> Biology Group. 2021 August 30;2(8):721-730. doi: 10.37871/jbres1303.
Breakthrough Infection among Fully Vaccinated Physicians Working in COVID-19 Treatment Centers; Prevalence, Presenting Symptoms, Co-Morbidities and Outcome in the Third Wave of Epidemics in Myanmar
Khin Phyu Pyar1, Sai Aik Hla2, Soe Min Aung3, Di Wunn4, Zar Ni Htet Aung5, Nyan Lin Maung6, Thurein Win7, Linn Htet Aung8, Aung Phyoe Kyaw9, Kyaw Zay Ya10, Thi Han Tun11, Myo Thant Kyaw12, Zaw Lin Oo9, Zay Phyo Aung12, Than Naing Lin13 and Soe Moe Htun14
2Senior Consultant Physician, Department of Medicine No (2) Defence Services General Hospital (1,000 Bedded) Naypyitaw, Myanmar
3Department of Medicine No (20) Military Hospital (100-Bedded), Tha Baik Kyin, Mandalay Division, Myanmar
4Senior Consultant Physician, Department of Medicine No (1) Military Hospital (700-Bedded), Pyin Oo Lwin, Mandalay Division, Myanmar
5Senior Consultant Physician, Department of Medicine No (4) Military Hospital (300-Bedded), Mon Ywar, Sagaing Division, Myanmar
6Senior Consultant Physician, Department of Medicine No (2) Military Hospital (700-Bedded), Aung Ban, Shan State, Myanmar
7Assistant Lecturer / Consultant physician, Department of Medicine, Defence Services Medical Academy, Myanmar
8Senior Consultant Physician, Department of Medicine No (5) Military Hospital (300-Bedded), Maw La Myaing, Mon State, Myanmar
9Phaung Gyi COVID Medical Centre (1000-Bedded), Yangon Division, Myanmar
10Assistant Lecturer/ Haematologist, Department of Medicine, Defence Services Medical Academy, Myanmar
11Public Health Specialist No (1) Defence Services General Hospital (1000-Bedded), Myanmar
12Assistant Lecturer, Department of Medicine, Defence Services Medical Academy, Myanmar
13Phaung Gyi COVID Medical Centre (1000-Bedded), Yangon Division, Myanmar
14Assistant Lecturer, Department of Medicine, Defence Services Medical Academy, Myanmar
- Breakthrough infection
- COVID-19
- Physicians
- Symptoms
- Co-morbidity
- Outcome
Abstract
Background: Coronavirus Disease 2019 (COVID-19), emerged in China at the end of 2019, became a major threat to health around the world. Breakthrough infection following COVID-19 vaccine has clinical and public health significance. The highest groups at risk of infection during the COVID-19 pandemic is health care workers; the physicians are the frontline workers. This study aimed to assess the prevalence of breakthrough COVID-19 infection and their clinical presentation, co-morbidities and outcome among physicians who were fully vaccinated, working in COVID-19 treatment centers in Myanmar.
Methods: A cross-sectional descriptive study was conducted among physicians, at least 14 days after receiving second dose, working at COVID-19 treatment centers in Myanmar, during the third wave from end of May to August 2021. Data were collected by using standardized forms and analysis was done.
Results: Among 410 physicians, 98.2% (221/225) received two dose of vaccination: Covaxin 90.0%, Covishield 9.5% and Sputink V 0.5%. They received first dose of vaccine in January/February 2021 and second dose in March/April 2021. In Myanmar, third wave started in end of May; the largest pandemic surge had reached its peak in July, 2021. In the third wave, most of them 72.9% (161/221) did not experience no infection. The prevalence of fully vaccinated break through infection was 27.1% (60/221); the majority 78.3% (47/60) were mild symptomatic infection. Severe infection was seen in 10% of physicians with breakthrough infection who required hospital admission and oxygen therapy. The common presenting symptoms in order of frequency were body aches and pain 62.6%, sneezing 56.6%, headache 53.5%, cough 52.5%, sore throat 45.5%, anosmia 33.3%, runny nose 23.2% and loose motion 27.3%. The uncommon symptoms were dyspnoea 9.1%, vertigo 6.1%, skin rash 5.1%, vomiting 5.1%, petechiae 3.0%, tinnitus 3.0% and silent hypoxia 3.0%, and non-per-os 1%. Most of them did not have any significant comorbidities. One out of six physicians having severe infection had diabetes mellitus and two were obese. The mean duration of hospital stay was 7 days. None of the cases was fatal.
Conclusions: In this study, over 98% of physicians were fully vaccinated; majority with Covaxin. One in four physicians had breakthrough infection in third wave; mainly mild form. Nearly half of them had possible delta symptoms; aches and pain, sneezing, runny nose, headache, cough, and sore throat. Awareness of rare but important symptoms like Non-per-Os and vertigo should be highlighted both to public and health care personnel. Ten percent of physicians with breakthrough infection were severe. Mortality rate was zero.
Background
Coronavirus Disease 2019 (COVID-19) has been spreading to the whole world since December 2019. As of 17th August, 2021, the total number of confirmed cases was over 207 million with approximately 4.36 million deaths around the world. Regarding immunization, 4,452 million vaccine doses have been administered world-wide [1].
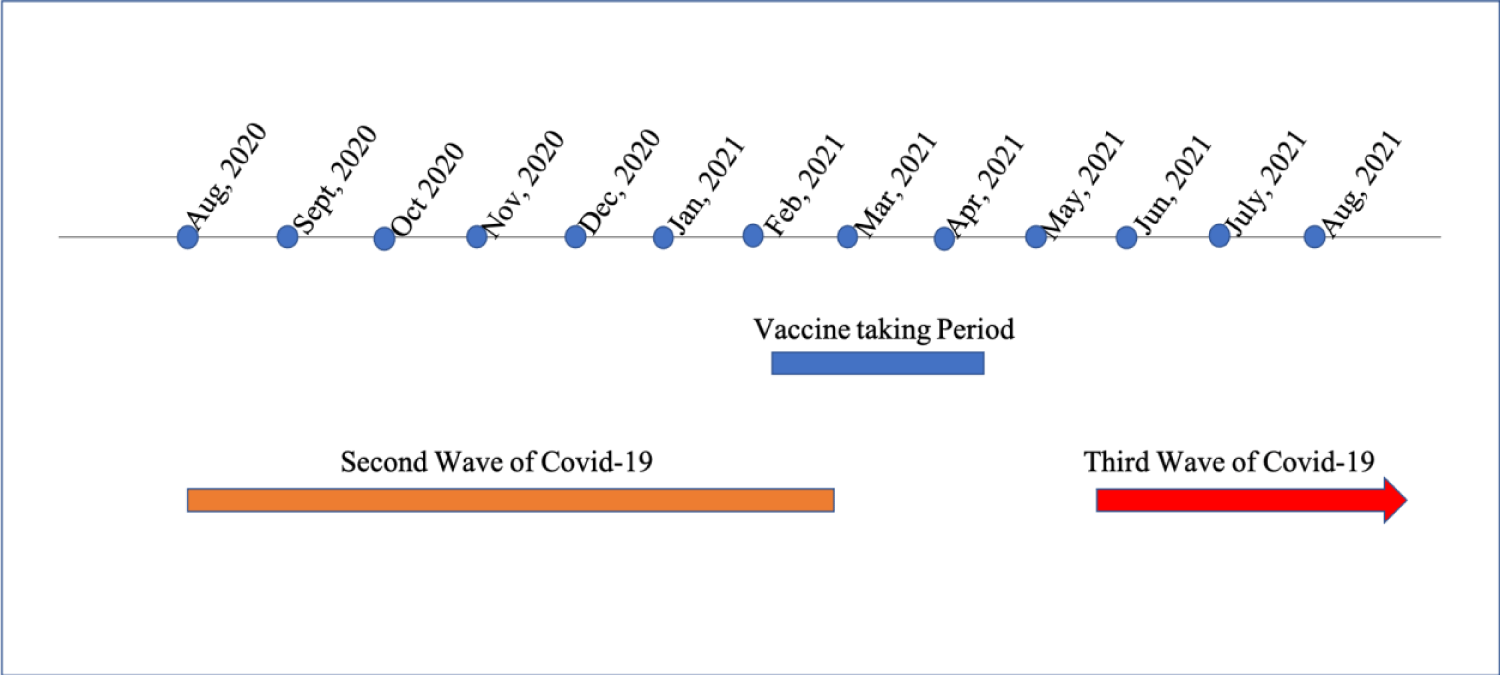
In Myanmar, the total population was estimated at 54.05 million in 2019, with about 70% of the population resided in rural areas [2]. In the first wave of epidemic, COVID-19 reported cases were identified on 23rd March 2020 and there were 379 confirmed cases and 6 deaths. In the second wave, began in October 2020 and the number gradually dropped in March 2021. Third wave began in end of May and reached peak in July/August. As of 17th August, 2021, the total number of confirmed cases was 357,000 with approximately 13,445 deaths [1].
Vaccine program was launched in Myanmar with a priority to over 65 in January 2021; Covaxin and Covishield were the two main vaccine. Two doses were given with the interval of 4-6 weeks. All the health care personnel were given vaccination at the same time; first dose of vaccine in January/February 2021 and second dose in March/April 2021. Figure 1 as of 5th June 2021, 3.5 million vaccine doses were given; people fully vaccinated was 1.53 million which covered 2.8% of total population [3].
Covaxin is whole-virion inactivated SARS-CoV-2 vaccine; it uses a complete infective SARS-CoV-2 viral particle consisting of RNA surrounded by a protein shell, but modified so that it cannot replicate [4]. In Covaxin’s phase I trial, 375 subjects who received the vaccine had notably elevated antibody response [5]; it proved safety and immunogenicity [6]. Its phase II trial result, a preprint paper on MedRxiv, highlighted enhanced immune response and safety [7]. In phase III trials, 25,800 participants have been enrolled; Bharat Biotech released interim efficacy data on 3 March 2021, showed a clinical efficacy of 81% [5]. The efficacy was 70% for Covaxin, 90% for Covisheld and 94% for Sputinex V. The immunity was said to be lasted for 6 months. With the development of new strain, the efficacy on particular strain varied with different vaccine. The effect of Covaxin on alpha & delta strain was mentioned in some studies [8]. Regarding Covaxin, efficacy at preventing disease and infection to D614G & B.1.1.7 were 78% and 69% respectively whereas efficacy at preventing disease and infection to B.1.351, P.1, B.1.617.2 were 68% and 60% respectively [9]. A large-scale study in India armed forces following completed Covishield vaccine revealed nearly 93% reduction in prevalence of Covid infections; and, reduction in COVID-related deaths by 98% [9]. Breakthrough infections were found to be related with COVID-19 variant of concern [10].
The clinical spectrum of COVID-19 could range from asymptomatic infection or mild upper respiratory tract illness to severe pneumonia with respiratory failure. The common symptoms were fever, cough, shortness of breath, fatigue, muscle aches, headache, loss of taste or smell, sore throat, runny nose, nausea or vomiting, and diarrhea [11-14]. Delta strain was said to give four common symptoms; headache, nasal stiffness, running nose and sore throat. The audio-vestibular symptoms like vertigo and tinnitus were not frequently mentioned [15] and these symptoms were known only by recalling retrospectively [16]. The clinical presentation, severity of disease and outcome [17] depended not only on race and ethnic groups [18,19] but also on viral strain- variant [20-22]. In the previous study done in Myanmar in second wave, the common initial symptoms reported by [23] were fever, loss of smell, cough, muscle ache and headache; thus, if common symptoms changed significantly in third wave, it would possibly be related with virus variant.
Certain comorbidities such as diabetes, heart diseases, chronic kidney disease, and obesity were strongly related to COVID-19 hospitalization and severity [24,25] and death [26]. Reported break through infections especially among health care workers were usually mild and non-fatal [27,28]. Nevertheless, the mortality in breakthrough infection was found to be related with mainly with age [29]. The clinical characteristics of 152 cases with breakthrough infection done in Israel revealed that 40% of them were in immune-compromised state; thus, the mortality rate was 22%, the highest among the studies on breakthrough infection [30]. The study pointed out the impact of comorbidities on severity and outcome as most of the cases had multiple comorbidities: hypertension, diabetes mellitus, congestive cardiac failure, chronic kidney disease, chronic lung disease, dementia and malignancy [30].
In Myanmar, non-communicable diseases were estimated to account for 68% of all deaths, with an exposure to potential risk factors such as raised blood pressure, diabetes mellitus, harmful use of alcohol, physical inactivity, tobacco use, and overweight or obesity. Cardiovascular diseases, diabetes mellitus, cancers and chronic respiratory diseases were reported to be the major contributing factors to the Non-Communicable Diseases (NCDs) burden in Myanmar.
Physicians are important actors in the critical infrastructure and they are under considerable risk of infection especially in COVID epidemic; thus, their physical fitness is extremely important both for their selves, health care system and nation-Myanmar. Studies suggested that COVID-19 vaccines protect against severe illness; however, breakthrough infections can occur because COVID-19 vaccines do not offer 100% protection. There were very few reports on breakthrough infection among health care workers [27,31,32]. The elevated risk of infection among health care workers, both clinical and non-clinical sectors, was seen in several studies [33] and it was the emerging problem in COVID era [34]. Some study highlighted that personnel protective measures were still essential in health care workers because those having breakthrough infection following fully vaccination were found to have high level of vaccine induced antibody [35]. In Iran study, prevalence of break through infection was 5.6% among the health care workers, the highest groups at risk of infection during the COVID-19 pandemic [31]; however, in the study done in India, it was 10% [36]. The reported prevalence of breakthrough infection varied from one country to another; it differed even within same country with same vaccine with different study site in the same study population- health care workers [27,28,37].
Data on the prevalence of breakthrough infections and the severity were limited. The efficacy of COVID vaccine in prevention of infection, prevention of severe disease and death especially in physicians working in highly infectious hospital environment was critical for health care system not only for man power management of health care worker but also for future planning of vaccine program. Analysis of symptoms particularly those due to delta virus strain would guide the effectiveness of vaccine over delta strain; thus, it would be useful in resource limited areas where molecular study could not be performed. Better understanding of vaccine efficacy, effectiveness over new strains- variant and preparedness for future vaccine program were required. In Myanmar, there is no previous study regarding the breakthrough infection among physicians caring patients with COVID-19 infection, its clinical presentation, severity and outcome. It is necessary to investigate them in Myanmar, where the prevalence of breakthrough infection may differ from other countries, using the same vaccine or different vaccine; any peculiar clinical presentation of breakthrough infection; the degree of clinical severity; and, mortality rate. The aim of the study was to detect the prevalence of breakthrough infection among physicians working in COVID treatment centers, their symptoms, co-morbidities, severity and outcome in Myanmar.
Methods
Study design and population
A cross-sectional study was conducted among physicians who had completed vaccination caring COVID-19 patients in various COVID-19 treatment center in Myanmar from March to August 2021. All physicians who had completed vaccination two weeks earlier, caring COVID-19 patients in various COVID-19 treatment center were included in this study. They had to do RT-PCR testing of a nasopharyngeal sample for confirmed SARS-CoV-2 infection if they suffered symptoms or they had exposure to COVID-19 patients under high risks; prolonged cardiopulmonary resuscitation, accidental tear of mask, difficult endotracheal tube intubation. They had to analyze their symptoms by their selves and also by the primary investigator; then, they took appropriate treatment by themselves and senior physicians. If required, second opinion was given by senior physician through Viber. Moderate and severe cases were hospitalized and treated according to national and local guideline; they were followed up till day 30 through Viber.
Sample size determination and sampling technique
Target population of this study was physicians working in COVID-19 treatment centers. There are 410 physicians currently working in Directorate of Medical Services. The required sample size was calculated by estimating a finite population mean. For 95 per cent confidence interval, 80 per cent power was set and two sided test, level of significance is 0.05 [38]. The estimating a finite population mean, the minimal required sample size was calculated as follows:
Sample size calculation
When two groups need to be compared, the following formula can be used.
N σ2 z21-α/2
n = d2 (N-1) + σ2 z21-α/2
where,
n = minimum required sample size for 95% confidence level.
N = Population size (400)
Z1-α = 1.96 (95% Confident Interval)
σ = The standard deviation (SD.) (5.0)
d = error (0.5)
n = 196
n = 217 (with 10% dropped out)
The minimum required sample size for 95% confidence level was 197 and it could be carried out with 216 samples to cover 10% dropout. The minimum required sample size for 95% confidence level was 197 and it was carried out with 216 samples to cover 10% dropout. All physicians working in COVID-19 treatment centers during the study (n = 222) were selected after getting informed consent.
Operational definitions
Unvaccinated received no COVID-19 vaccine doses. Partially vaccinated received one dose of COVID-19 vaccine. Fully vaccinated received two doses of COVID-19 vaccine. Fully vaccinated but not immune received two doses but <14 days had elapsed since receipt of second dose. Fully vaccinated with breakthrough infection received two doses and then received a positive SARS-CoV-2 test result ≥ 14 days after receipt of the second dose.
Prevention of infection was defined as the vaccine efficacy at stopping transmission of the virus from one person to another. Prevention of disease was defined as the vaccine efficacy at preventing an exposed individual who contracted the virus from developing disease which may be symptomatic or asymptomatic.
Body Mass Index (BMI) was a person’s weight in kilo- grams divided by the square of height in meters and it an indicator of body fatness. BMI was categorized as under- weight (<18.5 kg/m2), normal weight (18.5 to 24.9 kg/ m2), overweight (25.0 to 29.9 kg/m2) and (≥ 30.0 kg/m2) obese. Comorbidity was a presence of more or additional medical conditions or diseases in physicians. The initial presenting symptom was a symptom or group of symptoms at the time of confirmation of COVID-19 infection by positive nasopharyngeal swab for PCR for COVID-19 (such as runny nose, muscle ache, cough, sore throat, dyspnea, etc.) and it was categorized as asymptomatic and symptomatic.
The severity of COVID-19 was classified as mild, moderate and severe disease. Mild disease was symptomatic patients without evidence of viral pneumonia or hypoxia. Moderate disease was confirmed patients with clinical signs of pneumonia (fever, cough, dyspnea, and fast breathing). Severe disease was confirmed patient with clinical signs of pneumonia (fever, cough, dyspnea, and fast breathing) adding one of the following: respiratory rate > 30 breaths per min, severe respiratory distress and SpO2 < 93% on room air. For the final analysis, the severity was categorized as non-severe (asymptomatic, mild, and moderate) and severe disease.
Data collection and procedure
Demographic characteristics (sex, age, height, weight, blood group), initial presenting symptoms (asymptomatic and symptomatic patients with muscle aches, headache, sore throat, runny nose fever, chills, difficulty in breathing, fatigue, loss of smell, loss of taste, , nausea or vomiting, diarrhea), comorbidity (hypertension, diabetes mellitus) and severity of disease (mild, moderate, and severe) were collected using a standardized case report form. The name of the vaccine, date of first dose and date of second dose were recorded. The data were checked by two medical officers and then, supervision, completeness, and consistency of collected data were performed by the principle investigator.
Statistical analysis
The collected data were entered into Microsoft Excel 2016 and exported to IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY: IBM Corp) for analysis. Descriptive statistics were presented as frequency and percentages for categorical variables and mean (standard deviation, SD) for continuous variables.
Results
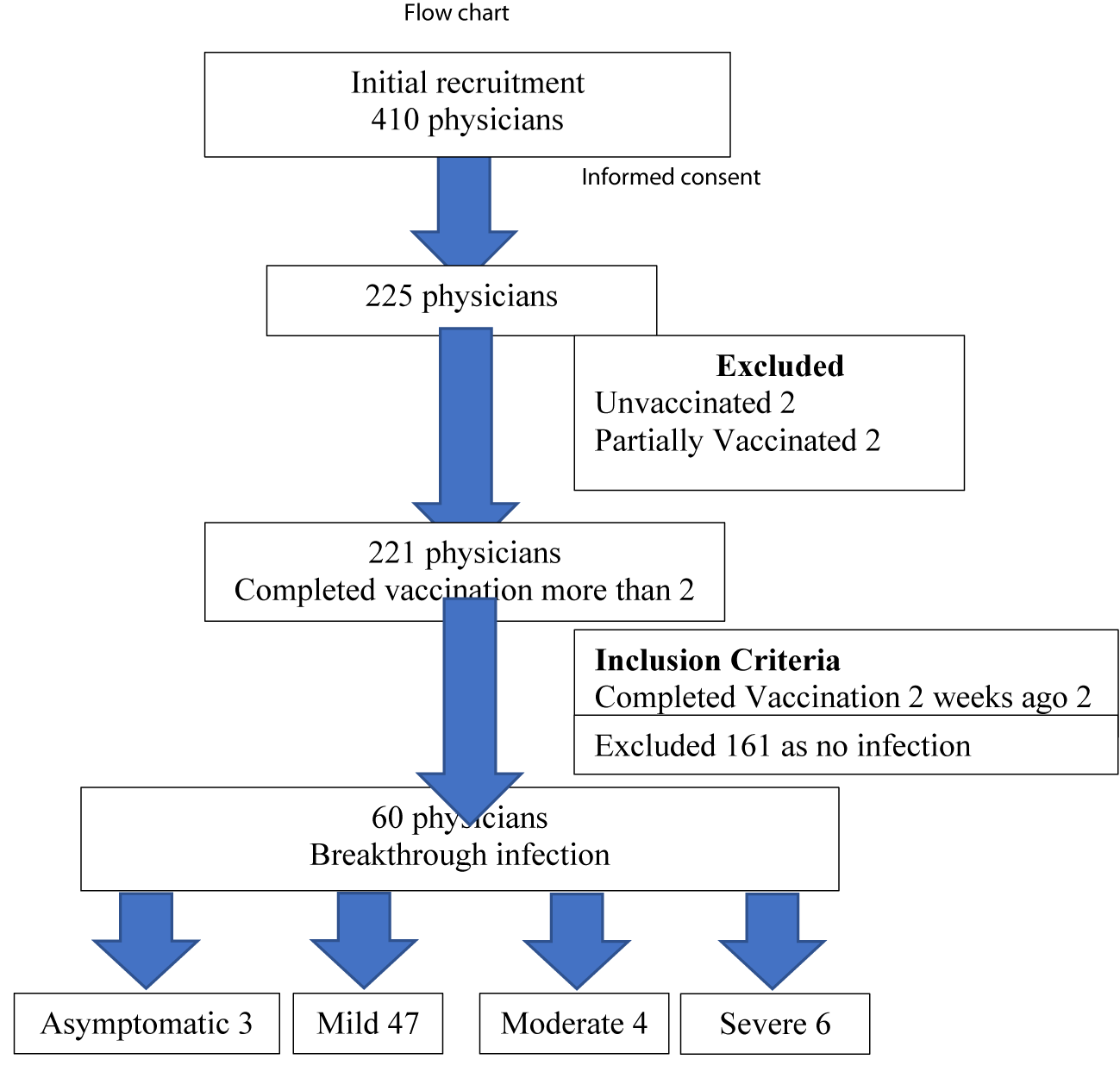
Among 410 physicians working in the medical field under Directorate of Medical Services, total of 225 physicians who were currently doing in COVID-19 treatment centers in different townships of 14 States and Divisions of Myanmar were included in this study. Most of physicians (221/225) had received a second dose of the Covaxin/Covisheld vaccine 14 days before the study which began on May 2021. Thus, the number of physicians who actually involved in this study was 221.The study ran for 14 weeks, during which Myanmar experienced its third and largest COVID-19 case surge figure 2.
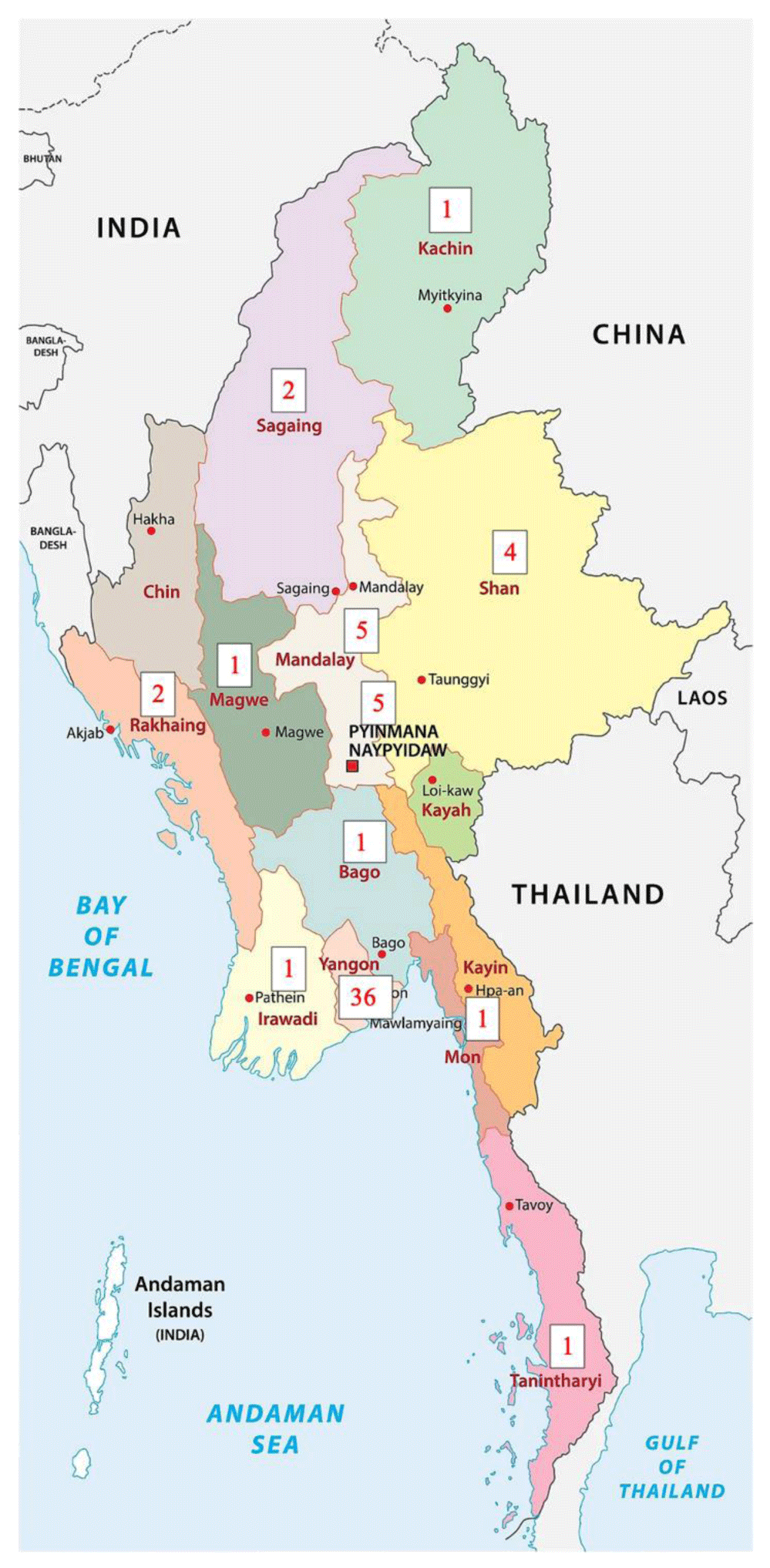
There are 14 States and Divisions in Myanmar. Seven States occupy hilly regions where they are less populated: Shan State, Kachin State, Chin State, Rachine State, Kayah State, Kayin State and Mon State. Seven Divisions are non-hilly areas; thus, they are densely populated favoring spread of epidemics: Yangon Division, Nay Pyi Daw Division, Mandalay Division, Sagaing Division, Bago Division, Magway Division and Irrawaddy Division. (Figure 2) The proportion of working site assignment for physicians for various COVID-19 treatment centers in whole country order of frequency was as follows: Yangon division 35%, Mandalay division 25%, Nay Pyi Daw division 20%, and the remaining states and division 20%. It was in accordance with population density as Yangon, commercial capital of Myanmar; Mandalay, second business capital of Myanmar; and, Nay Pyi Daw, Central Government Administration capital of Myanmar are very congested. The number of cases of breakthrough infection according to State and Division were as follows: Yangon Division (36/60), Nay Pyi Daw Division (5/60), Mandalay Division (5/60), Sagaing Division (2/60), Bago Division (1/60), Magway Division (1/60), Tanintharyi Division (1/60) and Irrawaddy Division (1/60), Shan State (4/60), Kachin State (1/60), Chin State (0/60), Rachine State (2/60), Kayah State (0/60), Kayin State (1/60) and Mon State (0/60). More than half of the cases with breakthrough infection were from Yangon Division.
Table 1 shows the demographic characteristics of COVID-19 caring physicians. Most of them 211 (95.5%) were males. Age ranged from 28 to 59 year; fifty percent of them were 30 to 35 years age group. BMI ranged from 17.7 to 35, and 62.8% were in normal BMI group. Blood group distribution was as follows: group “O” 37.1%, group “B” 29%, group “A” 24.9%, and group “AB” 9%; it was the same as frequency distribution of blood group in Myanmar. The majority 81% (179/221) of them did not have comorbidities; the common comorbidities found were hypertension 8.6%, obesity 3.1%, asthma/COPD 2.7%, diabetes mellitus 2.3%, smoking 6.8% and alcohol 3.2%.
| Table 1: Baseline characteristics of physicians (n = 221). | |||
| Baseline Characteristics | Mean | Maximum | Minimum |
| Age(years) | 35.28 | 59 | 28 |
| BMI (kg/m2) | 23.98 | 35 | 17.71 |
| Gender | No of Physicians | Percent | |
| Male | 211 | 95.5% | |
| Female | 10 | 4.5% | |
| Age Group | No. of Physicians | Percent | |
| 25-40 | 190 | 85.9% | |
| 41-60 | 31 | 14.1% | |
| BMI Group | No. of Physicians | Percent | |
| <18.5 underweight | 6 | 2.8% | |
| 18.5-24.9 healthy weight | 139 | 62.8% | |
| 25-29.9 overweight | 69 | 31.2% | |
| ≥ 30 obese | 7 | 3.2% | |
| Blood Group | No of Physicians | Percent | |
| A | 55 | 24.9% | |
| B | 64 | 29% | |
| AB | 20 | 9% | |
| O | 82 | 37.1% | |
| Comorbid Diseases | No of Physicians | Percent | |
| No Comorbid | 179 | 81% | |
| Hypertension | 19 | 8.6% | |
| DM | 5 | 2.3% | |
| Obesity | 7 | 3.1% | |
| Asthma / COPD | 6 | 2.7% | |
| Heart Disease | 1 | 0.4% | |
| Dyslipidemia | 1 | 0.4% | |
| Gouty Arthritis | 1 | 0.4% | |
| Inflammatory Bowel Disease | 1 | 0.4% | |
| old TB | 1 | 0.4% | |
| Smoking (current) Smoking (past) |
14 1 |
6.4% 0.4% |
|
| Alcohol Drinking | 7 | 3.2% | |
(1) Unvaccinated: received no COVID-19 vaccine doses;
(2) Partially vaccinated: received one dose;
(3) Vaccinated but not immune: received two doses but <14 days had elapsed since receipt of second dose; and
(4) Fully Vaccinated without Breakthrough Infection (FVNBTI): Received two doses and then received a negative SARS-CoV-2 test result ≥ 14 days after receipt of the second dose.
(5) Fully Vaccinated with Breakthrough Infection (FVBTI): Received two doses and then received a positive SARS-CoV-2 test result ≥14 days after receipt of the second dose.
Table 2 reveals COVID-19 vaccination status and type of vaccine in physicians having breakthrough infection. Among 225 physicians, 98% (221/225) were fully vaccinated. They had taken Covaxin (90%) 199/221, Covishield (9.5%) 20/221 and Sputnik V (0.5%) 1/221. They received first dose of vaccine in January/February 2021 and second dose in March/April 2021. The median interval between the first and second dose was 28 days. In Myanmar, second wave began in July 2020 and end in March 2021; third wave started in June 2021 and reached peak in July/August. Breakthrough infections FVBTI occurred 74 days (mean) after completion of second dose.
| Table 2: COVID-19 vaccination status and type of vaccine in physicians having breakthrough infection (n = 225; n = 221). | ||
| COVID vaccination status (n = 225) | No. of physicians | Percentage |
| Unvaccinated Partially vaccinated Vaccinated but not immune |
2 2 0 |
0.9% 0.9% 0 |
| Fully Vaccinated Without Breakthrough Infection (FVNBTI) Fully Vaccinated With Breakthrough Infection (FVBTI) |
161 60 | 72.8% 27.2% |
| Type of vaccine taken in fully vaccinated (n = 221) | No. of physicians | Percentage |
| COVAXIN | 199 | 90.0% |
| COVISHEILD | 21 | 9.5% |
| SPUTNIK V | 1 | 0.5% |
Tables 3,4 represent different types of COVID -19 Infection among physicians during three waves of pandemic. In first and second wave, nearly 90% (197/221) of physicians did not acquire COVID-19 infection; 10% of them had infection, none of them had severe or critical infection. In the third wave, following fully vaccination, most of them 72.8% (161/221) did not experience infection. Only 27.2% (60/221) had break through infection (FVBTI); the proportion of asymptomatic infection, mild symptomatic infection, moderate symptomatic infection and severe infection in order of frequency were 1.5% (3/221), 21.2% (47/221), 1.8% (4/221) and 2.7% (6/221). The percentage of severity of FVBTI in order of frequency was asymptomatic infection 5% (3/60), mild symptomatic infection 78.3 % (47/60), moderate symptomatic infection 6.7% (4/60) and severe infection 10% (6/60).
| Table 3: Different types of COVID-19 Infection among physicians during three waves of pandemic (n = 221). | ||
| Types of COVID-19 Infection | 1st and/or 2nd wave (n = 221) | 3rd wave (breakthrough infection) (n = 221) |
| No Infection | 197(89.1%) | 161(72.8%) |
| Asymptomatic infection | 4(1.8%) | 3(1.5%) |
| Symptomatic (mild) | 18(8.1%) | 47(21.2%) |
| Symptomatic (moderate) | 2(0.9%) | 4(1.8%) |
| Symptomatic (severe) | 0 | 6(2.7%) |
| Symptomatic (critical) | 0 | 0 |
| Table 4: Different types of fully vaccinated breakthrough infection (n = 60). | ||
| Types of COVID -19 infection | Number | Percent |
| Asymptomatic infection | 3 | 5 |
| Symptomatic (mild) | 47 | 78.3 |
| Symptomatic (moderate) | 4 | 6.7 |
| Symptomatic (severe) | 6 | 10 |
| Symptomatic (critical) | 0 | 0 |
| Total | 60 | 100 |
The initial presenting symptoms and comorbidities of physicians with COVID-19 breakthrough infection are described in table 5. In third wave, the most common presenting symptoms in order of frequency were body aches and pain 62.6%, sneezing 56.6%, headache 53.5%, cough 52.5%, sore throat 45.5%, anosmia 33.3% and loose motion 27.3%. The rare symptoms were dyspnoea 9.1%, vertigo 6.1%, skin rash 5.1%, vomiting 5.1%, petechiae 3.0%, tinnitus 3.0% and silent hypoxia 3.0%, and Non-per-Os 1%. Only 6 physicians (10.0%) (6/60) of them required hospital admission due to severe infection. The mean duration of hospital stay was 7 days.
| Table 5: Frequency distribution of symptoms in breakthrough infection during 3rd wave of pandemic among physicians. | |
| 3rd Wave Symptoms | Percent |
| Body Aches & Pain | 62.60% |
| Sneezing | 56.60% |
| Fever | 54.50% |
| Headache | 53.50% |
| Cough | 52.50% |
| Sore Throat | 45.50% |
| Anosmia | 33.30% |
| Runny Nose | 32.30% |
| Loose Motion | 27.30% |
| Prostration | 17.20% |
| Dyspnoea | 9.10% |
| Vertigo | 6.10% |
| Vomiting | 5.10% |
| Skin Rashes | 5.10% |
| Petechiae | 3% |
| Tinnitus | 3% |
| Silent Hypoxia | 3% |
| Non-Per Os | 1% |
Discussion
The COVID-19 mainly affects the respiratory system, and some patients required oxygen therapy due to severe pneumonia, respiratory failure and acute respiratory distress syndrome. This study investigated the prevalence of breakthrough infection, symptoms, comorbidities, severity, and the outcome in front line health leaders; physicians who were fully vaccinated, working in COVID-19 treatment center. In this study, most of the physicians were male; and, 80% of them were in normal BMI group.
In first and second wave, 90% (197/221) of physicians did not acquire COVID-19 infection and 10% were infected; none of them had severe or critical infection. In the third wave, following fully vaccination, most of them 72.8% (161/221) did not experience infection. The number of physicians infected with COVID-19 infection rose from 10% to 27.2% in third wave. The possible reasons were as follows: first, quadruple increased in total number of confirmed positive cases in third wave leading to more risky exposure in public places like shopping center; second, the development of variant of viral strain which was partially covered by current vaccine; third, the magnitude of exposure to increasing number of COVID-19 cases in their hospital; and, finally, the immune status of physicians may be low because of stress.
More than half of the participating physicians with breakthrough infection were from Yangon Division. The main reason for having larger number of breakthrough infection in Yangon Division was due to sudden rapid increase in number of patients. Other reasons were multifactorial; Yangon is the most populated area causing higher number of COVID-19 infection in public; it is very congested in terms of population density favoring rapid spread; and, 35% of physicians involved in this study were working in COVID-19 treatment centers in Yangon. They did have adequate supply of PPE.
Third wave pandemic surge in Myanmar had reached its peak on July, 2021. Nearly two-third of fully vaccinated physician, 72%, did not acquire COVID-19 infection. Thus, the prevalence of breakthrough infection was 27 % i.e., one in four chances; it was two times higher than that of India studies done in health care workers [27,32]. Over ten thousand cases with SARS-CoV-2 vaccine breakthrough infections had been reported to CDC from 46 U.S States and territories in April 30, 2021[38]. The prevalence of breakthrough infection in health care workers was less than 5% in studies done in Israel and Chicago; 39 of 1,497 health-care workers fully vaccinated with the BNT162b2 (Pfizer-BioNTech) vaccine in Israeli [39]; and, 22 of 627 in Chicago [40]. In the study done by Sharma, et al. the prevalence of breakthrough infection among health care workers was less than 10% (37/325); and, they alarmed the chance of breakthrough infection as “one in nine chance” [27]. The same prevalence of breakthrough infection was seen in Mumbai study, India [36]. The prevalence was more than 10% in another study of India, done in chronic care hospital dealing with cases of diabetes mellitus; symptomatic breakthrough infection was 13.5% (15/113) [32]. In addition, Eastern India study, 83% of 274 cases of breakthrough infection in health care personnel were symptomatic; only 10% of them required hospital treatment [41]. The findings highlighted to consider booster dose immunization or mixed vaccination in future vaccine program to reduce the prevalence of breakthrough infection.
In this study, only 27.2% (60/221) had break through infection; the frequency of severity of fully vaccinated break through infection in order of frequency was asymptomatic infection 5% (3/60), mild symptomatic infection 78.3% (47/60), moderate symptomatic infection 6.6% (4/60) and severe infection 10% (6/60). Nearly 80% of breakthrough infection in this study were mild; thus, it proved the findings from various countries. In the study from Israel, breakthrough infection in health care workers were mild or asymptomatic [39]. Furthermore, the study done in Delhi which included 325 health care workers, 95% of cases were mild and did not need oxygen therapy [27]. Moreover, the study carried out among health care workers in chronic care hospital dealing with cases of diabetes mellitus, prevalence of symptomatic breakthrough infection was 13.5% (15/113) and only one case (1/15) required treatment in hospital; mortality rate was zero [32]. In addition, in Chicago study, two-third of breakthrough infection were asymptomatic and 10% of cases (2/22) were hospitalized; one out of two hospitalized cases died because of co-morbidities and the contributing factor for death was multiple co-morbidities [40]. Reasons to be hospitalized in breakthrough infection were interesting; it may or may not be related with COVID-19. The report on breakthrough infection to CDC in 2021 April, based on preliminary data, 2,725 (27%) cases of vaccine breakthrough infections were asymptomatic, and 995 (10%) patients were known to be hospitalized; and, 160 (2%) patients died. Among the 995 hospitalized patients, 289 (29%) were asymptomatic or hospitalized for a reason unrelated to COVID-19 [38].
In third wave, the top presenting symptom was aches and pain (66.2%); half of the physicians suffered sneezing, headache, cough and sore throat. Possibly delta symptoms like aches and pain, sneezing, sore throat and headache were noticeable in half of them which suggested possibility of delta variant of COVID-19 infection. It was similar to preliminary report from India which included 274 cases of breakthrough infection; the common symptoms found were fever (88.5%), cough (77.6%) and sore throat (59.6%) [41]. It was the same as the India study on health care worker, done in 123 health care workers, where fever in all cases, sore throat and cough were seen in half of the cases [32]. In another finding, the most common symptoms were upper respiratory congestion (36%), muscle pain (28%), loss of smell or taste (28%), and fever or chills (21%). Fever, sore throat and myalgia were common symptoms among 108 breakthrough infection cases noted in South India study [28]. The study in Mumbi involving 441 health care workers, 40/441 had break through infection; conjunctivitis, diarrhea, sore throat, anosmia, and headache were common at presentation as compared to body ache, fever (36). The main clinical symptoms of COVID-19 patients were fever (77.6%), cough (64.8%), fatigue (27.2%), dyspnea (21.2%) and sputum production (18.0%); they were reported in most of the studies [11,12]. Therefore, symptoms of fully vaccinated breakthrough infection seen in this study were slightly different from that of second wave.
Anosmia and gastrointestinal symptoms which were common findings in second wave in Myanmar attributed nearly 30% [23]. Anosmia was recorded in 33% of physicians with breakthrough infections; it was a popular alarming symptom in public.
The rare symptoms were interesting. Although symptoms arising from nose and upper respiratory tract like sneezing, runny nose and anosmia were mentioned commonly, those symptoms from middle ear like vertigo and tinnitus were neglected. They showed involvement of Eustachian tube and middle ear. In this study, 3-6% of physicians with fully vaccinated breakthrough infection noticed them. In fact, they were very rarely reported previously [16].
Moreover, dermatological manifestation was seen in 3-5% of cases. Petechiae were seen with normal platelet count in full blood count examination indicating either vasculitis or platelet qualitative defect. In addition, vomiting and Non-per-Os state recorded in this study were not overlooked; and, recognition of these symptoms early were critical for treatment point as they were indication for remdesivir therapy and parenteral fluid replacement. As most of the physicians in this study were young and healthy, silent hypoxia and dyspnoea were seen in 3-9 %, requiring oxygen therapy.
Most of them involved in this study did not have any significant comorbidities. Although 6 severe cases in this study required oxygen therapy and hospitalization, none of them required ventilatory support or died. The mean duration of hospital stay was 7 days. Because of the mild nature of breakthrough infection among health care workers, most of them did not require oxygen therapy nor hospitalization in other studies [27,36]; treatment in hospital was required in 5% to 10% of cases [28].
One physician having severe infection in this study had diabetes mellitus; and, two were obese. According to the reports on vaccine related breakthrough infection, there was no deaths among them [27,28]. However, mortality rate was 22% (34/152) in Israel study [30]. Co-morbidities found in Israel study were hypertension (108; 71%), diabetes (73; 48%), congestive heart failure (41; 27%), chronic kidney and lung diseases (37; 24% each), dementia (29; 19%) and cancer (36; 24%). In Chicago study, one case died because of co-morbidity [40]. In the study done by Butt, et al. in Qatar where 10% of breakthrough infection were severe; they concluded that vaccination status was associated with a significantly lower risk of severe disease and old age was a risk factor for severe breakthrough infection [29]. Nevertheless, the mortality was related with associated co-morbidities like hypertension, diabetes, and cardiovascular disease, chronic lung disease, chronic kidney disease, and cancer [30]. The clinical characteristics of 152 cases with breakthrough infection done in Israel revealed that 40% were immune-compromised; the mortality rate was 22%, the highest among the studies on breakthrough infection [30]. The study pointed out the impact of comorbidities as most of the cases had multiple comorbidities: hypertension, diabetes mellitus, congestive cardiac failure, chronic kidney disease, chronic lung disease, dementia and malignancy [30].
There were some limitations in this study. Firstly, it was relatively difficult to establish the cause of breakthrough infection like variant strain which was not performed because of economic issue. Secondly, spike protein antibody level should be measured and the level should be correlated during the peri-infection period. Thirdly, the morbidity and mortality rate of breakthrough infection may be higher if vaccinated person is elderly, immune-deficient and he has co-morbidities like hypertension, chronic lung disease, chronic kidney disease, organ transplant recipient and connective tissue disorder. In this study, all physicians are generally healthy and in working age group- under 60 years. Further research using larger sample size including older age group would give different answer. Lastly, measuring viral load play important role in prevalence of breakthrough infection which could not be done at present.
Conclusion
In this study, 98% of physicians were fully vaccinated; mainly Covaxin. The prevalence of breakthrough infection among them was 27%. Thus, completely vaccinated persons should not be overlooked. Most of the breakthrough infection was mild form. Nearly half of them had delta symptoms: aches and pain, runny nose, headache, cough, and sore throat. Awareness of rare but important symptoms like Non-per-Os and vertigo should be emphasized both to public and health care personnel to get treatment timely. Only 10% of breakthrough infection was severe, requiring hospitalization and oxygen therapy. Two physicians with severe infection had co-morbidities; diabetes mellitus, hypertension and obesity. The mortality rate was zero. Therefore, expansion of COVID vaccination program is required to reduce morbidity and mortality of COVID-19 infection in Myanmar. Research in breakthrough infection in Myanmar should be extended.
Acknowledgement
The authors would like to thank all the candidates for giving informed consent to this study. The authors also acknowledged Prof Ko Ko Lwin, Prof Kyaw Zay Ya, Prof Myint Zaw for administrative support, and Professor Khine Khine Su and Dr Kyaw Wunna for laboratory support.
Ethical Approval
This study was approved by Hospital Research and Ethic Committee from Defence Services General Hospital (1,000 Bedded) Mingaladon, Myanmar. Informed consent was also taken from each physician.
References
- WHO. WHO Coronavirus (COVID-19). 2021 Aug. https://tinyurl.com/22s2tzcj
- United Nations Population Division. Burma (Myanmar). https://tinyurl.com/7vtfvx2p
- MOHS. Coronavirus Disease 2019 (COVID-19) Surveillance Dashboard (Myanmar). 2021 Aug. https://tinyurl.com/2bx5jym8
- Robertson S. India’s whole-virion inactivated SARS-CoV-2 vaccine shows promise. News Medical Life Sciences. 2020. https://tinyurl.com/pamrwrjr
- Thiagarajan K. What do we know about India’s Covaxin vaccine? BMJ. 2021 Apr 20;373:n997. doi: 10.1136/bmj.n997. PMID: 33879478.
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, Panda S, Gupta N, Reddy P, Verma S, Kumar Rai S, Singh C, Redkar SV, Gillurkar CS, Kushwaha JS, Mohapatra S, Rao V, Guleria R, Ella K, Bhargava B. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021 May;21(5):637-646. doi: 10.1016/S1473-3099(20)30942-7. Epub 2021 Jan 21. Erratum in: Lancet Infect Dis. 2021 Apr;21(4):e81. PMID: 33485468; PMCID: PMC7825810.
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv. 2020.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021 Aug 12;385(7):585-594. doi: 10.1056/NEJMoa2108891. Epub 2021 Jul 21. PMID: 34289274; PMCID: PMC8314739.
- Ghosh S, Shankar S, Chatterjee K, Chatterjee K, Yadav AK, Pandya K, Suryam V, Agrawal S, Ray S, Phutane V, Datta R. COVISHIELD (AZD1222) VaccINe effectiveness among healthcare and frontline Workers of INdian Armed Forces: Interim results of VIN-WIN cohort study. Med J Armed Forces India. 2021 Jul;77(Suppl 2):S264-S270. doi: 10.1016/j.mjafi.2021.06.032. Epub 2021 Jul 26. PMID: 34334892; PMCID: PMC8313084.
- Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, Caspi I, Levy R, Leshchinsky M, Ken Dror S, Bergerzon G, Gadban H, Gadban F, Eliassian E, Shimron O, Saleh L, Ben-Zvi H, Keren Taraday E, Amichay D, Ben-Dor A, Sagas D, Strauss M, Shemer Avni Y, Huppert A, Kepten E, Balicer RD, Netzer D, Ben-Shachar S, Stern A. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021 Aug;27(8):1379-1384. doi: 10.1038/s41591-021-01413-7. Epub 2021 Jun 14. PMID: 34127854.
- Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front Public Health. 2021 Jan 15;8:582932. doi: 10.3389/fpubh.2020.582932. PMID: 33520910; PMCID: PMC7844320.
- Tsai PH, Lai WY, Lin YY, Luo YH, Lin YT, Chen HK, Chen YM, Lai YC, Kuo LC, Chen SD, Chang KJ, Liu CH, Chang SC, Wang FD, Yang YP. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. 2021 Jan 1;84(1):3-8. doi: 10.1097/JCMA.0000000000000463. PMID: 33230062.
- Sheleme T, Bekele F, Ayela T. Clinical Presentation of Patients Infected with Coronavirus Disease 19: A Systematic Review. Infect Dis (Auckl). 2020 Sep 10;13:1178633720952076. doi: 10.1177/1178633720952076. PMID: 32973375; PMCID: PMC7495523.
- Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020 Mar 17;9(1):29. doi: 10.1186/s40249-020-00646-x. PMID: 32183901; PMCID: PMC7079521.
- Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front Public Health. 2021 Jan 15;8:582932. doi: 10.3389/fpubh.2020.582932. PMID: 33520910; PMCID: PMC7844320.
- Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol. 2021 Mar 22:1-11. doi: 10.1080/14992027.2021.1896793. Epub ahead of print. PMID: 33750252.
- SeyedAlinaghi S, Mirzapour P, Dadras O, Pashaei Z, Karimi A, MohsseniPour M, Soleymanzadeh M, Barzegary A, Afsahi AM, Vahedi F, Shamsabadi A, Behnezhad F, Saeidi S, Mehraeen E, Shayesteh Jahanfar. Characterization of SARS-CoV-2 different variants and related morbidity and mortality: a systematic review. Eur J Med Res. 2021 Jun 8;26(1):51. doi: 10.1186/s40001-021-00524-8. PMID: 34103090; PMCID: PMC8185313.
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT Jr, Skanderson M, Hauser RG, Schultze A, Jarvis CI, Holodniy M, Re VL 3rd, Akgün KM, Crothers K, Taddei TH, Freiberg MS, Justice AC. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. medRxiv [Preprint]. 2020 May 18:2020.05.12.20099135. doi: 10.1101/2020.05.12.20099135. Update in: PLoS Med. 2020 Sep 22;17(9):e1003379. PMID: 32511524; PMCID: PMC7273292.
- Townsend MJ, Kyle TK, Stanford FC. Outcomes of COVID-19: disparities in obesity and by ethnicity/race. Int J Obes (Lond). 2020 Sep;44(9):1807-1809. doi: 10.1038/s41366-020-0635-2. Epub 2020 Jul 9. PMID: 32647359; PMCID: PMC7347050.
- Danchin A, Timmis K. SARS-CoV-2 variants: Relevance for symptom granularity, epidemiology, immunity (herd, vaccines), virus origin and containment? Environ Microbiol. 2020 Jun;22(6):2001-2006. doi: 10.1111/1462-2920.15053. Epub 2020 May 19. PMID: 32367648; PMCID: PMC7267449.
- Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9;372:n579. doi: 10.1136/bmj.n579. PMID: 33687922; PMCID: PMC7941603.
- Graham MS, Sudre CH, May A, Antonelli M, Murray B, Varsavsky T, Kläser K, Canas LS, Molteni E, Modat M, Drew DA, Nguyen LH, Polidori L, Selvachandran S, Hu C, Capdevila J; COVID-19 Genomics UK (COG-UK) Consortium, Hammers A, Chan AT, Wolf J, Spector TD, Steves CJ, Ourselin S. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021 May;6(5):e335-e345. doi: 10.1016/S2468-2667(21)00055-4. Epub 2021 Apr 12. PMID: 33857453; PMCID: PMC8041365.
- Htun YM, Win TT, Aung A, Latt TZ, Phyo YN, Tun TM, Htun NS, Tun KM, Htun KA. Initial presenting symptoms, comorbidities and severity of COVID-19 patients during the second wave of epidemic in Myanmar. Trop Med Health. 2021 Aug 6;49(1):62. doi: 10.1186/s41182-021-00353-9. PMID: 34362468; PMCID: PMC8343344.
- Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KOA, Ahmed Z, Younas S. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020 Dec;13(12):1833-1839. doi: 10.1016/j.jiph.2020.07.014. Epub 2020 Aug 4. PMID: 32788073; PMCID: PMC7402107.
- Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, Miller F, Herbst H, Corman VM, Martin H, Radbruch H, Heppner FL, Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021 Feb 19;11(1):4263. doi: 10.1038/s41598-021-82862-5. PMID: 33608563; PMCID: PMC7895917.
- Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, Sempa JB, Burt FJ, Taylor A, Mahalingam S. Comorbidities in SARS-CoV-2 Patients: a Systematic Review and Meta-Analysis. mBio. 2021 Feb 9;12(1):e03647-20. doi: 10.1128/mBio.03647-20. PMID: 33563817; PMCID: PMC7885108.
- Sharma P, Mishra S, Basu S, Tanwar N, Kumar R. Breakthrough infection with SARS-CoV-2 and its predictors among healthcare workers in a medical college and hospital complex in Delhi, India. medRxiv. 2021. doi: 10.1101/2021.06.07.21258447
- Niyas VKM, Arjun R. Response to letter re Breakthrough COVID-19 infections among health care workers after two doses of ChAdOx1 nCoV-19 vaccine. QJM. 2021 Jul 23:hcab204. doi: 10.1093/qjmed/hcab204. Epub ahead of print. PMID: 34297117; PMCID: PMC8344452.
- Butt AA, Nafady-Hego H, Chemaitelly H, Abou-Samra AB, Khal AA, Coyle PV, Kanaani ZA, Kaleeckal AH, Latif AN, Masalmani YA, Bertollini R, Raddad LJA. Outcomes Among Patients with Breakthrough SARS-CoV-2 Infection After Vaccination. Int J Infect Dis. 2021 Aug 8;110:353-358. doi: 10.1016/j.ijid.2021.08.008. Epub ahead of print. PMID: 34375762; PMCID: PMC8349447.
- Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, Maor Y, Cohen R, Hussein K, Weinberger M, Zimhony O, Chazan B, Najjar R, Zayyad H, Rahav G, Wiener-Well Y. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021 Jul 7:S1198-743X(21)00367-0. doi: 10.1016/j.cmi.2021.06.036. Epub ahead of print. PMID: 34245907; PMCID: PMC8261136.
- Sabetian G, Moghadami M, Hashemizadeh Fard Haghighi L, Shahriarirad R, Fallahi MJ, Asmarian N, Moeini YS. COVID-19 infection among healthcare workers: a cross-sectional study in southwest Iran. Virol J. 2021 Mar 17;18(1):58. doi: 10.1186/s12985-021-01532-0. PMID: 33731169; PMCID: PMC7968574.
- Tyagi K, Ghosh A, Nair D, Dutta K, Singh Bhandari P, Ahmed Ansari I, Misra A. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr. 2021 May-Jun;15(3):1007-1008. doi: 10.1016/j.dsx.2021.05.001. Epub 2021 May 3. PMID: 33991805; PMCID: PMC8091733.
- Al-Kuwari MG, AbdulMalik MA, Al-Nuaimi AA, Abdulmajeed J, Al-Romaihi HE, Semaan S, Kandy M. Epidemiology Characteristics of COVID-19 Infection Amongst Primary Health Care Workers in Qatar: March-October 2020. Front Public Health. 2021 May 20;9:679254. doi: 10.3389/fpubh.2021.679254. PMID: 34095077; PMCID: PMC8173064.
- Barranco R, Ventura F. Covid-19 and infection in health-care workers: An emerging problem. Med Leg J. 2020 Jul;88(2):65-66. doi: 10.1177/0025817220923694. Epub 2020 May 22. PMID: 32441196.
- Schulte B, Marx B, Korencak M, Emmert D, Aldabbagh S, Eis-Hübinger AM, Streeck H. Case Report: Infection With SARS-CoV-2 in the Presence of High Levels of Vaccine-Induced Neutralizing Antibody Responses. Front Med (Lausanne). 2021 Jul 23;8:704719. doi: 10.3389/fmed.2021.704719. PMID: 34368197; PMCID: PMC8342944.
- Patil Y, Kesari M, Agrawal S, Dholpure M, Reheman, H. Post vaccination covid infection in health care workers at a tertiary care centre: A retrospective cohort study. Int J Contemp Med Res. 2021. https://tinyurl.com/5sxs8c
- Wayne WD, Daniel, Wayne W. Biostatistics-A Foundations for Analysis in the Health Sciences. Wiley & Sons, New York-Chichester-Brisbane-Toronto-Singapore, 6th ed. 1995;744-744.
- CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC-United States. 2021. https://tinyurl.com/j7euth28
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, Tal I, Zavitan M, Zuckerman N, Bar-Chaim A, Kreiss Y, Regev-Yochay G. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021 Jul 28:NEJMoa2109072. doi: 10.1056/NEJMoa2109072. Epub ahead of print. PMID: 34320281; PMCID: PMC8362591.
- Teran RA, Ghinai I, Gretsch S, Cable T, Black SR, Green SJ, Perez O, Chlipala GE, Cline MM, Kunstman KJ, Bleasdale SC, Fricchione MJ. COVID-19 Outbreak Among a University’s Men’s and Women’s Soccer Teams-Chicago, Illinois, July–August 2020. Morb Mo2rtal Wkly Rep. 2020;43:1591-1594. https://tinyurl.com/4vsfw5c
- Dash GC, Subhadra S, Turuk J, Parai D, Rath S, Sabat J, Rout UK, Kanungo S, Choudhary HR, Nanda RR, Pattnaik M, Pati S, Bhattacharya D. Breakthrough SARS-CoV-2 infections in an eastern state of India: A preliminary report. 2021. doi:10.21203/rs.3.rs-649914/v2
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.