Medicine Group . 2022 December 12;3(12):1478-1494. doi: 10.37871/jbres1622.
Regulation of Amino Acid Metabolism in Hematological Malignancies: Advances from Transcriptomics and Metabolomics
Donald Bajia1*, Gael Noe1 Neh Neba Ambe2 and Katarzyna Derwich1
2Department of Pharmacy, Faculty of Health and Life Sciences, De Montfort University, Gateway house, LE1 9BH, Leicester, United Kingdom
- Amino acid metabolism
- Metabolites
- Transcriptomics
- Metabolomics Hematological malignancies
Abstract
Tumor cells use amino acids to rewire metabolic pathways to meet increased demands for energy, reducing equivalents, and cellular biosynthesis. Aside acting as building blocks for protein synthesis, amino acids also function as metabolic intermediates for ATP generation and redox homeostasis, as well as fueling biosynthetic pathways. Tumor-related metabolic changes influence every stage of the interaction between cells and their metabolites. Over the years, advancements in molecular methods such as transcriptomics and metabolomics have emerged to provide in-depth knowledge into the functions, interactions, and actions of molecules in cells of organisms. These technologies surfaced as methods that provide a more complete picture of disease pathophysiology, facilitating the elucidation of disease mechanisms and identification of potential biomarkers (metabolites) and targets (genes) respectively. Though Omics in cancer research have been explored in different concepts, however, employing these methods in amino acid metabolism in hematological cancers still requires attention. Therefore, this mini review discusses an up-to-date knowledge of principal regulators and their role in amino acid metabolism in hematological malignancies. In that perspective, we cover relevant findings from transcriptomics and metabolomics, thereby constructing mechanistic insights associated with disease pathogenesis.
Abbreviations
2-HG: 2-hydroxyglutarate; AA: Amino Acid; AAT: Aspartate Aminotransferase; AC220: Quizartinib/ FLT3 Inhibitor; ALDH: Aldehyde Dehydrogenase; ALDH1A2: Aldehyde Dehydrogenase 1 Family Member A2; ALL: Acute Lymphoblastic Leukemic; ALT: Alanine Transferase; AML: Acute Myeloid/Myelogenous Leukemia; ASNS: Asparagine Synthetase; ASS1: Arginosuccinate Synthase; AST/GOT: Aspartate Transferases; ATF4: Activating Transcription Factor 4; ATP: Adenosine Triphosphate; BCAA: Branched Chain Amino Acids; BCAT1: Branched Chain Amino Acid Transaminase 1; BC-CML: Blast Crisis Phase-CML; BCKA Branched Chain α-keto Acids; BM: Bone Marrow; CML: Chronic Myeloid Leukemia; CpG: Cysteine-p-Glycine Oligonucleotides; CPS2: Carbamoyl Phosphate Synthetase II; EZH2: Enhancer of Zeste Homolog 2; FLT3: FMS-Like Tyrosine Kinase 3; FLT3-ITD: FLT3 Internal Tandem Duplication; GAC: Glutaminase A; GBC: Glutaminase B; GCN2: General control non-derepressible 2; GLDC: Glycine dehydrogenase; Gln: Glutamine; GLS1: Glutaminase 1; GSH: Glutathione; HSCs; Hematopoietic Stem Cells; IDH1 and IDH2: Isocitrate Dehydrogenase Isoforms 1 And 2; ITD: Internal Tandem Duplication; LC-HR-MS/MS: Liquid Chromatography--High Resolution Tandem Mass Spectrometry; L-DON: 6-diazo-5-oxo-L-norleucine; LSC: Leukemia Stem Cells; MDSCs: Myeloid-Derived Suppressor Cells; MRIAN: Metabolic Reprogrammed L-Phenylalanine Polymer; MRIAN-Dox: Metabolic Reprogramming Immunosurveillance Activation Nanomedicine assembled Doxorubicin; MS: Mass Spectrometry; NEAA: Non-Essential Amino Acids; NFE2L2: Nuclear Factor Erythroid 2-Related Factor 2; NMR: Nuclear Magnetic Resonance; Omics Transcriptomics/Metabolomics or Proteomics/ Genomics; OXPHOS: Oxidative Phosphorylation; P5C: D1-pyrroline-5-carboxylate; PPAT: Amidophosphoribosyltransferase; PSAT: Phosphohydroxythreonine Aminotransferase; PYCR1: Pyrroline-5-carboxylate Reductase; ROS: Reactive Oxygen Species; RTKs: Receptor Tyrosine Kinases; SLC: Solute Carrier; SLC1A5/ Slc38A1: Sodium Dependent Neutral Amino Acid Transporters; TAL1: T-Cell Acute Leukemia Protein 1; T-ALL: T-Cell Acute Lymphoblastic Leukemia; TCA: Tricarboxylic Acid Cycle; TKI: Tyrosine Kinase Inhibitor; ULK1: Unc-51 Like Autophagy Activating Kinase; xCT: Cystine/glutamate antiporter; α-KG: α-ketoglutarate
Introduction
The phrase "hematological malignancies" refers to a class of diseases that affect the blood, bone marrow, and organs and have a wide variety of prognoses and post-treatment relapse rates. Given its high prevalence (7 percent of all newly diagnosed cancers), leukemia remains one of the largest group of hematological malignancies that start in blood-forming cells in the bone marrow resulting in cumulation of abnormal blood cells in tissues [1].
Metabolism, the process by which organisms obtain the energy required to power their cellular functions as well as the building blocks needed to make new cells, has become a focal point for understanding disease pathogenesis and finding treatments. The malignant characteristics of cancer cells, including rapid proliferation and aggressive invasion into normal tissues, differ from normal non-proliferating cells and requires altered metabolism to meet increased nutritional and biosynthetic demands [2,3]. The primary carbon source for metabolism is provided by amino acids, which are also significant building blocks required for cellular functions. They are vital for the production of intermediate metabolites that power biosynthetic and bioenergetic pathways and have been conventionally categorized as either essential or nonessential [4].
Recent studies focused on deciphering mechanisms of chemo-resistance caused by abnormal Amino Acids (AA) metabolism [5-8]. To considerably inhibit hematological malignancies, therapies that target tumor AA metabolism are crucial for overcoming drug resistance and boosting the effectiveness of diagnosis and treatment [9,10]. Tumorigenesis-related metabolic changes have been shown to influence every stage of the interaction between cells and their metabolites, particularly how nutrients are acquired and preferentially assigned to metabolic pathways in order to support cellular tumorigenic properties. Studies on the metabolism of cancer cells have enhanced our understanding of tumor-associated metabolic changes influenced by genetic or epigenetic cues as well as components of tumor microenvironment [11-15].
As high-throughput technologies advance, a flood of data is generated in order to address clinical and translational research questions, necessitating the employment of bioinformatic and computational tools to organize, interpret, and analyse the data [16]. Metabolomics data is increasingly being integrated with other omics data, such as gene expression data, to fully utilize and improve the interpretability of metabolomic profiles [17]. More specifically, integrating metabolomics with transcriptomics aids in elucidating disease mechanisms as well as identifying potential biomarkers (metabolites) and targets (genes) [18].
The use of several omics platforms in cancer research has also been explored in a number of studies and provides a broad overview of the state of the field today [19], but the outcomes and relevance from employing these methods in amino acid metabolism in hematological cancers is poorly addressed. Thus, in this mini review, we summarize an up-to-date knowledge of key regulators and their role in amino acid metabolism in hematological malignancies. In that perspective, we cover the most relevant findings from transcriptomics and metabolomics, thereby constructing mechanistic insights related to disease pathogenesis.
Principal regulators and their role in AA metabolism associated with disease pathogenesis
TAL1 and ALDH1A2 deletion in T-cell acute lymphoblastic leukemia: According to gene expression and mutational patterns, T-ALL patients can be divided into distinct subgroups based on the expression of numerous transcription factors TAL1 [20-22]. TAL1-positive leukemia is the most prevalent subtype, representing majority of all primary cases [21,23-27]. ALDH1A2 [28,29] a member of the Aldehyde Dehydrogenase (ALDH) family of genes that encode oxidoreductases, is one of the recognised targets of TAL1 in ALL. ALDH detoxifies endogenous aldehydes generated by the metabolism of amino acids and other biomolecules [30].
TAL1 directly activates ALDH1A2, which guards against intracellular stress and promotes leukemia cell metabolism and survival by inhibiting apoptosis [31]. Global gene expression profiling by RNA-sequencing after ALDH1A2 deletion showed that several enzymes involved in amino acid metabolism, including ASS1 and ASNS, were increased following ALDH1A2 deletion compared to control samples, while a number of metabolic enzymes and transporters involved in the glycolysis pathway were down regulated [31].
After ALDH1A2 was deleted in the human T-lymphocyte Jurkat cell line, the relative amounts of metabolites implicated in the main metabolomics pathways were assessed using a capillary electrophoresis time-of-flight MS technique. Unexpectedly, acetyl-CoA, one of the major carbon sources that power the TCA cycle, as well as TCA subcomponents like citric acid, cis-aconitic acid, and isocitric acid, were reduced by ALDH1A2 loss [31]. It is important to note that the TCA cycle's levels of 2-oxoglutarate-derived metabolites remained unaltered. This suggests that the TCA cycle, which is reduced by the lack of ALDH1A2, may be augmented by glutaminolysis, which transforms exogenous glutamine into 2-oxoglutarate. The predominant carbon source for T-ALL cells with activated NOTCH1 is known to be glutamine [32,33]. In fact, after 24 hours of doxycycline treatment, glutamine depletion in culture induced apoptosis, which was amplified by ALDH1A2 loss. When exogenous glutamine was present, ALDH1A2 deletion alone did not cause apoptosis to occur until 48 hours after induction. In light of these findings, it is possible that exogenous glutamine may be used to replace downstream metabolites and maintain the TCA cycle in the absence of this gene transcript, and that ALDH1A2 and glutaminolysis pathways could work in concert to make up for one another [31].
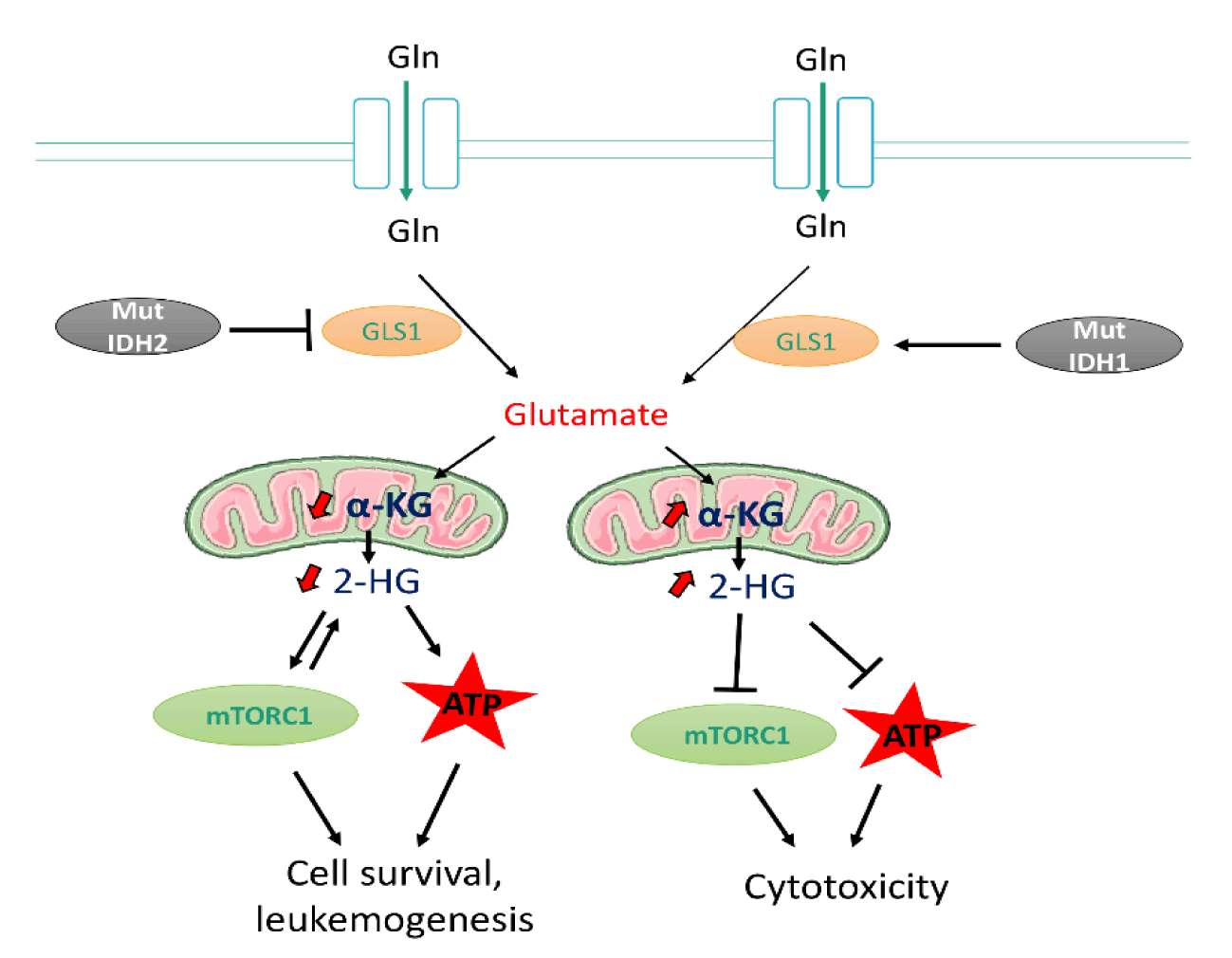
IDH1/IDH2 in leukemic transformation: Acute myeloid leukemia is most frequently associated with mutations in the Homologous Isocitrate Dehydrogenase Isoforms 1 And 2 (IDH1/IDH2) [34]. IDH1 and IDH2, localised in the cytoplasm and mitochondria respectively, are involved in a variety of metabolic processes in cells, such as Redox regulation, biosynthesis, and bioenergetics [34-37]. Active IDH enzymes take part in citrate metabolism by converting isocitrate to α-ketoglutarate (α-KG) and producing NADPH by reducing the cofactor NADP+ [34]. α-KG plays a vital role in metabolism a key intermediate in the TCA cycle and glutaminolysis [34].
Using Nuclear Magnetic Resonance (NMR) to access metabolic profile of the intracellular metabolite, 2-HG on mutant IDH1 and IDH2-expressing human primary leukemia cells from AML patients [38]. The Class III Receptor Tyrosine Kinase (FLT3) inhibitor quizartinib was observed to reduced 2-HG metabolite levels in mutant IDH1 cells. Thus, suggesting mutant IDH1 might be involved in activating Glutaminase 1 (GLS1) activity in the absence of FLT3 inhibitor. Treating mutant IDH2 with quizartinib, on the other hand, resulted in increased intracellular 2-HG levels [38], implying that mutant IDH2 may play a role in inhibiting GLS1 in the absence of the inhibitor.
Transcriptome-RNA-sequencing revealed elevated 2-HG induces cytotoxicity through regulation of several pathways including upregulation of apoptotic and P53 pathway, and downregulation of MYC pathway, mTOR affecting ATP synthesis [38,39]. In addition, studies have shown that accumulation of oncometabolites including 2-HG is associated with elevated glutamine metabolism hence supporting cancer transformation [40,41] (Figure 1).
Loss of PRMT7 cooperates with specific mediators to alter AA metabolism in CML: To investigate the role of the protein arginine methyltransferase PRMT7 in CML mice [42], RNAseq of Leukemia Stem Cells (LSCs) isolated from the bone marrow Prmt7-Knockout CML mice revealed that several genes were differentially regulated. Because PRMT7 has oncogenic epigenetic regulation activity, the authors focused their attention on the genes that were downregulated in Prmt7-KO cells. Glycine Decarboxylase (GLDC) was one of the top ten down regulated genes, as confirmed by qRT-PCR analysis [42].
Additionally, functional enrichment analysis of the deregulated transcripts showed that Prmt7-deficient cells had a high enrichment of genes associated to glycine and serine metabolism. Because the proliferation of tumor-initiating cells and cancer depend on glycine decarboxylase, a rate-limiting enzyme in the network that controls glycine and serine metabolism [43], Liu’s [42] group investigated this enzyme as a possible downstream target of Prmt7 in CML leukemia stem cells. In that perspective, they hypothesized that Prmt7 absence-mediated GLDC repression could be mediated by a downstream repressive transcription factor. As a result, RNA-seq results revealed GATA binding 1 (Trps1, a transcriptional repressor) among the upregulated genes in Prmt7-deleted cells.
According to Liu, et al. [42] the reduction in GLDC brought on by PRMT7-loss may allow the LSCs' glycine metabolism to be reprogrammed creating a hazardous metabolite, methylglyoxal. The outcome of glycine metabolism is determined by the balance of toxic product generation and clearance [44]. In terms of expulsion, glycine can be either converted by the decarboxylase into the nontoxic product 5,10-methylene-tetrahydrofolate (5,10-MTHF) or into toxic methylglyoxal by glycine C-acetyltransferase [44.45]. Zhang, et al. [43] suggested elevated glycine levels may be caused by excessive glycine generation from serine, mediated by Serine Hydroxymethyltransferase 2 (SHMT2). Furthermore, this transferase was found to be highly expressed in human CML-CD34 positive cells compared to normal cells and Prmt7 deletion had no effect on Shmt2 expression in leukemia mice. Most amino acids, including glycine and serine, have been reported to be higher in leukemia cells from newly diagnosed CML patients than in healthy individuals [46]. Following a time course assessment, High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) analysis showed that the intracellular contents of glycine and serine progressively increased as CML disease progressed. These findings demonstrate a potential link between altered glycine metabolism and the development of CML illness using transcriptomic and metabolomic methods [42].
FLT3ITD and glutamine metabolism in Leukemia: FLT3 Internal Tandem Duplication (FLT3-ITD) mutations are common in AML and probably correlate with poor prognosis. Albeit current FLT3 Tyrosine Kinase Inhibitors (TKI) has been quite promising in targeting this mutation, the consequences of this mutation in AML patients necessitate the identification of novel, specific and more effective therapeutic targets especially for the highly hostile AML subtype [47].
Combining metabolomics and gene expression analysis, studies have shown that by supporting both mitochondrial function and cellular redox function, glutamine metabolism becomes a metabolic dependence of FLT3ITD-leukemia, which is specifically revealed by treatment with FLT3 inhibitors.
Gallipoli, et al. [47] performed LC-MS analysis after incubation with stable isotope-labeled glutamine to understand the fate of glutamine metabolism in FLT3ITD cells after treatment with AC220 (FLT3 inhibitor). Intracellular levels of labeled glutamine were increased in treated cells, confirming that glutamine uptake was not impaired. However, given the antiproliferative properties of FLT3 inhibitors, incorporation of labeled glutamine in TCA intermediates was reduced likewise the total level of most TCA cycle intermediates as compared to control cells. Thus, despite a significant decrease in total TCA cycle activity, glutamine remains a major anaplerotic substrate in FLT3-treated cells.
In addition, glutamine (via glutamate) which supports the TCA cycle is also a precursor of glutathione, the primary cellular antioxidant [48]. It is noteworthy that the reduced/oxidized glutathione ratio (GSH/GSSG) was generally preserved in treated cells, and this treatment had no effect on glutathione metabolism genes, including the key regulator of antioxidant response NFE2L2. Taken together, these finding suggest glutamine supports the TCA cycle and glutathione production following FLT3 inhibition.
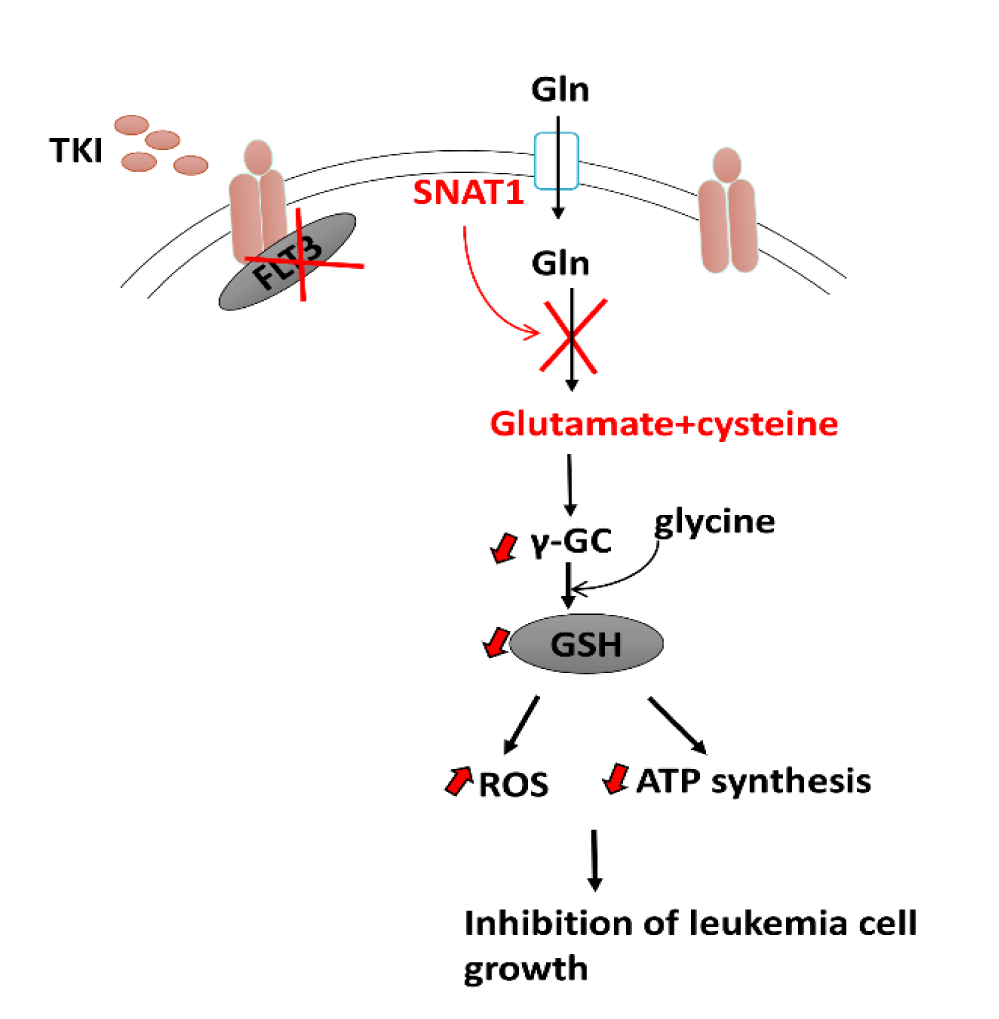
Following the work of Zarvoka Thomas, et al. [49] they sought to identify additional therapeutic targets that can be used to enhance the antileukemic effect of the tyrosine kinase inhibitor gilteritinib. Based on unbiased transcriptomic analyses, the glutamine transporter SNAT1 (SLC38A1) was identified as a potential target of gilteritinib that causes impaired glutamine uptake and utilization in leukemic cells. Furthermore, metabolomics and metabolic flux analyses in the presence of gilteritinib revealed reduced glutamine metabolism via the TCA cycle as well as cellular levels of the oncometabolite 2-HG. Finally, glutaminase inhibitor CB-839 improved gilteritinib antileukemic effect in ex vivo studies using human primary FLT3-ITD positive AML cells harboring mutations in the enzyme isocitrate dehydrogenase, which catalyses the oxidative decarboxylation of isocitrate to produce a-ketoglutarate. These findings have revealed a previously unknown gilteritinib-sensitive metabolic pathway downstream of SLC38A1 that causes decreased glutaminolysis and redox homeostasis disturbance [49] (Figure 2). Together, via transcriptomics and metabolomics, we can identify mechanistic insights connecting tyrosine kinase inhibition and glutaminolysis in AML treatment. Thus, providing a logical approach for the development and therapeutic investigation of targeted combinatorial treatment strategies for cases of relapse/refractory AML.
BCAT1 and reprogrammed Branched Chain Amino acids (BCAA) metabolism in leukemia: Blood AA levels in mouse models that replicate the chronic and blast crisis periods of human CML have been examined in order to better understand the role of AA metabolism in CML cancer growth [50,51].
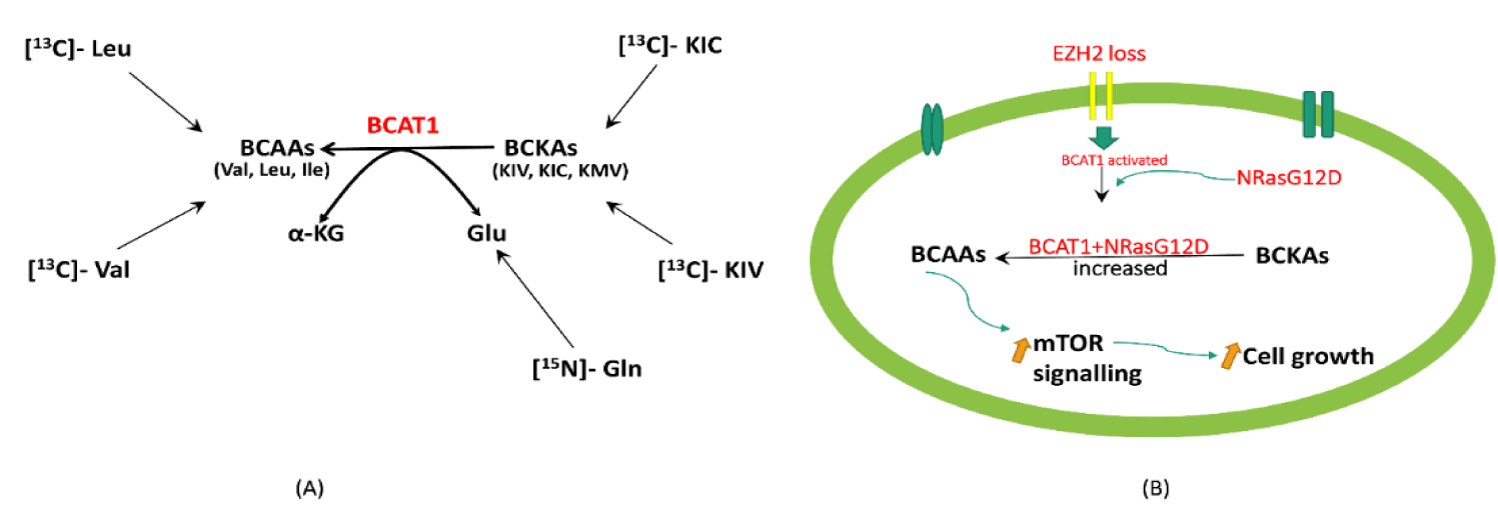
Although BCAA amino acid transaminase 1, BCAT1, catalyzes transamination in the forward and reverse direction, in most cell types, the breakdown of BCAAs is the predominant reaction [52]. BCAT1 requires the presence of Branched-Chain Keto Acids (BCKAs) as well as glutamate as substrates in order to generate BCAAs. Hattori’s group found the following BCKAs; keto-isovalerate, keto-isocaproate and keto-methylvalerate, in blood plasma and leukemia cells with significant amount of the corresponding BCAAs in blast crisis-CML cells, implying that intracellular BCKAs can serve as substrates for BCAA production. The authors then used stable-isotope tracer experiments with [13C]-valine or [13C]-KIV to see if BCAAs are produced in leukemia cells via BCAT1 transamination reactions. High-field NMR spectroscopy was used to examine intracellular 13C-labeled metabolites in K562 human BC-CML cells applying a one- and two-dimensional 1H-13C heteronuclear single bond correlation (HSQC) analysis. The production of strong [13C]-valine signals was observed, indicating that valine is efficiently generated from KIV intracellularly as compared to cells in non-labeled KIV in which [13C]-KIV was undetectable. This data indicated the transport of intracellular [13C]-valine in these leukemic cells [53]. In equal concentrations of KIV and Val, Val is produced from KIV but not the reverse reaction (Val to KIV) as well as it was impossible to detect the formation of KIC from [13C]-leucine. This suggest that BCAAs are not converted to BCKAs in leukemia cells [53].
Furthermore, other labeling studies have been executed to monitor the fate of the glutamate amine group providing additional evidence for intracellular BCAA production via transamination. Human myelogenous leukemia K562 cells were cultured with [15N]-amine-labeled glutamine, which is metabolized to [15N]-amineglutamate by glutaminase upon cellular intake, and the labeling of BCAAs was examined using NMR. The authors identified [15N]-amine-labeled BCAAs, indicating transamination from glutamine to BCAAs after 29h postlabeling. By 72 hours, the labeled BCAAs cumulated to a high abundance, suggesting that transamination made a significant contribution to the intracellular BCAA pool. These findings show that BCKA transamination by BCAT1 contributes to the pool of BCAAs in leukemia cells [53] (Figure 3a).
EZH2 and NRAS in BCAA Metabolism: EZH2, which is involved in histone-lysine methylation, is a frequent epigenetic regulator with high mutation rates in hematologic malignancies. Loss-of-function EZH2 mutations have been identified in several myeloproliferative neoplasms and juvenile myelomonocytic leukemia [47,54-56]. Overexpression or gain-of-function EZH2 mutations are also common in cancers [58,59], suggesting that EZH2 can cause tumors in both overactive and hypoactive states.
Alterations in EZH2 and RAS together promote the progression of myeloproliferative neoplasms to highly penetrant, transplantable, and lethal myeloid leukemias in mice [54-56,59-61] BCAT1, which catalyzes the reversible transamination of BCAAs, is normally repressed by EZH2 but abnormally activated in EZH2-deficient myeloid neoplasms in mice and humans. In addition, BCAT1 renewal collaborates with mutant NRAS to maintain intracellular BCAA pools, leading to increased mTOR signaling in leukemia cells lacking EZH2 [59]. Gu, et al. [59] used WT, G12D, E2-KO, and G12D/E2-Knockout mice Hematopoietic Progenitor Cells (HSPCs) and performed RNA-seq to unravel the molecular link between EZH2, BCAT1 and BCAA metabolism required for leukemogenesis. The levels of the BCAAs - Valine, Leucine and isoleucine were significantly higher in G12D/E2-KO compared to other genotypes, suggesting that increased BCAT1 in G12D/E2-KO HSPCs is associated with BCAA increase. These findings suggest that EZH2 deficiency with NRasG12D reactivates BCAT1 to improve BCKA to BCAA conversion, resulting in increased BCAA pools in HSPCs. Thus, modulating the enzyme and substrates for BCAA metabolism is critical for targeting the epigenetic and metabolic vulnerabilities of leukemia cells.
Another critical question is where BCKAs and Glu, the two substrates for BCAT1 transamination, come from. Gu, et al. [59] speculated that BCKAs were imported from extracellular sources via monocarboxylate transporters MCT1,2 or 4. In light of this, RNA-seq analysis revealed that MCT1 (or Slc16a1) was the most abundant transporter expressed in HSPCs. Strikingly, an MCT1 inhibitor AZD-3965 significantly reduced BCKAs and BCAAs in G12D/E2-KO HSPCs and mildly decreased BCAAs in wildtype and mutant NRAS HSPCs. In addition, NRAS activation resulted in elevated intracellular Glu in G12D and G12D/E2-KO cells. The authors then investigated whether NRasG12D increased Gln uptake and intracellular Glu pools via glutaminase (GLS)-mediated Gln to Glu conversion, which 'fuels' BCKA reamination catalyzed by BCAT1. Through [13C]-Gln tracing they found that NRasG12D increased intracellular Gln to Glu conversion in G12D and G12D/E2-KO, whereas glutaminase inhibition by CB-839 significantly decreased Glu and BCAAs in G12D/E2-KO cells. Together, these findings show that MCT1-dependent BCKA transport and GLS dependent Gln to Glu conversion are required for BCAT1 transamination in EZH2-deficient leukemia cells [59]. In a related study, BCAAs, particularly Leu, were found to activate mTORC1 and promote cell growth. Since BCAT1 activation increased BCAAs in EZH2-deficient cells, suggesting BCAT1-driven Leukemia is likely sensitive to mTOR inhibition [59]. In summary, these findings show that EZH2 loss and RAS activation promote leukemic transformation by modulating the enzyme and metabolic substrates of BCAA metabolism (Figure 3b).
KRAS regulates the cysteine/glutamate antiporter: Using B-ALL cell lines carrying KRASG12D mutation, Isotope tracing revealed KRASG12D mutation rewires methionine and arginine metabolism by boosting catabolism of these amino acids to enhance the anabolism of polyamines and proline, consequently inhibiting growth of the cells [62]. Overactivation of the AKT/mTOR pathway significantly increased the activity of KRAS12D. The effects of KRAS on amino acid metabolism were reversed when AKT/mTOR signaling was inhibited, and cell proliferation was restored [62]. According to the findings of Lim, et al. [63], the xCT gene expression was enhanced in KRAS transformed cells via the activation of ETS-1, which functions in conjunction with ATF4 to promote cell survival. On the other hand, knocking down this gene decreased tumor growth. This evidence suggests that the xCT (cysteine/glutamate antiporter) gene promotes tumor development, making it a possible therapeutic target.
TKTL1 gene in hypoxia-induced AML cells: The Transketolase (TKT) family's transketolase-like-1 gene has been linked to malignant transformation and a poor prognosis in many cancers [64-67]. The suppression of TKTL1 prevents THP-1 AML cells from switching to glycolysis in hypoxic circumstances by inhibiting hypoxia-induced transcription of genes that encode amino acid metabolism-related enzymes and transporters. In a study by Baptista and colleagues, metabolome data revealed considerable drop in concentrations of glutamate, glutamine, proline and ornithine in TKTL1-knockdown human monocytic leukemia THP-1 cells compared to wildtype cells [68,69]. Following transcriptome profiling, Proline dehydrogenase and arginase 1 were shown to have higher gene expression profiles in wildtype cells than in knockdown cells under hypoxic conditions. Furthermore, in control cells, Gln synthetase and SLC38A2 were modestly elevated, whereas glutaminase and SLC38A2 were dysregulated in THP-1 deficient cells. Likewise, still under hypoxic conditions, SLC1A3 and SLC17A7 were shown to be highly enhanced in the wildtype but decreased in the knockdown phenotype. Aside from the alterations in proline and ornithine, the investigators detected enhanced dimethylarginine secretion in the knockdown cells [69]. Taken together, this changes in metabolites and gene expression due to TKTL1 knockdown accounts for the amino acid metabolic adaptation observed in hypoxic induced AML cells.
DNMT3A, an epigenetic modulator controls AA metabolism: DNA Methyltransferase 3A (DNMT3A) alterations have been found in the majority of AML patients [70-73]. DNA methylation transforms the cytosine of CpG dinucleotides by attaching a methyl group, making it an essential component of the regulation of gene epigenetics. RNA microarrays on k562 cells indicated that this mutation influences glutathione metabolism [74]. PSPH, SLC7A11, PSAT1, and CTH were shown to be considerably upregulated, raising glutathione levels [74]. This data suggests that, targeting genes involved in glutathione synthesis is crucial for understanding the role of DNMT3A mutation in AML pathogenesis.
BCAT1 regulates αKG levels in AML cells: Through MS-BCAA tracing Raffel, et al. [75] found that when BCAT1 was knocked down, a quantity of 15N-labeled non-essential amino acids was significantly reduced, but the overall glutamate pool was unaffected, implying that leukaemia cells can compensate for the loss of BCAT1-dependent glutamate production from other sources. Given that BCAT1 uses αKG as a substrate, the authors speculated that BCAT1 could aid in regulating intracellular KG levels. Indeed, after BCAT1-Knockdown, KG levels increased significantly in several AML cell lines such as HL-60, SKM-1, and MOLM-13. In summary, this finding identifies BCAT1 as a critical regulator of αKG in distinct tumor cell types [75].
SLC1A5 cooperates with Pten and BCR-ABL: According to Ni, et al. [76] a constitutive deletion of SLC1A5 reduced leukemia initiation spurred by Pten deficiency while a deletion of SLC1A5 caused problems in bone marrow and mature blood cell formation. Interestingly, metabolomic analysis revealed that loss of this gene also impacted amino acid metabolism by impairing leucine influx which in turn interrupted mTOR signalling leading to cell death. BCR-ABL translocation mediates chronic leukemia [77-79]. Sontakkes’ [80] group found that in normoxic conditions, BCR-ABL-transduced newborn blood cord cells had increased expression of the importer SLC1A5. This suggests that glutamine metabolism is a key target in BCR CML since BCR cells need glutaminolysis to sustain citric cycle intermediates in glycolysis.
AA metabolic reprogramming and clinical treatments in hematological malignancies
Tumor cells obtain and use nutrients to meet their biosynthetic and energetic requirements. Different genetic and epigenetic modulators, nutrient specificity, tumor microenvironment, and cell-matrix interaction have been shown to influence metabolic reprograming in tumor growth [81-83]. Despite the fact that tumor cells preferentially use glucose, glutamine and other AA metabolites play an important role in tumor cell growth by feeding the TCA cycle. In recent decades, metabolome and transcriptome research have identified potent metabolic inhibitors (some of which are in clinical trials) that target AA metabolic shift via various mechanisms, hence influencing proliferation, growth, and survival of malignant cells (Table 1).
| Table 1: Metabolic targets in treatment of hematological malignancies. | |||
| Metabolic Inhibitors | Metabolite targets and off-targets | Mechanism of action | References |
| CB-839 (Telaglenastat) | GLS1,SLC7A11,c-MYC | Blocks GLS activity, decrease Gln utilization and flux into the TCA | [84-86] |
| V-9302 (GPNA derivative) | SLC38A2 (SNAT2),SLC1A5 | Selectively binds and target AA transporters hence inhibiting glutamine uptake | [87-89] |
| BPTES | GLS1 | Binding and stabilising the inactive state of GLS1 enzyme tetramer, Inhibiting tumor growth | [84,90-92] |
| Dibenzophenanthridine-968 | GLS, GLS2 | Selectively binds and inactivates the GAC and GBC tetramers in an inactive state | [93] |
| Alkyl benzoquinone AV-1 | GLS1/2, mTORC1, AMPK | Inhibits glutaminase activity, stimulates autophagy, ULK1 activation and mtorc1 inhibition | [93] |
| ASNS/ GCN2 inhibitors | L-asparaginase, GCN2/eIF2a, mTORC1, ATF4, ASS1, SLC1A4, SLC38A2, MTHFD1/2, and ATF5 | Induces stress-activated MAPK pathway hence triggering apoptosis | [94] |
| L-DON (JHU083) | Glutamine aminotransferases, glutaminases | Irreversibly competes with Gln for enzymes’ binding site, inhibiting Gln metabolism and enhance antitumor immunity | [95-98] |
| MRIAN-Dox | L-phenylalanine polymer and MRIAN | Degrades MRIAN into Phenylalanine, inhibits PKM2, reduces ROS in MDSCs | [99] |
| PYCR1 inhibitors | P5C | Competitively binds to target enzyme’s active site and inhibits proline utilisation | [100] |
| Aminooxyacetic acid/ hydrazinosuccinic acid | AST/GOT, ALT, AST | Inactivates pyridoxal phosphate-bound aminotransferases by reacting with the aldimine bond between these enzyme components | [101-104] |
AA metabolic reprograming and immunity
In fact, metabolic reprogramming is a feature of activated immune cells and is necessary for a vigorous anticancer immune response. Variations in AA metabolism can be found to affect a variety of cell types, including immune cells. Studies have demonstrated a link between AA metabolism, immunological control, and the impact on tumor cells [96-98,105].
Under normal circumstances, the tumor microenvironment harbors Myeloid-Derived Suppressor Cells (MDSCs) and Tumor-Associated Macrophages (TAM). By inhibiting GLS, ASNS, CPS2, and PPAT in malignant cells, MDSCs and DAMPs differentiate into pro-inflammatory macrophages, which improves antigen presentation to CD8+ CTLs. This causes a cascade of cancer-related immune responses, including increased cytokine production and decreased apoptosis, which inhibits tumor growth [97]. Furthermore, GLS inhibition has been demonstrated to boost CD4+ Th1 and CD8+ CTL differentiation, leading in increased cytokine production. Meanwhile, the same block reduces cytokine production in CD4 Th17 cells [106]. These changes have been connected to epigenetic markers of T cell subsets upon GLS inhibition [107].
Immunosuppressive cues must be eliminated in order to target hematological malignancies. Using isotope tracking, Hohtari, et al. [108] discovered that in T-ALL patients, MDSC levels were increased, impairing immune checkpoints, and contributing to chemoresistance. In this context, Li's group revealed that a metabolically reprogrammed L-phenylalanine polymer (MRIAN) degrades L-phenylalanine, triggering a chain of events that activate immune defence while suppressing MDSCs [99]. Given this knowledge, only a few metabolic targets have demonstrated therapeutic potential in modulating immune response and tumor progression. As a result, more effective approaches employing Omics methods to better understanding the mechanisms linking these processes will aid in identifying novel metabolic-associated immunological markers.
Conclusion
Hematological cancers often become dependent on particular amino acids for survival, and a lack of these amino acids sets up a metabolic threat and therapeutic advantage [109]. Over the years, omics have proven quite useful in medicine. Despite the promising outcomes or findings from using these molecular techniques, there still exist some challenges and limitations. The majority of metabolomics research has focused on patient plasma or serum samples, with the exception of investigations needing invasive tumor biopsy samples. Measurements from one omics approach may not adequately correlate with data from other approaches when there is no considerable overlap between distinct omics datasets. It takes time and money to generate multi-omics data, and the value of the data generated is heavily reliant on the availability of biopsy samples and suitable tissue samples that can be effectively analyzed for their tissue transcriptome and metabolome [110].
Nonetheless, despite the challenges, transcriptomics and metabolomics remain among the most advanced techniques used today, with numerous applications spanning multiple fields of science. Findings have shown that these techniques have the potential to unlock ground-breaking discoveries in medicine, particularly in the treatment of life-threatening diseases such as cancer [19,111,112].
Presently, only in a few clinical settings have omics technologies (RNA-seq) been fully employed compared to traditional clinical tests. However, because the technologies allows for a more complete picture of health and disease, integration of these technologies should be a milestone in future clinical practice [111].
Most studies' findings have revealed the significant AA metabolites, TCA intermediates, pathways, and gene transcripts that, when altered, cause disease pathogenesis. The changes in particular metabolites can caused by specific mutations in genes encoding enzymes that regulate metabolism and other cellular processes including apoptosis, cell proliferation and differentiation and should be targeted against drug resistance in future studies.
Transcriptomics have revealed significant transcripts that drive AA metabolism including PSAT1, ASNS, ASS1, and SLC1A5, ALDH1A2, GLS1, SLC38A1, GLDC, BCAT1 and others [31,38,42,49,52,53,74]. The dysregulation of these genes influenced by genetic and epigenetic mediators can either support or eradicate LSCs environment and leukemia development and progression. Because of their impact on multiple metabolic pathways, SLC1A5 and xCT solute-carrier gene have been shown to interrupt AA metabolism via the mTOR signalling pathways [63,76].
Furthermore, the observed effects or changes in AA metabolism is associated with the cooperative effect of the downstream targets such as Trps1, Ets1,Atf4 on the AA biosynthesis [42,63] that drives the progression of the disease via mTOR and RAS signalling.
Among the non-essential AAs, glutamine has been shown to most prominent in refuelling (anaplerotic substrate) the Krebs cycle. As a result, it is well known to support mitochondrial function and redox mechanisms, making it an important metabolic target particularly in leukemia cells with FLT3-ITD mutations [47].
The BCAAs have been studied for their role in reprogramming cancer metabolism [53]. More importantly, BCAT1 was shown to cooperate with NRAS of RAS signalling causing activation of mTOR signalling resulting in the cumulation of BCAAs [59]. Thus, BCAAs’ metabolism should be exploited in future research as they can reveal mechanistic insights that targets drug resistance and aid in developing effective chemotherapy against hematological cancers.
Metabolic inhibitors that target metabolites in the tumor stroma may produce encouraging results and should be evaluated for future clinical trial investigations.
The difficulty in immune-linked metabolic reprogramming is to always design metabolism-targeted therapies that are tumor-selective while protecting the host immune system. This will almost certainly necessitate a tailored therapeutic approach based on understanding the tumor and immune response determinants to certain metabolic disturbances.
To sum up, genetic and epigenetic modulators play crucial role in hematological cancer pathogenesis by altering amino acid metabolism. Employing an integrated system of transcriptomics and metabolomics has the potential to significantly advance understanding of the therapeutic mechanisms and metabolic pathways required for biomarker discovery, cancer diagnosis, and treatment.
Author Contributions
Conceptualization, original draft preparation: DB. Data collection and preparation of tables and figures: DB and GNN Neba Ambe. Supervision, revision, and manuscript editing: DB, GNN Neba Ambe and KD. Intellectual input and manuscript review: DB, GNN Neba Ambe, and KD. All authors have read and agreed to the published version of the manuscript.
Acknowledgement
The authors acknowledge the Poznan University of Medical Sciences and the Polish National Agency for academic exchange for their support in promoting research and internationalisation. DB is a participant of STER Internationalisation of Doctoral Schools Programme from NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.”
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflict of Interest: The authors declare no conflict of interest
Funding: Not applicable
References
- Auberger P, Tamburini-Bonnefoy J, Puissant A. Drug Resistance in Hematological Malignancies. Int J Mol Sci. 2020 Aug 24;21(17):6091. doi: 10.3390/ijms21176091. PMID: 32847013; PMCID: PMC7503602.
- DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016 May 27;2(5):e1600200. doi: 10.1126/sciadv.1600200. PMID: 27386546; PMCID: PMC4928883.
- Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020 Apr 10;368(6487):eaaw5473. doi: 10.1126/science.aaw5473. PMID: 32273439; PMCID: PMC7227780.
- ROSE WC, OESTERLING MJ, WOMACK M. Comparative growth on diets containing ten and 19 amino acids, with further observations upon the role of glutamic and aspartic acids. J Biol Chem. 1948 Nov;176(2):753-62. PMID: 18889930.
- Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018 Dec;18(12):744-757. doi: 10.1038/s41568-018-0074-8. PMID: 30425336.
- Casero RA, Murray Stewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018 Nov;18(11):681-695.
- Stäubert C, Bhuiyan H, Lindahl A, Broom OJ, Zhu Y, Islam S, Linnarsson S, Lehtiö J, Nordström A. Rewired metabolism in drug-resistant leukemia cells: a metabolic switch hallmarked by reduced dependence on exogenous glutamine. J Biol Chem. 2015 Mar 27;290(13):8348-59. doi: 10.1074/jbc.M114.618769. Epub 2015 Feb 19. PMID: 25697355; PMCID: PMC4375488.
- Wolfson RL, Sabatini DM. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab. 2017 Aug 1;26(2):301-309. doi: 10.1016/j.cmet.2017.07.001. PMID: 28768171; PMCID: PMC5560103.
- Liu D, Chen B, Mo Y, Wang Z, Qi T, Zhang Q, Wang Y. Redox-Activated Porphyrin-Based Liposome Remote-Loaded with Indoleamine 2,3-Dioxygenase (IDO) Inhibitor for Synergistic Photoimmunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Nano Lett. 2019 Oct 9;19(10):6964-6976. doi: 10.1021/acs.nanolett.9b02306. Epub 2019 Sep 24. Erratum in: Nano Lett. 2020 Jan 17;: PMID: 31518149.
- Tabe Y, Lorenzi PL, Konopleva M. Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood. 2019 Sep 26;134(13):1014-1023. doi: 10.1182/blood.2019001034. Epub 2019 Aug 15. PMID: 31416801; PMCID: PMC6764269.
- Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Löwenberg B, Licht JD, Godley LA, Delwel R, Valk PJ, Thompson CB, Levine RL, Melnick A. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010 Dec 14;18(6):553-67. doi: 10.1016/j.ccr.2010.11.015. Epub 2010 Dec 9. PMID: 21130701; PMCID: PMC4105845.
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, Jackson E, Aiello NM, Haas NB, Rebbeck TR, Judkins A, Won KJ, Chodosh LA, Garcia BA, Stanger BZ, Feldman MD, Blair IA, Wellen KE. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014 Aug 5;20(2):306-319. doi: 10.1016/j.cmet.2014.06.004. Epub 2014 Jul 3. PMID: 24998913; PMCID: PMC4151270.
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016 Jan 12;23(1):27-47. doi: 10.1016/j.cmet.2015.12.006. PMID: 26771115; PMCID: PMC4715268.
- Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022 Dec;13(12):877-919. doi: 10.1007/s13238-021-00846-7. Epub 2021 May 29. PMID: 34050894; PMCID: PMC9243210.
- Yuan Y, Li H, Pu W, Chen L, Guo D, Jiang H, He B, Qin S, Wang K, Li N, Feng J, Wen J, Cheng S, Zhang Y, Yang W, Ye D, Lu Z, Huang C, Mei J, Zhang HF, Gao P, Jiang P, Su S, Sun B, Zhao SM. Cancer metabolism and tumor microenvironment: fostering each other? Sci China Life Sci. 2022 Feb;65(2):236-279. doi: 10.1007/s11427-021-1999-2. Epub 2021 Nov 26. PMID: 34846643.
- van Karnebeek CDM, Wortmann SB, Tarailo-Graovac M, Langeveld M, Ferreira CR, van de Kamp JM, Hollak CE, Wasserman WW, Waterham HR, Wevers RA, Haack TB, Wanders RJA, Boycott KM. The role of the clinician in the multi-omics era: are you ready? J Inherit Metab Dis. 2018 May;41(3):571-582. doi: 10.1007/s10545-017-0128-1. Epub 2018 Jan 23. PMID: 29362952; PMCID: PMC5959952.
- Gomez-Cabrero D, Abugessaisa I, Maier D, Teschendorff A, Merkenschlager M, Gisel A, et al. Data integration in the era of omics: current and future challenges. BMC Syst Biol. 2014;8(Suppl 2):I1.
- Bjelosevic S, Gruber E, Newbold A, Shembrey C, Devlin JR, Hogg SJ, Kats L, Todorovski I, Fan Z, Abrehart TC, Pomilio G, Wei A, Gregory GP, Vervoort SJ, Brown KK, Johnstone RW. Serine Biosynthesis Is a Metabolic Vulnerability in FLT3-ITD-Driven Acute Myeloid Leukemia. Cancer Discov. 2021 Jun;11(6):1582-1599. doi: 10.1158/2159-8290.CD-20-0738. Epub 2021 Jan 12. PMID: 33436370.
- Gallo Cantafio ME, Grillone K, Caracciolo D, Scionti F, Arbitrio M, Barbieri V, Pensabene L, Guzzi PH, Di Martino MT. From Single Level Analysis to Multi-Omics Integrative Approaches: A Powerful Strategy towards the Precision Oncology. High Throughput. 2018 Oct 26;7(4):33. doi: 10.3390/ht7040033. PMID: 30373182; PMCID: PMC6306876.
- Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland DG, Lander ES, Golub TR, Look AT. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002 Feb;1(1):75-87. doi: 10.1016/s1535-6108(02)00018-1. PMID: 12086890.
- Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, McCastlain K, Edmonson M, Pounds SB, Shi L, Zhou X, Ma X, Sioson E, Li Y, Rusch M, Gupta P, Pei D, Cheng C, Smith MA, Auvil JG, Gerhard DS, Relling MV, Winick NJ, Carroll AJ, Heerema NA, Raetz E, Devidas M, Willman CL, Harvey RC, Carroll WL, Dunsmore KP, Winter SS, Wood BL, Sorrentino BP, Downing JR, Loh ML, Hunger SP, Zhang J, Mullighan CG. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017 Aug;49(8):1211-1218. doi: 10.1038/ng.3909. Epub 2017 Jul 3. PMID: 28671688; PMCID: PMC5535770.
- Soulier J, Clappier E, Cayuela JM, Regnault A, García-Peydró M, Dombret H, Baruchel A, Toribio ML, Sigaux F. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL). Blood. 2005 Jul 1;106(1):274-86. doi: 10.1182/blood-2004-10-3900. Epub 2005 Mar 17. PMID: 15774621.
- Bornschein S, Demeyer S, Stirparo R, Gielen O, Vicente C, Geerdens E, Ghesquière B, Aerts S, Cools J, de Bock CE. Defining the molecular basis of oncogenic cooperation between TAL1 expression and Pten deletion in T-ALL using a novel pro-T-cell model system. Leukemia. 2018 Apr;32(4):941-951. doi: 10.1038/leu.2017.328. Epub 2017 Nov 20. PMID: 29151585; PMCID: PMC5886055.
- Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA, Winter SS, Larson R, Zhang J, Protopopov A, Chin L, Pandolfi PP, Silverman LB, Hunger SP, Sallan SE, Look AT. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009 Jul 16;114(3):647-50. doi: 10.1182/blood-2009-02-206722. Epub 2009 May 20. PMID: 19458356; PMCID: PMC2713461.
- O'Neil J, Calvo J, McKenna K, Krishnamoorthy V, Aster JC, Bassing CH, Alt FW, Kelliher M, Look AT. Activating Notch1 mutations in mouse models of T-ALL. Blood. 2006 Jan 15;107(2):781-5. doi: 10.1182/blood-2005-06-2553. Epub 2005 Sep 15. PMID: 16166587; PMCID: PMC1895623.
- O'Neil J, Shank J, Cusson N, Murre C, Kelliher M. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell. 2004 Jun;5(6):587-96. doi: 10.1016/j.ccr.2004.05.023. PMID: 15193261.
- Tremblay M, Tremblay CS, Herblot S, Aplan PD, Hébert J, Perreault C, Hoang T. Modeling T-cell acute lymphoblastic leukemia induced by the SCL and LMO1 oncogenes. Genes Dev. 2010 Jun 1;24(11):1093-105. doi: 10.1101/gad.1897910. PMID: 20516195; PMCID: PMC2878648.
- Ono Y, Fukuhara N, Yoshie O. TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3. Mol Cell Biol. 1998 Dec;18(12):6939-50. doi: 10.1128/MCB.18.12.6939. PMID: 9819382; PMCID: PMC109277.
- Sanda T, Lawton LN, Barrasa MI, Fan ZP, Kohlhammer H, Gutierrez A, Ma W, Tatarek J, Ahn Y, Kelliher MA, Jamieson CH, Staudt LM, Young RA, Look AT. Core transcriptional regulatory circuit controlled by the TAL1 complex in human T cell acute lymphoblastic leukemia. Cancer Cell. 2012 Aug 14;22(2):209-21. doi: 10.1016/j.ccr.2012.06.007. PMID: 22897851; PMCID: PMC3422504.
- Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004 May;36(2):279-99. doi: 10.1081/dmr-120034001. PMID: 15237855.
- Zhang C, Amanda S, Wang C, King Tan T, Zulfaqar Ali M, Zhong Leong W, Moy Ng L, Kitajima S, Li Z, Eng Juh Yeoh A, Hao Tan S, Sanda T. Oncorequisite role of an aldehyde dehydrogenase in the pathogenesis of T-cell acute lymphoblastic leukemia. Haematologica. 2021 Jun 1;106(6):1545-1558. doi: 10.3324/haematol.2019.245639. PMID: 32414855; PMCID: PMC8168519.
- Herranz D, Ambesi-Impiombato A, Sudderth J, Sánchez-Martín M, Belver L, Tosello V, Xu L, Wendorff AA, Castillo M, Haydu JE, Márquez J, Matés JM, Kung AL, Rayport S, Cordon-Cardo C, DeBerardinis RJ, Ferrando AA. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat Med. 2015 Oct;21(10):1182-9. doi: 10.1038/nm.3955. Epub 2015 Sep 21. PMID: 26390244; PMCID: PMC4598309.
- O'Neil J, Grim J, Strack P, Rao S, Tibbitts D, Winter C, Hardwick J, Welcker M, Meijerink JP, Pieters R, Draetta G, Sears R, Clurman BE, Look AT. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007 Aug 6;204(8):1813-24. doi: 10.1084/jem.20070876. Epub 2007 Jul 23. PMID: 17646409; PMCID: PMC2118656.
- Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013 Jul;3(7):730-41. doi: 10.1158/2159-8290.CD-13-0083. Epub 2013 Jun 24. PMID: 23796461.
- Inoue S, Lemonnier F, Mak TW. Roles of IDH1/2 and TET2 mutations in myeloid disorders. Int J Hematol. 2016 Jun;103(6):627-33. doi: 10.1007/s12185-016-1973-7. Epub 2016 Mar 15. PMID: 26980223.
- Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, Schmidt S, Metallo CM, Dranka BP, Schwartz B, DeBerardinis RJ. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016 Apr 14;532(7598):255-8. doi: 10.1038/nature17393. Epub 2016 Apr 6. PMID: 27049945; PMCID: PMC4860952.
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011 Nov 20;481(7381):380-4. doi: 10.1038/nature10602. PMID: 22101433; PMCID: PMC3710581.
- Chen D, Xia S, Zhang R, Li Y, Famulare CA, Fan H, Wu R, Wang M, Zhu AC, Elf SE, Su R, Dong L, Arellano M, Blum WG, Mao H, Lonial S, Stock W, Odenike O, Le Beau M, Boggon TJ, He C, Chen J, Gao X, Levine RL, Chen J. Lysine acetylation restricts mutant IDH2 activity to optimize transformation in AML cells. Mol Cell. 2021 Sep 16;81(18):3833-3847.e11. doi: 10.1016/j.molcel.2021.06.027. Epub 2021 Jul 20. PMID: 34289383; PMCID: PMC8455438.
- Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, Pai MY, Li S, Ta L, Fazlollahi F, Chen C, Prins RM, Teitell MA, Nathanson DA, Lai A, Faull KF, Jiang M, Clarke SG, Cloughesy TF, Graeber TG, Braas D, Christofk HR, Jung ME, Reue K, Huang J. 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab. 2015 Sep 1;22(3):508-15. doi: 10.1016/j.cmet.2015.06.009. Epub 2015 Jul 16. PMID: 26190651; PMCID: PMC4663076.
- Sharma S, Agnihotri N, Kumar S. Targeting fuel pocket of cancer cell metabolism: A focus on glutaminolysis. Biochem Pharmacol. 2022 Apr;198:114943. doi: 10.1016/j.bcp.2022.114943. Epub 2022 Feb 5. PMID: 35131295.
- Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013 Sep;123(9):3652-8. doi: 10.1172/JCI67228. Epub 2013 Sep 3. PMID: 23999438; PMCID: PMC3754247.
- Liu C, Zou W, Nie D, Li S, Duan C, Zhou M, Lai P, Yang S, Ji S, Li Y, Mei M, Bao S, Jin Y, Pan J. Loss of PRMT7 reprograms glycine metabolism to selectively eradicate leukemia stem cells in CML. Cell Metab. 2022 Jun 7;34(6):818-835.e7. doi: 10.1016/j.cmet.2022.04.004. Epub 2022 May 3. PMID: 35508169.
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012 Jan 20;148(1-2):259-72. doi: 10.1016/j.cell.2011.11.050. Epub 2012 Jan 5. Erratum in: Cell. 2012 Mar 2;148(5):1066. Mitchell, Wayne [added]. PMID: 22225612.
- Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015 Apr 16;520(7547):363-7. doi: 10.1038/nature14363. Epub 2015 Apr 8. PMID: 25855294; PMCID: PMC4533874.
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013 Aug;13(8):572-83. doi: 10.1038/nrc3557. Epub 2013 Jul 4. PMID: 23822983; PMCID: PMC3806315.
- Karlíková R, Široká J, Friedecký D, Faber E, Hrdá M, Mičová K, Fikarová I, Gardlo A, Janečková H, Vrobel I, Adam T. Metabolite Profiling of the Plasma and Leukocytes of Chronic Myeloid Leukemia Patients. J Proteome Res. 2016 Sep 2;15(9):3158-66. doi: 10.1021/acs.jproteome.6b00356. Epub 2016 Aug 9. PMID: 27465658.
- Gallipoli P, Giotopoulos G, Tzelepis K, Costa ASH, Vohra S, Medina-Perez P, Basheer F, Marando L, Di Lisio L, Dias JML, Yun H, Sasca D, Horton SJ, Vassiliou G, Frezza C, Huntly BJP. Glutaminolysis is a metabolic dependency in FLT3ITD acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood. 2018 Apr 12;131(15):1639-1653. doi: 10.1182/blood-2017-12-820035. Epub 2018 Feb 20. PMID: 29463564; PMCID: PMC6061932.
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009 Apr 9;458(7239):762-5. doi: 10.1038/nature07823. Epub 2009 Feb 15. PMID: 19219026; PMCID: PMC2729443.
- Zavorka Thomas ME, Lu X, Talebi Z, Jeon JY, Buelow DR, Gibson AA, et al. Gilteritinib Inhibits Glutamine Uptake and Utilization in FLT3 -ITD–Positive AML. Molecular Cancer Therapeutics. 2021 Nov 1;20(11):2207–17.
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824-30. doi: 10.1126/science.2406902. PMID: 2406902.
- Dash AB, Williams IR, Kutok JL, Tomasson MH, Anastasiadou E, Lindahl K, Li S, Van Etten RA, Borrow J, Housman D, Druker B, Gilliland DG. A murine model of CML blast crisis induced by cooperation between BCR/ABL and NUP98/HOXA9. Proc Natl Acad Sci U S A. 2002 May 28;99(11):7622-7. doi: 10.1073/pnas.102583199. PMID: 12032333; PMCID: PMC124303.
- Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr. 2005 Jun;135(6 Suppl):1557S-64S. doi: 10.1093/jn/135.6.1557S. Erratum in: J Nutr. 2005 Aug;135(8):2009. PMID: 15930469.
- Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, Tayyari F, McSkimming D, Kannan N, Tojo A, Edison AS, Ito T. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017 May 25;545(7655):500-504. doi: 10.1038/nature22314. Epub 2017 May 17. PMID: 28514443; PMCID: PMC5554449.
- Caye A, Strullu M, Guidez F, Cassinat B, Gazal S, Fenneteau O, Lainey E, Nouri K, Nakhaei-Rad S, Dvorsky R, Lachenaud J, Pereira S, Vivent J, Verger E, Vidaud D, Galambrun C, Picard C, Petit A, Contet A, Poirée M, Sirvent N, Méchinaud F, Adjaoud D, Paillard C, Nelken B, Reguerre Y, Bertrand Y, Häussinger D, Dalle JH, Ahmadian MR, Baruchel A, Chomienne C, Cavé H. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat Genet. 2015 Nov;47(11):1334-40. doi: 10.1038/ng.3420. Epub 2015 Oct 12. PMID: 26457648.
- Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, Hochhaus A, Drexler HG, Duncombe A, Cervantes F, Oscier D, Boultwood J, Grand FH, Cross NC. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010 Aug;42(8):722-6. doi: 10.1038/ng.621. Epub 2010 Jul 4. PMID: 20601953.
- Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tönnissen ER, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010 Aug;42(8):665-7. doi: 10.1038/ng.620. Epub 2010 Jul 4. PMID: 20601954.
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010 Feb;42(2):181-5. doi: 10.1038/ng.518. Epub 2010 Jan 17. PMID: 20081860; PMCID: PMC2850970.
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010 Feb;42(2):181-5. doi: 10.1038/ng.518. Epub 2010 Jan 17. PMID: 20081860; PMCID: PMC2850970.
- Gu Z, Liu Y, Cai F, Patrick M, Zmajkovic J, Cao H, Zhang Y, Tasdogan A, Chen M, Qi L, Liu X, Li K, Lyu J, Dickerson KE, Chen W, Ni M, Merritt ME, Morrison SJ, Skoda RC, DeBerardinis RJ, Xu J. Loss of EZH2 Reprograms BCAA Metabolism to Drive Leukemic Transformation. Cancer Discov. 2019 Sep;9(9):1228-1247. doi: 10.1158/2159-8290.CD-19-0152. Epub 2019 Jun 12. PMID: 31189531; PMCID: PMC6726547.
- Beer PA, Delhommeau F, LeCouédic JP, Dawson MA, Chen E, Bareford D, Kusec R, McMullin MF, Harrison CN, Vannucchi AM, Vainchenker W, Green AR. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010 Apr 8;115(14):2891-900. doi: 10.1182/blood-2009-08-236596. Epub 2009 Dec 11. PMID: 20008300.
- Stieglitz E, Taylor-Weiner AN, Chang TY, Gelston LC, Wang YD, Mazor T, Esquivel E, Yu A, Seepo S, Olsen S, Rosenberg M, Archambeault SL, Abusin G, Beckman K, Brown PA, Briones M, Carcamo B, Cooper T, Dahl GV, Emanuel PD, Fluchel MN, Goyal RK, Hayashi RJ, Hitzler J, Hugge C, Liu YL, Messinger YH, Mahoney DH Jr, Monteleone P, Nemecek ER, Roehrs PA, Schore RJ, Stine KC, Takemoto CM, Toretsky JA, Costello JF, Olshen AB, Stewart C, Li Y, Ma J, Gerbing RB, Alonzo TA, Getz G, Gruber T, Golub T, Stegmaier K, Loh ML. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015 Nov;47(11):1326-1333. doi: 10.1038/ng.3400. Epub 2015 Oct 12. Erratum in: Nat Genet. 2015 Nov;47(11):1333. Erratum in: Nat Genet. 2016 Jan;48(1):101. PMID: 26457647; PMCID: PMC4626387.
- Xu Y, Fang H, Chen Y, Tang Y, Sun H, Kong Z, Yang F, Kirschner-Schwabe R, Zhu L, Toker A, Xiao N, Zhou BS, Li H. The KRAS-G12D mutation induces metabolic vulnerability in B-cell acute lymphoblastic leukemia. iScience. 2022 Feb 7;25(3):103881. doi: 10.1016/j.isci.2022.103881. PMID: 35243242; PMCID: PMC8861657.
- Lim JKM, Delaidelli A, Minaker SW, Zhang HF, Colovic M, Yang H, et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci USA. 2019 May 7;116(19):9433–42.
- Fritz P, Coy JF, Mürdter TE, Ott G, Alscher MD, Friedel G. TKTL-1 expression in lung cancer. Pathol Res Pract. 2012 Apr 15;208(4):203-9. doi: 10.1016/j.prp.2012.01.007. Epub 2012 Mar 24. PMID: 22445516.
- Chen H, Yue JX, Yang SH, Ding H, Zhao RW, Zhang S. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res. 2009 Mar 30;28(1):43. doi: 10.1186/1756-9966-28-43. PMID: 19331662; PMCID: PMC2669053.
- da Costa IA, Hennenlotter J, Stühler V, Kühs U, Scharpf M, Todenhöfer T, Stenzl A, Bedke J. Transketolase like 1 (TKTL1) expression alterations in prostate cancer tumorigenesis. Urol Oncol. 2018 Oct;36(10):472.e21-472.e27. doi: 10.1016/j.urolonc.2018.06.010. Epub 2018 Aug 16. PMID: 30119993.
- Li J, Zhu SC, Li SG, Zhao Y, Xu JR, Song CY. TKTL1 promotes cell proliferation and metastasis in esophageal squamous cell carcinoma. Biomed Pharmacother. 2015 Aug;74:71-6. doi: 10.1016/j.biopha.2015.07.004. Epub 2015 Aug 3. PMID: 26349965.
- Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020 Sep;52(9):1496-1516. doi: 10.1038/s12276-020-00504-8. Epub 2020 Sep 17. PMID: 32943735; PMCID: PMC8080614.
- Baptista I, Karakitsou E, Cazier JB, Günther UL, Marin S, Cascante M. TKTL1 Knockdown Impairs Hypoxia-Induced Glucose-6-phosphate Dehydrogenase and Glyceraldehyde-3-phosphate Dehydrogenase Overexpression. Int J Mol Sci. 2022 Mar 25;23(7):3574. doi: 10.3390/ijms23073574. PMID: 35408935; PMCID: PMC8999113.
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, Harris CC, Lichti CF, Townsend RR, Fulton RS, Dooling DJ, Koboldt DC, Schmidt H, Zhang Q, Osborne JR, Lin L, O'Laughlin M, McMichael JF, Delehaunty KD, McGrath SD, Fulton LA, Magrini VJ, Vickery TL, Hundal J, Cook LL, Conyers JJ, Swift GW, Reed JP, Alldredge PA, Wylie T, Walker J, Kalicki J, Watson MA, Heath S, Shannon WD, Varghese N, Nagarajan R, Westervelt P, Tomasson MH, Link DC, Graubert TA, DiPersio JF, Mardis ER, Wilson RK. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010 Dec 16;363(25):2424-33. doi: 10.1056/NEJMoa1005143. Epub 2010 Nov 10. PMID: 21067377; PMCID: PMC3201818.
- Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, Levine RL. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012 Mar 22;366(12):1079-89. doi: 10.1056/NEJMoa1112304. Epub 2012 Mar 14. PMID: 22417203; PMCID: PMC3545649.
- Thol F, Damm F, Lüdeking A, Winschel C, Wagner K, Morgan M, Yun H, Göhring G, Schlegelberger B, Hoelzer D, Lübbert M, Kanz L, Fiedler W, Kirchner H, Heil G, Krauter J, Ganser A, Heuser M. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011 Jul 20;29(21):2889-96. doi: 10.1200/JCO.2011.35.4894. Epub 2011 Jun 13. PMID: 21670448.
- Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, Shi JY, Zhu YM, Tang L, Zhang XW, Liang WX, Mi JQ, Song HD, Li KQ, Chen Z, Chen SJ. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011 Mar 13;43(4):309-15. doi: 10.1038/ng.788. PMID: 21399634.
- Yang L, Liu Y, Zhang N, Ding X, Zhang W, Shen K, Huang L, Zhou J, Cui S, Zhu Z, Hu Z, Xiao M. Novel impact of the DNMT3A R882H mutation on GSH metabolism in a K562 cell model established by TALENs. Oncotarget. 2017 May 2;8(18):30395-30409. doi: 10.18632/oncotarget.16449. PMID: 28418922; PMCID: PMC5444751.
- Raffel S, Falcone M, Kneisel N, Hansson J, Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A, Bahr C, Zeisberger P, Przybylla A, Sohn M, Tönjes M, Erez A, Adler L, Jensen P, Scholl C, Fröhling S, Cocciardi S, Wuchter P, Thiede C, Flörcken A, Westermann J, Ehninger G, Lichter P, Hiller K, Hell R, Herrmann C, Ho AD, Krijgsveld J, Radlwimmer B, Trumpp A. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature. 2017 Nov 16;551(7680):384-388. doi: 10.1038/nature24294. Epub 2017 Nov 8. Erratum in: Nature. 2018 Aug;560(7718):E28. PMID: 29144447.
- Ni F, Yu WM, Li Z, Graham DK, Jin L, Kang S, Rossi MR, Li S, Broxmeyer HE, Qu CK. Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression. Nat Metab. 2019 Mar;1(3):390-403. doi: 10.1038/s42255-019-0039-6. Epub 2019 Mar 11. PMID: 31535081; PMCID: PMC6750232.
- NOWELL PC, HUNGERFORD DA. Chromosome studies in human leukemia. II. Chronic granulocytic leukemia. J Natl Cancer Inst. 1961 Nov;27:1013-35. PMID: 14480645.
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290-3. doi: 10.1038/243290a0. PMID: 4126434.
- Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13-19;315(6020):550-4. doi: 10.1038/315550a0. PMID: 2989692.
- Sontakke P, Koczula KM, Jaques J, Wierenga AT, Brouwers-Vos AZ, Pruis M, Günther UL, Vellenga E, Schuringa JJ. Hypoxia-Like Signatures Induced by BCR-ABL Potentially Alter the Glutamine Uptake for Maintaining Oxidative Phosphorylation. PLoS One. 2016 Apr 7;11(4):e0153226. doi: 10.1371/journal.pone.0153226. PMID: 27055152; PMCID: PMC4824381.
- Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012 Feb 8;15(2):157-70. doi: 10.1016/j.cmet.2011.12.015. PMID: 22326218; PMCID: PMC3282107.
- Dunphy MPS, Harding JJ, Venneti S, Zhang H, Burnazi EM, Bromberg J, Omuro AM, Hsieh JJ, Mellinghoff IK, Staton K, Pressl C, Beattie BJ, Zanzonico PB, Gerecitano JF, Kelsen DP, Weber W, Lyashchenko SK, Kung HF, Lewis JS. In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of 18F-(2S,4R)-4-Fluoroglutamine. Radiology. 2018 May;287(2):667-675. doi: 10.1148/radiol.2017162610. Epub 2018 Jan 31. PMID: 29388903; PMCID: PMC5929369.
- Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, Wahlig S, Chiba L, Kim SH, Morse J, Pradeep S, Nagaraja AS, Haemmerle M, Kyunghee N, Derichsweiler M, Plackemeier T, Mercado-Uribe I, Lopez-Berestein G, Moss T, Ram PT, Liu J, Lu X, Mok SC, Sood AK, Nagrath D. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016 Nov 8;24(5):685-700. doi: 10.1016/j.cmet.2016.10.011. PMID: 27829138; PMCID: PMC7329194.
- Zimmermann SC, Duvall B, Tsukamoto T. Recent Progress in the Discovery of Allosteric Inhibitors of Kidney-Type Glutaminase. J Med Chem. 2019 Jan 10;62(1):46-59. doi: 10.1021/acs.jmedchem.8b00327. Epub 2018 Jul 3. PMID: 29969024; PMCID: PMC6335185.
- Vogl DT, Younes A, Stewart K, Orford KW, Bennett M, Siegel D, et al. Phase 1 Study of CB-839, a First-in-Class, Glutaminase Inhibitor in Patients with Multiple Myeloma and Lymphoma. Blood. 2015 Dec 3;126(23):3059–3059.
- Wang ES, Frankfurt O, Orford KW, Bennett M, Flinn IW, Maris M, et al. Phase 1 Study of CB-839, a First-in-Class, Orally Administered Small Molecule Inhibitor of Glutaminase in Patients with Relapsed/Refractory Leukemia. Blood. 2015 Dec 3;126(23):2566–2566.
- Esslinger CS, Cybulski KA, Rhoderick JF. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005 Feb 15;13(4):1111-8. doi: 10.1016/j.bmc.2004.11.028. PMID: 15670919.
- Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JC, Sharick JT, Skala MC, Smith JA, Berlin J, Washington MK, Nickels ML, Manning HC. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018 Feb;24(2):194-202. doi: 10.1038/nm.4464. Epub 2018 Jan 15. PMID: 29334372; PMCID: PMC5803339.
- Scopelliti AJ, Font J, Vandenberg RJ, Boudker O, Ryan RM. Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nat Commun. 2018 Jan 2;9(1):38. doi: 10.1038/s41467-017-02444-w. PMID: 29295993; PMCID: PMC5750217.
- Lee EJ, Duggirala KB, Lee Y, Yun MR, Jang J, Cyriac R, Jung ME, Choi G, Chae CH, Cho BC, Lee K. Novel allosteric glutaminase 1 inhibitors with macrocyclic structure activity relationship analysis. Bioorg Med Chem Lett. 2022 Nov 1;75:128956. doi: 10.1016/j.bmcl.2022.128956. Epub 2022 Aug 28. PMID: 36038117.
- Robinson MM, McBryant SJ, Tsukamoto T, Rojas C, Ferraris DV, Hamilton SK, Hansen JC, Curthoys NP. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem J. 2007 Sep 15;406(3):407-14. doi: 10.1042/BJ20070039. PMID: 17581113; PMCID: PMC2049044.
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010 Sep 14;18(3):207-19. doi: 10.1016/j.ccr.2010.08.009. Erratum in: Cancer Cell. 2010 Oct 19;18(4):397. PMID: 20832749; PMCID: PMC3078749.
- Lee YZ, Yang CW, Chang HY, Hsu HY, Chen IS, Chang HS, Lee CH, Lee JC, Kumar CR, Qiu YQ, Chao YS, Lee SJ. Discovery of selective inhibitors of Glutaminase-2, which inhibit mTORC1, activate autophagy and inhibit proliferation in cancer cells. Oncotarget. 2014 Aug 15;5(15):6087-101. doi: 10.18632/oncotarget.2173. PMID: 25026281; PMCID: PMC4171615.
- Nakamura A, Nambu T, Ebara S, Hasegawa Y, Toyoshima K, Tsuchiya Y, Tomita D, Fujimoto J, Kurasawa O, Takahara C, Ando A, Nishigaki R, Satomi Y, Hata A, Hara T. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7776-E7785. doi: 10.1073/pnas.1805523115. Epub 2018 Jul 30. PMID: 30061420; PMCID: PMC6099884.
- MAGILL GB, MYERS WP, REILLY HC, PUTNAM RC, MAGILL JW, SYKES MP, ESCHER GC, KARNOFSKY DA, BURCHENAL JH. Pharmacological and initial therapeutic observations on 6-diazo-5-oxo-1-norleucine (DON) in human neoplastic disease. Cancer. 1957 Nov-Dec;10(6):1138-50. doi: 10.1002/1097-0142(195711/12)10:6<1138::aid-cncr2820100608>3.0.co;2-k. PMID: 13489662.
- Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, Tam A, Blosser RL, Prchalova E, Alt J, Rais R, Slusher BS, Powell JD. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019 Nov 22;366(6468):1013-1021. doi: 10.1126/science.aav2588. Epub 2019 Nov 7. PMID: 31699883; PMCID: PMC7023461.
- Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, Collins SL, Tam AJ, Blosser RL, Patel CH, Englert JM, Arwood ML, Wen J, Chan-Li Y, Tenora L, Majer P, Rais R, Slusher BS, Horton MR, Powell JD. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020 Jul 1;130(7):3865-3884. doi: 10.1172/JCI131859. PMID: 32324593; PMCID: PMC7324212.
- Nabe S, Yamada T, Suzuki J, Toriyama K, Yasuoka T, Kuwahara M, Shiraishi A, Takenaka K, Yasukawa M, Yamashita M. Reinforce the antitumor activity of CD8+ T cells via glutamine restriction. Cancer Sci. 2018 Dec;109(12):3737-3750. doi: 10.1111/cas.13827. Epub 2018 Nov 2. PMID: 30302856; PMCID: PMC6272119.
- Li C, You X, Xu X, Wu B, Liu Y, Tong T, Chen J, Li Y, Dai C, Ye Z, Tian X, Wei Y, Hao Z, Jiang L, Wu J, Zhao M. A Metabolic Reprogramming Amino Acid Polymer as an Immunosurveillance Activator and Leukemia Targeting Drug Carrier for T-Cell Acute Lymphoblastic Leukemia. Adv Sci (Weinh). 2022 Mar;9(9):e2104134. doi: 10.1002/advs.202104134. Epub 2022 Jan 26. PMID: 35080145; PMCID: PMC8948613.
- Christensen EM, Bogner AN, Vandekeere A, Tam GS, Patel SM, Becker DF, Fendt SM, Tanner JJ. In crystallo screening for proline analog inhibitors of the proline cycle enzyme PYCR1. J Biol Chem. 2020 Dec 25;295(52):18316-18327. doi: 10.1074/jbc.RA120.016106. Epub 2020 Oct 27. PMID: 33109600; PMCID: PMC7939384.
- Antti H, Sellstedt M. Metabolic effects of an aspartate aminotransferase-inhibitor on two T-cell lines. PLoS One. 2018 Dec 7;13(12):e0208025. doi: 10.1371/journal.pone.0208025. PMID: 30532126; PMCID: PMC6285999.
- Yamada RH, Wakabayashi Y, Iwashima A, Hasegawa T. Inhibition of aspartate aminotransferase by hydrazinosuccinate. Biochimica et Biophysica Acta (BBA) - General Subjects. 1984 Sep;801(1):151–4.
- Yamada RH, Wakabayashi Y, Iwashima A, Hasegawa T. Inhibition of aspartate aminotransferase by hydrazinosuccinate. Biochim Biophys Acta. 1984 Sep 7;801(1):151-4. doi: 10.1016/0304-4165(84)90224-1. PMID: 6547859.
- Yamada R hei, Wakabayashi Y, Iwashima A, Hasegawa T. In vivo inhibition of aspartate aminotransferase in mice by l-hydrazinosuccinate. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1987 Feb;911(3):372–5.
- El Sayed R, Haibe Y, Amhaz G, Bouferraa Y, Shamseddine A. Metabolic Factors Affecting Tumor Immunogenicity: What Is Happening at the Cellular Level? Int J Mol Sci. 2021 Feb 21;22(4):2142. doi: 10.3390/ijms22042142. PMID: 33670011; PMCID: PMC7927105.
- Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, Maseda D, Liberti MV, Paz K, Kishton RJ, Johnson ME, de Cubas AA, Wu P, Li G, Zhang Y, Newcomb DC, Wells AD, Restifo NP, Rathmell WK, Locasale JW, Davila ML, Blazar BR, Rathmell JC. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell. 2018 Dec 13;175(7):1780-1795.e19. doi: 10.1016/j.cell.2018.10.001. Epub 2018 Nov 1. PMID: 30392958; PMCID: PMC6361668.
- Britt EC, John SV, Locasale JW, Fan J. Metabolic regulation of epigenetic remodeling in immune cells. Current Opinion in Biotechnology. 2020 Jun;63:111–7.
- Hohtari H, Brück O, Blom S, Turkki R, Sinisalo M, Kovanen PE, et al. Immune cell constitution in bone marrow microenvironment predicts outcome in adult ALL. Leukemia. 2019 Jul;33(7):1570–82.
- Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, Pei S, Khan N, Adane B, Ye H, Krug A, Reinhold D, Smith C, DeGregori J, Pollyea DA, Jordan CT. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell. 2019 Feb 11;35(2):333-335. doi: 10.1016/j.ccell.2019.01.013. Erratum for: Cancer Cell. 2018 Nov 12;34(5):724-740.e4. PMID: 30753831; PMCID: PMC6389327.
- Olivier M, Asmis R, Hawkins GA, Howard TD, Cox LA. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int J Mol Sci. 2019 Sep 26;20(19):4781. doi: 10.3390/ijms20194781. PMID: 31561483; PMCID: PMC6801754.
- Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018 May;19(5):299-310. doi: 10.1038/nrg.2018.4. Epub 2018 Feb 26. PMID: 29479082; PMCID: PMC5990367.
- Patt A, Siddiqui J, Zhang B, Mathé E. Integration of Metabolomics and Transcriptomics to Identify Gene-Metabolite Relationships Specific to Phenotype. In: Haznadar M, editor. Cancer Metabolism [Internet]. New York, NY: Springer New York; 2019 [cited 2022 Sep 30]. p. 441–68. (Methods in Molecular Biology; vol. 1928).
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.