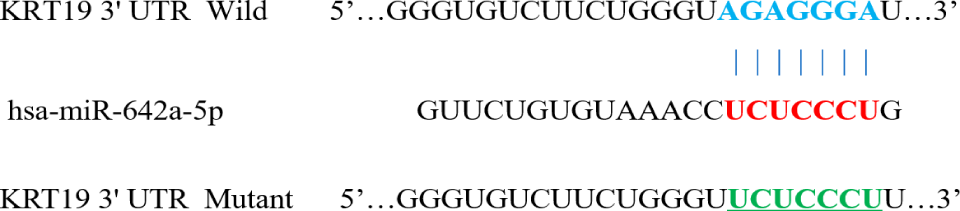Biology Group . 2023 April 03;4(4):579-589. doi: 10.37871/jbres1713.
Overexpression miR-642 Inhibits Cell Migration and Proliferation by Regulation of KRT19 and Clinical Prognosis Value in Gastric Cancer
Lan Mi1, Yu Wu3, Xing Wei4, Xiaohua He2, Xuanping Wang1, Shaoheng Li2 and Yongping Mu1,2*
2Department of Clinical Test Center, Peking University Cancer Hospital (Inner Mongolia Campus), Affiliated cancer hospital of inner Mongolia medical university. Hohhot 010020, Inner Mongolia, China
3Department of Nuclear Medicine, Peking University Cancer Hospital (Inner Mongolia Campus), Affiliated cancer hospital of inner Mongolia medical university. Hohhot 010020, Inner Mongolia, China
4Department of Abdominal Surgery, Peking University Cancer Hospital (Inner Mongolia Campus); Affiliated cancer hospital of inner Mongolia medical university. Hohhot 010020, Inner Mongolia, China
- Gastric cancer
- miR-642
- KRT19 gene
- >Cell proliferation
- >Prognosis
- >Therapeutic target
Abstract
Objective: To investigate the expression of miR-642 in gastric cancer and its mechanism in the proliferation and migration.
Methods: qRT-PCR was used to detect the expression of miR-642 in gastric cancer. Dual luciferase gene reporting system was used to verify the direct regulatory effect of miR-642 on KRT19 3' UTR. The mRNA and protein levels of KRT19 gene after transfection were detected by qRT-PCR and Western blot. The proliferation of miR-642 was detected by IncucyteS long term dynamic cell observation. Kaplan-Meier method was used to analyze the relationship between miR-642 expression level and prognosis in patients with gastric cancer.
Results: The expression of miR-642 in gastric cancer tissues was lower than that in paracancer tissues. The fluorescence intensity of MGC803 cells was significantly decreased after co-transfection of miR-642 mimics and wild-type KRT19-3 'UTR recombinant vector. The expression levels of KRT19 mRNA and protein were significantly decreased after transfected with miR-642 mimics and KRT19 siRNA. Western blot analysis of PTEN, Akt and mTOR proteins showed different expression. Prognostic survival analysis showed that gastric cancer patients with low expression of miR-642 had shorter survival.
Conclusion: miR-642 expression can negatively regulate the target gene KRT19.
Introduction
Worldwide, gastric cancer is the second leading cause of cancer-related death. In 2022, China reported more than 509,421 new cases of gastric cancer and about 400,415 deaths related to gastric cancer [1]. Due to the atypical early symptoms of gastric cancer and the lack of early diagnostic indicators, most patients have reached the middle and late stages of the disease when diagnosed [2]. Therefore, it is very important to explore the pathogenesis of gastric cancer and find effective therapeutic targets.
According to studies, miRNAs are involved in various biological processes in vivo, and have been proved to be potential tumor biomarkers or therapeutic targets in many cancer types, which are associated with a variety of tumor characteristics, such as cell proliferation, apoptosis, metastasis, invasion and prognostic survival [3,4]. Previous laboratory work, using high-throughput miRNA chip differential expression technology, detected that the expression of miR-642 in gastric cancer tissues was down-regulated by 6.4 times compared with paracancer tissues (p < 0.05) [5]. It is suggested that miR-642 may play a role as a tumor suppressor gene in the occurrence of gastric cancer. miR - 642 in the human genome 19 q13. 32 position, namely chromosome 19, by bioinformatics prediction and forecast experiments, speculated that miR-642 in gastric cancer by regulating cytokeratin 19, (cytokeratin 19 fragment), also named cytokeratin 21-1 (cytokeratin 19 fragment antigen21 - 1,CYFRA 21-1,) gene. CYFRA 21-1 can be used as a tumor diagnostic marker in lung cancer [6] and gastric cancer [7]. This study verified that KRT19 was the direct and functional target of miR-642 in gastric cancer cells, involved in the proliferation and migration of tumor cells. miR-642 had an impact on the survival, and had a prognosis value of patients with gastric cancer.
Materials and Methods
Clinical research samples
A total of 48 paired samples of gastric cancer and adjacent tissues from the Department of General Surgery, Affiliated Hospital of Inner Mongolia Medical University from March 2009 to March 2010 were collected, including 37 males and 11 females, aged 43-79 years, with an average of 60.5 years. Patients staged according to the 7th American Joint Committee on Cancer (AJCC), Tumor-Lymph Node-Metastasis (TNM) staging system. Each patient was followed up for 5 years after surgery. All participants gave written informed consent, and the study was approved by the Investigation and Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University in accordance with the current Chinese rules and all specimens were handled anonymously according to the ethical and legal standards of the Helsinki Declaration. Excision samples are immediately rinsed with cold phosphate buffer PBS, cut to the size of soybean grains, quickly frozen in liquid nitrogen, stored overnight in -80°C freezer, and extracted total RNA inverted to cDNA for later use.
Cell and material reagents
In the experiment, normal Gastric Mucosal Cells (GES-1) were provided by the Institute of Peking University Cancer Hospital, and the different differentiated cell lines: undifferentiated BGC-823, poorly differentiated MGC-803, and moderately differentiated SGC-7901 gastric cancer cell lines were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and the gastric cancer cell lines were stored in liquid nitrogen. Fetal bovine serum FBS and RPMI-1640 medium were purchased from GBICO in the United States, trypsin and Trizol reagent were purchased from Thermo Fisher in the United States, transfection reagent lipo2000 was purchased from Jima Pharmaceutical Technology (Suzhou, China), miR-642 mimics were purchased from Tiangen Biotechnology (Beijing, China), KRT19 3' UTR-Wild, KRT19 3'UTR- Mut reporter plasmid and its no-load plasmid diluciferase reporter kit, KRT19 siRNA was purchased from Ribo Biotechnology (Guangzhou, China ), and reverse transcription reagent and real-time PCR reagent were purchased from Tiangen Biochemical Technology (Beijing, China). KRT19 (#94369), PTEN (#8552s), AKT (#9272), mTOR (#2983), BAX (#2774), PARP (#5625), GAPDH (#5174) antibodies were purchased from cell-signaling (Boston, MA) and IRDye®800w conjugated secondary antibodies were purchased from Bio Rad (CA, USA).
Instrument
Carbon dioxide incubator, ultra-clean workbench, medical refrigeration freezer (Qingdao Haier Bio-Medical , China), fluorescence quantitative 7500 PCR instrument (ABI, USA), automaticenzyme label instrument (Thermo Fish, Singapore), Incucyte long time dynamic cell observation and functional analysis system Senci, (Wantong Technology, Beijing), inverted fluorescence microscope (Sainyu Instrument, Ningbo), infrared protein imprinting scanner (Odyssey, USA).
Methods
Cell culture and transfection: GES-1, BGC-823, MGC-803 and SGC-7901 were inoculated in the Medium containing 10% fetal bovine serum RPMI Medium 1640, and cultured in carbon dioxide incubator with 5% CO2, 37℃ and 95% humidity. Transfection was performed routinely when the cells' growth density reached about 60%, as specified in the Jima-transfection reagent Lipo 2000. Transfected cells were routinely cultured for 2 days, and transfected cells were digested and collected for subsequent experiments. First, miRNA-642 mimics and NC reagents were transfected into gastric cancer cells, and fluorescence microscopy was used to observe the transfection line efficiency. Cell transfection group: no transfection sequence was used as blank control group (blank). Cells transfected with meaningless sequences were used as Negative Control (NC). Cells transfected with miR-642 mimics were treated as miR-642 overexpression group. Transfected KRT19 siRNA as KRT19 knockout group.
Double luciferase reporter gene experiment: According to the experimental design, the plasmids carrying KRT19 3 'UTR-Wt and KRT19 UTR-Mut (Figure 1), the negative control plasmid and miR-642 Mimics plasmid were transfected into MGC-803 gastric cancer cell line, respectively. Conventional culture for 2 days; the cells were treated according to the steps suggested by the double luciferase reporting system, and the luciferase activity was measured with an enzyme label. According to the experimental design, the following formula was used to calculate: relative luciferase activity = firefly luciferase activity/renilla luciferase activity ×100%.
Real-time quantitative fluorescence PCR: qRT-PCR was used to detect RNA levels RNA was extracted from tissues and cells, RNA concentration was detected, mRNA was reversely transcribed to generate cDNA, which was used as a template for PCR amplification according to the instructions of fluorescence quantitative reaction kit and its reaction system. The reaction conditions of miR-642 were as follows: 95°C 15 min, 94°C 20 s, 64°C 30 s, 72°C 34s 5 cycles, 94°C 20 s, 60°C 34s 40 cycles; Reaction conditions of KRT19 detection: 95°C for 2 min, 95°C for 15 s, 62°C for 30 s, 72°C for 1 min, a total of 35 cycles. The relative expression of miR-642 (U6 as the internal reference) was calculated by 2-Ct. Primer sequence of KRT19 (GAPDH as internal reference) (Table 1). miR-642 reaction conditions: denaturation at 95°C for 15 min, 94°C for 20 s, 64°C for 30 s, 72°C for 34s for 5 cycles of pre-amplification, 94°C for 20 s, 60°C for 34s, 40 cycles of amplification. The relative expression levels of each group were calculated by 2-Ct, with miR-642 (U6 as internal reference) and KRT19 (GAPDH as internal reference).
| Table 1: Sequences of primers used in qRT-PCR. | |
| Gene | Primer sequence |
| miR-642 F | 5’- GTCCCTCTCCAAATGTGTCTTG - 3’ |
| miR-642 R | 5’- GGCTGTCGTTGACTGCGTG - 3’ |
| U6 F | 5’- CTCGCTTCGGCAGCACA - 3’ |
| U6 R | 5’- AACGCTTCACGAATTTGCGT - 3’ |
| KRT19 F | 5’- AGAATTGAACCGGGAGGTCG - 3’ |
| KRT19 R | 5’- CAGGTCAGTAACCTCGGACC - 3’ |
| GAPDH F | 5’- CGGGAAATCGTGCGTGAC - 3’ |
| GAPDH R | 5’- GGGCGACAATTTCTGGTTCA - 3’ shRNAi-KRT19 |
| 5’-ACCGGAAGACACACTGGCAGAAACCGAAGTTTCTGCCAGTGTGTCTTCC - 3’ | |
The relative expression level of protein in transfected cells was detected by Westernblot: The stable cells of each group were collected, total proteins were extracted, and the cells were lysised with RIPA lysis buffer containing protease and acidase inhibitor mixture. The protein concentration was quantified by BCA protein quantification kit. 40ug total protein was isolated by 10% SDS-PAGE gel and transferred to PVDF membrane. The membrane was sealed with 10% skim milk powder for 1h and incubated overnight at 4°C in a dilution ratio of 1:1000 with primary antibody. After washing by d2, TBST, Labeled IRDye®800w conjugate secondary antibody was added at room temperature and incubated at 1:10,000 for 1h. After TBST washing, the protein bands were detected by near-infrared scanner. The gray values of the protein bands were analyzed by ImageJ software, with GAPDH as the internal reference.
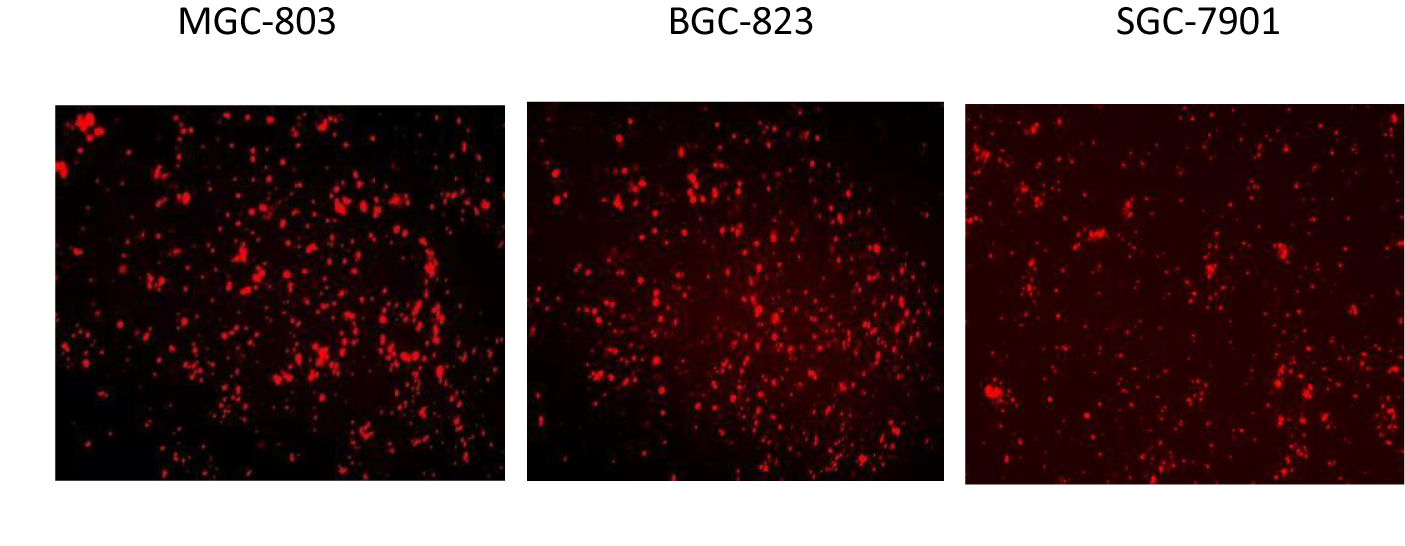
Incucyte S3 long-term dynamic live cell imaging and data analysis system to observe cell proliferation and scratch experiments: Incucyte S3 long-term dynamic live cell imaging and data analysis system was used to observe the changes and trends of cell growth and proliferation in each group in a long-term, real-time dynamic and non-invasive manner, and the cell proliferation was observed at 0, 24, 48, 72, 96 h time points, respectively. Each group was set up with 6 repeat holes, the results were recorded and cell growth curves were plotted. miR-642 mimics and KRT19 siRNA were transfected into 24-well MGC803 cell culture plate and placed in the incubator for static culture. 6h later, the culture medium in the hole was sucked out and replaced with complete medium. The transfection efficiency was observed under fluorescence microscope (Figure 2) when the cell confluence reached about 80%. Vertical lines were drawn in the middle of each hole, and cell migration at the lines was detected at 24h and 48h. Image-ProPlus software was used to process the pictures and calculate cell mobility (cell mobility = 0h scratch width -48h scratch width /0h scratch width ×100%). Three duplicate wells were set up in each group and repeated 3 times.
Prognostic analysis: Patients with gastric cancer were followed up for (15-54) months. After follow-up, the survival period of patients was recorded, and the relationship between the expression level of miR-642 and the survival rate of patients with gastric cancer was observed.
Statistical analysis
Experimental results analysis by SPSS 25.0 statistical software, p < 0.05 for the difference was statistically significant, the experiment data in the form of standard deviation of mean and subtract. T-test or Mann-Whitney U test were used to compare the expression of miR-642 in gastric cancer tissues and adjacent tissues, and cell proliferation in miR-642 mimics and NC groups in cell function tests. Chi-square test was used to evaluate the relationship between miR-642 expression and clinical variables. The survival rate was analyzed by Kaplan-Meier survival curve and tested by Log rank.
Results
Expression of miR-642 in gastric cancer patients and cells
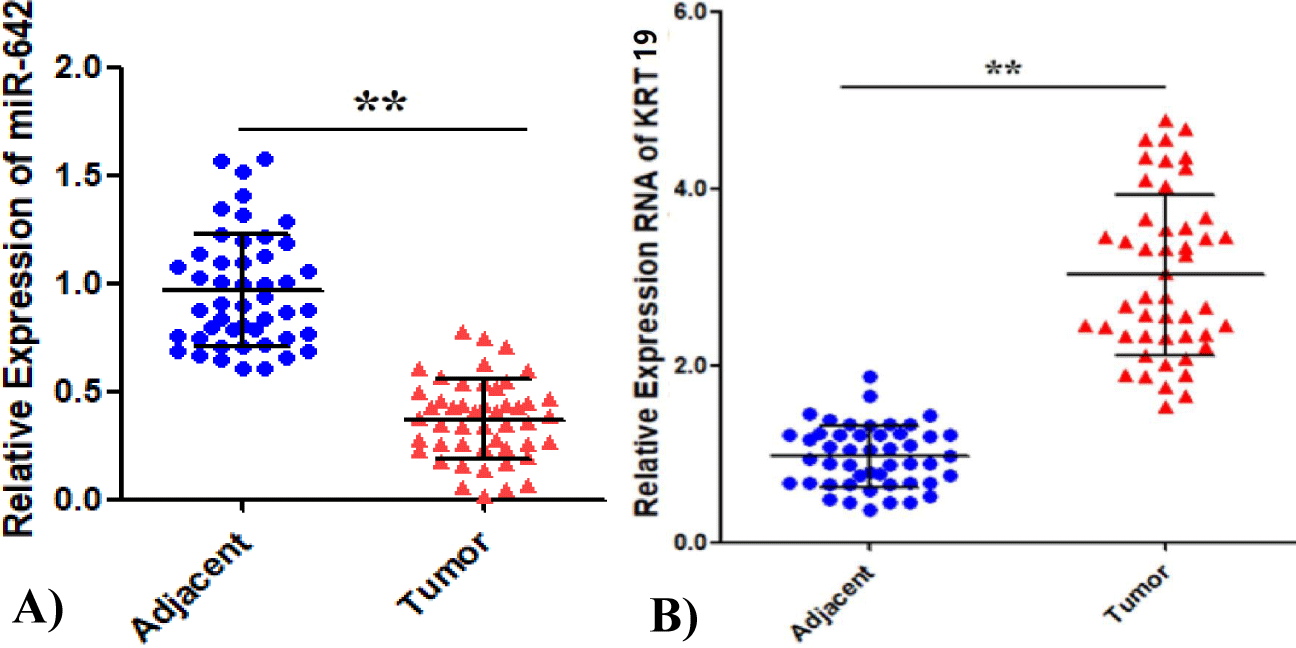
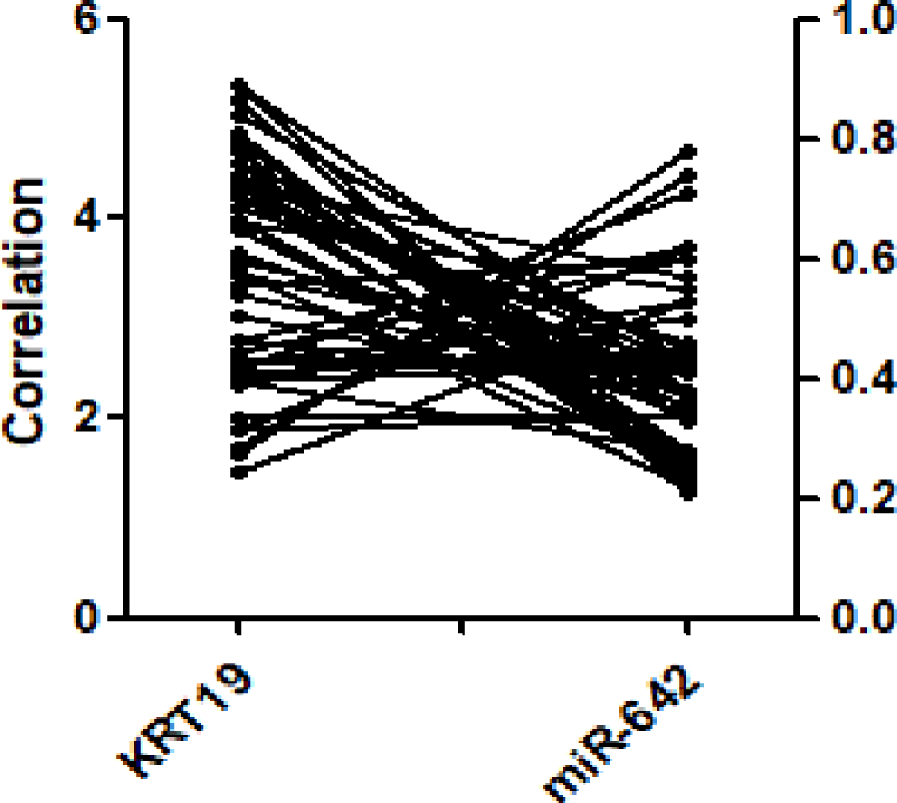
The relative expression of miR-642 was 0.37 ± 0.18, 95% CI (0.32 - 0.42) in 48 gastric cancer tissues and paracancer tissues. The paracancer tissues as control group was 0.97 ± 0.26, 95%CI (0.99 - 1.04), and the gastric cancer group had a low expression level (F = 2.06, p < 0.05), as shown in figure 2A, among which 23 samples were below the mean value and 25 were above the mean value. The mRNA expression level of KRT19 was significantly higher than that of the control group (t = -14.558, p < 0.01), as shown in figure 2B. Pearson correlation analysis showed that miR-642 was negatively correlated with KRT expression, and the regression coefficient was r = -0.577 (p < 0.01), as shown in figure 3.
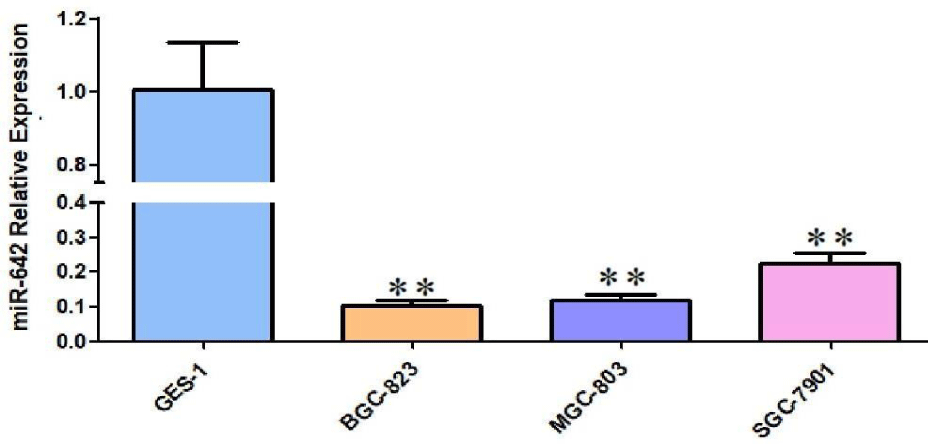
The expression level of miR-642 in normal gastric epithelial cell lines GES-1 and gastric cancer cell lines MGC-803, BGC-823, SGC-7901 was detected by qRT-PCR. Compared with GES-1 cells, the relative expression of miR-642 in MGC-803, BGC-823, SGC-7901 cell lines showed different degrees of low expression respectively, and the difference was statistically significant (F = 93, p < 0.01; F = 102, p < 0.01; F = 17.82, p < 0.01), as shown in figure 4. Fluorescence microscopy was used to observe transfection efficiency. As show in figure 5, MGC-803 cell with high transfection efficiency was selected as the subsequent experimental research object.
Relationship between miR-642a expression in gastric cancer tissues and clinicopathological features
The expression of miR-642 in 48 gastric cancer samples was compared with that in paired paracuncinoma samples. The expression of miR-642 in the gastric cancer group was down-regulated by 2.6 times, including 23 samples less than 0.37 and 25 samples greater than the mean. There were no statistical differences in the expression level of miR-642, gender, age, and degree of tumor differentiation in the distant metastasis group (p > 0.05), and there were statistical differences in TNM clinical staging and lymph node metastasis group (p < 0.05), as shown in table 2.
| Table 2: Relationship between expression of miR-642 in gastric cancer tissues and clinicopathological features. | |||||
| Relative expression of miR-642 |
|||||
| Clinicopathologic features | Total cases (n = 48) |
Low expression (n = 25) |
High expression (n = 23) |
χ2 | p -value |
| Gender | |||||
| male | 37 | 19 | 18 | 0.035 | 0.852 |
| female | 11 | 6 | 5 | ||
| Age | |||||
| <60 | 25 | 10 | 15 | 3.052 | 0.081 |
| ≥60 | 23 | 15 | 8 | ||
| Difrential level | |||||
| High and medium | 29 | 12 | 17 | 3.364 | 0.067 |
| low | 19 | 13 | 6 | ||
| TNM stage | |||||
| T1+T2 | 27 | 10 | 17 | 5.598 | 0.018 |
| T3+T4 | 21 | 15 | 6 | ||
| Lymphnode metastasis | |||||
| yes | 37 | 23 | 14 | 6.572 | 0.016 |
| no | 11 | 2 | 9 | ||
| Distant matatasis | |||||
| yes | 13 | 9 | 4 | 2.101 | 0.20 |
| no | 35 | 16 | 19 | ||
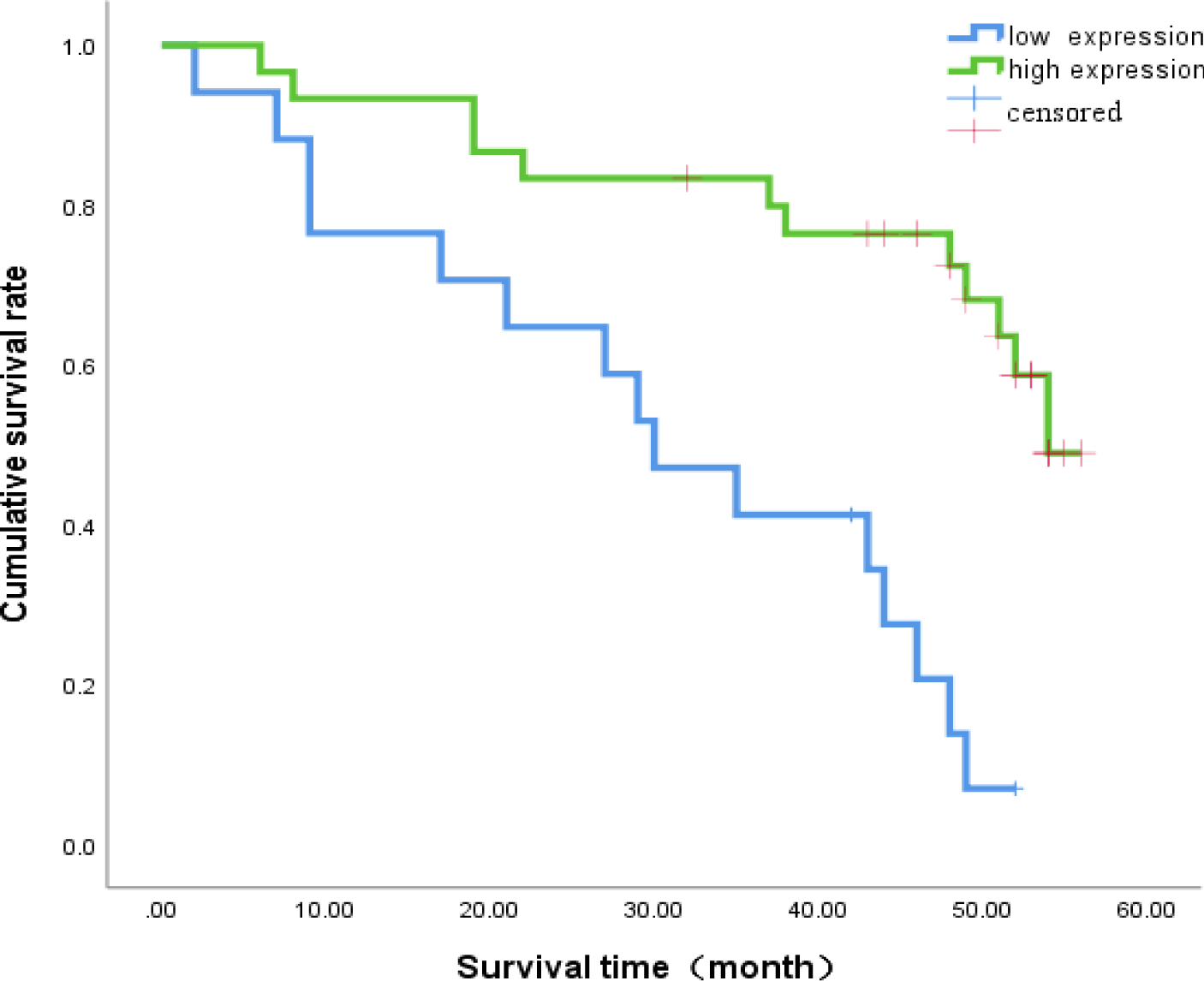
Relationship between miR-642 expression and prognostic survival
The median survival time of miR-642 low expression group was (30.3 ± 3.98) months, 95% CI: (22.5-38.1) months. miR-642 median survival was relatively high expression group (46.6 ± 2.80) months, 95% CI:(44.1- 52.1) months, Log - Rank value 2 to 16.30 for two groups comparing (p < 0.001). The survival of patients in the high expression group was higher than that in the low expression group, as shown in figure 6.
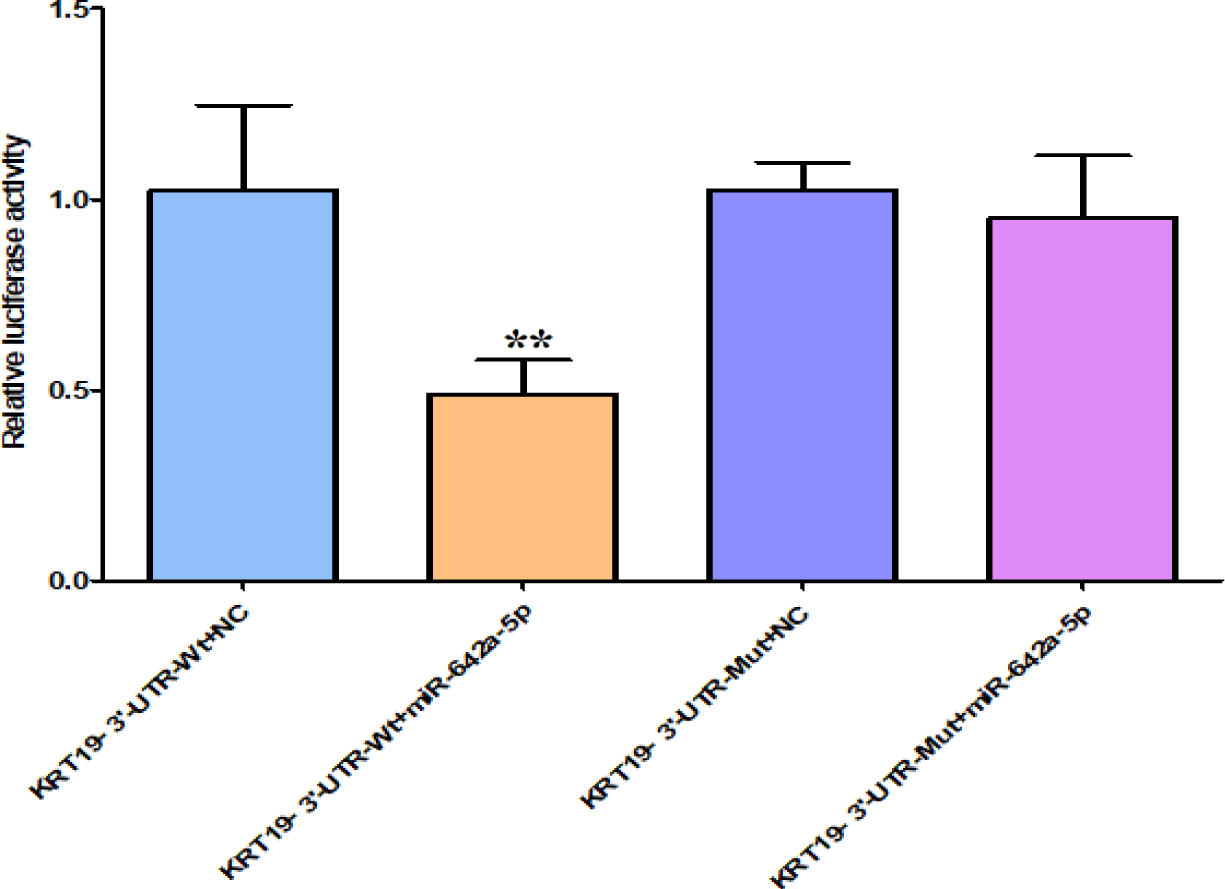
Verify the targeting relationship between miR-642 and KRT
Based on the biological information database (Targetscan, miRanda, miRDB) screening analysis, it is speculated that there is a targeted binding site between KRT19's 3’UTR and miR-642. Double luciferase reporter gene verification experiment showed that: The luciferase activity of KRT19 3 'UTR-Wt with miR-642 mimics in cells group was significantly different from that in the negative control group transfected with meaningless sequences (p < 0.01). However, there was no significant difference in the luciferase activity of miR-642 mimics and KRT3 'UTR-mut cells group compared with the negative control group transfected with meaningless sequences (p > 0.05), as shown in figure 7, indicating that KRT19 is the target gene of miR-642.
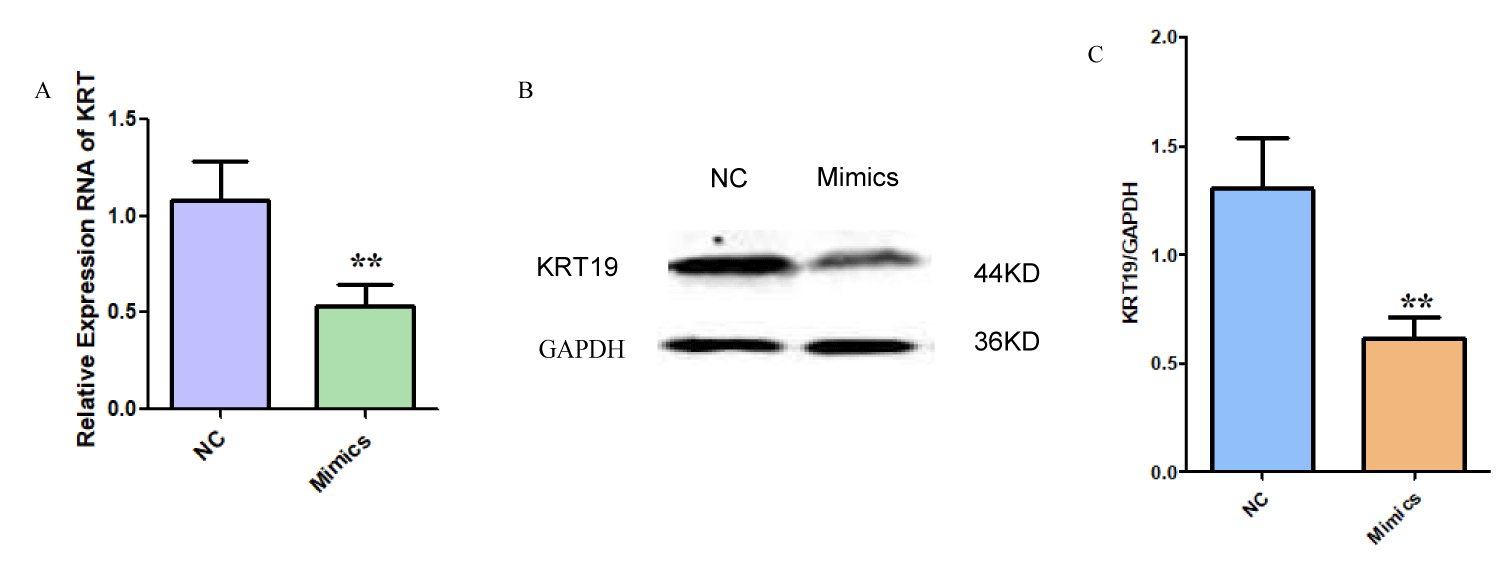
Transfected miR-642 mimics and KRT19 siRNA inhibited KRT19 expression
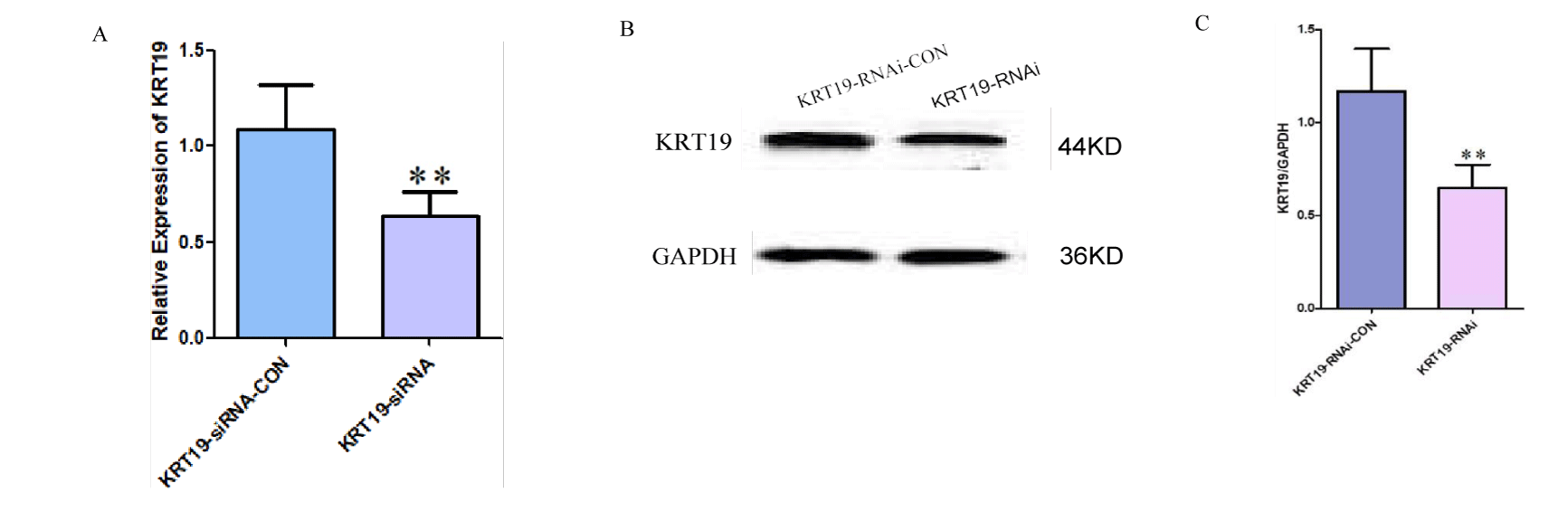
RT-PCR and Western blot experiments showed that the mRNA and protein levels of KRT19 in miR-642 mimics overexpression group (0.487 ± 0.08) and 0.532 ± 0.10) were significantly lower than those in negative control group (1.021 ± 0.10 and 1.102 ± 0.11). The mRNA and protein of KRT19 were statistically significant (t = 7.254, p < 0.01; t = 8.190, p < 0.01), as shown in figure 8. It was proved that miR-642 could inhibit the expression of KRT19 at the post-transcriptional level. Transfection with KRT19 siRNA inhibited mRNA and protein expression of KRT19, and the difference was statistically significant (t = 5.31, p < 0.01; t = 5.99, p < 0.01) as shown in figure 9.
Effects of miR-642 on proliferation of gastric cancer cells
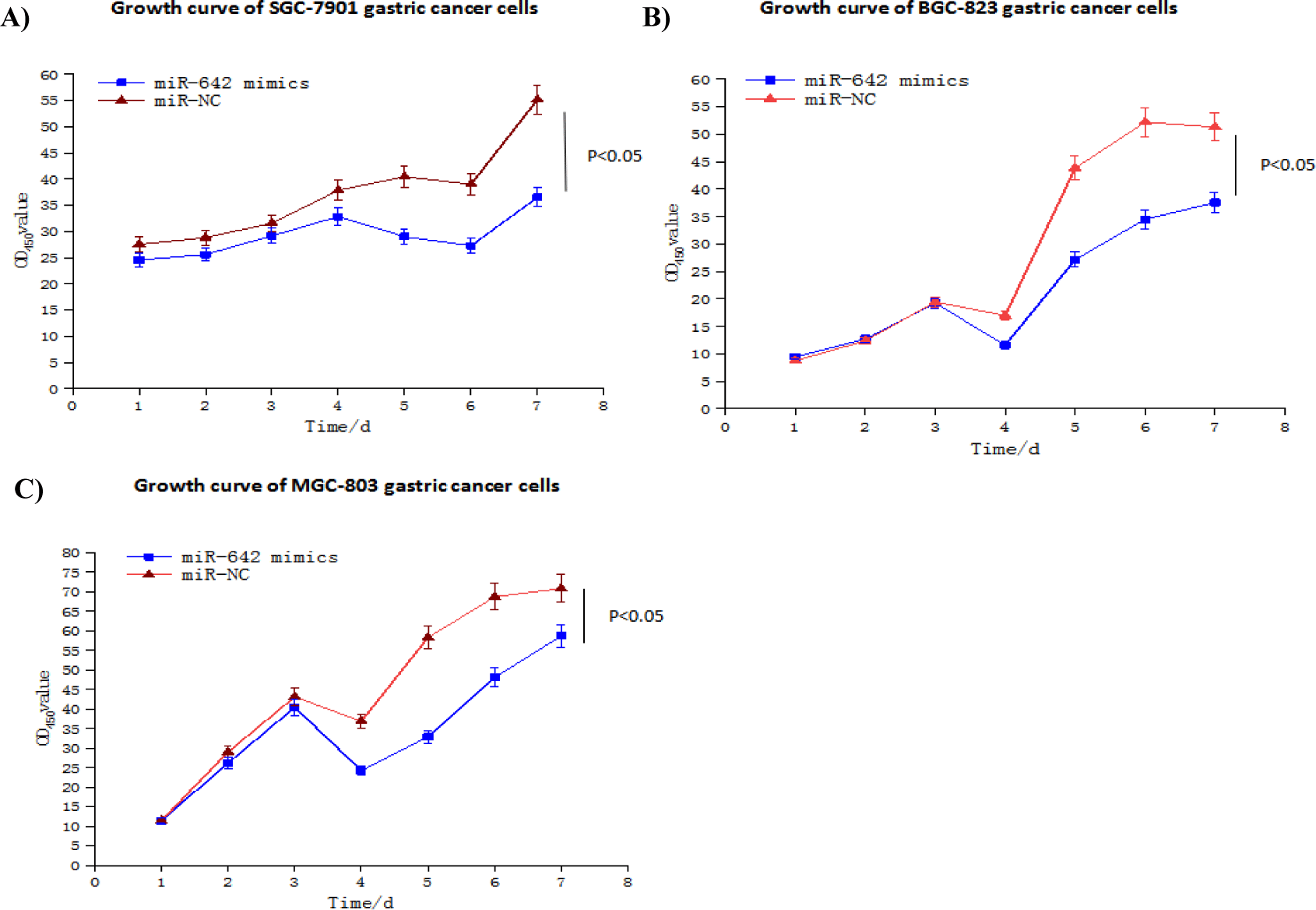
We studied the effects of miR-642 on the proliferation of BGC-823, MGC-803 and SGC-7901 gastric cancer cell lines. The change of confluence degree of three gastric cancer cell lines MGC- 803, BGC-823 and SGC-7901 at different time points was analyzed by Incucyte long-term dynamic cell observation and functional analysis system to observe their proliferation ability and plot their growth curve. The results showed that the cell proliferation ability of miR-642 mimics group was lower than that of control group, and the difference was statistically significant (t = -2.98, p < 0.05; t = -2.49, p < 0.05; t = -3.41, p < 0.05) (Figure 10).
Effects of miR-642 on migration of gastric cancer cells
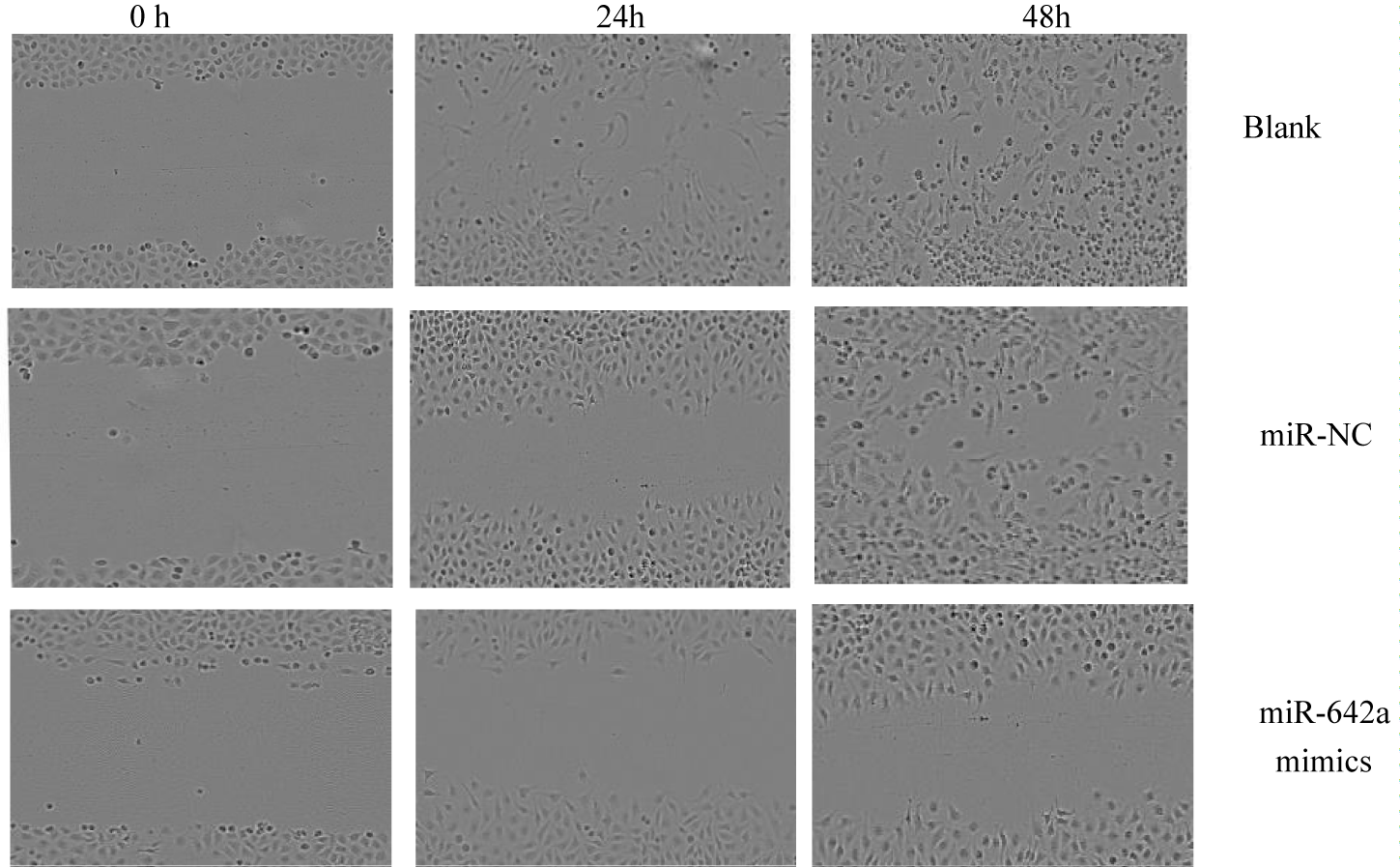
The effect of miR-642 on the migration of MGC-803 gastric cancer cells was studied. Scratch test results showed that the mobility of MGC-803 gastric cancer cells in miR-642 mimics group at 48h was (42.85 ± 2.28) %, NC group was (79.88 ± 1.08) %, and that of blank group was (88.17 ± 2.04) % at 48h. Among the three groups (F = 20.326, p < 0.01), further comparison showed that compared with the negative control group and the blank group, the miR-642 mimics group was p < 0.01 (t=- 16.23, p < 0.01; t =-18.67, p < 0.01) The comparison between the blank group and the control group (p > 0.05) suggested that the high expression of miR-642 could inhibit the migration of gastric cancer cells, as shown in figure 11.
Effects of transfection of miR-642 mimics and KRT19 siRNA on PTEN/ AKT/ mTOR pathway proteins and apoptosis proteins in gastric cancer
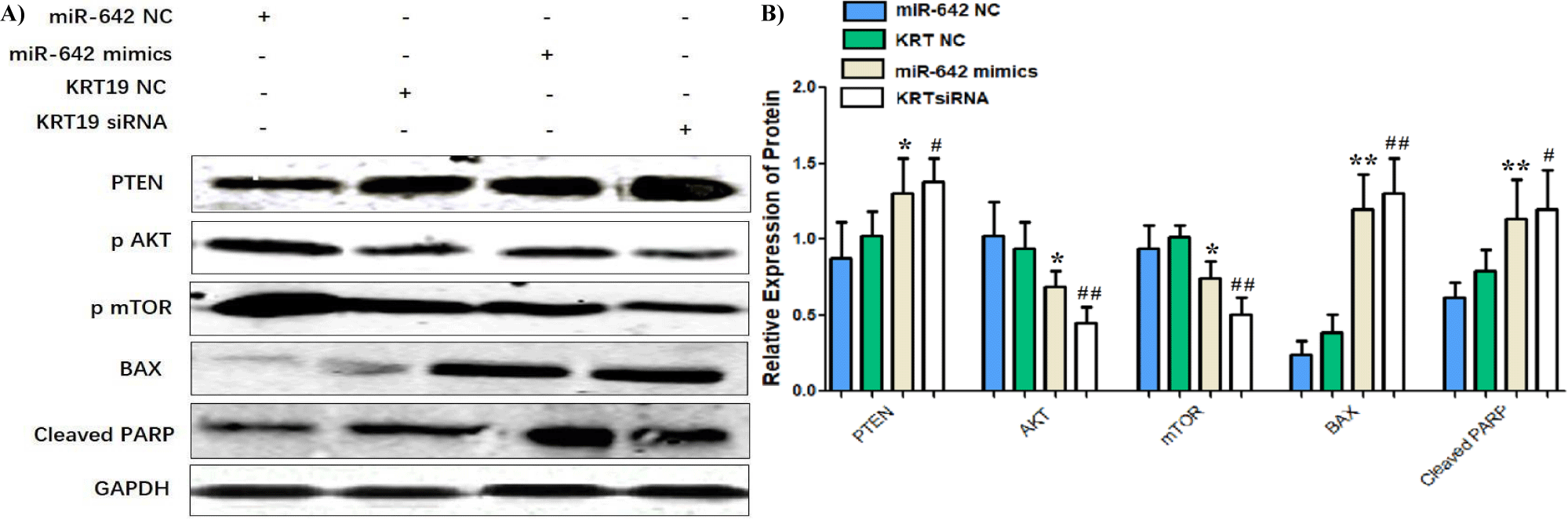
Western blot results showed that PTEN protein expression in miR-642 mimics group compared with miR-642-NC group and KRT19 siRNA group compared with KRT19-NC group was up- regulated (t = -0.39, p < 0.01; t = -2.816, p = 0.012, p < 0.05)); AKT(t = 4.177, p < 0.01; t = -7.189, p < 0.01)); The expression of mTOR protein decreased (t = 3.069, p < 0.01; t = 5.925, p < 0.01)), apoptosis-related protein BAX(t = -11.688, p < 0.01; t = -10.678, p < 0.01) and PARP expression were up-regulated (t=-5.724, p < 0.01; t = -4.157, p < 0.01). No statistically significant differences in PTEN, AKT, mTOR, BAX, PARP of miR-642⁃NC group and KRT19 siRNA-NC group (p > 0.05), as shown in figure 12.
Discussion
Gastric cancer lacks early specific symptoms and is usually diagnosed in late stage, resulting in low survival rate of gastric cancer [8]. Therefore, it is necessary to further elucidate the pathogenesis of gastric cancer so as to find more effective diagnosis and treatment methods. Some studies have pointed out that miRNAs, as endogenous non-coding genes, play the role of tumor suppressor genes or oncogenes by finely regulating their targeted genes after transcription, and participate in the mechanism of tumor occurrence and development [9]. Li S, et al. [10] reported that miR-885-5p was highly expressed in gastric cancer and promoted cell proliferation and invasion by regulating its target gene Yippee-like 1 (YPEL1). It is suggested that miR-885-5p may be a promising therapeutic target. Sheng Chunhai's research found that [11], heat shock protein gp96 is overexpressed in liver tumors, and gp96 3 'UTR inhibits the expression of miR-642a as a ceRNA(competing endogenous RNA). Upregulation of miR-642a target gene deoxyhypusine hydroxylase (DOHH) is significantly correlated with the malignity and prognosis of patients with hepatitis B disease and liver tumors, providing a new idea for the study of the occurrence and progression of liver cancer and how to regulate the occurrence of liver cancer.
In this study, it was found that the expression of miR-642 in gastric cancer cell lines MGC-803, BGC-823 and SGC-7901 was significantly lower than that of normal gastric epithelial cell lines GES-1. In gastric cancer samples, it was found that the relative expression level of miR-642 in gastric cancer tissues was lower than that in adjacent tissues, and the TNM stage was Ⅲ+Ⅳ. The expression level of miR-642 in gastric cancer patients with lymph node metastasis was lower than that in gastric cancer patients with clinical stage Ⅰ+Ⅱ and no lymph node metastasis. Survival curve analysis showed that the survival time of patients with relatively high expression was higher than that of the group with relatively low expression. miR-642 may play a role as a tumor suppressor gene in the development of gastric cancer. The predicted target of miR-642, KRT19, is the fragment of cytokeratin 19, encoding the protein CYFRA 21-1, which is a polypeptide tumor marker and can be produced by almost all human cells for tumor recurrence or therapeutic evaluation [12]. The expression level of KRT19 mRNA in gastric cancer tissues was higher than that in adjacent tissues. The proliferation ability of miR-642 mimics transfected cell lines was significantly inhibited in cell lines with different degrees of differentiation. MGC-803 cell model was selected to study the mechanism of miR-642 in gastric cancer. Dual luciferase assay and Western blot results confirmed that miR-642 targeted KRT19, and overexpression of miR-642 inhibited KRT19 expression. In vitro experiments clarified that miR-642 could interact with KRT19 through 3'-UTR. Overexpression of miR-642 could inhibit the migration ability of MGC-803 gastric cancer cells.
PTEN/AKT/mTOR [13] is a signal transduction pathway involved in the regulation of cell growth, proliferation and differentiation. Gene of Phosphate and Tension homology deleted on chromosome ten (PTEN) is a tumor suppressor. Phosphorylation of PTEN dephosphorizes PIP3 to form PIP2, reduces PIP3 level, blocks PI3K/Akt pathway, leads to G1 phase stop and causes cell apoptosis. mTOR is mammalian target of rapamycin (mTOR). mTOR is over-activated during the development of tumors [14]. Transfected miR-642 mimics and KRT19 siRNA plasmid to investigate the expression of related proteins in the PTEN/AKT/mTOR signaling pathway. Western-blot experiment showed that the expression of PTEN protein was up-regulated, while the expression of Akt and mTOR protein was down. The levels of BAX and cleaved PARP were increased. Zhou H, et al. [15] found that down-regulation of miR-21 promoted apoptosis, inhibited the process of cell cycle, targeted the PTEN/Akt signaling pathway and regulated cell proliferation, migration and apoptosis in human gastric cancer cells.
In this study, we demonstrated that miR-642 may be a novel gastric cancer gene. The overexpression of miR-642 can significantly inhibit the proliferation and migration of gastric cancer cells. This study may contribute to providing new diagnostic and therapeutic targets for the treatment of gastric cancer.
The Research was Supported by Foundations
Science and Technology Million Fund of Inner Mongolia Medical University [No. YKD2018KJBW (LH) 017]; 2. Zhi Yuan Talent Project of Inner Mongolia Medical University (No. ZY0202036) and 3. Inner Mongolia Natural Science Foundation (No.2019LH08014) for Yongping Mu; 4. Inner Mongolia Natural Science Foundation (No.2022MS08054) for Yu Wu.
References
- Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022 Feb 9;135(5):584-590. doi: 10.1097/CM9.0000000000002108. PMID: 35143424; PMCID: PMC8920425.
- Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020 Jun 4;21(11):4012. doi: 10.3390/ijms21114012. PMID: 32512697; PMCID: PMC7312039.
- Wei W, Liu C, Yao R, Tan Q, Wang Q, Tian H. miR‑486‑5p suppresses gastric cancer cell growth and migration through downregulation of fibroblast growth factor 9. Mol Med Rep. 2021 Nov;24(5):771. doi: 10.3892/mmr.2021.12411. Epub 2021 Sep 7. PMID: 34490480; PMCID: PMC8436225.
- Zhong Ke, Deng Tianzhi, and Huang Dacui. miRNA-30b regulated ITGB 3 expression and promoted apoptosis in breast cancer cells. Clinical Oncology in China. 2021;48(15):761-766.
- Relative correlation of MicroRNA-30c and prognosis of gastric cancer. Chinese Journal of Cancer Prevention and Treatment. 2014.21 (20): 1598-1601.
- Yoshimura A, Uchino J, Hasegawa K, Tsuji T, Shiotsu S, Yuba T, Takumi C, Yamada T, Takayama K, Hiraoka N. Carcinoembryonic antigen and CYFRA 21-1 responses as prognostic factors in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019 Jun;8(3):227-234. doi: 10.21037/tlcr.2019.06.08. PMID: 31367536; PMCID: PMC6626854.
- Gwak HK, Lee JH, Park SG. Preliminary evaluation of clinical utility of CYFRA 21-1, CA 72-4, NSE, CA19-9 and CEA in stomach cancer. Asian Pac J Cancer Prev. 2014;15(12):4933-8. doi: 10.7314/apjcp.2014.15.12.4933. PMID: 24998567.
- Walker R, Poleszczuk J, Mejia J, Lee JK, Pimiento JM, Malafa M, Giuliano AR, Enderling H, Coppola D. Toward early detection of Helicobacter pylori-associated gastric cancer. Gastric Cancer. 2018 Mar;21(2):196-203. doi: 10.1007/s10120-017-0748-z. Epub 2017 Jul 19. PMID: 28725964; PMCID: PMC7810132.
- Tang Yunyun, Tang Hailin, Su Qi. The biological role of microRNA in gastric cancer. China. 2014;41(02): 131-133.
- Li S, Sun MY, Su X. MiR-885-5p promotes gastric cancer proliferation and invasion through regulating YPEL1. Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):7913-7919. doi: 10.26355/eurrev_201909_19005. PMID: 31599416.
- Sheng Chunhai, et al. Heat shock protein gp96 3 ′ UTR acting as a ceRNA regulatesbDOHH expression via miR-642a. Biochemistry and Biophysical advances. 2016;43(10): 990-996.
- Nakata B, Takashima T, Ogawa Y, Ishikawa T, Hirakawa K. Serum CYFRA 21-1 (cytokeratin-19 fragments) is a useful tumour marker for detecting disease relapse and assessing treatment efficacy in breast cancer. Br J Cancer. 2004 Aug 31;91(5):873-8. doi: 10.1038/sj.bjc.6602074. PMID: 15280913; PMCID: PMC2409884.
- Wang Z, Zhou H, Cheng F, Zhang Z, Long S. miR-21 Negatively Regulates the PTEN-PI3K-Akt-mTOR Signaling Pathway in Crohn's Disease by Altering Immune Tolerance and Epithelial-Mesenchymal Transition. Discov Med. 2022 Jul-Aug;34(171):45-58. PMID: 36494326.
- The expression and clinical significance of mTOR, and PTEN genes in colorectal cancer tissues. Occupational and Health. 2015;31(08):1041-1044.
- Zhou H, Liu H, Jiang M, Zhang S, Chen J, Fan X. Targeting MicroRNA-21 Suppresses Gastric Cancer Cell Proliferation and Migration via PTEN/Akt Signaling Axis. Cell Transplant. 2019 Mar;28(3):306-317. doi: 10.1177/0963689719825573. Epub 2019 Jan 30. PMID: 30700111; PMCID: PMC6425105.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.