Biology Group . 2023 January 20;4(1):041-049. doi: 10.37871/jbres1647.
Improvement of Bacterial Transformation Efficiency Using Biopolymers as Plasmid Complements
Jailson de A Santos1*, Fabio OS Ribeiro2, Roosevelt DS Bezerra3, Durcilene A da Silva2, and Daniel B Liarte4
2Research Center on Biodiversity and Biotechnology, Federal University of Piauí, Parnaíba, PI, Brazil
3Federal Institute of Education, Science and Technology of Piauí, Teresina-Central Campus, IFPI, Teresina 64000-040, PI, Brazil
4Biology Department-Federal University of Piauí, UFPI, Teresina 64049-550, PI, Brazil
- Cloning
- Gene transfer
- Cellulose
- Babassu mesocarp
- Orbignya speciosa
Abstract
The process of bacterial transformation results from the introduction of specific genetic material into bacteria cells and subsequent gene expression. This technology has significant applications in gene cloning technology, and allows new possibilities for genetic breeding. The use of cellulose and babassu mesocarp to improve the process was not found in the literature. Thus, the objective of this work was to evaluate the effect of biopolymers (cellulose and biopolymer rich in starch extracted from the babassu mesocarp) on the efficiency of this process. The biopolymers were characterized by XRD, FTIR, zeta potential, thermal analysis, agarose gel electrophoresis and DLS. The transformation was carried out using the plasmid pGEM and E. coli. It was investigated the cytotoxicity and the effect of different concentrations of the materials and plasmid DNA on the transformation. The study demonstrated that 0.050 mg/mL of both materials increased the efficiency of bacteria transformation, and small amount of DNA combining with the biopolymers allowed obtaining satisfactory results. Therefore, the biopolymers can be used in genetic studies involving the transfer of DNA into bacterial cells.
Introduction
The bacterial transformation results from the introduction of specific genetic material into bacterial cells and subsequent gene expression, adding to the host cell a new genetic trait. This technology is one of the main techniques in molecular biology with significant applications in gene cloning technology, and allows new possibilities for genetic breeding of many species [1]. This technology can be applied to understand the mechanisms of replication and gene expression, determinate gene sequences of interest, encode proteins, or develop microbial cultures capable of producing useful substances such as human insulin [2], growth hormone [3], vaccines [4], and enzymes in large quantities [5].
In some prokaryotic systems, genetic transformation can occur naturally through the homologous recombination of free DNA, abundant in many environments. In addition, transformation can occur by transduction, in which a bacteriophage is required as a transfer vector; the transformation also occurs by direct contact between two bacteria with transfer of genetic material, process called conjugation [6]. There are several limitations in the use of in vitro transformation techniques that open space for future research aimed at overcoming these barriers [7]. Among these limitations, there is the slow diffusion of hydrophilic DNA entry through the hydrophobic lipid bilayer membrane and to the slight electrostatic repulsion between the anionic DNA and the anionic groups of the cellular membrane. Furthermore, in natural processes, the DNA can undergo hydrolysis or be degraded by any enzymatic process during its transformation course [1].
One strategy to circumvent these limitations is to make the target cells more competent through treating then with chemicals that provide Ca2+ ions to the cell membrane, and perform thermal shock treatment. Competent cells have physiological characteristics that allows them to bind and incorporate exogenous high molecular weight DNA. It is assumed that the divalent cations (Ca2+, Mg2+) bind first with anionic DNA and that these DNA molecules are transferred through the pores in the cell membrane formed during thermal shock [8].
On this, many DNA vector complements have been developed [1], these methods mainly involve the encapsulation of DNA within lipids [9,10], polymeric nanoparticles [11], mesoporous structure [12], cationic surfactants [13], and copolymers [14] have been reported in the literature. Being that, in studies related to different types of cationic lipids and cationic polymers, it can be observed that these materials provide electrostatic interaction due to their strong opposite charge and spontaneously form interpolylectrolyte complexes with negatively charged nucleic acids, called lipoplexes and polypeptides, respectively [15]. However, recent studies have shown a toxicity of these positively charged carriers because of their strong positive charge, thus, the tendency has been changed for the production of carriers with a slightly anionic or zwitterionic surface charge [16].
In this context, two natural anionic biopolymers that are used in several applications are cellulose and babassu mesocarp. Cellulose, the most abundant natural biopolymer on earth, is considered one of the most important organic compounds produced in the biosphere. Cellulose is a linear carbohydrate polymer with long chains of β-(1→4)-linked D-anhydroglucopyranose moieties repeating units. Each cellulose manomeric unit has three carbon atoms attached to the hydroxyl groups: one primary C-6 atom in the methylol group (–CH2OH) and two secondary carbons (C-2 and C-3) of the anhydroglucose unit [17]. The presence of the OH groups allows this biopolymer to be capable of forming hydrogen bonds, inter and intramolecular, which causes cellulose to have very specific physicochemical and structural properties [17,18].
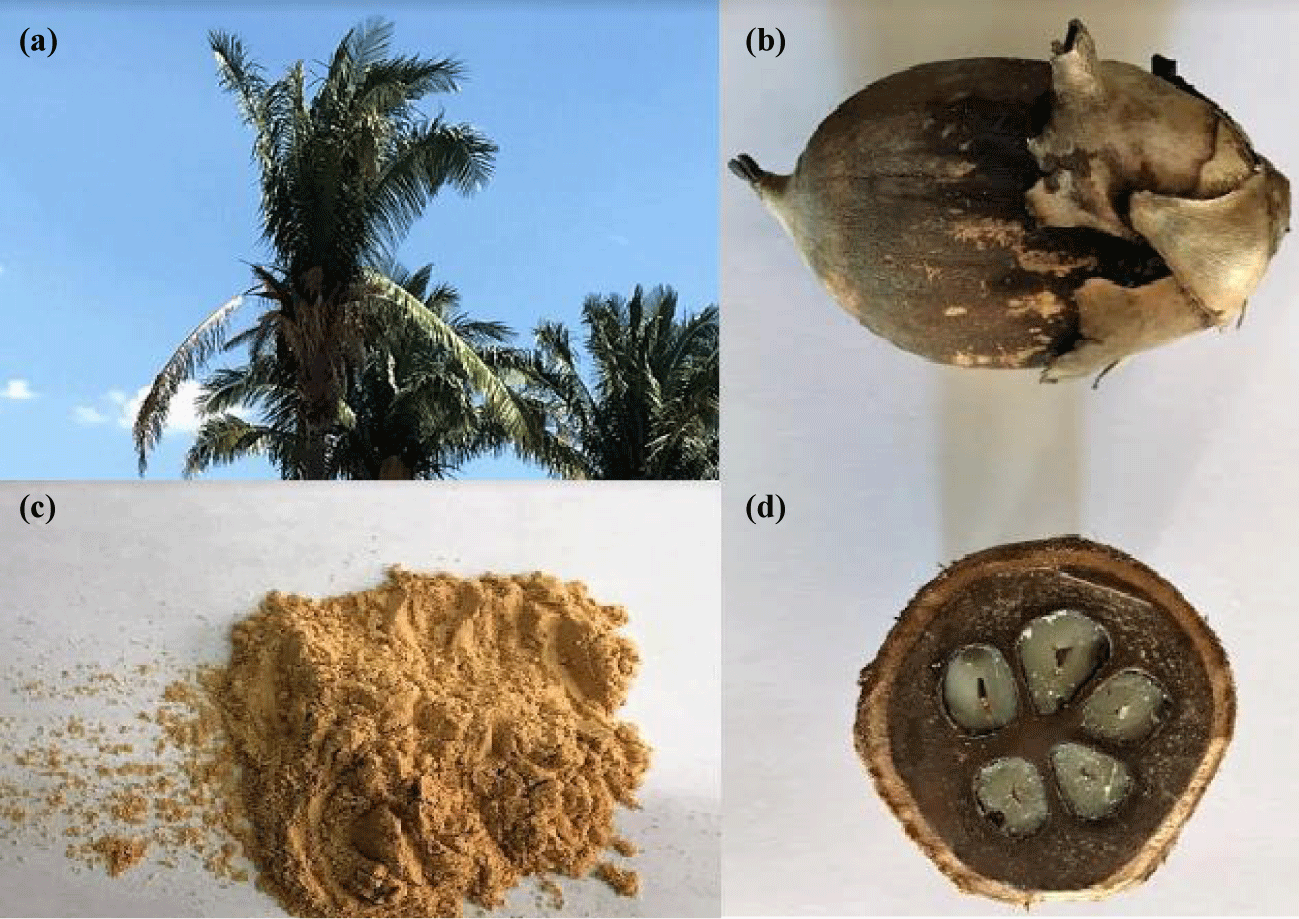
Already the babassu mesocarp (Figure 1) is obtained from babassu (Orbignya speciosa), which is a native palm tree that grows in the northern and northeastern states of Brazil [19]. This biopolymer is composed mostly of starch, 60 to 90%, moreover, it contains phenolic compounds which give it antioxidant properties [20]. The babassu mesocarp is characterized by being a non-toxic and renewable biomaterial, besides having a great potential due to the presence of reactive hydroxyl (OH) groups that are available in its surface [21-23]. In addition, the use of babassu mesocarp is extremely important in economic terms because it is very cultivated in the regions of Brazil mentioned above and it is possible to use its different parts in the most diverse applications. Being that, each tree gives 2.2–15.6 tons of fruit per hectare per year [24]. Therefore, studies using babassu mesocarp are necessary to enable the application of this natural material to increase the application of this product, which is a source of income for many people from poor regions of Brazil.
The use of cellulose and babassu mesocarp as plasmid complements in bacterial transformation was not found in the literature. Therefore, a study is necessary to observe the capacity of these biopolymers as transfer vectors in bacterial genetic transformation. According to what was presented, the aim of this study is to characterize the cellulose and babassu mesocarp by zeta potential (ζa), X-ray diffraction (XRD), infrared spectroscopy (FTIR), thermogravimetric analysis (TG/DTG), agarose gel electrophoresis, Dynamic Light Scattering (DLS) and evaluate the effect of these biopolymers in the genetic transformation of bacterial cells.
Material and Methods
Materials
Microcrystalline cellulose (C0) (Fagron) and the babassu mesocarp (M0) flour was purchased at a drug store located in Teresina-PI, Brazil. The Luria-Bertani (LB) culture medium, CaCl2 and MgCl2 were purchased from Invitrogen®. In addition, E. coli TOP 10 F' bacterial cells and a recombinant strain with p-GEM-Teasy (SOD 282-col 3) were obtained from the functional genomics laboratory of the Research Center Renê Rachou-Fiocruz, Belo Horizonte, MG, Brazil. All reagents were analytical grade and no previous purification was need. The only exception was babassu mesocarp which was washed several times with Milli-Q water and acetone (Dinâmica) to remove impurities.
Characterization of the biopolymers
The X-rays diffraction (XRD) measurements were carried out with a D600-XR instrument (Shimadzu) with Cu-target tube at wavelength λ = 0.1540 nm. Diffractograms were collected in a 2θ ranging from 5° to 50° at the rate of 5° min-1. The absorption spectra in the infrared (FTIR) were obtained using a Vertex 70 spectrophotometer (Bruker), with an accumulation of 64 scans over the range of 4000–400 cm−1. Thermogravimetric analyses (TG/DTG) were obtained on a Q600 V20.9 Build 20 SDT instrument, in a temperature range of 200 to 1400°C using nitrogen as the entrainment gas at a flow rate of 100 mL min−1. The potential-ζ (zeta) of the samples in distilled water (cpolymer = 1 mg/mL) and the dynamic light scattering (DLS) analyzes of the functional structures of DNA-C0 and DNA-M0 were carried out using the Nano-ZS ZS90 equipment (Malven Instruments) at 25°C.
Evaluation of the DNA interaction with C0 and M0
Agarose gel electrophoresis was performed to monitor the interaction between DNA and C0 or M0. Initially, 1% (w/v) C0/DNA and M0/DNA solutions were prepared by adding a suitable volume of C0 or M0 solution (the biopolymers were dispersed in 0.1 mol/L NaCl solution) [25] to an aqueous plasmid p-GEM solution (40 ng). The complexes were incubated at 37°C for 30 min. The electrophoresis was carried out on a 1% (w/v) agarose gel containing GelRed at a ratio of 1: 50,000. To estimate the size of the fragments was used the standard 1kb DNA Ladder (Promega®). Electrophoresis was performed in Tris-borate-EDTA buffer (89 mM Tris-Borate; 2 mM EDTA, pH 8.0) at 80 V for 120 min. Images were obtained using ImageQuant Las 4000 (GE) equipment.
Cytotoxicity studies of the DNA-C0 and DNA-M0 structures in E. coli cells
Before the transformation studies, the cytotoxicity of the materials in non-competent bacterial cells was determined to estimate the threshold concentration of C0 and M0 which did not show any significant toxicity to the cells. Different concentrations of the materials ranging from 0 to 0.1 mg/mL were added to 3 mL of bacteria cells. Then, the solution was incubated at 37°C for 6 hours under stirring. Prior to the incubation, the optical density (OD600) of the bacteria cells was determined. After 3 and 6 hours of incubation, two other OD600 measurements were carried out. The data analysis were carried out based on the mean value for each OD600 measuring for the control compared to the mean value for each OD600 measuring for the different concentrations over 6 hours of incubation. In addition, the effect of C0 and M0 on bacterial growth was also observed on solid agar plates.
Preparation of competent cells
TOP 10 F' E. coli cells were plated in LB medium culture at 37°C for 16 h under shaking (fisatom shaker - 753A) at 200 rpm (100 μL of cells to 10 mL of liquid LB medium). After incubation, 1 mL of cell culture was added to 100 mL of fresh LB medium. Cells were incubated at 37°C with shaking at 200 rpm for 15 minutes (OD600 between 0.4-0.5). After incubation, the cultures were centrifuged at 4000 rotations (1431 rcf) for 7 minutes at 4°C and the supernatant was removed. Subsequently, the cells were again suspended in 25 mL of ice cold CaCl2 (100 mM/Hepes 10%) and incubated on ice for 30 minutes. Then, the centrifugation was repeated and the cells were resuspended in 1 mL of CaCl2/glycerol solution (100 mM/10% Hepes). Aliquots of 50 μL of competent cells were prepared and stored at -70°C.
Transformation of competent cells
Solutions of DNA mixed with C0 or M0 were prepared by adding appropriate volumes of the materials and DNA in 1.5 mL tubes, which were incubated at 37°C for 30 min. Then, 50 μL of the competent cells were homogenized with plasmid DNA, DNA-C0 and DNA-M0. The concentrations of C0, M0 and DNA were tested in different ranges on two concentration assays. Cells were incubated on ice for 30 min and were then heated at 42°C for 45 sec. After the heat shock, the cells were put on ice for 2 min. Right after, 1 mL of liquid LB medium was added into the tubes that were incubated on an orbital shaker (fisatom shaker - 753A) at 37°C/200 rpm for 1 h. The culture cells (100 μL) was plated on solid LB medium plate containing 1 μg/mL ampicillin and placed in growing during the night for a maximum of 16 h. Plate images were obtained using the ImageQuant Las 4000 (GE) equipment and the number of colonies per plaque was determined using the free software ImageJ. The data analysis was carried out using the software Microsoft Excel.
Transformation efficiency
The Colony Forming Unit (CFU) per nanogram of DNA was calculated to determine the transformation efficiency. The number of cells transformed with and without the DNA-C0 and DNA-M0 structures, on LB agar plates supplemented with 100 μg/mL ampicillin, was determinated by using the software free ImageJ. Subsequently, data analysis was performed using the software Microsoft Excel.
Results and Discussion
Characterization of the biopolymers
The results from the zeta potential showed a medium superficial charge value of -23.0 mV for C0 and -26.6 mV for M0. These negative zeta potential values for the two biopolymers are compatible with values already presented in the literature [22,26], where a negative zeta potential value indicates the existence of negatively charged functional groups in the biopolymer structure [26]. Being that, the hydroxyl groups (-OH) are the groups responsible for the negative charge on the surface of C0 and M0, giving these biopolymers an anionic character [22,26-28].
Figure 2a shows the C0 diffraction pattern, where can be observed four characteristic peaks at 2θ = 14.5°, 16.3°, 22.3° and 34.3°, ascribed to the primary diffraction of (101), (101’), (002) and (040) planes of the microcrystalline cellulose, respectively [29-31]. The microcrystalline cellulose has both crystalline and amorphous domains in its structure, these domains are originated from the inter and intramolecular hydrogen interactions that hydroxyl groups (-OH) can perform [32-34].
Figure 2b shows the diffractogram of M0. The figure shows six peaks at 2θ = 5.8°, 15.1°, 17.2°, 18.1°, 23.1° and 26.6°. This Figure shows a typical type A starch pattern, with intense reflections at 2θ = 15.1° and 23.1°, and an unresolved doublet at 17.2° and 18.1° [35]. The A-Type crystallines occur due to the double-helical arrangement of amylopectin chains [22,36]. Already the peak present at 2θ = 5.8°, is related to the characteristic of the B-type starch pattern [34]. Finally, the diffractoframa also shows the presence of the peak at 2θ = 26.6°, this peak is related to the presence of starch amylose that forms the babassu mesocarp, being that, the intensity of this peak depends on the degree of starch hydration [35].
The FTIR spectra of C0 and M0 are shown in figure 3. From figure 3a, characteristic absorption bands of the microcrystalline cellulose structure can be observed. Where, the main absorption bands of C0 are: 1) the band relates the stretching vibrations of the OH bonds from the aromatic ring at 3352 cm-1; 2) the band at 2895 cm-1, related to the stretching vibrations of the C-H groups; 3) the band corresponds the bending vibrations of the primary and secondary OH groups at 1647 cm-1; 4) the bands between 1500 and 1200 cm-1, are related to the deformation of primary and secondary OH groups; 5) The stretching vibrations of alcoholic groups (C-O) that occur in the region between 1200 and 1000 cm-1; and 6) Absorption bands present at values less than 1000 cm-1 are related to the absorption of alcohol groups [18,29,31,32,34].
By figure 3b, it is possible to observe the characteristic absorption bands of the babassu messocarp. In this spectrum is observed a band at 3348 cm-1, which corresponds to stretching vibrations of the O-H groups. Another band is observed at 2926 cm-1, this band correspond to stretching CH groups present in M0. The band at 1627 cm-1 is related to the OH deformation vibrations of the M0 structure. In addition, bands between 1200 and 600 cm-1 are attributed to carbohydrate vibrations. Finally, the bands in the region of 1110 and 900 cm-1 are associated with the COH bond stretching that occurs due to the presence of starch that composes the babassu mesocarp [19,22,23].
Figure 4 shows the thermal stability analyzes (TG/DTG) of C0 and M0. From figure 4a, two characteristic thermal decomposition events of microcrystalline cellulose can be observed. The first event occurred between 35-120°C, with a mass loss of 1.61%, and corresponds to the physisorbed water molecules. The second event occurred with an 89% mass loss, between 300-380°C (maximum decomposition temperature = 360°C), and is related to C2 and C3 dehydroxylation and decomposition of cellulose units [18,29,34].
Figure 4b shows two characteristic decomposition events of babassu mesocarp thermal decomposition. The first step of decomposition can be attributed to the release of water physically sorbed on the surface. This stage corresponds to a mass loss of 4.2%, in the temperature range of 48-129°C (Tpeak at 90°C). The second stage is related to the decomposition of organic material and occurs in the 247 to 340°C temperature range (Tpeak at 325°C). The mass loss that corresponds to this interval of temperature is 70.8% [23].
Evaluation of the interaction between DNA, C0 and M0
Figure 5 shows the result of the agarose gel electrophoresis, in which the fragments of pure DNA and DNA in solution with the C0 or the M0 were separated according to their sizes. The migration pattern is proportional to size and charge. Since DNA has a negative charge, the direction of the migration is toward the positive pole.
The electrophoresis showed two fragments in common between the plasmid DNA, DNA in solution with C0, and DNA with M0, one at 3000 bp and another at 10000 bp. These fragments were attributed to DNA that did not interact with the materials in solution. The migration pattern of the DNA fragments in solution with the M0 showed a different band with a higher molecular weight (10000 bp) which was attributed to the interaction between the DNA and the M0.
Dynamic Light Scattering (DLS) was carried out to study changes in the hydrodynamic radius of plasmid DNA before and after its interaction with C0 and M0. DLS studies demonstrated that the hydrodynamic radius of the plasmid DNA was estimated at 220 ± 97 nm, the C0 radius was estimated as 404 ± 116 nm and the estimated radius of the M0 was 2740 ± 383 nm. After interaction with C0 or M0, the hydrodynamic radius of the DNA in solution with C0 was estimated at 620 ± 89 nm, and the radius of the DNA with M0 was estimated at 3548 ± 158 nm (Figure 6).
From the results of electrophoresis and DLS, it can be observed that the interaction of DNA with C0 and M0 occurred. C0 and M0 have similar structures as shown in item 3.1, i.e., the interactions that the two biopolymer perform with DNA are similar. The two biopolymers interact with DNA through hydrophobic interaction and hydrogen bonds, this is because DNA and biopolymers have got hydrophilic and hydrophobic regions. Hydrophobic interactions occur between the carbons present in the biopolymers rings with the aromatic moieties in the bases of the DNA, while hydrogen interactions occur through the hydroxyl (-OH) groups of the biopolymers with the proton donor (-NH2) and proton acceptor (C=O) groups At the DNA bases [37].
Considering the results of the agarose gel electrophoresis (Figure 5) and the particle sizes (Figure 6), the difference of transformation between DNA-C0 and DNA-M0 can be explained as result of the higher interaction between DNA and M0, which resulted on the formation of structures with high molecular weight that allowed increasing the bacterial transformation process efficiency. This may be related to two factors: the negative charge and structure of the biopolymer. An increase in the negative charge of the system may be responsible for the increased interaction of the biopolymer with DNA [37]. Regarding the structure of the two biopolymers, it can be observed that C0 and M0 have different structures, because the C0 is formed the union of β-glucose molecules through β-1,4-glycosidic bonds [17,18,31,34], while M0 is mainly composed of starch, which is formed by amylose and amylopectin [20,22,35,36]. Thus, the intensity of the negative charge on the biopolymer surface and the manner in which the polymer chain is organized promotes the incorporation of biopolymer, by DNA molecules, in different amounts.
Cytotoxicity study
In the cytotoxicity assay, E. coli TOP 10 F' bacteria were incubated with and without the biopolymers (C0 and M0) in different concentrations ranging from 0 to 0.1 mg/mL (Figure 7).The OD600 readings indicated that after 3 hours of incubation (Figure 7(A.a)), no significant cytotoxic effect of the materials was observed at all concentrations tested. After 6 hours of incubation (Figure 7(A.b)), differences on the bacterial growth were observed using C0 at the concentration of 0.025 mg/mL and M0 at concentrations ≥ 0.050 mg/mL.
It was observed variation from one experiment to another and, in some cases, large deviations from the control. Such results may have occurred due the fact that the materials are not 100% soluble in the solutions we tested. Both materials presented solubility in NaCl solution, but babassu mesocarp was shown to form a small amount of precipitate.
Figure 7 shows that the OD600 readings were essentially the same in the absence of cellulose and babassu mesocarp (control) and in 0.1 mg/mL of the materials after 6h. These results indicate that for up to a 0.1 mg/mL quantity of cellulose and babassu mesocarp, no toxicity was found. Subsequently, the role of varying amounts of the materials with a constant amount of DNA and their effect on the transformation efficiency were studied.
In addition to the OD600 readings, the effect of different C0 and M0 concentrations on bacterial growth in agar plates was studied (Figure 7B). The results showed that there is no cytotoxic effect of biopolymers on the concentrations tested after bacterial incubation, once bacterial growth was observed throughout the length of the plates.
Bacterial genetic transformation
The experiment was carried out in order to investigate the ability of cellulose and mesocarp to enhance the transformation efficiency of plasmid DNA into the bacterial cell. Thus, it was investigated the influence of varying amounts of C0 and M0 solutions with a constant amount of plasmid DNA on bacterial genetic transformation efficiency. In this assay, the DNA-C0 and DNA-M0 mixtures were prepared by adding variable concentrations of C0 and M0 from 0.015 to 0.1 mg/mL while maintaining the DNA concentration (0.008 mg/mL). Figure 8 shows the transformed colonies unit obtained from the assay in which the concentrations of the materials were varied and the volume of the DNA kept constant. From these assays, it was observed that C0 at concentrations of 0.050 and 0.1 mg/mL resulted in the maximum transformation efficiency of E. coli Top 10 F'. For the M0, it was observed that the maximum transformation efficiency was obtained using the concentration of 0.050 mg/mL.
Analyzing the result from figure 8, it was determined that the concentration of 0.050 mg/mL of C0 and M0 resulted in a higher number of transformed cells visually compared to the control. Thus, the concentration of 0.050 mg/mL was used in the bacterial transformation efficiency assay, varying the concentrations of the biopolymers.
A new bacterial transformation assay was carried out by keeping the material concentration constant and varying the amount of DNA in order to verify the effect of different DNA concentrations on the transformation efficiency. We decided to test lower concentrations based on the results found by other authors [1] that described that the enhancement of transformation efficiency as a function of higher DNA concentration became saturated. The DNA-C0 and DNA-M0 mixtures in the transformation assay with decreasing concentrations of DNA (8 to 0.8 ng) was carried out by maintaining constant the material concentrations (0.050 mg/mL).
The C0 and M0 concentrations were determined from the assay carried out with different concentrations of the biopolymers. Plasmid DNA without biopolymers were used as control for the transformation. The results are showed in figure 9. From the graphical representation (Figure 9), it is possible to verify that both C0 and the M0 at concentrations lower than 8 ng did not enhanced the transformation efficiency as it was tested. The concentration of 0.8 ng of DNA provided a quantitative result superior to the control. However, there is no statistical difference. The variation observed from experiment showed in figure 8 to the experiment showed in figure 7 may have occurred because the materials are not 100% soluble in the solutions we tested.
The DNA concentration assay allowed verifying that small amounts of DNA combined to C0 and/or M0 did not resulted in a higher number of transformed colonies compared to the control.
Therefore, this study showed an improvement of bacterial transformation efficiency by using C0 and M0 as plasmid complements. In terms of efficiency, considering the highest number of transformed colonies obtained using 0.050 mg/mL with 8 ng of DNA, the biopolymers showed satisfactory results and allowed improving bacterial transformation, which can improve the detection limit of DNA.
The relevance of the results from this study lies in the fact that the biopolymers used as plasmid DNA complements did not need to be chemically modified, as most studies reported in the literature recommend, which provides an improvement in the bacterial transformation efficiency without increase the expenses. Regarding the strains used in the study, these cultures were obtained from non-commercial strains, which usually have low transformation efficiencies.
Conclusion
The two biopolymers (C0 and M0) were successfully characterized by zeta potential, XRD, FTIR and TG/DTG, where by these results the characteristics of these biopolymers were observed. In addition, the study showed through electrophoresis and DLS that DNA has incorporated C0 and M0 through hydrophobic interactions and hydrogen bonds. Being that, the interaction between DNA and M0 was the most efficient. Furthermore, it was demonstrated a new biological application of C0 and M0 as non-viral plasmid complements to the transport of genes into bacterial cells. The increase in the number of transformed colonies after the bacterial transformation process, using C0 and M0 at concentrations of 0.050 mg/mL with a DNA concentration of 8ng, demonstrated that both materials can be used as adjuncts to the plasmid vector being an alternative to improve the transformation process efficiency. The use of biopolymers without prior structural chemical modifications provided good results in genetic studies involving the transfer of DNA into bacterial cells.
Acknowledgement
The authors thank CAPES, CNPq, FAPEPI and UFPI for financial and/or structural support.
References
- Soni SK, Sarkar S, Mirzadeh N, Selvakannan PR, Bhargava SK. Self-Assembled Functional Nanostructure of Plasmid DNA with Ionic Liquid [Bmim][PF₆]: Enhanced Efficiency in Bacterial Gene Transformation. Langmuir. 2015 Apr 28;31(16):4722-32. doi: 10.1021/acs.langmuir.5b00402. Epub 2015 Apr 15. PMID: 25843437.
- Johnson IS. Human insulin from recombinant DNA technology. Science. 1983 Feb 11;219(4585):632-7. doi: 10.1126/science.6337396. PMID: 6337396.
- Flodh H. Human Growth Hormone Produced with Recombinant DNA Technology: Development and Production. Acta Paediatrica. 1986;75. doi: 10.1111/j.1651-2227.1986.tb10356.x.
- Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect. 1986 Jul;13 Suppl A:39-45. doi: 10.1016/s0163-4453(86)92668-x. PMID: 2943814.
- Arbige MV, Shetty JK, Chotani GK. Industrial Enzymology: The Next Chapter. Trends Biotechnol. 2019 Dec;37(12):1355-1366. doi: 10.1016/j.tibtech.2019.09.010. Epub 2019 Nov 1. PMID: 31679826.
- Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, Pallett M, Brady J, Baldwin GS, Lea SM, Matthews SJ, Pelicic V. Specific DNA recognition mediated by a type IV pilin. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3065-70. doi: 10.1073/pnas.1218832110. Epub 2013 Feb 5. PMID: 23386723; PMCID: PMC3581936.
- Das M, Raythata H, Chatterjee S. Bacterial Transformation: What? Why? How? And When? Annual Research & Review in Biology. 2017;16:35872. doi: 10.9734/ARRB/2017/35872.
- Singh M, Yadav A, Ma X, Amoah E. Plasmid DNA transformation in Escherichia coli: effect of heat shock temperature, duration, and cold incubation of CaCl2 treated cells. International Journal of Biotechnology & Biochemistry. 2010;6.
- Xu L, Feng L, Dong S, Hao J, Yu Q. Carbon nanotubes modified by a paramagnetic cationic surfactant for migration of DNA and proteins. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018;559:201-208.
- Gallego I, Villate-Beitia I, Martínez-Navarrete G, Menéndez M, López-Méndez T, Soto-Sánchez C, Zárate J, Puras G, Fernández E, Pedraz JL. Non-viral vectors based on cationic niosomes and minicircle DNA technology enhance gene delivery efficiency for biomedical applications in retinal disorders. Nanomedicine. 2019 Apr;17:308-318. doi: 10.1016/j.nano.2018.12.018. Epub 2019 Feb 18. PMID: 30790710.
- Zamboni CG, Kozielski KL, Vaughan HJ, Nakata MM, Kim J, Higgins LJ, Pomper MG, Green JJ. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. J Control Release. 2017 Oct 10;263:18-28. doi: 10.1016/j.jconrel.2017.03.384. Epub 2017 Mar 27. PMID: 28351668; PMCID: PMC5636223.
- Li X, Xie QR, Zhang J, Xia W, Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 2011 Dec;32(35):9546-56. doi: 10.1016/j.biomaterials.2011.08.068. Epub 2011 Sep 8. PMID: 21906804.
- Costa D, Briscoe WH, Queiroz J. Polyethylenimine coated plasmid DNA-surfactant complexes as potential gene delivery systems. Colloids Surf B Biointerfaces. 2015 Sep 1;133:156-63. doi: 10.1016/j.colsurfb.2015.06.005. Epub 2015 Jun 8. PMID: 26099970.
- Kuang W, Zhao X. Synthesis, characterization, and properties of novel hydrophobically associating fluorinated copolymers for DNA delivery. Reactive and Functional Polymers. 2013;73(5):703-709. doi: 10.1016/j.reactfunctpolym.2013.02.
- Fayazpour F, Lucas B, Alvarez-Lorenzo C, Sanders NN, Demeester J, De Smedt SC. Physicochemical and transfection properties of cationic Hydroxyethylcellulose/DNA nanoparticles. Biomacromolecules. 2006 Oct;7(10):2856-62. doi: 10.1021/bm060474b. PMID: 17025362.
- Goldshtein M, Shamir S, Vinogradov E, Monsonego A, Cohen S. Co-assembled Ca2+ Alginate-Sulfate Nanoparticles for Intracellular Plasmid DNA Delivery. Mol Ther Nucleic Acids. 2019 Jun 7;16:378-390. doi: 10.1016/j.omtn.2019.03.006. Epub 2019 Mar 28. PMID: 31003172; PMCID: PMC6475713.
- Trache D, Hussin MH, Hui Chuin CT, Sabar S, Fazita MR, Taiwo OF, Hassan TM, Haafiz MK. Microcrystalline cellulose: Isolation, characterization and bio-composites application-A review. Int J Biol Macromol. 2016 Dec;93(Pt A):789-804. doi: 10.1016/j.ijbiomac.2016.09.056. Epub 2016 Sep 16. PMID: 27645920.
- Bezerra RDS, Silva MMF, Morais AIS, Santos MRMC, Airoldi C, Silva Filho EC. Natural cellulose for ranitidine drug removal from aqueous solutions. Journal of Environmental Chemical Engineering. 2014;2(1):605-611. doi: 10.1016/j.jece.2013.10.016.
- Maniglia BC, Tapia-Blácido DR. Isolation and characterization of starch from babassu mesocarp. Food Hydrocolloids. 2016;55: doi: 10.1016/j.foodhyd.2015.11.001.
- Maniglia BC, Tessaro L, Ramos AP, Tapia-Blácido DR. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocolloids. 2018. doi: 10.1016/j.foodhyd.2018.10.038.
- Teixeira PRS, Teixeira ASDNM, Farias EAO, da Silva Filho EC, da Cunha HN, Dos Santos Júnior JR, Nunes LCC, Lima HRS, Eiras C. Development of a low-cost electrochemical sensor based on babassu mesocarp (Orbignya phalerata) immobilized on a flexible gold electrode for applications in sensors for 5-fluorouracil chemotherapeutics. Anal Bioanal Chem. 2019 Jan;411(3):659-667. doi: 10.1007/s00216-018-1480-1. Epub 2018 Dec 5. PMID: 30515537.
- Teixeira PRS, Teixeira AS do NM, Farias EA, de O da Silva DA, Nunes LCC, da Silva Leite CM, da Silva Filho EC, Eiras C. Chemically modified babassu coconut (Orbignya sp.) biopolymer: characterization and development of a thin film for its application in electrochemical sensors. Journal of Polymer Research. 2018;25(5). doi: 10.1007/s10965-018-1520-8.
- Vieira AP, Santana SAA, Bezerra CWB, Silva HAS, de Melo JCP, da Silva Filho EC, Airoldi C. Copper sorption from aqueous solutions and sugar cane spirits by chemically modified babassu coconut (Orbignya speciosa) mesocarp. Chemical Engineering Journal. 2010;161(1-2):99-105. doi: 10.1016/j.cej.2010.04.036.
- Ghosh A, Razzino C do A, Dasgupta, A, Fujisawa K, Vieira LHS, Subramanian S, Costa, RS, Lobo AO, Ferreira OO, Robinson J, Terrones M, Terrones H, Viana BC. Structural and electrochemical properties of babassu coconut mesocarp-generated activated carbon and few-layer graphene. Carbon. 2019. doi: 10.1016/j.carbon.2018.12.114
- Song Y, Sun Y, Zhang X, Zhou J, Zhang L. Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules. 2008 Aug;9(8):2259-64. doi: 10.1021/bm800429a. Epub 2008 Jul 19. PMID: 18637686.
- Ahsan HM, Zhang X, Li Y, Li B, Liu S. Surface modification of microcrystalline cellulose: Physicochemical characterization and applications in the Stabilization of Pickering emulsions. International Journal of Biological Macromolecules. 2019. doi: 10.1016/j.ijbiomac.2019.04.051.
- Wang Z, Huang W, Yang G, Liu Y, Liu S. Preparation of cellulose-base amphoteric flocculant and its application in the treatment of wastewater. Carbohydrate Polymers. 2019;215:179-188. doi: 10.1016/j.carbpol.2019.03.097.
- Xu D, Zhang J, Cao Y, Wang J, Xiao J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT - Food Science and Technology. 2016;66:590-597. doi: 10.1016/j.lwt.2015.11.002.
- Silva LS, Ferreira FJL, Silva MS, Citó AMGL, Meneguin AB, Sábio RM, Barud HS, Bezerra RDS, Osajima JA, Silva Filho EC. Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. International Journal of Biological Macromolecules. 2018;116:1282-1295. doi: 10.1016/j.ijbiomac.2018.05.034.
- Ciolacu D, Ciolacu F, Popa VI. Amorphous cellulose – structure and characterization. Cellulose Chemistry and Technology. 2011;45:13-21.
- Bezerra RDS, Leal RC, da Silva MS, Morais AIS, Marques THC, Osajima JA, Meneguin AB, da S Barud H, C da Silva Filho E. Direct Modification of Microcrystalline Cellulose with Ethylenediamine for use as Adsorbent for Removal Amitriptyline Drug from Environment. Molecules. 2017 Nov 22;22(11):2039. doi: 10.3390/molecules22112039. PMID: 29165380; PMCID: PMC6150279.
- Silva LS, Carvalho J, Bezerra RDS, Silva MS, Ferreira FJL, Osajima JA, da Silva Filho EC. Potential of Cellulose Functionalized with Carboxylic Acid as Biosorbent for the Removal of Cationic Dyes in Aqueous Solution. Molecules. 2018 Mar 23;23(4):743. doi: 10.3390/molecules23040743. PMID: 29570648; PMCID: PMC6017135.
- Liu Y, Liu A, Ibrahim SA, Yang H, Huang W. Isolation and characterization of microcrystalline cellulose from pomelo peel. International Journal of Biological Macromolecules. 2018;111:717-721. doi: 10.1016/j.ijbiomac.2018.01.098.
- Bezerra RDS, Morais AIS, Osajima JA, Nunes LCC, Silva Filho EC. Development of new phosphated cellulose for application as an efficient biomaterial for the incorporation/release of amitriptyline. International Journal of Biological Macromolecules. 2016;86:362-375. doi: 10.1016/j.ijbiomac.2016.01.063.
- Cheetham NW, Tao L. Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydrate Polymers. 1998;36(4):277-284. doi: 10.1016/s0144-8617(98)00007-1.
- Paiva D, Pereira AM, Pires AL, Martins J, Carvalho LH, Magalhães FD. Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles. Polymers (Basel). 2018 Sep 4;10(9):985. doi: 10.3390/polym10090985. PMID: 30960910; PMCID: PMC6403725.
- Mujtaba M, Kaya M, Akyuz L, Erdonmez D, Akyuz B, Sargin I. Detailed adsorption mechanism of plasmid DNA by newly isolated cellulose from waste flower spikes of Thypa latifolia using quantum chemical calculations. International Journal of Biological Macromolecules. 2017;102:914-923. doi: 10.1016/j.ijbiomac.2017.04.104.
- Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J Cell Biol. 2007 Apr 9;177(1):7-11. doi: 10.1083/jcb.200611141. PMID: 17420288; PMCID: PMC2064100.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.