> Biology Group. 2021 August 30;2(8):715-720. doi: 10.37871/jbres1302.
Phytochemical Evaluation and Acute Toxicity Study of Ethanol Leaf Extract of Acalypha wilkesiana
Anthony Osamuyi Iyamu1, Uwaifoh Akpamu2* and Karen Uwarobehi Iyamu3
2Gastrointestinal Secretory and Inflammatory Unit, Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Ibadan-Nigeria
3Medical Officer, Mary-Martha Hospital, Delta State, Nigeria
- Plant-derived medicines
- Acalypha wilkesiana leaf
- Proximate analysis
- Phytochemical screening
- Acute toxicity
Abstract
Increased curiosity on natural plant products has been raised due to problems of cost, unavailability, and after-effects of countless synthetic drugs. Worrisome, many plant-derived formulations lack phytochemically or toxicological screening. Hence, this study phytochemical and elemental screened the ethanolic leaf extract of Acalypha wilkesiana and as well as determining acute toxicity in adult male Wistar rats. The leaves were obtained in Benin City, Nigeria. Ethanol extraction was carried out on leaves and the extract was subjected to proximate, qualitative, and quantitative phytochemical screening and elemental analysis. Acute toxicity was determined on 12 adult male Wistar rats following Lork’s method. Proximate analysis revealed a high presence of carbohydrate, ash, fiber, and moisture. The qualitative and quantitative evaluation showed the abundance of alkaloids (68.7 ± 0.120%), flavonoids (34.7 ± 0.001%) and minute (<1mg/g) saponins, tannins, phenol, and terpenes. The extract contain nutritive (vitamin E = 1.184 ± 0.055µg/g; vitamin A = 0.0066 ± 0.003µg/g; vitamin C = 0.046 ± 0.037µg/g) and anti-nutritive (oxalates = 229.780 ± 16.93mg/100g; cyanide=0.162 ± 0.006 mg/100g; phytate = 0.131 ± 0.01mg/100g) elements. The elemental evaluation showed an abundance of potassium, sodium, and chloride with traces of cadmium and lead and the absence of manganese and copper. There was no sign of acute toxicity or mortality at an extract dose of 5000mg/kg. These findings indicate the ethanol leaf extract of A. wilkesiana as a rich source of phytochemicals and major macro elements and high safety at 5000mg/kg dose. Considering the several components in the leaves extract, Acalypha wilkesiana leaf might be pharmacological significant for the biological system.
Background
Herbal medicine or botanical medicine or phytomedicine sometimes referred to as herbalism which is the use of herbs for their therapeutic or medicinal values; refers to using plants seeds, flowers, roots for the medicinal purpose [1]. It is the oldest form of health care known to mankind. About 80% of the world population particularly in developing countries depend on herbal medicines for primary health care because of better cultural acceptability, safety, efficacy, potent, inexpensive, and lesser side effects [2]. It is now frequently been considered that plant drugs are less toxic compared with synthetic drugs [3]. In most of the world today medicinal plants are increasingly used as contraceptives, abortifacients, emmenagogues or oxytocic [4], antihypertensive [5], hypoglycemic [6-8], hypolipidemic [7], antibacterial and antifungal [9-11]), treatment of skin diseases [12], One of such plant is Acalypha wilkesiana.
Acalypha wilkesiana (A. wilkesiana) (other names include A. amentacea and A. tricolor) with common names like “copperleaf”, “Joseph’s coat”, “fire dragon”, “match me if you can” belongs to the family Euphorbiaceae (spurge family) [13,14]. The Yorubas of Southern Nigeria calls it “aworoso” while the Hausas of Northern Nigeria call it “Jinwinini” [15]. According to Al-Attar [14], the plant is native to Fiji and nearby islands in the South Pacificans. It is a popular outdoor and indoor spreading evergreen shrub with branches that tend to originate near the base and propagated by stem cuttings at any time of the year. It grows up to 3.1 m tall, with leaves (12.7-20.3 cm long) that are alternate, elliptic to oval, serrate and multi-coloradans small inconspicuous flowers (10.2-20.3 cm) that hangs in catkin-like racemes beneath the foliage [14].
A. wilkesiana is used in the traditional medicines for the treatment of bacterial and fungal skin infections, neonatal jaundice, and gastrointestinal disorders [16] and as antimicrobial [17], antihypertension [18], and anticarcinogenic properties [19] as well as exhibit antiparasitic and analgesic properties [20]. The methanolic leaves extract of A. wilkesiana has been documented to exhibit hypoglycemic activities and positively affected the hematopoietic system, the integrity of liver and kidney functions, and improved the lipid profile [21].
With the discovery that most plant extracts and products have significant antioxidant activity their significance in the treatment of several diseases is now been investigated. For this reason, Scartezzini and Speroni, documented that herbal plants are now considered useful means for the prevention, and amelioration of many disorders and complications [21,22]. Although ethnobotanical information indicates the use of over ten of thousand plants and plant products and formula as traditional remedies for the treatment of several diseases and Al-Attar [11] reported that many of these plants do not have scientific scrutiny. Not much pharmacological research has been carried out on A. wilkesiana despite its importance in traditional medicine [23]. Therefore, this study aims to evaluate the phytochemical and elemental component of the ethanolic leaves extract of Acalypha wilkesiana and determine the acute toxicity in adult male Wistar rats.
Materials and Methods
Collection, processing, and extraction of plant materials
Samples of Acalypha wilkesiana leaves were collected from Benin City in Edo State, Nigeria, and authenticated at the Herbarium Unit of Forestry Research Institute, Ibadan, Oyo State, Nigeria. The leaves were plucked, sorted, air-dried for 7 days, and then pulverized and packaged in polyethylene airtight bags. 200g of the powder leaf was added into a container containing 1.5L of 70% ethanol and used to prepare the ethanolic extract as described in Majekodunmi and Nubani with few modifications [24].
Extract screening
Proximate analysis was carried out using the method and procedure described in the Association of Official Analytical Chemists [25]. Qualitative and quantitative phytochemical screening was done using the method of Harborne [26] and Trease and Evans [27], to identify active constituents and simple chemical tests to detect the presence of secondary plant constituents such as alkaloids, tannins, flavonoids, saponins, sterols, phenols, glycosides and reducing sugars. Anti-nutrients constituents were determined following standard methods. The phytate content was as described by Lucas and Markaka [28], oxalate as described by Munro [29], and hydrogen cyanide and haemagglutinin as in Boyle and Shepiengh [30]. Elemental contents were determined by atomic absorption spectrometry, flame photometry, and spectrophotometry according to the method of AOAC [25].
Experimental animals
Twelve adult male Wistar rats with an average weight of 145-160g were obtained from the animal house of the College of Medicine, Ambrose Alli University, Ekpoma. The rats were housed at room temperature on a 12-hour dark-light cycle and acclimatized for 14 days with ad libitum access to food (rat chow; Vital Feed Nig. Ltd, Jos, Nigeria) and water.
Acute toxicity study
Oral acute toxicity (LD50) study was carried out as the method described by Lorke with few modifications in two stages [31]:
• Stage I: Nine rats were divided into 3 groups (A1, B1, and C1) of 3 rats each. Groups A1, B1, and C1 received 10, 100 and 1000 mg/kg body weight of ethanolic leaves extract of A. wilkesiana respectively. With no death recorded, the experiment proceeded to state two.
• Stage II: Three rats were divided into three groups (A2, B2, and C2) and treated as followed; Groups A2, B2, and C2 received 1500, 3000 and 5000 mg/kg body weight of ethanolic leaves extract of A. wilkesiana respectively. In each stage, the animals were observed hourly for the 1st 6 hours and then 6 hourly for the next 18 hours for changes in physical and behavioral signs and mortality.
Analysis
The obtained data were analyzed for Mean ± SD and presented in suitable tables and figures.
Results
Phytochemical evaluations
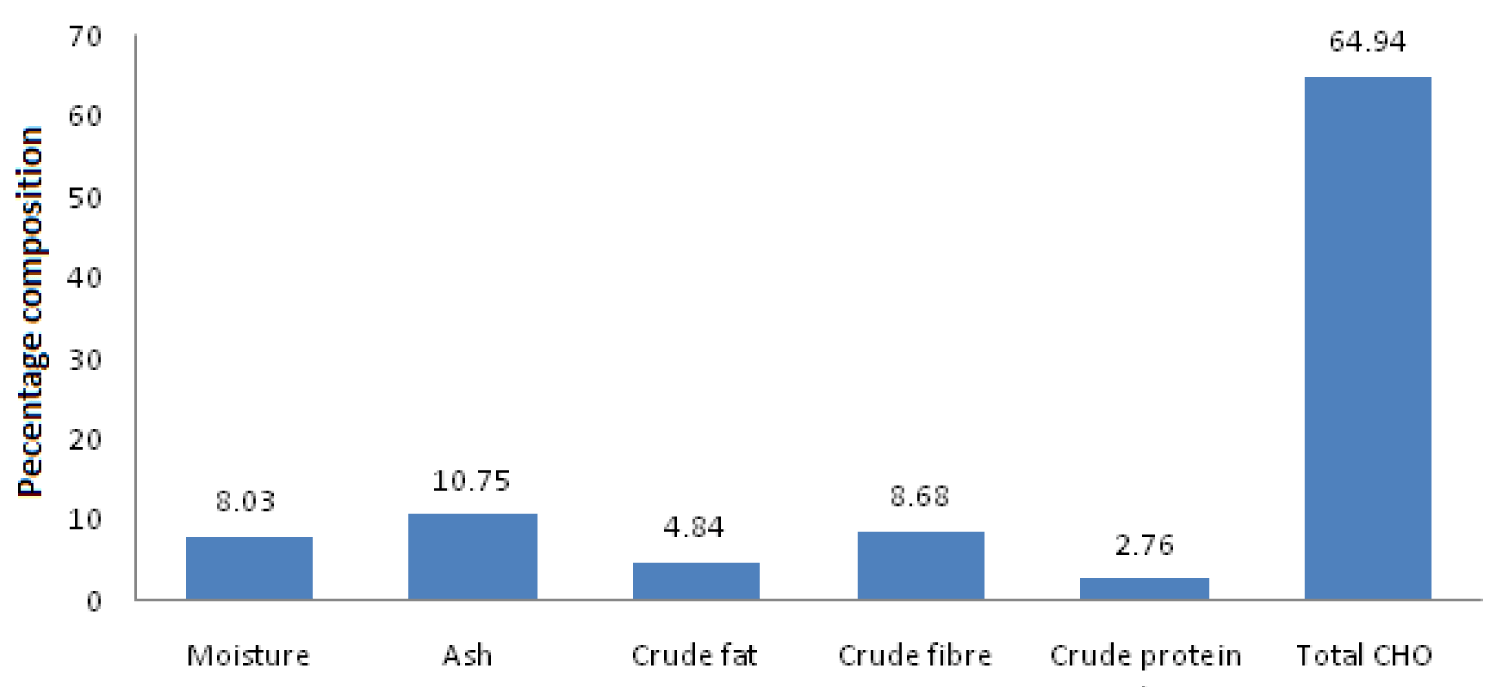
Figure 1 presents the proximate analysis of the leaf extract of A. wilkesiana to contains abundant carbohydrates, ash, crude fiber, and moisture, and little crude fat and protein. Qualitative and quantitative phytochemical screenings of leaf extract of A. wilkesiana are presented in tables 1,2 respectively. There were the presence of alkaloids, flavonoids, tannins, phenol, terpenoids, saponins, and glycosides as well as reducing sugar (Table 1) and abundance of alkaloids (68.7 ± 0.120%), and flavonoids (34.7 ± 0.001%) while saponins and tannins were between 1 and 0.5 mg/100g with a little number of terpenes, steroids, and glycosides (Table 2).
| Table 1: Qualitative analysis of the phytochemical compositions of the ethanolic leaves extract of A. wilkesiana. | ||
| S/N | Phytochemicals | Bioavailability |
| 1 | Steroids | + |
| 2 | Terpenoids | + |
| 3 | Saponins | + |
| 4 | Flavonoids | ++ |
| 5 | Glycosides | + |
| 7 | Alkaloids | +++ |
| 8 | Total phenolics | ++ |
| 9 | Total tannins | ++ |
| 10 | Reducing sugar | + |
| Key: + = low, ++ = moderate and +++ = high | ||
| Table 2: Quantitative compositions of the phytochemical analysis of the ethanolic leaves extract of A. wilkesiana (Values are amount ± standard deviation). | ||
| S/N | Phytochemical | Quantitative value |
| 1 | Alkaloids (%) | 68.7 ± 0.120 |
| 2 | Saponins ( mg/100g) | 0.864 ± 0.016 |
| 3 | Flavonoids (%) | 34.7 ± 0.001 |
| 4 | Total tannins (mg/100g) | 0.562 ± 0.023 |
| 5 | Terpenes (µg/g) | 0.221 ± 0.147 |
| 6 | Steroids (μg/mg) | 0.27 ± 0.021 |
| 7 | Total glycosides (g%) | 0.391 ± 0.015 |
| 8 | Reducing sugar (g%) | 0.672 ± 0.083 |
Nutritive and anti-nutritive evaluation
The nutritive and anti-nutritive components are shown in table 3. Nutritive components were more of vitamin E (1.184 ± 0.055 µg/g) and low levels of vitamin A (0.0066 ± 0.003 µg/g) and C (0.046 ± 0.037 µg/g). Anti-nutritive analysis showed the presence of oxalates (229.780 ± 16.93 mg/100g), cyanide (0.162 ± 0.006 mg/100g ± SD) and phytate (0.131 ± 0.01 mg/100g ± SD).
| Table 3: Nutritive and anti-nutritive components of the ethanolic leaves extract of A. wilkesiana (Values are amount ± standard deviation). | |
| Vitamin type | Content ( µg/g) |
| Vitamin A | 0.0066 ± 0.003 |
| Vitamin C | 0.046 ± 0.037 |
| Vitamin E | 1.184 ± 0.055 |
| Antinutrient | Concentration in extract (mg/100g) |
| Phytate | 0.131 ± 0.01 |
| Oxalate | 229.780 ± 16.93 |
| Cyanide | 0.162 ± 0.006 |
| N = 3 | |
Elemental composition evaluation
The elemental composition of the Leaf extract of A. wilkesiana is presented in table 4 and showed the abundance of potassium (406.08 mg/kg), sodium (118.06 mg/kg), and Chloride (41.18 mg/L) with a trace amount of magnesium (7.29 ppm) and zinc (0.5541ppm) but absent of manganese and copper. There were traces of cadmium (4.67ppm) and lead (0.25ppm) in the extract of A. wilkesiana.
| Table 4: Elemental compositions of the ethanolic leaves extract of A. wilkesiana (Values are mean ± SD). | ||
| S/N | Element | Concentration |
| 1 | Sodium | 118.06 (mg/kg) |
| 2 | Potassium | 406.08 (mg/kg) |
| 3 | Chloride | 41.18 (mg/L) |
| 4 | Magnesium | 7.29 (ppm) |
| 5 | Manganese | Nil |
| 6 | Copper | Nil |
| 7 | Zinc | 0.5541 (ppm) |
| 8 | Cadmium | 4.67 (ppm) |
| 9 | Lead | 0.25 (ppm) |
Acute toxicity study
Table 5 shows the acute toxicity (LD50) of the ethanolic leaf extract of A. Wilkesiana in adult male Wistar rats. All the oral doses of the extract of A. wilkesiana administered showed no signs of toxicity and no death occur at the highest dose of 5000 mg/kg body weight. All the rats displayed normal behavioral, neurological, autonomic, and physical signs.
| Table 5: Acute toxicity of the ethanolic leaves extract of Acalypha Wilkesiana in adult male Wistar rats. | |||||
| Groups | Number of rats | Ethanolic Leaves extract of A. wilkesiana (mg/kg body weight) | Mortality | Percentage mortality | Other symptoms |
| Phase 1 A1 B1 C1 |
3 3 3 |
10 100 1000 |
0 0 0 |
0% 0% 0% |
No sign No sign No sign |
| Phase 2 A2 B2 C2 |
1 1 1 |
1500 3000 5000 |
0 0 0 |
0% 0% 0% |
No sign No sign No sign |
Discussion
The study of the chemical constituents and the active principles of medicinal plants have acquired a lot of importance all over the world (1). The proximate analysis of the leaf extract of A. wilkesiana in the present study revealed the extract to be carbohydrate, ash, crude fiber, and moisture-rich but diminutive in crude fat and protein. There was the presence of alkaloids, flavonoids, tannins, phenol, terpineol, saponins, glycosides, and reducing sugar in the qualitative phytochemical screening of the extract while quantitative analysis revealed the abundance of alkaloids, flavonoids with a small number of saponins and tannins, and traces of terpenes, steroids, and glycosides. In line with these findings, Odoh, et al. [32] have reported alkaloids and terpineol to be abundant with the lowest glycosides in the methanolic root extract of A. wilkesiana. El-Khateeb, et al. [21] has also documented methanolic A. wilkesiana leaves extract to reveal the presence of alkaloids, flavonoids, saponins, and tannins, and Ikewuchi, et al. [15] has obtained similar phytoconstituents from the leaves of A. wilkesiana. However, Fonkoua, et al. [33] reported aqueous and hydroethanolic extracts of A. wilkesiana leaves to be rich in bioactive compounds like phenols, tannins, and flavonoids. These variations in phytochemicals in A. wilkesiana extracts may be due to variation in the location where they were obtained, solvent and method of extractions, as well as arts of the plant used.
Also shown in the present study is the rich elemental composition of the leaf extract of A. wilkesiana which showed the abundance of potassium, sodium, and Chloride. Of a problem is the presence of toxic heavy metals like cadmium and lead; although traces but long high intake may raise health concerns. The presence of several phytochemicals, nutritive and elemental constituents in the leaf of A. Wilkesiana are indicative of the plant’s nutritious and pharmacological significance. For example, carbohydrates and their derivatives are known to take part in the immune system, fertilization, pathogenesis, blood clotting, and development [34]. Alkaloids are pharmacologically useful as a local anesthetic, CNS stimulant, analgesic [35]. Tannins on the other hand have been reported to inhibitor microbial proliferation and possess antibacterial anti-parasitic, anticancer and improve wound healing properties [36-41]. Saponins also have been documented to have antibacterial activities and expectorant properties and these abilities support their use in upper respiratory tract infection. The presence of flavonoids in the leaf extract is also very important because flavonoids act as natural modifiers of biological response and have anti-allergic, anti-inflammatory, anti-microbial, and anti-cancer potentials [27,42,43].
On the elemental significance, macro elements like sodium, potassium, and calcium are known to regulate fluid balance and influence cardiac output while restriction of sodium intake or increase Potassium intake exert an antihypertensive effect [44,45]. Calcium ions play important physiological and biochemical processes like neuromuscular excitability, blood coagulation, secretary processes, the proper extracellular fluid concentration of calcium and phosphate ions are required for bone mineralization [45,46]. Elements such as iron, zinc, and manganese are essential for enzyme reactions as co-factors [46]. It may be possible therefore that the extract of A. wilkesiana might be useful in the treatment of several diseases considering its several phytochemicals and elements with health benefits.
Acute toxicity studies are conducted to ascertain the safety of plant extracts and Ashafa and Olunu noted that toxicity studies of herbal extract in animals are commonly used to assess potential health risks in humans that can cause intrinsic adverse effects of chemical compounds or plant extracts [47]. In the present study, the highest dose of 5000mg/kg was safe in the adult male Wistar rats and there was no sign of toxicity or death. In agreement with this study, Iniaghe, et al. [48] documented that at an administered dose of 10,000 mg/kg body weight of aqueous leaf extract of A. wilkesiana, all rats displayed normal behavioral, neurological, and autonomic profiles.
Conclusively, this study showed that the ethanolic leaves extract of A. wilkesiana is a rich source of phytochemicals and major macro elements that can be pharmacological significant for the biological system. Also shown in this study is the safety of this extract at doses as high as 5000mg/kg. However, the need for chronic toxicity study with this extract is needed and their potentials in several disease conditions are recommendations for further studies.
References
- Brahma K, Tripura N, Meena P, Siddika SK, Ravi A. Preliminary phytochemical and antimicrobial activity of Acalypha indica, Acalypha wilkesiana milk white and Acalypha wilkesiana tricolor. International Journal of Research in Pharmacy and Chemistry. 2015;5(1):176-182. https://tinyurl.com/28ccdj5p
- Pullaiah T, Chandrasekhar Nadiu K. Ant diabetic plants in india and herbal based antidiabetic research. Regency publications. New Delhi. 2003. https://tinyurl.com/ehmr9c
- Pari L, Umamaheswari J. Antihyperglycaemic activity of Musa sapientum flowers: Effect on lipid peroxidation in alloxan diabetic rats. Phytother Res. 2000 Mar;14(2):136-138. doi: 10.1002/(sici)1099-1573(200003)14:2<136::aid-ptr607>3.0.co;2-k. PMID: 10685115.
- Ritchie HE. The safety of herbal medicine use during pregnancy. Frontiers Fetal Health. 2001;3:259-266.
- Nworgu ZAM, Onwukaeme DN, Afolayan AJ, Amaechina FC, Ayinde BA. Preliminary studies of blood pressure lowering effect of nauclea latifolia in rats. Afr J Pharm Pharmacol. 2008;2(2):37-41. https://tinyurl.com/tkymbdjy
- Patel VS, Chitra V, Prasanna PL, Krishnaraju V. Hypoglycemic effect of aqueous extract of parthenium hysterophorus L. in normal and alloxan induced diabetic rats. Indian J Pharmacol. 2008 Aug;40(4):183-185. doi: 10.4103/0253-7613.43167. PMID: 20040954; PMCID: PMC2792614.
- Yadav JP, Saini S, Kalia AN, Dangi AS. Hypoglycemic and hypolipidemic activity of ethanolic extract of salvadora oleoides in normal and alloxan-induced diabetic rats. Indian J Pharmacol. 2008 Jan;40(1):23-27. doi: 10.4103/0253-7613.40485. PMID: 21264157; PMCID: PMC3023117.
- Odoh UE, Ezugwu CO, Ezejiofor M. Anti-diabetic and toxicological studies of the alkaloids of acanthus montanus (acanthaceae) leaf. Int J Curr Res. 2013;5(12):4249-4255. https://tinyurl.com/ych5zmfd
- Adesina SK, Idowu O, Ogundaini AO, Oladimeji H, Olugbade TA, Onawunmi GO, Pais M. Antimicrobial constituents of the leaves of acalypha wilkesiana and aacalypha hispida. Phytother Res. 2000 Aug;14(5):371-4. doi: 10.1002/1099-1573(200008)14:5<371::aid-ptr625>3.0.co;2-f. Erratum in: Phytother Res 2000 Dec;14(8):661. PMID: 10925407.
- Ogundainio A. From green into medicine: Taking a lead from Nature. An inaugural lecture delivered at Oduduwa Hall, Obafemi Awolowo University, Nigeria. 2005;12-15. https://tinyurl.com/a6b8zp4t
- Oladunmoye MK. Comparative evaluation of antimicrobial activities and phytochemical screening of two varieties of acalypha wilkesiana. Trends in Appl Sci Res. 2006;1:538–541. https://tinyurl.com/k3awj4ak
- Ajose FO. Some nigerian plants of dermatologic importance. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55. doi: 10.1111/j.1365-4632.2007.03466.x. PMID: 17919209.
- Christman S. Acalypha wilkesiana. Florida. 2004. https://tinyurl.com/4hc9vm4z
- Attar AM. Physiological study on the effect of acalypha wilkesiana leaves extract on streptozotocin-induced experimental diabetes in male mice. Am Med J. 2010;1(1):51-58. https://tinyurl.com/kd4zcymh
- Ikewuchi CJ, Ikewuchi CC, Onyeiken EN, Uwakwe AA. Nutritional potential of the leaves of acalypha wilkesiana ‘godseffiana’ muell. Arg J Appl Sci Environ Manage. 2010;14(3):21-24. https://tinyurl.com/22472458
- Ogundaini AO. An inaugural lecture delivered at Oduduwa Hall, Obafemi Awolowo University, Ile‐Ife, Nigeria. Inaugural Lecture Series 176, OAU Press Limited. Nigeria. 2005:12‐15. https://tinyurl.com/b25u5njt
- Akinyemi KO, Oluwaans OK, Omomigbehin EO. Antimicrobial activity of crude extracts of three medicinal plants used in south-western Nigerian folk medicine on some food borne bacterial pathogens. Afr J Tradit Compl Altern Med. 2013;3(4):13‐22. https://tinyurl.com/778ajr9b
- Ikewuchi JC. Moderation of hematological and plasma biochemical indices of sub-chronic salt-loaded rats, by an aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae). Asian Pac J Trop Med. 2013 Jan;6(1):37-42. doi: 10.1016/S1995-7645(12)60197-7. PMID: 23317883.
- Lim SW, Loh H, Ting KN, Bradshaw TD, Zeenathul NA. Acalypha wilkesiana ethyl acetate extract enhances the in vitro cytotoxic effects of α-tocopherol in human brain and lung cancer cells. Int J Biosci Biochem Bioinforma. 2013;3(4):335‐340. https://tinyurl.com/wet3k3wb
- Udobang JA, Nwafor PA, Okokon JE. Analgesic and antimalarial activities of crude leaf extract and fractions of Acalypha wilkensiana. J Ethnopharmacol. 2010 Feb 3;127(2):373-378. doi: 10.1016/j.jep.2009.10.028. Epub 2009 Nov 3. PMID: 19892007.
- Khateeb YA, Azzaz AN, Mahmoud IH. Phytochemical constituents, hypoglycemic and haematological effects of methanolic Acalypha wilkesiana leaves extract on streptozotocin‐induced diabetic rats. European Journal of Chemistry. 2014;5(3):430‐438. https://tinyurl.com/5f85zmwb
- Anjali P, Manoj KM. Some comments on diabetes and herbal therapy. Ancient Sci Life. 1995;15:27-29. https://tinyurl.com/2j4usn32
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000 Jul;71(1-2):23-43. doi: 10.1016/s0378-8741(00)00213-0. PMID: 10904144.
- Majekodunmi SO, Nubani SE. Formulation of acalypha wilkesiana muell. Arg. Ethanol leaf extract into creams for the treatment of microbial microbial skin infections. International Journal of Pharma Pharmacycological Science. 2014;31(10):45-53. https://tinyurl.com/4p4dfxp8
- AOAC. Official method of analysis of the association of official analytical chemists. Arlington. 1990. https://tinyurl.com/4b7dwc
- Harbone J. Phytochemical methods: A guide to modern techniques of plant analysis. 1998. https://tinyurl.com/ewddheb3
- Trease GE, Evans WC. Pharmacognosy. 11th Edition. Macmillan Publishers, London, United Kingdom. 1989:116-180. https://tinyurl.com/45557dx4
- Lucas GM, Markakas. Phytic acid and other phosphorus compounds of beans (Phaseolus vulgaris). Advanced Journal of Food Science. 1975; 23(23):13-15. https://tinyurl.com/5dd36jvf
- Munro AB. Oxalate in Nigeria vegetables. W Afri J Biol Appl Chem. 2000;12:14-18. https://tinyurl.com/5hhnf4jf
- Boyd WC, Shapleigh E. Specific precipitating activity of plant agglutinins (lectins). Science. 1954 Mar 26;119(3091):419. doi: 10.1126/science.119.3091.419. PMID: 17842730.
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983 Dec;54(4):275-287. doi: 10.1007/BF01234480. PMID: 6667118.
- Odoh UE, Ndubuokwu RI, Agha SI, Osadebe PO, Uzor PF, Ezejiofor M. Antidiabetic activity and phytochemical screening of Acalypha wilkesiana (Euphorbiaceae) Mull Arg. roots in alloxan-induced diabetic rats. Sci Res Essays. 2014;9(7):204-212.
- Fonkoua M, Ngaluh J, Edoun EFL, Mbah NL, Mbouobda HD, Ngondi JL. Screening of antioxidant and antidiabetic properties of aqueous and hydroethanolic extracts of the leaves of Acalypha wilkesiana Muel. American Journal of Pharmacy & Health Research. 2017;5(6):78-88. https://tinyurl.com/df4ve3e7
- Maton A, Mclaughlin JCW, Johnson S, Warner MQ, Lattart D, Wright JD. Human biology and health. Englewood Cliffs, New Jersey, USA: Practice Hall. 1993:52–59. https://tinyurl.com/yzf77se7
- Madziga HA, Sanni S, Sandabe UK. Phytochemical and elemental analysis of acalypha wilkesiana leaf. J American Sci. 2010;6(11):510-514. https://tinyurl.com/wtex77aj
- Awosika F. Local medicinal plants and health of consumers. Pharm Herbal Medicine. 1991;28–29. https://tinyurl.com/9ddjjznt
- Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001 Oct;48(4):487-491. doi: 10.1093/jac/48.4.487. PMID: 11581226.
- Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol. 2004;48(4):251-261. doi: 10.1111/j.1348-0421.2004.tb03521.x. PMID: 15107535.
- Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999 Mar 1;136(2):215-221. doi: 10.1016/s0304-3835(98)00323-1. PMID: 10355751.
- Li H, Wang Z, Liu Y. [Review in the studies on tannins activity of cancer prevention and anticancer]. Zhong Yao Cai. 2003 Jun;26(6):444-8. Chinese. PMID: 14528687.
- Tyler VE, Braddyu LR, Roberts J. A textbook of pharmacology Lea and Pernoager, Philadelphia. 1998;85-90. https://tinyurl.com/cbt23rm8
- Liener IE. Toxic constituents of plant foodstuffs. Proc Nutr Soc. 1970 May;29(1):56-57. doi: 10.1079/pns19700010. PMID: 5529217.
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001 Jan;107(2):135-142. doi: 10.1172/JCI11914. PMID: 11160126; PMCID: PMC199180.
- Sanni S. Pharmacological and toxicological effects of olimum basilicum linn. Aqueous extract in rats. PhD thesis, Usman Danfodiyo University Sokoto. 2007;16.
- Schroever HA. In trace element and nutrition 1st Ed. Faber London. 1976;30-46.
- Roberts KM, Daryl KG, Peter AM, Victor WK. Harper’s biochemistry. 25th edition Large Medicial Book. 2000;209-210. https://tinyurl.com/2u3uwsxx
- Ashafa AT, Olunu OO. Toxicological evaluation of ethanolic root extract of Morinda lucida (L.) Benth. (Rubiaceae) in male Wistar rats. J Nat pharm. 2011;2:108-114. https://tinyurl.com/e99rh4ap
- Iniaghe MO, Egharevba, Oyewo BE. Effect of aqueous leaf extract of acalypha wilkesiana on hematological parameters in male wistar albino rats. British Journal of Pharmaceutical Research. 2013;3(3):465-471. https://tinyurl.com/44kmfm9f
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.