Environmental Sciences . 2023 January 24;4(1):064-076. doi: 10.37871/jbres1649.
Thevetia peruviana Seed Oil Transesterification for Biodiesel Production: An Optimization Study
Inam Ullah Khan, Han Wei, Ying Li, Abourehab Elmanshawy, Manqing Li, Huiting Chen and Yaowei Yu*
- Thevetia peruviana
- Non-edible
- Feedstock’s
- Transesterification
- Biodiesel
Abstract
Biodiesel production has been studied using non-edible seed oil from Thevetia peruviana. The non-edible oil from Thevetia peruviana seed could be used as a feedstock for biodiesel manufacturing. In this paper, the production of biodiesel utilizing Thevetia peruviana Seed Oil (TPSO) has been investigated. The ripe seeds of Thevetia peruviana were gathered from China and Pakistan, and after drying, its oil was extracted using the Soxhlet procedure. The seeds' highest oil content was discovered to be between 48-62%. Investigations were done into the TPSO's physicochemical characteristics. Transesterification was used to make biodiesel. The methanol to oil molar ratio, the duration, temperature, rate of stirring, and the amount of catalyst loaded were all studied in order to optimize the biodiesel synthesis process and get the highest yield. Under the ideal conditions, which included a methanol-to-oil molar ratio of 6:1, a catalyst loading of 0.42 weight percent, a temperature of 70°C, a stirring rate of 700 rpm, and an 80-minute reaction period, the biodiesel yield was 98.4%. Gas Chromatography (GC) analysis was used to identify the fatty acid configuration of the oil. Nuclear Magnetic Resonance Spectroscopy (NMR, 1H NMR and 13C NMR) and Fourier Transform Infrared Spectroscopy (FTIR) were used to characterize the biodiesel product. The ASTM & EN test technique were used to determine the biodiesel's fuel characteristics. Thevetia peruviana seed oil is used to make biodiesel that satisfies the requirements of ASTM D6751 and EN standards. As a result, this seed oil is a possible feedstock for the production of biodiesel.
Introduction
The creation of new forms of energy, such as biodiesel, is becoming more popular throughout the world as a means of avoiding rising oil prices and potential shortages. The bioenergy has attracted full attention throughout the last 15 to 20 years. The two main fuels used as additions or replacements for the fossil fuels made from petroleum in this scenario are bioethanol and biodiesel made from vegetable oils [1,2]. The use of biodiesel has many benefits over the use of fossil fuels since it is a fuel that is both safe and environmentally friendly, that comes from a renewable energy source, has lower pollutant emission profiles than mineral diesel, and is suitable for diesel engines right away because of its similar physical properties. Vegetable oils, animal fats, or used cooking oil can all be converted into biodiesel, a clean, long-lasting bioenergy source, by reacting them with alcohol to create esters (biodiesel) and glycerol [1]. Biodiesel is rapidly being researched by scientists looking into alternative fuels due to the rising expense of fuel and environmental concerns over exhaust emissions [1]. The transesterification process can be used to create this. New raw materials are eagerly anticipated by researchers, particularly non-edible ones for the manufacture of biodiesel [2-4]. Because they are more affordable and less polluting than fossil fuels, renewable energy sources are receiving increased attention [5,6]. Researchers have examined the criteria to discover what conditions are ideal for the manufacture of biodiesel. A mono-alkyl ester mixture of long-chain saturated and unsaturated fatty acids makes up the majority of biodiesel and is completely miscible with diesel fuel derived from petroleum [7,8]. Biodiesel's composition by weight is 77% carbon, 12% hydrogen, and 11% oxygen [9]. Benefits of biodiesel as a fuel include its high flash point, excellent lubricity, enhanced oxygen content, lack of sulfur and aromatic Hydrocarbons (HC), absence of sulfur, and improved biodegradability [10,11]. Two well-known downsides of biodiesel fuels are their low calorific values and greater cloud point.
Thermal cracking (pyrolysis), microemulsions, and transesterification are only a few of the processes used to generate biodiesel. The process of changing one chemical into another using heat and a catalyst is known as thermal cracking. Heat in the lack of oxygen or air causes chemical bonds to break, resulting in the formation of trivial molecules. A microemulsion is described as the spontaneous formation of two liquids that are generally incompatible, the presence of one or more ionic or nonionic amphiphiles, and a colloidal equilibrium dispersion of optically isotropic fluid microstructures with diameters typically between 1 and 150 nm [12]. Transesterification, which involves swapping an ester molecule's alkoxy group for another alcohol, is the most well-known technique for creating biodiesel. For these reactions to be catalyzed, a base and an acid are typically introduced. A proton can be donated to the carbonyl group by acids to catalyze the reaction, but a proton can also be withdrawn from the alcohol by bases to catalyze the reaction, making the alcohol more reactive. Additionally, biodiesel can be made via a supercritical alcohol transesterification [13,14]. As a sustainable, low-carbon, and ecologically friendly alternative to traditional transportation fuels, biodiesel—a fuel made from non-edible oils—has received praise. In comparison to fossil fuel, studies conducted in the past have found that biodiesel emits significantly less Sulfur oxides (SOx), Carbon monoxide (CO), Carbon dioxide (CO2), Particulate Matter (PM), Poly-Aromatic Hydrocarbons (PAH), Nitrated Poly-Aromatic Hydrocarbons (nPAH), and Hydrocarbons (HC) [15-17].
The plant oils typically include water, odorants, sterols, phospolipids, free fatty acids, and other contaminants. These factors prevent the oil from being utilized as fuel directly [18]. A minor chemical alteration of the oil is necessary to solve these issues [19]. An enzyme, a strong acid, and a strong alkaline catalyst are the three different kinds of catalysts are usable in the transesterification reaction. Shorter transesterification reaction times and less catalyst are used during production, which are the foremost advantages of utilizing a powerful alkali as a catalyst. In order to produce biodiesel in large quantities, a powerful alkaline catalyst is frequently utilized in the commercial production.
Because edible oils account for more than 95% of biodiesel production, there are worries that a variety of issues with feedstock availability could develop. Recent years have seen a rise in public concern around the use of first-generation feedstocks or vegetable oils that can be consumed for the creation of biodiesel. The conversion of food resources into fuels occurs during the biodiesel synthesis process from edible oils. Therefore, there may be an imbalance between the supply and demand for food around the world as a result of the large-scale manufacturing of biodiesel from edible oils. Additionally, as nations all over the world start clearing forests for plantations, this issue may result in significant ecological issues. So, using edible oils as a feedstock for the manufacture of biodiesel could result in the destruction of forests and harm to wildlife. As a result, feedstocks made from non-edible vegetable oils are more appealing for the generation of biodiesel [5,8]. Non-edible oil feedstock’s have a variety of advantages over edible oils when it comes to the production of biodiesel. Non-edible oils are inappropriate for human consumption because of their potentially dangerous ingredients [11]. The creation of non-edible oils requires less ideal environmental conditions. Because of this, non-edible oil crops may be produced on barren ground, fields, roadways, damaged forests, and irrigation canals. Therefore, producing biodiesel from non-edible oils could play an important role in projects that boost the economies of rural areas that are still undeveloped [14].
As an alternative feedstock for the manufacturing of biodiesel fuel, TPSO seed oil was examined in the current study. In order to produce biodiesel, the crude oil was transesterified with methanol using the TPSO seed in the presence of KOH as a catalyst. ASTM and EN standards were met by the TPSO seed oil biodiesel's properties after transesterification.
Plant Description
Small, perennial, and evergreen Thevetia peruviana, sometimes referred to as milk bush or yellow oleander is mostly grown for ornamental purposes. The Apocynaceae family includes this plant, which is widely distributed on the continents of America, Asia, and Africa. The plant, which is loathed by herbivorous animals, produces seeds with high oil content (60-65%) [20], and it can be cultivated on expressway road barriers and roadside ditches for aesthetic purposes, environmental protection, and biodiesel generation. The plants may be gathered continuously for years on end and can be grown on places that would otherwise not appropriate for use in conventional agriculture. Figure 1 shows Thevetia peruviana Plant and seed photographs.
Materials and Methods
Feedstock
Thevetia peruviana seeds were used as a non-edible feedstock in the current investigation to make biodiesel. Thevetia peruviana seeds were first collected from ripe trees in Pakistan and China, following which the seeds were taken out once the stems had dried. A Thevetia peruviana plant and the seeds are shown in figure 1.
Laboratory resources
Sigma-Aldrich Chemical Suppliers, Shanghai, China, provided analytical-grade methanol and Potassium hydroxide (KOH) for this research.
Oil extraction
Through numerous visits from various locations in China and Pakistan, ripe seeds of the Thevetia peruviana plant were collected. After field collection, the Thevetia peruviana seeds were cleaned of dust and other contaminants by washing in warm distilled water. After that, at 60°C the seeds were dried until their weight remained consistent. Soxhlet equipment was used to extract the oil in order to determine its precise composition [21]. This experiment used lactic acid, nitrogen, potassium bromide, potassium hydroxide, sodium hydroxide, magnesium oxide, and calcium oxide as chemical reagents. Chloroform, petroleum ether, n-hexane, phenolphthalein, isopropyl alcohol, sulphuric acid, anhydrous sodium sulfate, oxalic acid, methyl heptadecanoate, and tetramethylsilane were some of the additional chemicals.
Process of extracting and measurement of oil content
The seeds of Thevetia peruviana were removed from the stalks and mashed into ten grams in a grinder. The crushed seeds were placed inside of a filter paper. Oil extraction started when Thevetia peruviana seeds on filter paper were crushed and petroleum ether (150 mL) was added to the Soxhlet extraction apparatus. The filter paper containing the seeds was taken out of the Soxhlet extraction system after 7 hours, and it was left to air dry for 24 hours. The oil content was determined using Eq. (1) [22].
Transesterification process
It was necessary to measure the acid value before the transesterification reaction. As a result, titration was used in this investigation to measure the TPSO's free fatty acid content. To calculate the acid value of the TPSO, the [23] approach was utilized. The results showed that TPSO has an acid value of less than 0.137%. The oil had a low acid value, therefore the acid-catalyzed esterification step was not required and the transesterification reaction was started immediately away. Using a magnetic stirrer, methanol and potassium hydroxide were swirled for five minutes to make potassium methoxide, which was then utilized to perform the transesterification process. In a conical flask, the generated methoxide mixture and TPSO were combined. The mixture was then moved into a reaction room where ultrasonic waves were then applied. The controller was used to change the response time, amplitude, and pulse. The settings for the transesterification process were 50°C, 70% amplitude, and 70% pulse, respectively. In response to a variety of variables, including the reaction temperature, stirring rate, time, methanol to oil molar ratio, and catalyst loading, the rate of TPSO conversion to methyl esters was investigated. Biodiesel and glycerol were the two phases of the final product that were created during the process. After the glycerol was taken out, the biodiesel was refined.
FT-IR spectroscopy
The transesterification reaction was observed using FT-IR spectroscopy. Thevetia peruviana seed oil biodiesel was examined using FT-IR technology between 4000-400 cm-1 on a Bio-Rad Excalibur Model FTS3000MX. There were 15 scans at a resolution of 1 cm-1.
NMR analyses
Avan CE 300 MHz, 7.05 T, and 5 mm BBO probe-equipped spectrometers were used for the NMR investigation. The internal reference standard was TMS, and the solvent was Deuterated chloroform (CDCl3). We captured the 1H (300 MHz) spectra using 8 scans, a recycle delay of 1 second, and pulse widths of 30 degrees. With a pulse duration of 30 degrees and a recycle delay of 1.89 seconds, 160 scans of the 13C (75 MHz) spectra were made.
Analysis of TPSO FAMEs using GC-MS
By converting the oil sample into its methyl esters using the ASTM D-1983 standard procedure, the fatty acid content of TPSO was ascertained. All of the evaluated FAMEs were subjected to GC-MS analysis using a Varian 450-GC/240-MS gas chromatography/mass spectrometer (Varian, Santa Clara, CA, USA) coupled with a DBWAX capillary column (30 m x 250 lm x 0.25 um film thickness). Following are the precise operational conditions that were used: 3 mL/min helium gas flow rates, a 1:10 split ratio, a 250°C injector temperature, a 1 uL injection volume, an oven temperature progression of 150 to 210°C at a rate of 15°C/min, and from 210 to 226°C at a rate of 1°C/min; a 300°C detector temperature; a 200°C ion source temperature; the use of electronic impact mode at The mass spectra fragmentation patterns of the FAMEs were compared to those in the mass spectral library NIST 02 using the software of the GC-MS instrument, and their identities were then further verified by comparing the data to those of recognized standards. By dividing the total mass of the FAMEs by the total mass of TPSO used in each experiment, the percentage of biodiesel yield (%, g/g) is computed (10 g).
FAMEs purification
After the two layers were separated, the remaining methanol was heated to 60°C and used to distill, purifying the upper biodiesel layer. Apply 1-2 drops of acetic acid on the catalyst to remove it, consecutive rinses with distilled water were used to eliminate any leftover catalyst. Anhydrous sodium sulfate (Na2SO4) treatment and filtration were used to get rid of any leftover water.
TPSO-produced biodiesel's fuel characteristics
After being generated in line with ASTM biodiesel requirements, the physical and chemical characteristics of pure biodiesel were assessed. The following physical and chemical properties were measured: acid value, cetane number, oxidation stability at 110°C (EN 14112), sulfur content, density at 15°C (ASTM D4052), kinematic viscosity at 40°C (ASTM D445), cloud point, pour point, flash point, and IV (AOAC CD1-25).
Elemental analysis of biodiesel
The presence of metals in the FAMEs of the sources from non-edible plants was checked using an elemental analyzer (Vario EL CUBE, Hanau, Germany) and an ICP-OES (Spectro-blue, Kleve, Germany) [24].
Result and Discussion
Variables effecting TPBD yield
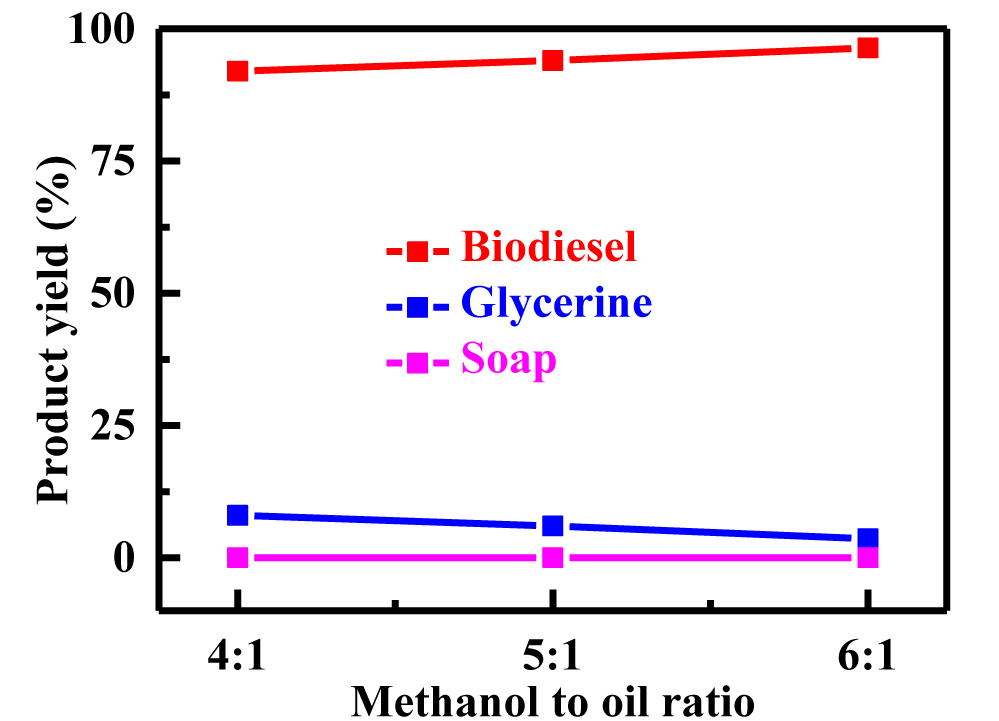
Biodiesel yield is impacted by the methanol/ oil ratio: The molar ratio of alcohol to triglycerides is one of the most crucial factors impacting the ester yield. For alkali catalyzed transesterification processes, a methanol: oil rate of 6:1 is recommended for maximizing conversion [25]. The methyl esters' acid, peroxide, saponification, and iodine levels are unaffected by the molar ratio. The variety of catalyst employed is related to the molar ratio. The feasible range of the methanol to oil ratio was 4:1-6:1 [26]. In table 1 and figure 2, the influence of the methanol to oil ratio on TPBD production is depicted. Maximum biodiesel output (96.4%) was attained at a molar ratio of 6:1.
| Table 1: Shows detail process of optimization. | |||||||||
| Amount of Oil Used (ML) | Molar Ratio of Oil to Alcohol | Temperature (°C) | Stirring Intensity (rpm) | Reaction Time (min) | KOH Conc. Used (g) | Methanol Used (ML) | Biodiesel (%) | Glycerol (%) | Soap (%) |
| 50 | 4:01 | 65 | 700 | 60 | 0.22 | 12.5 | 92 | 8 | 0 |
| 50 | 5:01 | 65 | 700 | 60 | 0.32 | 10.0 | 94 | 6 | 0 |
| 50 | 6:01 | 65 | 700 | 60 | 0.26 | 8.3 | 96.4 | 3.6 | 0 |
| 50 | 6:01 | 65 | 700 | 60 | 0.22 | 8.3 | 88 | 8 | 4 |
| 50 | 6:01 | 65 | 700 | 60 | 0.32 | 8.3 | 94.4 | 5.6 | 0 |
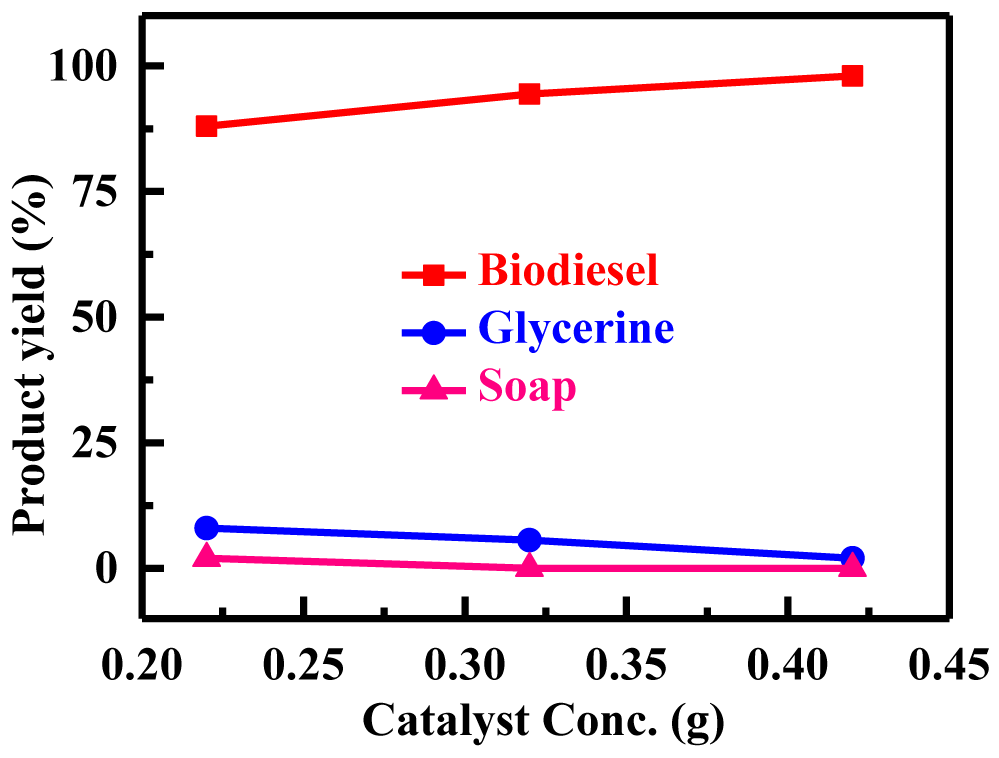
| 50 | 6:01 | 65 | 700 | 60 | 0.42 | 8.3 | 98 | 2 | 0 |
| 50 | 6:01 | 60 | 700 | 60 | 0.42 | 8.3 | 94.6 | 5.4 | 0 |
| 50 | 6:01 | 65 | 700 | 60 | 0.42 | 8.3 | 95.6 | 4.4 | 0 |
| 50 | 6:01 | 70 | 700 | 60 | 0.42 | 8.3 | 98.2 | 1.8 | 0 |
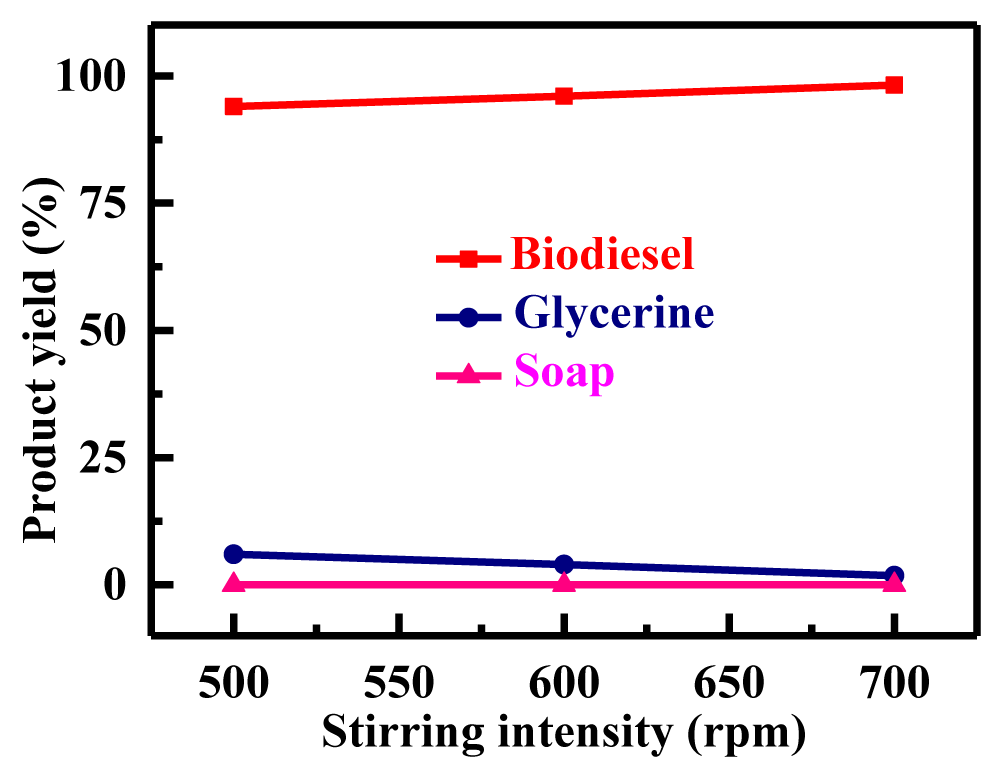
| 50 | 6:01 | 70 | 500 | 60 | 0.42 | 8.3 | 94 | 6 | 0 |
| 50 | 6:01 | 70 | 600 | 60 | 0.42 | 8.3 | 96 | 4 | 0 |
| 50 | 6:01 | 70 | 700 | 60 | 0.42 | 8.3 | 98.2 | 1.8 | 0 |
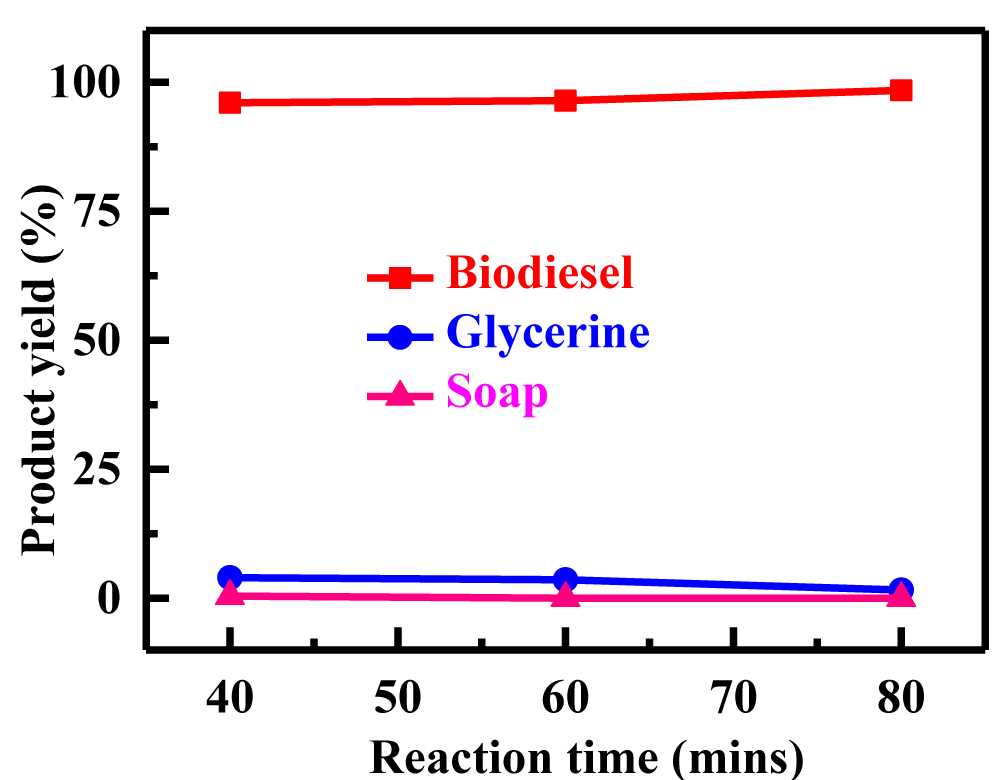
| 50 | 6:01 | 70 | 700 | 40 | 0.42 | 8.3 | 96 | 4 | 0 |
| 50 | 6:01 | 70 | 700 | 60 | 0.42 | 8.3 | 96.4 | 3.6 | 0.4 |
| 50 | 6:01 | 70 | 700 | 80 | 0.42 | 8.3 | 98.4 | 1.6 | 0 |
Influence of catalyst ratio on biodiesel yield: Alkalis, acids, enzymes, or heterogeneous catalysts can be employed to transesterify triglycerides. Alkali catalysts, such as potassium methoxide (KOMe), sodium methoxide (NaOMe), and alkali catalysts like KOH, are the most efficient of these [25]. By KOH as catalysts, the methanolysis of beef tallow was investigated. KOH was clearly superior to NaOH when compared to the two catalysts [27]. Mostly potassium hydroxide has been employed in alkali catalyzed methanolysis at concentrations ranging from 0.22%, 0.32, to 0.42% weight/weight of oil. Figure 3 depicts the reaction's three distinct catalyst concentrations used with 50 ml of oil: 0.22, 0.32, and 0.42 g KOH. By increasing the catalyst concentration up to 0.42 g, the synthesis of FAMEs was improved; however, as the KOH ratio rise beyond 0.42 g, the methyl esters' yield declined. Figure 3 shows that the yield achieved 98% at the optimum catalyst concentration of 0.42%. The yield of biodiesel was unchanged despite the additional catalyst. This was probably caused by the slurry thickening to an unmanageable degree, which made stirring more difficult and energy-intensive.
Impact of temperature and time on FAMEs: Reaction rate is greatly influenced by temperature. However, given enough time and ambient temperature, the reaction advances to almost completion [25]. We investigated the methanolysis of castor oil to methyl ricinoleate. Between 20 and 35°C, the reaction operated most efficiently. The reactions were generally conducted at atmospheric pressure at methanol's boiling point (60-70°C). A 6:1 methanol: oil ratio, 0.42% KOH, and a temperature of 60°C were used to study the transesterification of linseed, tung seed, safflower, and Rhus typhina oil. After 1 hour, the conversion rate for all four oils was nearly same (93-98%). Depending on the oil utilized, transesterification was performed at various temperatures (60, 65, and 70°C). At three different temperatures, 60, 65, and 70°C, the transesterification of Thevetia peruviana oil with methanol (6:1) and 0.42% KOH was studied. Figure 4 illustrates how the production of biodiesel increased as temperature raised up to 70°C. At 70°C, the maximum yield (98.2%) was attained. Ma F, et al. [12] and Lee I, et al. [29] looked into how reaction time affected the methanol-beaf tallow transesterification process. The first minute of the reaction was somewhat slow because of the mixing of methanol and beef tallow. The production of beef tallow methyl esters peaked after around 15 minutes. Currently, transesterification was performed utilizing various reaction times (40, 60 and 80 min). At 40 minutes, 96% yield was obtained, but as time passed, biodiesel yield increased, and at 60 minutes, 96.6% yield was obtained, but the maximum yield was obtained at 80 minutes (98.4 %), which is shown in figure 5 and table 1. These studies showed that, in the case of the alkali catalyst, transesterification proceeded satisfactorily at room temperature. Temperature was a determining factor in both the reaction rate and esters yield [30]. Higher reaction times bring the conversion closer to equilibrium rate.
Stirring rate's impact on biodiesel production: The potassium hydroxide methanol solution and oils or fats cannot mix, because of this stirring is critical for the transesterification reaction, as shown in figure 6. A slow pace is caused by insufficient diffusion between the phases. The reaction might become diffusion regulated. Stirring is no longer required once the two phases have been blended and the reaction has begun. Ma F, et al. [12] investigated the impact of mixing on beef tallow's transesterification. If there was no mixing, there was no reaction. The speed of the churning was quite slow when KOH-MeOH was introduced to the melted beef tallow in the reactor. This implied that the evaluated stirring rates were faster than the mixing threshold. The stirring intensity was varied between 500, 600, and 700 rpm. Starting with 500 rpm, the yield was only 94%; however, with 600 rpm, the yield increased to 96%. However, at 700 rpm resulted in the maximum output. Thevetia peruviana biodiesel required 700 rpm of stirring at its best.
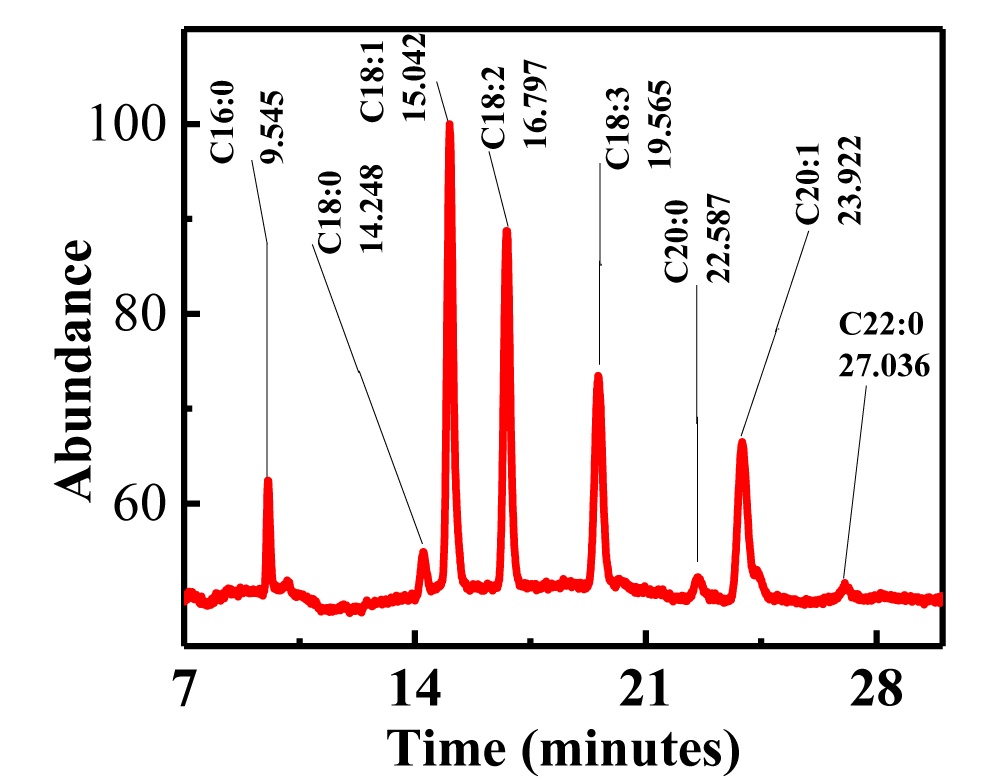
Thevetia peruviana FAMEs were sketched using GC-MS analysis
Due to its typically greater precision in quantifying small components, the most widely used method for analyzing biodiesel has been gas chromatography. To accurately determine the rate at which the synthetic Thevetia peruviana oil is converted into biodiesel, we also used gas chromatography and mass spectrometry techniques in this experiment. Figure 7 displays data pertaining to GC-MS spectra. In agreement with our earlier study [31], we have reported seven main peaks utilizing GC spectra. Using library match tools (NO. NIST 02), the peaks were each determined to be fatty acid methyl ester compounds. Table 2 displays the recognized FAMEs and their retention times. Fatty acid methyl esters were identified using retention time data, and this was later validated by mass spectrometric analysis. An EI ion source was used to obtain the mass spectrum.
| Table 2: Fatty acids detected in TPBD FAMEs. | ||||||
| S/ No | Fatty Acids | Retention Time | Number of Carbons and Double Bonds | Chemical Name | Chemical Structure | Molecular Weight |
| 1 | Palmitic acid | 9.545 | C16:0 | Hexadecanoic acid, methyl ester | 270 | |
| 2 | Stearic acid | 14.248 | C18:0 | methyl stearate | 298 | |
| 3 | Oleic acid | 15.042 | C18:1 | 9-Octadecenoic acidZ-, methyl Ester | 296 | |
| 4 | Linoleic acid | 16.797 | C18:2 | 9, 12-Octadecadienoic Acid (Z, Z)-, methyl ester | 294 | |
| 5 | α-Linolenic acid | 19.565 | C18:3 | α-Linolenic Acid | ||
| 6 | Arachidic acid | 22.587 | C20:0 | Eicosanoic Acid, methyl ester | 326 | |
| 7 | Gondoic acid | 23.922 | C20:1 | CiS- 11- Eicosenoic Acid, methyl ester | 324 | |
Elemental analysis by (ICP-OES & EA)
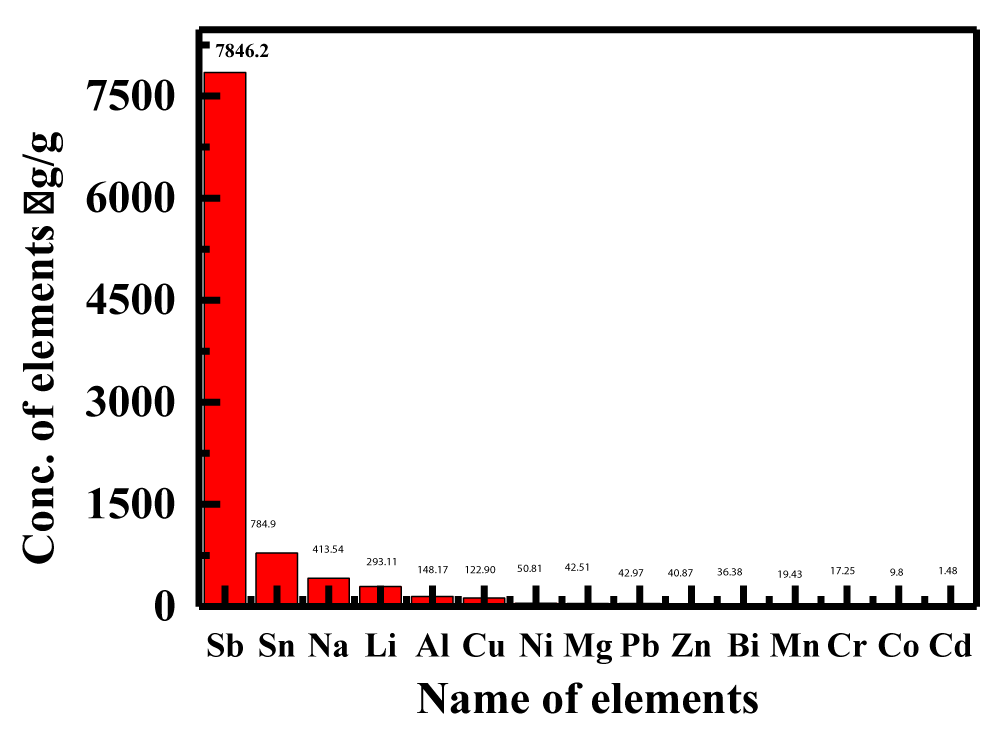
Elemental content in biodiesel is disagreeable because they contribute to a number of problems, including those that promote biodiesel breakdown, engine corrosion, operability issues, social issues like ecological contamination, and negative impacts on physical health [32]. Sodium and potassium, which are components of the appliance in the production of biodiesel, are the essentials whose quantity in biodiesel needs to be managed. The quantity of metal Phosphorus (P), which essentially comes from the raw materials, is also crucial. Thevetia peruviana Biodiesel (TPBD) elementals concentrations were matched with high-speed diesel (Petrodiesel). Results show that Thevetia peruviana Biodiesel (TPBD) has a lower concentration of numerous components than High Speed Diesel (HSD), which is shown in figure 8.
Essentials like potassium, sodium, magnesium, and calcium are present in biodiesel and serve as injector guides, drain stimulators, wear indicators for pistons and circles, indications for locomotive deposits, and filter plugging [33]. The levels of C, H, N, and O that were measured and C% was (76.25), H (13.01), N (1.41), and O (9.33%), while K, Na, Mg, and Ca were (6.14, 412.54, 42.51, and 14.90 g/g), which relative to HSD, were low (213.3, 868.3, and 45.6 g/g). Na, K, and P can all is included in biodiesel at a maximum allowable concentration of 5 mg kg-1 and 10 mgkg-1 each [34]. The raw materials needed to make biodiesel contain additional components like Ca, Mg, and P. Soap is created when sodium levels are too high. To lower the salt concentration below the standard amount, more washing will be necessary.
The physical and chemical characteristics
Using the listed biodiesel (ASTM-D6751) and petro-diesel values (ASTM-D6751), the synthetic TPBD's physical and fuel properties were evaluated using ASTM procedures. The results are shown in table 3 (ASTM-D975).
| Table 3: Shows TPSOBD (Thevetia peruviana seed oil biodiesel) physiochemical characterization. | |||||
| Studied Parameters | ASTM D6751-02 | EN 14214 | ASTM D6751 | Petrodiesel | Experimental Result |
| Density @ 15°C (g/cm3) | - | 860-900 | 0.86-0.90 | 809.1 | 0.887 |
| Kinematic Viscosity @ 40°C (mm2/s) | 1.9-6.0 | 3.5-5.0 | 1.9-6.0 | 1.3-4.1 | 3.9 |
| Flash Point, °C | 130.0 Min | 120.0 Min | 130 Min | 60-80 | 160 |
| Fire Point, °C | - | - | - | - | 190 |
| Acid Value, mg KOH/g oil | 0.80 Max | 0.50 Max | 0.5 Max | - | 0.54 |
| Saponification Value, mg KOH/g oil | - | - | - | - | 169.8 |
| Iodine Value, gI2/100 mg | - | Max. 120 | Max. 120 | - | 79.6 |
| Refractive index @ 20°C | - | - | - | - | 1.3502 |
| Cloud Point, °C | - | -3 to 12 | -3 to 12 | -15 to 5 | 4 |
| Pour Point, °C | - | -15 to 16 | -5 to 5 | -2.0 | 1 |
| Cetane number | 47 Min | 51 Min | Min. 47 | 49.7 | 48.5 |
| Acid number | - | - | - | - | 0.137 |
| HHV | - | - | - | - | 23.39 |
| Oxidation stability (110°C, h) | 3 Min | 6 Min | Min. 3 | 25.8 | 72.60 |
| Ash content | - | - | - | - | 0.03 |
| Specific gravity | - | - | - | - | 0.891 |
| Cold filter plug point (CFPP, °C) | – | Max.19 | Max.19 | -16 | -9 |
| Sulfated ash content (wt%) | 0.02 Max | 0.02 Max | - | - | 0.003 |
| Phosphorous (mg/kg) | - | 4 mg/kg Max | 0.001% mass Max | - | 0.105 ug/g |
| Sulfur (mg/kg) | - | 10 mg/kg Max | 0.0015% mass (ppm) Max | - | 2.073 ug/g |
At 40°C, TPBD's density was found to be 0.887 gcm-3, in comparison to petrodiesel (0.834 gcm-3) and within the biodiesel ASTM D6751 restrictions (Table 3). The current investigation determined that the specific gravity of TPBD was equivalent to the ASTM values for petro-diesel and biodiesel (Table 3).
Viscosity, which impacts the fuel's fluidity and how fuel injection equipment operates, particularly at low temperatures, is the most crucial characteristic of biodiesel. The determined viscosity of TPBD was 3.9 mm2/s, which is close to the ASTM D6751 value for biodiesel and the ASTM values for petro-diesel (Table 3). The measured TPBD kinematic viscosity (3.9 mm2/s) at 40°C is within the range of biodiesel and petroleum-based diesel ASTM values.
In contrast to the cloud point (cp), which is the temperature at which wax first becomes visible after the fuel has cooled, the pour point (pp) is the temperature at which there is enough wax out of solution to acquire the fuel. As a result, the pour point is the lowest temperature at which fuel can flow. Testing revealed that Thevetia peruviana Seed Oil Biodiesel (TPSOBD) pp and cp values are 1 and 4°C, respectively, and are within the ASTM requirements for diesel fuels (Table 3).
When managing, storing, and maintaining the safety of fuels and flammable materials, the flash point is a consideration. Even though it is higher than petro-diesel, the claimed flash point of TPBD (160°C) is within ASTM limits. This illustrates how safer TPBD fuel is compared to petroleum fuel. A higher flash point may, however, result in less combustibility when compared to petro-diesel.
The biodiesel's level of free fatty acids is indicated by the acid number. If it contains more than 0.50 mg KOH per gram, corrosion to engines is possible. A lot less than the ASTM standards for diesel fuels, 0.137 mg KOH g-1 was found to be the acid number of TPBD (Table 1). Sulfur levels in the TPBD were found to be 2.037 g/g, which is within the permitted limits for diesels (Table 3).
NMR spectroscopy
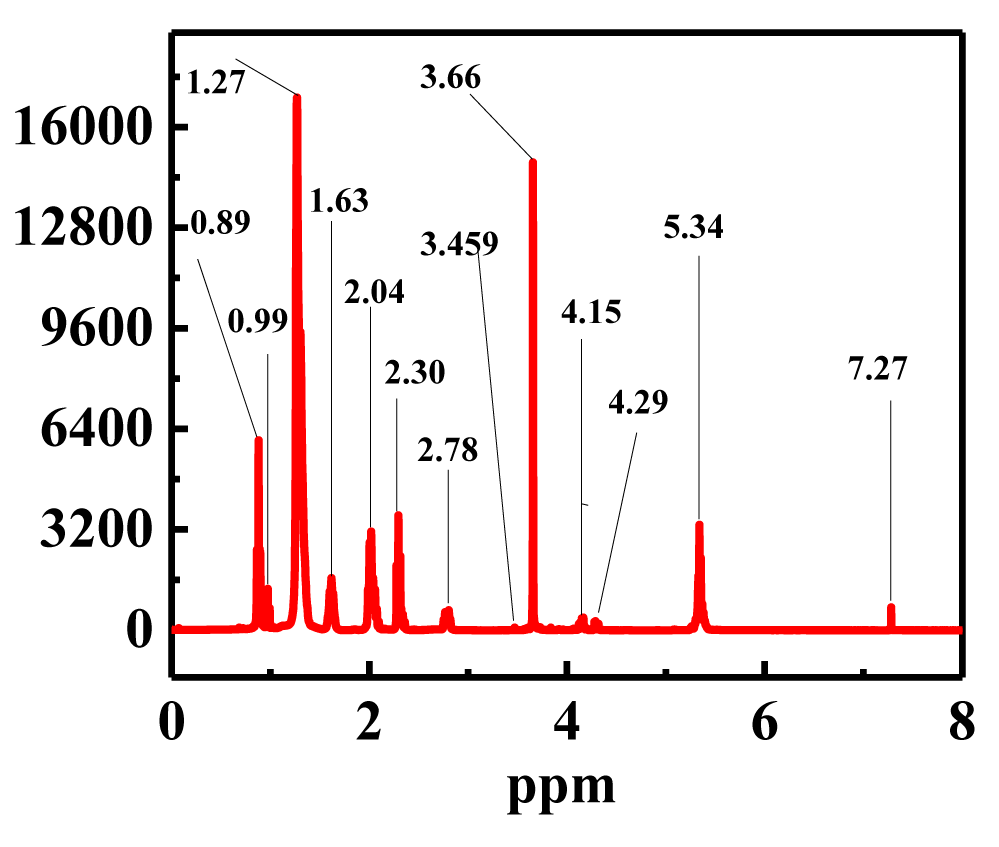
1H NMR analysis: The transesterification process is demonstrated to go through its various spectrums using the 1H NMR method to calculate biodiesel yield. By measuring the protons in the alcohol moiety of the resulting methyl esters and the protons in the methylene group next to the ester moiety in TAG, the yield was determined by 1H NMR. The 1H NMR spectroscopy used to assess the Thevetia peruviana oil-based biodiesel is shown in figure 9. α-CH2 protons were found in a triplet at 2.30 ppm, and methoxy protons were discovered in a singlet at 3.66 ppm. These two separate peaks demonstrate that methyl esters are present in biodiesel. Additionally, there were peaks for the terminal methyl protons at 0.89 ppm and a robust signal for the carbon chain-containing methylene protons at 1.27 ppm. Additional signals were observed for olefinic hydrogen and β-carbonyl methylene protons at concentrations of 1.63 ppm and 5.34 ppm, respectively. Using a variety of calibration curves based on 1H NMR spectroscopy, several studies have previously assessed the reaction yield during the transesterification of fatty acid mixtures and methanol to produce biodiesel [35-38]. The amount of the transesterification reaction's triglyceride that was converted to methyl esters can be deduced from the 1H NMR spectra of the biodiesel produced from Thevetia peruviana oil, which are consistent with earlier studies [39,40]. Thevetia peruviana oil is used to make biodiesel, and this study's revelation that the methyl esters in that biodiesel exhibit unique 1H NMR spectrum from one another allowed for a new investigation of the biodiesel spectra. The methoxy group in the methyl esters, at 3.66 ppm, and the α-carbonyl methylene protons, at 2.30 ppm, were the pertinent signals for integration.
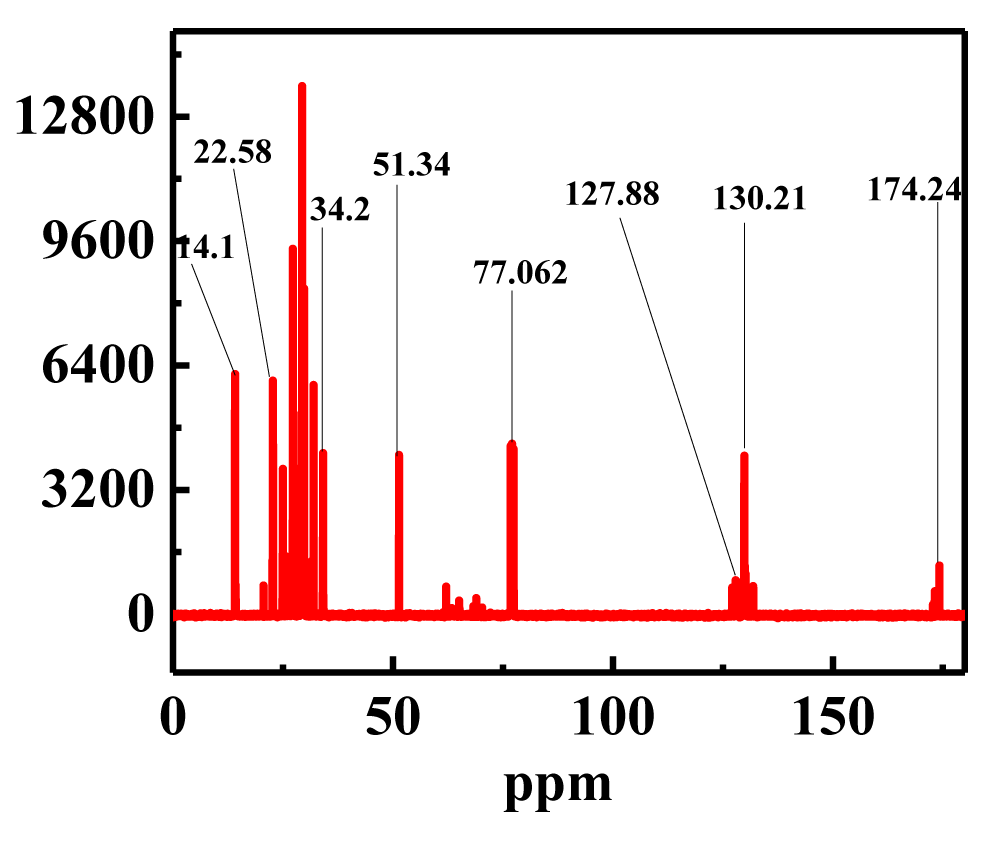
13C NMR analysis: The typical ester carbonyl peaks of the 13C NMR spectra are shown at 174.24 and 51.34 ppm, respectively. Figure 10 depicts the 13C NMR spectra. Peaks at 130.21 and 127.88 ppm showed that methyl esters were unsaturated. Additional peaks are connected to the terminal carbon of methyl groups and the long-chain methylene carbon, respectively, around 14.1 ppm and between 22.5 and 34.2 ppm. Our findings are supported by the fact that these peaks were closely related to previous studies [41].
FT-IR spectroscopy
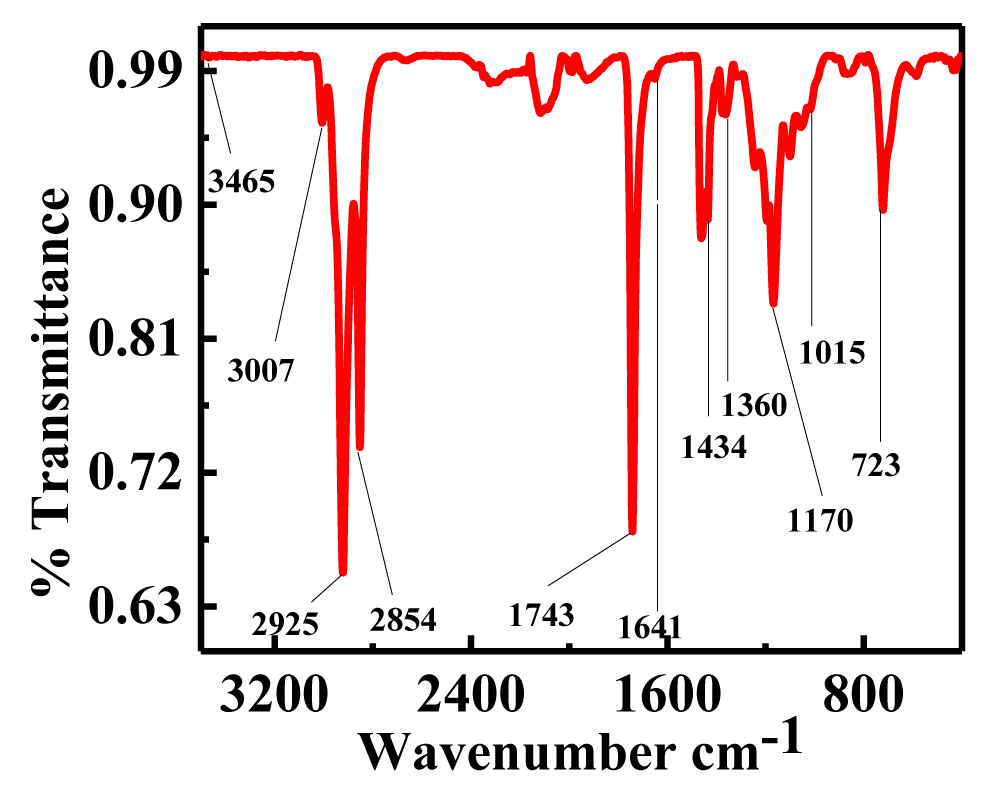
The functional groups and bands in the samples of Thevetia peruviana oil biodiesel were located using the mid-infrared area of the FT-IR spectrum, which correlates to various stretching and bending vibrations. The position of the carbonyl group in FT-IR is determined by the molecule's structure and the effect of substituents [42]. Esters can be identified by two distinct, powerful absorption bands from carbonyl (ʋC = O) at about 1750-1730 cm-1 and from C-O (anti symmetric axial stretching and asymmetric axial stretching) at about 1300-1000 cm-1. While the bending vibrations of these groups may be found at 1475-1350, 1350-1150, and 723 cm-1 (ρCH2), respectively, the stretching vibrations of CH3, CH2, and CH emerge at 2980-2950, 2950-2850, and 3050-3000 cm-1, respectively [43]. In the biodiesel created from Thevetia peruviana oil, figure 11 demonstrates that the methoxy ester carbonyl group seems to be present at a wavelength of 1743 cm-1. The overtone of the ester functional group was visible in the band, which was detectable at 3465 cm-1 [44]. The vC-C( = O)-O and O-C-C vibrations were two asymmetrically coupled vibrations that were present in the C-O stretching vibration at 1170 cm-1 and 1016 cm-1, respectively. At 3007, 2925, and 2854 cm-1, respectively, the methylene and methane stretching bands were seen. The bending vibrations of methyl groups were seen at 1434 and 1360 cm-1, while methylene's vibrations were seen at 723 cm-1. The biodiesel from the Thevetia peruviana plant displayed C = C unsaturation in the band at 1641 cm-1. The use of methods likes chromatography and spectroscopy to evaluate the quality, production, and monitoring of biodiesel is widely known. The GC-MS, infrared spectroscopy, and 1H & 13C NMR techniques are the most effective, user-friendly, and quick ways to identify FAMEs, their properties, the presence of functional groups, and their distinctive structures. For accurately understanding biodiesel data, it is therefore appropriate and trustworthy to utilize.
Conclusion
The seeds of the Milk Bush (Thevetia peruviana) were found to contain 48-62% oil after extraction. Due to the fatty acid makeup of TPO, this seed oil can be used as a feedstock for the production of biodiesel. The transesterification reaction involved methanol and Potassium hydroxide (KOH) as a catalyst. The process input variables, including the methanol to oil molar ratio, reaction time (min), catalyst loading, reaction temperature, and stirring intensity, were tuned to provide the highest FAMEs yield. After optimizing the input variables, which included a catalyst loading of 0.42 weight percent, a methanol to oil molar ratio of 6:1, a reaction time of 80 minutes, a temperature of 70°C, and a stirring intensity of 700 rpm, the rate of biodiesel generation was reached at 98.4%. When the physical and chemical characteristics of the biodiesel produced from TPBD were evaluated, it was discovered to meet all ASTM and EN criteria, proving that this seed oil can be used as a feedstock for the production of biodiesel. Thevetia peruviana seed oil has been identified by the exploration as a very promising feedstock for the production of biodiesel.
Acknowledgment
Funding
This work was supported by National Natural Science Foundation of China under grant no. (51974182), Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning under grant No. (TP2015039), National 111 Project (The Program of Introducing Talents of Discipline to University), Grant Award Number: D17002, China Baowu Low Carbon Metallurgy Innovation Foudation-BWLCF202112 and Independent Research Project of State Key Laboratory of Advanced Special Steel, Shanghai Key Laboratory of advanced Ferrometallurgy, Shanghai University (SKLASS 2022-Z01) and the Science and Technology Commission of Shanghai Municipality, under grant No.(19DZ2270200).
Authors’ contributions
Inam Ullah Khan present the idea, carried out the experiments and wrote the first draft of the manuscript. Yaowei Yu revised the data and manuscript carefully.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
This is to certify that study has been undertaken by keeping in preview and adherence to research ethics.
References
- Ozcanli MA, Keskin A, Aydın K. Biodiesel production from terebinth (Pistacia terebinthus) oil and its usage in diesel engine. International Journal of Green Energy. 2011a;8:518-528. doi: 10.1080/15435075.2011.588766.
- Khan IU, Haleem A, Khan AU. Non-edible plant seeds of Acacia farnesiana as a new and effective source for biofuel production. RSC Adv. 2022 Aug 2;12(33):21223-21234. doi: 10.1039/d2ra03406a. PMID: 35975044; PMCID: PMC9345019.
- Saribiyik OY, Ozcanli M, Serin H, Serin S, Aydin K. Biodiesel production from Ricinus communis oil and its blends with soybean biodiesel. Strojniški vestnik-Journal of Mechanical Engineering. 2010;56:811-816.
- Altun S. Fuel properties of biodiesels produced from different feedstocks. Energy Education Science and Technology Part A. 2011;26:165-174.
- Khan IU, Yan Z, Chen J. Optimization, transesterification and analytical study of Rhus typhina non-edible seed oil as biodiesel production. Energies. 2019;12(22):1-21. doi: 10.3390/en12224290.
- Ozcanli M, Keskin A, Serin H, Yamacli S, Ustun D. Effects of soybean biodiesel on engine vibration and noise emission. Energy Education Science and Technology Part A: Energy Science and Research. 2012;28(2):949-956.
- Benjumea P, Agudelo J, Agudelo A. Basic properties of palm oil biodiesel-diesel blends. Fuel. 2008;87:2069-2075. doi: 10.1016/j.fuel.2007.11.004.
- Khan IU, Shah SAH. Optimization and characterization of novel & non-edible seed oil sources for biodiesel production. IntechOpen. 2021;39-56. doi: 10.5772/intechopen.97496.
- Alptekin E, Canakci M. Determination of the density and the viscosities of biodiesel-diesel fuel blends. Renewable Energy. 2008;33:2623-2630. doi: 10.1016/j.renene.2008.02.020.
- Jeong GT, Yang HS, Park DH. Optimization of transesterification of animal fat ester using response surface methodology. Bioresource Technology. 2009;100:25-30. doi: 10.1016/j.biortech.2008.05.011.
- Khan IU, Yan Z, Chen J. Production and characterization of biodiesel derived from a novel source Koelreuteria paniculata seed oil. Energies. 2020;13:791. doi: 10.3390/en13040791.
- Ma F, Clements LD, Hanna MA. The effects of catalyst, fatty acids and water on transesterification of beef tallow. Transactions of the American Society of Agricultural Engineers. 1998;41:1261-1264.
- Khan IU, Chen H, Yan Z, Chen J. Extraction and quality evaluation of biodiesel from six familiar non-edible plants seeds sources. Processes. 2021;9:840. doi: 10.3390/pr9050840.
- Ahmad M, Teong LK, Sultana S, Khan IU, Zuhairi AA, Zafar M, Hassan F. Optimization of biodiesel production from Carthamus tinctorius L. Cv. Thori 78: A novel cultivar of safflower crop. Int J Green Energy. 2015;5:447-452.
- Saydut A, Duz MZ, Kaya C, Kafadar AB, Hamamci C. Transesterified sesame (Sesamum indicum L.) seed oil as a biodiesel fuel. Bioresour Technol. 2008 Sep;99(14):6656-60. doi: 10.1016/j.biortech.2007.11.063. Epub 2008 Feb 21. PMID: 18178427.
- Khan IU, Ahmad M, Khan AU. Chemistry and elemental analysis of Carthamus tincturoius Cv. Thori-78. J Am Acad Res. 2015;3(5):81-90.
- Kaygusuz K. Biomass as a renewable energy source for sustainable fuels. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2009;31:535-545. doi: 10.1080/15567030701715989.
- Marinkovic SS, Tomasevic A. Transesterification of sunflower oil in situ. Fuel. 1998;77:1389-1391. doi: 10.1016/S0016-2361(98)00028-3.
- Canakci M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour Technol. 2007 Jan;98(1):183-90. doi: 10.1016/j.biortech.2005.11.022. Epub 2006 Jan 18. PMID: 16412631.
- Oluwaniyi OO, Ibiyemi SA. Extractability of Thevetia peruviana glycosides with alcohol mixture. Afr J Biotechnol. 2007;6:2166-2170. doi: 10.5897/AJB2007.000-2339.
- Liu XJ, He HY, Wang YJ, Zhu SL, Piao XL. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel. 2010;87:216-221.
- Khan IU, Haleem A. A seed of Albizzia julibrissin wild plant as an efficient source for biodiesel production. Biomass Bioenerg. 2022;158:106381. doi: 10.1016/j.biombioe.2022.106381.
- Van Gerpen J. Biodiesel processing and production. Fuel Process Technol. 2005;86(10):1097-1107. doi: 10.1016/j.fuproc.2004.11.005.
- Khan IU, Yan Z, Chen J, Chen H, Shah SAH. Acacia farnesiana seed oil: A promising source of biodiesel production. Int J Sci Eng Res. 2021;12(1):277-295.
- Pramanik K. Properties and use of Jatropha curcas oil and diesel fuel blends incompression ignition engine. Renewable Energy. 2003;28:239-248. doi: 10.1016/S0960-1481(02)00027-7.
- Shay EG. Diesel fuel from vegetable oils: Status and opportunities. Biomass and Bioenergy. 1993;4:227-242.
- Hossain AK, Davies PA. Plant oils as fuels for compression ignition engines: A technical review and life-cycle analysis. Renewable Energy. 2010;35:1-13.
- Freedman B, Pryde EH, Mounts TL. Variables affecting the yields of fatty esters from transesterified vegetable oils. Journal of the American Oil Chemists’ Society. 1984;61(10):1638-1643.
- Lee I, Johnson LA, Hammond EG. Use of branched-chain esters to reduce the crystallization temperature of biodiesel. Journal of the American Oil Chemists’ Society. 1995;72:1155-1160.
- Ramadhas AS, Jayaraj S, Muraleedharan C. Use of vegetable oils as I.C. engine fuels: A review. Renewable Energy. 2004;29:727-742.
- Ahmad M, Ullah K, Khan MA, Zafar M, Tariq M, Ali S, Sultana S. Physico-chemical analysis of hemp oil biodiesel: A promising nonedible new source for bioenergy. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2011;33:1365-1374. doi: 10.1080/15567036.2010.499420.
- Schober S, Mittelbach M. Influence of diesel particulate filter additives on biodiesel quality. Eur J Lipid Sci Technol. 2005;107:268-271.
- McCormick RL, Alleman TL, Ratcliff M, Moens L, Lawrence R. Survey of the quality and stability of biodiesel and biodiesel blends in the United States in 2004, Technical report of National Renewable Energy Laboratory. USA. 2005.
- Betha R, Balasubramanian R. Emissions of particulate-bound elements from biodiesel and ultra low sulfur diesel: Size distribution and risk assessment. Chemosphere. 2013;90:1005-1015. doi: 10.1016/j.chemosphere.2012.07.052.
- Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, Wang J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energy. 2010;87:2589-2596.
- Mello VM, Oliveira FCC, Fraga GW, Nascimento CJD, Suarez PAZ. Determination of the content of Fatty Acid Methyl Esters (FAMEs) in biodiesel samples using 1H NMR spectroscopy. Magn Reson Chem. 2008;46:1051-1054.
- Monterio MR, Ambrozin ARP, Liao LM, Ferreira AG. Determination of biodiesel blends levels in different diesel samples by 1H NMR. Fuel. 2009;88:691-696.
- Samios D, Pedrotti F, Nicolau A, Martini DD, Dalcen FM. A transesterification double process-TDSP for biodiesel preparation from fatty acid tryglycerides. Fuel Process. 2009;90:599-605.
- Liu C, Lv P, Yuan Z, Yan F, Luo W. Biodiesel from different oil using fixed-bed and flow reactors. Renew Energy. 2010;35:1531-1536.
- Gelbard G, Bres O, Vargas RM, Vielfaure F, Schuchardt UF. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. Am Oil Chem Soc. 1995;72:1239-1241. doi: 10.1007/BF02540998.
- Knothe G. Monitoring a progressing transesterification reaction by fiber optic NIR spectroscopy with correlation to 1H NMR spectroscopy. Am Oil Chem Soc. 2000;77:489-493. doi: 10.1007/s11746-000-0078-5.
- Pasto D, Johnson C, Miller M. Experiments and Techniques in Organic Chemistry. 1st ed. New Jersey, Prentice Hall: Upper Saddle River; 1992.
- Guillen MD, Cabo N. Infra-red spectroscopy in the study of edible oils and fats. Sci Food Agric. 1997;75:1-11.
- Safar M, Bertrand D, Robert P, Devaux MD, Genut C. Characterization of edible oil, butter and margarines by Fourier Transfer Infra-Red spectroscopy with attenuated total reflectance. Am Chem Soc. 1994;71:371-377. doi: 10.1007/BF02540516.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.