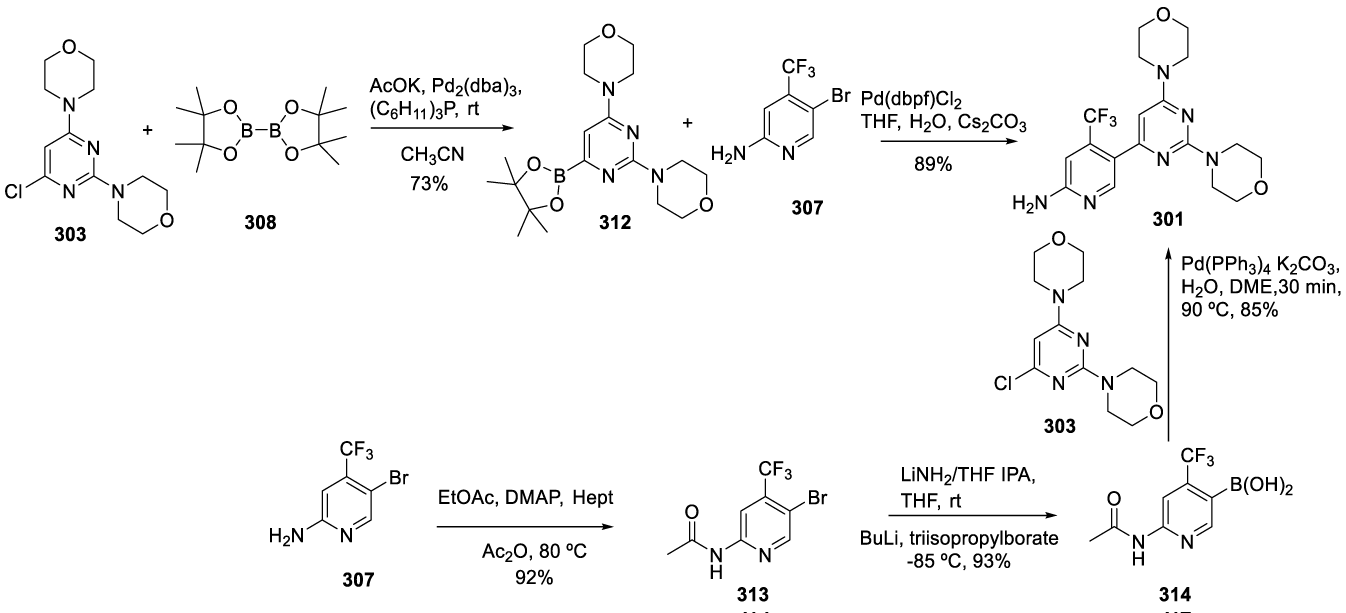
Medicine Group. 2024 February 15;5(2):159-213. doi: 10.37871/jbres1883.
Drug Design Strategies, Modes of Action, Synthesis and Industrial Challenges Behind Trifluoromethylated New Chemical Entities
Leitão EPT* and Sobral LMS
- Trifluoromethyl
- Drug design
- New chemical entities
- Therapeutic applications
- Manufacturing industrial challenges
Abstract
Medicinal plants have been an inspiration source for developing new chemical entities, providing diverse chemical structures and modes of action for medicinal chemistry strategies. Modern medicinal chemistry approaches promote functional group changes to improve NCE’s biological activity. Incorporating the trifluoromethyl (-CF3) group into organic compounds is crucial in modern drug design. It may affect the compound's lipophilicity, solubility, stability, molecular conformation, and pKa, changes that may lead to the discovery of new medicines to treat diseases of our times. For this reason, this chemical transformation is currently a hot topic in the organic chemistry field. Chemists are competing to develop better methods to carry out this transformation efficiently, less hazardously, and cost-effectively. The high number of annual scientific publications on trifluoromethylation evidences the competition. This review profiles 21 trifluoromethylated New Chemical Entities (NCE) selected in December 2020 from the GlobalData database, mainly in late stages of clinical trials (Phase II and III) or already in the commercial phase, containing one or two -CF3 groups in para or meta positions of benzene, or a pyridine ring. Synthetic approaches, therapeutic applications, and manufacturing industrial challenges are provided.
Introduction
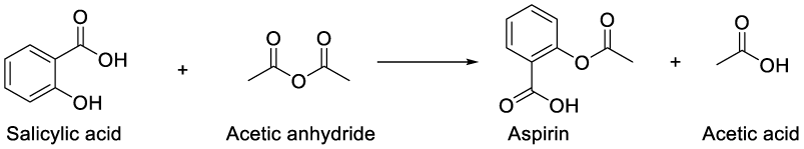
Medicinal plants have been a resource for healing in local communities around the world for thousands of years [1]. The oldest written evidence of medicinal plants’ usage for the preparation of drugs is approximately 5000 years old and was found on a Sumerian clay slab from Nagpur, describing 12 recipes using over 250 different plants, some of them alkaloids such as poppy, henbane, and mandrake [2]. So, plants are the most significant natural source for potential new drugs, due to their chemical, structural diversity and biodiversity of its components [3]. There are several examples of common drugs derived from plants. Aspirin an analgesic [4], was obtained by acetylation of the natural product salicylic acid [5] (from willow tree bark) [6].
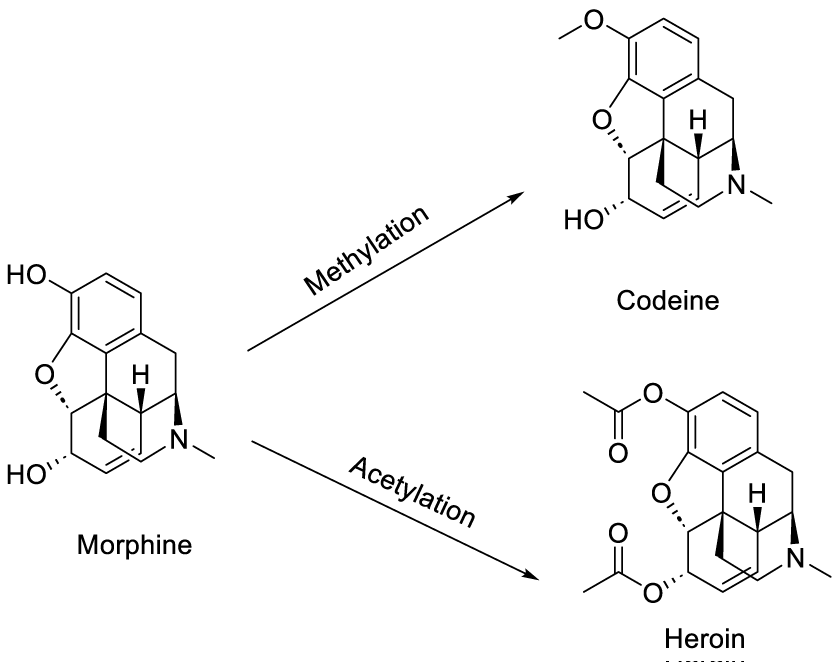
Morphine is an opiate alkaloid isolated from the opium plant (present in poppy seeds) [7,8] used for pain relief and illegal recreation [9,10]. Codeine is a naturally opium alkaloid morphine derivative with less analgesic and sedative effects than morphine. It is usually prepared for medicinal use by methylation of morphine [11,12]. Heroin is a powerful synthetic opiate analgesic synthesized from morphine by acetylation [13]and a potent illegal addictive drug [14].
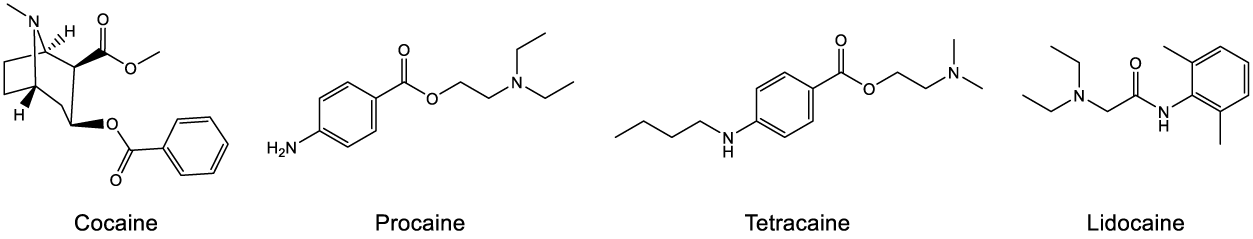
Cocaine derived from the coca plant (Erythroxylon coca) is an illegal recreational drug. It is also used to treat medical conditions [15]. Besides being favored by many surgeons for sinonasal surgery due to its superior vasoconstrictive and anesthetic properties, historical reports suggested cocaine was associated with an increased risk of cardiac events [16]. For this reason, cocaine was modified structurally to produce other anesthetic drugs with different properties such as procaine [17], tetracaine [18] and lidocaine [19].
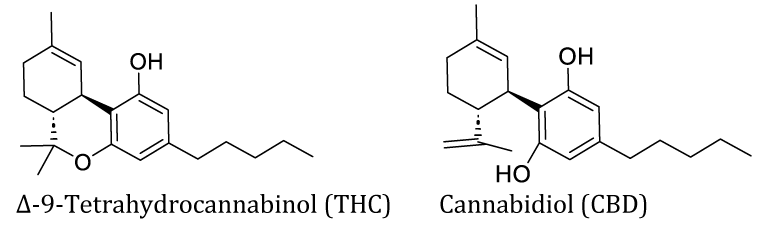
Cannabis, contains more than 460 known products, of which more than 60 are cannabinoids. The main psychoactive ingredient of marijuana and hashish is delta-9-Tetrahydrocannabinol (THC), which holds a methyl group instead of a methylene group compared to cannabidiol [20,21]. Cannabis is used for illegal recreation while cannabidiol was approved by the FDA for treating severe forms of epilepsy [22-24].
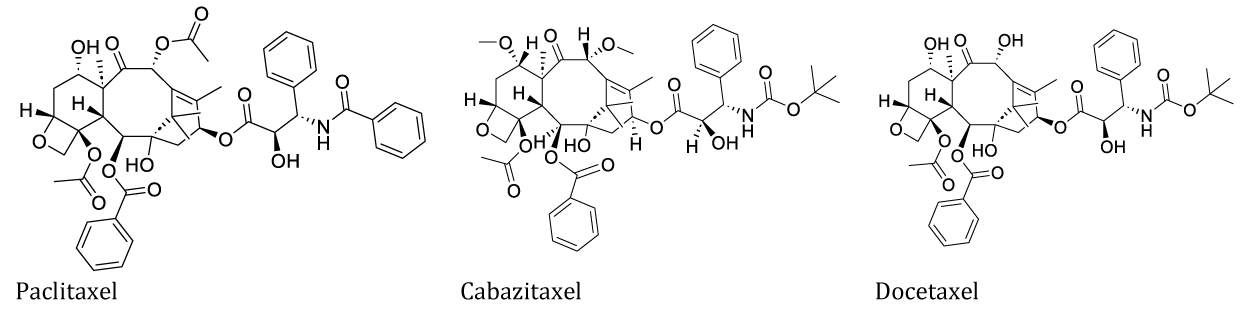
Paclitaxel a more complex molecule, is a broad-spectrum anti-cancer compound (from the bark of the yew tree Taxus brevifolia Nutt) [25]. Cabazitaxel and docetaxel are derived from Paclitaxel. Cabazitaxel (brand name: Jevtana®) was approved in the USA in 2010 for hormone-refractory prostate cancer. Docetaxel (brand name: Taxotere®) was approved in the USA (1995) for breast, head and neck, stomach, prostate, and non-small-cell lung cancers [26].
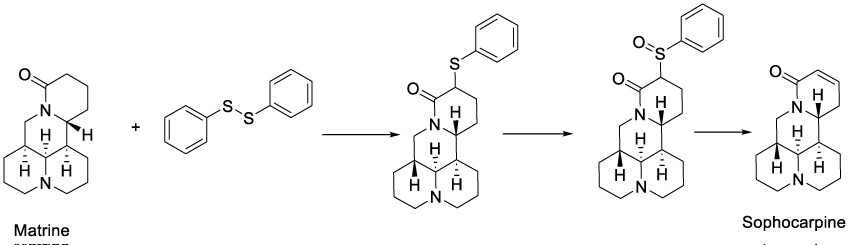
Sophocarpine, a tetracyclic quinolizidine alkaloid, is one of the most abundant active ingredients in Sophora alopecuroides L. and exerts anti-inflammatory activity in an animal model [27]. This molecule can be synthesized from matrine, an alkaloid found in plants from the genus Sophora. It has a variety of pharmacological effects, including anti-cancer effects [28].
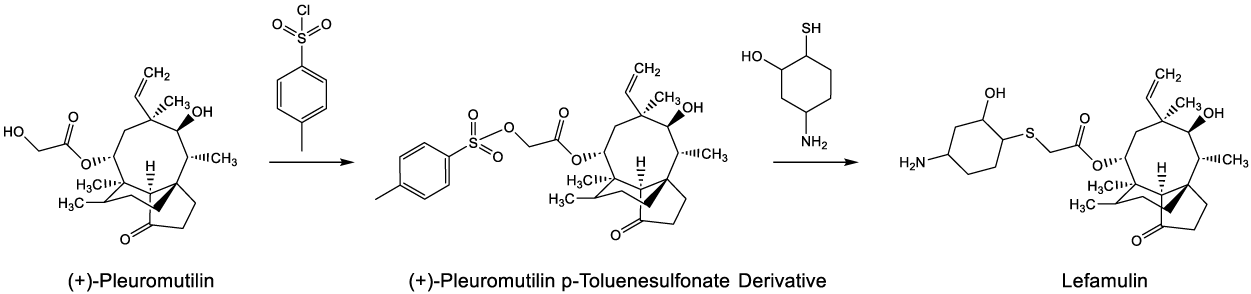
Lefamulin, derived from pleuromutilin, a naturally occurring antibacterial isolated from an edible mushroom (Pleurotus mutilus), is a novel antibiotic for Community-acquired Bacterial Pneumonia (CABP) [29]. The antibiotic was synthesized from (+)-pleuromutilin by SN2 displacement of the p toluenesufonate group of (+)-pleuromutilin p-toluenesulfonate derivative with 5-amino-2-mercaptocyclohexan-1-ol [30].
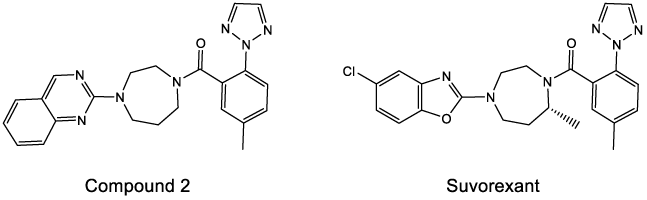
From a medicinal chemistry perspective, functional groups provide specific properties and behaviors that allow drug molecules to exert their desired biological activity. Among the relevant functional groups in modulating biological activity are the methyl groups [31]. An example is the discovery of Suvorexant, a medicine approved by the FDA for the treatment of insomnia [32]. A high-throughput program identified previously compound 2 as having enhanced potency and improved physicochemical properties; further studies demonstrated that replacing the quinazoline in compound 2 with a chlorobenzoxazole afforded a dramatic improvement in pharmacokinetic and suppression of reactive metabolites with potential safety risk for the molecule. Then, substitutions on the seven-membered diazepane ring were studied to assess impacts on binding affinity, pharmacokinetics properties, receptor potency, brain penetration, and in vivo efficacy. The introduction of a 7-methyl substituent on the diazepane core provided additional improvements in potency and pharmacokinetics.
Previous examples showed that nature is still the inspiration and source of new drugs. It also showed that modifying or introducing a chemical group might change the drug's physical and biological properties. However, drug screening and design can be done from scratch using computational methods [33], and incorporating specific functional groups into a molecule, such as a trifluoromethyl ( CF3) group, to obtain specific properties.
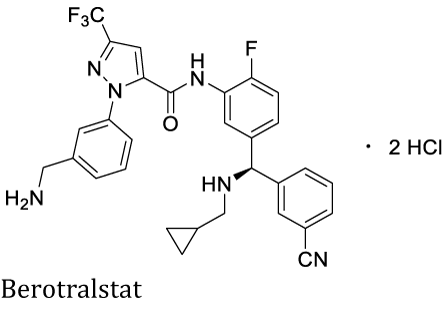
The Incorporation of the -CF3 group into organic compounds may benefit their pharmacological profile affecting the compounds' lipophilicity, solubility, stability, molecular conformation, and pKa [34]. Therefore, the -CF3 substituted building blocks are crucial in modern drug design. In 2020 the FDA approved 53 New Molecular Entities (NMEs). 68% (36 molecules) were small molecules, (19%) were fluoro-compounds containing one or two fluorines, and one of them held the -¬CF3 group, Berotralstat (ORLADEYO™) [35]. Berotralstat is an orally administered kallikrein inhibitor developed by BioCryst Pharmaceuticals for Hereditary Angioedema (HAE).
This review describes the profile of 21 New Chemical Entities (NCE), which are small molecules containing the ‑CF3 group, mainly in advanced stages of clinical trials (Phase II and III) or already in the commercial phase. It details therapeutic applications, mechanisms of action, synthetic approaches followed, and manufacturing industrial challenges.
Compounds containing trifluoromethyl group in benzene ring para position
CORT 108297: (1) (alternative name: ADS-108297) is an investigational drug developed by Corcept Therapeutics to treat severe metabolic and psychiatric disorders [36,37]. Unlike Mifepristone (2) [38], which is a Corcept first-generation compound that directly blocks the Glucocorticoid Receptor (GR) and the progesterone (PR) receptor,36 CORT 108297 (1) had no activity at the progesterone receptor, a condition associated to weight gain, and showed to attenuate weight gain induced by the antipsychotic medication Olanzapine (Figure 1) [3,38,39].
(2) was also examined for Retrograde Amnesia (RA) [40], a memory loss for information acquired before the onset of amnesia. It is caused by a brain injury or by the start of a neurological disease [41]. CORT 108297 (2) was also tested for acute stress [40,42-44]. This compound is still in phase II trials for mild cognitive impairment, Alzheimer’s disease, memory impairment and post-traumatic stress disorder in the USA.
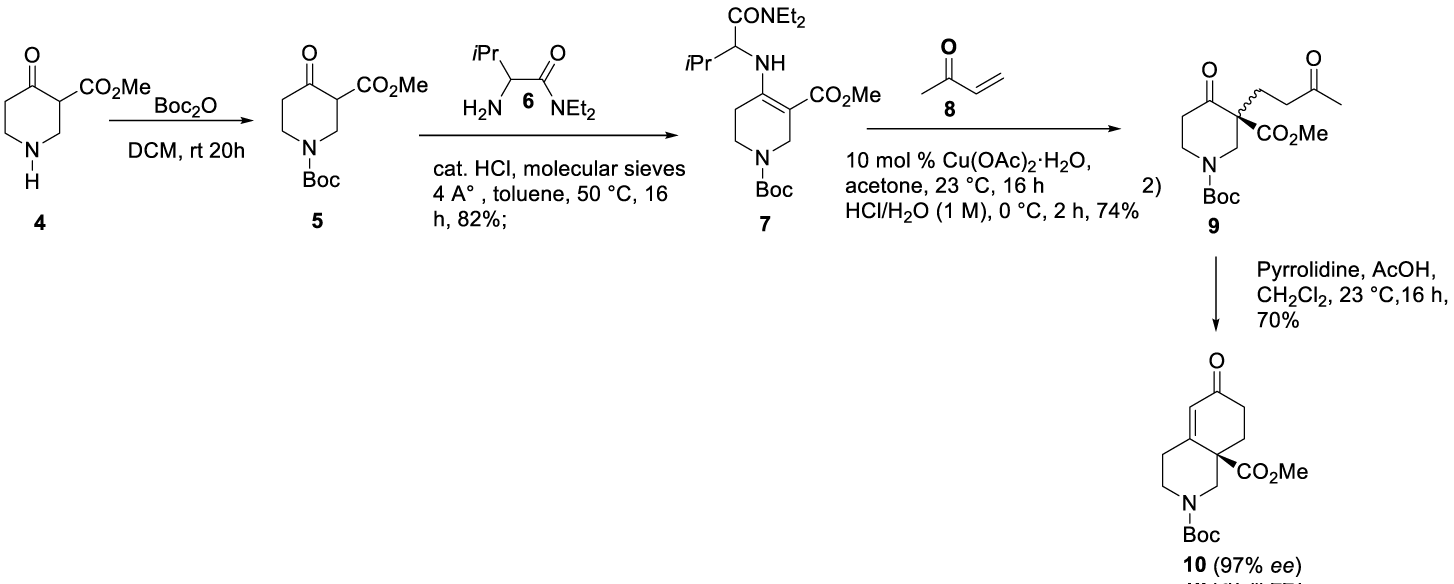
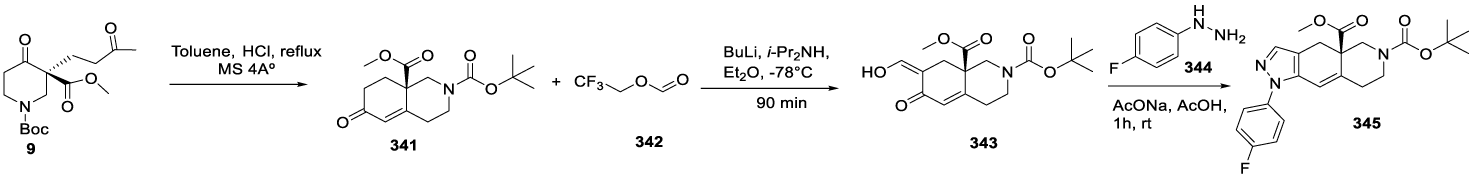
CORT 108297 synthesis: (1) (CAS number: 1018679-79-2) starting raw material (5) may be prepared as reported by Zhou and co-workers with 100% yield [45]. CORT 108297 synthesis can be done starting from optically active chiral building block piperidine 5, as reported by Christoffers and Scharl [46] in 2002, followed by the process described by Clark and co-workers [47] in 2008, starting from intermediate 9. The acid-catalyzed conversion of β-ketocarboxylate 5 with equimolar amounts of the auxiliary L valine diethylamide 6 yielded the enamino ester 7 with 82% yield. Treatment of the Michael donor 7 with a catalytic amount of copper (II) acetate and a slight excess of methyl vinyl ketone 8 gave the optically active product 9 in 74% yield after acidic hydrolysis. The reaction was performed at 23 °C in acetone, and excluding moisture or air was unnecessary. According to the authors, the carbamate protective group of 9 seemed to be stable under workup conditions (1 M HCl). The auxiliary could be recovered from the aqueous layer by extraction. The piperidine 9 was cyclized to afford 10 in 70% yield with 97% ee Scheme 1.
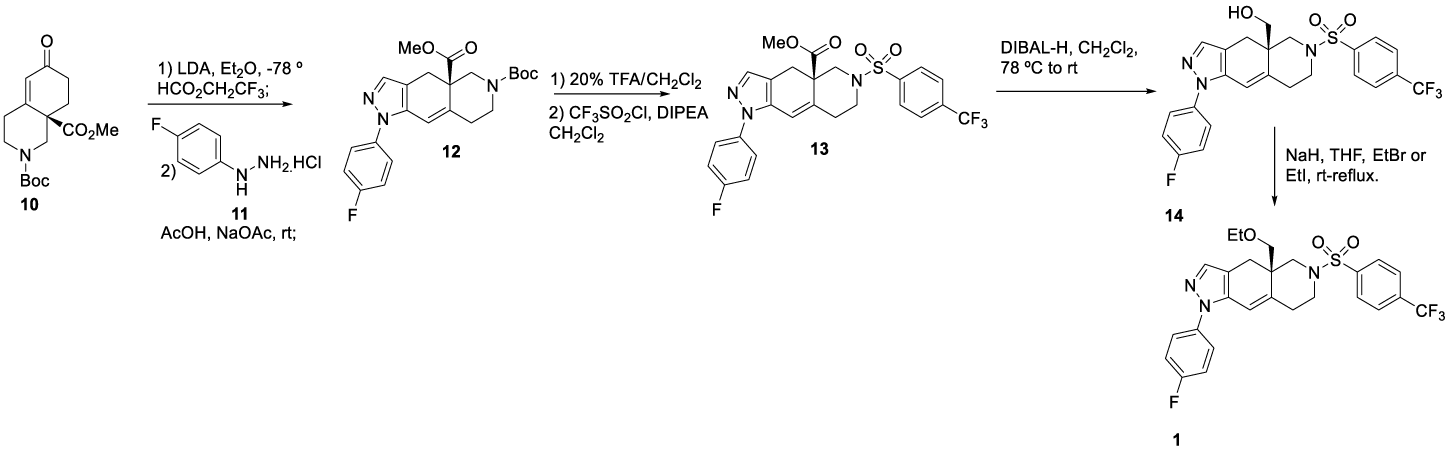
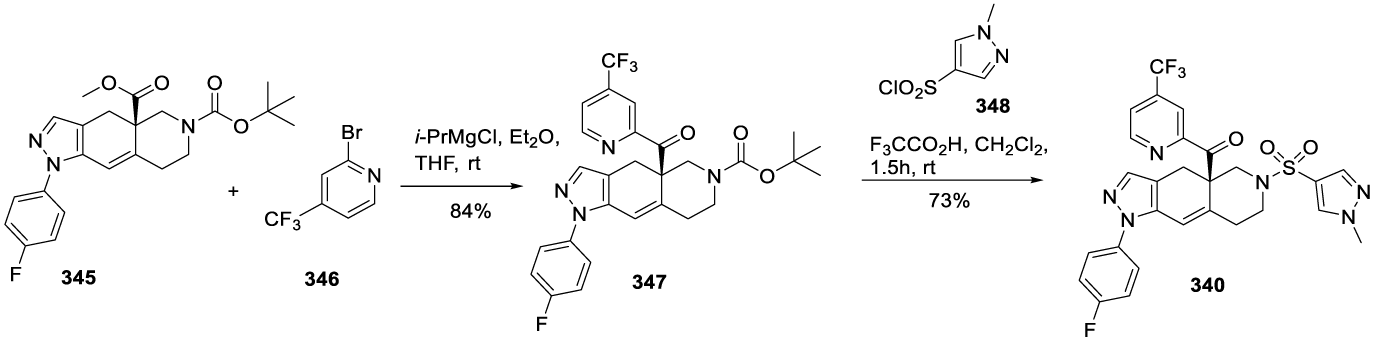
1CORT108297: (1) may also be synthesized starting from homochiral enone 10 prepared according to Christoffers and Scharl46, as reported by Clark and co-workers [47], scheme 2. The formylation of 10 was accomplished by conversion of the enolate generated by the LDA, followed by a reaction of 2,2,2-trifluoroethyl formate and then with (4-fluorophenyl)hydrazine hydrochloride 11 to afford pyrazole intermediate 12. After removal of the N Boc protecting group with TFA, the amine was sulfonated with trifluoromethanesulfonyl chloride (source of trifluoromethyl group) in the presence of N,N-diisopropylethylamine (DIPEA) to yield 13, which was then reduced with DIBAL-H (diisobutylaluminum hydride) to form 14. The esterification of 14 with ethyl bromide or iodide provided 2, Scheme 2.
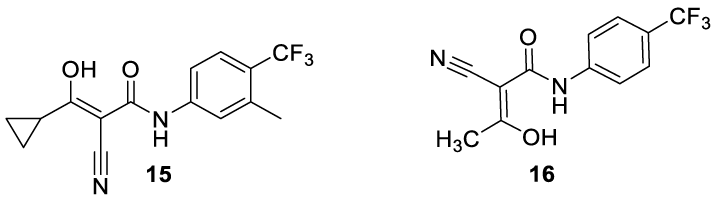
Laflunimus: (15) (alternative names: aflunimus; AP325) is an investigational drug developed by Aventis [48]. The compound was under investigation as an immunosuppressive agent to prevent the progression of vascular injury in hypertension and lower blood pressure [49]. In vitro and in vivo studies demonstrated that Laflunimus (15) inhibits the secretion of immunoglobulin, an effect consistent with the hypothesis that this compound exerts its immunosuppressive effect by interfering with pyrimidine metabolism [48]. More recently, a clinical trial was reported to evaluate the efficacy and safety of AP-325 (15) in subjects with peripheral post-surgical neuropathic pain. The compound acts as an inhibitor of the enzyme Dihydroorotate Dehydrogenase (DDOH) and such inhibitors have been used to treat autoimmune diseases like rheumatoid arthritis and multiple sclerosis [50]. The inhibitors of dihydroorotate dehydrogenase have also been investigated for the treatment of cancer [51,52]. Laflunimus (15) is an analog of Teriflunomide (16) which contains a cyclopropyl moiety instead of a methyl group, as depicted in figure 2. Laflunimos is still in the Phase IIa trial to evaluate the efficacy in subjects with peripheral post-surgical neuropathic pain [53].
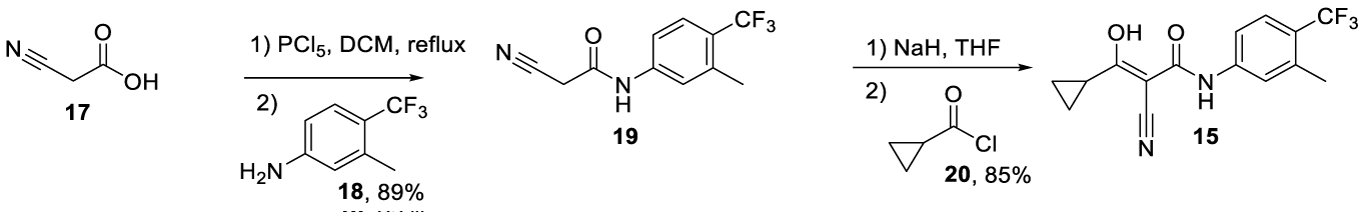
Laflunimus synthesis: Laflunimus (CAS number: 147076-36-6) may be prepared as reported by Kuo54 and co-workers. The process starts with the acylation of cyanoacetic acid 17 with PCl5, in dichloromethane, at reflux temperature, or with Diisopropylcarbodiimide (DCI) in THF, followed by the coupling with 3-methyl-4-(trifluoromethyl)aniline to afford the amide 19 with 89% yield.The acylation of 19 with cyclopropanecarbonyl chloride 20 in the presence of NaH affords the desired product 15 as colorless crystals, Scheme 3.
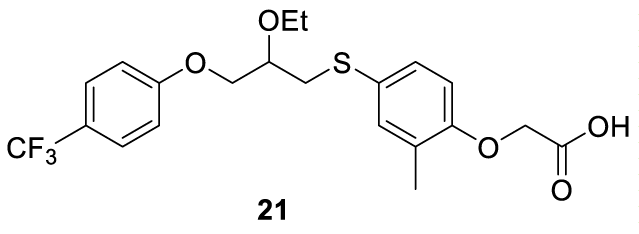
Seladelpar: Seladelpar (21) (alternative names: MBX 8025 and RWJ 800025; (Figure 3) is an investigational drug first reported by Johnson & Johnson in 2007 as a selective and potent peroxisome proliferator-activated receptor-delta (PPARδ) agonist, but originally discovered by Ortho-McNeil Pharmaceutical (a subsidiary of Johnson & Johnson) [55]. Now is being developed by CymaBay Therapeutics [56]. Seladelpar (21) was under Phase III clinical trials for the treatment of primary biliary cholangitis, a chronic liver disease with a higher prevalence in women than in men [57-59]. Primary biliary cholangitis is an autoimmune disease characterized by progressive destruction of the small bile ducts of the liver, the cholangiocytes, causing bile to build up in the liver, a condition designated by cholestasis [60]. Cholangiocytes are the epithelial cells of the bile duct and are involved in the first line of defense of the biliary system against foreign substances. Seladelpar (21) is a potent selective agonist for the PPAR-δ, a class of proteins expressed in hepatocytes and cholangiocytes that controls genes involved in bile acid homeostasis [57,61,62].
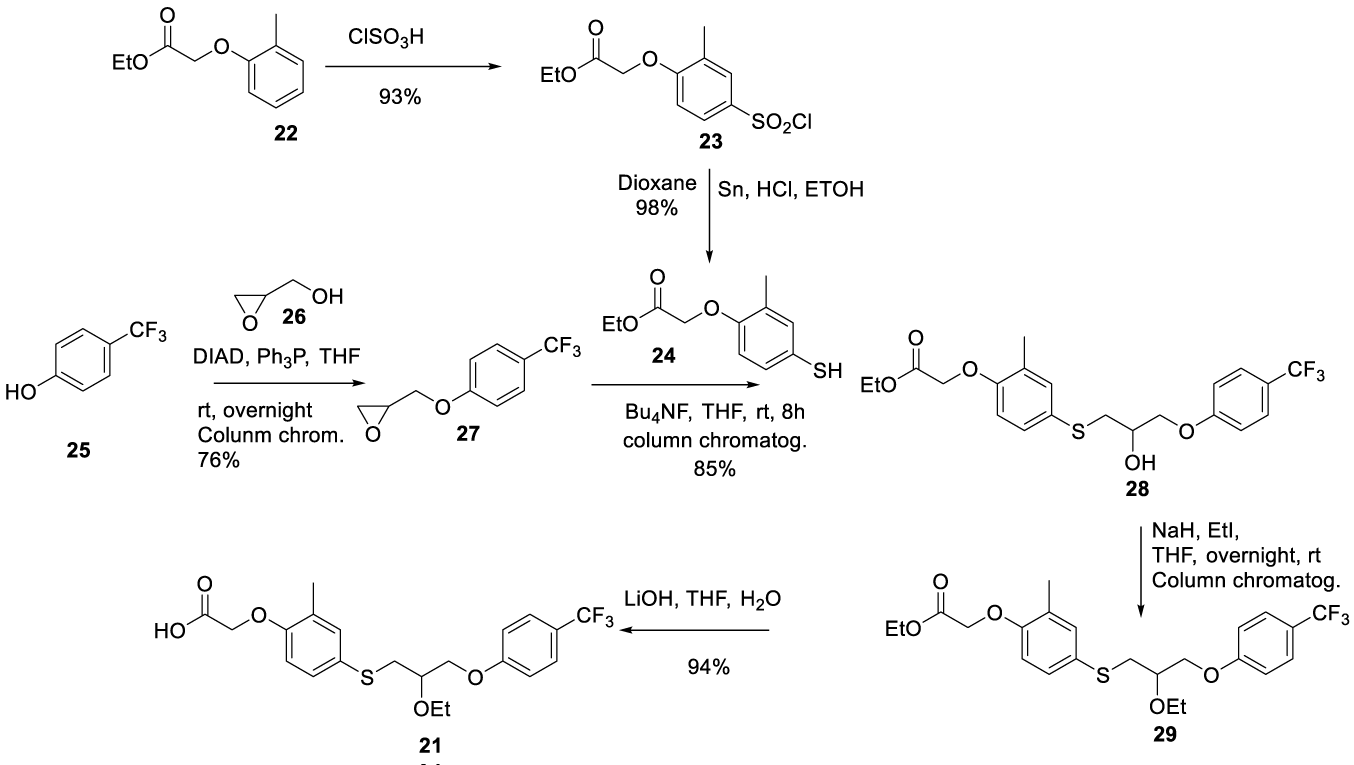
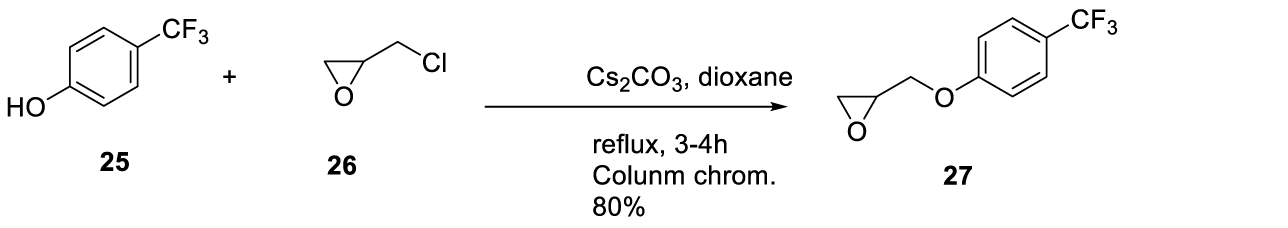
Seladelpar synthesis: In 2005, Kuo and co-workers [63] from Janssen Pharmaceutica N.V. filed a patent (WO2005042478 A2) claiming the preparation of 4-((phenoxyalkyl)thio)-phenoxyacetic acids and analogs, which includes Seladelpar (21) (CAS number: 851528-79-5). Seladelpar (21) was prepared by a convergent synthesis starting from intermediates 24 and 27. Intermediate 24 was prepared by sulfonating the benzenic ring of (2-methylphenoxy) acetate 22 with chlorosulfonic acid to afford 23. The reaction of 23 with Sn and HCl in ethanol provided 24 in excellent yield. Intermediate 27 was prepared by the Mitsunobu reaction of 4-fluoromethyl)phenol 25 (fluoromethyl source) with oxiran-2-ylmethanol 26 in the presence of Diisopropyl Azodicarboxylate (DIAD) and triphenylphosphine (Ph3P) in THF. The product obtained was purified by chromatography to provide pure 27. The epoxide ring opening of 27 with 24 in the presence of a catalytic amount of tetrabutylammonium fluoride in THF for 8 hours furnished pure alcohol 28 after column chromatography. The hydroxyl group from 28 was alkylated with iodoethane in the presence of sodium hydride to afford 29, which was then purified by column chromatography. The ester group of 29 was hydrolyzed with aqueous lithium hydroxide and THF to provide the desired product 21, Scheme 4.
Intermediate 27 may also be synthesized starting from 4-(trifluoromethyl)phenol but with (2-chloromethyl)oxirane 26, in the presence of cesium carbonate and dioxane, with a higher yield (80%) [64],as described in WO 2006/032023 patent, also from Janssen Pharmaceutica N.V., Scheme 5.
Tasquinimod: Tasquinimod [30]; (Figure 4) (alternative names: ABR-215050 and TASQ) is an experimental drug developed by Active Biotech and licensed by Ipsen, initially tested for castration-resistant prostate cancer, and currently under test for the treatment of myeloma [65-68]. According to studies performed during clinical trials on prostate cancer, the molecule inhibits tumor angiogenesis needed for cancer cell survival by targeting Histone Deacetylase 4 (HDAC4). The studies conducted during myeloma clinical trials showed that the molecule inhibits the growth and metastasis of tumor cells by enhancing the host immune response and inhibiting the angiogenic response through binding to the calcium-binding protein S100A9 [69,70].
Tasquinimod is currently being evaluated in Phase I multiple myeloma to establish the maximum tolerated dose (MTD) and optimal schedule, alone and in combination with a standard oral myeloma regimen of ixazomib, lenalidomide, and dexamethasone (IRd) [71].
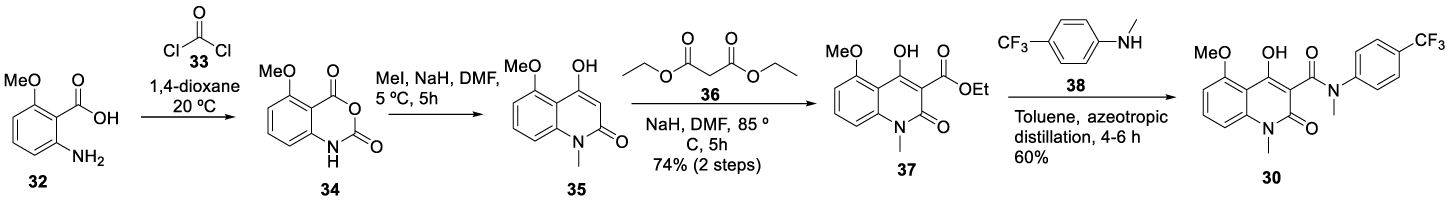
Tasquinimod synthesis: Jönsson, and co-workers [72] reported in 2004, the synthesis of Tasquinimod (30) (CAS Number: 254964-60-8) starting from commercially available 2-amino-6-methoxybenzoic acid (32). The 2-amino-6-methoxybenzoic acid 32 was transformed into an isatoic anhydride 34 with phosgene 33 in 1,4-dioxane, at 20 ºC. The isatoic anhydride 34 was methylated with methyl iodide, in the presence of sodium hydride in DMF at 5 ºC for 5 hours to give 35, which was condensed with diethylmalonate 36 to afford the corresponding methyl ester 37. The methyl ester 37 was condensed with the appropriate aniline 38 in toluene to provide the desired final compound 30, Scheme 6.
Later on, in 2012, Bock and co-workers,[73] from Active Biotech, filed a patent (WO 2012004338 A1) claiming a method to prepare quinoline 3-carboxamides such as Paquinimod (38), Laquinimod (39), and Tasquinimod (30), by reacting the appropriate alkyl ester and an aniline derivative (Figure 5).
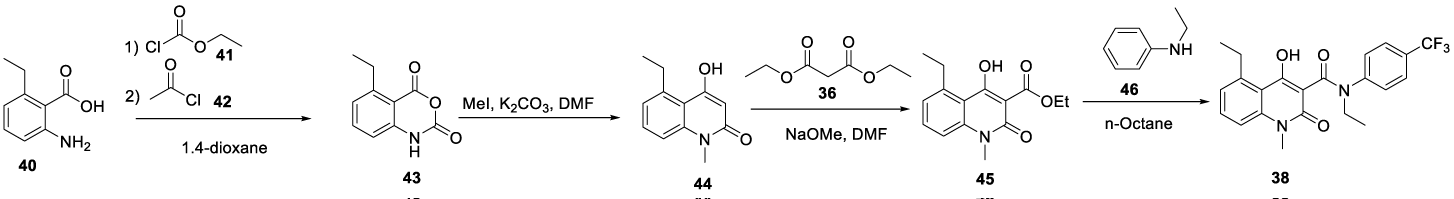
According to the authors, the new process was short and avoided using expensive reagents. All intermediates were stable and easy to isolate in high purity by precipitation and filtration. The synthesis route presented in the patent was exemplified for Paquinimod (38). The route started with an anthranilic acid 40 which was transformed into an isatoic anhydride 43 with ethyl chloroformate 41, followed by acetyl chloride 42 in 1,4-dioxane. The isatoic anhydride 43 formed was methylated with MeI in the presence of K2CO3 and DMF to give the N-methyl 44. Compound 44 was condensed with dimethylmalonate 36 to give the corresponding methyl ester 45. The methyl ester 45 was subsequently condensed with the appropriate aniline 46 in n-octane to provide the desired final compound 38. The main impurity in the last condensation step was the remaining alkyl ester 45, Scheme 7.
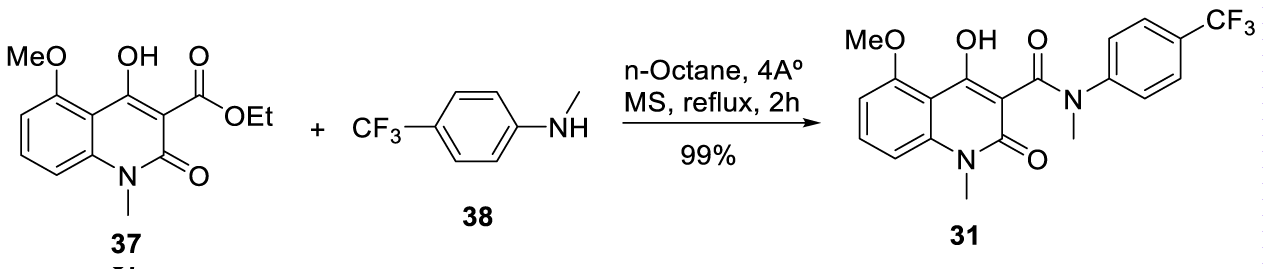
According to the authors, when Tasquinimod was prepared from intermediate 37 and using the prior art conditions, 94% yield was obtained, with 4% of unreacted ester (37). Using optimized conditions (4Å molecular sieves in n-octane for 2 hours), the yield increased to 99%, with 1% of ester (37), Scheme 8.
Several methods are described in the literature to prepare 34 starting from 32 and phosgene; in NaOH and water, with 90% yield (reported by Wang and co-workers in 2007) [74], and in NaOH, water and toluene, for 30 minutes at room temperature, with 88% yield (reported by Morgentin and co-workers in 2012) [75]. This conversion can also be performed with bis(trichloromethyl) in ethyl acetate and K2CO3 (reported by Chai and co-workers in 2007) [76] with 99% yield, in THF at 50 ºC for 12 hours (reported by Crew and co-workers in 2012) [77], in THF at room temperature overnight, with 87% yield (reported by Suzuki and co-workers in 2013) [78], in THF at -5 ºC for 5 hours with 95% yield (reported by Chen and co-workers 2019) [79] or in THF at 70 ºC for 6 hours, with 97% yield (reported by Lou and co-workers) [80].
The methylation of 34 may also be prepared with MeI in the presence of NaH in DMF, at room temperature for 30 min, with 53% yield, as reported by Lou and co-workers in 2018 [80].
Teriflunomide: Teriflunomide (16), commercially available under the tradename AUBAGIO® by Sanofi, [81] is a once-daily oral immunomodulatory Disease-Modifying Therapy (DMT) presently approved in several regions, including Europe, North America, Latin America and Australia, for the treatment of Relapsing Forms of Multiple Sclerosis (RMS; RRMS). The therapeutic mode of action of Teriflunomide in Multiple Sclerosis (MS) continues to be investigated [82]. Multiple sclerosis is an autoimmune inflammatory disorder of the Central Nervous System (CNS) of unknown etiology, characterized by demyelination and variable degrees of axonal loss. The disease affects mostly young women (between ages 20 and 40) and is one of the leading causes of disability in young adults in the United States [83]. Teriflunomide received its first approval for the same indication in pediatric patients aged ≥ 10 years in the EU on 18 June 2021 [84]. Teriflunomide (16) is the active metabolite of leflunomide (46) [85], formed by in-vivo isoxazole ring opening (Figure 6).
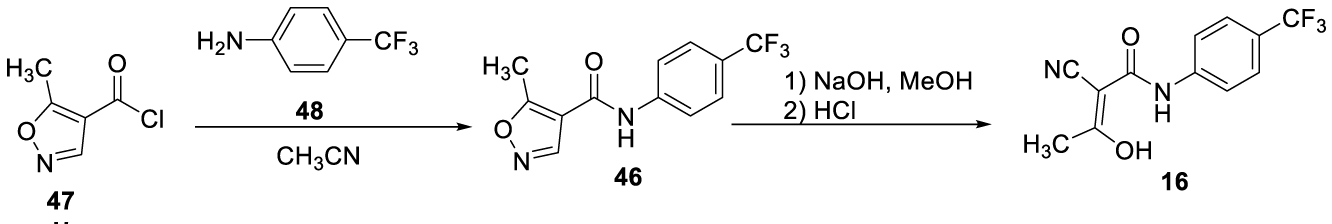
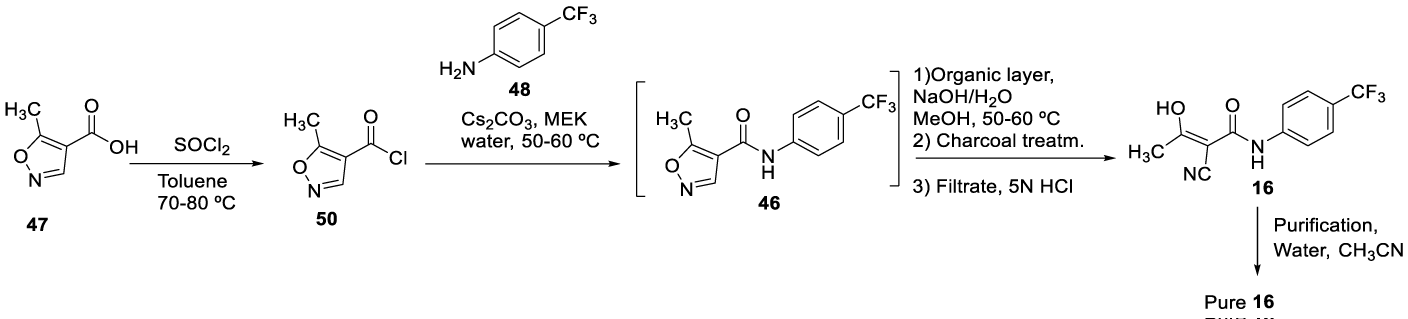
Teriflunomide synthesis: Several methods are described in the literature to prepare Teriflunomide (16) (CAS number: 163451-81-8) and some of them are presented herein. In 1996, Bartlett and Kammerer [86], from Hoechst Aktiengesellschaft,filed the patent U.S. Patent No. 5,494,911 for the synthesis of Teriflunomide (16). The process consisted of reacting 5-methyl isoxazole-4-carbonyl chloride 47 with 4-(trifluoromethyl) aniline 48 in acetonitrile to obtain leflunomide (46). Subsequent hydrolysis with aqueous sodium hydroxide solution in methanol gives Teriflunomide (16), which was then purified in a mixture of acetonitrile and water at 60-65 ºC to obtain a pure product Scheme 9, [86].
In the same year, Morris and Bartlett filed a patent (US 5,519,042) [87] claiming a new method to treat hyperproliferative vascular disease comprising the administration of Teriflunomide or leflunomide or derivatives thereof, and they used the same process described above. This process was also followed by Rajan and Eswaraiah [88], from MSM Laboratories Private Limited, in a patent filed in 2015 (WO2015/029063) to prepare a new polymorphic form of Teriflunomide.
Later, in 2016, Palle and co-workers [89] from Biocon Limited filed the patent WO2016203410A1 claiming a safe, simple, economical and commercially practical process providing a product with high yield and chemical purity. The process consisted of chlorinating 5-methylisoxazole-4-carboxylic acid 47 with thionyl chloride, in toluene, at 70 80 ºC to afford 50, which reacts with 4-(trifluoromethyl) aniline 48 in the presence of cesium carbonate, in a biphasic mixture composed of ethyl methyl ketone and water, at 45 ± 2°C, to afford 46. The compound 46 was not isolated and was readily hydrolyzed with aqueous sodium hydroxide solution in methanol to give pure 16, after recrystallization from water acetonitrile at 60-65 ºC. According to the authors, this process eliminated the Leflunomide (46) intermediate isolation, which was a time-consuming and often led to impurities in the product mixture, Scheme 10.
In 1999, Hirth and co-workers [90], from Sugen Inc., filed a patent (US patent 5,990,141) entitled “Treatment of platelet-derived growth factor-related disorders such as cancer” which describes the synthesis of Teriflunomide (16), starting from the reaction of 51 with 4-(trifluoromethyl) aniline 48, under reflux, to afford acetoacetyl-(4-trifluoromethyl)aniline 52. Compound 52 reacts with acetic anhydride in triethoxymethane 53, under reflux, to give 54. The reaction of 54 with hydroxylamine hydrochloride in ethanol, at reflux for 1 hour, afforded Leflunomide (46), which after treating with DBU in EtOH at room temperature, provided the final product (16), Scheme 11.
In 2017, Subramaniam and co-workers [91] reported the same synthetic route starting from 52. The main difference of this route synthesis was the conversion of Leflunomide (46) to Teriflunomide (16) with HCl in methanol.
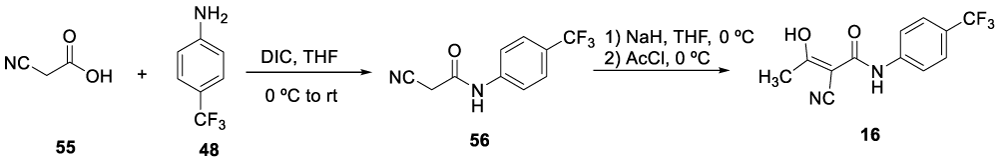
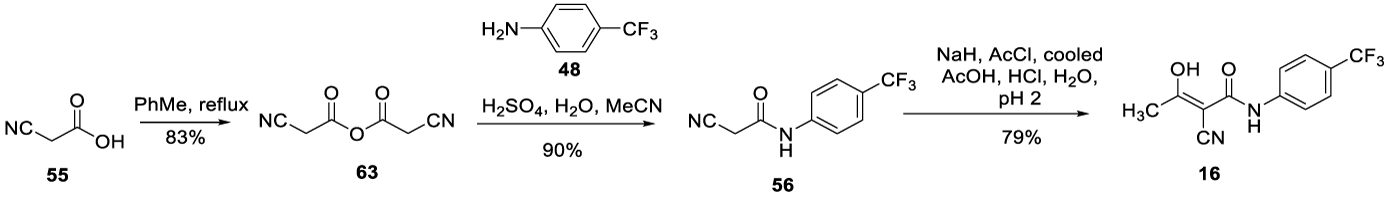
In 1999, Mahajan and co-workers [92], reported the synthesis of Teriflunomide (16), starting from cyanoacetic acid 55. The process involved the coupling of cyanoacetic acid 55 with 4-(trifluoromethyl) aniline 48, in the presence of diisopropylcarbodiimide to form pure 56, after recrystallization from ethanol. Intermediate 56 was treated with NaH and acylated with acetyl chloride to afford pure product 16. Uckun and co-workers also described this process in US 6,355,678 B1 patent, filed in 2002, Scheme 12 [93].
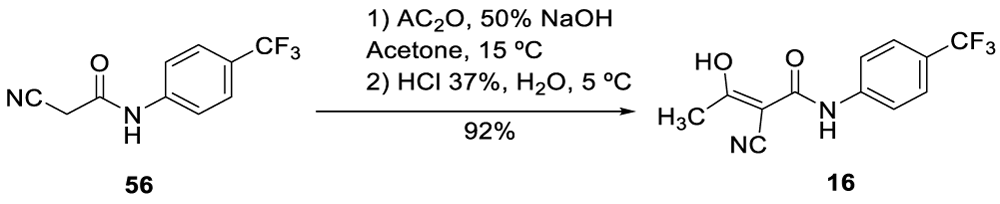
In 2004, Hachtel and co-workers from Aventis Pharma Deutschland GmbH filed the US 2004/0186173 A1 patent [94] describing the preparation of Teriflunomide (16)by reacting phenyl-substituted 2-cyano-N-(phenyl)acetamide 56 in the presence of acetic anhydride and sodium hydroxide, Scheme 13. According to the authors, the disadvantages of the processes described in US 5,519,042 and US patent 5,700,822 patents were low yield and purity.
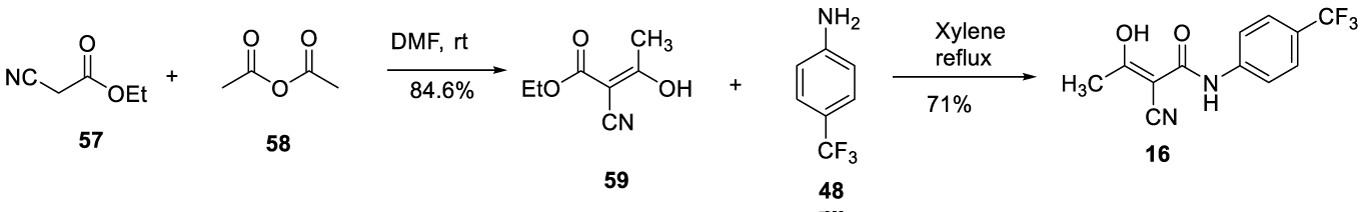
In 2009, Keshav and co-workers, from Alembic Limited, filed a patent (WO 2009/147624 A) claiming a process to prepare Teriflunomide (16). Ethyl cyanoacetate 57 reacts with acetic anhydride 58 in the presence of K2CO3 in DMF at room temperature to afford 59. The compound 59 reacts with 4-(trifluoromethyl) aniline 48 in xylene, under reflux, for 48 hours to provide the desired product (16), Scheme 14.
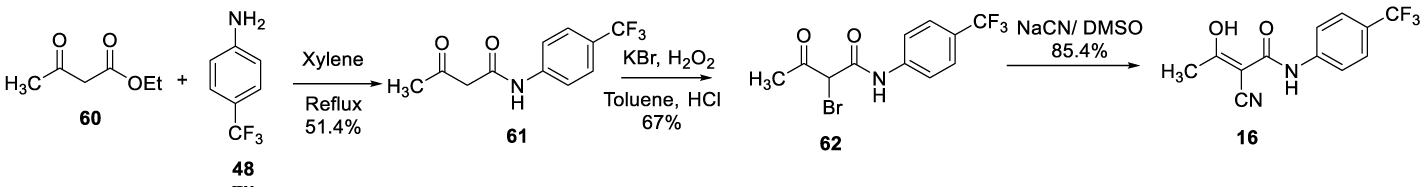
In 2010, Keshav and co-workers [95], reported in the patent WO 2010013159 that the processes described in the patents US 5,494,91, US 5,990,141 and US 6,365,626 require chromatographic purification, which in turn results in low yield. According to the authors, the process presented in WO 2010013159 had less steps and was feasible at a commercial scale. The process consisted of refluxing the ethyl acetoacetate 60 with 4 (trifluoromethyl phenyl) aniline 48 in xylene to give pure 3-oxo-N-(4-trifluoromethylphenyl)butanamide 61, after column chromatography purification. At room temperature, the 61 was brominated with KBr, H2O2 and HCl in toluene to afford pure 2-bromo-3-oxo-N-[4-(trifluoromethyl)phenyl]butanamide 62, after column chromatography purification. Finally, 62 reacts with NaCN in DMSO to give Teriflunomide (16), Scheme 15.
In 2012, Chen and Sun, from China Pharmaceutical University, filed the patent CN 102786437 claiming a process for preparing Teriflunomide (16) [96], The process starts by reacting 2-cyanoacetic acid 55 in toluene, under reflux, to form 2-cyanoacetic anhydride 63, which reacts with 4-trifluoroaniline 48 to afford 2-cyano-N-(4-(trifluoromethyl)phenyl)acetamide 56. Treatment of 56 with NaH, followed by acylation yields Teriflunomide (16), Scheme 16.
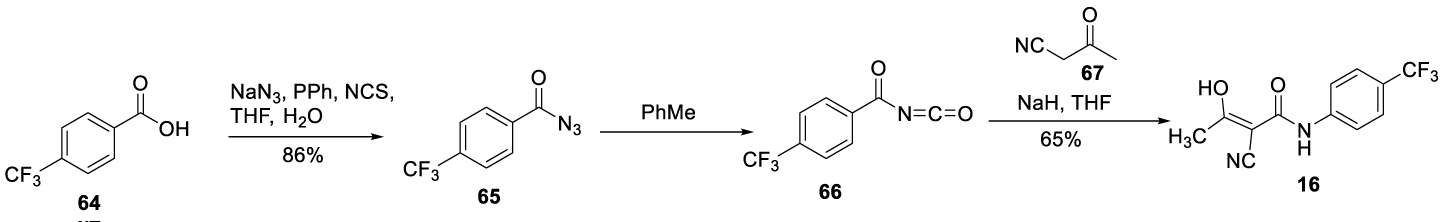
In 2014, Xinliang Chen and co-workers [97], from Hybio Pharmaceuticals (Wuhan), filed a patent CN 103848756 to prepare Teriflunomide (16) and its intermediate. The process starts by using a different source of fluorine, 4-(trifluoromethyl)benzoic acid 64. Compound 64 reacts with sodium azide to afford 4-(fluoromethyl)benzoyl azide 65, which after Curtius rearrangement produces the 4 (trifluoromethyl)benzoyl isocyanate 66. Finally, the isocyanate 66 was treated with NaH, followed by the reaction with 3-oxobutanenitrile 67 to afford 16, Scheme 17.
One year later, in 2015, Li and co-workers [98], also from Hybio Pharmaceutical Co., filed another patent (CN 104693070) claiming a new synthetic method for preparing teriflunomide (16). The process consists of chlorinating 2-cyanoacetic acid 55 with thionyl chloride to afford 2-cyanoacetyl chloride 67, which reacts with 4-trifluoroaniline 48, in the presence of triethylamine, in dichloromethane to afford 56. The treatment of 56 with sodium followed by acylation provided (16) in 91% yield, Scheme 18.
Vicriviroc: Vicriviroc (68); (Figure 7) (alternative names: SCH-417690 and SCH-D) is an investigational drug originally from Schering-Plough, for the treatment of immunodeficiency virus type 1 (HIV-1) infection. Vicriviroc (68)is a next-generation antiretroviral compound that blocks HIV from entering uninfected cells by binding to the virus's cellular co-receptor chemokine receptor 5 (CCR5) [99,100]. It was shown that vicriviroc is a potent antagonist of the CCR5 receptor, possessing broad-spectrum antiviral activity [100].
Vicriviroc (68) was acquired by Merck as part of its merger with Schering-Plough, which had been developing the drug for over ten years. In 2010 Merck announced that it was to halt all development of the CCR5 inhibitor Vicriviroc (68), following disappointing data from a Phase II study of the drug in people with HIV with no previous history of treatment [101]. However, in 2011, the pharmacokinetic interaction of Vicriviroc in regimens containing the most common antiretroviral agents (protease inhibitors atazanavir, darunavir, fosamprenavir, indinavir, nelfinavir, saquinavir and tipranavir) was assessed with positive results [102]. It also assessed the effect of Vicriviroc with or without ritonavir on oral contraceptive pharmacokinetics [103]. In 2015, a Phase I study was performed to evaluate the pharmacokinetics and safety of two Intravaginal rings (IVRs) containing different doses of a combination of two HIV ARV drugs, Vicriviroc (VCV) (MK-4176) and MK-2048, in healthy, HIV-uninfected women [104]. The safety and pharmacokinetics of IVRs containing Vicriviroc, MK-2048, and a combination of Vicriviroc/MK-2048, in healthy, HIV-uninfected women were assessed in the following year [105]. The safety and efficacy of Vicriviroc (MK-7690), at 2 dose levels in combination with pembrolizumab (MK-3475), in participants with advanced/Metastatic Microsatellite Stable (MSS) Colorectal Cancer (CRC) was evaluated in 2018 [106].
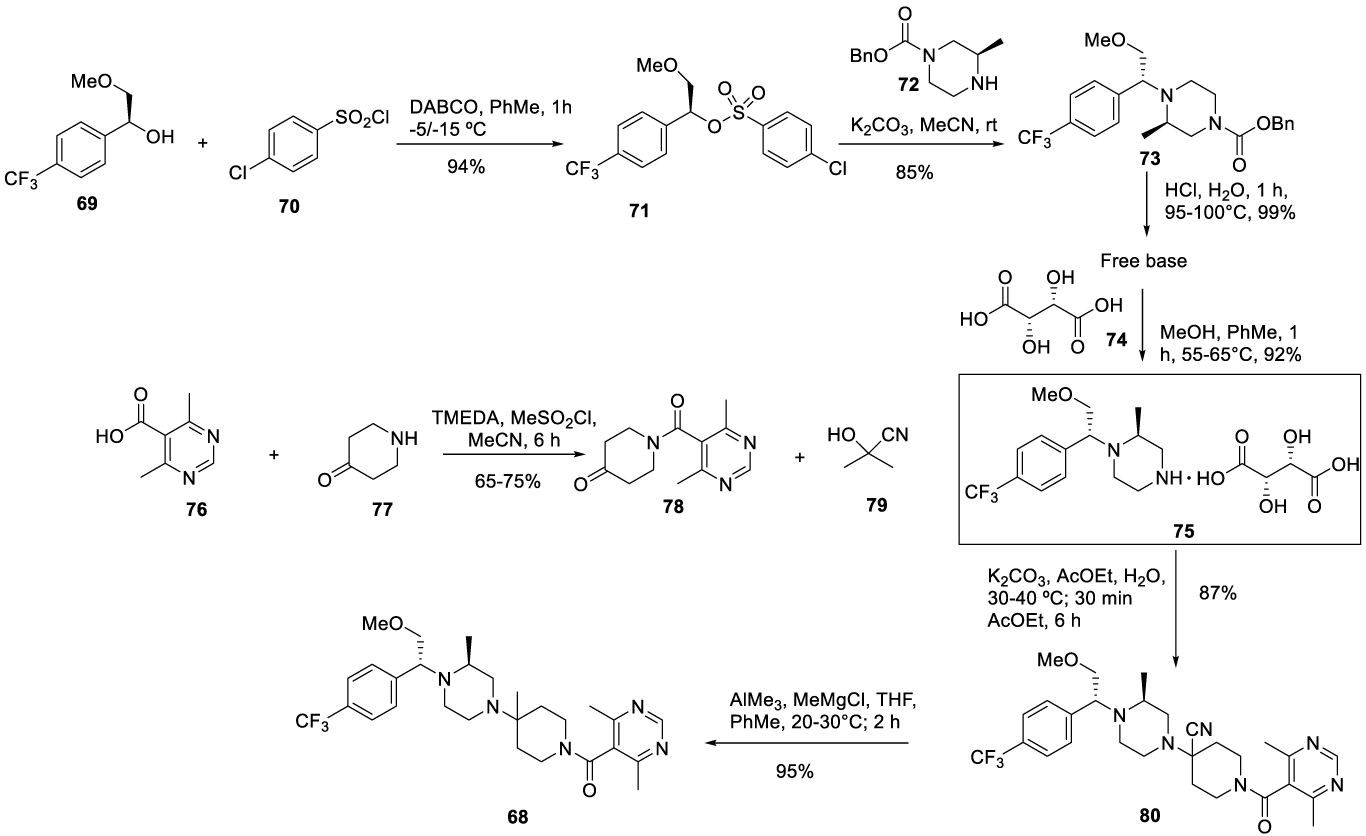
Vicriviroc synthesis: In 2003, Leong and co-workers [107] from Schering Corporation filed a patent (WO2003084950 A1) claiming the preparation of Vicriviroc (68) (CAS number. 541503-81-5) starting from intermediates 75 and 78 in the presence of a cyanate compound 79, according to Scheme 19. Intermediate 75 was prepared by reacting 69 with 4 chlorobenzenesulfonyl chloride 70 in the presence of 1,4-diazabicyclo[2,2,2]octane (DABCO), in toluene, at -5/-15 ºC, for 1 hour to afford 71. Compound 71 reacts with 72 in the presence of potassium carbonate in acetonitrile at room temperature to give 73. The hydrolysis of 73 in acidic media, at 95-100 ºC for 1 hour, affords an oily free base, which was then converted to its corresponding salt as white solid 75, in the presence of D-tartrate acid 74, in a mixture of methanol and toluene. Intermediate 78 was prepared by reacting 76 with 4 piperidone monohydrate hydrochloride 77 in the presence of N,N,N',N'-tetramethylethylenediamine (TMEDA) and acetonitrile at -10 ºC to 0°C, followed by the addition of methanesulfonyl chloride and more TMEDA to afford 78. The reaction of tartrate salt 75 with 78 and cyanohydrin 79 yields 80, which reacts with AIMe3 and MeMgCl at 20-30°C to give 68, Scheme 19.
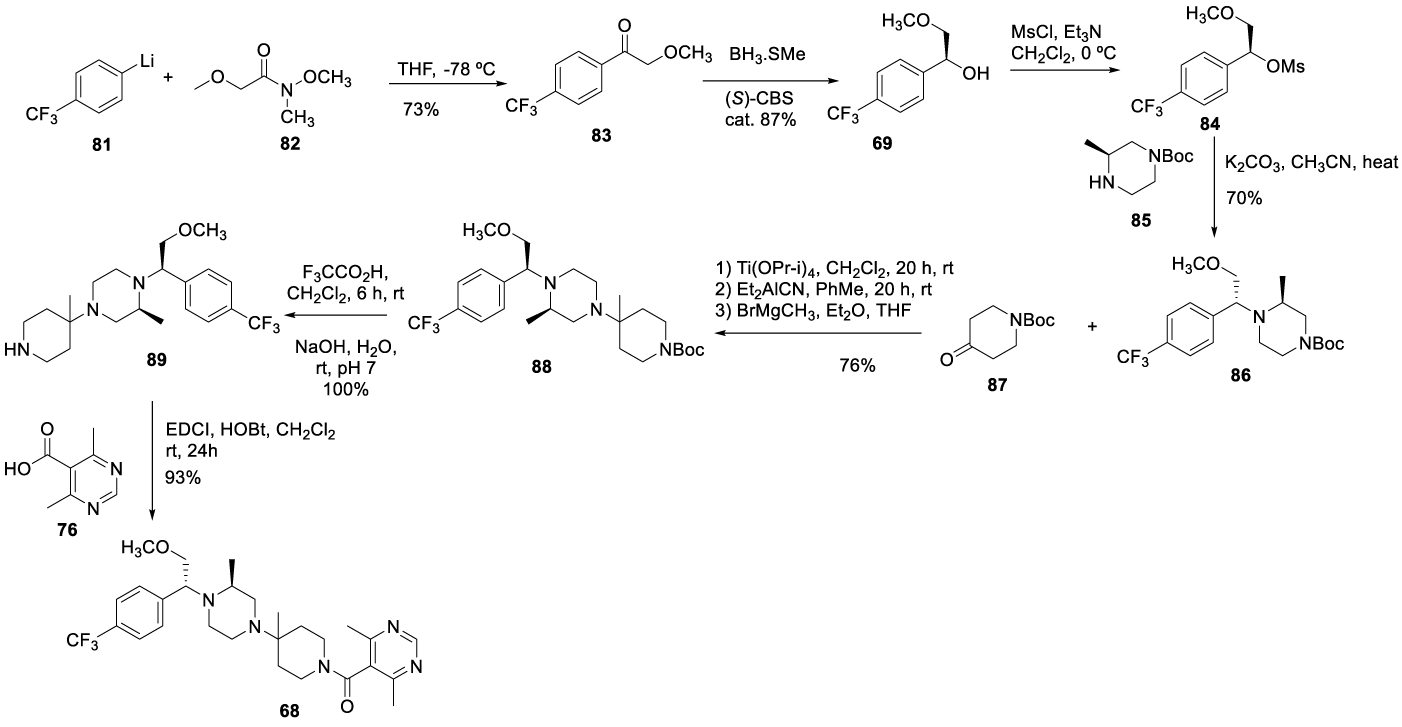
In 2004, Tagat and co-workers [108] from Schering-Plough Research Institute reported the synthesis of piperazine-piperidine compounds, which includes Vicriviroc (68), according to a route designated as the SN2 displacement route (Scheme 20). The process starts by adding aryllithium 81 to the Weinreb amide 82 to afford α-alkoxyacetophenone 83 as an amber oil. The α alkoxyacetophenone 83 was reduced by (S)-CBS (or Corey–Bakshi–Shibata catalyst) in borane dimethylsulfide to furnish chiral benzylic alcohol 69 as a yellow oil. Activation via mesylate yields 84 (colorless oil) which, upon reaction with the chiral piperazine 85 led to the desired (R,S) diastereomeric 86, after flash chromatography treatment. The N-Boc deprotection of 87, followed by the reaction with titanium isopropoxide and diethylaluminum cyanide and then magnesium bromide, was afforded 88. At room temperature the Boc deprotection of 88 with TFA in dichloromethane afforded 89, which condensed with 4,6 dimethylpyrimidine-5-carboxylic acid 76 to give the final target 68, Scheme 20.
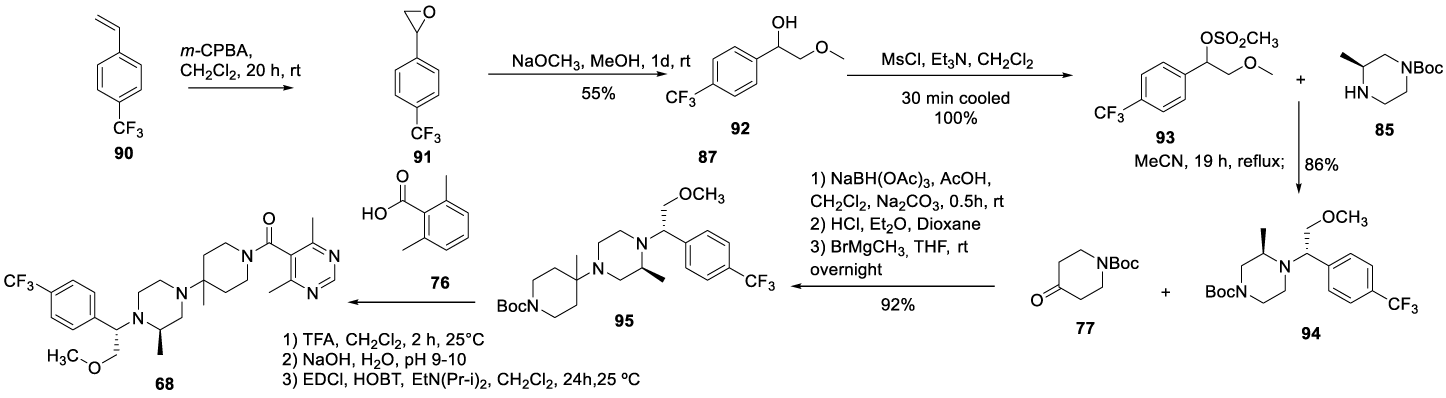
In 2006, Ramanathan and co-workers [109] from Schering Corporation USA filed a patent (US 20060105964 A1) claiming the preparation of piperazine derivatives, which included Vicriviroc (68), Scheme 21. The process starts by oxidizing 1-(trifluoromethyl)-4-vinylbenzene 90 with m-chloroperoxybenzoic acid (m-CPBA) to form 2-(4-(trifluoromethyl)phenyl)oxirane 91 as a colorless oil. The epoxide 91 formed was opened with NaOCH3 in methanol to afford 92 as a colorless oil, which is mesylated in the presence of triethylamine in dichloromethane to afford 93. The coupling of 93 with 85 in acetonitrile, under reflux, afforded a racemic mixture. After flash chromatography on silica gel, afforded pure 94. The reductive amination of the free N-Boc-piperidin-4-one with the installation of the ipso-methyl group provided the Boc-protected piperidinyl 95. The Boc protecting group was removed from the piperidine nitrogen with TFA. The resultant compound was coupled with acid 76 in the presence of EDCI/HOBt to provide 68.
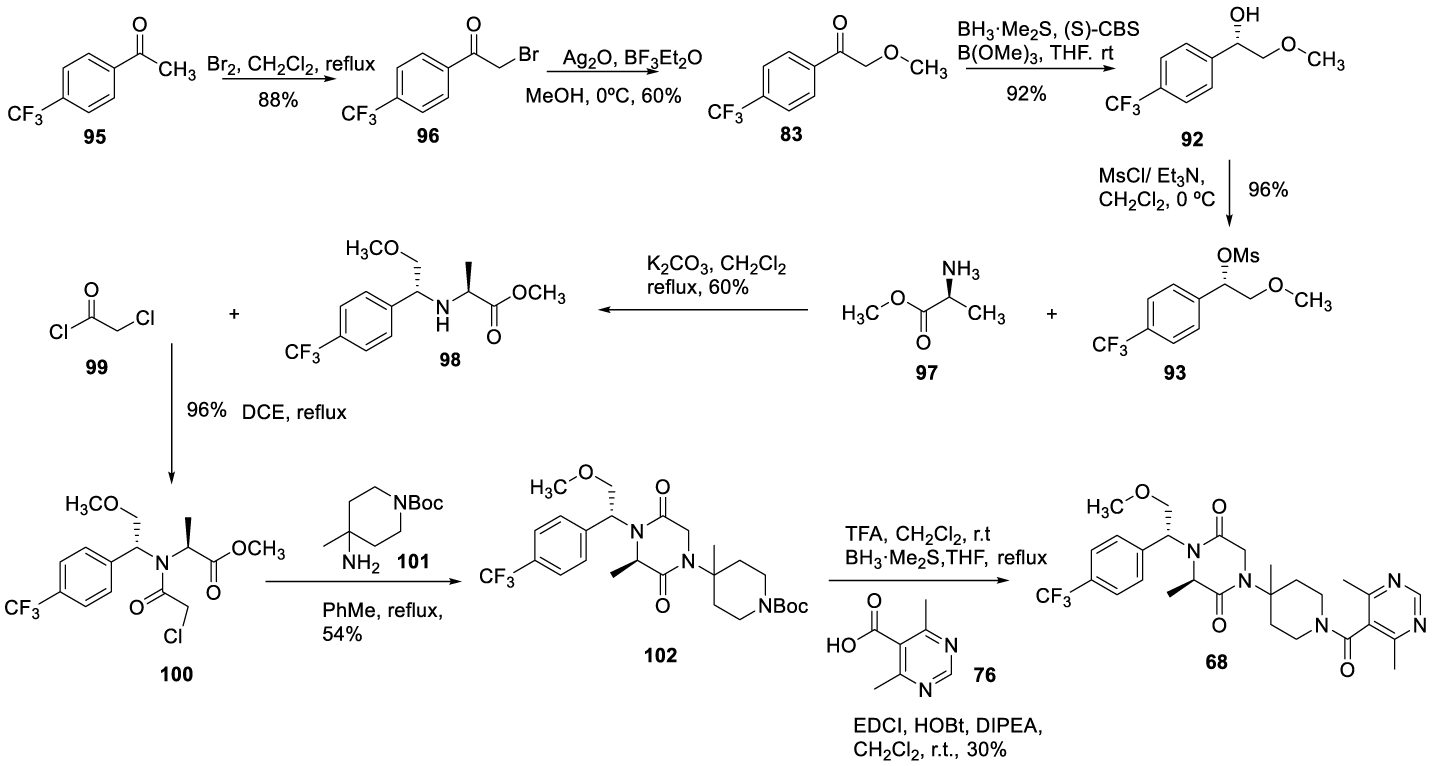
Vicriviroc (68), may also be prepared as reported in 2007 by Jiang (Feng) and co-workers [110]. The process consists of 8 steps, starting with the bromination of 1-(4-(trifluoromethyl)phenyl)ethan-1-one 95 in dichloromethane, at reflux temperature, to afford 96 as white crystals. The bromo from the α bromoacetophenone 96 is displaced by nucleophilic substitution with methanol in the presence of Ag2O, BF3.Et2O, at room temperature to afford 83 as white crystals. The asymmetric reduction of 83 with borane dimethylsulfide (BMS) catalyzed by CBS afforded 92 as a yellowish oil. Compound 92 leads to 93 by mesylation with mesyl chloride in the presence of triethylamine. The condensation with L alanine methyl ester 97 yielded 98 which, by acetylation with 2 chloroacetyl chloride 99, gives 100. The condensation with 101 affords 102, which after N-Boc deprotection with TFA in dichloromethane at room temperature and coupling with 76 in the presence of EDCl, HOBt and DIPEA affords Vicriviroc (68), Scheme 22.
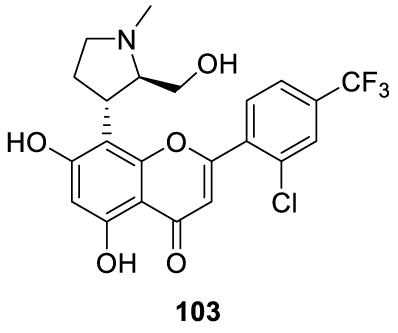
Voruciclib: Voruciclib (103) (alternative name: P-1446A-05) is an investigational drug originally from Piramal Life Sciences and developed by MEI Pharma Piramal Enterprise [111,112]. Voruciclib initiated in 2018 a Phase I clinical trial to determine the safety and efficacy in patients with relapsed/refractory B cell malignancies like chronic lymphocytic leukemia and acute myeloid leukemia [113]. Approximately 35% to 40% of patients with diffuse large B-cell lymphoma and who did not cure with standard frontline therapy, will exhibit primary refractory or relapsed disease [114]. Novel biological targets, enabled by biological advances in the characterization of B cell lymphomas, are being evaluated in clinical trials [115]. Inhibition of Cycline-Dependent Kinase 9 (CDK9), a member of the CDK proteins family, results in the repression of short-life cells like Mcl-1 which are associated with apoptosis implicated in the pathogenesis of malignancies like lymphomas and leukemias. Voruciclib (103) (Figure 8) acts as an inhibitor of CDK9, indirectly inhibiting the apoptotic function of Mcl-1.
The approach of targeting Mcl-1 by inhibiting specific CDK isoforms that regulate transcription, particularly CDK9, was conducted after failure in the preclinical development of Mcl-1 inhibitors, due to their toxicity [116].
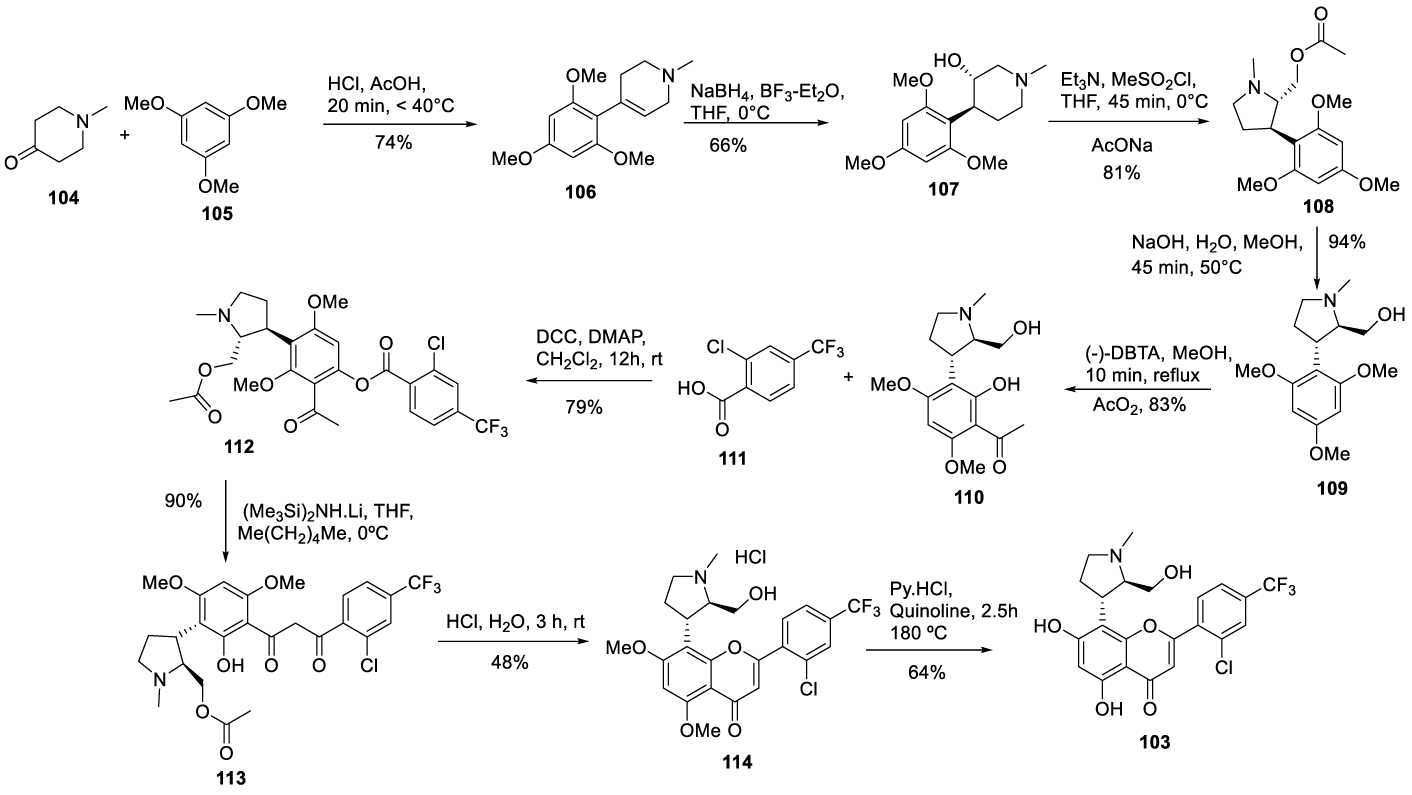
Voruciclib synthesis: In 2007 Sivakumar and co-workers [117], from Piramal India Limited, filed a patent (WO 2007148158 A1) claiming the preparation of the enantiomerically pure compound and its intermediates which includes Voruciclib (103) (Cas number. 1000023-04-0), Scheme 23. The process starts by reacting 1-methyl-4-piperidone 104 with 1,3,5-trimethoxybenzene [105] in glacial acetic acid, at a temperature below 40 ºC, to afford [106], which is reduced to [107] with NaBH4 in the presence of BF3.Et2O, in THF. The hydroxyl group on the piperidine ring is activated with methanesulfonyl chloride and triethylamine. The following step is the ring contraction with sodium acetate to afford [108], which after hydrolysis with an aqueous NaOH solution in methanol yields [109]. Resolving [109] with (-)-DBTA (dibenzoyl tartaric acid) followed by acylation with acetic anhydride affords [110], which reacts with [111] in the presence of a base DCC-DMAP combination in dichloromethane to afford [112]. The Baker-Venkataraman rearrangement of the resolved 112 with a lithium hexamethyl disilazide in THF led to the corresponding β-diketone [113], which cyclizes to give hydrochloride flavone [114] after acid treatment. Subjecting the compound of formula [114] to dealkylation by heating it with a dealkylating agent pyridine hydrochloride at a temperature ranging from 120-180 °C to obtain the {+)-trans enantiomer of the compound of formula [103]. Rathos and co-workers, [118] also from Piramal Life Sciences Limited, described the same process in a patent (WO2012066508 A1) filed in 2012.
Compounds containing one trifluoromethyl group in benzene ring meta position
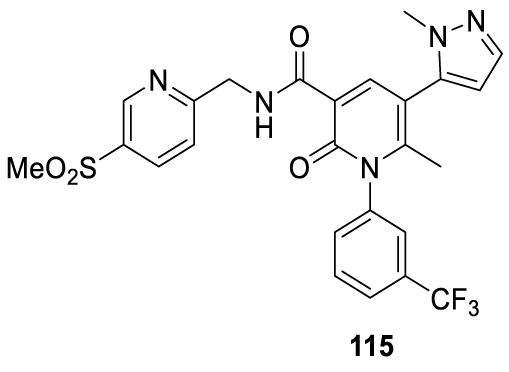
Alvelestat: MPH-966, previously AZD-9668 and also known as Alvelestat (115; (Figure 9)), is an oral small molecule that selectively and reversibly inhibits Neutrophil Elastase (NE), an aggressive and cytotoxic protease enzyme that is associated with the destruction of lung tissue. NE has long been known to be implicated in the signs, symptoms, and disease progression in many lung disorders through its role in inflammatory processes, mucus over-production, and lung tissue damage. Normally NE activity is tightly regulated by endogenous protease inhibitors including α1-antitrypsin (AAT), secretory leukoprotease inhibitor, and α2-macroglobulin. AstraZeneca initially developed Alvelestat for treating COPD, Cystic Fibrosis (CF) and bronchiectasis, where these NE pathways are known to be disrupted [119,120].
More recently, in 2020, Phase II clinical trials were conducted to reduce lung damage and slow the progression of lung disease in patients with AAT deficiency [121]. Alvelestat was also tested for Covid treatment as reported by Erin in 2022 [122].
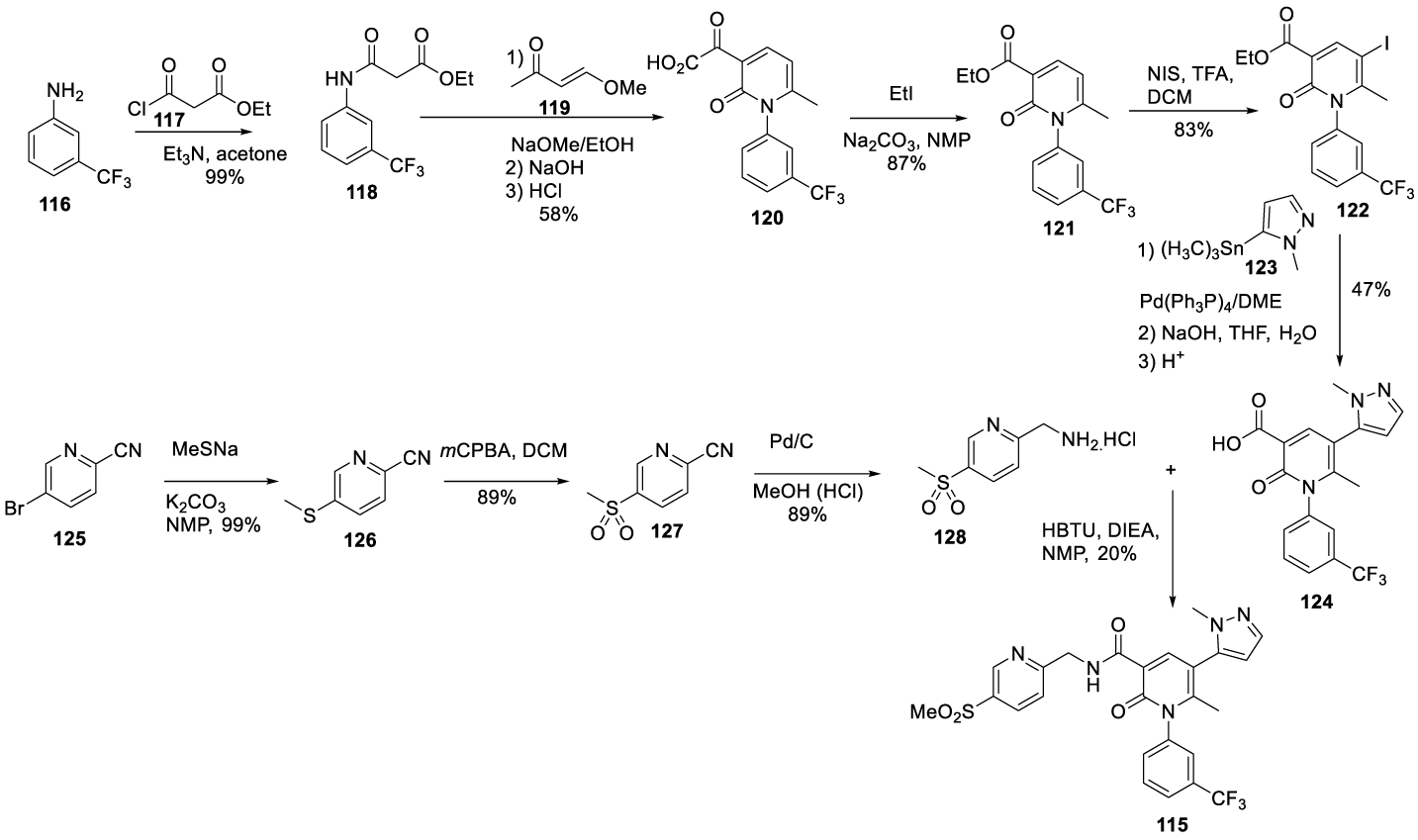
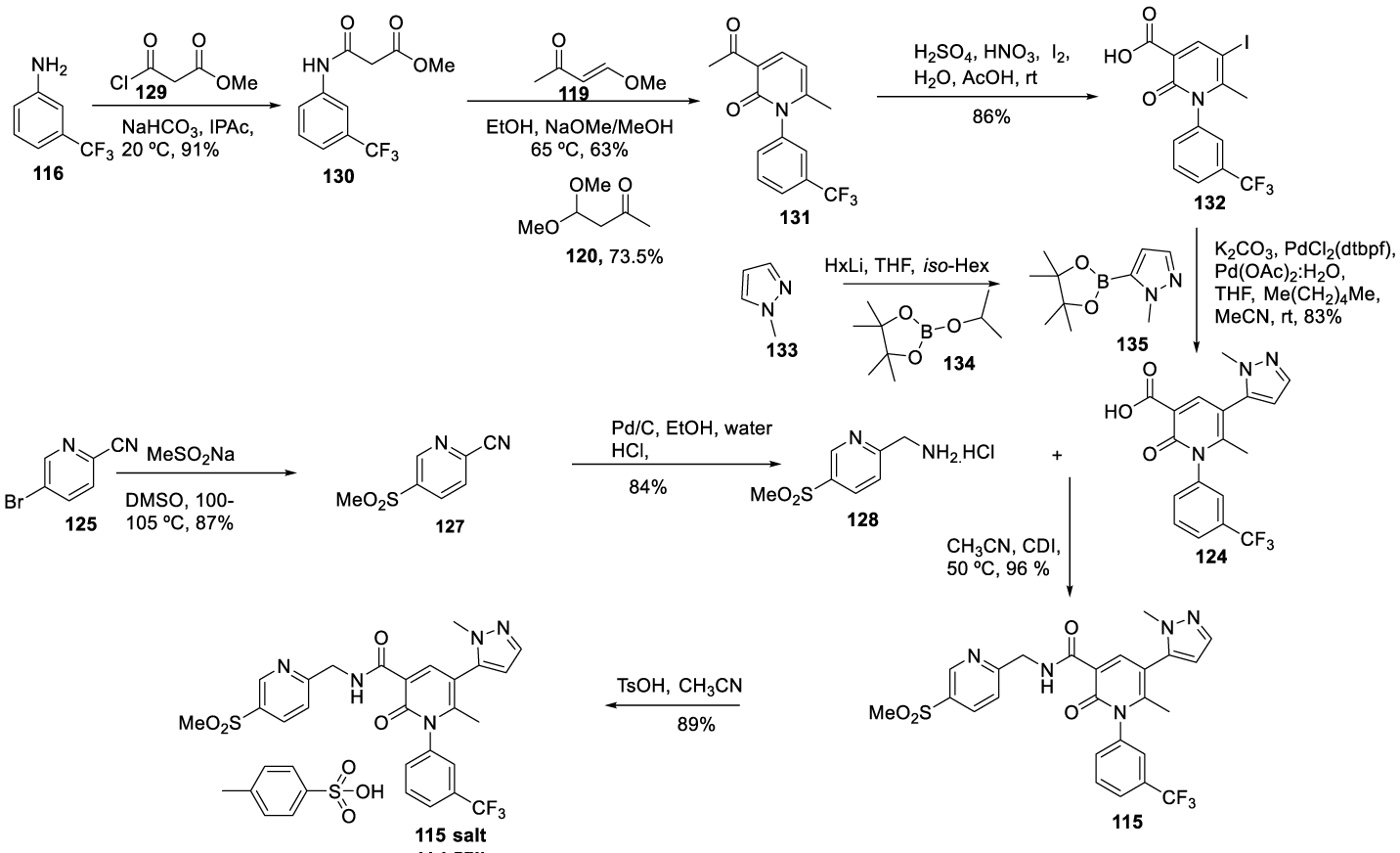
Alvelestat synthesis: In 2005, Anderson and co-workers123 from AstraZeneca AB, filed a patent (WO 2005/026 123) claiming the preparation of novel 2-pyridone derivatives, which included Alvelestat (115) (CAS number 848141-11-7), Scheme 25. Alvelestat (115) is prepared by a convergent synthesis starting from intermediates 124 and 128. The intermediate 124 preparation process starts by coupling 3-trifluoromethyl aniline 116 with ethyl 3-chloro-3-oxopropanoate 117, in acetone with triethylamine, to afford 118. The reaction of 118 with 119 in the presence of sodium methoxide in ethanol, followed by cyclization and ethyl ester hydrolysis with HCl, provided the correspondent acid 120, which was recrystallized twice from heptane/ ethyl acetate. The esterification of 120 with iodoethane in the presence of disodium carbonate in NMP, afforded [121]. The iodination of the pyridine ring with N-iodosuccinimide (NIS) with TFA and dichloromethane afforded [122]. Compound [122], after the Stille palladium-catalyzed coupling with [123] afforded [124]. Intermediate 128 was prepared by reacting 5-bromopicolinonitrile [125] with sodium methanethiolate to provide [126], which after oxidation with 3-chloroperbenzoic acid followed by hydrogenation afforded [127]. The amide formation by coupling 128 with 124, in NMP, with HBTU and DIEA, provided crude 115 which was purified by preparative HPLC and freeze-dried to give the free base as a white solid, Scheme 24.
Alcaraz and co-workers [124],also from AstraZeneca UK Limited, filed a patent (WO2010094964A1) in 2010 claiming a process which, according to the authors, had significant advantages compared to previous routes. This process had less steps and afforded significantly improved yields. The route also minimized the use of potentially toxic reagents such as ethyl iodide and organotin compounds, Scheme 25. The route also provides the final compound with improved purity.
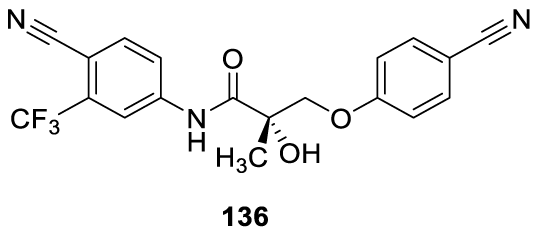
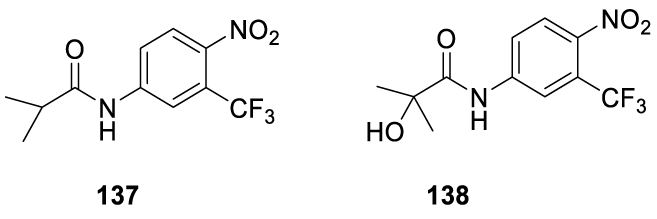
Enobosarm: Enobosarm (136) (alternative names: ostarine, GTx-024, S-22) is an investigational drug developed by GTx, Inc., formerly under development by Merck & Company [125], 126 GTx-024 is an orally bioavailable nonsteroidal selective androgen receptor modulator (SARM), with tissue-selective anabolic and androgenic pharmacologic activity [127-131]. Enobosarm (136) is under clinical trials for treating cancer cachexia, sarcopenia and muscle wasting diseases [132-134]. It was designed to work like testosterone, promoting muscle growth. Muscle wasting, with or without fat mass loss, is a complex metabolic syndrome designated by cachexia [135]. It is a common condition in cancer, characterized by systemic inflammation, negative protein and energy balance, and an involuntary loss of lean body mass. Enobosarm (136) is a member of the class of organic compounds known as trifluoromethylbenzenes (Figure 10) and has been shown to stimulate the growth of lean muscle mass in humans in Phase I, II, and III clinical trials [136].
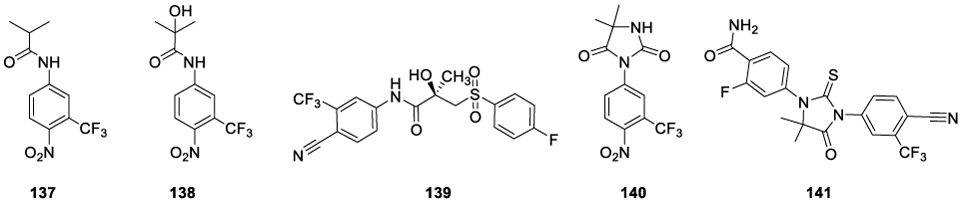
The rational design of Enobosarm (136) was based on the anilide molecular structures of non-steroidal androgen drugs approved for the treatment of prostate cancer, namely, Flutamide (137), 2 Hydroxyflutamide (138), Bicalutamide (139), Nilutamide (140) and Enzalutamide (141) (Figure 11) [137].
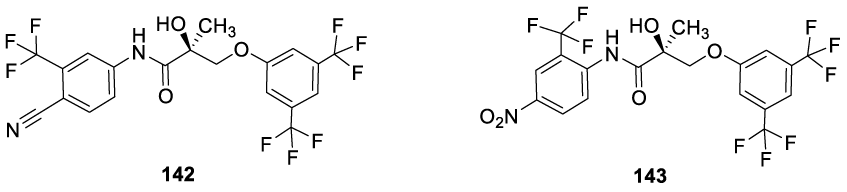
Interestingly, novel Enobosarm analogs (Figure 12) were designed by the rational addition of bis trifluoromethyl groups into the phenol ether ring, resulting in an improved binding affinity for the androgen receptors.138 SK33 (142) and SK51 (143) analogs showed in vitro to be 100-fold more potent than Enobosarm (136), using a panel of LNCaP prostate cancer cell lines [138].
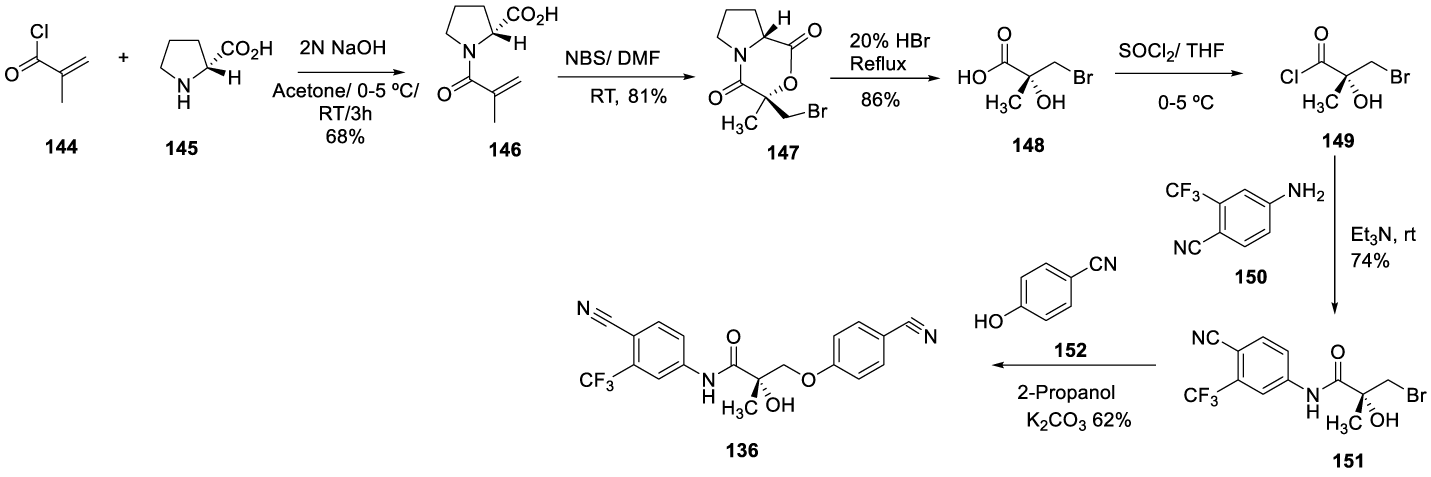
Enobosarm synthesis: In 2007 Dalton and co-workers filed a patent (US 2007/0123563 A1) describing the synthesis of Enobosarm (136) (CAS number 841205-47-8) [139]. More recently, in 2019, Narayanan and co-workers [140-143] from Oncternal Therapeutics filed a patent (US 2020/028593 A1), describing the same synthesis of Enobosarm (136) (enantiomer S), as illustrated in Scheme 26. 2-Methyl-acroloy chloride [144] is coupled with pyrrolidine 2-carboxylic acid [145]in a mixture of acetone with aqueous NaOH 2N, at 0 – 5 ºC to rt, for 3 hours, affording crude 146 as a colorless oil, which after recrystallization from ethyl ether and hexanes afforded [146] as colorless crystals. Recrystallized [146] reacts with N-Bromo Succinimide (NBS) in Dimethylformamide (DMF) at room temperature to afford [147]. Acidic hydrolysis with aqueous HBr produces the crude carboxylic acid 148, which after workup, isolation and recrystallization from toluene afforded pure [148]. The chlorination with thionyl chloride afforded the acid chloride [149], which was coupled with [150] in triethylamine at room temperature to give the pure amide [151], after the column chromatography and recrystallization. The coupling with 4-hydroxybenzonitrile 152 in the presence of K2CO3 and 2-propanol yielded Enobosarm (136).
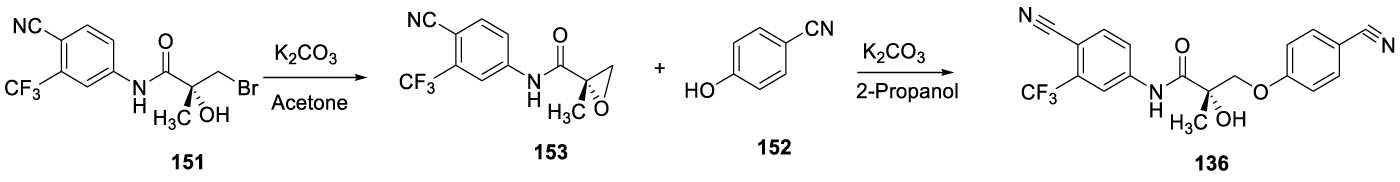
According to Dalton and co-workers 139, alternatively, intermediate [151] may be treated with K2CO3 in acetone to afford an oxirane [152], which reacts with 4-hydroxybenzonitrile [153], in the presence of K2CO3 and in 2-propanol (as presented in Scheme 27), to afford the desire 136.
In 2013, Schragl [141] and co-workers reported the synthesis of a new, simple route for the efficient preparation of racemic O-dephenylostarine [154], a potential intermediate for the synthesis of Enobosarm (136), Scheme 28.
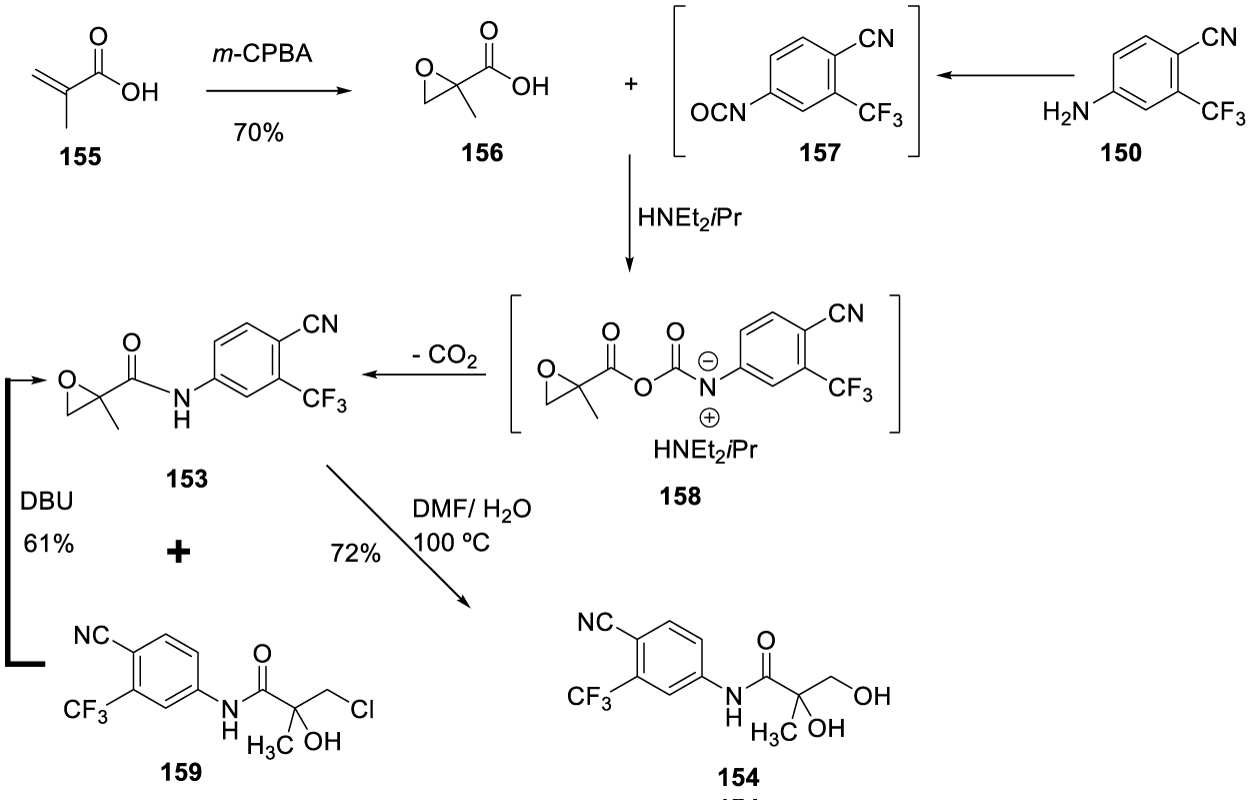
According to the authors, a short, highly cost-effective pathway providing [154] in 31% yield over five reaction steps was developed (Scheme 29). The synthesis starts with the epoxidation of methacrylic acid [155] with m-CPBA to give epoxide 156 in 70% yield. The DIPEA-salt of epoxy acid [156] was reacted with in-situ generated isocyanate [157] to provide intermediate [158], which after decarboxylation yielded the desired epoxyamide [153] together with the corresponding chlorohydrins [159]. The authors explained that the formation of the chlorohydrins could be due to the presence of chloride ions in the reaction mixture, resulting from triphosgene. The conversion of [15-159] was easily achieved by treatment of the corresponding chlorohydrin with DBU to give [153] in good yield over three steps. The best result for the epoxide opening reaction was obtained by refluxing [153] in a mixture of DMF/water and O dephenylostarine [154] in 72% yield.
2-Hydroxyflutamide: 2-Hydroxyflutamide (138) (alternative names: SCH-16423) is an investigational drug originally from LIDDS; Uppsala University, Inc. [142]. 2 Hydroxyflutamide (138) is the active metabolite of Flutamide (137) and its molecular structure holds an additional hydroxyl group, as depicted in figure 13.
Analyses of plasma, urine and feces, following oral dose administration of Flutamide (137), showed rapid conversion of Flutamide (137) to Hydroxyflutamide (138), being observed high plasma levels of Hydroxyflutamide (138), which suggested Hydroxyflutamide (138) be the active form of Flutamide (137) [143]. 2-Hydroxyflutamide (138) acted as a nonsteroidal antiandrogen and was used in Phase II clinical trials to treat prostate cancer [144]. Reduction in serum prostate-specific antigen and prostate volume were observed in most patients participating in the clinical trials. It was thought that the drug might have controlled cancer by inducing apoptosis or inhibiting cell growth through cell cycle arrest, resulting in long-term cancer suppression. In vitro prostate cancer cell studies suggested that 2-Hydroxyflutamide (138) modulates the phosphorylation of cadherin, catenin and androgen receptors [145]. Cadherins are a family of cell surface proteins, and catenins are a family of proteins found in complexes with cadherin in cell adhesion molecules of animal cells [146]. The complex cadherin/catenin is essential in maintaining epithelial integrity through cell-to-cell adherence. Any dysfunction or destabilization of the cadherin-catenin complex may result in loss of intercellular adhesion and possible tumor progression [147]. The cadherin/catenin complex stability is related to its phosphorylation status because phosphorylation regulates cellular functions like cell growth, differentiation, apoptosis, and alterations in phosphorylation pathways may result in cancer disease.
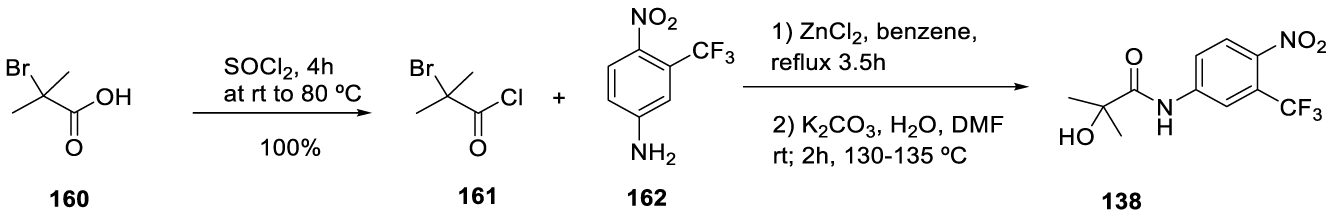
2-Hydroxyflutamide synthesis: 2-Hydroxyflutamide (CAS number: 52806-53-8) may be prepared using the conditions reported by Bal'on and co-workers [148] in 2009. Intermediate 161 is commercially available but may be synthesized in 100% yield with thionyl chloride, at room temperature, for 4 hours, starting from 2-bromo-2-methyl propanoic acid (160), as described in the Chinese patent by Jiang and co-workers in 2017 [149]. The intermediate 161 is condensed with4 nitro-3-(trifluoromethyl)aniline 162 in the presence of ZnCl2, in benzene, at reflux temperature, to yield 138 (Scheme 30).
Lifirafenib: Lifirafenib (163) (alternative names: BGB-283) is an investigational drug originally from BeiGene [150]. Lifirafenib (163) is a novel small molecule under Phase I clinical trials for the treatment of solid tumors in patients with mutated B-Raf (Rapidly accelerated fibrosarcoma) and K-Ras/N-Ras (Rat Sarcoma) kinase proteins [151]. Raf kinases initiate the Mitogen-Activated Protein Kinase (MAPK) pathway, which regulates cell proliferation by transmitting signals from the membrane receptors to the cell nucleus [152,153]. Raf and Ras mutations have been found in melanomas and cancers, providing a better understanding of the mechanisms underlying resistance and driving specific therapies for mutant forms mediating malignant transformation. [154,155]. Lifirafenib (163) is a fused tricyclic compound (Figure 14) that inhibits Raf's activities, showing potential for treating cancers with aberrations in the MAPK pathway [156].
The compound also inhibited the Epidermal Growth Factor Receptor (EGFR) at the biochemical and cellular levels. EGFR is a trans-membrane protein acting as a receptor for extracellular protein ligands of the epidermal growth factor family [157]. It regulates signaling pathways to control cellular proliferation. Mutations affecting EGFR expression or activity may lead to cancer. Results on in vitro and in vivo K RAS models support a new strategy to target K RAS-mutated cancers in the clinic. This strategy was currently being tested in a Phase I-b study evaluating Lifirafenib (163) (BGB-283) in combination with Mirdametinib (165) (PD-0325901; figure 15) in patients with advanced or refractory solid tumors harboring RAS mutations, RAF mutations, and other MAPK pathway aberrations [158].
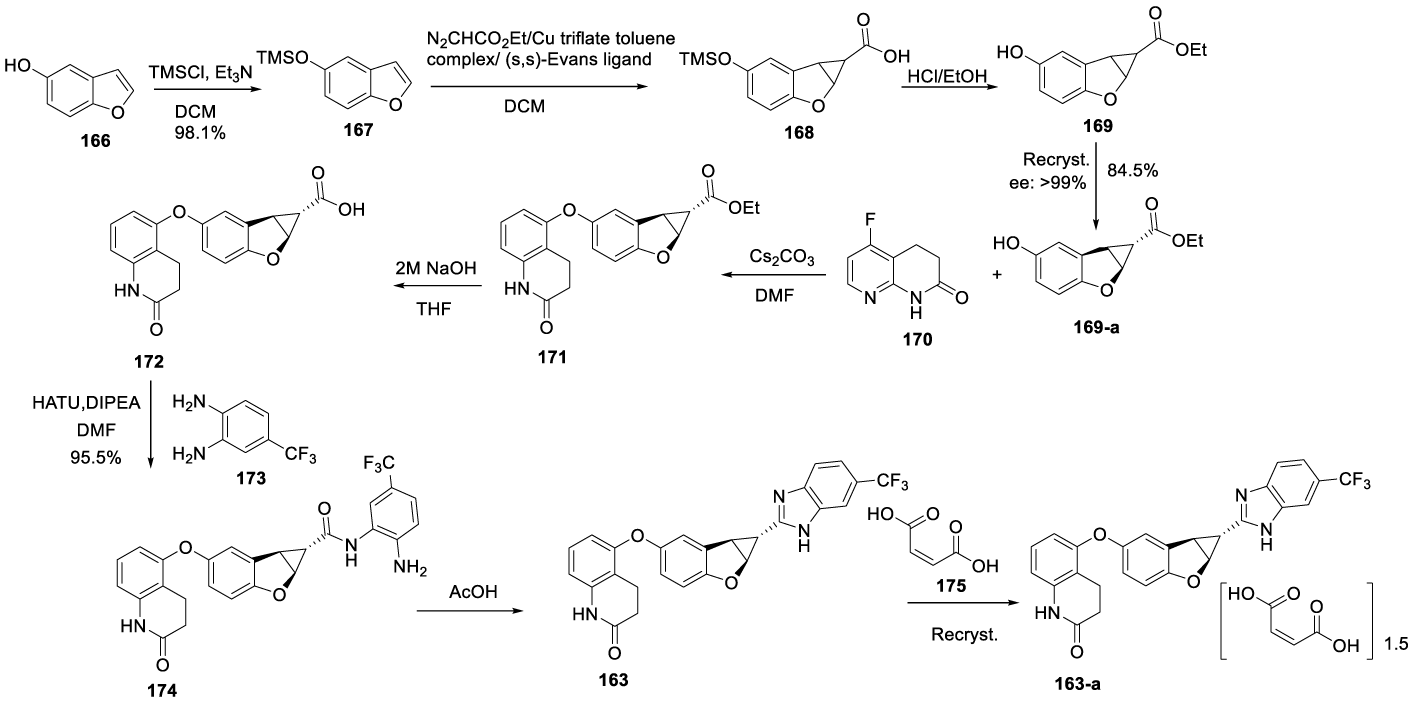
Lifirafenib synthesis: Lifirafenib maleate (163-a) (CAS number: 1446090-79-4) may be prepared using the process described in a patent filed in 2016 by Zhang and Zhou, from Biopharmaceutical company BeiGene Ltd (WO2016165626A), Scheme 31 [159]. The process starts by the tosylation of the alcohol group from the benzofuran 5 ol 166 with TMSCl, in dichloromethane, at -5 to 0 ºC. The tosylate residue 167 obtained after the workup, was directly used in the next step. The tosylate 167 reacted with ethyl diazoethanoate in the presence of copper triflate complex with toluene and (S,S)-Evans Ligand in DCM to afford the crude residue 168, which was collected by distillation at 120~140 ºC. Treatment of 168 with HCl in methanol afforded 169, which, after recrystallization from n-heptane/EtOAc, gave 169-a with 99.3% chiral purity. The coupling of 169-a with 5-fluoro-3,4-dihydro-1,8-naphthyridin-2(1H)-one 170 in the presence of cesium carbonate in DMF afforded 171, which after hydrolysis with aqueous sodium hydroxide and THF gave 172. The coupling of 172 with 173 in the presence of HATU and DIPEA, in DMF, yielded 174, which cyclized to 163 free base by treatment with acetic acid, and afterwards, was converted to a crystalline form of maleate salt 163-a.
Ligandrol: Ligandrol (176) (alternative names: VK5211, LGD-4033) was initially developed to address conditions associated with the loss of muscle mass with age. Phase I clinical trials were found to act as a Selective Androgen Receptor Modulator (SARM), binding to the androgen receptor with high affinity and selectivity [160]. The clinical trial showed that the compound stimulated preferentially skeletal muscle while sparing the prostate, indicating that its pharmacologic actions were tissue-selective. The androgenic activity was confirmed in this study by significant gains in muscle mass and strength, and by the suppression of testosterone, sex hormone-binding globulin and High-Density Lipoprotein (HDL) cholesterol levels. It has been hypothesized that non-steroidal selective androgen modulators like Ligandrol (176) provide the beneficial effects of androgens, like increased muscle mass and bone density, and have reduced anabolic side effects associated with androgenic steroids, like prostate stimulation in men and virilizing activity in women [161]. Ligandrol (176) is a small nonsteroidal molecule exhibiting two trifluoromethyl moieties in its molecular structure (Figure 16).
In vitro and animal model studies conducted with a combination of Ligandrol (176) and bisphosphonate alendronate, provided positive results regarding the potential use of such combination therapy for osteoporosis and bone frailty [162]. Interestingly, a review article about osteosarcopenia, a condition where osteoporosis and sarcopenia occur together, published in a geriatric maganize, referred that clinical Phase II trials with Ligandrol (176), showed a significant increase in lean muscle mass and a significant improvement in procollagen type 1 N propeptide (P1NP), a marker of bone formation, suggesting a dual effect on bone and muscle (unpublished results) [163]. In 2020, Barbara [164] reported the case of a 32-year-old man who developed severe drug-induced liver injury after using Ligandrol (176). In the same year, Roch and co-workers [165] reported the results of a study to investigate the effect of Ostarine (136; figure 17) and Ligandrol (176) on the muscle tissue of ovariectomized rats as the standard model for postmenopausal conditions. A beneficial effect on muscle vascularization was observed for both SARMs, with a more substantial impact on Ostarine (136). Ligandrol (176) showed more effect on muscle metabolism. However, higher intramuscular fat content was observed after Ligandrol (176) treatment, and a uterotrophic effect of both SARMs at higher dosages, which could be considered unfavorable side effects, and a limitation for applying the dosages used.
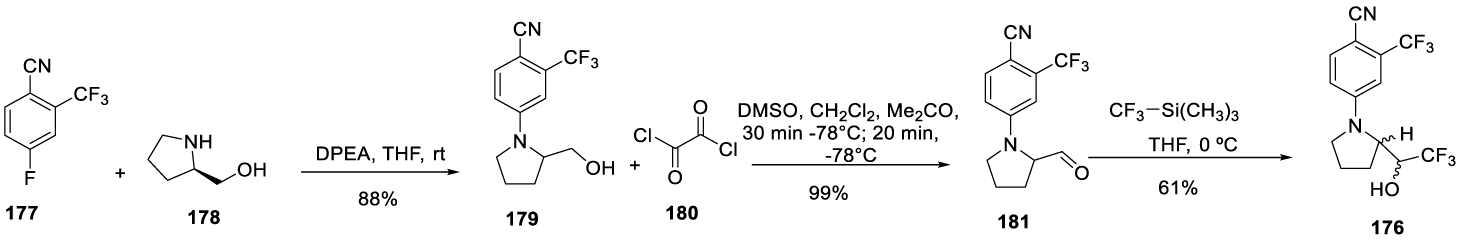
Ligandrol synthesis: Recently, in 2019, Grill and co-workers [166] reported the synthesis of Ligandrol (176) starting from the reaction of 4-fluoro-2-(trifluoromethyl)benzonitrile 177 with D-prolinol 178 in THF, and with Hünig base, at room temperature, for 2 days, to afford 179. The reaction of 179 with oxalyl chloride 180 in dichloromethane at -78 ºC afforded 181. The reaction of 181 with trimethyl(trifluoromethyl)silane in THF at 0 ºC for 15 hours afforded 176 (Scheme 32).
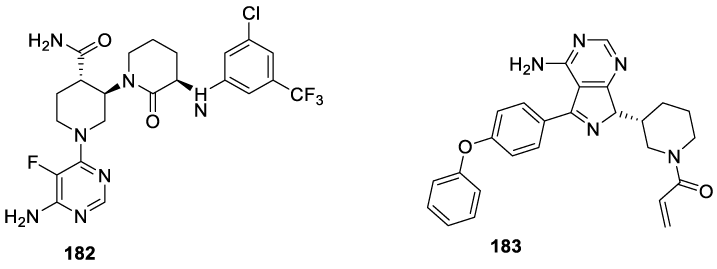
Vecabrutinib: Vecabrutinib (182; figure 18) (alternative name: SNS-062) is a second-generation, noncovalent (reversible) Bruton's Tyrosine Kinase (BTK) inhibitor developed by Sunesis Pharmaceuticals as a therapeutic for B cell malignancies that have developed resistance to first-generation BTK inhibitors such as Ibrutinib (183), through the acquisition of the Cys481Ser mutation in the enzyme's active site [167].
In 2016, Newman and co-workers reported the results of a randomized, double-blind placebo-controlled Phase I study and the results were encouraging, with favorable pharmacokinetic and safety properties [168]. In 2018, Jebaraj and co-workers investigated the efficacy of Vecabrutinib (182) using the Eµ-TCL1 adoptive transfer model. Vecabrutinib was efficacious in vivo in a preclinical Chronic Lymphocytic Leukemia (CLL) adoptive transfer model, decreasing tumor burden in different organs and significantly improving survival [169].In 2019, Alan and co-workers reported an open-label modified 3+3 dose-escalation, cohort expansion Phase I trial in patients with advanced B-cell malignancies. According to the results, Vecabrutinib's (182) safety profile at 25 and 50 mg BID dose levels was acceptable and the dose escalation was ongoing [170]. In 2020, Sunesis Pharmaceuticals announced that the Company would not advance its non-covalent BTK inhibitor Vecabrutinib (182) into a planned Phase II trial in adults with relapsed/refractory Chronic Lymphocytic Leukemia (CLL) and other B-cell malignancies [171].
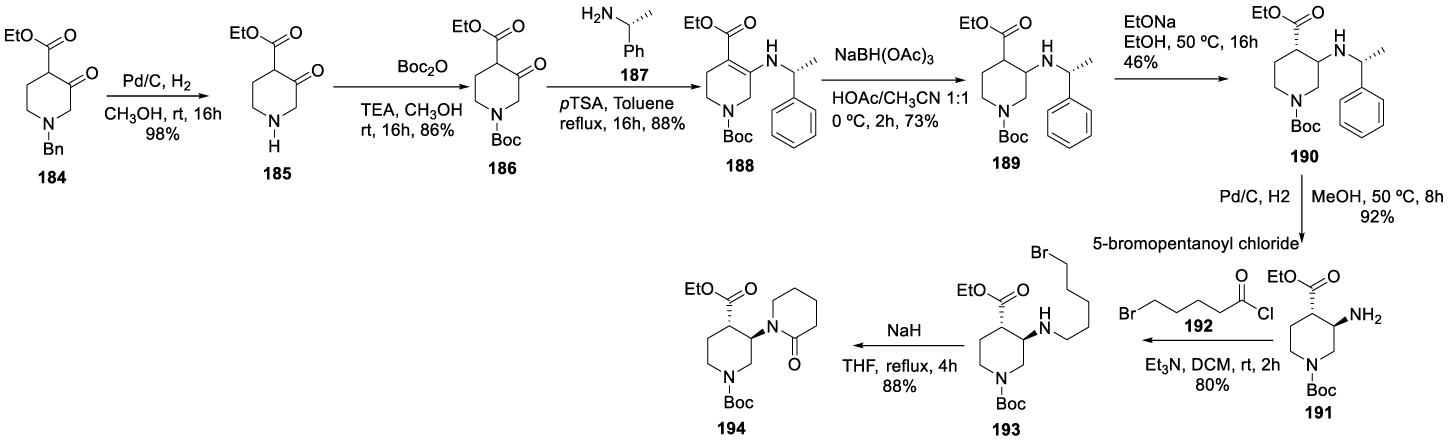
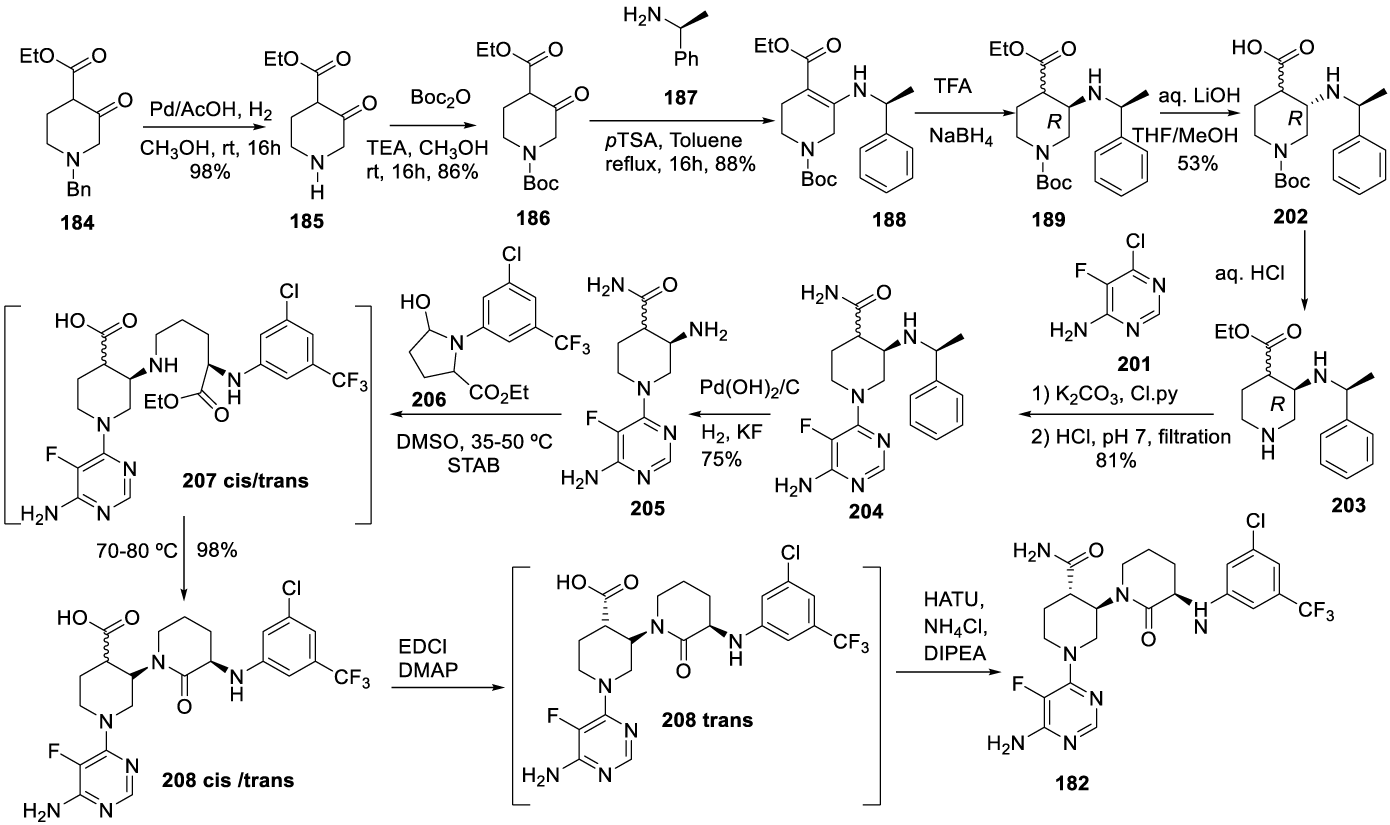
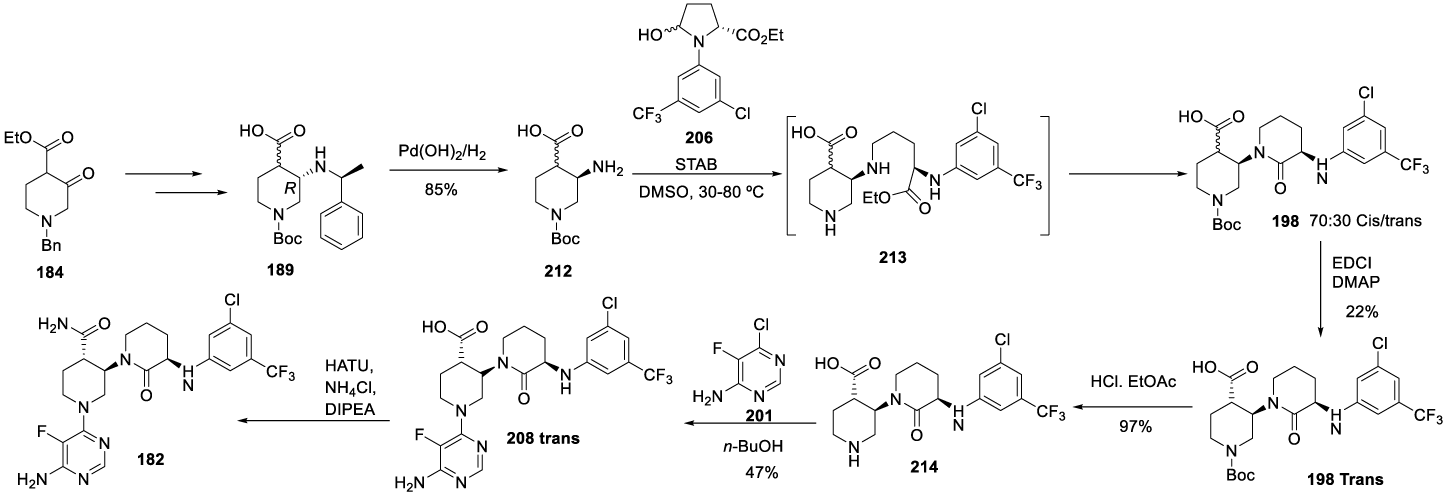
Vecabrutinib synthesis: In 2013, Hopkins and co-workers167 from Biogen IDEC MA INC. filed a patent (WO2013185084 A1) on pyrimidinyl tyrosine kinase inhibitors which include Vecrabrutinib (182) (CAS number: 1510829-06-7). The synthesis of the key intermediate 194 is presented in Scheme 33. The process starts with N-Bn protecting group removal of 184 with Pd/C catalyst under hydrogen atmospheric pressure, in methanol, for 16 hours, to afford 185, which is protected with Boc anhydride and triethylamine in methanol, at room temperature, for 16 hours to afford 186. The reaction of 186 with (R)-1-phenylethan-1-amine with p-TSA 187, in toluene, at reflux, afforded 188, which is reduced with NaBH(OAc)3 in a mixture of acetonitrile and acetic acid at 0 ºC, for 2 hours, to yield 189. The treatment of 189 with aqueous sodium ethoxide in ethanol afforded enantiomer 190, which after hydrogenation with Pd/C under hydrogen pressure, in methanol, at 50 ºC, provided 191. The coupling with 5-bromopentanoyl chloride 192 with triethylamine, in dichloromethane, at room temperature, for 2 hours, afforded 193, which cyclizes in the presence of sodium hydride in methanol for 8 hours, at 50 ºC, to afford intermediate 194.
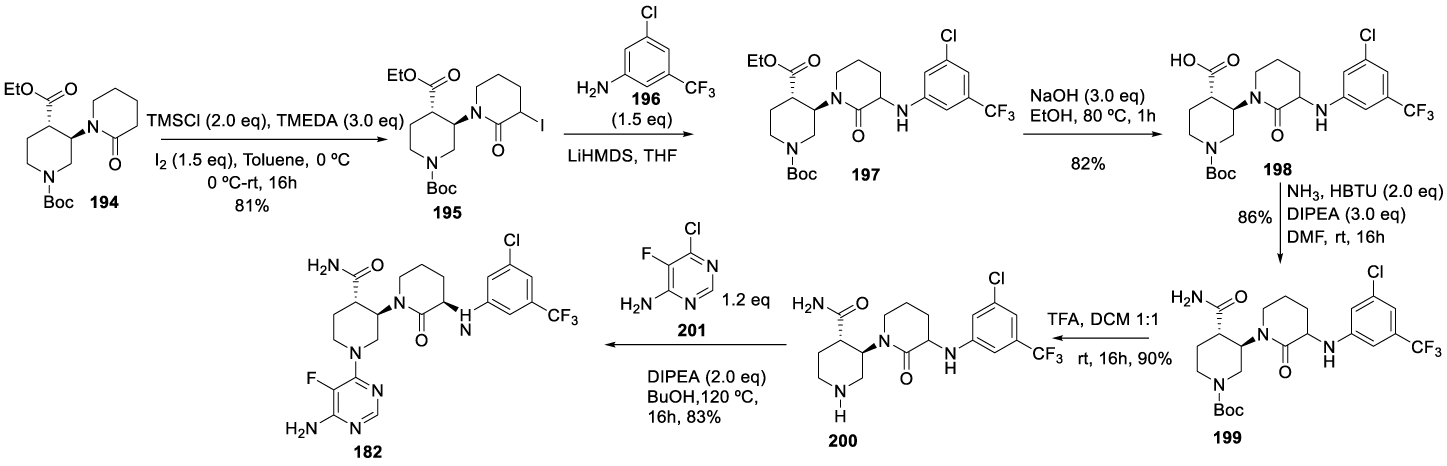
The intermediate 194 previously prepared was subjected to α-halogenation with I2 in the presence of TMSCl and TMEDA to afford 195, which was purified by column chromatography. Displacement of iodide atom by 3-chloro-5-(trifluoromethyl) aniline 196 with lithium bis(trimethylsilyl)amide in THF afforded 197, hydrolysis of the ethyl ester with sodium hydroxide in ethanolic solution produced the corresponding carboxylic acid 198 as an oil, after purification. The amide formation with HBTU in the presence of DIPEA, in DMF, at room temperature, afforded 199, which after N-Boc protecting group removal with TFA in dichloromethane provided 200. Finally, the coupling with 6-chloro-5-(trifluoromethyl)aniline 201 in the presence of DIPEA, in butanol, at 120 ºC, provided Vecrabrutinib (182), after purification by preparative HPLC (Scheme 34).
The alternative process (Scheme 35), also disclosed in the same patent, starts with removing the N-Bn protecting group of 184 with palladium in acetic acid and replacement with the N-Boc protecting group to afford 186 as an oil. The reaction of 186 with (R)-1-phenylethan-1-amine 187 and p-TSA, in toluene at reflux, afforded 188 as a thick oil with 3-10% of amide byproduct. The reduction of 188 with NaBH4 in the presence of TFA afforded 189. The treatment of 189 with aqueous LiOH in THF and MeOH afforded 202. Treatment of 202 with HCl yielded 203, which after coupling with Cl-pyridine 201 afforded 204. The reduction of 204 with palladium hydroxide and hydrogen in the presence of potassium fluoride gave 205. The coupling of 205 with 206 provided 207 (cis/trans), which was cyclized at 90 ºC to form 208 (cis/trans). The reaction of 208 (trans) with l-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) in the presence of 4-dimethylaminopyridine, in DMF, afforded 208 (trans) which was not isolated and was converted to 182 by the reaction with ammonium chloride, in the presence of HATU and DIPEA.
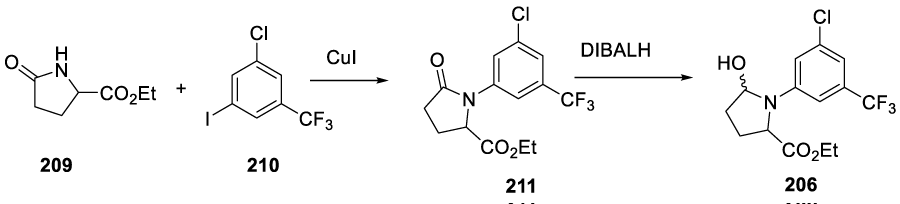
The same patent also describes a process for the preparation of Intermediate 206. The process starts with the reaction of ethyl 5-oxopyrrolidine-2-carboxylate 209 with 1-choro-3-iodo-5(trifluoromethyl)benzene to prepare 211, which after reduction with DIBALH gives 206 (Scheme 36).
Another alternative process is also disclosed in this patent, Scheme 37. The process starts with the hydrogenation of 189 with palladium hydroxide under hydrogen pressure to afford 212. The 212 reacts with 3-chloro-5-(trifluoromethyl) aniline 206 in DMSO, with sodium triacetoxyborohydride and Et3N, at 50 ºC for 3 hours to afford 213, which after the workup provided the racemic mixture 198. The 198-trans isomer is obtained by treating the mixture with EDAC and DMAP in DMF at 20 ºC. The following step is the N-Boc deprotection with HCl in ethyl acetate to afford 214, which after coupling with 6-chloro-5-fluoropyrimidin-4-amine 201 in the presence of n-butanol affords 208-trans. The reaction of 208-trans with ammonia chloride provides the desired compound 182.
More recently, in 2018, Macphee and Dreis [172], also from Biogen IDEC MA INC., filed a patent of succinate forms and compositions of Bruton's tyrosine kinase inhibitors which described the process for the preparation of Vecrabrutinib (182) disclosed by Hopkins and co-workers167 (Scheme 34).
Compounds containing two trifluoromethyl groups in meta positions of the benzenic ring
Eltanexor: Eltanexor (215; figure 19) (alternative names: ATG-016, KPT-8602 and ONO-7706) is an investigational second-generation oral Selective Inhibitor of Nuclear Export (SINE) developed by Antengene Corporation. In May 2018, Antengene and Karyopharm entered into a strategic collaboration for the development, manufacturing and commercialization of XPOVIO® (Selinexor (216)), aka ATG-010) and Eltanexor (215) for all human oncology indications in mainland China and Macau. Eltanexor (215) binds to and inhibits the nuclear export protein XPO1 (also called chromosomal region maintenance 1, CRM1), accumulating tumor suppressor proteins in the cell nucleus. Eltanexor (215) has demonstrated minimal animal brain penetration, associated with reduced toxicities in preclinical studies, while maintaining potent anti-tumor effects [173].
Eltanexor (215) is orally bioavailable and has similar pharmacokinetic properties to Selinexor (216) but has markedly reduced (approximately 30-fold less) penetration across the blood−brain barrier [174]. The FDA approved an IND for KPT-8602 (IND Number 126583) and KPT-8602 entered into a Phase I/II open-label study of safety, tolerability and efficacy in patients with Relapsed/Refractory Multiple Myeloma, in January 2016.174 The Phase I/II open-label study was ongoing in 2020 to evaluate Eltanexor (216) in myelodysplastic syndrome, colorectal cancer and castrate-resistant prostate cancer [173].
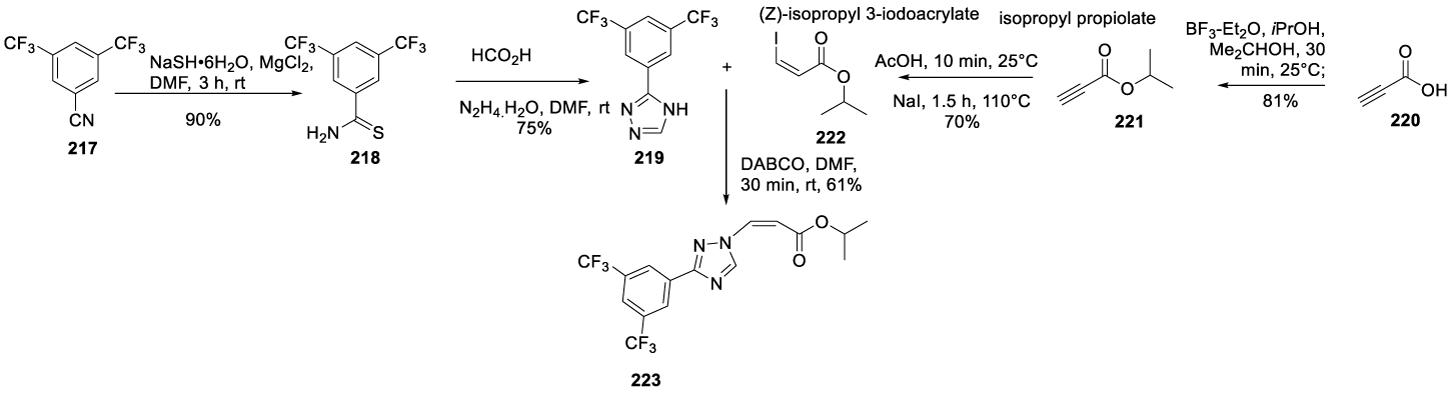
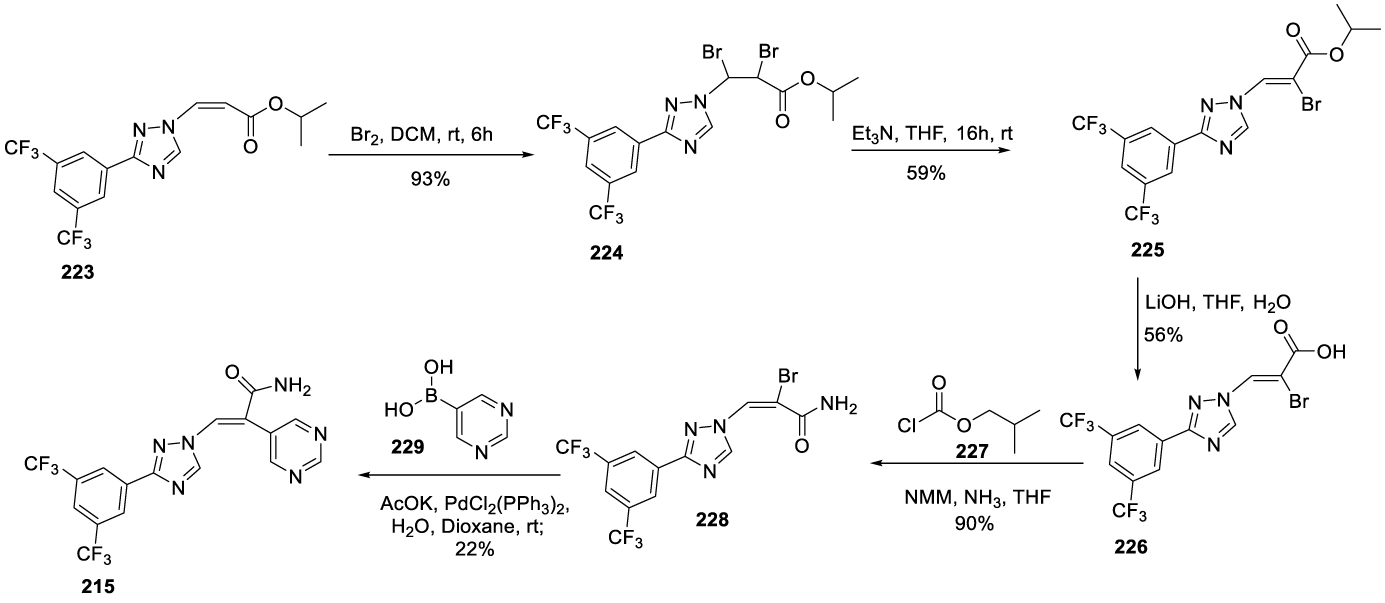
Eltanexor synthesis: In 2014, Baloglu and co-workers, from Karyopharm Therapeutics Inc. USA, filed a patent (WO2014205393 A1) comprising the preparation of intermediate 223 by a convergent synthesis in 5 steps, Scheme 38 [175] The process begins with the reaction of 3,5-bis(trifluoromethyl)benzonitrile 217 with sodium hydrosulphide hydrate in the presence of magnesium chloride in DMF at room temperature, for 3 hours, to afford 3,5-bis(trifluoromethyl)benzothioamide 218, which reacts with hydrazine hydrate monohydrate and formic acid in DMF at 90 ºC for 3 hours, to afford 219. The reaction of 219 with 222 in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO), at room temperature, for 30 minutes, afforded the intermediate 223.
The same authors filed another patent (WO 2014/205389 A1) describing the preparation of Eltanexor (215) starting from intermediate 223 (Scheme 39) [176]. The process starts with the bromination of the double bond in intermediate 223 with bromine in dichloromethane for 6 hours at room temperature to afford 224, which, by elimination in the presence of triethylamine in DMF, at room temperature for 16 hours, provides 225. The ester hydrolysis with lithium hydroxide in a mixture of THF and water gives 226, which by reaction of isobutyl chloroformate 227 followed by ammonia reaction provides 228. The Suzuki reaction between 228 and pyrimidin-5-ylboronic acid 229 provides the desired compound 215.
Serlopitant: Serlopitant (230; figure 20) (alternative names: JTS 661, MK-0594 and VPD-737) was discovered by Merck in January 2005 and was included in thirteen Phase I studies and two Phase II studies in patients with overactive bladder and alcohol dependence. In 2012, the Serlopitant (230) clinical development program was licensed to Menlo Therapeutics, formerly Tigercat Pharma, where Phase II clinical studies have been completed in patients with chronic pruritus, pruritus associated with PN (Prurigo nodularis), pruritus associated with atopic dermatitis, pruritus associated with psoriasis and chronic cough. In addition, researchers at Stanford University completed an exploratory Phase II investigator-initiated study in patients with epidermolysis bullosa [177]. Neurokinin-1 antagonists (Serlopitant (230) and Aprepitant (231)) have been found to relieve chronic pruritus [178,179].
The neurokinin-1 receptor targets substance P (SP), a mediator of itch and a probable pathogenic agent in PN179 (Prurigo nodularis, characterized by papules and nodules with primary intense pruritus) [180]. Binding by these agents likely disrupts substance P signaling, halting PN pathogenesis.. Chronic pruritus treatment includes antihistamines, topical and systemic corticosteroids, or certain antidepressants. However, their efficacy is limited, and systemic application of corticosteroids and antidepressants may be associated with severe side effects.
In 2018, Yosipovitch reported the assessment results of the safety and efficacy of P/neurokinin 1 Serlopitant (230) in treating chronic pruritus (Phase II clinical trial – NCT01951274). Although the results showed a statistically significant reduction in chronic pruritus and was well tolerated, according to the authors, the sample size was insufficient for subgroup analysis of the efficacy of Serlopitant (230) for chronic pruritus based on the conditions used [181].
In 2019, Chiou and co-workers reported the results of a study to evaluate the safety and efficacy of oral NK1 receptor antagonist Serlopitant (230) in treating moderate-severe pruritus in Epidermolysis Bullosa (EB). The serlopitant (230) was well-tolerated. However, the small sample size of the study was considered a limitation [182].
In the same year Ständer and co-workers reported a phase II placebo-controlled study to evaluate Serlopitant in adults with alcohol dependence (ClinicalTrials.gov identifier: NCT00835718). However, the study was prematurely terminated by Merck, the study sponsor, for business reasons [177].
In 2020, Pariser and co-workers reported the effects of Serlopitant (230) in oral, once-daily treatment of psoriatic pruritus in a Phase II clinical trial. Serlopitant (230) was well tolerated and could be a beneficial addition to treatments directed at psoriatic lesions [183]. However in 2022 it was reported that due to a corporate decision to no longer pursue an indication of treatment for pruritus, the phase II trials of this drug were terminated prematurely [184].
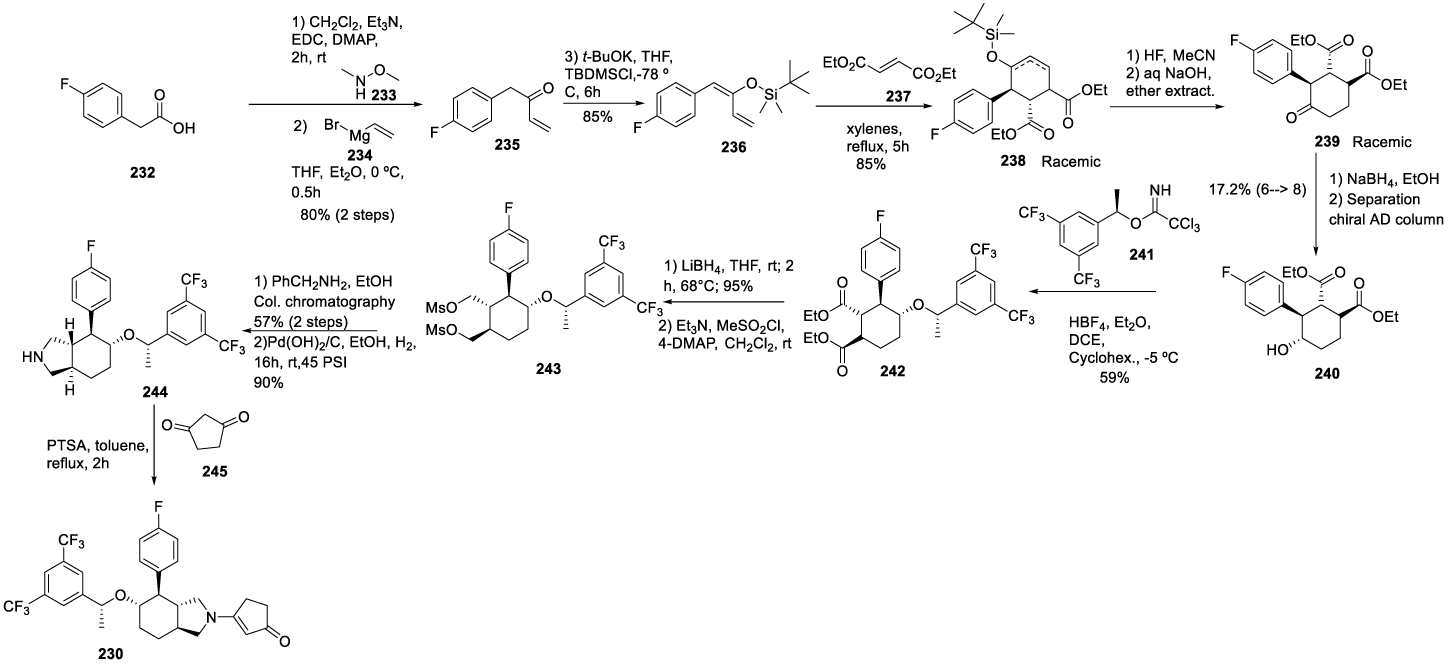
Serlopitant synthesis: In 2005, Bunda and co-workers [185] from Merck & CO. Inc. filed a patent describing the preparation of Serlopitant (230) (CAS number: 860642-69-9), Scheme 40. The process starts with the coupling reaction between 4-fluorophenyl acetic acid 232 and N,O-dimethylhydroxyl amine 233 in acetonitrile, with EDC, Et3N and catalyzed by DMAP, followed by the reaction with vinylmagnesium bromide 234 in Et2O and THF to afford 235. Finally, the reaction of compound 235 with TBDMSCl in the presence of t BuOK in THF provided 236. The Diels-Alder reaction of the crude 236 with diethyl fumarate 237 in xylenes at 160 ºC provides adduct 238 as a mixture of the two phenyl isomers. Without purification, the silyl group in 238 was removed with hydrogen fluoride in acetonitrile and gave a mixture of two ketone isomers 239, which were epimerized with sodium hydroxide in acetonitrile to provide the desired enantiomer 240, after chiral column chromatography. SN2 reaction of the enantiomerically pure alcohol 240 with 241 catalyzed with tetrafluorohydroboric acid gave ether 242 in 59% yield with about 15% of the undesired more polar (1S)-diastereomer, which was readily removed by silica gel column chromatography. Treatment of the product obtained with lithium borohydride in refluxing THF afforded the alcohol, which was then converted into dimethanesulfonate 243, by reaction with methanesulfonyl chloride. The crude dimethylsulfonate 243 was heated at 150 °C with benzylamine to provide bicyclic amine protected with an N-benzyl group, which was then removed by hydrogenolysis to give compound 244. Finally, the reaction of 244 with cyclopentane-1,3-dione 245 and PTSA for 2 hours at reflux afforded 230.
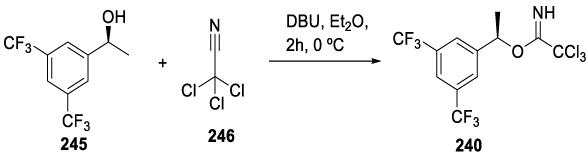
Intermediate 241 may be prepared as depicted in Scheme 41, by the reaction of (S)-1-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol with trichloroacetonitrile in the presence of DBU in diethyl ether, at 0 ºC, for 2 hours.
Later, the same authors and other colleagues [186] reported the same process in an article issued in 2009.
In 2007, Kuethe and co-workers [187], also from MERCK & CO. INC filed a patent (WO 2007/008564 A1) claiming a process for preparing hydroisoindole compounds. According to the authors, the previous process has significant disadvantages. The route produces racemic products and requires chromatographic separation of enantiomers and diastereomers after reducing racemic ketone 252. In addition, the synthesis requires 5 steps to bismesylate VIE from ketone 252 (reduction, separation, etherification, reduction, and mesylation). This route utilized a benzyl-protecting group of the octahydroisoindole.
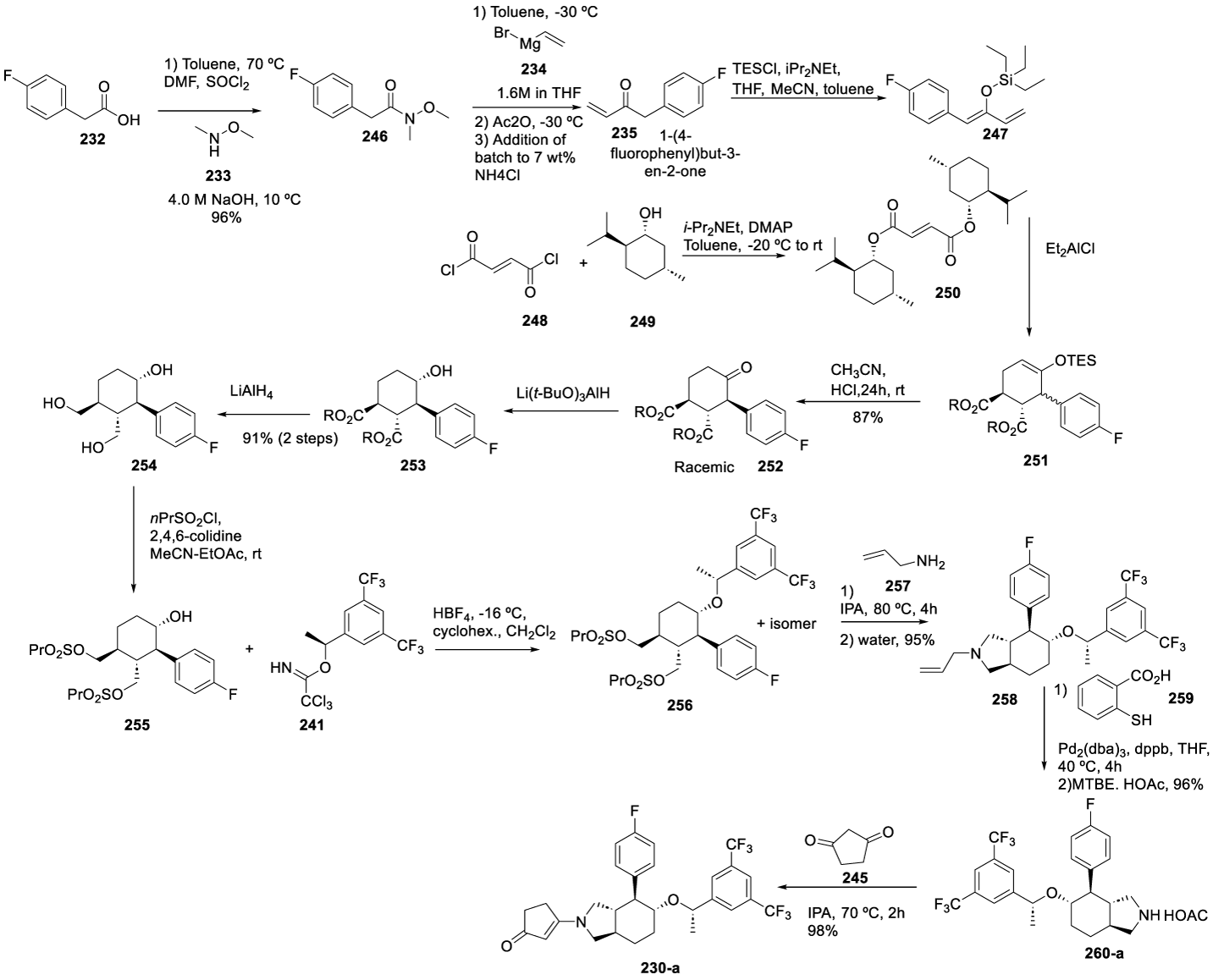
According to the authors, the conversion of 232 to 246 gives a consistently high yield and high purity of 246, with no significant side products identified. The product is an oil, typically transparent or slightly yellow. The conversion of 246 to 235 is a reaction susceptible to the quality of the Grignard reagent and the quench method. The major side products were identified. When concentrated in an oil, the product is unstable and has moderate stability in solution. The TES diethyl ether 247 is prepared with TES chloride in isopropyl ether, in a mixture of acetonitrile, toluene and THF. The di-(-)-methylfumarate 250 is prepared by reacting 248 with 249 in the presence of Hünig's base and DMAP, in toluene, from -20 ºC to room temperature. The Diels-Alder reaction between 247 and 250 provides 251. The following reaction is the deprotection and epimerization to afford 252, which was then reduced to triol with Li(t BuO)3AlH, followed by lithium aluminum hydride to afford 254. The sulfonylation of 254 provided 255 as an oil. The reaction of 255 with 241 afforded 256. The etherification of 255 with 241 provided 256, which cyclized with allylamine 257 to afford 258. The deprotection of 258 was performed in the presence of 2-mercaptobenzoic acid 259, Pd2(dba)3 and dppb in THF at 40 ºC for 4 hours to afford 260-a. The reaction of cyclopentane-1,3-dione 245 in isopropanol, at 70 ºC, for 2 hours, afforded 230-a, Scheme 42.
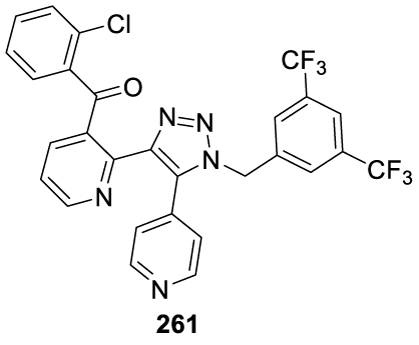
Tradipitant: Tradipitant (261; figure 21) (alternative names: LY-686017 and VLY 686) is an experimental drug developed by Eli Lilly and Company (Lily) and licensed by Vanda from Lilly, in April 2012. Tradipitant was designed to focus on novel therapies for alcohol dependence [188]. Currently, it is in clinical development for chronic pruritus in patients with atopic dermatitis. Tradipitant (261) (VLY-686) is a potent selective inhibitor of NK1R and is hypothesized to treat gastroparesis by acting centrally in the nausea-vomiting centers of the brain and peripherally in the smooth muscle of the intestines. Gastroparesis is a severe illness characterized by delayed gastric emptying and chronic symptoms of nausea, vomiting, bloating, fullness, and abdominal pain [189]. NK1 receptor (NK1R) antagonists are approved to treat nausea and vomiting in chemotherapy and the mechanism has been investigated in gastroparesis, which showed positive effects on nausea [189]. In 2020, Welsh and co-workers reported the efficacy and safety of Tradipitant (261) in the reduction of chronic pruritus in adults with mild to severe Atopic Dermatitis (AD) (Phase III clinical trial). According to the authors, future studies are needed to confirm the efficacy results and refine treatment recommendations for AD patients who despite having mild lesions, experience significant pruritus [190]. In 2021, Carlin and co-workers investigated the safety and efficacy of Tradipitant (261), in patients with idiopathic or diabetic gastroparesis [189]. The results of this Phase II study further validate the NK1 receptor as a useful target for symptom relief in diabetic and idiopathic gastroparesis [189].
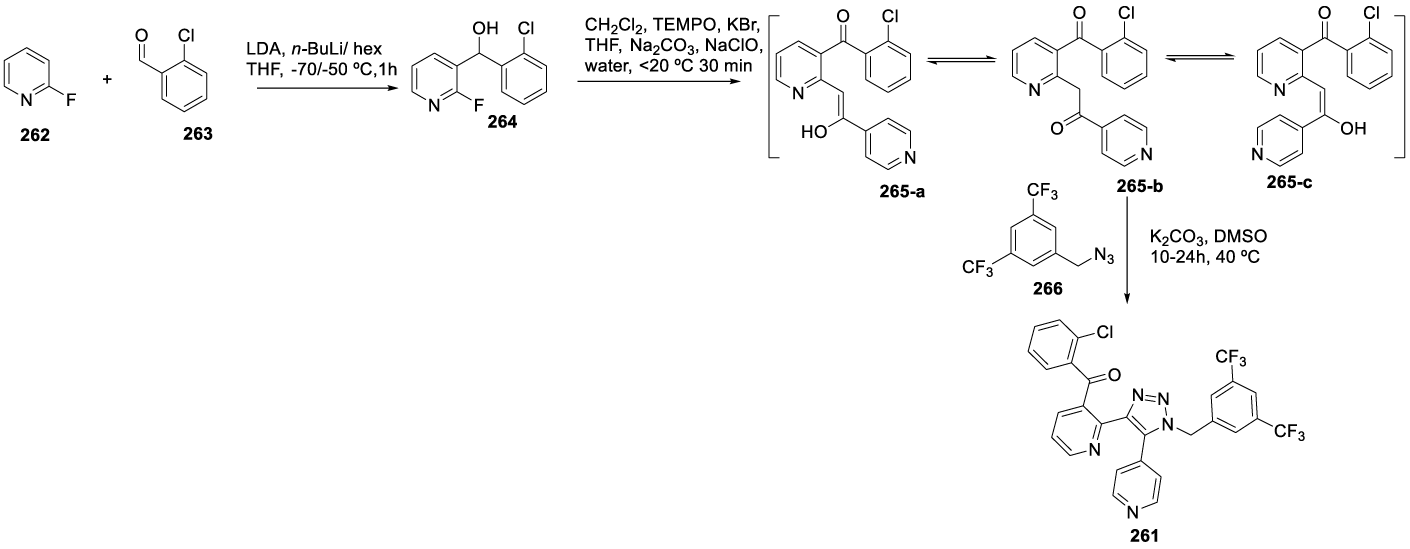
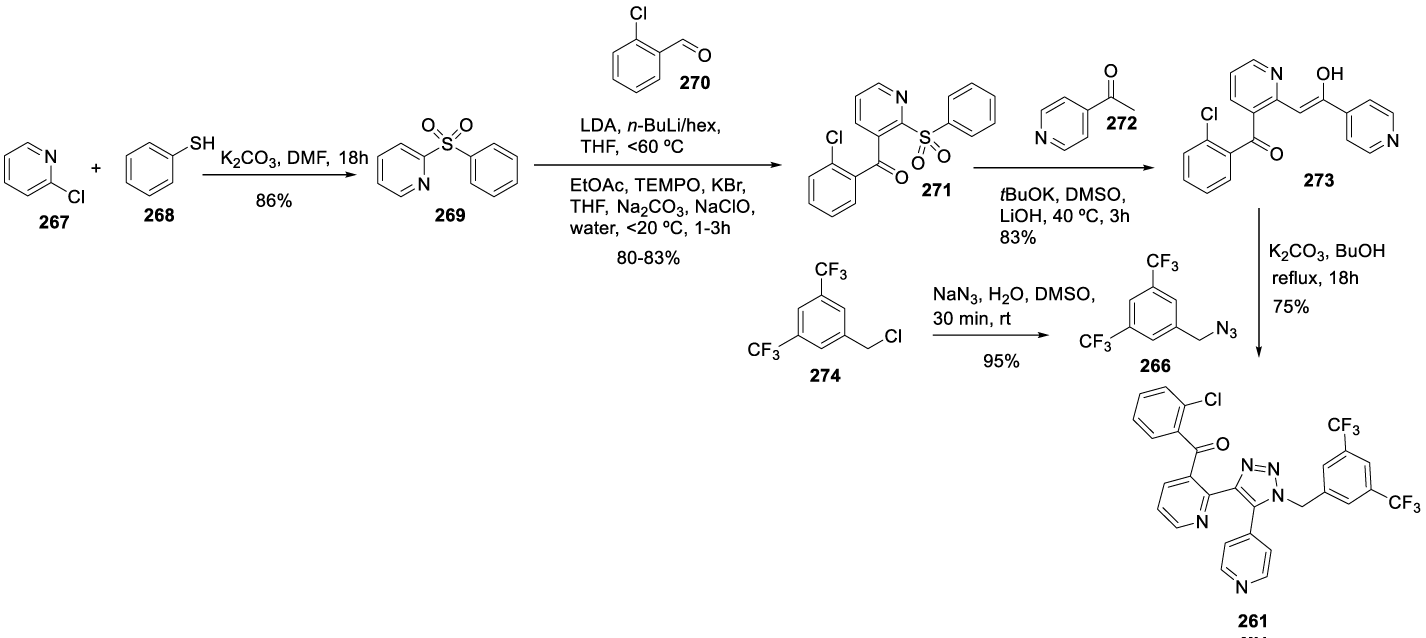
Tradipitant synthesis: In 2005, Borghese and co-workers [191] from Lilly filed a patent (WO 2005042515 A1) claiming the preparation of Tradipitant 261 (CAS number: 622370-35-8) by reacting (2-chlorophenyl)-[2-(2-hydroxy-2-pyridin-4-yl-vinyl)pyridine-3-yl]methanone 265-a or a phosphate salt thereof with 1-azidomethyl-3,5-bistrifluoromethylbenzene 266 in the presence of K2CO3 and DMSO as a solvent. The authors also presented in the same patent the preparation of 266 starting from 2-fluoropyridine 262 and 2-chlorobenzaldehyde 263, as shown in Scheme 43.
In 2008, Kobierski and co-workers [192] from Vanda Pharmaceuticals filed a patent (WO 2008079600 A1) reporting an improved process (Scheme 44). According to the authors, the use of this procedure results in several shortcomings for synthesis on a commercial scale. For example, the use of DMSO as a solvent, with (2-chlorophenyl)-2-(2-hydroxy-2-pyridin-4-yl-vinyl)pyridin-3-yl)methanone phosphate, required a complex work-up that had the propensity to emulsify. This process also required extraction with CH2Cl2, which is discouraged due to its potential as an occupational carcinogen, as well as the use of MgSO4 and acid-washed carbon, which can generate large volumes of waste on a commercial scale. The improved process would control 1-azidomethyl-3,5- bistrifluoromethylbenzene impurity level and improve the yield. According to the authors, using benzoate salt and tert-butanol as the reaction solvent improves reaction times and final yield while decreasing impurities in the final product. The novel process for the preparation of (2-chlorophenyl)-2-(2-hydroxy-2-pyridin-4-yl-vinyl)pyridin-3-yl)methanone benzoate, in which a pre-formed enolate of 4-acetyl pyridine is added to (2-phenylsulfonyl-pyridine-3-yl)-(2-chlorophenyl) methanone, results in an overall improved yield, improved purity and is helpful on a commercial scale.
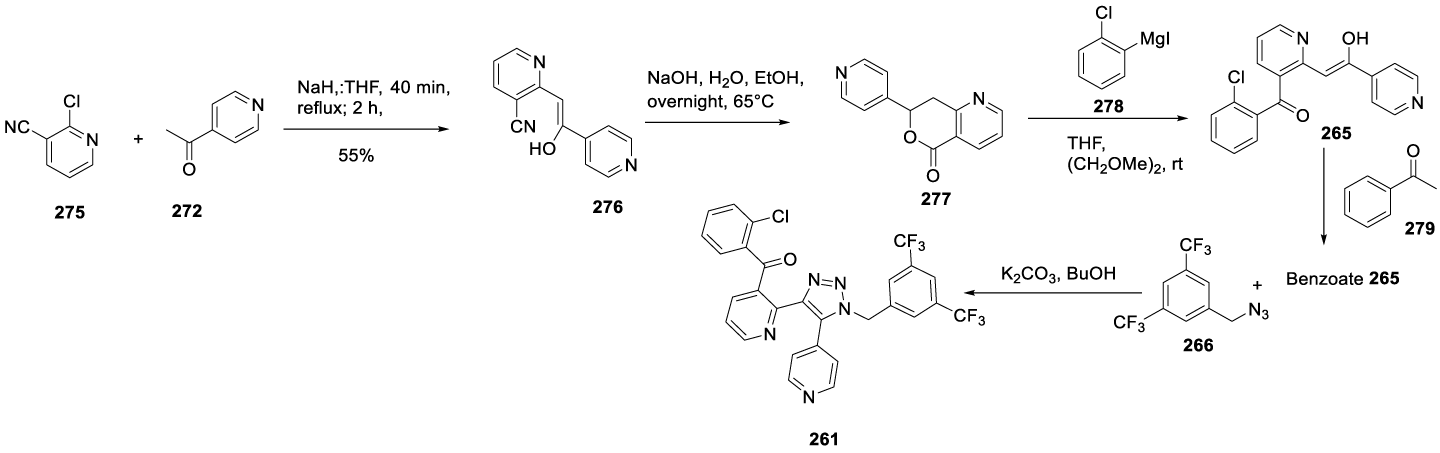
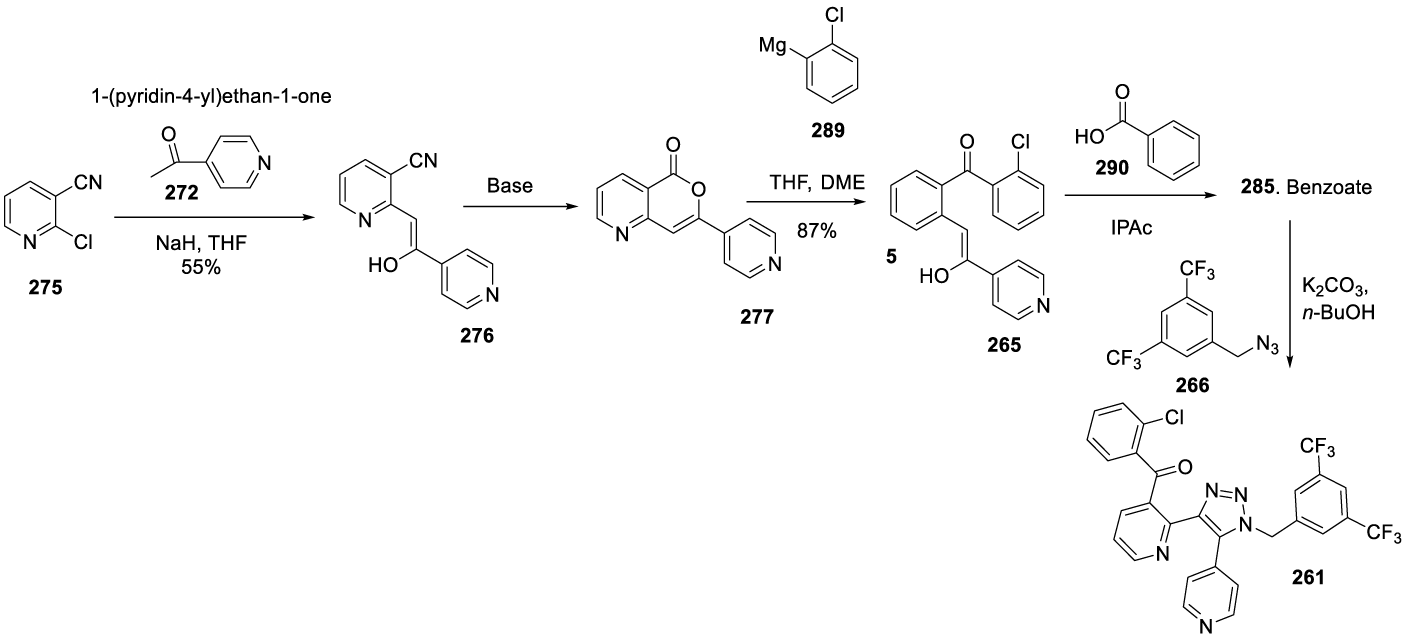
In 2017, the same authors filed a new patent (US 9708291) claiming the preparation of useful intermediates for the synthesis of 261, Scheme 45 [193]. In this synthetic route, 2-chloronicotinitrile reacts with 1-(pyridin-4-yl)ethan-1-one 272 in the presence of NaH, in THF, to afford 276 (Z)-2-(2-hydroxy-2-(pyridin-4-yl)vinyl)nicotinonitrile. Compound 276 cyclizes with NaOH in water and ethanol at 65 ºC to afford 277. The reaction of 277 with (2-chlorophenyl)magnesium iodide 278 provided the 265, which by the addition of acetophenone 279 formed the benzoate salt 265. The reaction of the benzoate salt 265 with 1-azidomethyl-3,5-bistrifluoromethylbenzene 266 provided the desired product 261.
In 2017, Kopach and co-workers [194] from Lilly filed a patent (WO 2017/031215 A1) claiming the preparation of Tradipitant (261) using two different processes and the preparation of the intermediates 284. Compound 281 was prepared from the reaction of 4-bromopyridine hydrochloride 280 in the presence of Zinc bromide, triphenylphosphine and palladium (II) chloride. The intermediate 281 obtained was combined with 266 at 100°C for 18 hours to afford a 92:8 mixture of regioisomers, which after crystallization from heptane, afforded 282-a in 62-72% yield, with no need for chromatography. The 282-a was converted to 283 in the presence of iodine monochloride in 1,2-dichloroethane, at 75 ºC, for 4 hours. Zinc dust combined with dimethylformamide was treated with 1,2-dibromoethane and heated to 65°C. The mixture obtained was cooled to ambient temperature and treated with trimethylsilyl chloride. Zinc chloride 1M in diethyl ether was added to the mixture followed by 283. The mixture was heated to 65°C and further treated with 1,2-dibromoethane and trimethylsilyl chloride. After 2.5 hours, via HPLC chromatogram, the reaction showed some formation of the zincate and was allowed to stir at ambient temperature for 16 hours. At this time, tetrakis(triphenylphosphine)palladium(0) and 284 were added to the reaction and the mixture was heated to 65°C to afford 261 (Scheme 46).
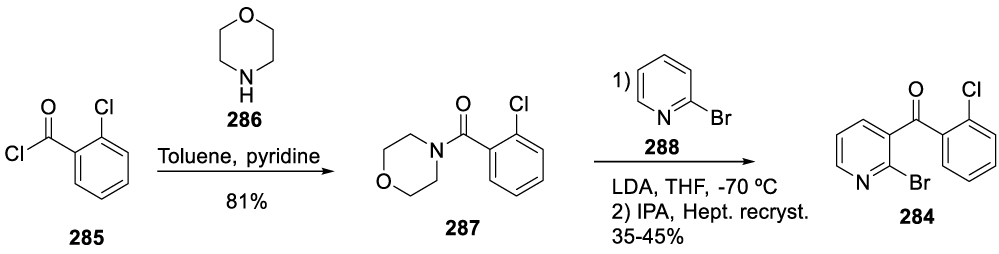
Intermediate 284 was prepared by coupling 2-chlorobenzoyl chloride 285 with morpholine 286 in the presence of pyridine and in toluene to afford (2-chlorophenyl)(morpholino)methanone 287. The reaction of 287 with 2-bromopyridine 288 and LDA in THF at -70 ºC afforded intermediate 284, after recrystallization (Scheme 47).
As an alternative, chloronicotinonitrile 275 may react with 1-(pyridin-4-yl)ethan-1-one 272 in the presence of NaH, in THF, at reflux temperature, to afford 276 as a yellow solid with an approximate 95:5 enol-ketone mixture in CDCI3. In the presence of a base, 276 may form 277.The Grignard reaction of 277 with 289 in THF and DME may afford 265 as a free base, which may be converted into its benzoate salt in isopropanol and then combined with 266 to afford the desired compound 261 (Scheme 48).
Verdinexor: Verdinexor (291; figure 22) (alternative names: ATG-527 and KPT-335) is a first-in-class, oral Selective Inhibitor of Nuclear Export (SINE) compound being investigated by Antegengene Therapeutics and Karyopharm Therapeutics for a variety of non-oncology indications in humans with an initial focus as a potential broad-spectrum treatment for viral diseases. Verdinexor (291) acts by binding to and inhibiting the nuclear export protein XPO1 (also called CRM1), which is believed to be responsible for the movement of the critical host cell and pathogen-encoded cargoes across the nuclear membrane into the cytoplasm. Inhibition of this process with Verdinexor (291)results in the accumulation of these cargoes in the nucleus, where they promote an anti-inflammatory state and prevent key steps in pathogen replication from occurring [173].
In 2014, Perwitasari and co-workers demonstrated that Verdinexor (291) was efficacious against influenza virus infection in vitro and in vivo [195]. In 2019 Jorquera and co-workers evaluated the efficacy of KPT-335 as an anti-Respiratory Syncytial Virus (RSV) drug, a respiratory leading cause of hospitalization of infants and young children that may lead to chronic respiratory conditions such as asthma, wheezing, and bronchitis [196]. According to the authors the results obtained were promising [196].
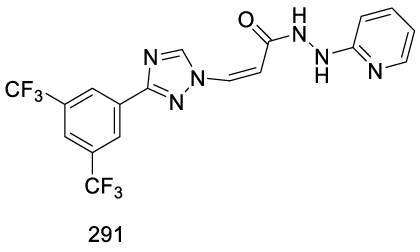
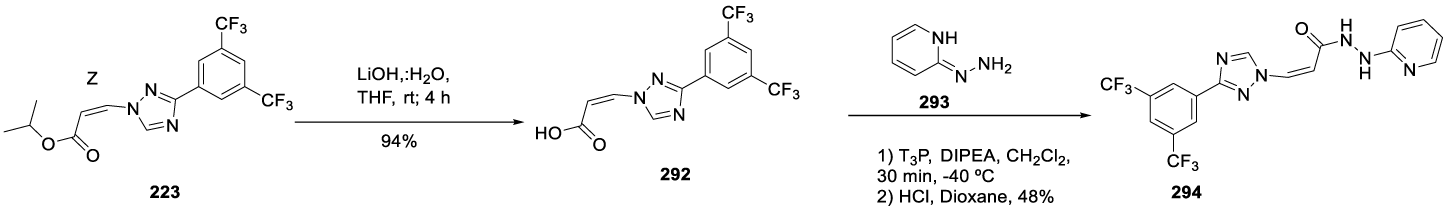
Verdinexor synthesis: In 2013, Sandanayaka and co-workers [197] from Karyopharm Therapeutics Inc. USA, filed a patent (WO2013019548 A1) claiming the synthesis of hydrazide containing nuclear transport modulators, which included Verdinexor (291) (CAS number 1392136-43-4) starting from intermediate 223 (Scheme 50). Baloglu and co-workers synthesized intermediate 223 according to Scheme 49 [175]. Intermediate 223 is hydrolyzed with LiOH to afford (Z)-3 -(3 -(3,5-bis(trifluoromethyl)phenyl)- 1H-1,2,4-triazol- 1-yl)acrylic acid 292. The final step is the coupling of 292 with 293, in the presence of T3P and DIPEA, in dichloromethane, at -40 ºC, for 30 minutes, to afford 291.
Compounds containing trifluoromethyl group in pyridine ring
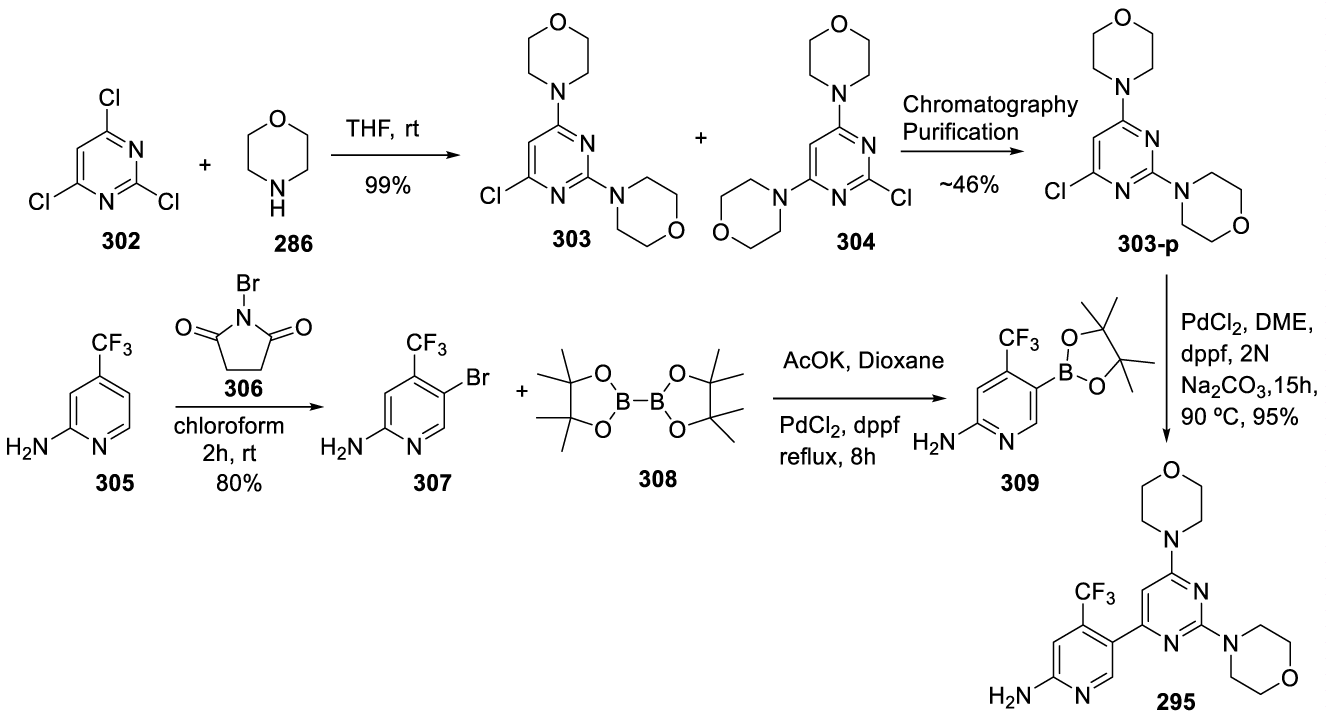
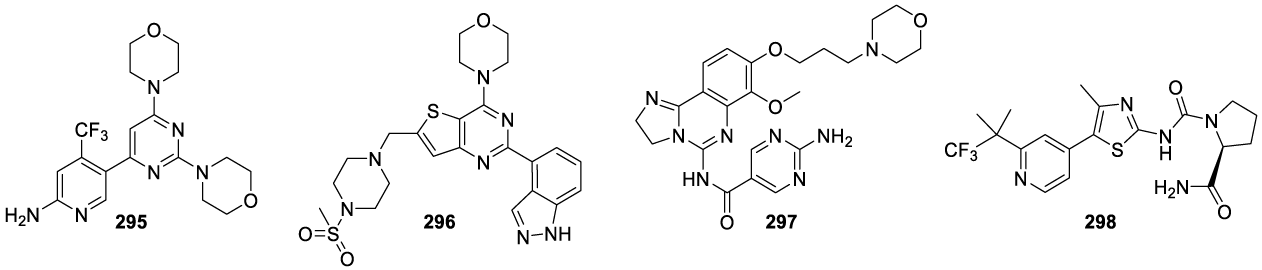
Buparlisib: Buparlisib (295) (alternative names: BKM120, BKM120-AAA, AN-2025) was developed by Novartis Pharma AG (Basel, Switzerland) and is an oral pan-phosphatidylinositol 3-kinase (pan-PI3K) inhibitor with demonstrated antiproliferative, pro-apoptotic and antitumor activity in cancer cell lines and tumor xenograft models, as a single agent and in combination with other anticancer therapies [198]. PI3K (phosphoinositide-3-kinase) inhibitors are a family of lipid kinases involved in diverse cellular functions like cell growth, proliferation, motility, differentiation, glucose transport, survival, intracellular trafficking, and membrane ruffling [199]. They are important oncology targets due to the deregulation of this signaling pathway in a wide variety of human cancers. PI3K inhibitors are divided into pan-PI3K inhibitors (Buparlisib (295), Pictilisib (296), Copanlisib (297)) and isoform-specific PI3K inhibitors (Alpelisib (298)). Pan-PI3K inhibitors target all four isoforms (α, β, γ and δ) of the class-IA PI3K p110 catalytic subunit (Figure 23).
In 2014, Ando and co-workers reported a first-in-man Phase I study, in predominantly European and US patients with advanced solid tumors (NCT01068483), the Maximum Tolerated Dose (MTD) of single-agent Buparlisib (295) given on a continuous daily schedule (100 mg). Buparlisib (295) was well tolerated with a minority of patients experiencing Grade 3/4 Adverse Events (AE) [198]. In the same year, Rodon and co-workers reported that Buparlisib (295) was well tolerated using the same dose and showed preliminary activity in patients with advanced cancers [200]. Bedard and co-workers reported the results of Phase I-b to determine MTD and/or RP2D for Buparlisib (295) combined with Trametinib (299; figure 24), when administered orally to adult patients with selected advanced solid tumors, and then to evaluate the safety and preliminary antitumor activity of MTD and/or RP2D in patients with advanced NSCLC, ovarian cancer, or pancreatic cancer with RAS or BRAF mutations in the expansion part of the study (NCT01155453). Promising results were obtained in patients with Ras or BRAF mutant NSCLC [201]. However, in 2015, the Phase II clinical trials results reported by Vansteenkiste and co-workers, with Buparlisib (295) in NSCLC patients with PI3K pathway activation, did not show any benefit [202].
Buparlisib (295) was also assessed for Glioblastomas (GBM) by Netland and co-workers, in 2016. GBMs are aggressive brain tumors with a dismal prognosis, despite combined surgery, radio- and chemotherapy. The results showed that Buparlisib has anti-tumour efficacy as monotherapy in preclinical studies [203]. The efficacy of Buparlisib (295) plus Fulvestrant (300; figure 25) in postmenopausal with advanced breast cancer was assessed in a Phase III clinical trial with positive results, as reported by Baselga (295) and co-workers in 2017. The most common adverse events were increased liver transaminases, hyperglycemia, and rash [204].
In 2017, Martin and co-workers reported that Buparlisib in combination with Paclitaxel (301; figure 26) did notimprove PPFS in the full or PI3K pathway-activated study population. For this reason, the trial was stopped at the end of Phase II [205].
In 2020, Garrido and co-workers reported a Phase II study of Buparlisib (295). The primary objective of this Phase II study was to evaluate the clinical activity of Buparlisib (295) monotherapy in patients with metastatic triple-negative breast cancer. According to the authors, although the results did not support additional testing of single-agent PI3K inhibitors in triple-negative breast cancer, they do not preclude the potential benefit for the efficacy of rational combinations including selective PI3K pathway inhibitors [206].
Buparlisib synthesis: Buparlisib (295) (CAS number: 944396-07-0) may be prepared by a convergent synthesis as described by Pick and co-workers [207] in a patent filed in 2007, by Novartis Vaccines and Diagnostics (WO 2007084786 A1, Scheme 50).
One arm of the synthesis is the preparation of dimorpholine-pyrimidine 303-p. The process starts with the nucleophilic substitution of cyanuric chloride 302 with morpholine 286 in THF, at room temperature, followed by the separation of 303 and 304 by chromatography to afford 303-p in 46% yield. The second arm is the preparation of 309. The process consists of bromination of 305 with NBS 306 in chloroform, for 2 hours at room temperature, to afford 5-bromo-4-(trifluoromethyl)-2-pyridylamine 307, which after Miyaura borylation reaction with 1,1’-bis(diphenylphosphino)ferrocene (dppf) 308 and PdCl2 in acetic acid and dioxane at reflux for 8 hours affords 309. The borate 309 is used in a Suzuki coupling reaction between 303-p and 309, catalyzed by PdCl2 and dppf, in 1,2-dimethoxiethane, to provide the desired Buparlisib 301.
Alternatively, dimorpholine-pyrimidine 303 may be synthesized, as described in the same patent,207 starting from the reaction of morpholinoformamidine hydrobromide 311 with diethyl malonate 36 to afford 311. The compound 311 is chlorinated with POCl3 at 120 °C, for 16 h, to afford 312, which by nucleophilic substitution with morpholine 286 provides 303 (Scheme 51).
In 2011, Burgers and co-workers from Novartis Institutes for Biomedical Research reported the synthesis of Buparlisib 301 with minor changes [208]. The preparation of 307 was performed in dichloromethane instead of chloroform with a similar yield. The reaction of 307 with 308 was performed with the same catalyst but in dichloromethane and with KOAc, with 48% yield. The dimorpholine-pyrimidine 303 was prepared with morpholine 286 and DIEA, in ethanol, with 80% yield. The process used by Pick and co-workers was described by Zhao and co-workers, from Dana-Farber Cancer Institute Inc., in a patent filed in 2012 [209]. In 2011, Calienni and co-workers [210], from Novartis, described an alternative route of compound 301 (Scheme 52), starting from dimorpholine-pyrimidine 303 which after Miyaura borylation reaction afforded 312. The Suzuki coupling of boronate 312 and bromopyridine 307 afforded 301 in 89% yield. An alternative route synthesis starts with the acetylation of 307 with acetic anhydride to afford 313, followed by borylation to afford 314 and finally by Suzuki reaction with 304 to provide 301.
Flubacher and co-workers from Novartis [211] filed in 2014 a patent (WO2014064058 A1) claiming a new process for manufacturing 301 (Scheme 53). By reacting N-(5-bromo-4-(trifluoromethyl)pyridin-2-yl)acetamide 313 with isopropylmagnesium chloride and lithium chloride in tetrahydrofuran followed by the borylation reaction by adding tris(isopropylborate) to afford compound 314, which is then converted to 315 by adding 2,2'-azanediyldiethanol. The Suzuki coupling of 315 with 303 with a palladium catalyst is generated in-situ using a mixture of palladium(ll) acetate, triphenylphosphine and an aqueous base to afford 316, which is then hydrolyzed under acidic conditions to form 301. Alternatively, 301 can be prepared via a palladium-catalyzed Kumada coupling reaction, coupling 307 with 303.
Hebeisen and co-workers [212] from Basel University, Switzerland filed a patent in 2015 (WO2015162084 A1), using a different process as depicted in Scheme 54.
In 2016, Xu [213] filed a patent (US 20160264546 A1) claiming a new process for preparing 301. According to the author, the reaction route is simple and easy to control for industrial production (Scheme 55). An example is presented for the preparation of compound 301 by reacting cyanuric acid with morpholine, in TEA and dichloromethane. The yields obtained were 91% and 92%. The Buparlisib (301) is prepared by borylation of 307 with 308, in DMSO, catalyzed by PdCl2(PPh3)2, in the presence of KOAc, in DMSO, for 3 hours at 15 ºC, to afford 301 with 93% yield.
In 2016, Wu [214] from Xuanzhu Pharma described the synthesis of Buparlisib 301. The process starts by reacting cyanuric chloride 302 with morpholine 266 in a biphasic mixture of toluene and water at room temperature to afford 303 in 92% yield. Coupling of aryl halide 303 with bis(pinacolato)diboron 308, in the presence of palladium catalyst yields 312, which reacts with 307 to yield the desired compound 301. The same patent also describes the synthesis of compound 307, as presented in Scheme 56.
Pradigastat: Pradigastat (324; figure 27) (alternative names: ANJ908; LCQ 908; LCQ908-NXA; LCQ908A), developed by Novartis and licensed by Anji Pharma is a novel Diacylglycerol Acyltransferase 1 (DGAT1) inhibitor that substantially reduces extremely high Triglycerides (TGs) levels and seems to be promising in the treatment of the rare Familial Chylomicronemia Syndrome (FCS). Since DGAT1 catalyzes the final step in TGs synthesis and is highly expressed in the small intestine enterocytes, where it plays a key role in the absorption of dietary fat, DGAT1 inhibition is an attractive strategy to reduce the synthesis and secretion of TGs, and thereby lower plasma TGs [215].
In 2014, Yan and co-workers reported a study to determine whether Pradigastat (324) alters the clinical pharmacokinetics of Warfarin (325) or Digoxin(326), two drugs with narrow therapeutic windows, which are used in patients with cardiometabolic disease [216]. The study also assessed the potential Pharmacodynamic (PD) drug-drug interaction (DDI) effects of Pradigastat (324) on Warfarin (325)The safety of dosing Pradigastat (324), Warfarin (325), and Digoxin (326; figure 28) to healthy subjects as mono or combined treatment was also evaluated in the study [216]. The authors concluded that Pradigastat (324) and Digoxin (326) or Warfarin (325) had no relevant clinical Pharmacokinetic (PK) or Pharmacodynamic (PD) drug–drug interactions. Administration of Pradigastat (324) and warfarin (325) or Pradigastat (324) and digoxin (326) as mono or combined treatment appeared to be safe and tolerated [216].
In 2015, Meyers and co-workers reported a study (Phase I/II) to assess the safety, tolerability, and effects of Pradigastat (324) on fasting and postprandial plasma TG in patients with Family Chylomicronomia Syndrome (FCS) and severe hypertriglyceridemia. The authors concluded that Pradigastat (324) substantially reduced plasma TG levels in FCS patients and could be a promising new treatment for this orphan disease [217]. In the same year, they also reported a first-in-human study to evaluate the pharmacokinetics, pharmacodynamics, safety, and tolerability of Pradigastat (324) administered at single and multiple doses to overweight or obese healthy subjects. According to the authors, Pradigastat (324) was safe and tolerated in healthy subjects at single and multiple doses. These results support that Pradigastat (324) was a potential therapeutic agent for conditions in which postprandial hypertriglyceridemia has a pathophysiological role [218]. In 2015, Ayalasomayajula and co-workers reported two studies that evaluated the effect of food on the oral bioavailability of Pradigastat (324). The study concluded that pradigastat (324) could be administered with or without food [219]. In 2016, Gane and co-workers reported that Pradigastat (324) had been envisaged as a plausible strategy to control Hepatitis C Virus (HCV) infection. However, despite its potent antiviral effect in vitro, the clinical trial of Pradigastat (324) was prematurely terminated due to a lack of antiviral efficacy.
Pradigastat synthesis: The intermediate 334 of Pradigastat (324) (CAS number: 956136-95-1) may be prepared as reported by Zhou and co-workers in 2014 [220], from Merck Research Laboratories, Scheme 57. The process begins with reacting 4-(4-hydroxyphenyl)cyclohexanone 327 with trimethyl phosphonoacetate 328 and NaH in THF, at a temperature below 10 ºC, to afford 329. Compound 329 is hydrogenated with Pd in ethyl acetate, at room temperature, to yield 330. Compound 330 reacts with trifluoromethanesulfonic anhydride and triethylamine in dichloromethane at 0 ºC for 5 hours to afford 331, which by Miyaura borylation provides intermediate 332. The synthesis of Pradigastat 324 starting from intermediate 334, was described by Serrano-Serrano-Wu and co-workers [221] from Novartis Pharma G.m.b.H. in a 2007 patent. The process starts by coupling the Suzuki reaction of 334 with 335 catalyzed with Pd(PPh3)4 in the presence of K2CO3 in H2O and DME, at room temperature, for 1 day, to afford 336. Compound 336 is hydrogenated with Pd/C and ammonium formate in ethanol at reflux for 4 hours to afford 337. The following step is the Suzuki coupling with 338 catalyzed by Pd2dba3 and Xantphos in dioxane at 80 ºC, for 18 hours, to afford 339, which after hydrolysis with LiOH in water, at room temperature, overnight, provided 324.
Relacorilant: Relacorilant (340; figure 29) (alternative name: CORT-125134), an experimental drug developed by Corcept Therapeutics [222], is an orally active, high-affinity antagonist of the Glucocorticoid Receptor (GR) with excellent selectivity for the GR over other steroid receptors. The clinical relevance of GR modulation has been demonstrated in several settings, most notably in Cushing syndrome [223]. which is caused by excessive cortisol activity. Excess cortisol activity leads to a plethora of severe symptoms, including the typical body habitus of patients with Cushing’s syndrome, as well as diabetes mellitus, hypertension, dermatologic manifestations, and psychiatric disturbances [224]. Mifepristone (2) (KorlymR ®) is marketed in the United States to control hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing syndrome. Unlike Mifepristone (2), Relacorilant (340) does not bind to the progesterone receptor and is therefore anticipated to be devoid of antiprogesterone effects, such as termination of pregnancy, endometrial hypertrophy, and irregular vaginal bleeding.223 Relacorilant (340) has received orphan drug designation in the United States for treating Cushing’s syndrome and pancreatic cancer [225].
In 2017, Hunt and co-workers reported a first-in-human study to evaluate the dose-related safety, tolerability, pharmacokinetics and pharmacological effects of CORT125134 and its active metabolite CORT125201 with positive results [223]. More recently, in 2021, Auchus and co-workers reported a Phase II study in patients with endogenous Cushing Syndrome (CS) demonstrating improvements in glycemic control and hypertension with no instances of drug-related hypokalemia or antiprogesterone effects. They also reported an international, multicenter Phase III clinical trial to evaluate the efficacy and safety of Relacorilant (340) in CS of various etiologies with evidence of hypercortisolism documented through two independent biochemical tests and at least two clinical signs and symptoms of CS [226].
Relacorilant synthesis: The intermediate 9 of Relacorilant (340) (CAS number: 1496510-51-0) may be prepared as reported by Christoffers and Scharl [227] in 2002, previously presented in Scheme 1. In 2012, Clark and co-workers [228], from Corcept Therapeutics Inc., reacted 341 with 2,2,2-trifluoroethyl formate 342 in the presence of n butyl lithium and diisopropylamine in diethyl ether, at -78 ºC, for 90 minutes, to afford 343, which reacts with 344 in the presence of AcONa, in acetic acid, for 1 hour, at room temperature, to provide 345 as pure solid after chromatographic treatment (Scheme 58).
In 2017, Hunt and co-worker [224] reported the synthesis of Relacorilant (340) in 2 steps starting from 345. The Grignard reaction between 345 and 346 in the presence of isopropyl magnesium chloride in THF and dry ether at room temperature afforded 347, which reacts with 1-methyl-1H-pyrazole-4-sulfonyl chloride 348 in the presence of trifluoroacetic acid in dichloromethane, at room temperature, to yield 340 in 73% yield (Scheme 59).
Tavapadon: Tavapadon (349) (alternative names: CVL 751, PF 6649751 and PF-06649751) originally from Pfizer was later developed by Cerevel Therapeutics to target Parkinson’s disease treatment [229-231] Parkinson’s disease is characterized by disruption of brain signaling via the neurotransmitter dopamine. Dopamine replacement can partly alleviate the symptoms of Parkinson’s disease but is associated with side effects that limit its use [232]. Parkinson’s disease is currently treated with Levodopa (L-DOPA) (350; figure 30). However, prolonged treatment for 3 or more years can lead to motor fluctuations in up to 40% of treated patients, representing a substantial source of disability [233].
The results of two Phase I studies to evaluate the safety pharmacokinetics, and pharmacodynamics of PF-06649751 were assessed by Sohur and co-workers in 2018 [234].In the same year Gurrell and co-workers reported a Phase I clinical trial (NCT02565628) to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-06669571 (349) in subjects with idiopathic Parkinson’s disease [232]. Riesenberg, in 2019, reported the results of Phase II to assess the efficacy and safety of flexible-dose of PF-06649751 (349) in subjects with early-stage Parkinson’s disease (NCT02847650) [235]. In October 2020, Cerevel Therapeutics reinitiated the Phase III TEMPO-3 trial in Parkinson's disease (Adjuvant therapy) in the USA, Germany, Czech Republic, Hungary and Spain (PO) (EudraCT2019-002951-40). Tavapadon is in a Phase III trial for Parkinson's disease [236].
Tavapadon synthesis: Tavapadon (349) (CAS number 1643489-24-0) may be prepared as described by Brodney and co-workers, from Pfizer Inc. USA, in a patent filed in 2014 (WO 2014207601A1, Scheme 60) [237]. The synthesis starts from 1 methylurea 351, which is combined with ethyl 2-cyanopropanoate 352 in the presence of NaOMe in methanol, at reflux temperature, for 18 hours to afford the 6-amino-1,5-dimethylpyrimidine-2,4(1H,3H)-dione 353. Bromination of 353 in the presence of sodium nitrite and copper(ll) bromide, in a biphasic mixture of water and acetonitrile, at room temperature, for 66 hours, afforded 354. Protection of 354 with BOC anhydride 355, in the presence of triethylamine and DMAP in THF, at 70 ºC, for 1 hour, afforded 356, which reacted by Suzuki coupling with boronic acid 357 to afford 358. The selective deprotection of Boc and Bn with HBr and acetic acid afforded 359. Compound 359 reacted with 360 to provide a racemic mixture, which gave the desired 349 after chromatographic treatment.
Conclusion
This review describes the synthesis and biological activity of 21 trifluoromethyl-containing drugs that were recently approved (after 2020) in the late stages of clinical studies (Phase II/III). The NCE herein described have been tested for several therapeutic applications, such as cancer, diabetes, Parkinson’s, autoimmune diseases like rheumatoid arthritis and multiple sclerosis, HIV-1, respiratory diseases such as bronchiectasis and chronic obstructive pulmonary disease, alcohol dependence and psoriasis. Only a few compounds were abandoned due to adverse effects. Synthetic routes describing different ways of incorporating the trifluoromethyl group into the target molecule were discussed, highlighting the most advantageous methods leading to more accessible paths and/or higher yields. Mechanisms of action on biological targets are briefly presented, describing whenever possible interactions drug-target at the molecular level, with emphasis on the trifluoromethyl participation in the therapeutic activity. Collecting this data is important to help in the growing demand for new drugs, that are more effective and have fewer side effects.
Acknowledgment
We thank Dr. Poulina Qazi Uddin Md. Cardiology for medical terms revision.
References
- Fitzgerald M, Heinrich M, Booker A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front Pharmacol. 2020 Jan 9;10:1480. doi: 10.3389/fphar.2019.01480. PMID: 31998121; PMCID: PMC6962180.
- Kelly K. History of medicine. Facts on file. New York; 2009.
- Mahidol C, Ruchirawat S, Prawat H, Pisutjaroenpong S, Engprasert S, Chumsri P, Tengchaisri T, Sirisinha S, Picha P. Biodiversity and natural product drug discovery. Pure Appl Chem. 1998;70(11):2065-2072. doi:10.1351/pac199870112065.
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003 Jun 15;110(5-6):255-8. doi: 10.1016/s0049-3848(03)00379-7. PMID: 14592543.
- Montinari MR, Minelli S, De Caterina R. The first 3500 years of aspirin history from its roots - A concise summary. Vascul Pharmacol. 2019 Feb;113:1-8. doi: 10.1016/j.vph.2018.10.008. Epub 2018 Nov 2. PMID: 30391545.
- Li J, Larregieu CA, Benet LZ. Classification of natural products as sources of drugs according to the biopharmaceutics drug disposition classification system (BDDCS). Chin J Nat Med. 2016 Dec;14(12):888-897. doi: 10.1016/S1875-5364(17)30013-4. PMID: 28262115.
- Sproll C, Perz RC, Lachenmeier DW. Optimized LC/MS/MS analysis of morphine and codeine in poppy seed and evaluation of their fate during food processing as a basis for risk analysis. J Agric Food Chem. 2006 Jul 26;54(15):5292-8. doi: 10.1021/jf0608975. PMID: 16848508.
- Yuan H, Ma Q, Ye L, Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016 Apr 29;21(5):559. doi: 10.3390/molecules21050559. PMID: 27136524; PMCID: PMC6273146.
- Hoffmann JP. The historical shift in the perception of opiates: from medicine to social menace. J Psychoactive Drugs. 1990 Jan-Mar;22(1):53-62. doi: 10.1080/02791072.1990.10472197. PMID: 2182806.
- Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004 Dec;67(12):2141-53. doi: 10.1021/np040106y. Erratum in: J Nat Prod. 2006 Jan;69(1):172. PMID: 15620274.
- Bowery NG. Codeine. In: XPharm: The Comprehensive Pharmacology Reference. Elsevier. 2007:1-4. doi: 10.1016/B978-008055232-3.61504-1.
- Malm H, Borisch C. Analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, and antigout medications. In: Drugs During Pregnancy and Lactation. Elsevier. 2015:27-58. doi: 10.1016/B978-0-12-408078-2.00002-0.
- Wright C, XLIX. On the action of organic acids and their anhydrides on the natural alkaloids. Part I. J Chem Soc Trans. 1874;27:1031-1043.
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001 Jan 1;61(2):195-206. doi: 10.1016/s0376-8716(00)00162-9. PMID: 11137285.
- Petersen RC. Cocaine: an overview. NIDA Res Monogr. 1977 May;Series 13:5-15. PMID: 408703.
- MacNeil SD, Rotenberg B, Sowerby L, Allen B, Richard L, Shariff SZ. Medical use of cocaine and perioperative morbidity following sinonasal surgery-A population study. PLoS One. 2020 Jul 30;15(7):e0236356. doi: 10.1371/journal.pone.0236356. PMID: 32730351; PMCID: PMC7392254.
- Reuter URM, Oettmeier R, Nazlikul H. Procaine and Procaine-Base-Infusion: A Review of the Safety and Fields of Application after Twenty Years of Use. Clin Res Open Access. 2018;4(1):1-7. doi: 10.16966/2469-6714.127.
- ADRIANI J, CAMPBELL D. Fatalities following topical application of local anesthetics to mucous membranes. J Am Med Assoc. 1956 Dec 22;162(17):1527-30. doi: 10.1001/jama.1956.02970340017006. PMID: 24544163.
- Gordh T. Lidocaine: the origin of a modern local anesthetic. 1949. Anesthesiology. 2010 Dec;113(6):1433-7. doi: 10.1097/ALN.0b013e3181fcef48. PMID: 21068652.
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. doi: 10.1016/j.jep.2006.02.001. Epub 2006 Mar 15. PMID: 16540272.
- Amar M Ben. Pharmacologie du cannabis et synthèse des analyses des principaux comités d ’ experts. 2009;2. doi:10.7202/008535ar.
- Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012 Feb;87(2):172-86. doi: 10.1016/j.mayocp.2011.10.003. PMID: 22305029; PMCID: PMC3538401.
- Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014 Mar-Apr;37(2):41-4. doi: 10.1097/WNF.0000000000000016. PMID: 24614667.
- Abu-Sawwa R, Stehling C. Epidiolex (Cannabidiol) Primer: Frequently Asked Questions for Patients and Caregivers. J Pediatr Pharmacol Ther. 2020 Jan-Feb;25(1):75-77. doi: 10.5863/1551-6776-25.1.75. PMID: 31897080; PMCID: PMC6938286.
- Walsh V, Goodman J. From taxol to Taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002 Jul-Dec;21(3-4):307-36. doi: 10.1080/01459740214074. PMID: 12458837.
- Sarker SD, Nahar L, Miron A, Guo M. Anticancer natural products. Vol 55. 1st ed. Elsevier Inc. 2020. doi: 10.1016/bs.armc.2020.02.001.
- Gao Y, Jiang W, Dong C, Li C, Fu X, Min L, Tian J, Jin H, Shen J. Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling pathways. Toxicol In Vitro. 2012 Feb;26(1):1-6. doi: 10.1016/j.tiv.2011.09.019. Epub 2011 Sep 29. PMID: 21978812.
- Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009 Apr;59(3):191-200. doi: 10.1007/s10616-009-9211-2. Epub 2009 Aug 2. PMID: 19649719; PMCID: PMC2774570.
- Nainu F, Permana AD, Djide NJN, Anjani QK, Utami RN, Rumata NR, Zhang J, Emran TB, Simal-Gandara J. Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges. Antibiotics (Basel). 2021 Aug 14;10(8):981. doi: 10.3390/antibiotics10080981. PMID: 34439031; PMCID: PMC8388863.
- Mang R, Heilmayer W, Badegruber R, et al. US Patent 8,071,643 B2. 2011.
- Sun S, Fu J. Methyl-containing pharmaceuticals: Methylation in drug design. Bioorg Med Chem Lett. 2018 Nov 1;28(20):3283-3289. doi: 10.1016/j.bmcl.2018.09.016. Epub 2018 Sep 14. PMID: 30243589.
- Uslaner JM, Herring WJ, Coleman PJ. The Discovery of Suvorexant: Lessons Learned That Can Be Applied to Other CNS Drug Development Efforts. ACS Pharmacol Transl Sci. 2020 Jan 28;3(1):161-168. doi: 10.1021/acsptsci.9b00110. PMID: 32259095; PMCID: PMC7088936.
- Lin X, Li X, Lin X. A Review on Applications of Computational Methods in Drug Screening and Design. Molecules. 2020 Mar 18;25(6):1375. doi: 10.3390/molecules25061375. PMID: 32197324; PMCID: PMC7144386.
- Artamonov OS, Slobodyanyuk EY, Volochnyuk DM, Komarov I V, Tolmachev AA, Mykhailiuk PK. Synthesis of trifluoromethyl-substituted 3-azabicyclo[n.1.0]alkanes: Advanced building blocks for drug discovery. European J Org Chem. 2014;2014(17):3592-3598. doi: 10.1002/ejoc.201402158.
- https://cen.acs.org/content/cen/sections/drugs-approved-in-2020.html, accessed on 2021.02.01.
- Corcept Therapeutics. Corcept Therapeutics Initiates a Phase 1 Study of Its Lead Selective Cortisol Receptor (GR-II) Antagonist - CORT 108297. 23 févr 2010 09h05 HE.
- Nasdaq C. Corcept Therapeutics, Inc. History. 2010;5(5):4-6.
- Belanoff JK, Blasey CM, Clark RD, Roe RL. Selective glucocorticoid receptor (type II) antagonist prevents and reverses olanzapine-induced weight gain. Diabetes Obes Metab. 2010 Jun;12(6):545-7. doi: 10.1111/j.1463-1326.2009.01185.x. PMID: 20518810.
- https://adisinsight.springer.com/drugs/800028115, assessed on 26.04.2021.
- Andrade C, Shaikh SA, Narayan L, Blasey C, Belanoff J. Administration of a selective glucocorticoid antagonist attenuates electroconvulsive shock-induced retrograde amnesia. J Neural Transm (Vienna). 2012 Mar;119(3):337-44. doi: 10.1007/s00702-011-0712-8. Epub 2011 Sep 16. PMID: 21922193.
- Hunkin N. Focal retrograde amnesia following closed head injury: A case study and theoretical account. Neuropsychologia. 1995;33(4):509-523. doi: 10.1016/0028-3932(94)00136-D.
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18459-64. doi: 10.1073/pnas.1111746108. Epub 2011 Oct 27. PMID: 22032926; PMCID: PMC3215005.
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008 Aug;90(2):236-49. doi: 10.1016/j.pbb.2007.10.014. Epub 2007 Nov 28. PMID: 18045671.
- Butts KA, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol. 2013 Sep;16(8):1799-807. doi: 10.1017/S1461145713000187. Epub 2013 Apr 16. PMID: 23590841.
- Zhou Y, Vourloumis D, Gregor VE, et al. PCT Int. Appl. WO 2005/028467 A1. 2005;(12).
- Christoffers J, Scharl H. Copper-catalyzed asymmetric Michael reactions with α-amino acid amides: Synthesis of an optically active piperidine derivative. European J Org Chem. 2002;(9):1505-1508. doi: 10.1002/1099-0690(200205)2002:9<1505::AID-EJOC1505>3.0.CO;2-K.
- Clark RD, Ray NC, Williams K, Blaney P, Ward S, Crackett PH, Hurley C, Dyke HJ, Clark DE, Lockey P, Devos R, Wong M, Porres SS, Bright CP, Jenkins RE, Belanoff J. 1H-Pyrazolo[3,4-g]hexahydro-isoquinolines as selective glucocorticoid receptor antagonists with high functional activity. Bioorg Med Chem Lett. 2008 Feb 15;18(4):1312-7. doi: 10.1016/j.bmcl.2008.01.027. Epub 2008 Jan 11. PMID: 18226897.
- Thomson TA, Spinella-Jaegle S, Francesconi E, Meakin C, Millet S, Flao KL, Hidden H, Ruuth E. In vitro and in Vivo inhibition of immunoglobulin secretion by the immunosuppressive compound HR325 is reversed by exogenous uridine. Scand J Immunol. 2002 Jul;56(1):35-42. doi: 10.1046/j.1365-3083.2002.01107.x. PMID: 12100469.
- Uehara Y, Hirawa N, Kawabata Y, Akie Y, Ichikawa A, Funahashi N, Omata M. Immunosuppressant HR-325 attenuates progression of malignant arteritis in the kidney of Dahl salt-sensitive rats. Hypertens Res. 1997 Jun;20(2):91-7. doi: 10.1291/hypres.20.91. PMID: 9220272.
- Munier-Lehmann H, Vidalain PO, Tangy F, Janin YL. On dihydroorotate dehydrogenases and their inhibitors and uses. J Med Chem. 2013 Apr 25;56(8):3148-67. doi: 10.1021/jm301848w. Epub 2013 Mar 20. PMID: 23452331.
- Kuo EA, Hambleton PT, Kay DP, Evans PL, Matharu SS, Little E, McDowall N, Jones CB, Hedgecock CJ, Yea CM, Chan AW, Hairsine PW, Ager IR, Tully WR, Williamson RA, Westwood R. Synthesis, structure-activity relationships, and pharmacokinetic properties of dihydroorotate dehydrogenase inhibitors: 2-cyano-3-cyclopropyl-3-hydroxy-N-[3'-methyl-4'-(trifluoromethyl)phenyl ] propenamide and related compounds. J Med Chem. 1996 Nov 8;39(23):4608-21. doi: 10.1021/jm9604437. PMID: 8917650.
- Abdel-Magid AF. Use of Dihydroorotate Dehydrogenase Inhibitors for Treatment of Autoimmune Diseases and Cancer. ACS Med Chem Lett. 2020 Sep 4;11(11):2072-2074. doi: 10.1021/acsmedchemlett.0c00466. PMID: 33214811; PMCID: PMC7667645.
- AP-325 in Subjects With Peripheral Post-Surgical Neuropathic Pain. Identifier NCT04429919. National Library of Medicine. US. 2020.
- Kuo EA, Hambleton PT, Kay DP, et al. Synthesis, Structure−Activity Relationships, and Pharmacokinetic Properties of Dihydroorotate Dehydrogenase Inhibitors: 2-Cyano-3-cyclopropyl-3-hydroxy- N -[3‘-methyl-4‘-(trifluoromethyl)phenyl]propenamide and Related Compounds. J Med Chem. 1996;39(23):4608-4621. doi: 10.1021/jm9604437.
- Zhang R, Wang A, DeAngelis A, Pelton P, Xu J, Zhu P, Zhou L, Demarest K, Murray WV, Kuo GH. Discovery of para-alkylthiophenoxyacetic acids as a novel series of potent and selective PPARdelta agonists. Bioorg Med Chem Lett. 2007 Jul 15;17(14):3855-9. doi: 10.1016/j.bmcl.2007.05.007. Epub 2007 May 10. PMID: 17524639.
- Seladelpar (MBX-8025) in Subjects With Primary Biliary Cholangitis (PBC). Identifier NCT02955602. National Library of Medicine (US). (2016, November – 2019, September).
- Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, Doerffel Y, Gitlin N, Gordon SC, Odin JA, Sheridan D, Wörns MA, Clark V, Corless L, Hartmann H, Jonas ME, Kremer AE, Mells GF, Buggisch P, Freilich BL, Levy C, Vierling JM, Bernstein DE, Hartleb M, Janczewska E, Rochling F, Shah H, Shiffman ML, Smith JH, Choi YJ, Steinberg A, Varga M, Chera H, Martin R, McWherter CA, Hirschfield GM. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017 Oct;2(10):716-726. doi: 10.1016/S2468-1253(17)30246-7. Epub 2017 Aug 14. PMID: 28818518.
- Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019 Jan;69(1):394-419. doi: 10.1002/hep.30145. Epub 2018 Nov 6. PMID: 30070375.
- Gerussi A, Lucà M, Cristoferi L, Ronca V, Mancuso C, Milani C, D'Amato D, O'Donnell SE, Carbone M, Invernizzi P. New Therapeutic Targets in Autoimmune Cholangiopathies. Front Med (Lausanne). 2020 Apr 7;7:117. doi: 10.3389/fmed.2020.00117. PMID: 32318580; PMCID: PMC7154090.
- Lleo A, Maroni L, Glaser S, Alpini G, Marzioni M. Role of cholangiocytes in primary biliary cirrhosis. Semin Liver Dis. 2014;34(3):273-284. doi: 10.1055/s-0034-1383727.
- Wong VWS, Singal AK. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol. 2019;4(July). doi: 10.21037/tgh.2019.06.06.
- Bahar R, Wong KA, Liu CH, Bowlus CL. Update on New Drugs and Those in Development for the Treatment of Primary Biliary Cholangitis. Gastroenterol Hepatol (N Y). 2018 Mar;14(3):154-163. PMID: 29928160; PMCID: PMC6004046.
- Kuo GH, Zhang R, Wang A, Deangelis AR. PCT Int. Appl. WO2005042478A2. 2005:146.
- Deangelis A, Demarest KT, Kuo GH, Pelton P, Wang A, Zhang R. PCT Int. Appl. WO2006032023 A2. 2006:91.
- Jennbacken K, Welén K, Olsson A, Axelsson B, Törngren M, Damber JE, Leanderson T. Inhibition of metastasis in a castration resistant prostate cancer model by the quinoline-3-carboxamide tasquinimod (ABR-215050). Prostate. 2012 Jun 1;72(8):913-24. doi: 10.1002/pros.21495. Epub 2011 Oct 5. PMID: 22287276.
- Olsson A, Björk A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010 May 17;9:107. doi: 10.1186/1476-4598-9-107. PMID: 20470445; PMCID: PMC2885345.
- Bratt O, Häggman M, Ahlgren G, Nordle O, Björk A, Damber JE. Open-label, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br J Cancer. 2009 Oct 20;101(8):1233-40. doi: 10.1038/sj.bjc.6605322. Epub 2009 Sep 15. PMID: 19755981; PMCID: PMC2768463.
- Active biotech and Ipsen enter into a broad partnership for the co-development and commercialization of TASQ in uro-oncology. Chief Exec. Active Biotech. 2011:4-7.
- Isaacs JT, Antony L, Dalrymple SL, Brennen WN, Gerber S, Hammers H, Wissing M, Kachhap S, Luo J, Xing L, Björk P, Olsson A, Björk A, Leanderson T. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013 Feb 15;73(4):1386-99. doi: 10.1158/0008-5472.CAN-12-2730. Epub 2012 Nov 13. PMID: 23149916; PMCID: PMC3578133.
- Raymond E, Dalgleish A, Damber JE, Smith M, Pili R. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother Pharmacol. 2014 Jan;73(1):1-8. doi: 10.1007/s00280-013-2321-8. Epub 2013 Oct 27. PMID: 24162378; PMCID: PMC3889691.
- Vogl DT, Nefedova Y, Wileyto EP, Sembhi H, Strakovsky I, Nguyen C, Taneja R, Bondesson E, Eriksson H, Tuvesson H. A Phase 1 Study of Tasquinimod in Patients with Relapsed or Refractory Multiple Myeloma. Blood. 2020;136(Suplement 1):17.
- Jönsson S, Andersson G, Fex T, Fristedt T, Hedlund G, Jansson K, Abramo L, Fritzson I, Pekarski O, Runström A, Sandin H, Thuvesson I, Björk A. Synthesis and biological evaluation of new 1,2-dihydro-4-hydroxy-2-oxo-3-quinolinecarboxamides for treatment of autoimmune disorders: structure-activity relationship. J Med Chem. 2004 Apr 8;47(8):2075-88. doi: 10.1021/jm031044w. PMID: 15056005.
- Bock LM, HOLMBERG PH, Jansson KE. PCT Pat. Appl. WO2012004338 A1. 2012:25.
- Wang GT, Mantei RA, Kawai M, Tedrow JS, Barnes DM, Wang J, Zhang Q, Lou P, Garcia LA, Bouska J, Yates M, Park C, Judge RA, Lesniewski R, Sheppard GS, Bell RL. Lead optimization of methionine aminopeptidase-2 (MetAP2) inhibitors containing sulfonamides of 5,6-disubstituted anthranilic acids. Bioorg Med Chem Lett. 2007 May 15;17(10):2817-22. doi: 10.1016/j.bmcl.2007.02.062. Epub 2007 Feb 25. PMID: 17350258.
- Morgentin R, Barlaam B, Foote K, et al. Two-Directional Approach for the Rapid Synthesis of 2,4-Bis-Aminoaryl Pyridine Derivatives. Synth Commun. 2012;42(1):8-24. doi: 10.1080/00397911.2010.520403.
- Chai D, Colon M, Duffy, Kevin J, Fitch, Duke M, Tedesto R, Zimmerman MN. PCT Int. Appl. WO 2007038571 A2. 2007.
- Crew, Andrew P, Dong H, Ferraro C, Sherman D, Siu KW. PCT Int. Appl. WO2012074951 A1. 2012;11735(12).
- Suzuki M, Kondo K, Kurimura M, et al. PCT Int. Appl. WO2013003586. 2013;00(12).
- Chen X, Jia H, Li Z, Xu X. Synthesis and nematicidal evaluation of 1,2,3-benzotriazin-4-one derivatives containing piperazine as linker against Meloidogyne incognita. Chinese Chem Lett. 2019;30(6):1207-1213. doi: 10.1016/j.cclet.2019.02.033.
- Luo H, Sheng C, Lai Z, Chen J. PCT Int. Appl. WO2018188446 A 1.; 2018.
- Mehta B, Gohil Y. Development and validation of stability indicating rp-hplc method for estimation of teriflunomide in active pharmaceutical ingredient development and validation of stability indicating rp-hplc method for estimation of teriflunomide in active pharmaceutica. Pharma Innov J. 2017;6(9):440-449.
- Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014 Apr;74(6):659-74. doi: 10.1007/s40265-014-0212-x. PMID: 24740824; PMCID: PMC4003395.
- Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015 Sep;5(9):e00362. doi: 10.1002/brb3.362. Epub 2015 Aug 3. PMID: 26445701; PMCID: PMC4589809.
- Paik J. Teriflunomide: Pediatric First Approval. Paediatr Drugs. 2021 Nov;23(6):609-613. doi: 10.1007/s40272-021-00471-1. PMID: 34595696.
- Fragoso YD, Brooks JB. Leflunomide and teriflunomide: altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expert Rev Clin Pharmacol. 2015 May;8(3):315-20. doi: 10.1586/17512433.2015.1019343. Epub 2015 Feb 24. PMID: 25712857.
- Bartlett RR, Kammerer FJ. U.S. Patent 5494911. 1996;(19).
- Morris RE, Bartlett RR. U.S. Patent 5519042. New York. 1996;1(19):1-29.
- Rajan ST, Eswaraiah S. PCT Int. Appl. WO 2015029063 A2. 2015;(12).
- Palle RV, Bhat, Shanmughasamy R, Babu JR, Shanmughasamy R. PCT Int. Appl. WO2016203410 A1. 2016;(12).
- Hirth KP, Schwartz DP, Mann E, et al. U.S. Patent 5990141. 1999;222(19):213-222.
- Subramaniam P, Ramasubbu C, Athiramu S. Exploiting intramolecular hydrogen bonding for the highly (z)-selective & metal free synthesis of amide substituted β-aminoenones. Green Chem. 2017;19(11):2541-2545. doi: 10.1039/C7GC00909G.
- Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide]. J Biol Chem. 1999 Apr 2;274(14):9587-99. doi: 10.1074/jbc.274.14.9587. PMID: 10092645.
- Uckun FM, Zheng Y, Ghosh S. U.S. Patent 6355678 B1. 2002;1(12).
- Hachtel J, Neises B, Schwab W, Utz R, Zahn M. U.S. Patent 20040186173 A1. 2004;1(19).
- Keshav D, Samir P, Snehal D, Sunil S, Vishal R. PCT Int. Appl. WO 2010013159 A1. 2010;390003(390003).
- Chen G, Sun L. PCT Int. Appl. WO 2009147624 A2. Faming Zhuanli Shenqing. 2012;2009(CN102786437A):6pp.
- Chen X, Liu B, Ma Y, Yuan J. C.N. Patent 103848756. Published online 2014.
- Li G, Liu B, Ma Y, Yuan J. C.N. Patent 104693070. Published online 2015.
- Klibanov OM. Vicriviroc, a CCR5 receptor antagonist for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2009 Aug;10(8):845-59. PMID: 19649929.
- Strizki JM, Tremblay C, Xu S, Wojcik L, Wagner N, Gonsiorek W, Hipkin RW, Chou CC, Pugliese-Sivo C, Xiao Y, Tagat JR, Cox K, Priestley T, Sorota S, Huang W, Hirsch M, Reyes GR, Baroudy BM. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005 Dec;49(12):4911-9. doi: 10.1128/AAC.49.12.4911-4919.2005. PMID: 16304152; PMCID: PMC1315929.
- Alcorn K. Merck stops development of CCR5 inhibitor vicriviroc/ aidsmap. 2021:3-5.
- Kasserra C, O'Mara E. Pharmacokinetic interaction of vicriviroc with other antiretroviral agents: results from a series of fixed-sequence and parallel-group clinical trials. Clin Pharmacokinet. 2011 Apr;50(4):267-80. doi: 10.2165/11584560-000000000-00000. PMID: 21348539.
- Kasserra C, Li J, March B, O'Mara E. Effect of vicriviroc with or without ritonavir on oral contraceptive pharmacokinetics: a randomized, open-label, parallel-group, fixed-sequence crossover trial in healthy women. Clin Ther. 2011 Oct;33(10):1503-14. doi: 10.1016/j.clinthera.2011.08.012. Epub 2011 Oct 19. PMID: 22015327.
- Phase 1 Pharmacokinetic Trial of Two Intravaginal Rings (IVRs) Containing Different Dose Strengths of Vicriviroc (MK-4176) and MK-2048. Identifier: NCT02419456.National Library of Medicine (US). 2016.
- Phase 1 Safety and Pharmacokinetics Study of MK-2048/Vicriviroc (MK-4176)/MK-2048A Intravaginal Rings. Identifier: NCT02356302. National Library of Medicine (US). 2016.
- A Phase 2 Trial to Evaluate the Safety and Efficacy of Vicriviroc (MK-7690) in Combination With Pembrolizumab (MK-3475) in Participants With Advanced/Metastatic Microsatellite Stable (MSS). National Library of Medicine (US). 2021.
- Leong W, Chen M, D’Sa BA, et al. PCT Int. Appl. WO2003084950 A1. Published online 2003.
- Tagat JR, McCombie SW, Nazareno D, Labroli MA, Xiao Y, Steensma RW, Strizki JM, Baroudy BM, Cox K, Lachowicz J, Varty G, Watkins R. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]- 4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J Med Chem. 2004 May 6;47(10):2405-8. doi: 10.1021/jm0304515. PMID: 15115380.
- Ramanathan R, Ghosal A, Miller MW, Chowdhury SK, Alton KB. U.S. Patent 20060105964 A1. 2006;1(19).
- Feng DZ, Song YL, Jiang XH, Chen L, Long YQ. Forward- and reverse-synthesis of piperazinopiperidine amide analogs: a general access to structurally diverse 4-piperazinopiperidine-based ccr5 antagonists. Org Biomol Chem. 2007;5(16):2690. doi:10.1039/b707175b.
- Zhao L, Yuan X, Wang J, Feng Y, Ji F, Li Z, Bian J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg Med Chem. 2019 Mar 1;27(5):677-685. doi: 10.1016/j.bmc.2019.01.027. Epub 2019 Jan 26. PMID: 30733087.
- Tolcher AW, Peng W, Calvo E. Rational Approaches for Combination Therapy Strategies Targeting the MAP Kinase Pathway in Solid Tumors. Mol Cancer Ther. 2018 Jan;17(1):3-16. doi: 10.1158/1535-7163.MCT-17-0349. PMID: 29295962.
- Gupta P, Zhang YK, Zhang XY, Wang YJ, Lu KW, Hall T, Peng R, Yang DH, Xie N, Chen ZS. Voruciclib, a Potent CDK4/6 Inhibitor, Antagonizes ABCB1 and ABCG2-Mediated Multi-Drug Resistance in Cancer Cells. Cell Physiol Biochem. 2018;45(4):1515-1528. doi: 10.1159/000487578. Epub 2018 Feb 19. PMID: 29486476.
- Sarkozy C, Sehn LH. New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma. Ann Lymphoma. 2019;3:10-10. doi: 10.21037/aol.2019.09.01.
- Dey J, Deckwerth TL, Kerwin WS, Casalini JR, Merrell AJ, Grenley MO, Burns C, Ditzler SH, Dixon CP, Beirne E, Gillespie KC, Kleinman EF, Klinghoffer RA. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci Rep. 2017 Dec 21;7(1):18007. doi: 10.1038/s41598-017-18368-w. PMID: 29269870; PMCID: PMC5740070.
- Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013 Sep;12(9):1691-700. doi: 10.1158/1535-7163.MCT-13-0058. Epub 2013 Aug 23. PMID: 23974697.
- Sivakumar M, Mascarenhas M, Sarde A, et al. PCT Int. Appl. WO2007148158 A1. 2010.
- Rathos MJ, Joshi KS. PCT Int. Appl. WO2012066508 A1. 2012;(12).
- Stevens T, Ekholm K, Gränse M, Lindahl M, Kozma V, Jungar C, Ottosson T, Falk-Håkansson H, Churg A, Wright JL, Lal H, Sanfridson A. AZD9668: pharmacological characterization of a novel oral inhibitor of neutrophil elastase. J Pharmacol Exp Ther. 2011 Oct;339(1):313-20. doi: 10.1124/jpet.111.182139. Epub 2011 Jul 26. PMID: 21791628.
- Mereo BioPharma. Trinity Delta. Outlook 31 January 2019.
- Lo Bello F, Hansbro PM, Donovan C, Coppolino I, Mumby S, Adcock IM, Caramori G. New drugs under development for COPD. Expert Opin Emerg Drugs. 2020 Dec;25(4):419-431. doi: 10.1080/14728214.2020.1819982. Epub 2020 Sep 29. PMID: 32882146.
- Taylor EB. Casting a wide NET: an update on uncontrolled NETosis in response to COVID-19 infection. Clin Sci (Lond). 2022 Jul 15;136(13):1047-1052. doi: 10.1042/CS20220039. PMID: 35791847; PMCID: PMC9264284.
- Andersson M, Hansen P, Lonn H, Nikitidis A, Sjolin P. PCT Int. Appl. WO2005026123 A1. Published online 2005:101.
- Alcaraz ML, Briggner LE, Klingstedt PT, et al. PCT Int. Appl. WO 2010/094964 A1. 2010;(12).
- Srinath R, Dobs A. Enobosarm (GTx-024, S-22): a potential treatment for cachexia. Future Oncol. 2014 Feb;10(2):187-94. doi: 10.2217/fon.13.273. PMID: 24490605.
- Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011 Sep;2(3):153-161. doi: 10.1007/s13539-011-0034-6. Epub 2011 Aug 2. PMID: 22031847; PMCID: PMC3177038.
- Bohl CE, Chang C, Mohler ML, Chen J, Miller DD, Swaan PW, Dalton JT. A ligand-based approach to identify quantitative structure-activity relationships for the androgen receptor. J Med Chem. 2004 Jul 15;47(15):3765-76. doi: 10.1021/jm0499007. PMID: 15239655; PMCID: PMC2080780.
- Chen J, Hwang DJ, Chung K, Bohl CE, Fisher SJ, Miller DD, Dalton JT. In vitro and in vivo structure-activity relationships of novel androgen receptor ligands with multiple substituents in the B-ring. Endocrinology. 2005 Dec;146(12):5444-54. doi: 10.1210/en.2005-0732. Epub 2005 Sep 15. PMID: 16166218; PMCID: PMC2121105.
- Kim J, Wu D, Hwang DJ, Miller DD, Dalton JT. The para substituent of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamides is a major structural determinant of in vivo disposition and activity of selective androgen receptor modulators. J Pharmacol Exp Ther. 2005 Oct;315(1):230-9. doi: 10.1124/jpet.105.088344. Epub 2005 Jun 29. PMID: 15987833.
- Gao W, Kim J, Dalton JT. Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm Res. 2006 Aug;23(8):1641-58. doi: 10.1007/s11095-006-9024-3. PMID: 16841196; PMCID: PMC2072875.
- Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem. 2009 Jun 25;52(12):3597-617. doi: 10.1021/jm900280m. PMID: 19432422.
- Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, Dalton JT. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr Oncol Rep. 2016 Jun;18(6):37. doi: 10.1007/s11912-016-0522-0. PMID: 27138015; PMCID: PMC4853438.
- Dubois V, Simitsidellis I, Laurent MR, Jardi F, Saunders PT, Vanderschueren D, Claessens F. Enobosarm (GTx-024) Modulates Adult Skeletal Muscle Mass Independently of the Androgen Receptor in the Satellite Cell Lineage. Endocrinology. 2015 Dec;156(12):4522-33. doi: 10.1210/en.2015-1479. Epub 2015 Sep 22. PMID: 26393303.
- Leciejewska N, Pruszynska-Oszmalek E, Bien J, Nogowski L, Kolodziejski PA. Effect of ostarine (enobosarm/GTX024), a selective androgen receptor modulator, on adipocyte metabolism in Wistar rats. J Physiol Pharmacol. 2019 Aug;70(4). doi: 10.26402/jpp.2019.4.04. Epub 2019 Oct 19. PMID: 31642815.
- Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015 Apr 15;7(4):17-29. doi: 10.4251/wjgo.v7.i4.17. PMID: 25897346; PMCID: PMC4398892.
- Jones A, Coss CC, Steiner MS, Dalton JT. An overview on selective androgen receptor modulators: Focus on enobosarm. Drugs Future. 2013;38(5):309. doi: 10.1358/dof.2013.038.05.1970866.
- Bassetto M, Ferla S, Pertusati F, Kandil S, Westwell AD, Brancale A, McGuigan C. Design and synthesis of novel bicalutamide and enzalutamide derivatives as antiproliferative agents for the treatment of prostate cancer. Eur J Med Chem. 2016 Aug 8;118:230-43. doi: 10.1016/j.ejmech.2016.04.052. Epub 2016 Apr 22. PMID: 27131065.
- Dart DA, Kandil S, Tommasini-Ghelfi S, Serrano de Almeida G, Bevan CL, Jiang W, Westwell AD. Novel Trifluoromethylated Enobosarm Analogues with Potent Antiandrogenic Activity In Vitro and Tissue Selectivity In Vivo. Mol Cancer Ther. 2018 Sep;17(9):1846-1858. doi: 10.1158/1535-7163.MCT-18-0037. Epub 2018 Jun 12. PMID: 29895558.
- Dalton JT, Miller DD. U.S. Patent 2007123563 A1. Published online 2007.
- Narayanan R, Ponnusamy T. PCT Int. Appl. WO 2020/028593 A1. 2019;167(51).
- Schragl KM, Forsdahl G, Gmeiner G, Enev VS, Gaertner P. Novel pathway for the synthesis of arylpropionamide-derived selective androgen receptor modulator (sarm) metabolites of andarine and ostarine. Tetrahedron Lett. 2013;54(18):2239-2242. doi: 10.1016/j.tetlet.2013.02.065.
- https://adisinsight.springer.com/drugs/800034692. Accessed on 25.05.2021.
- Katchen B, Buxbaum S. Disposition of a new, nonsteroid, antiandrogen, alpha,alpha,alpha-trifluoro-2-methyl-4'-nitro-m-propionotoluidide (Flutamide), in men following a single oral 200 mg dose. J Clin Endocrinol Metab. 1975 Aug;41(2):373-9. doi: 10.1210/jcem-41-2-373. PMID: 1159048.
- Klotz L, Grudén S, Axén N, Gauffin C, Wassberg C, Bjartell A, Giddens J, Incze P, Jansz K, Jievaltas M, Rendon R, Richard PO, Ulys A, Tammela TL. Liproca Depot: A New Antiandrogen Treatment for Active Surveillance Patients. Eur Urol Focus. 2022 Jan;8(1):112-120. doi: 10.1016/j.euf.2021.02.003. Epub 2021 Feb 12. PMID: 33583762.
- Górowska-Wójtowicz E, Hejmej A, Kamińska A, Pardyak L, Kotula-Balak M, Dulińska-Litewka J, Laidler P, Bilińska B. Anti-androgen 2-hydroxyflutamide modulates cadherin, catenin and androgen receptor phosphorylation in androgen-sensitive LNCaP and androgen-independent PC3 prostate cancer cell lines acting via PI3K/Akt and MAPK/ERK1/2 pathways. Toxicol In Vitro. 2017 Apr;40:324-335. doi: 10.1016/j.tiv.2017.01.019. Epub 2017 Feb 2. PMID: 28163245.
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002 Apr;109(8):987-91. doi: 10.1172/JCI15429. PMID: 11956233; PMCID: PMC150951.
- Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P. Role of Cadherins in Cancer-A Review. Int J Mol Sci. 2020 Oct 15;21(20):7624. doi: 10.3390/ijms21207624. PMID: 33076339; PMCID: PMC7589192.
- Bal’on YG, Simurov A V. Synthesis and antiandrogenic activity of 2-hydroxy-2-methylpropionic acid N-[4-nitro-3-(trifluoromethyl)phenyl]amide and its O-acyl derivatives. Zhurnal Org ta Farmatsevtichnoi Khimii. 2009;7(4):60-63.
- Jiang C. C.N. Patent 106946650. Published online 2017.
- BeiGene and SpringWorks Announce Presentation of Preclinical Data Combining RAF Dimer Inhibitor Lifirafenib with MEK Inhibitor Mirdametinib and Provide Update on Ongoing Phase 1b / 2 Clinical Trial. Source: SpringWorks Therapeuticcs, Inc. June 22, 2020.
- Desai J, Gan H, Barrow C, Jameson M, Atkinson V, Haydon A, Millward M, Begbie S, Brown M, Markman B, Patterson W, Hill A, Horvath L, Nagrial A, Richardson G, Jackson C, Friedlander M, Parente P, Tran B, Wang L, Chen Y, Tang Z, Huang W, Wu J, Zeng D, Luo L, Solomon B. Phase I, Open-Label, Dose-Escalation/Dose-Expansion Study of Lifirafenib (BGB-283), an RAF Family Kinase Inhibitor, in Patients With Solid Tumors. J Clin Oncol. 2020 Jul 1;38(19):2140-2150. doi: 10.1200/JCO.19.02654. Epub 2020 Mar 17. PMID: 32182156; PMCID: PMC7325368.
- Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012 Jan;16(1):103-19. doi: 10.1517/14728222.2011.645805. Epub 2012 Jan 12. PMID: 22239440; PMCID: PMC3457779.
- Khazak V, Astsaturov I, Serebriiskii IG, Golemis EA. Selective Raf inhibition in cancer therapy. Expert Opin Ther Targets. 2007 Dec;11(12):1587-609. doi: 10.1517/14728222.11.12.1587. Erratum in: Expert Opin Ther Targets. 2009 Sep;13(9):1135. PMID: 18020980; PMCID: PMC2720036.
- Li ZN, Zhao L, Yu LF, Wei MJ. BRAF and KRAS mutations in metastatic colorectal cancer: future perspectives for personalized therapy. Gastroenterol Rep (Oxf). 2020 Jun 15;8(3):192-205. doi: 10.1093/gastro/goaa022. PMID: 32665851; PMCID: PMC7333923.
- Brose MS, Volpe P, Feldman M. Braf and ras mutations in human lung cancer and melanoma. 2003.
- Tang Z, Yuan X, Du R, Cheung SH, Zhang G, Wei J, Zhao Y, Feng Y, Peng H, Zhang Y, Du Y, Hu X, Gong W, Liu Y, Gao Y, Liu Y, Hao R, Li S, Wang S, Ji J, Zhang L, Li S, Sutton D, Wei M, Zhou C, Wang L, Luo L. BGB-283, a Novel RAF Kinase and EGFR Inhibitor, Displays Potent Antitumor Activity in BRAF-Mutated Colorectal Cancers. Mol Cancer Ther. 2015 Oct;14(10):2187-97. doi: 10.1158/1535-7163.MCT-15-0262. Epub 2015 Jul 24. PMID: 26208524.
- Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010 Mar;2(1):48-51. PMID: 22263017; PMCID: PMC3256436.
- Yuan X, Tang Z, Du R, Yao Z, Cheung SH, Zhang X, Wei J, Zhao Y, Du Y, Liu Y, Hu X, Gong W, Liu Y, Gao Y, Huang Z, Cao Z, Wei M, Zhou C, Wang L, Rosen N, Smith PD, Luo L. RAF dimer inhibition enhances the antitumor activity of MEK inhibitors in K-RAS mutant tumors. Mol Oncol. 2020 Aug;14(8):1833-1849. doi: 10.1002/1878-0261.12698. Epub 2020 May 18. PMID: 32336014; PMCID: PMC7400788.
- Zhang G, Zhou C. PCT Int. Appl. WO20165626 A1. 2016;3(12).
- Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, Miciek R, Ulloor J, Zhang A, Eder R, Zientek H, Gordon G, Kazmi S, Sheffield-Moore M, Bhasin S. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013 Jan;68(1):87-95. doi: 10.1093/gerona/gls078. Epub 2012 Mar 28. PMID: 22459616; PMCID: PMC4111291.
- Fonseca GWP Da, Dworatzek E, Ebner N, Von Haehling S. Selective Androgen Receptor Modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin Investig Drugs. 2020;29(8):881-891. doi: 10.1080/13543784.2020.1777275.
- Vajda EG, Hogue A, Griffiths KN, Chang WY, Burnett K, Chen Y, Marschke K, Mais DE, Pedram B, Shen Y, van Oeveren A, Zhi L, López FJ, Meglasson MD. Combination treatment with a selective androgen receptor modulator q(SARM) and a bisphosphonate has additive effects in osteopenic female rats. J Bone Miner Res. 2009 Feb;24(2):231-40. doi: 10.1359/jbmr.081007. PMID: 18847323.
- Kirk B, Al Saedi A, Duque G. Osteosarcopenia: A case of geroscience. AGING Med. 2019;2(3):147-156. doi: 10.1002/agm2.12080.
- Barbara M, Dhingra S, Mindikoglu AL. Ligandrol (LGD-4033)-Induced Liver Injury. ACG Case Reports J. 2020;7(6):e00370. doi: 10.14309/crj.0000000000000370.
- Roch PJ, Henkies D, Carstens JC, Krischek C, Lehmann W, Komrakova M, Sehmisch S. Ostarine and Ligandrol Improve Muscle Tissue in an Ovariectomized Rat Model. Front Endocrinol (Lausanne). 2020 Sep 17;11:556581. doi: 10.3389/fendo.2020.556581. PMID: 33042018; PMCID: PMC7528560.
- Grill M, Patt M, Odermatt A. Novel Protective Group Synthesis of Androgen Receptor Modulators with Steroidal and Nonsteroidal Scaffolds. 2019. doi: 10.26434/chemrxiv.11346962.
- Hopkins BT, Conlon P, Chan TR, et al. PCT Int. App. WO2013185084 A1. 2013;02493.
- Neuman LL, Ward R, Arnold D, Combs DL, Gruver D, Hill W, Kunjom JM, Miller LL, Fox JA. First-in-Human Phase 1a Study of the Safety, Pharmacokinetics, and Pharmacodynamics of the Noncovalent Bruton Tyrosine Kinase (BTK) Inhibitor SNS-062 in Healthy Subjects. Blood. 2016;128(22):2032-2032. doi: 10.1182/blood.V128.22.2032.2032.
- Jebaraj BMC, Scheffold A, Tausch E, Fox JA, Taverna P, Stilgenbauer S. Vecabrutinib is efficacious in vivo in a preclinical call adoptive transfer model. Blood. 2018;132(Supplement 1):1868-1868. doi: 10.1182/blood-2018-99-116664.
- Allan JN, Patel K, Mato AR, et al. PS1148 Preliminary results of a phase 1B/2 dose escalation and cohort-expansion study of the noncovalent, reversible Bruton’S Tyrosine Kinase Inhibitor (BTKI), Vecabrutinib, in B-CELL malignancies. 24th Congress of the European Hematology Association. Hem. 2019;3:S1.
- Sunesis Pharmaceuticals Announces Clinical Update on Vecabrutinib Program - Company Shifting Resources from Vecabrutinib to Development of PDK-1 Inhibitor SNS-510. 2020.
- Macphee JM, Neuman LL. PCT Int. Appl. WO2018017153 A1. 2017;(12).
- Antengene announces expansion of partnership with karyopharm in Asia Pacific Markets. Antengene Corporation. 2020.
- Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017;31(1):143-150. doi: 10.1038/leu.2016.145.
- Baloglu E, Shacham S, Mccauley D, et al. PCT Int. Appl. WO2014205393 A1. 2014;(12).
- Baloglu E, Shacham S, Mccauley D, et al. PCT Int. Appl. WO2014205389 A1. 2014;(12).
- Ständer S, Spellman MC, Kwon P, Yosipovitch G. The NK1 receptor antagonist serlopitant for treatment of chronic pruritus. Expert Opin Investig Drugs. 2019;28(8):659-666. doi: 10.1080/13543784.2019.1638910.
- Grundmann S, Ständer S. Chronic pruritus: Clinics and treatment. Ann Dermatol. 2011;23(1):1-11. doi: 10.5021/ad.2011.23.1.1.
- Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS One. 2010;5(6):3-7. doi:10.1371/journal.pone.0010968.
- Lotti T, Buggiani G, Prignano F. Prurigo nodularis and lichen simplex chronicus. Dermatol Ther. 2008;21(1):42-46. doi: 10.1111/j.1529-8019.2008.00168.x.
- Yosipovitch G, Ständer S, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, Zhang X, Tang JY, Luger T, Steinhoff M. Serlopitant for the treatment of chronic pruritus: Results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J Am Acad Dermatol. 2018 May;78(5):882-891.e10. doi: 10.1016/j.jaad.2018.02.030. Epub 2018 Feb 17. PMID: 29462657.
- Chiou AS, Choi S, Barriga M, Dutt-Singkh Y, Solis DC, Nazaroff J, Bailey-Healy I, Li S, Shu K, Joing M, Kwon P, Tang JY. Phase 2 trial of a neurokinin-1 receptor antagonist for the treatment of chronic itch in patients with epidermolysis bullosa: A randomized clinical trial. J Am Acad Dermatol. 2020 Jun;82(6):1415-1421. doi: 10.1016/j.jaad.2019.09.014. Epub 2019 Sep 18. PMID: 31541747.
- Pariser DM, Bagel J, Lebwohl M, Yosipovitch G, Chien E, Spellman MC. Serlopitant for psoriatic pruritus: A phase 2 randomized, double-blind, placebo-controlled clinical trial. J Am Acad Dermatol. 2020;82(6):1314-1320. doi: 10.1016/j.jaad.2020.01.056.
- Dodson J, Lio PA. Biologics and Small Molecule Inhibitors: an Update in Therapies for Allergic and Immunologic Skin Diseases. Curr Allergy Asthma Rep. 2022;22(12):183-193. doi: 10.1007/s11882-022-01047-w.
- Bunda JL, DeVita RJ, Jiang J, Mills SG. U.S. Patent 20050165083 A1. 2005;1(19).
- Jiang J, Bunda JL, Doss GA, Chicchi GG, Kurtz MM, Tsao KL, Tong X, Zheng S, Upthagrove A, Samuel K, Tschirret-Guth R, Kumar S, Wheeldon A, Carlson EJ, Hargreaves R, Burns D, Hamill T, Ryan C, Krause SM, Eng W, DeVita RJ, Mills SG. Potent, brain-penetrant, hydroisoindoline-based human neurokinin-1 receptor antagonists. J Med Chem. 2009 May 14;52(9):3039-46. doi: 10.1021/jm8016514. PMID: 19354254.
- Kuethe JT, Yin J, Huffman MA, Journet M. PCT Int. Appl. WO 2007008564 A1. 2007.
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008 Mar 14;319(5869):1536-9. doi: 10.1126/science.1153813. Epub 2008 Feb 14. PMID: 18276852.
- Carlin JL, Lieberman VR, Dahal A, Keefe MS, Xiao C, Birznieks G, Abell TL, Lembo A, Parkman HP, Polymeropoulos MH. Efficacy and Safety of Tradipitant in Patients With Diabetic and Idiopathic Gastroparesis in a Randomized, Placebo-Controlled Trial. Gastroenterology. 2021 Jan;160(1):76-87.e4. doi: 10.1053/j.gastro.2020.07.029. Epub 2020 Jul 18. PMID: 32693185.
- Welsh SE, Xiao C, Kaden AR, Brzezynski JL, Mohrman MA, Wang J, Smieszek SP, Przychodzen B, Ständer S, Polymeropoulos C, Birznieks G, Polymeropoulos MH. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: results from EPIONE, a randomized clinical trial. J Eur Acad Dermatol Venereol. 2021 May;35(5):e338-e340. doi: 10.1111/jdv.17090. Epub 2021 Jan 7. PMID: 33330999; PMCID: PMC8248080.
- Borghese A, Coffey DS, Footman PK, et al. PCT Int. Appl. WO2005042515 A1. 2005.
- Kobierski ME, Kopach ME, Chen P. PCT Int. Appl. WO 2008079600 A1. 2008.
- Kobierski ME, Kopach ME, Chen P. U.S. Patent 9708291 B2. October. 2017.
- Kopach, Michael E, Wilson, Thomas M, Kobierski, Michael E. PCT Int. Appl. WO2017031215 A1. 2017;(12).
- Perwitasari O, Johnson S, Yan X, Howerth E, Shacham S, Landesman Y, Baloglu E, McCauley D, Tamir S, Tompkins SM, Tripp RA. Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo. J Virol. 2014 Sep 1;88(17):10228-43. doi: 10.1128/JVI.01774-14. Epub 2014 Jun 25. PMID: 24965445; PMCID: PMC4136318.
- Jorquera PA, Mathew C, Pickens J, Williams C, Luczo JM, Tamir S, Ghildyal R, Tripp RA. Verdinexor (KPT-335), a Selective Inhibitor of Nuclear Export, Reduces Respiratory Syncytial Virus Replication In Vitro. J Virol. 2019 Feb 5;93(4):e01684-18. doi: 10.1128/JVI.01684-18. PMID: 30541831; PMCID: PMC6364025.
- Sandanayaka VP, Shacham S, Dilara M, Shechter S. PCT Int. Appl. WO2013019548 A1. 2016;3(12).
- Ando Y, Inada-Inoue M, Mitsuma A, Yoshino T, Ohtsu A, Suenaga N, Sato M, Kakizume T, Robson M, Quadt C, Doi T. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2014 Mar;105(3):347-53. doi: 10.1111/cas.12350. Epub 2014 Feb 13. PMID: 24405565; PMCID: PMC4317947.
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006 Aug;7(8):606-19. doi: 10.1038/nrg1879. PMID: 16847462.
- Rodon J, Braña I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32(4):670-681. doi: 10.1007/s10637-014-0082-9.
- Bedard PL, Tabernero J, Janku F, Wainberg ZA, Paz-Ares L, Vansteenkiste J, Van Cutsem E, Pérez-García J, Stathis A, Britten CD, Le N, Carter K, Demanse D, Csonka D, Peters M, Zubel A, Nauwelaerts H, Sessa C. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res. 2015 Feb 15;21(4):730-8. doi: 10.1158/1078-0432.CCR-14-1814. Epub 2014 Dec 10. PMID: 25500057.
- Vansteenkiste JF, Canon JL, De Braud F, Grossi F, De Pas T, Gray JE, Su WC, Felip E, Yoshioka H, Gridelli C, Dy GK, Thongprasert S, Reck M, Aimone P, Vidam GA, Roussou P, Wang YA, Di Tomaso E, Soria JC. Safety and Efficacy of Buparlisib (BKM120) in Patients with PI3K Pathway-Activated Non-Small Cell Lung Cancer: Results from the Phase II BASALT-1 Study. J Thorac Oncol. 2015 Sep;10(9):1319-1327. doi: 10.1097/JTO.0000000000000607. PMID: 26098748; PMCID: PMC4646607.
- Netland IA, Førde HE, Sleire L, Leiss L, Rahman MA, Skeie BS, Miletic H, Enger PØ, Goplen D. Treatment with the PI3K inhibitor buparlisib (NVP-BKM120) suppresses the growth of established patient-derived GBM xenografts and prolongs survival in nude rats. J Neurooncol. 2016 Aug;129(1):57-66. doi: 10.1007/s11060-016-2158-1. Epub 2016 Jun 9. PMID: 27283525; PMCID: PMC4972854.
- Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, Awada A, Chia S, Jagiełło-Gruszfeld A, Pistilli B, Tseng LM, Hurvitz S, Masuda N, Takahashi M, Vuylsteke P, Hachemi S, Dharan B, Di Tomaso E, Urban P, Massacesi C, Campone M. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 Jul;18(7):904-916. doi: 10.1016/S1470-2045(17)30376-5. Epub 2017 May 30. Erratum in: Lancet Oncol. 2019 Feb;20(2):e71-e72. PMID: 28576675; PMCID: PMC5549667.
- Martín M, Chan A, Dirix L, O'Shaughnessy J, Hegg R, Manikhas A, Shtivelband M, Krivorotko P, Batista López N, Campone M, Ruiz Borrego M, Khan QJ, Beck JT, Ramos Vázquez M, Urban P, Goteti S, Di Tomaso E, Massacesi C, Delaloge S. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol. 2017 Feb 1;28(2):313-320. doi: 10.1093/annonc/mdw562. PMID: 27803006.
- Garrido-Castro AC, Saura C, Barroso-Sousa R, Guo H, Ciruelos E, Bermejo B, Gavilá J, Serra V, Prat A, Paré L, Céliz P, Villagrasa P, Li Y, Savoie J, Xu Z, Arteaga CL, Krop IE, Solit DB, Mills GB, Cantley LC, Winer EP, Lin NU, Rodon J. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020 Nov 2;22(1):120. doi: 10.1186/s13058-020-01354-y. PMID: 33138866; PMCID: PMC7607628.
- Pick T, Barsanti P, Iwanowicz E, et al. PCT Int. Appl. WO 2007084786 A1. 2007;2007(July).
- Burger MT, Pecchi S, Wagman A, Ni ZJ, Knapp M, Hendrickson T, Atallah G, Pfister K, Zhang Y, Bartulis S, Frazier K, Ng S, Smith A, Verhagen J, Haznedar J, Huh K, Iwanowicz E, Xin X, Menezes D, Merritt H, Lee I, Wiesmann M, Kaufman S, Crawford K, Chin M, Bussiere D, Shoemaker K, Zaror I, Maira SM, Voliva CF. Identification of NVP-BKM120 as a Potent, Selective, Orally Bioavailable Class I PI3 Kinase Inhibitor for Treating Cancer. ACS Med Chem Lett. 2011 Aug 26;2(10):774-9. doi: 10.1021/ml200156t. PMID: 24900266; PMCID: PMC4017971.
- Zhao JJ, Wang Q. PCT Int. Appl. WO2012109423 A1. 2012;48104(12).
- Calienni JV, Cruz MD La, Flubacher D, et al. PCT Int. Appl. WO2012044727 A2. 2012;(12).
- Flubacher D, Bieri N, Acemaglu M, et al. PCT Int. Appl. WO2014064058 Al. 2014;(12).
- Hebeisen P, Beaufils F, Langlois JB. PCT Int. Appl. WO2015162084 A1. 2015;3(12).
- Yong X. U.S. Patent 20160264546 A1. Published online 2016:6. doi:10.26434/chemrxiv.11346962
- Wu F. PCT Int. Appl. WO2016050201. 2016;(12).
- Reiner Ž. Triglyceride-Rich Lipoproteins and Novel Targets for Anti-atherosclerotic Therapy. Korean Circ J. 2018 Dec;48(12):1097-1119. doi: 10.4070/kcj.2018.0343. PMID: 30403015; PMCID: PMC6221868.
- Yan JH, Meyers D, Lee Z, Danis K, Neelakantham S, Majumdar T, Rebello S, Sunkara G, Chen J. Pharmacokinetic and pharmacodynamic drug-drug interaction assessment between pradigastat and digoxin or warfarin. J Clin Pharmacol. 2014 Jul;54(7):800-8. doi: 10.1002/jcph.285. Epub 2014 Mar 18. PMID: 24619917.
- Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 2015;14(1):1-9. doi: 10.1186/s12944-015-0006-5.
- Meyers CD, Amer A, Majumdar T, Chen J. Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J Clin Pharmacol. 2015 Sep;55(9):1031-41. doi: 10.1002/jcph.509. Epub 2015 May 27. PMID: 25854859.
- Müller AC, Kanfer I. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm Drug Dispos. 2011 Nov;32(8):458-70. doi: 10.1002/bdd.775. PMID: 22024968.
- Zhou G, Zorn N, Ting P, Aslanian R, Lin M, Cook J, Lachowicz J, Lin A, Smith M, Hwa J, van Heek M, Walker S. Development of novel benzomorpholine class of diacylglycerol acyltransferase I inhibitors. ACS Med Chem Lett. 2014 Mar 1;5(5):544-9. doi: 10.1021/ml400527n. PMID: 24900877; PMCID: PMC4027756.
- Serrano-Wu MH, Kwak YS, Liu W. PCT Int. Appl. WO 2007126957 A2. 2008;2008(September):50-80.
- Pivonello R, Bancos I, Feelders RA, Kargi AY, Kerr JM, Gordon MB, Mariash CN, Terzolo M, Ellison N, Moraitis AG. Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study. Front Endocrinol (Lausanne). 2021 Jul 14;12:662865. doi: 10.3389/fendo.2021.662865. Erratum in: Front Endocrinol (Lausanne). 2022 Apr 27;13:899616. PMID: 34335465; PMCID: PMC8317576.
- Hunt H, Donaldson K, Strem M, Zann V, Leung P, Sweet S, Connor A, Combs D, Belanoff J. Assessment of Safety, Tolerability, Pharmacokinetics, and Pharmacological Effect of Orally Administered CORT125134: An Adaptive, Double-Blind, Randomized, Placebo-Controlled Phase 1 Clinical Study. Clin Pharmacol Drug Dev. 2018 May;7(4):408-421. doi: 10.1002/cpdd.389. Epub 2017 Oct 2. PMID: 28967708; PMCID: PMC5947602.
- Hunt HJ, Belanoff JK, Walters I, Gourdet B, Thomas J, Barton N, Unitt J, Phillips T, Swift D, Eaton E. Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist. J Med Chem. 2017 Apr 27;60(8):3405-3421. doi: 10.1021/acs.jmedchem.7b00162. Epub 2017 Apr 17. PMID: 28368581.
- Low ZY, Farouk IA, Lal SK. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses. 2020 Sep 22;12(9):1058. doi: 10.3390/v12091058. PMID: 32972027; PMCID: PMC7551028.
- Auchus RJ, Grauer A, Moraitis A. MON-163 A Phase 3, Double-Blind, Randomized, Placebo-Controlled Study to Assess the Efficacy and Safety of a Selective Glucocorticoid Receptor Modulator, Relacorilant, in Patients with Autonomous Cortisol Secretion Due to Cortisol-Secreting Adrenal Adenoma(s)/Hyperplasia. J Endocr Soc. 2020 May 8;4(Suppl 1):MON-163. doi: 10.1210/jendso/bvaa046.1409. PMCID: PMC7208039.
- Christoffers J, Scharl H. Copper-Catalyzed Asymmetric Michael Reactions with α-Amino Acid Amides: Synthesis of an Optically Active Piperidine Derivative. European J Org Chem. 2002;2002(9):1505-1508. doi: 10.1002/1099-0690(200205)2002:9<1505::AID-EJOC1505>3.0.CO;2-K.
- Clark RD, Ray NC, Blaney PM, Urley C, Williams K. PCT Int. Appl. WO2005087769A1. 2005.
- Tavapadon - Cerevel Therapeutics. Adis Insight. Springer Nature Switzerland AG.
- Cerri S, Blandini F. An update on the use of non-ergot dopamine agonists for the treatment of Parkinson's disease. Expert Opin Pharmacother. 2020 Dec;21(18):2279-2291. doi: 10.1080/14656566.2020.1805432. Epub 2020 Aug 17. PMID: 32804544.
- Hall A, Provins L, Valade A. Novel Strategies To Activate the Dopamine D1 Receptor: Recent Advances in Orthosteric Agonism and Positive Allosteric Modulation. J Med Chem. 2019 Jan 10;62(1):128-140. doi: 10.1021/acs.jmedchem.8b01767. Epub 2018 Dec 27. PMID: 30525590.
- Gurrell R, Duvvuri S, Sun P, DeMartinis N. A Phase I Study of the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Novel Dopamine D1 Receptor Partial Agonist, PF-06669571, in Subjects with Idiopathic Parkinson's Disease. Clin Drug Investig. 2018 Jun;38(6):509-517. doi: 10.1007/s40261-018-0632-6. PMID: 29478239.
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain. 2000 Nov;123 ( Pt 11):2297-305. doi: 10.1093/brain/123.11.2297. PMID: 11050029.
- Sohur US, Gray DL, Duvvuri S, Zhang Y, Thayer K, Feng G. Phase 1 Parkinson's Disease Studies Show the Dopamine D1/D5 Agonist PF-06649751 is Safe and Well Tolerated. Neurol Ther. 2018 Dec;7(2):307-319. doi: 10.1007/s40120-018-0114-z. Epub 2018 Oct 25. PMID: 30361858; PMCID: PMC6283789.
- Riesenberg R, Werth J, Zhang Y, Duvvuri S, Gray D. PF-06649751 efficacy and safety in early Parkinson’s disease: A randomized, placebo-controlled trial. Ther Adv Neurol Disord. 2020;13:1-11. doi: 10.1177/1756286420911296.
- A Phase 1, Randomized, Multiple-Dose, Crossover Trial in Participants With Parkinson’s Disease to Evaluate the Clinical Bioequivalence Between Tavapadon Tablets. Identifier NCT05610189. National Library of Medicine (US). 2022.
- Brodney MA, Davoren JE, Dounay AB, et al. PCT Int. Appl. WO 2014207601. 2014;(12).
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.