> Medicine Group. 2021 August 31;2(8):731-740. doi: 10.37871/jbres1304.
Prevention of Opioid Addiction
Stephanie A Ihezie and Nachum Dafny*
- Opioid
- Addiction
- Interferon
- Cyclosporine
- Cortisol
Abstract
Opioid addiction is classified as a Substance Use Disorder (SUD), a complex and chronic health condition with physical, social, and psychological consequences. While there is no cure for it, we present a novel approach towards preventing a hallmark feature of addiction-- the opiate withdrawal syndrome. Opioids exert numerous effects, acutely and chronically, on the nervous system with physical dependence, tolerance, and withdrawal being the most adverse chronic features. The degree of opioid dependence can be quantified by the frequency and/or intensity of the behavioral expression of withdrawal seen after abrupt termination of opioid consumption or after treatment with an opioid antagonist such as naloxone. Although the Central Nervous System (CNS) is the primary area of opioid impact, the involvement of the immune system in modifying CNS phenomena was suggested nearly two centuries ago and proved by several groups within the last few decades. Through a series of studies with immunomodulators alpha interferon, cyclosporine A, and cortisol, preclinical experiments show that administration of these agents prior to chronic morphine exposure prevents the expression of opiate withdrawal a hallmark feature of addiction. This review provides updates on current developments in the management of the opioid epidemic and an overview of studies on preventative immunomodulation prior to repetitive opioid administration as a means of addressing one of the underlying symptomatology driving the epidemic.
Introduction
The rise of the opioid epidemic
Morphine is a legally prescribed opioid analgesic, and as the first alkaloid isolated from opium by German pharmacist Friedrich Sertürner in the early 1800s, it is the standard by which other opioids are tested [1,2]. The current opioid epidemic began its precipitous incline in the 1990s when opioids were widely prescribed for post-surgical and chronic pain [1]. The rationale for this boom is that opioids were known to be potent but falsely projected as not having any serious adverse effects. It was during this decade that the incidence of first time opioid analgesic abuse rose from 628,000 individuals in 1990 to 2.4 million in 2001 [3]. One of the more recent attempts to curb this trend was when the FDA encouraged the reformulation of OxyContin in order to make it more difficult to abuse, but this has been associated with a subsequent rise in heroin use [4,5].
About 20-30% of patients who are prescribed opioids for chronic pain misuse them, and about 80% of heroin users first misuse prescription opioids [6,7]. More than 210 million opiate prescriptions were filled in 2010 with close to 12 million people admitting to abusing these drugs by taking them for non-medical reasons. Results from a 2014 National Survey on Drug Use and Health revealed that about 4.3 million Americans aged 12 or older had used opioid for non-medical purposes the month prior to the survey interview [8]. Other factors highlighting the gravity of the opioid epidemic are overdose-related emergency room visits and deaths. In 2015, an estimated 20,101 deaths occurred due to prescription painkillers and 12,990 deaths due to heroin use specifically, but even more people-- 591,000 – were reported to have a substance use disorder [9,10]. In 2016, the number of drug overdose deaths was 63,600, then 70,237 in 2017, and in 2018, the number dipped to 67,367 but opioid overdose deaths comprised ~70% (46,802) of all drug overdose deaths [11,12]. Furthermore in 2017 the National Safety Council reported that the lifetime odds of dying from an accidental opioid overdose exceeded that from dying of a motor vehicle accident and gun assault, pushing accidental overdose into the top five causes of death behind heart disease, cancer, chronic lower respiratory disease, and suicide. Pitt estimate that on the current course, just over 500,000 Americans will die of opiate overdose from 2016 to 2025 [13].
As the opioid crisis continues to devastate the USA and its communities, it is essential to investigate “out of the box” treatment approaches. Although there are a couple treatment options such as naloxone for rescue therapy or methadone for long-term users, preventative options are minimal. Opioids exert their effect not only on the Central Nervous System (CNS) but also on the immune and endocrine systems. If the immune system facilitates the effect of opioids, then perhaps immunomodulation before opioid administration can prevent tolerance and withdrawal. Thus, this review presents studies using immunomodulators alpha interferon, cyclosporine A, and cortisol before repetitive morphine administration to curb opioid withdrawal.
Opioid addiction: Dependence, Tolerance, and Withdrawal
Opioid receptors are primarily found in the CNS but are also present on other organs. Opioids produce their rewarding effects through disinhibition of dopamine neurons and their analgesic effects through binding of opioid receptors in several brain regions and the spinal cord [14]. Prolonged opioid use, however, leads to harmful neural adaptations in the brain that facilitate tolerance, so that over time the drug dose needs to be increased to achieve the same desired effects. When the drug is withdrawn, it produces severe physical and behavioral symptoms prompting continued use to avoid these effects, thereby eventually creating dependence. The opiate withdrawal syndrome is dependent upon the integrity of specific brain sites within the mesocorticolimbic system and associated brain regions including the thalamus, hypothalamus, and several midbrain regions. Morphine is the most used opiate in experimental procedures, so several experimental procedures have used morphine to elicit dependence and withdrawal in animal models. Perhaps the most widely used method is the subcutaneous implantation of morphine pellets under light inhalation anesthesia. With this procedure, a marked degree of physical dependence and tolerance develop within 12-48 hours [15,16]. The degree of opiate dependence can be assessed by injecting the animals with the opiate antagonist naloxone which induces a measurable withdrawal syndrome of various behavioral signs including locomotor hyperactivity, teeth chattering, wet dog shakes diarrhea and scream to touch [17].
Side effects and long-term effects of opioid use
Opioid addiction can arise from both the abuse of illicit opiates such as heroin and the misuse of prescription pain relief medications such as morphine, hydrocodone, oxycodone, and codeine, and misuse can result in life-threatening health problems. During the acute period of use, opioids elicit a sense of well-being that can become addictive. However, increased doses or inadequate systemic clearance can induce respiratory depression, constipation, urinary retention, drowsiness, nausea, vomiting, and hypothermia or more fatally cardiac arrest and death [2,18]. Long-term use can also elicit opioid-induced hyperalgesia, a phenomenon most often seen with higher doses of parenteral morphine and hydromorphone, in which there is increased sensitivity to pain despite increased opioid dosing and diffuse extension of the pain and allodynia [19,20].
Current management of opioid addiction
Presently, no treatment regimen prevents the development of opiate dependence. Management of opioid abuse is mostly reactive rather than proactive. Therapy is limited to treating overdose with naloxone. However, reversing an overdose does not stop relapse. When discussing opioid abuse and addiction, it is important to consider that there are non-medical and medical uses of opioids. Non-medical uses refer to consuming opioids when they are not indicated in order to get the feelings of euphoria whereas medical uses refer to prescription pain relievers. It is this latter group of prescription opiates that contribute more to the opioid crisis, but strategies have focused on preventing non-medical use at the expense of preventing and treating opioid addiction in both medical and non-medical users [21].
Pharmacological management of opioid addiction
In the mid-1960s, a methadone maintenance treatment was initiated to manage heroin dependence long-term [22]. Methadone acts as an agonist at the opioid receptor and, along with partial agonist buprenorphine, works to control cravings [21]. Alternatively, naltrexone is an antagonist that blocks the effects of opioids. At first glance it may appear as the best pharmacological option out of the three, but relapse occurs very often. Therefore, not every patient is suited for naltrexone. For acute cases of opioid overdose, antagonist naloxone is a solution to life-threatening respiratory depression. It can reverse opioid overdose within minutes and has proven to be an effective strategy for rescue therapy.
Long treatment periods are required for most addiction therapies including Suboxone, Zubsolv, Probuphine, Sublocade, Bunavail, naltrexone, methadone, buprenorphine, CAM2038, and lofexidine (an alpha-2 adrenergic agonist that minimizes the symptoms of naloxone-induced withdrawal) [23,24]. Buprenorphine implants are relatively new, but initial findings show promise for therapeutic effectiveness and safety [25,26]. However, because most of the previously mentioned drugs belong to the opioid family there is concern that the above treatments are simply substituting one opioid for another which may prolong a type of dependence. Many of these drugs are for opiate withdrawal management, so they can lead to stronger cravings and relapse. Furthermore, there is little evidence for patient willingness to comply with these long-term treatments, and patients treated with these drugs may be at increased risk for subsequent overdose. Thus, an out of the box non-opioid regimen to prevent the induction of opioid withdrawal syndrome, a key contributor to the opioid epidemic, is needed. Additional novel treatments are under development including vaccines, trans-cranial current stimulations, and methods for improving treatment delivery [23].
Non-pharmacological management of opioid addiction
In addition to the above drugs, counseling, behavioral therapies, and social/spiritual support for long periods of time are essential. Psychosocial approaches, policies, and regulations are established methods of non-pharmacologic management. Residential treatment and rehabilitation, mutual-help programs such as Narcotics Anonymous, and 12-Step regimens are valuable options that can be used alone or in conjunction with medication [21].
Neuroimmune effects of opioids
Wybran showed that morphine does not only impact neuronal function but also affects the immune system, a finding that spearheaded investigations revealing that many neuropeptides, hormones, and neurotransmitters can alter components of the immune response [27,28]. In their study, they employed both endogenous opioids and exogenous opioids and found the former led to an increase in active T-cell rosettes and the latter led to a decrease in active T-rosettes [27]. Immune cells, such as T-cells, secrete endogenous opioid peptides which bind opioid receptors to alleviate pain and reduce inflammation, but exogenous opioids like fentanyl and morphine impair the function of T-cells, macrophages, and Natural Killer (NK) cells and circumvent them in order to bind opioid receptors [29]. Additionally, Kohno noted the role of neuroinflammation in the genesis, maintenance and treatment of SUD, and concluded that the pathologic activity induced by drugs of abuse contributes to the immune response [30]. Other studies have confirmed these results that endogenous opioids activate the immune system, and exogenous opioids suppress it [2,31]. Yet the level of immunosuppression by exogenous opioids is dependent on the type of opiate.
The endogenous opioid system is composed of widely scattered neurons that produce endorphins, enkephalins, and dynorphins which all function both as neurotransmitters and/or neuromodulators at the mu (µ), delta (δ), and kappa (κ) opioid receptors [32]. Exogenous opioids include morphine, buprenorphine, fentanyl, and several others. Morphine takes a dual approach to immunosuppression in that it binds directly to µ-Opioid Receptors (MORs) on macrophages, monocytes, NK lymphocytes, B-cells, and T-cells and indirectly to MORs in the CNS. Downstream pathways of the Hypothalamic-Pituitary-Adrenal (HPA) and sympathetic nervous system are activated and release glucocorticoids and noradrenalin, respectively, which both act on leukocytes to negatively modify immune function [33]. Buprenorphine is a partial MOR agonist and κ-Opioid Receptor (KOR) antagonist [34,35]. Fentanyl is a synthetic opioid that is 30-50 times more potent than heroin and 80-100 times stronger than morphine. These different exogenous opioids have varying levels of immunomodulation whether stimulatory, suppressive, or both [22,33]. For example, in rodent studies, buprenorphine did not affect splenic NK-lymphocyte, T-cell, or macrophage function but morphine significantly suppressed immunity [33,36]. In another rodent study on the development of tumors and surgery-induced immunosuppression underlying metastasis, tramadol increased NK Lymphocyte (NKL) activity while morphine suppressed it [37]. Fentanyl also decreases NK lymphocyte cytotoxicity thereby increasing the risk of tumor metastasis [38]. These findings were consistent with Sacerdote results from a study of surgery-induced immunosuppression in rats: buprenorphine returned NKL activity to preoperative baseline, morphine partially returned NKL activity, and fentanyl failed to return NKL activity [33]. In a mouse model of long-term opioid administration, after 7 days of infusion of fentanyl, there was reduced lymphoproliferation, interleukin-2, and interferon-gamma, but after 7 days of buprenorphine, there were no immune alterations [39]. Therefore, putting all these findings together it can be posited that tramadol is immunostimulatory, buprenorphine is immune-protective, and fentanyl and morphine are immunosuppressive.
Various hypotheses have been offered to explain the mechanisms underlying opioid withdrawal and tolerance. Most withdrawal behaviors have been accepted as primarily CNS-mediated phenomena, but further investigations have shown that opioid dependence has an immune system involvement. As early as the 1980s the question arose of using immunomodulatory agents to test for a possible link between the immune system and the expression of opiate withdrawal [17,41,42]. It was found that immune ablated rats treated with chronic morphine did not exhibit withdrawal behavior following naloxone injection [43]. Thereby demonstrating that an intact immune system is essential to the expression of opiate withdrawal. Soon after that, studies revealed that opioids change the percentage of T-lymphocytes forming active rosettes and their reactivity to mitogenic stimulation [44]. Opioids also affect cytotoxicity of natural killer cells, decrease the capability of cells to produce α-interferon, and decrease the levels of endogenous circulating α-interferon [45]. An alternative hypothesis is that the function of T-cells and natural killer cells is suppressed by stress which is mediated by endogenous opioids [46]. When associated with immunosuppression, opioid use can enhance tumor progression and increase susceptibility to infection [33]. Indeed, clinical studies have demonstrated that opioid abusers are more susceptible to opportunistic infections including pneumonia and HIV which can develop from lowered levels of α-IFN, retrovirus restriction factors TRIM22 and TRIM5a, and APOBEC3G [45,47,48].
Glial cells and opiate addiction
Traditionally, glial cells were considered passive accessories to neurons. However, it was demonstrated that these cells actively participate in synaptogenesis, neuronal excitability, and neurotransmission [49]. Additionally, the glial system exhibits robust synaptic plasticity via changes in its morphology and physiology in response to opioid exposure within key brain sites contributing to addiction [50-52]. Synaptic plasticity is a hallmark of neurons and involves changes in synaptic strength which are believed to be the basis of learning and memory.
Recent studies have increased understanding of the interactions between the CNS and immune system that likely play causal roles in the pathophysiology of multiple psychiatric illnesses [14]. Microglia in the CNS regulate both addiction and analgesia, and due to their general role in modulating inflammation they, along with astrocytes, are a source of cytokine up regulation in the brain after opioid exposure [14,50,53,54]. Additionally, since opioid receptors have been identified outside of the CNS, it has been shown that opioids modify both the innate and acquired immune responses at several levels [55]. Glial cells express receptors for most neurotransmitters and release neuroactive substances. As a result, glial cells have been shown to modulate synaptic plasticity in many ways from changes in synaptic coverage to release of chemokines and cytokines. Moreover, the glial cells are part of the immune system. Glia cells are nomadic immune cells of the brain and active participants in the generation of innate immune response [56]. The Toll-Like Receptor 4 (TLR4) is a potential site for opioid-induced glial activation [57]. By binding the same binding site as bacterial lipopolysaccharide on the TLR4-Myeloid Differentiation Factor 2 (TLR4-MD2) complex, opioids can stimulate signaling downstream to TLR4. It has been suggested that opioid induced immune signaling does not occur through classical opioid receptors because the opioid receptor active metabolite of morphine, M6G, cannot bind and activate TLR4 whereas the metabolite without opioid receptor activity, M3G, can. Morphine modifies gene expression profiling in glial cells and promotes the release of factors such as IFN-γ, IL1-β, IL-6, IL-10, CCL4, and CCL17. Through the release of pro-inflammatory cytokines and chemokines, glial cells contribute to opioid reward, and through regulation of synaptic transmission and plasticity contribute to opioid-elicited addictive behaviors [57].
Indeed, acute and chronic morphine and psycho stimulant use activate specific components of the innate immune system [56]. Given the known involvement of the immune system, immunotherapy is now being considered in the management of addiction [56]. It has been suggested that the effects of drugs of abuse on the immune cells in the brain can be summarized into three steps: 1) opioids act on glia cells to generate and release proinflammatory cytokines, 2) these cytokines induce activation of quiescent astrocytes and microglia which in turn enhance the inflammatory response, and 3) the significant pathways that are initiated by these cytokines activate the immune cells and alter function [53]. Opioids activate CNS microglia to release various factors, which in turn contribute to opioid tolerance, dependence and alleviation of the withdrawal symptoms. Thus, Evans and Cahill suggest that repeated opioid consumption induces adaptive changes that modify neuronal circuitry, alter transcription, and spur dendritic spine changes, all of which create an altered “normality” pushing the individual into a new allostatic state or “drug dependent” state [52].
Immunomodulators attenuate behavioral opiate withdrawal
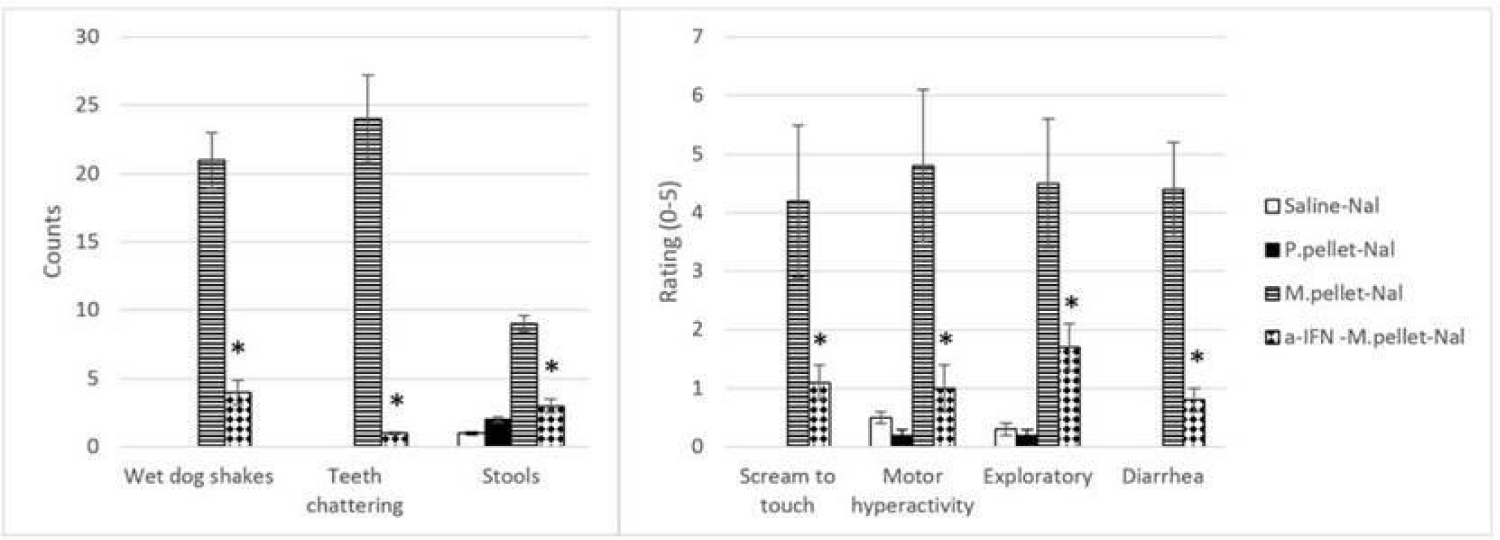
Further evidence for immune system implication in the expression of opioid withdrawal come from studies showing that its obliteration significantly reduced, if not eliminated, withdrawal behaviors [58-61]. Key immunomodulators that have been shown to diminish the opioid effect include α-interferon (α-IFN), cortisol, and cyclosporine A though there are several others under current investigation as well such as minocycline and ibudilast, but for the purposes of this review we will focus on the former three α-interferon (α-IFN) (Figure 1).
As a biological response modifier, α-IFN is often used as immunotherapy to regulate functions of the immune cells. It is part of an endogenous family of proteins found in vertebrates. α-IFN possess non-specific, potent immunomodulatory activity and is the most rapidly produced defense against foreign macromolecules [62,63]. α-IFN stimulates cells to produce other proteins, and it enhances the proliferation of human B cells and activates NK cells. It activates dendritic cells, initiating immune responses, and induces the expression of inducible-protein 10 (IB-10), a chemokine that promotes a TH1 inflammatory response [64].
In addition to immunologic properties, α-IFN poses both neurologic and endocrine activity and elicits corticosteroid secretion [65,66]. It has been argued that the immune system is the source for α-IFN, ACTH, and endorphins, but experimental studies divide this association with descriptions of α-IFN linking the endocrine and immune systems or linking the immune system and the CNS [68-73].
Blalock and Smith suggest that the action of α-IFN on neurons operates through opiate receptors [68]. α-IFN was responsible for modulating opiate mediated phenomenon by direct action on the brain and participated in pain, temperature, and food intake [71,74,75]. Inflammatory changes have also been implicated in modifying dopamine signaling and decreasing dopamine synthesis by affecting important cofactors and possibly dopamine transporter function [14]. For example, patients receiving infusions of pro-inflammatory cytokine α-IFN had altered activity of the basal ganglia and reduced dopamine [76].
Since opioid addiction stems from increased dopamine neurotransmission, applying α-IFN before drug administration could lower the effects of opiates arising from activated dopaminergic pathways. Therefore, α-IFN was utilized in our lab’s studies to determine if it can prevent the opiate withdrawal syndrome. It was found that α-IFN treatment prior to chronic morphine exposure attenuated naloxone-precipitated withdrawal in morphine dependent rats [77-81] (Figure 1). Because opioid use decreases circulating levels of α-IFN, it is likely that application of α-IFN before repetitive opioid administration stimulated the immune response leading to changes that reduced dopamine signaling thereby diminishing subsequent opioid effects [45,47,48,82-89].
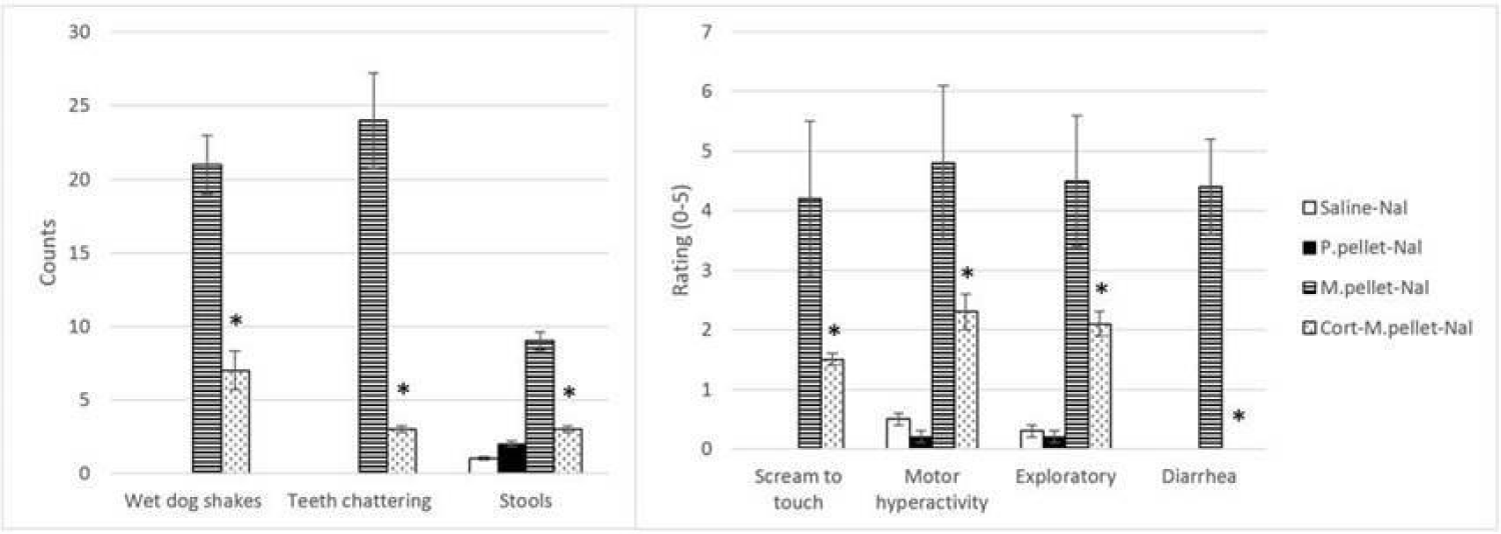
Corticosteroids are nonspecific immunosuppressors because they affect macrophages and monocytes as well as T-cells and B-cells, and hence cell-mediated and humoral-mediated immune processes [90]. Aside from inducing a lymphopenia by redistributing circulating lymphocytes to other lymphoid compartments [91-97], glucocorticoids suppress type 2 cytokines such as IL-2 and IL-4 which are responsible for memory T cell differentiation as well as helper T cell differentiation and B-cell isotype switching to IgE [98]. Cortisol was also able to reduce the naloxone- precipitated withdrawal syndrome [99] (Figure 2). Cortisol is produced in the adrenal glands and is responsible for regulating a wide range of processes including immunomodulation and stress response. Almost every cell contains receptors for cortisol, so it is not just an anti-inflammatory agent but also controls salt and water balance, influences blood pressure, controls glycemic levels and thus regulates metabolism, and influences cognitive function [100-102]. Activation of the HPA axis arises from IL-1 stimulating hypothalamic Corticotrophin Releasing Hormone (CRH) thereby activating Adrenocorticotrophic Hormone (ACTH) in the pituitary which then activates the adrenal cortex to release glucocorticoids, and increased plasma concentrations of glucocorticoids depress the immune system [103-105].
In rodents, acute opioid administration increases ACTH and glucocorticoids levels which in turn leads to immunosuppression, but chronic opioids either decrease or have no effect on these hormone levels. Cortisol is anti-inflammatory acutely and pro-inflammatory in the long-term [14,106]. In humans, exogenous glucocorticoids are given to decrease inflammation and pain but long-term can cause immunosuppression. Although both α-IFN and cortisol decrease the occurrence of withdrawal behaviors when given prior to repetitive morphine exposure, they have opposing functions, cortisol is anti-inflammatory and α-IFN is pro-inflammatory. However, cortisol also directly operates as part of the HPA with a feedback mechanism. As a result, it is likely that administration of cortisol before repetitive morphine provided negative feedback to the HPA axis preventing further release of glucocorticoids and, subsequently, immune suppression.
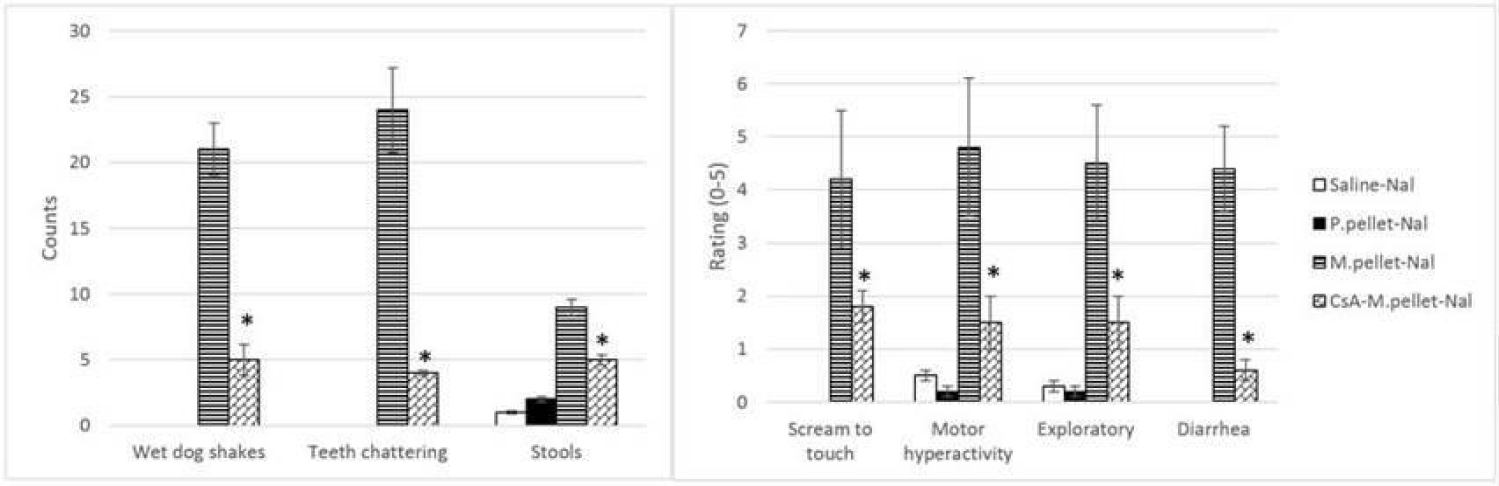
Cyclosporine A (CsA) is a cyclic, undeca-polypeptide extracted from soil fungus and used in organ and tissue transplantation to prevent graft rejection [107,108]. CsA utility in transplants comes from its immunosuppressive properties with preferential action against helper T-lymphocytes through the arrest of the cell in the G0 or G1-phase. [107,109,110].
Although cyclosporine A is primarily used as an immunosuppressant, it also affects the CNS. In one of our previous studies we directly measured the cyclosporine levels in rat brain tissue 1 hour following intraperitoneal injection. Significant levels of the drug were observed in comparison to the control group. This indicates that the drug does reach the brain area where it conceivably may have a direct effect on the CNS [41]. Another study revealed that a 3uM dose of CsA was found to reduce the electroconvulsive threshold and increase electroconvulsive activity in a hippocampal slice [111]. Granted, there are also positive effects of cyclosporine A on the CNS. Tsutsumi, et al. [112] reported how a patient who was receiving cyclosporine after a stem cell transplant had improved pain relief from transdermal fentanyl but developed withdrawal symptoms after fentanyl discontinuation. However, when this patient was switched to morphine, she did not develop withdrawal symptoms after morphine discontinuation. This finding is consistent with our studies showing that cyclosporine. A administered before repetitive morphine administration significantly reduces naloxone-induced withdrawal syndrome [82-89] (Figure 3). A potential explanation of this phenomenon is provided and the study in which they describe how Transporter Organic Anion Transporting Polypeptide 2B1 (OATP2B1) is inhibited by CsA. OATP2B1 mediates blood-brain barrier transport of morphine and one of its potent metabolites Morphine-6-Glucuronide (M6G). M6G is known to induce tolerance much easier than morphine and M3G, another morphine metabolite. CsA reduced intracellular accumulations of morphine and M6G, and subsequent alteration of µ-opioid receptor and Calcium/Calmodulin-Dependent Protein Kinase IIα (CaMKIIα) expression and phosphorylation led to tolerance suppression [113]. Another mechanism for reducing side effect of morphine and its metabolites is through induction of p-glycoprotein which mediates morphine brain efflux and decreases morphine brain uptake [113,114]. With decreased morphine uptake, the likelihood of developing tolerance is reduced, and withdrawal is curbed.
Interaction of immunomodulators and opioids
Either ablating the immune system--which would only be indicated in humans in very rare cases-- or stimulating it before repeated opioid administration resulted in attenuated opioid withdrawal. Two possible explanations for this finding is that 1) since opioids negatively affect the immune response, if there is no immune system for the drug to impact, the effects are negligible, or 2) stimulation of the immune system before repetitive drug administration can counter the immunosuppressive effects of opioids so that the physiological effects of withdrawal are diminished. Moreover, it has been reported that the interplay between the CNS and immune system is active during the immune response, a response that is altered by drugs of abuse [14]. The reciprocal interaction between the CNS and the immune system has gained traction because of the demonstration of a putative pathway between these two systems. This interaction occurs essentially at two levels: 1) cell-to-cell contact and 2) release of soluble mediators that bind to cell surface receptors [91,92]. Thus, immunomodulators such as α-IFN, cyclosporine, and cortisol possess both immune and neural actions, permitting their modulation of the behavioral expression of opioid addiction. These treatments carry potential therapeutic implications for substance use disorders.
Conclusion
Using immunomodulators to prevent opioid adverse effects
Opioid abuse can lead to addiction. Our previous studies on the neurophysiological mechanisms underlying drug dependence have established that repetitive administration of morphine and other drugs of abuse modulate the behavioral and neuronal Baseline (BL) activities. These changes correlate closely with the development of tolerance and withdrawal. In other words, when the drug is used repeatedly and then withdrawn, it results in a biochemical chain of events that produces changes in the neuronal BL firing patterns of involved brain regions, and it is these electrophysiological changes that are involved with causing the subsequent behavioral expressions of withdrawal [95,96,115-118]. Although addiction is primarily a CNS process, it is linked to the immune system. Several studies recounted above show that if we can modulate the immune system before repeated opioid administration, then the analgesic properties of the opioid remain but the adverse effect of withdrawal is prevented. Despite current, multi-pronged tactics to combat opioid abuse, the statistics remain grim. In the USA alone, in early 2019, 2 million individuals were misusing first-time opioid prescriptions, around 130 people were dying from opioid-related drug overdoses each day, and nearly 33,000 deaths were linked to overdosing with synthetic non-methadone opioids. Since standard efforts have had little effect over the years in decreasing mortality and morbidity from the disease, we believe that new approaches to this problem are required for a solution and that a different non-opioid method is warranted.
In summary, preclinical studies using immunomodulatory substances α-interferon, cyclosporine, or cortisol given prior to repetitive morphine were able to significantly reduce the severity of opiate withdrawal behavior precipitated by naloxone injection [119-124]. Preventing opiate withdrawal syndrome will decrease relapse and toxicity and potentially prolong lives. These immunomodulators are safe and effective therapies that have long been on the market and used for other disorders as well [125-129]. They hold promise in combating the on-going opioid epidemic. Given the discussions in this review of the underlying basis of opioid withdrawal and proposed alternative approach of using non-opioid immunomodulators to prevent it, it may be beneficial to utilize preventative immunomodulation in the management of opioid abuse in an effort to overcome the current opioid epidemic.
Acknowledgment
Supported in part by NIH grant RO1 DA 00803.
References
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008 Oct;16(5):405-416. doi: 10.1037/a0013628. PMID: 18837637; PMCID: PMC2711509.
- Khademi H, Kamangar F, Brennan P, Malekzadeh R. Opioid Therapy and its Side Effects: A Review. Arch Iran Med. 2016 Dec;19(12):870-876. PMID: 27998163.
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006 Feb 1;81(2):103-107. doi: 10.1016/j.drugalcdep.2005.05.009. Epub 2005 Jul 14. PMID: 16023304.
- Alpert A, Powell D, Pacula RL. Supply-side drug policy in the presence of substitutes: Evidence from the introduction of abuse-deterrent opioids. American Economic Journal: Economic Policy. 2018;10(4):1–35. Doi: 10.1257/pol.20170082
- Schnell M. The Opioid Crisis : Tragedy, Treatments and Trade-offs. SIEPR Report. 2019:1-4.
- Muhuri P, Gfroerer J, Davies M. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United States. CBHSQ Data Rev. 2013.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015 Apr;156(4):569-576. doi: 10.1097/01.j.pain.0000460357.01998.f1. PMID: 25785523.
- Hedden SL, Kennet J, Lipari R, Medley G, Tice P, Copello EA, Kroutil LA. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015.
- Hedden SL, Kennet J, Lipari R, Medley G, Tice P, Copello EA, Kroutil LA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016.
- Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(50-51):1445-1452. doi: 10.15585/mmwr.mm655051e1. PMID: 28033313.
- Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999-2016. 2017.
- Hedegaard H, Miniño AM, Warner M. Drug Overdose Deaths in the United States, 1999-2018. 2020.
- Pitt AL, Humphreys K, Brandeau ML. Modeling Health Benefits and Harms of Public Policy Responses to the US Opioid Epidemic. Am J Public Health. 2018 Oct;108(10):1394-1400. doi: 10.2105/AJPH.2018.304590. Epub 2018 Aug 23. PMID: 30138057; PMCID: PMC6137764.
- Hofford RS, Russo SJ, Kiraly DD. Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur J Neurosci. 2019 Aug;50(3):2562-2573. doi: 10.1111/ejn.14143. Epub 2018 Sep 26. PMID: 30179286; PMCID: PMC6531363.
- Cicero TJ, Meyer ER. Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther. 1973 Feb;184(2):404-408. PMID: 4734581.
- Wei E, Way E. Application of the pellet implantation technique for the assessment of tolerance and physical dependence in the rodent. Marcel Dekker New York. 1975:243-259.
- Dafny N. Modification of morphine withdrawal by interferon. Life Sci. 1983 Jan 24;32(4):303-305. doi: 10.1016/0024-3205(83)90074-7. PMID: 6681856.
- Kalant H. Opium revisited: a brief review of its nature, composition, non-medical use and relative risks. Addiction. 1997 Mar;92(3):267-77. PMID: 9219389.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011 Mar-Apr;14(2):145-161. PMID: 21412369.
- Groninger H, Vijayan J. Pharmacologic management of pain at the end of life. Am Fam Physician. 2014 Jul 1;90(1):26-32. PMID: 25077499.
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015 Mar 18;36:559-574. doi: 10.1146/annurev-publhealth-031914-122957. Epub 2015 Jan 12. PMID: 25581144.
- Liang X, Liu R, Chen C, Ji F, Li T. Opioid System Modulates the Immune Function: A Review. Transl Perioper Pain Med. 2016;1(1):5-13. PMID: 26985446; PMCID: PMC4790459.
- NIDA. How effective are medications to treat opioid use disorder? National Institute on Drug Abuse (NIDA). 2020.
- Weiss S, Volkow ND. 129th Meeting Minutes | National Institute on Drug Abuse (NIDA). 2018.
- Lofwall MR, Walsh SL, Nunes EV, Bailey GL, Sigmon SC, Kampman KM, Frost M, Tiberg F, Linden M, Sheldon B, Oosman S, Peterson S, Chen M, Kim S. Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine With Naloxone for Treatment of Opioid Use Disorder: A Randomized Clinical Trial. JAMA Intern Med. 2018 Jun 1;178(6):764-773. doi: 10.1001/jamainternmed.2018.1052. PMID: 29799968; PMCID: PMC6145749.
- Tompkins CNE, Neale J, Strang J. Opioid users’ willingness to receive prolonged-release buprenorphine depot injections for opioid use disorder. J Subst Abuse Treat. 2019 Sep;104:64-71. doi: 10.1016/j.jsat.2019.06.007. Epub 2019 Jun 9. PMID: 31370986.
- Wybran J, Appelboom T, Famaey JP, Govaerts A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J Immunol. 1979 Sep;123(3):1068-1070. PMID: 224107.
- Sacerdote P, Limiroli E, Gaspani L. Experimental evidence for immunomodulatory effects of opioids. Adv Exp Med Biol. 2003;521:106-16. PMID: 12617569.
- Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018 Jul;175(14):2717-2725. doi: 10.1111/bph.13750. Epub 2017 Mar 23. PMID: 28213891; PMCID: PMC6016673.
- Kohno M, Link J, Dennis LE, McCready H, Huckans M, Hoffman WF, Loftis JM. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol Biochem Behav. 2019 Apr;179:34-42. doi: 10.1016/j.pbb.2019.01.007. Epub 2019 Jan 26. PMID: 30695700; PMCID: PMC6637953.
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008 Mar;11(2 Suppl):S105-120. PMID: 18443635.
- Holden J, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management. AACN Clinical Issues. 2005;16(3):291-301. https://tinyurl.com/dwkvjfym
- Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20 Suppl 1:s9-15. PMID: 16764216.
- Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav Immun. 2008 May;22(4):606-613. doi: 10.1016/j.bbi.2007.12.013. Epub 2008 Feb 21. PMID: 18294814.
- Ling W. Buprenorphine for opioid dependence. Expert Rev Neurother. 2009 May;9(5):609-616. doi: 10.1586/ern.09.26. PMID: 19402772; PMCID: PMC4151622.
- Gomez-Flores R, Weber RJ. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology. 2000 Jul 20;48(2):145-156. doi: 10.1016/s0162-3109(00)00198-3. PMID: 10936512
- Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002 Aug;129(1-2):18-24. doi: 10.1016/s0165-5728(02)00165-0. PMID: 12161016.
- Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11(4):255-260. doi: 10.1159/000078444. PMID: 15249732.
- Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: Similar analgesic profile but different effects on immune responses. Pain. 2004;110(1–2):385-392. doi: 10.1016/j.pain.2004.04.020
- Dafny N, Burks TF, Bergmann F. Dose effects of morphine on the spontaneous unit activity recorded from the thalamus, hypothalamus, septum, hippocampus, reticular formation, central gray, and caudate nucleus. J Neurosci Res. 1983;9(2):115-126. doi: 10.1002/jnr.490090203. PMID: 6842623.
- Dougherty PM, Aronowski J, Samorajski T, Dafny N. Opiate antinociception is altered by immunemodification: the effect of interferon, cyclosporine and radiation-induced immune suppression upon acute and long-term morphine activity. Brain Res. 1986 Oct 22;385(2):401-404. doi: 10.1016/0006-8993(86)91091-7. PMID: 3779401.
- Dougherty PM, Dafny N. Cyclosporine affects central nervous system opioid activity via direct and indirect means. Brain Behav Immun. 1988 Sep;2(3):242-253. doi: 10.1016/0889-1591(88)90026-8. PMID: 3242657.
- Dafny N, Pellis NR. Evidence that opiate addiction is in part an immune response. Destruction of the immune system by irradiation-altered opiate withdrawal. Neuropharmacology. 1986 Aug;25(8):815-818. doi: 10.1016/0028-3908(86)90003-1. PMID: 3774111.
- Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clinical and Diagnostic Laboratory Immunology. American Society for Microbiology (ASM). 2000. Doi: 10.1128/CDLI.7.5.719-723.2000
- Zhu JW, Liu FL, Mu D, Deng DY, Zheng YT. Heroin use is associated with lower levels of restriction factors and type I interferon expression and facilitates HIV-1 replication. Microbes Infect. 2017 Apr-May;19(4-5):288-294. doi: 10.1016/j.micinf.2017.01.002. Epub 2017 Jan 16. PMID: 28104465.
- Eisenstein TK. The Role of Opioid Receptors in Immune System Function. Front Immunol. 2019 Dec 20;10:2904. doi: 10.3389/fimmu.2019.02904. PMID: 31921165; PMCID: PMC6934131.
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998 Mar 15;83(1-2):77-87. doi: 10.1016/s0165-5728(97)00224-5. PMID: 9610676.
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998 Mar 15;83(1-2):4-18. doi: 10.1016/s0165-5728(97)00216-6. PMID: 9610668.
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005 Dec;28(12):661-669. doi: 10.1016/j.tins.2005.10.001. Epub 2005 Oct 24. PMID: 16246435.
- Hidalgo JJ. The role of glial cells in drug abuse. Current Drug Abuse Reviews. 2009;2(1):76–82. Doi: 10.2174/1874473710902010076
- Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012 May;134(2):219-245. doi: 10.1016/j.pharmthera.2012.01.008. Epub 2012 Feb 1. PMID: 22316499.
- Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 2016 Jul 19;5:F1000 Faculty Rev-1748. doi: 10.12688/f1000research.8369.1. PMID: 27508068; PMCID: PMC4955026.
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014 Apr;20(2):160-172. doi: 10.1177/1073858413504466. Epub 2013 Oct 8. PMID: 24106265.
- Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017 Sep 8;23(9):1018-1027. doi: 10.1038/nm.4397. PMID: 28886007.
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001 Apr 1;62(2):111-123. doi: 10.1016/s0376-8716(00)00181-2. PMID: 11245967.
- Harricharan R, Abboussi O, Daniels WMU. Addiction: A deregulation of satiety inflammation processes. Progress in Brain Research. 2016:235. Doi10.1016/bs.pbr 2017.07.012
- Zhang H, Largent-Milnes TM, Vanderah TW. Glial neuroimmune signaling in opioid reward. Brain Res Bull. 2020 Feb;155:102-111. doi: 10.1016/j.brainresbull.2019.11.012. Epub 2019 Nov 29. PMID: 31790721; PMCID: PMC6946383.
- Dafny N, Dougherty PM, Pellis NR. The immune system and opiate withdrawal. Int J Immunopharmacol. 1989;11(4):371-375. doi: 10.1016/0192-0561(89)90083-0. PMID: 2674032.
- Dougherty PM, Dafny N. Muramyl-dipeptide, a macrophage-derived cytokine, alters neuronal activity in hypothalamus and hippocampus but not in the dorsal raphe/periaqueductal gray of rats. J Neuroimmunol. 1990 Aug;28(3):201-208. doi: 10.1016/0165-5728(90)90013-d. PMID: 2373761.
- Dougherty PM, Aronowski J, Drath D, Dafny N. Evidence of neuro-immunologic interactions: cyclosporine modifies opiate withdrawal by effects on the brain and immune components. J Neuroimmunol. 1987 Jan;13(3):331-342. doi: 10.1016/0165-5728(87)90068-3. PMID: 3793881.
- Dougherty PM, Drath DB, Dafny N. Evidence of an immune system to brain communication axis that affects central opioid functions: muramyl peptides attenuate opiate withdrawal. Eur J Pharmacol. 1987 Sep 11;141(2):253-260. doi: 10.1016/0014-2999(87)90270-6. PMID: 2824218.
- Pestka S, Baron S. Definition and classification of the interferons. Methods Enzymol. 1981;78(Pt A):3-14. doi: 10.1016/0076-6879(81)78091-1. PMID: 6173606.
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251-82. doi: 10.1146/annurev.bi.51.070182.001343. PMID: 6180680.
- Dafny N, Lincoln J. The Role of Interferons on the Central Nervous System in Health and Disease. In Reference Module in Biomedical Sciences. Elsevier. 2016. Doi: 10.1016/b978-0-12-801238-3.99446-7.
- Roosth J, Pollard RB, Brown SL, Meyer WJ 3rd. Cortisol stimulation by recombinant interferon-alpha 2. J Neuroimmunol. 1986 Oct;12(4):311-316. doi: 10.1016/0165-5728(86)90037-8. PMID: 3760157.
- Dafny N. Is interferon-α a neuromodulator? Brain Research Reviews. Elsevier BV. 1998. doi: 10.1016/S0165-0173(97)00029-5
- Blalock JE, Smith EM. Human leukocyte interferon: Structural and biological relatedness to adrenocorticotropic hormone and endorphins. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5972-5974. doi: 10.1073/pnas.77.10.5972. PMID: 6160589; PMCID: PMC350194.
- Dafny N, Rigor BM. Characterization of unit activity recorded from septum, thalamus, and caudate following incremental opiate treatment. J Neurosci Res. 1980;5(2):117-127. doi: 10.1002/jnr.490050203. PMID: 7401192.
- Dafny N, Reyes-Vazquez C. Three different types of alpha-interferons alter naloxone-induced abstinence in morphine-addicted rats. Immunopharmacology. 1985 Feb;9(1):13-17. doi: 10.1016/0162-3109(85)90041-4. PMID: 4039302.
- Dafny N, Wagle VG, Drath DB. Cyclosporine alters opiate withdrawal in rodents. Life Sci. 1985 May 6;36(18):1721-1726. doi: 10.1016/0024-3205(85)90554-5. PMID: 4039025.
- Dafny N, Zielinksi M, Reyes-Vazquez C. Alteration of morphine withdrawal to naloxone by interferon. Neuropeptides. 1983 Oct;3(6):453-463. doi: 10.1016/0143-4179(83)90036-7. PMID: 6420721.
- Vázquez, Cruz, Gómez, Dafny N. Interferon modulates central nervous system function. Brain Research. 2012. Doi: 10.1016/j.brainres.2011.09.061
- Reyes-Vazquez C, Mendoza-Fernandez V, Herrera-Ruiz M, Dafny N. Interferon modulates glucose-sensitive neurons in the hypothalamus. Exp Brain Res. 1997 Oct;116(3):519-524. doi: 10.1007/pl00005780. PMID: 9372301.
- Reyes-Vázquez C, Prieto-Gómez B, Dafny N. Alpha-interferon suppresses food intake and neuronal activity of the lateral hypothalamus. Neuropharmacology. 1994 Dec;33(12):1545-1552. doi: 10.1016/0028-3908(94)90128-7. PMID: 7760977.
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012 Oct;69(10):1044-1053. doi: 10.1001/archgenpsychiatry.2011.2094. PMID: 23026954; PMCID: PMC3640298.
- Claussen M, Chong SL, Dafny N. Nucleus accumbens activity correlates to the animal’s behavioral response to acute and chronic methylphenidate. Physiol & Behav. 2014;129:85-94. https://tinyurl.com/rbc8pd27
- Claussen CM, Dafny N. Caudate neuronal recording in freely behaving animals following acute and chronic dose response methylphenidate exposure. Pharmacol Biochem Behav. 2015 Sep;136:21-30. doi: 10.1016/j.pbb.2015.06.003. Epub 2015 Jun 20. PMID: 26101057; PMCID: PMC4743873.
- Dafny N. Multiunit recording from medial basal hypothalamus following acute and chronic morphine treatment. Brain Res. 1980 May 26;190(2):584-592. doi: 10.1016/0006-8993(80)90304-2. PMID: 7189436.
- Dafny N. The hypothalamus exhibits electrophysiologic evidence for morphine tolerance and dependence. Exp Neurol. 1982 Jul;77(1):66-77. doi: 10.1016/0014-4886(82)90143-1. PMID: 7200913.
- Dafny N, Dong WQ, Prieto-Gomez C, Reyes-Vazquez C, Stanford J, Qiao JT. Lateral hypothalamus: Site involved in pain modulation. Neuroscience. 1996 Jan;70(2):449-460. doi: 10.1016/0306-4522(95)00358-4. PMID: 8848153.
- Dafny N, Gildenberg P. Morphine effects on spontaneous, nociceptive, antinociceptive and sensory evoked responses of parafasciculus thalami units in morphine naive and morphine dependent rats. Brain Res. 1984 Dec 3;323(1):11-20. doi: 10.1016/0006-8993(84)90260-9. PMID: 6543146.
- Dafny N, Marchand J, McClung R, Salamy J, Sands S, Wachtendorf H, Burks TF. Effects of morphine on sensory-evoked responses recorded from central gray, reticular formation, thalamus, hypothalamus, limbic system, basal ganglia, dorsal raphe, locus ceruleus, and pineal body. J Neurosci Res. 1980;5(5):399-412. doi: 10.1002/jnr.490050505. PMID: 7441794.
- Dafny N, Qiao JT. Habenular neuron responses to noxious input are modified by dorsal raphe stimulation. Neurol Res. 1990 Jun;12(2):117-121. doi: 10.1080/01616412.1990.11739929. PMID: 1974700.
- Dafny N, Rigor BM, Burks TF. Dependence and tolerance: multiunit recording from central gray, mesencephalic reticular formation, and medial thalamus in freely behaving rats. Exp Neurol. 1980 May;68(2):217-227. doi: 10.1016/0014-4886(80)90080-1. PMID: 7363992.
- Dafny N, Rigor BM. Characterization of unit activity recorded from septum, thalamus, and caudate following incremental opiate treatment. J Neurosci Res. 1980;5(2):117-127. doi: 10.1002/jnr.490050203. PMID: 7401192.
- Dafny N, Reyes-Vazquez C. Three different types of alpha-interferons alter naloxone-induced abstinence in morphine-addicted rats. Immunopharmacology. 1985 Feb;9(1):13-17. doi: 10.1016/0162-3109(85)90041-4. PMID: 4039302.
- Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133-155. doi: 10.1111/j.1600-065x.1982.tb00431.x. PMID: 6214495.
- Fauci AS, Dale DC. The effect of hydrocortisone on the kinetics of normal human lymphocytes. Blood. 1975 Aug;46(2):235-243. PMID: 1139040.
- Jones Z, Dafny N. Dose response effect of methylphenidate on ventral tegmental area neurons and animal behavior. Brain Res Bull. 2013 Jul;96:86-92. doi: 10.1016/j.brainresbull.2013.03.004. Epub 2013 May 4. PMID: 23651545.
- Jones Z, Dafny N. Acute and chronic dose-response effect of methylphenidate on ventral tegmental area neurons correlated with animal behavior. J Neural Transm (Vienna). 2014;121(3):327-345. doi: 10.1007/s00702-013-1101-2. Epub 2013 Nov 20. PMID: 24249696; PMCID: PMC4743876
- Karim TJ, Vázquez C, Dafny N. VTA neurons responding to MPD similarity to LC neurons: a concomitant behavioral and neuronal study of adolescent male rats. J of Neurophysiology. 2017;118:1501-1514.
- Karim TJ, Aksel C, Kharas N, Reyes-Vasquez C, Dafny N. Caudate nucleus neurons participate in methylphenidate function: Behavioral and neuronal recordings from freely behaving adolescent rats. Brain Res Bull. 2018 Sep;142:241-252. doi: 10.1016/j.brainresbull.2018.07.008. Epub 2018 Aug 10. PMID: 30016725.
- Kharas N, Whitt H, Reyes-Vasquez C, Dafny N. Methylphenidate modulates dorsal raphe neuronal activity: Behavioral and neuronal recordings from adolescent rats. Brain Res Bull. 2017 Jan;128:48-57. doi: 10.1016/j.brainresbull.2016.10.011. Epub 2016 Nov 23. PMID: 27889580; PMCID: PMC5224521.
- Kharas N, Reyes-Vazquez C, Dafny N. Locus coeruleus neuronal activity correlates with behavioral response to acute and chronic doses of methylphenidate (Ritalin) in adolescent rats. J Neural Transm (Vienna). 2017 Oct;124(10):1239-1250. doi: 10.1007/s00702-017-1760-5. Epub 2017 Jul 20. PMID: 28730316.
- Kanagalingam T, Solomon L, Vijeyakumaran M, Palikhe NS, Vliagoftis H, Cameron L. IL-2 modulates Th2 cell responses to glucocorticosteroid: A cause of persistent type 2 inflammation? Immun Inflamm Dis. 2019 Sep;7(3):112-124. doi: 10.1002/iid3.249. Epub 2019 Apr 17. PMID: 30994266; PMCID: PMC6688076.
- Montgomery SP, Dafny N. Cyclophosphamide and cortisol reduce the severity of morphine withdrawal. Int J Immunopharmacol. 1987;9(4):453-457. doi: 10.1016/0192-0561(87)90019-1. PMID: 3623770.
- Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, Golden SH. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2015 Dec;62:327-335. doi: 10.1016/j.psyneuen.2015.08.021. Epub 2015 Aug 28. PMID: 26356041; PMCID: PMC4637243.
- Kelly J, Mangos G, Williamson P, Whitworth J. CORTISOL AND HYPERTENSION. Clinical and Experimental Pharmacology and Physiology. 1998;25(S1):S51–S56. Doi: 10.1111/j.1440-1681.1998.tb02301.x
- Law R, Clow A. Stress, the cortisol awakening response and cognitive function. Int Rev Neurobiol. 2020;150:187-217. doi: 10.1016/bs.irn.2020.01.001. Epub 2020 Mar 11. PMID: 32204832.
- Jefferies WMK. Cortisol and immunity. Medical Hypotheses. 1991. Doi: 10.1016/0306-9877(91)90212-H
- Eisenstein TK, Rahim RT, Feng P, Thingalaya NK, Meissler JJ. Effects of opioid tolerance and withdrawal on the immune system. J Neuroimmune Pharmacol. 2006 Sep;1(3):237-249. doi: 10.1007/s11481-006-9019-1. Epub 2006 May 23. PMID: 18040801.
- Al-Hashimi M, Scott SW, Thompson JP, Lambert DG. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013 Jul;111(1):80-88. doi: 10.1093/bja/aet153. PMID: 23794649.
- Manetti L, Cavagnini F, Martino E, Ambrogio A. Effects of cocaine on the hypothalamic-pituitary-adrenal axis. J Endocrinol Invest. 2014 Aug;37(8):701-708. doi: 10.1007/s40618-014-0091-8. Epub 2014 May 23. PMID: 24852417.
- Borel JF, Feurer C, Magnee C, Stahelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32(6):1017–1025. https://tinyurl.com/mj8tzpr3
- Kahan BD. Cyclosporine: a powerful addition to the immunosuppressive armamentarium. Am J Kidney Dis. 1984 May;3(6):444-455. doi: 10.1016/s0272-6386(84)80009-8. PMID: 6372448.
- Andrus L, Lafferty KJ. Inhibition of T-cell activity by cyclosporin A. Scand J Immunol. 1981 May;15(5):449-458. doi: 10.1111/j.1365-3083.1982.tb00670.x. PMID: 6808656.
- Colombani PM, Robb A, Hess AD. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science. 1985 Apr 19;228(4697):337-339. doi: 10.1126/science.3885394. PMID: 3885394.
- Choi BJ, Whang KT. Change of hyperexcitability of hippocampus by cyclosporin A and its modulatory action by fentanyl. J Korean Med Sci. 2002 Feb;17(1):96-102. doi: 10.3346/jkms.2002.17.1.96. PMID: 11850597; PMCID: PMC3054821.
- Tsutsumi Y, Kanamori H, Tanaka J, Asaka M, Imamura M, Masauzi N. Withdrawal symptoms from transdermal fentanyl (TDF) after an allogeneic peripheral blood stem cell transplant (PBSCT). Pain Med. 2006 Mar-Apr;7(2):164-165. doi: 10.1111/j.1526-4637.2006.00107.x. PMID: 16634729.
- Yang ZZ, Li L, Wang L, Xu MC, An S, Jiang C, Gu JK, Wang ZJ, Yu LS, Zeng S. siRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: a mechanism for acute morphine tolerance suppression. Sci Rep. 2016 Sep 15;6:33338. doi: 10.1038/srep33338. PMID: 27629937; PMCID: PMC5024137.
- Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Zhang Y, Laracuente ML, DeMarco KM, Ronaldson PT, Davis TP. P-glycoprotein modulates morphine uptake into the CNS: a role for the non-steroidal anti-inflammatory drug diclofenac. PLoS One. 2014 Feb 10;9(2):e88516. doi: 10.1371/journal.pone.0088516. PMID: 24520393; PMCID: PMC3919782.
- Venkataraman SS, Claussen C, Joseph M, Dafny N. Concomitant behavioral and PFC neuronal activity recorded following dose-response protocol of MPD in adult male rats. Brain Res Bull. 2017 Apr;130:125-137. doi: 10.1016/j.brainresbull.2017.01.008. Epub 2017 Jan 19. PMID: 28111275.
- Venkataraman SS, Joseph M, Dafny N. Concomitant behavioral and prefrontal cortex neuronal responses following acute and chronic methylphenidate exposure in adolescent and adult rats. Brain Res Bull. 2019 Jan;144:200-212. doi: 10.1016/j.brainresbull.2018.11.004. Epub 2018 Nov 28. PMID: 30502401.
- Venkataraman SS, Joseph M, Dafny N. Concomitant behavioral and prefrontal cortex neuronal responses following acute and chronic methylphenidate exposure in adolescent and adult rats. Brain Res Bull. 2019 Jan;144:200-212. doi: 10.1016/j.brainresbull.2018.11.004. Epub 2018 Nov 28. PMID: 30502401.
- Venkataraman SS, Claussen CM, Kharas N, Dafny N. The prefrontal cortex and the caudate nucleus respond conjointly to methylphenidate (Ritalin). Concomitant behavioral and neuronal recording study. Brain Res Bull. 2020 Apr;157:77-89. doi: 10.1016/j.brainresbull.2019.10.009. Epub 2020 Jan 24. PMID: 31987926.
- Tang B, Dafny N. Behavioral and dorsal raphe neuronal activity following acute and chronic methylphenidate in freely behaving rats. Brain Res Bull. 2013 Sep;98:53-63. doi: 10.1016/j.brainresbull.2013.06.004. Epub 2013 Jul 22. PMID: 23886570.
- Tang B, Dafny N. Locus coeruleus neuronal and behavioral activity following acute and chronic methylphenidate. J of Brain Sciences. 2015;1:24-42.
- Tang B, Dafny N. Dorsal raphe neuronal activities are modulated by methylphenidate. J Neural Transm (Vienna). 2013 May;120(5):721-31. doi: 10.1007/s00702-012-0917-5. Epub 2012 Dec 27. PMID: 23269378; PMCID: PMC4036810.
- Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972 Dec 22;178(4067):1290-1292. doi: 10.1126/science.178.4067.1290. PMID: 4640066.
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136-142. doi: 10.1016/0167-5699(92)90111-J. PMID: 1374612
- Chong SL, Claussen CM, Dafny N. Nucleus accumbens neuronal activity in freely behaving rats is modulated following acute and chronic methylphenidate administration. Brain Res Bull. 2012 Mar 10;87(4-5):445-456. doi: 10.1016/j.brainresbull.2012.01.004. Epub 2012 Jan 13. PMID: 22248440; PMCID: PMC3295903.
- Owens T, Khorooshi R, Wlodarczyk A, Asgari N. Interferons in the central nervous system: a few instruments play many tunes. Glia. 2014 Mar;62(3):339-355. doi: 10.1002/glia.22608. PMID: 24588027.
- Dong WQ, Wilson OB, Skolnick MH, Dafny N. Hypothalamic, dorsal raphe and external electrical stimulation modulate noxious evoked responses of habenula neurons. Neuroscience. 1992 Jun;48(4):933-940. doi: 10.1016/0306-4522(92)90281-6. PMID: 1630629.
- Frolov A, Reyes-Vasquez C, Dafny N. Behavioral and neuronal recording of the nucleus accumbens in adolescent rats following acute and repetitive exposure to methylphenidate. J Neurophysiol. 2015 Jan 1;113(1):369-379. doi: 10.1152/jn.00633.2013. Epub 2014 Oct 15. PMID: 25318764; PMCID: PMC4294568.
- National Academies of Sciences, Engineering, and M., Health and Medicine Division, Board on Health Sciences Policy, Abuse, & Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid. Pain Management and the Opioid Epidemic. Pain Management and the Opioid Epidemic. National Academies Press. 2017.Doi: 10.17226/24781
- Priority A, Action G, Giroir B, Mccance, Collins FS, Volkow ND. Reducing Opioid Morbidity and Mortality. 2018.
- Ruhm CJ, Batten F, Doleac J, Leive A, Reshef A, Simon D, Skinner J. Deaths of Despair or Drug Problems? 2018.
- Qiao JT, Dafny N. Dorsal raphe stimulation modulates nociceptive responses in thalamic parafascicular neurons via an ascending pathway: further studies on ascending pain modulation pathways. Pain. 1988 Jul;34(1):65-74. doi: 10.1016/0304-3959(88)90183-2. PMID: 3405622.
- Sarpatwari A, Avorn J, Kesselheim AS. Using a drug-safety tool to prevent competition. N Engl J Med. 2014 Apr 17;370(16):1476-1478. doi: 10.1056/NEJMp1400488. PMID: 24738666.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.