Medicine Group . 2022 December 12;3(12):1495-1499. doi: 10.37871/jbres1623.
Application of Umbilical Cord Tissue Allografts in Reduction Mammoplasty Wound: A Case Study
Allen Meglin2, John Shou3, Kevin Welch4, Eric Vinke1, Naomi Lambert1* and Tyler Barrett1
2Advanced Regenerative Therapy, USA
3Baylor College of Medicine Escambia, USA
4County Medical Association, USA
Abstract
Nearly 15 million patients undergo cosmetic surgery in the United States each year, with breast augmentations such as implants, lifts, or reductions being some of the most common procedures. The most common complications of these procedures are scarring and infection at the incision site, which often necessitates expensive corrective surgery.
After significant weight loss, the patient in this study underwent an elective lower body lift in conjunction with a breast reduction and nipple-areolar transplant. The patient experienced postoperative wound dehiscence, creating a large open wound, warranting rapid wound closure to avoid further pain and infection. The patient was treated for eight weeks with conservative measures. After eight weeks of failed attempts to close her wound, she was referred for specialist care.
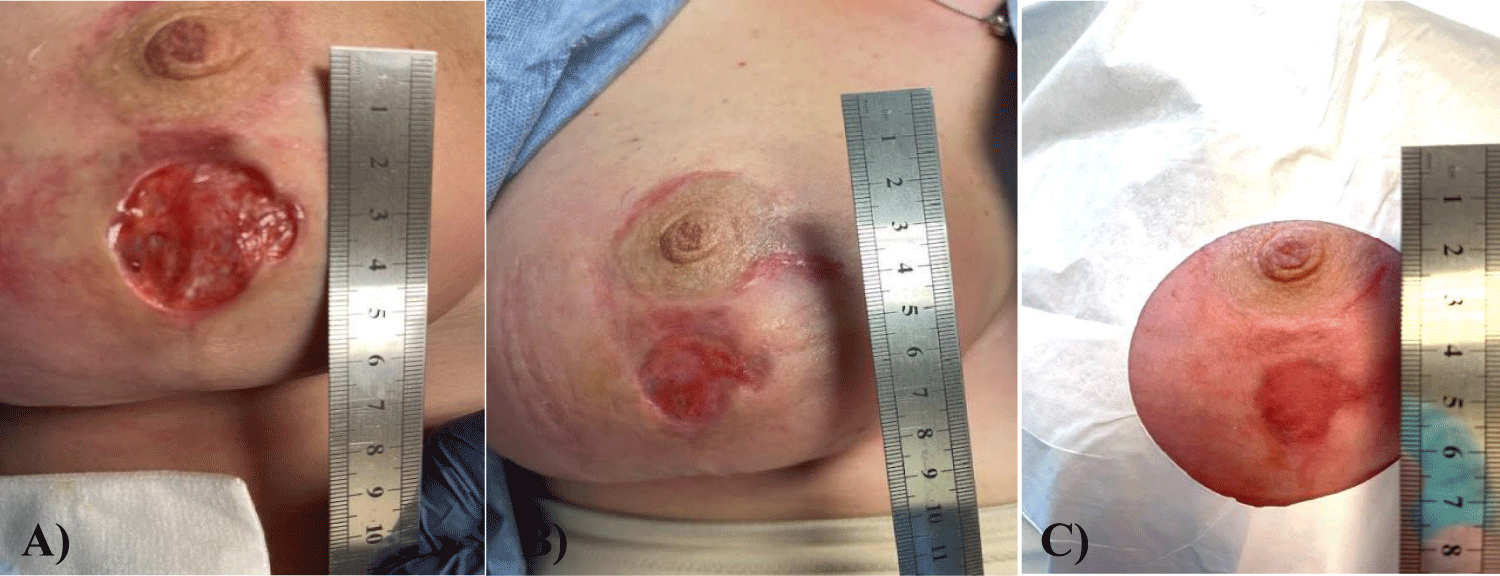
Upon initial examination, the donor site wound measured 3.5 cm x 3.5 cm x 1.25 cm deep with no sign of epithelialization. The patient received a single dose of Wharton’s jelly flowable perinatal tissue allograft and twelve hyperbaric oxygen therapy treatments over seven weeks. Upon inspection at the final examination, the wound was closed entirely with 100% epithelialization.
This case study demonstrates a precedent for the application of Wharton’s jelly flowable allografts in complicated cosmetic post-surgical wounds. Future efforts will be directed at applying Wharton’s jelly allografts on a preventative basis. Preventative applications could be in stage 2 pressure sores, in the region of recurrent chronic venous insufficiency ulcers between stages of tenuous healing, prior to joint replacement in patients with immune compromised states, or prior to surgical intervention in patients with a known history of postoperative infections. The obvious goal is to prevent needless patient suffering and reduce unnecessary healthcare costs.
Introduction
Reduction mammoplasty, commonly known as a breast reduction, is a procedure in which the overall volumetric abatement of the breast is performed to assuage the painful symptoms and the emotional distress associated with macromastia [1,2]. Reduction mammoplasty has increased in popularity by 49% in patients from 2020 to 2021 [3,4]. Typically, the surgery takes around three to four hours to remove the excess glandular tissue and fat, create an aesthetically pleasing shape, and close the wound in a way that minimizes scarring. Breast reduction surgery is indicated for women experiencing negative symptoms from natural or cosmetic breast hypertrophy [5,6]. The patient in this study experienced post-partum dysthymia, symptoms of body dysmorphic disorder, and suffered from declining overall mental and physical health. After losing considerable weight post-pregnancy, she requested an elective body lift and reduction mammoplasty. Due to the complex nature of these surgeries, longer operating room times are typical, increasing the risk and likelihood of surgical site complications and infection.
A study conducted in 2014 examined the risk factors for complications within 30 days following breast reduction surgery in a group of 512 patients. The study found a 32% complication rate, including minor and major complications. Minor surgical complications included hematoma, wound infection, and delayed wound healing. Major complications included wound dehiscence, flap necrosis, and nipple-areolar necrosis [7]. The risk of post-operative infections nearly tripled in surgeries lasting more than 120 minutes compared to operations lasting less than one hour [8]. At an average of three hours, reduction mammoplasty is already at a higher risk for post-operative complications.
The patient in this study received one Wharton's Jelly (WJ) allograft application and twelve HBOT treatments. Initially, the wound was convex, with the breast tissue swelling out of the wound. The patient received six HBOT treatments to reduce the inflammation. After the initial HBOT treatments, the wound measured 3.5 cm in diameter, 1.25 cm deep and was wet and open. The patient received one Wharton's jelly flowable allograft application, which infiltrated throughout the dermal tissue around and beneath the wound bed. The patient was given six HBOT treatments post-WJ application. Upon examination at the final office visit, the prior donor site wound dehiscence was entirely closed and completely epithelialized. While Wharton's jelly is known for its function as a micro-architectural framework or extracellular matrix, there are very few reports of WJ being successfully applied in complicated wounds [9]. This case study demonstrates that the application of WJ allografts has the potential to accelerate wound closure time. Future non-randomized and randomized controlled trials may further clarify protocols for WJ application in wounds that are stage 2, intra-operative application to prevent post-operative wound dehiscence, application between wound closures in patients with recurrent chronic venous insufficiency wounds, and to accelerate wound closure in deep or irregular cosmetic wounds that are not amenable to standard dehydrated amniotic membrane allograft application.
Case Presentation Section
Umbilical cord tissue allografts
All methods were completed in compliance with the United States Food and Drug Administration (FDA) and American Association of Tissue Banks (AATB) standards.
Donation and collection: Human umbilical cords were obtained from consenting mothers following full-term caesarian section deliveries. Prior to delivery, birth mothers underwent comprehensive medical, social, and blood testing. An independent certified laboratory tested all donations for infectious disease in accordance with Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493 and FDA regulations. Each birth mother was tested for Hepatitis B Core Antibody (HBcAb), Hepatitis B Surface Antigen (HBsAg), Hepatitis C Antibody (HCV), Human Immunodeficiency Virus Antibody (HIV-1/HIV-2 Plus O), Human T-Lymphotropic Virus Antibody (HLTV-I/11), Syphilis (RPR), Cyto Megalo Virus (CMV), HIV-1/HCV/HBV, NAT and West Nile Virus (WNV). Each test was performed with an FDA-Approved testing kit. All test results were negative or non-reactive.
Preparation of processed umbilical cord tissue samples product: Wharton’s jelly was aseptically dissociated from the rinsed umbilical cord. After dissociation, 100 mg of Wharton’s jelly was suspended in approximately 2 mL of sterile Sodium Chloride 0.9% solution (normal saline). The sample was not combined with cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10 (a) (3) (human cells, tissues, and cellular and tissue-based product regulation).
Allograft application: The patient had a flow able allograft application performed in a private medical setting. Before application, the skin around the wound was cleaned and dried, biofilm abatement was performed, and marks were made in 0.5 cm increments around the wound’s circumference. 2 cc’s of Wharton’s jelly flow able allograft was diluted in 1 cc of normal saline and applied parallel to the dermal layer around the circumference of the wound at each pre-marked location.
Patient history: In this case study, the patient is a 43 year-old female who received an elective reduction mammoplasty after losing 35 lbs post pregnancy. The patient’s pre-operative breast measurements were 44 DD. This patient had no prior medical conditions and was a nonsmoker. Post-operative measurements determined the patient’s breast size to be 44 C. The patient’s nipple-areola complex was transplanted cephalad, and sutures were used to close the donor location. Unfortunately, the patient suffered post-operative donor wound dehiscence, leaving a 3.5 x 3.5 x 1.25 cm deep wound. Although this wound was not yet infected, rapid wound closure was necessary due to the high-risk location. The patient received one WJ allograft application and twelve hyperbaric oxygen therapy treatments.
Results
The patient’s wound failed to close after eight weeks of conservative treatment. The wound measured 3.5 cm in diameter and was 1.5 cm deep. The wound closed with a single Wharton’s jelly allograft application and twelve HBOT treatments in 7 weeks, evident in figures 1,2. Upon application on September 2, 2022, the wound measured 3.50 cm x 3.50 cm and 1.25 cm deep. Upon the first follow-up on September 10, 2022, the wound measured 2.0 cm x 2.0 cm and 0.5 cm deep with 43% epithelialization. At the second follow-up visit, the wound was entirely closed with some discoloration. At the final visit on October 20, 2022, the wound area had returned to normal coloration with no scarring. These results demonstrate a significant, favorable clinical outcome compared to the prior eight weeks of failed conservative management. The application of Wharton’s jelly proved a critical component in timely wound epithelialization and closure. Lateral and deep infectious involvement of the breast tissue was avoided.
Discussion
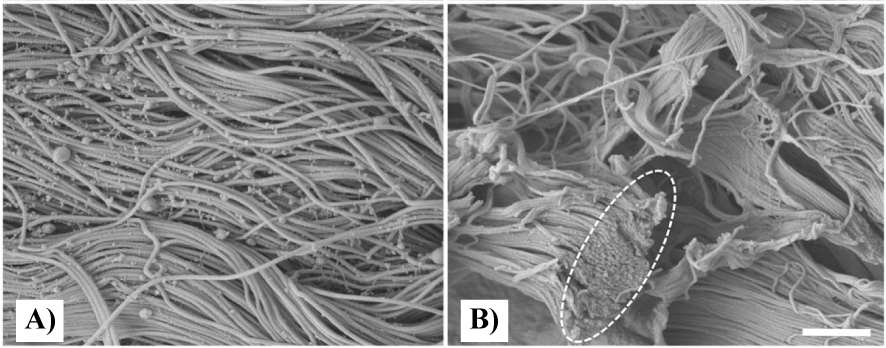
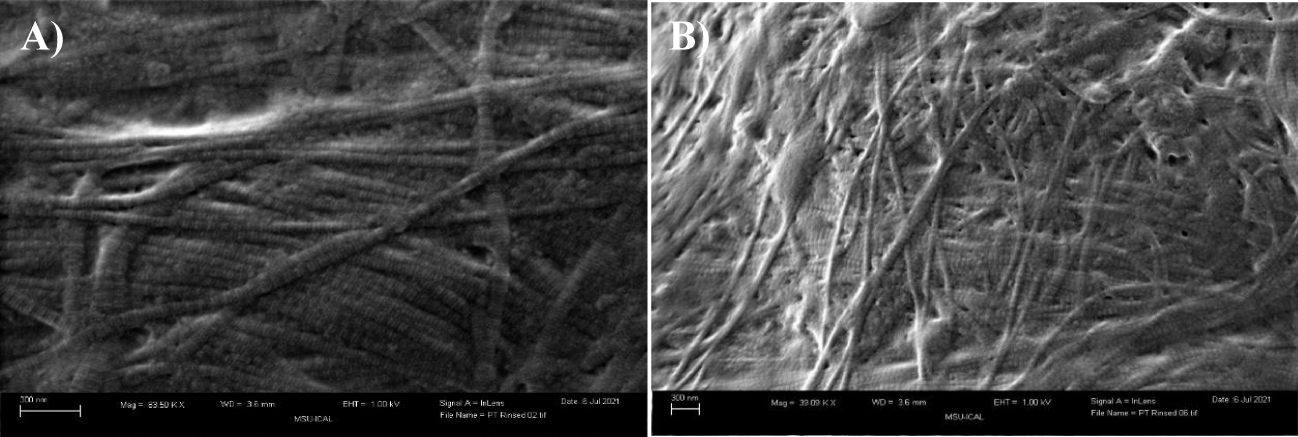
As observed in the present case study, the application of Wharton’s jelly for the closure of wounds following reduction mammoplasty significantly improved wound closure time. After eight weeks of failed conservative measures, Wharton’s jelly was administered to the wound. Prior work has demonstrated the role of WJ as an extracellular matrix analog, sharing comparative quantitative and qualitative structure to collagen structural tissue matrices in load-bearing joints, intervertebral discs, and dermis. The collagen seen in the Extra Cellular Matrix (ECM) of dermal tissue, as shown in figure 2, has a homologous structure to WJ in the formation pattern, as well as the diameter of fibers. In figure 2, the Scanning Electron Microscope (SEM) photo of native and cellular skin shows the entangled structure of collagen fibers, which is analogous to all other anatomical locations of ECM in the body, notably in articular cartilage and the fibers in WJ [10]. The average diameter of the skin collagen fibers is about 60 nm, and the average diameter of the WJ fibers is 65 nm (Figures 2,3). These similarities suggest that WJ is a suitable allograft alternative for the repair of structural tissue defects in the dermis. Although WJ is a viable tissue allograft, the primary function is the structural tissue supplementation made possible by the collagen matrix that contains hyaluronic acid, growth factors, cytokines, proteoglycans, and other GAGs.
The present case provides a foundation for the application of Wharton’s jelly allografts for refractory wounds following reduction mammoplasties. WJ is comparable in cost to the standard of care for non-healing surgical wounds, which averages approximately $6,931 for wound-related costs and $20,752 for the total treatment cost [12]. Not only is the standard of care less effective, but when used alone, it may result in prolonged stay and increased morbidity. In addition, expenses involved in treating non-healing surgical wounds, patient travel to and from a wound care clinic, wound care supplies not covered by insurance, lost wages from time off work, and caregiver fees are all out-of-pocket expenditures for patients.
Conclusion
The application of Wharton’s jelly to improve the rate of cosmetic surgical wound closure demonstrated clinically favorable results. In the presented case, Wharton’s jelly accelerated wound contracture and epithelization following eight weeks of failed conservative care. The use of Wharton’s jelly allografts in combination with HBOT exponentially improved the overall closure rate of this surgical wound dehiscence. Nonrandomized and randomized controlled trials may help to refine further the frequency of flowable WJ allograft application protocols, prevention-based protocols for stage 2 wounds, and application of WJ in the wound region of recurrent chronic venous insufficiency ulcers.
The presented study also postulates a prevention-based application of Wharton’s jelly allografts at the time of instrumented, large joint or spine surgery to improve postoperative outcomes. Reduced hospital length of stay, decreased surgical site infection rates, lower health care expenses from protracted postoperative wound care, and improved patient and ancillary caregiver quality of life are all reasons to pursue WJ applications during intra-operative care.
Funding
This research received no external funding. Regenative labs is responsible for all APC charges.
Institutional Review Board Statement
The study was conducted in accordance with the declaration of helsinki and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (protocol code IRCM-2022-311 and approved on 12 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be found in Appendix A.
Acknowledgment
The authors would like to thank Advanced Regenerative Therapy for their collaboration in data collection. The authors would also like to thank Catherine Becker for her collaboration in reviewing the manuscript.
Conflicts of Interest
John Shou, Naomi Lambert, Eric Vinke, and Tyler Barrett are associated with Regenative Labs. Regenative Labs was involved in the design of the study, data analysis, and writing. An independent physician performed the treatment and data collection at advanced regenerative therapy. Regenative Labs donated product for the present case and influenced the decision to publish.
References
- Purohit S. Reduction mammoplasty. Indian J Plast Surg. 2008 Oct;41(Suppl):S64-79. PMID: 20174545; PMCID: PMC2825129.
- Dabbah A, Lehman JA Jr, Parker MG, Tantri D, Wagner DS. Reduction mammaplasty: an outcome analysis. Ann Plast Surg. 1995 Oct;35(4):337-41. doi: 10.1097/00000637-199510000-00001. PMID: 8585673.
- Aesthetic Plastic Surgery National Databank Statistics. 2020-2021.
- Aesthetic Plastic Surgery National Databank Statistics 2020-2021. Aesthet Surg J. 2022 Jun 22;42(Suppl 1):1-18. doi: 10.1093/asj/sjac116. PMID: 35730469.
- Nava MB, Rancati A, Angrigiani C, Catanuto G, Rocco N. How to prevent complications in breast augmentation. Gland Surg. 2017 Apr;6(2):210-217. doi: 10.21037/gs.2017.04.02. PMID: 28497025; PMCID: PMC5409896.
- Lewin R, Göransson M, Elander A, Thorarinsson A, Lundberg J, Lidén M. Risk factors for complications after breast reduction surgery. J Plast Surg Hand Surg. 2014 Feb;48(1):10-4. doi: 10.3109/2000656X.2013.791625. Epub 2013 Apr 29. PMID: 23627557.
- Sachs D, Szymanski KD. Breast reduction. In StatPearls. 2022.
- Kompatscher P, von Planta A, Spicher I, Seifert B, Vetter S, Minder J, Beer GM. Comparison of the incidence and predicted risk of early surgical site infections after breast reduction. Aesthetic Plast Surg. 2003 Jul-Aug;27(4):308-14. doi: 10.1007/s00266-003-3010-5. PMID: 15058556.
- Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Tien CH, Jeschke MG. Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014 Feb 24;5(1):28. doi: 10.1186/scrt417. PMID: 24564987; PMCID: PMC4055091.
- Davis MJ, Purita RJ, Shou J, Barrett CT. Three-dimensional electron microscopy of human umbilical cord tissue allograft pre and post processing: A literature comparison. J Biomed Res Environ Sci. 2022;3(8):934–940. doi: 10.37871/jbres1535.
- Barton SP, Marks R. Measurement of collagen-fibre diameter in human skin. J Cutan Pathol. 1984 Feb;11(1):18-26. doi: 10.1111/j.1600-0560.1984.tb00345.x. PMID: 6699234.
- Joszt L. Comparing costs of wound therapy in the post-acute setting. AJMC. 2022.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.