Biology Group . 2023 March 31;4(3):567-578. doi: 10.37871/jbres1712.
Garlic Extracts Up-regulate IL-4 and IL-13 Cytokines and Increase the Goblet Cell Numbers in Eimeria vermiformis Infected Mice
Atef Mohammed Hussein Khalil1,2* and Marwa Mohamed Fawaz3
2Center for Animal Disease Control, University of Miyazaki, Gakuen-Kibanadai-Nishi 1-1, Miyazaki, 889-2192, Japan
3Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, 83523 Qena, Egypt
- Eimeria vermiformis
- Garlic extracts
- Mouse
- >Antioxidant
- >Goblet cells
- >Interleukin expression
Abstract
We investigated the immune regulation, goblet cell responses and the anti-oxidative role of garlic extracts in the Eimeria vermiformis parasitized ileum in mice. Garlic extracts up-regulate IL-4 and IL-13 cytokines and increase the goblet cell numbers in Eimeria vermiformis-infected mice. The results showed that garlic extract is protective in response to E. vermiformis infection. Experiment was formed of three groups. Control group, mice were delivered distilled water. Infected group, rats were inoculated orally with E. vermiformis in a dose of 300 mature oocysts. The infected + garlic group was given garlic extracts at a dose of 500 mg/kg. b.w every day for ten days before infection, thereafter was inoculated orally with E. vermiformis in a dose of 300 mature oocysts, and the garlic treatment was continued to the end of the study day 9 post infection. Ileum tissues were harvested for immune and histological analyses. Compared to the infected group, garlic extracts upregulated the expressions of IL-13 and IL-4 in the parasitized section. Garlic extracts decreased the fecal oocyst numbers as well as tissue oocyst numbers. In contradiction to decreasing the parasitic oocysts numbers, goblet cell numbers in ten successive Villus-Crypt Units (VCU) in the ileal tissues were significantly increased. Furthermore, garlic treatment caused a significant decrease in the lipid peroxidation (MDA) level with an increased concentration of Glutathione Reductase (GR) enzyme. In histopathology, ileum exhibited a significant reduction in the advancement of the reproductive stages of E. vermiformis with improvement to the pathological damage caused by E. vermiformis infection.
Introduction
Coccidiosis of herbivorous livestock and birds is a widespread parasitic infection mainly caused by the genus Eimeria, protozoan parasites, with 800 known Eimeria species [1]. Coccidia spp., are intracellular obligatory parasites belonging to the phylum Apicomplexa infects the epithelium of the intestine in livestock and rodents. Infected animals often show slow growth rates with high morbidity, and mortality [2]. During the intestinal infection caused by various pathogens such as parasites, bacteria, and viruses, goblet cell feedback and mucus production are observed. Goblet cell-produced mucin has been reported and described in several parasitic infections, including Ascaridia galli [3], Trichinella spiralis [4,5], Nippostrongylus Brasiliense [6,7], and Trichuris muris [8,9]. The produced intestinal mucin has a defensive role against parasitic infestation and infection by catching the parasites and preventing their motility and feeding capability [10]. Moreover, goblet cells possess a central role in immune system activation by donation of the demonstrated irritants or pathogens to the dendritic cells of the intestinal lamina propria [11]. Functions of the goblet cells are regulated by Type 2 helper (Th2) cells. Type 2 helper cells have a vital role in enhancing the immune system in fighting extracellular and intracellular pathogens, by overproduction of important cytokines such as IL-13 and IL-4 [12,13]. Using antiparasitic drugs (chemoprophylaxis) in the strategies of control and prevention of coccidiosis is expensive, especially for developing countries [14]. Frequent treatment and the misuse of anti-parasitic medicines have induced drug-resistant in parasites [15]. Medicinal plants are used in the development of alternative safe and effective, as well as low-cost strategies for control and prevention of coccidiosis [16]. Oils and extracts of different medicinal plants can be used as substitutes for chemical antiparasitic drugs [17]. In the ancient decades, Garlic (Allium sativum L.) has been described as a common medicinal herb. It has different medicinal impacts including antiparasitic [18], and immunomodulatory responses against parasitic infections [19]. Garlic has antiparasitic certain efficacies against Cryptosporidium spp. [20], leishmaniasis [21], and coccidia infection in rabbits [22] and in the goats [23]. In addition, garlic exhibited activity in the therapy of giardia infection, entamoebasis, and Trichomonas vaginalis [24]. Several experimental models and reports have shown the anti-oxidative characterization of garlic as well as its protective effects on the site of injury. Garlic and its compounds have shown a variety of antioxidant activities. Numbers of organosulfur compounds have been found to have antioxidant activity and can stimulate the synthesis of glutathione enzyme, an important intracellular antioxidant [25]. Moreover, aqueous and methanol bulb extracts of garlic induced free radical inhibitory effects evidenced by inhibition of 2, 2'-diphenyl-1-picrylhydrazyl, both extracts exhibited strong antioxidant activity [26]. This presented report was conducted to evaluate the impact of garlic extract treatment on the iliac cytokines expression, oxidative system response, and the goblet cells population in the Illum tissue infected with Eimeria vermiformis in mice.
Materials and Methods
Materials
Experimental animals: Fifteen male ICR mice aged between 5-6 weeks and weight between 33-38 gs were brought from Charles River Company, Yokohama, Japan). Experimental animals were accommodated in sterile and clean cages in ventilated and air-conditioned rooms and given a balanced diet and clean water freely. Mice were housed for two weeks before the beginning of the study for acclimatization and were confirmed free from infection by fecal analysis using a McMaster chamber. The designated experiment is authorized by the Institutional Review Board for Animal Experiments of Miyazaki University, Japan with allowance No. 2011-007-5, under the accepted laws of Japan and the rights of the welfare and management of animals.
Parasite and infection: Eimeria vermiformis were used in this experiment and were propagated and sporulated as reported by Rose ME, et al. [27]. Sporulation and maturity were conducted using the light microscope. Three hundred oocysts were orally inoculated per mouse diluted in 100 µl of distilled water by oral gavage.
Methods
Garlic extract preparation: Garlic bulbs were well macerated and incubated in 100 % ethanol at room temperature for 24 hours with frequent mixing. Then, the homogenate went under purification by filtering. After the process of filtration, the obtained mixture extracted using soxhlet apparatus to obtain complete solubility to the garlic contents finally the extracted powder was obtained using dry freezing [28]. Eighty (80) gram of ethanol extracts were dissolved in one liter of distilled water resulting in an 80 mg/ml final concentration and incubated overnight [29]. Mixed water has undergone centrifugation at 3500 rpm for 10 minutes and the obtained supernatants were kept in a refrigerator at 4ºC. Then it was given at a dose of 500 mg/kg b.w orally to mice by stomach tube according to preliminary experimental results.
Experimental design: Fifteen male ICR mice were allocated to 3 groups (n = 5/group, as follows: Control group the mice were given 200 μl of distilled water by stomach tube. The infected group received 300 active and fully sporulated oocysts of E. vermiformis diluted in 200 μl of distilled water by stomach tube. Infected + garlic group, mice were administrated garlic extract (500 mg/kg b.w) orally for successive 10 days before infection then infected with 300 oocysts per mouse and the administration of extracts continued to day 9 post-infection (p.i). On day 9 p.i, mice were sacrificed. The ileum was harvested and well washed with cold Phosphate Buffer Saline (PBS), and then divided into two parts. The first part was frozen at -20°C for lipid peroxidation (MDA) and Glutathione Reductase (GR) analysis. The second part was used for histopathological examination as well as goblet cells and oocysts count in randomly selected ten Villous Crypt Units (VCU).
PCR analysis of mRNA expression of ileum IL-1e and IL-4: The genes of Interleukin-13 (IL-13) and Interleukins-4 (IL-4) of ileum were expressed by real-time polymerase chain reaction RT-PCR technique using the specific reverse and forward primers on day 9 p.i. RNA was isolated and extracted from the illum using an RNeasy Mini kit purchased from Qiagen, Valencia, CA, and the USA. By using the Power SYBR Green kit (Applied Biosystems, Foster City, CA, USA) the Real-time PCR test was performed.
Primers sets were as the following each primer is with sense and anti-sense gene: IL-13 sense 5′TGTAGCCCTGGATTCCCTGA-3′ and antisense 5′GGTTACAGAGGCCATGCAAT-3′, IL-4 sense 5′GGTCTCACCTCCCAACTGCT, antisense 5′ GTTTTCCAACGTACTCTGGTTGGC 3′. β-actin was used as a housekeeping gene, sense 5′ ATGGAGCCACCATCCACA-3′ and antisense 5′-CATCCG TAAAGACCT CTATGCCA-3′). The Real-time PCR test was operated on a real-time PCR device and software (7300, Applied Biosystems, Foster City, CA, USA). Calculation of the expressed values was performed using the 2-ΔΔ Ct equation given by the real-time PCR methodology note, where ΔCt indicates the cycle threshold changes in IL-4 1nd IL-13 genes when compared withβ-actin gene [30].
Oxidative stress and antioxidant analysis: From collected, frozen ileum tissue extract, Lipid peroxidation Malondialdehyde (MDA) (CAT NO: MD 95035) and Glutathione Reductase (GR) (CAT NO: GR 95035) kits were brought from (Cayman Co, Ann Arbor, USA) and analyzed by colorimetric method using mindary chemical analyzer (Mindray Ba-88A, Mindray, China).
Fecal oocyst count: Single individual fecal specimens were collected from both groups on day 9 p.i., fecal specimens were counted for E. vermiformis oocysts Output per Day (OPD), using a McMaster chamber [31]. In passing, each mouse was housed in a clean separate cage. A fecal specimen of each mouse was collected in a 50 ml tube that was marked with the number of the mouse, then fecal samples were diluted and well homogenized by adding 15 ml of distilled water. Fifty-eight milliliters of saturated sodium chloride solution of low density (311 g/l, specific gravity 1.2) were transferred to 2 grams of well homogenized fecal samples. After good homogenization, the suspension was transferred to fill McMaster counting chambers.
Histopathological analysis and histology index score: Collected ileum specimens were rapidly cleaned, washed, trimmed, and transferred to 10% neutral buffered formalin for fixation. The processed sections passed in ascending series of ethanol, immersed in paraffin for sectioning (4-5 μm), and the obtained paraffin sections were then launched on slides. Staining with Haematoxylin and Eosin (H&E) was performed for routine histopathological examinations [32]. The histopathological findings were scored according to Dommels YE, et al. [29], as shown in table 1. The total sum of the histopathological injuries was multiplied by 2 to give more weight to this value and the total Histological Injury Score (HIS) was expressed.
| Table 1: Criteria for scoring microscopic damages of mice ileum. | |
| Score | Criteria |
| 1 | Inflammatory injuries |
| Hemorrhagic areas, lymphocytes’ cell aggregation, plasma cell aggregation, and eosinophil aggregation) | |
| 2 | Tissue damage |
| Loss of the intestinal cells and mucosal shrinking | |
| 3 | Same the normal tissue |
| 5 | Mild injuries |
| The injury included a few zones of the intestinal tissues | |
| 7 | Severe injuries |
| Lesions involved in mucosa and submucosa | |
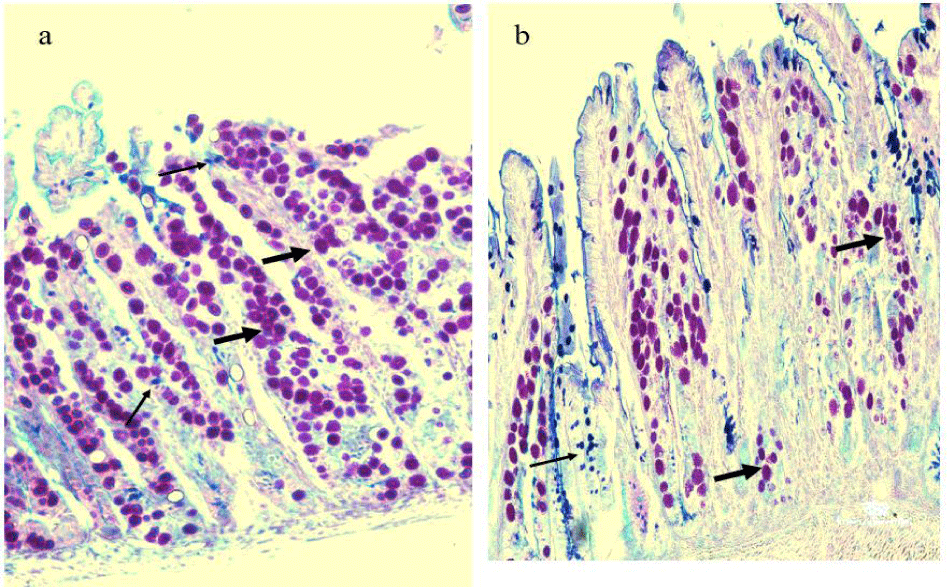
Oocyst and goblet cell counts in 10 Villus-Crypt Units (VCUs): Paraffin sections were stained with Alcian blue-PAS stain for counting goblet cells and oocysts. Counting was made from randomly selected ten well-oriented Villus-Crypt Units (VCU), according to Ishikawa N, et al. [33]. Paraffin sections were placed on glass slides and passed in a series of xylene and ethanol for 3-5 minutes each, followed by distilled water washing for 5 minutes. For 5 minutes later, tissue sections were stored in acetic acid, then placed in Alcian Blue for 30 minutes and rewashed by acetic acid for a moment, and ended with distilled water washing. Next, tissue sections were rinsed in periodic acid for 5 minutes followed by distilled water washing for another 2 minutes, then stained by Schiff stain for 15 minutes and washed with distilled water. Finally, tissue sections were soaked in Hematoxylin and washed in distilled water, then passed into a series of ethanol and xylene.
Correlation coefficient: Correlation a coefficient was computed in garlic treated group to assess the linear relationship as shown in table 2 between.
| Table 2: The correlation coefficient between variables in the infected + garlic group. | |||
| Infected + Garlic Treated Group | |||
| 1 | Oocysts count in 10 VCU | Versus | Goblet cells count in 10 VCU |
| 2 | IL-13 | Versus | Goblet cells number |
| 3 | IL-4 | Versus | Histology Injury Score (HIS) |
| 4 | MDA | Versus | Histology Injury Score (HIS) |
| 5 | GR | Versus | Histology Injury Score (HIS) |
Statistical analysis: The obtained results were statistically analyzed using the SPSS computer software (version 21.0; SPSS Inc., Chicago, USA). One-way ANOVA test was used to compare results between and within groups. The Results were presented as mean ± SE. p < 0.05 was considered significant.
Results
Cytokines gene expression
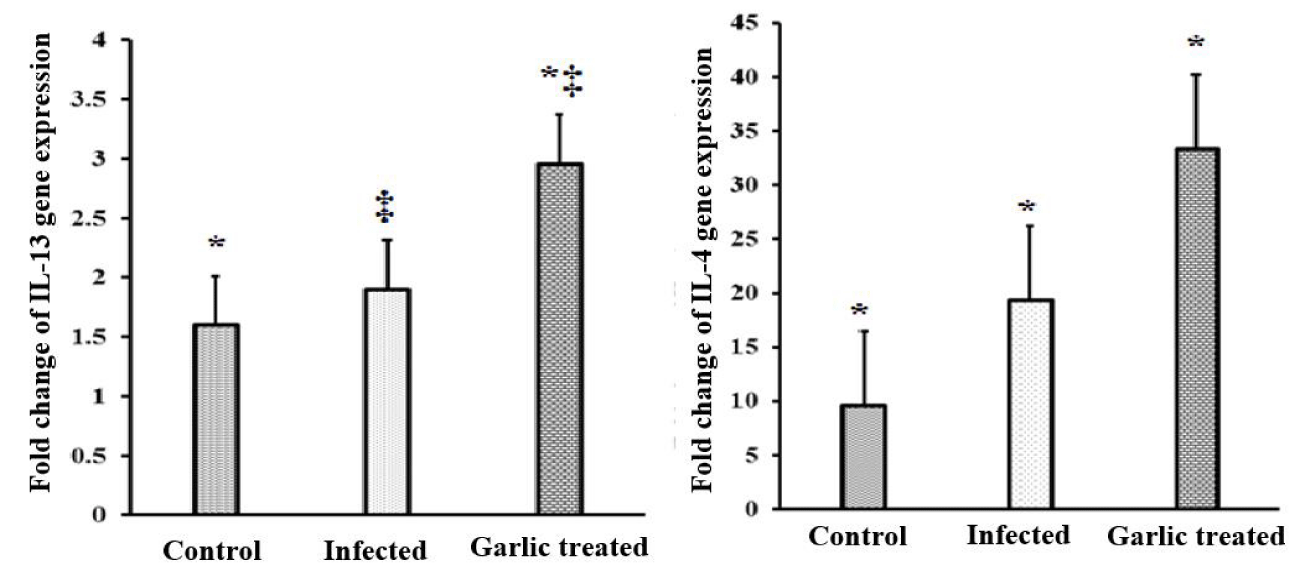
The cytokines in the ileum pieces collected from sacrificed animals were explored by qRT-PCR for IL-4 and IL-13 gene expression. The qRT-PCR was performed on day 9 p.i. specimens. E. vermiformis infection induced a non-significant increase in IL-13 in the site of infection when compared with the normal animals. Garlic extract treatment significantly increased (p < 0.001) the level of IL-13 in comparison to both infected and control groups. Furthermore, infected mice with E. vermiformis had a significant elevation in the expression of IL-4 (p < 0.01) in comparison to the control mice, while IL-4 expression became much higher when garlic extract was orally administrated 10 days and continued to day 9 post-infection in comparison to control and infected groups as shown in figure 1.
Oxidative stress and antioxidant activity
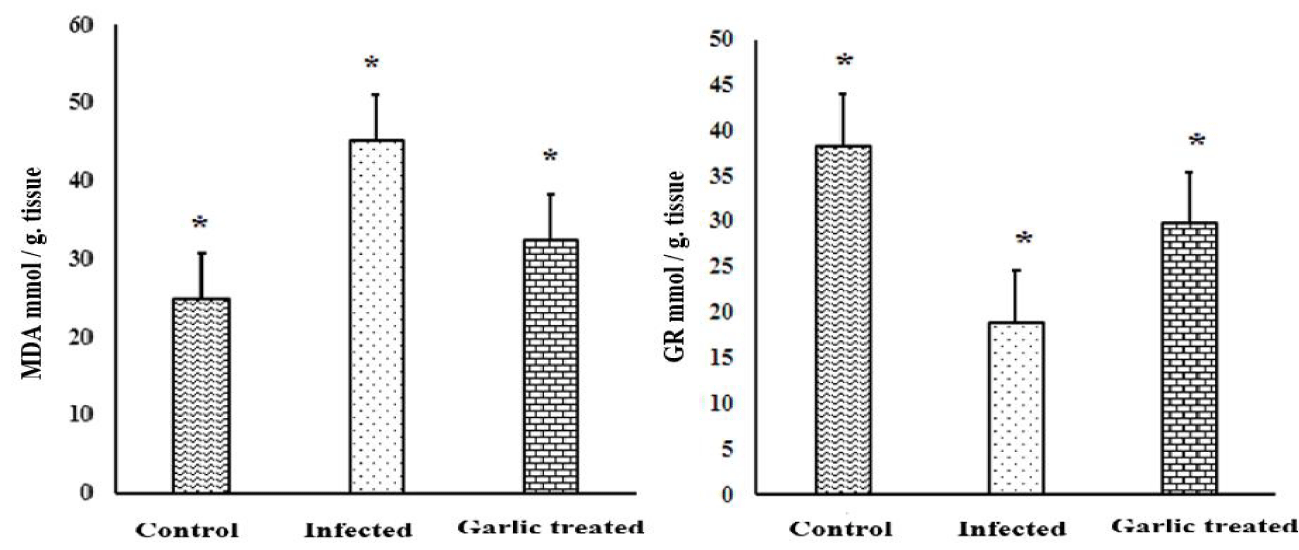
Garlic extract treatment exhibited great effects on oxidative stress and anti-oxidant activity, evidenced by differences observed in lipid peroxidation (Malondialdehyde) and glutathione reductase. Lipid peroxidation activity significantly increased (p < 0.01), in infected mice at day 9 p.i regarding the control group. Treatment with garlic extract caused a significant decrease in lipid peroxidation activity when compared with the infected group. In accordance, infection with E. vermiformis oocysts induced a significant decrease (p < 0.01), in the activity of glutathione reductase enzyme on day 9 post-infection. The level of GR enzyme increased significantly after garlic extract administration in comparison to infected mice as shown in figure 2.
Fecal oocyst count
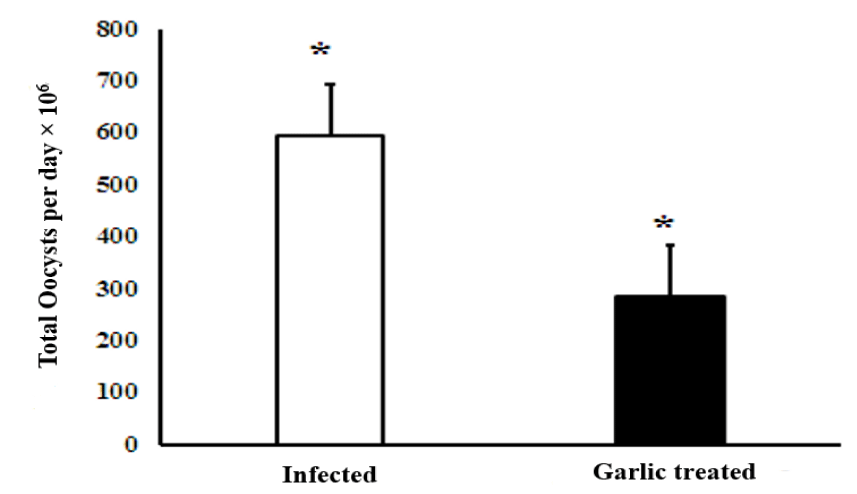
The oocysts count was performed on day 9 post-infection and the Oocysts Output per Day (OPD) in Eimeria vermiformis infected mice was very high. Administration of the infected with garlic extracts significantly (p < 0.001) declined the number of produced oocysts per day by approximately 68% in comparison to the infected garlic group as shown in figure 3.
Oocysts and goblet cells count in tissue sections of the ileum
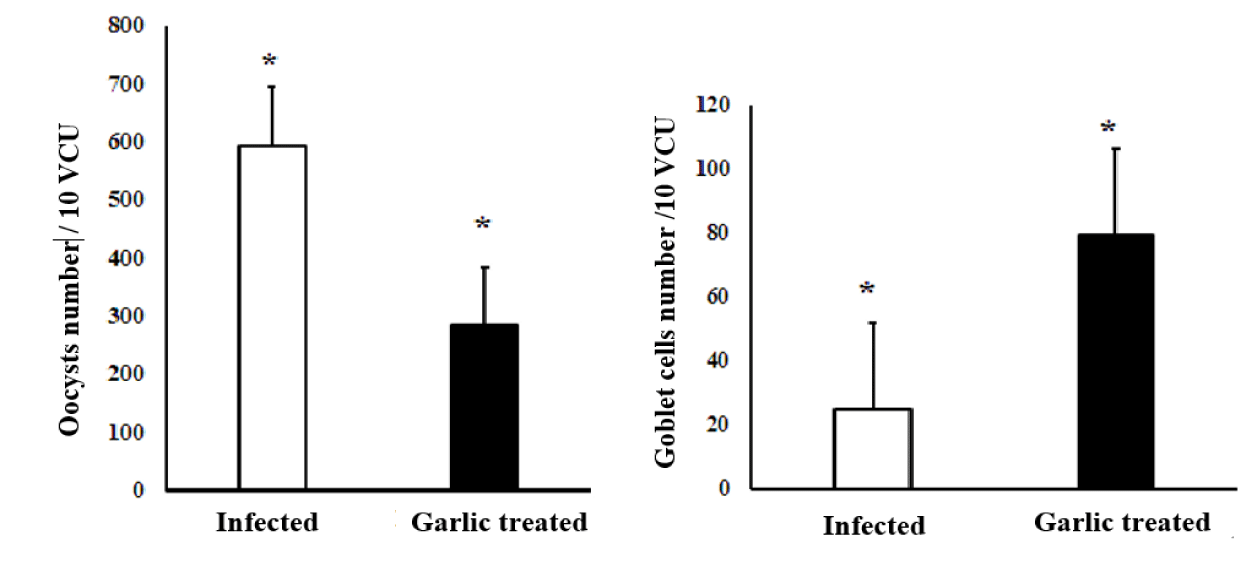
Goblet cells and parasitic oocysts were counted in 10 random Villous Crypt Units (VCU) stained with Alcian blue -PAS stain in infected and garlic-treated groups. Parasitic oocysts were seen in the intestinal villi and intestinal crypts on day 9 p.i. in both groups. In the infected group higher number of parasitic oocysts, was associated with a significantly (p < 0.01) reduced number of the goblet cell population at the site of infection, which was confirmed by Alcian Blue-PAS stain. Treatment of the infected mice with garlic extracts revealed a significant decrease in the oocysts while the number of goblet cells was significantly increased as shown in figures 4,5.
Histopathological findings and histology index score
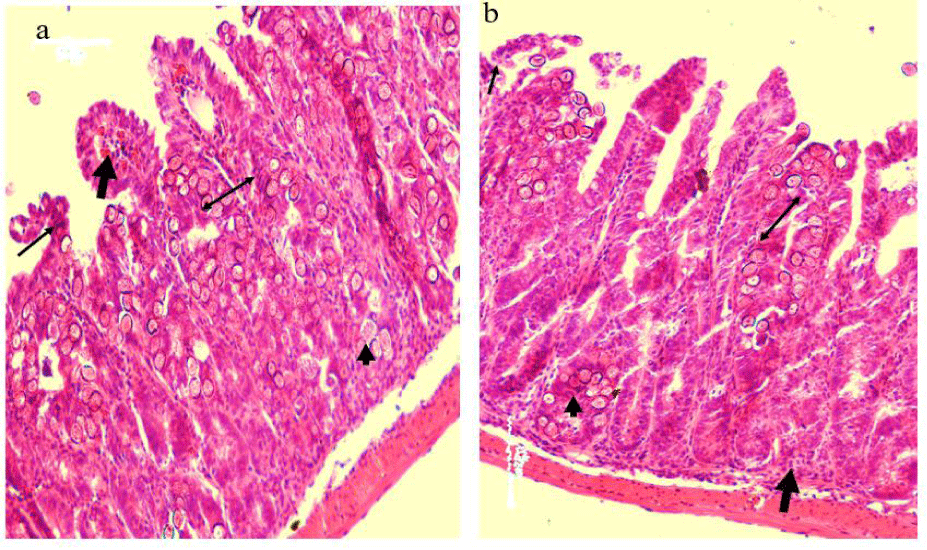
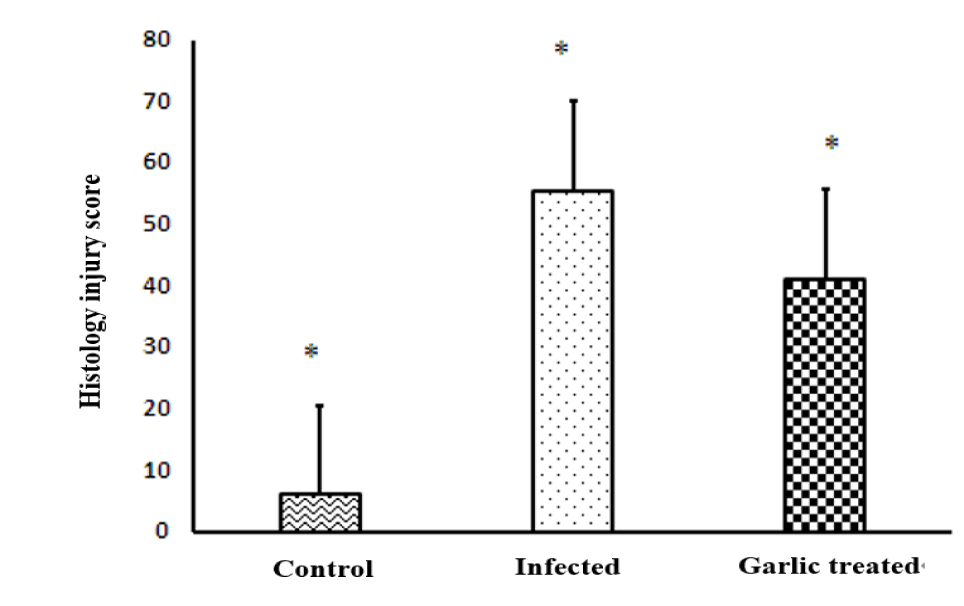
Compared to normal control mice, E. vermiformis infection induced severe histopathological damage revealed by necrosis of the intestinal crypt epithelium. In the due course, there was a noticed villous atrophy associated with lymphocytes and plasma cells infiltration as well a few eosinophils cells were aggregated in the site of infection (intestinal lamina propria and crypts). Different stages of the parasite were seen in the intestinal crypts and intestinal epithelium (Figure 6a). The oral administration of the garlic extracts to the infected mice prevented the histological damage caused by Eimeria vermiformis infection at the site of infection manifested by a decrease in the incidence of necrosis and villous destruction associated with an observed reduction in the aggregation of the inflammatory cells as well as the different parasitic stages in the intestinal crypts as shown in figure 6b. The Histopathology Injury Score (HIS) in ileum on day 9 p.i was significantly elevated by E. vermiformis infection, while it was significantly decreased (p < 0.01) in the infected + garlic treated group as shown in figure 7.
Correlation coefficient
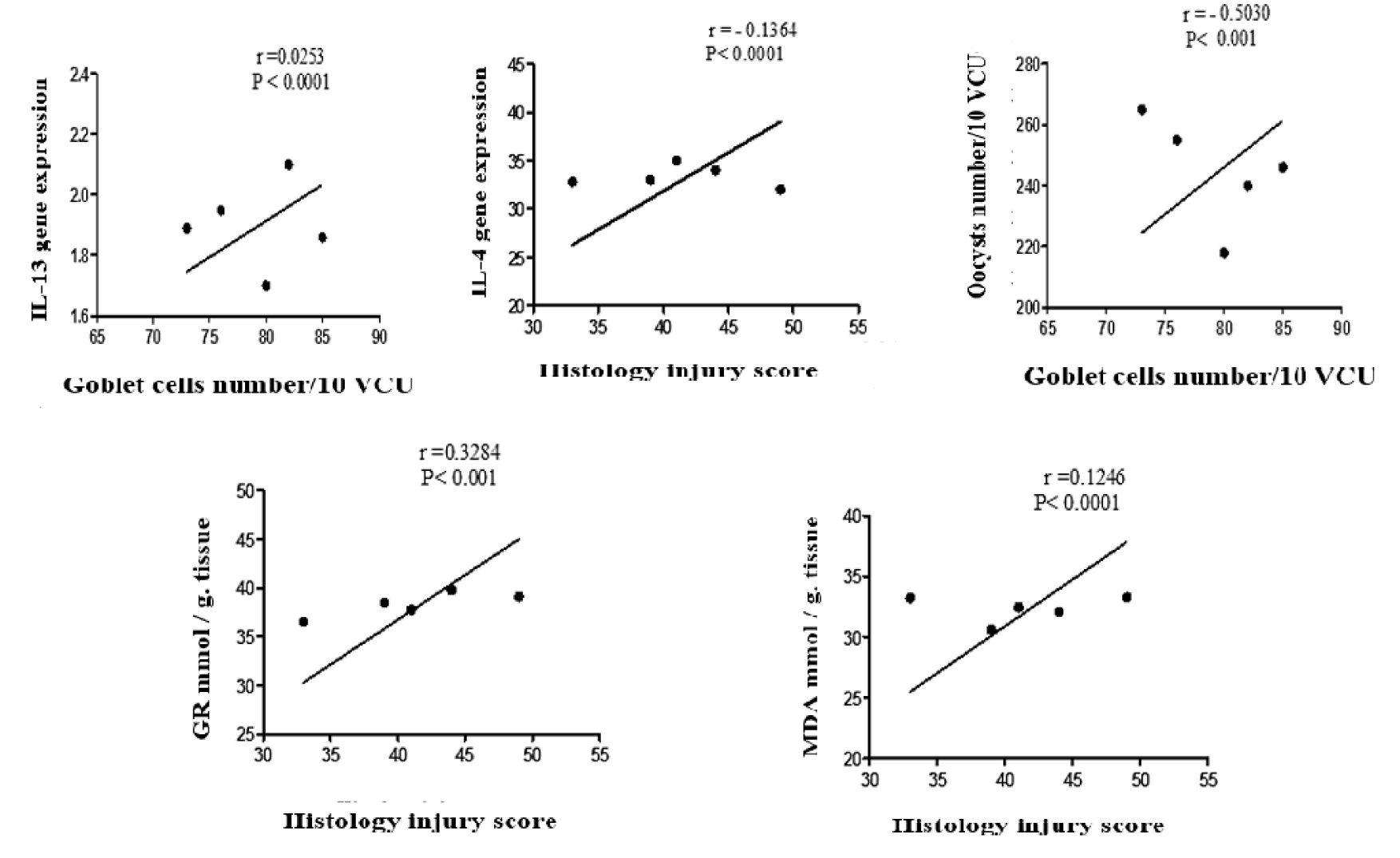
The Pearson correlation coefficient was analyzed to evaluate the linear relationship between tested variables in the infected + garlic group. Between the Oocysts count and goblet cells number at 10 VCU, there was a strong negative correlation between the two variables r = - 0.5030. The relationship was significant p < 0.0001. The goblet cell population is associated with a decrease in the number of oocysts. In the relation between the IL-13 and goblet cells population, there was a strong positive correlation between the two variables r = 0.0253, and the relationship was significant p < 0.0001. The increase in the goblet cell population is associated with an increase in the expression of IL-13. Furthermore, computing the correlation between the expression of IL-14 and HIS exhibited that there was a negative correlation between the two variables r = - 0.1364, and the relationship was significant p < 0.001. In this correlation, decreased intestinal pathological injuries were associated with an increase in the expression of IL-4 at the site of infection. Analysis of the linear relationships between the MDA and GR concentrations with HIS, indicated a positive correlation between the MDA, GR, and HIS and r = 0.1246, r = 0.8234 respectively and the relationships were significant p < 0.001 in both correlations. Regarding this statistical analysis of the correlation ships, increased intestinal pathological damages are associated with an increase in the concentration of MDA and decreased concentration of GR in the site of infection as shown in figure 8.
Discussion
This report assessed the immune regulation and anti-parasitic impacts of garlic extracts against E. vermiformis in infected mice. This study indicated that garlic extract administration has immune regulatory, and anti-coccidial impacts, in addition, to increasing the number of goblet cells in the ileum epithelium.
One of the main purposes of this scientific report was to estimate the effects of garlic extract treatment on IL-13 and IL-4 expression and their relation to the goblet cell population in the site of replication of E. vermiformis parasite. Interleukin-4 (IL-4) and IL-13 are considered essential immune regulatory cytokines, as their dysregulation may involve a series of inflammatory conditions processing such as Ulcerative Colitis (UC) and bronchial asthma. Even though both IL-4 and IL-13 are T helper Type 2 (Th2) cytokines, however, they have well-defined effective functional tasks. IL-4 is a criticizing Th2-managing cytokine its function to drive the immune consequences and feedback, while the IL-13 is an effector cytokine, directing the epithelial and fibrotic responses during injuries [34].
Intestinal parasites enhance the expression of some cytokines. Among the enhanced cytokines is IL-13, which is upregulated by Eimeria magna infection in chicken [35], and IL-4, which is upregulated by Eimeria bovis in cattle [36]. In our study, administration of garlic extracts to mice infected with E. vermiformis enhanced the expression of Il-4 and IL-13. This result comes in agreement with results reported by Ota N, et al. [28], organ Sulphur compounds alliin and DADS enhanced the production of IL-4 when it was given orally to mice.
Multiplication of E. vermiformis at the site of infection reduced the number of goblet cells [37]. In our study, E.vermiformis infection decreased goblet cell number, which significantly increased after garlic extract treatment. The increase in the goblet cell numbers by garlic extract administration may be attributed to the upregulation of IL-4 and Il-13. Concerning our results intestinal parasitic infection with helminth resulted in goblet cell hyperplasia and mucus hypersecretion under the direct regulation by the immune system. During the parasitic infestation of the intestine T helper type 2 (Th2) responses were generated with increased expression of some cytokines such as interleukin IL-4, IL-5, IL-9, and IL-13, where IL-13 is designed as the most effector cytokine against infection [38,39]. Moreover, our data agreed with Lee JJ, et al. [40], who reported that IL-13 is overexpressed in the intestinal tissues of mice infected with Gymnophalloides seoi which in turn it causes goblet cell hyperplasia and development. In another study done by Sharba S, et al. [41], giving IL-4 to the mice infected with C. Rodentium parasites increased the thickness and facet of the mucus while it decreased the pathogenic contact with the injured epithelium, in addition, to decreasing the colitis. Moreover, IL-13 and IL-4 up-regulate the expression of specified goblet cell outcomes such as Trefoil Factor Family 3 (TFF3) and Mucous component mucin 2 (MUC2), through signaling the transcription factor named Signal transducer and activator of transcription 6 (STAT6) [42], and through A Mitogen-activated Protein Kinase (MAPK) pathway [43].
The increase in the goblet cell number induced by garlic extract administration to the infected mice was associated with a decrease in oocysts count at 10 VCU as well as decreases in fecal oocysts count in feces. The same results were reported by Khalil AM, et al. [44]. The decrease of oocysts counts in feces and at the site of infection is related to IL-13 and IL-4 up-regulation. It is well known that both IL-4 and IL-13 are related cytokines that belong to the same -helix superfamily [45], and was reported also that IL-13 Rα2 is predominantly an intracellular molecule its responses can be regulated by IFN-γ [46]. Both CD4 and CD8 T cells are considered the main sources of IFN-gamma production [47], and Natural Killer (NK) T cells [48]. In the study done by Khalil AM, et al. [44], garlic extract treatment induced upregulation of IFN-γ in the ileum with CD8 T cell overexpression in the Intraepithelial Lymphocytes (IEL), where CD8 T cells act directly on the parasitized enterocytes while producing IFN-γ, activate the IL-13, resulting in a decrease in the fecal oocysts counts resulting in an increase in the goblet cells populations at the site of infection.
The antioxidant effects of garlic extracts have been studied by many researchers. It was due to the presence of organosulfur combinations in its chemical compositions such as diallyl sulfide, thiosulfinates, sulfoxides, and S-allyl Cysteine (SAC) [49]. Importantly, superoxide dismutase and glutathione reductase are enzymes effectively sharing in the systemic antioxidant defense mechanisms for neutralization and removal of the toxic reactive oxygen species ROS [50]. Our result showed that Eimeria vermivormis infection enhanced the oxidative stress at the site of infection manifested by high MDA levels. The high MDA levels in infected animals resulted from increased ROS production, leading to damage to the lipid part in the cell membrane structures in a process known as lipid peroxidation [51]. Garlic extract treatment decreased MDA concentration on the opposite side the GR concentration was increased. The increment in GR activity probably confirms the protective role of garlic extract in the inhibition and impairment of ROS production. The lower serum concentrations of MDA in garlic-treated mice indicate the protective and anti-oxidant effects of garlic extract against oxidative stress [52].
Our data concerning the histological injuries and the Histology Injury Scores (HIS) showed that on day 9 post-infection, E. vermivormis induced significant damage to the intestinal mucosa in comparison to control and garlic-treated animals which were confirmed by the same results reported by Rose ME, et al. [53]. Garlic extract administration significantly decreased the histological damage. The improvement in the intestinal epithelium histology was attributed to the upregulation of IL-4 at the site of infection. Previously, was found that Interleukin (IL)-4 has anti-inflammatory effects [54], as well as its antioxidative impacts against Eimeria vermivormis infection, resulting in the recovery of ileal tissue damage and increased goblet cell number.
For the statistical analysis of the linear relationships between the parameters examined in the infected and garlic-treated group. Administration of garlic extract increases the expression of IL-13 and decreased the oocysts count, in association with elevation in the population of the goblet cells in the epithelium of the ileum. In addition, garlic extract treatment decreased the histological damage by increasing the expression of IL-4 and decreasing GR concentration in the parasitized ileac tissues.
Conclusion
In conclusion, garlic extract treatment exhibited anti-oxidant, and anti-parasitic effects and increased the goblet cell population without significantly increasing IL-13 production. In accordance, its role is to rise the goblet cell number associated with decreasing the oocyst count at the site of infection.
Declarations
Authors' contributions
All authors participated in the conception and design of the study. Preparation of the materials, result collection and analysis were done by Atef M Khalil and Marwa Mohamed Fawaz. The preliminary form of the manuscript was written by Atef Khalil and Marwa Mohamed Fawaz made the specific and required comments on the old draft of the manuscript. Finally, all participating authors read and approved the manuscript.
Funding
This study was approved with the support of the Project for Zoonoses Education and Research, University of Miyazaki, Japan, and the research center of Pathology and Clinical Pathology Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt by using equipment, devices, and machines for sample analyses.
Acknowledgment
Special thanks to the Egyptian government for its financial support for Atef Khalil’s living expenses as a member of the post-doctor scholarship system.
Ethics approval
The rules of the Ethical Committee of Miyazaki University, Japan, were used as a guide for applying all the experimental protocols and procedures, and methodology including the rules for animal welfare and uses for experiments, sampling, and analyses.
Conflicts of interest
The authors declare no competing interests.
References
- Mehlhorn H. Encyclopedic Reference of Parasitology. Volume 1. 3rd ed. Berlin: Springer; 2008.
- Jenkins MC. Advances and prospects for subunit vaccines against protozoa of veterinary importance. Vet Parasitol. 2001 Nov 22;101(3-4):291-310. doi: 10.1016/s0304-4017(01)00557-x. PMID: 11707303.
- FRICK LP, ACKERT JE. Further studies on duodenal mucus as a factor in age resistance of chickens to parasitism. J Parasitol. 1948 Jun;34(3):192-206. PMID: 18867394.
- Ishikawa N, Wakelin D, Mahida YR. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology. 1997 Aug;113(2):542-9. doi: 10.1053/gast.1997.v113.pm9247474. PMID: 9247474.
- Khan WI, Collins SM. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 2004 Aug-Sep;26(8-9):319-26. doi: 10.1111/j.0141-9838.2004.00715.x. Erratum in: Parasite Immunol. 2004 Oct;26(10):423. PMID: 15679628.
- Miller HR, Nawa Y. Nippostrongylus brasiliensis: intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol. 1979 Feb;47(1):81-90. doi: 10.1016/0014-4894(79)90010-9. PMID: 421768.
- Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol. 1995 Sep;17(9):485-91. doi: 10.1111/j.1365-3024.1995.tb00919.x. PMID: 8552418.
- Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010 May;138(5):1763-71. doi: 10.1053/j.gastro.2010.01.045. Epub 2010 Feb 4. PMID: 20138044; PMCID: PMC3466424.
- Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011 May 9;208(5):893-900. doi: 10.1084/jem.20102057. Epub 2011 Apr 18. PMID: 21502330; PMCID: PMC3092342.
- Miller HR. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology. 1987;94 Suppl:S77-100. doi: 10.1017/s0031182000085838. PMID: 3295692.
- McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012 Mar 14;483(7389):345-9. doi: 10.1038/nature10863. PMID: 22422267; PMCID: PMC3313460.
- Grencis RK. Th2-mediated host protective immunity to intestinal nematode infections. Philos Trans R Soc Lond B Biol Sci. 1997 Sep 29;352(1359):1377-84. doi: 10.1098/rstb.1997.0123. PMID: 9355130; PMCID: PMC1692029.
- Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998 Aug;28(8):1145-58. doi: 10.1016/s0020-7519(98)00087-3. PMID: 9762559.
- Dalloul RA, Lillehoj HS. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005 Mar;49(1):1-8. doi: 10.1637/7306-11150R. PMID: 15839405.
- Mortensen LL, Williamson LH, Terrill TH, Kircher RA, Larsen M, Kaplan RM. Evaluation of prevalence and clinical implications of anthelmintic resistance in gastrointestinal nematodes in goats. J Am Vet Med Assoc. 2003 Aug 15;223(4):495-500. doi: 10.2460/javma.2003.223.495. PMID: 12930089.
- Hady MM, Zaki MM. Efficacy of some herbal feed additives on performance and control of Cecal coccidiosis in broilers. APCBEE Procedia. 2012;4:163-168. doi: 10.1016/j.apcbee.2012.11.028.
- Anthony JP, Fyfe L, Smith H. Plant active components - a resource for antiparasitic agents? Trends Parasitol. 2005 Oct;21(10):462-8. doi: 10.1016/j.pt.2005.08.004. PMID: 16099722.
- Duke JA. Handbook of Medicinal Herbs. Boca Raton, FL, USA: CRC Press; 2002.
- Clement F, Pramod SN, Venkatesh YP. Identity of the immunomodulatory proteins from garlic (Allium sativum) with the major garlic lectins or agglutinins. Int Immunopharmacol. 2010 Mar;10(3):316-24. doi: 10.1016/j.intimp.2009.12.002. Epub 2010 Jan 6. PMID: 20004743.
- Sréter T, Széll Z, Varga I. Attempted chemoprophylaxis of cryptosporidiosis in chickens, using diclazuril, toltrazuril, or garlic extract. J Parasitol. 1999 Oct;85(5):989-91. PMID: 10577746.
- Ghazanfari T, Hassan ZM, Khamesipour A. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J Ethnopharmacol. 2006 Feb 20;103(3):333-7. doi: 10.1016/j.jep.2005.08.026. Epub 2005 Oct 5. PMID: 16213117.
- Toulah FH, Al-Rawi MM. Efficacy of garlic extract on hepatic coccidiosis in infected rabbits (Oryctolagus cuniculus): histological and biochemical studies. J Egypt Soc Parasitol. 2007 Dec;37(3):957-68. PMID: 18383795.
- Worku M, Franco R, Baldwin K. Efficacy of garlic as anthelmintic in adult boar goats. Arch of Biological Science. 2009;61(1):135-140. doi: 10.2298/ABS0901135W.
- Lun ZR, Burri C, Menzinger M, Kaminsky R. Antiparasitic activity of diallyl trisulfide (Dasuansu) on human and animal pathogenic protozoa (Trypanosoma sp., Entamoeba histolytica and Giardia lamblia) in vitro. Ann Soc Belg Med Trop. 1994 Mar;74(1):51-9. PMID: 8024350.
- Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003 Feb;17(2):97-106. doi: 10.1002/ptr.1281. PMID: 12601669.
- Meriga B, Mopuri R, MuraliKrishna T. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Trop Med. 2012 May;5(5):391-5. doi: 10.1016/S1995-7645(12)60065-0. PMID: 22546657.
- Rose ME, Owen DG, Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984 Feb;88 ( Pt 1):45-54. doi: 10.1017/s0031182000054330. PMID: 6709395.
- Ota N, Takano F, Muroga S, Kawabata T, Ishigaki Y, Yahagi N, Ohta T. Garlic extract and its selected organosulphur constituents promote ileal immune responses ex vivo. Journal of Functional Foods. 2012;4:243-252. doi: 10.1016/j.jff.2011.11.003
- Dommels YE, Butts CA, Zhu S, Davy M, Martell S, Hedderley D, Barnett MP, McNabb WC, Roy NC. Characterization of intestinal inflammation and identification of related gene expression changes in mdr1a(-/-) mice. Genes Nutr. 2007 Nov;2(2):209-23. doi: 10.1007/s12263-007-0051-4. Epub 2007 Sep 27. PMID: 18850176; PMCID: PMC2474946.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101-8. doi: 10.1038/nprot.2008.73. PMID: 18546601.
- Long PL, Rowell JG. Counting oocysts of chicken coccidia. Lab Pract. 1958;7:515-518.
- Culling CF. Handbook of histological and histochemical techniques. 3rd ed. Butterworth, London, Boston: 1983.
- Ishikawa N, Horii Y, Nawa Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology. 1993 Feb;78(2):303-7. PMID: 8473019; PMCID: PMC1421791.
- Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008 Jul 15;76(2):147-55. doi: 10.1016/j.bcp.2008.04.002. Epub 2008 Apr 16. PMID: 18502398.
- Hong YH, Lillehoj HS, Lillehoj EP, Lee SH. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet Immunol Immunopathol. 2006 Dec 15;114(3-4):259-72. doi: 10.1016/j.vetimm.2006.08.006. Epub 2006 Oct 12. PMID: 17045659.
- Alcala-Canto Y, Ibarra-Velarde F. Cytokine gene expression and NF-kappaB activation following infection of intestinal epithelial cells with Eimeria bovis or Eimeria alabamensis in vitro. Parasite Immunol. 2008 Mar;30(3):175-9. doi: 10.1111/j.1365-3024.2007.01015.x. PMID: 18251972.
- Linh BK, Hayashi T, Horii Y. Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res. 2009 Mar;104(4):789-94. doi: 10.1007/s00436-008-1256-1. Epub 2008 Nov 13. PMID: 19005680.
- Marillier RG, Michels C, Smith EM, Fick LC, Leeto M, Dewals B, Horsnell WG, Brombacher F. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008 Mar 28;9:11. doi: 10.1186/1471-2172-9-11. PMID: 18373844; PMCID: PMC2329604.
- Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 2015 May;8(3):672-82. doi: 10.1038/mi.2014.101. Epub 2014 Oct 22. PMID: 25336167.
- Lee JJ, Kim D, Pyo KH, Kim MK, Kim HJ, Chai JY, Shin EH. STAT6 expression and IL-13 production in association with goblet cell hyperplasia and worm expulsion of Gymnophalloides seoi from C57BL/6 mice. Korean J Parasitol. 2013 Oct;51(5):589-94. doi: 10.3347/kjp.2013.51.5.589. Epub 2013 Oct 31. PMID: 24327788; PMCID: PMC3857510.
- Sharba S, Navabi N, Padra M, Persson JA, Quintana-Hayashi MP, Gustafsson JK, Szeponik L, Venkatakrishnan V, Sjöling Å, Nilsson S, Quiding-Järbrink M, Johansson MEV, Linden SK. Interleukin 4 induces rapid mucin transport, increases mucus thickness and quality and decreases colitis and Citrobacter rodentium in contact with epithelial cells. Virulence. 2019 Dec;10(1):97-117. doi: 10.1080/21505594.2019.1573050. PMID: 30665337; PMCID: PMC6363059.
- Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol. 2004 Mar 15;172(6):3775-83. doi: 10.4049/jimmunol.172.6.3775. PMID: 15004182.
- Iwashita J, Sato Y, Sugaya H, Takahashi N, Sasaki H, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003 Aug;81(4):275-82. doi: 10.1046/j.1440-1711.2003.t01-1-01163.x. PMID: 12848848.
- Khalil AM, Yasuda M, Farid AS, Desouky MI, Mohi-Eldin MM, Haridy M, Horii Y. Immunomodulatory and antiparasitic effects of garlic extract on Eimeria vermiformis-infected mice. Parasitol Res. 2015 Jul;114(7):2735-42. doi: 10.1007/s00436-015-4480-5. Epub 2015 Apr 18. PMID: 25895065.
- Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994 Jan;15(1):19-26. doi: 10.1016/0167-5699(94)90021-3. PMID: 7907877.
- Daines MO, Hershey GK. A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses. Evidence for intracellular stores of IL-13 receptor alpha -2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002 Mar 22;277(12):10387-93. doi: 10.1074/jbc.M108109200. Epub 2002 Jan 10. PMID: 11786536.
- Ngai P, McCormick S, Small C, Zhang X, Zganiacz A, Aoki N, Xing Z. Gamma interferon responses of CD4 and CD8 T-cell subsets are quantitatively different and independent of each other during pulmonary Mycobacterium bovis BCG infection. Infect Immun. 2007 May;75(5):2244-52. doi: 10.1128/IAI.00024-07. Epub 2007 Feb 16. PMID: 17307945; PMCID: PMC1865770.
- Rodig S, Kaplan D, Shankaran V, Old L, Schreiber RD. Signaling and signaling dysfunction through the interferon gamma receptor. Eur Cytokine Netw. 1998 Sep;9(3 Suppl):49-53. PMID: 9831186.
- Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001 Mar;131(3s):1010S-5S. doi: 10.1093/jn/131.3.1010S. PMID: 11238807.
- McCord JM. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983 Sep;94(3):412-4. PMID: 6310808.
- Davies KJ, Goldberg AL. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220-6. PMID: 3597372.
- Mannervik B. The enzymes of glutathione metabolism: an overview. Biochem Soc Trans. 1987 Aug;15(4):717-8. doi: 10.1042/bst0150717. PMID: 3315772.
- Rose ME, Millard BJ, Hesketh P. Intestinal changes associated with expression of immunity to challenge with Eimeria vermiformis. Infect Immun. 1992 Dec;60(12):5283-90. doi: 10.1128/iai.60.12.5283-5290.1992. PMID: 1452361; PMCID: PMC258307.
- Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803-7. doi: 10.1073/pnas.86.10.3803. PMID: 2786204; PMCID: PMC287229.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.