> General Science. 2021 May 15;2(5):323-327. doi: 10.37871/jbres1235.
Facial Palsy, Hypoacusis and Vertigo as Inaugural Symptoms of Neuroborreliosis - Case Report
Joana Borges da Costa1*, Joana Barreto1, Miguel Neno2, Delfim Duarte3 and Miguel Viana1
2Infectious Diseases Department, Hospital Pedro Hispano, Portugal
3Director of Otolaryngology Department, Hospital Pedro Hispano, Portugal
- Borrelia
- Neuroborreliosis
- Facial palsy
- Meningopolyneuritis
- Lyme disease
Abstract
Introduction: Facial Palsy (FP) is the most common acute mononeuropathy and consists of a decrease in facial muscle strength due to facial nerve damage. Peripheral FP (PFP) can result from a wide variety of disorders and aetiologies, with Lyme Disease (LD) being considered one of the most common causes of FP. LD is an infectious disease that affects the central nervous system, causing Neuroborreliosis (NB) in 15% of cases. Cranial neuropathy is the most common form of presentation of NB, as uni or bilateral PFP.
We describe a case of PFP with hypoacusis and vertigo as the inaugural presentation of neuroborreliosis.
Case Report: We present a case report of a 75-year-old female patient, referred to ENT consultation due to a 3-day course of a grade 4 right PFP, moderate right sensorineural hearing loss and right vestibular hypofunction. The patient underwent cranioencephalic Computed Tomography (CT-CE) which excluded a central event and was treated with 2 cycles of oral corticosteroids, without any clinical improvement. A Magnetic Resonance Imaging (MRI) was performed, which showed an abnormal inflammatory uptake of the right facial nerve. An analytical positivity for Borrelia IgM was found and the diagnosis of polyneuropathic NB with involvement of the VII and VIII right cranial pairs was assumed.
The patient completed 28 days of doxycycline, with FP and vertigo improvement and normalization of hearing acuity.
Discussion/Conclusion: In the presented case, the absence of the classical migratory erythema or painful meningopolyneuritis didn’t exclude the diagnosis of NB. FP associated to signs of other cranial nerves involvement raised the hypothesis of a systemic polyneuropathic disease, which motivated the etiological investigation carried out.
Abbreviations
Bb: Borrelia burgdorferi; BP: Bell’s Palsy; CE-CT: Cranioencephalic Computed Tomography; CSF: Cerebrospinal Fluid; ENT: Ear, Nose and Throat Specialist; FN: Facial Nerve; FP: Facial Palsy; IAC: Internal Auditory Canal; LD: Lyme Disease; MRI: Magnetic Resonance Imaging; NB: Neuroborreliosis; PCR: Polymerase Chain Reaction; PFP: Peripheral Facial Palsy
Introduction
Peripheral facial palsy may be secondary or idiopathic, which is usually designed by Bell’s Palsy (BP) [1]. BP is the most common acute mononeuropathy and is the most common diagnosis associated with FN weakness/paralysis, with an incidence of 20-25/100.000 cases [2].
Secondary FP is generally less prevalent than BP (25 vs. 75%) [2] and can be caused by metabolic, neurologic or autoimmune diseases and infections [3]. Treatment of secondary peripheral facial weakness is based on therapy for the underlying disorder, unlike the treatment of BP that is controversial due to the lack of large, randomized, controlled, prospective studies [1].
One of the most common FP infectious causes is Lyme Disease (LD) that is a tick-bite transmitted bacterial infection caused by Spiroquete Borrelia burgdorferi (Bb) [4].
The spirochete has a tropism for peripheral and/or central nervous systems, resulting in Neuroborreliosis (NB) in up to 15% of the affected patients [4]. The clinical course of NB is highly variable, being the meningoradiculitis the most frequent manifestation in Europe [5]. In endemic areas for Bb, NB is estimated to be responsible for 2-25% of PFP cases and although at least 80% of European NB patients present with FP and radiculitis, NB symptoms may be quite unspecific or even mimic other neurological diseases [5].
The NB diagnosis is based on medical history, clinical findings, serological and Cerebrospinal Fluid (CSF) analysis. Identification of pleocytosis, intrathecal production of immunoglobulins and detection of Bb antibodies in CSF are the best indicators for definite diagnosis The NB prognosis is usually favourable after antibiotic treatment, although a small number of patients remain with residual symptoms [6].
The present work serves to describe a case of PFP associated with hypoacusis and vertigo as the inaugural presentation of NB.
Case Report
A 75-year-old white woman presented with a 3-day course of a right unilateral PFP, right hearing loss, persistent poor balance and rotatory vertigo lasting several minutes to hours, aggravated by body movements and associated nausea, without fever or other otological or neurological complaints. On inspection, there was a House-Brackmann grade 4 right PFP, the otoscopy and pharyngeal inspection were normal, the Rinne test was bilaterally positive and the Webber test lateralized to left ear. The patient presented a right vestibular hypofunction, with an Unterberger stepping test positive for the right side.
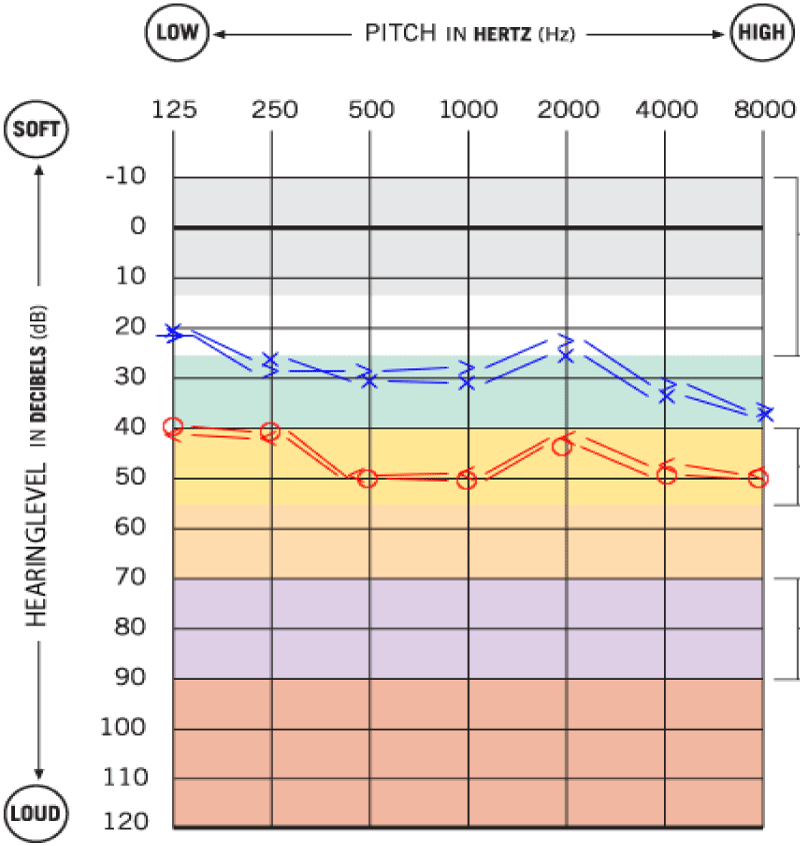
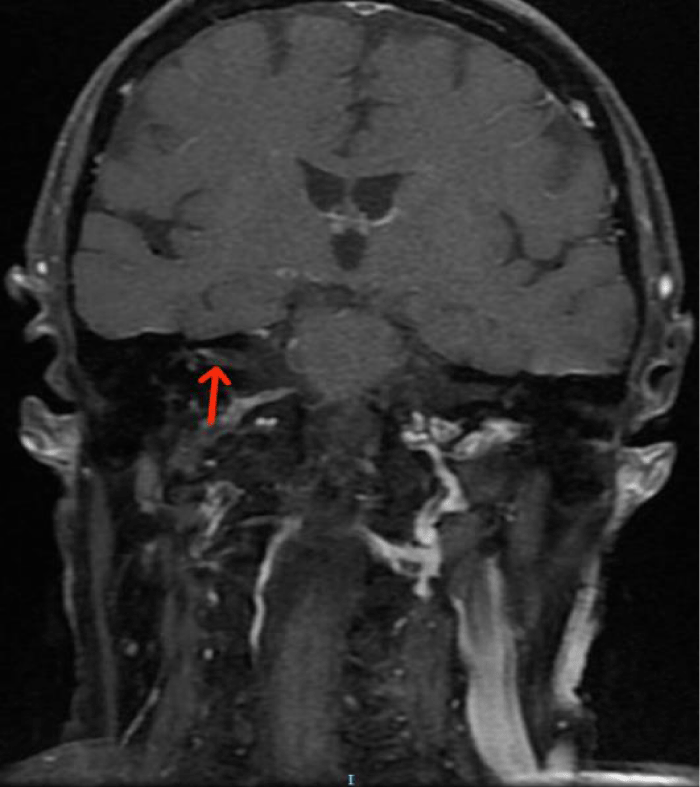
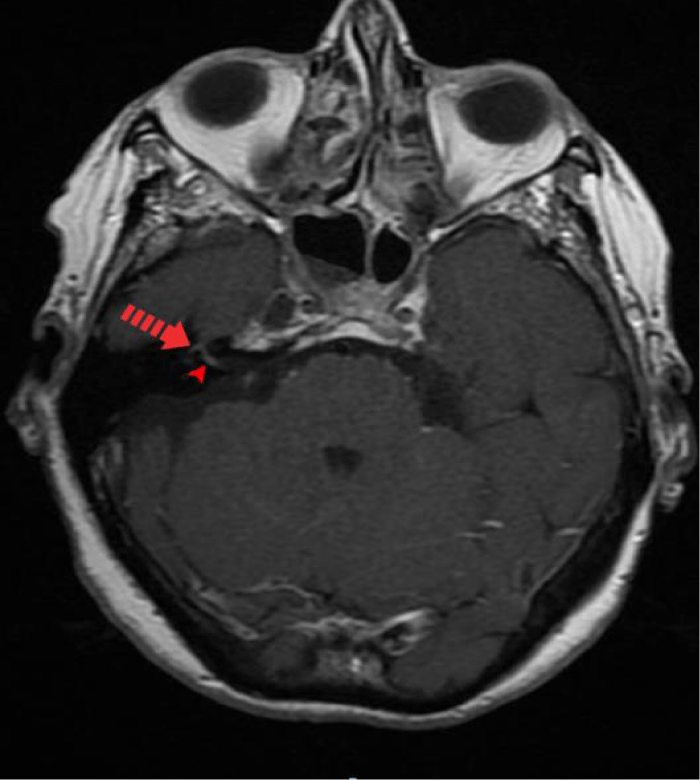
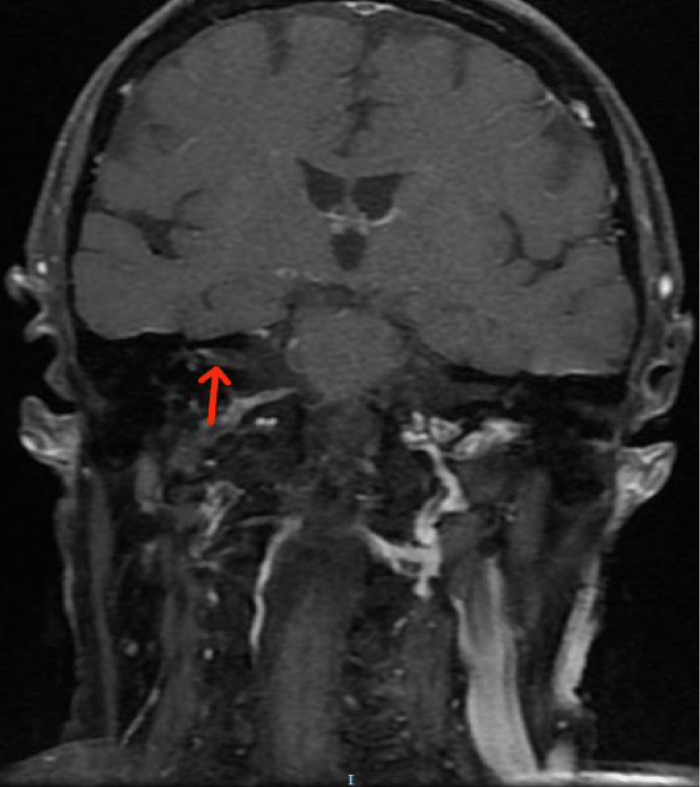
A pure-tone audiometry showed a moderate right sensorineural hearing loss of all tested frequencies, with a Pure Tone Average (PTA) of 45dB (Figure 1). A Cranioencephalic Computed Tomography (CE-CT) excluded the presence of an acute central event or the presence of an occupying-space lesion and the patient was medicated with two cycles of oral corticosteroids (1mg/Kg/day of methylprednisolone) for 2 weeks each. Due to the lack of clinical improvement of FP, hypoacusis and vertigo, a cranioencephalic and an Internal Auditory Canal (IAC) Magnetic Resonance Imaging (MRI) were performed, which showed incipient gliotic changes of admissible ischemic nature due to arteriosclerotic microangiopathy and slight reduction in brain parenchyma volume, to an expected degree for the patient’s age group. In the study directed at the ears, an anomalous uptake of the right facial nerve at the bottom of the IAC was identified, with an extension to its labyrinthine segment and to the geniculate ganglion, without evident nodularity and without destruction of the surrounding bone. The tympanic and mastoid segment of the nerve tenuously highlighted, in a symmetrical way to that observed in the left facial nerve, without evident pathological value. Taken together, these findings suggested an inflammatory alteration involving the right FN, without images suggestive of a neoformative process (Figures 2,3).
Looking for a cause of secondary FP, an exhaustive analytical investigation was performed and went negative. It included haematological, renal, hepatobiliary, cardiovascular, immunological (antibody anti DNA, anti-nuclear, anti SSA/SSB) and serological analysis (Hepatitis B and C, HIV, syphilis, brucellosis, HSV, CMV, EBV). The Borreliosis serology was positive to IgM and negative to IgG. This result was confirmed by Western blot test and the patient was referred to an Infectious Diseases consultation. A lumbar puncture was performed, and the CSF analysis showed elevation of protein (32mg/dL) and a glycorrhachia of 67mg/dL, with a peripheral blood glucose of 125mg/dL. The detection of Bb by Polymerase Chain Reaction (PCR) and of specific antibodies by immunoblotting were both negative. A presumptive diagnosis of polyneuropathic NB was assumed, with involvement of right VII and VIII cranial nerves and the patient was treated with a 100mg of oral doxycycline twice daily for 28 days.
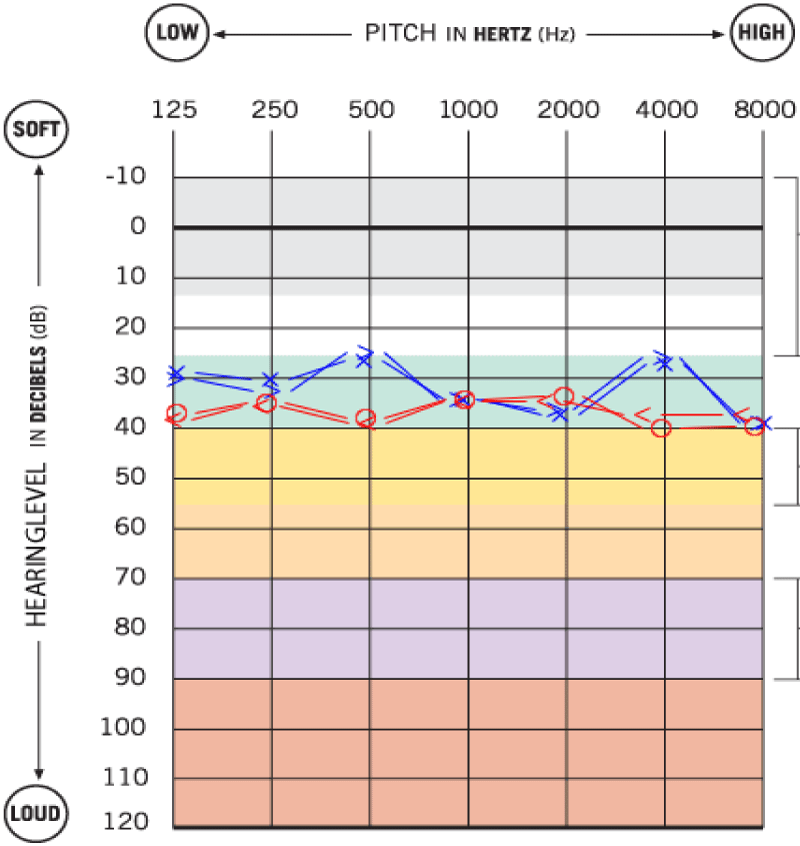
Three months after treatment, there was a clinical improvement of FP from grade 4 to grade 2-3, the hearing acuity normalized to pre-borreliosis levels (a bilateral and symmetrical mild sensorineural hearing loss) and imbalance complaints improved (Figure 4). The ELISA test was repeated and showed positivity for Bb IgM and negativity for IgG and a new MRI was performed, exhibiting a decrease in contrast uptake in the right FN, with disappearance of contrast uptake in the nerve segment at the bottom of the IAC, in its labyrinthine segment and in the geniculate ganglion, persisting a linear contrast uptake in the tympanic and mastoid segments of the nerve (Figure 5).
The patient was also submitted to a right enlarged excision of a grade 2 nasal pterygium with conjunctiva autotransplantation.
The patient is currently followed up in the ENT and Infectious Diseases consultations. To date, the patient remains without any clinical worsening of disease.
Discussion
Lyme disease is a vector-borne illness transmitted to the human via Ixodes ticks harbouring the spirochete Bb. Humans contract Bb via the tick bite and if it remains attached more than 24 hours on the host, the spirochete is transferred to the bloodstream of the host [7].
The clinical course of LD is subdivided in 3 stages and, in nearly 80% of cases, LD begins as erythema chronicum migrans at the site of the tick bite that is pathognomonic [7]. A disseminated form characterizes the disease second stage and usually occurs days to weeks after onset of the rash. Cranial neuropathy remains the most frequent sign in early NB, where uni/bilateral PFP is the most common symptom. Less frequently, neuroborreliosis cause III, VI and VIII nerve palsy. During this period of the infection, Bb antibodies are produced, being the IgM response associated to polyclonal activation of B cells. The IgG response develops slowly over weeks to months [8]. Late or persistent infection (stage 3) occurs months to years after the initial onset of symptoms, being the chronic progressive encephalomyelitis and cerebral vasculitis the most frequent syndrome in this form [8].
The identification of Bb antibodies in blood and CSF remains the diagnostic mainstay and the ELISA and immunoblot are currently the best sensitivity and specificity rated tests [8]. The serologic reactions are insensitive during the first weeks of infection and only half of patients with early-stage LD have positive serology test results [8]. IgM and IgG antibodies respectively appear 2 to 4 weeks and 4 to 6 weeks after the onset of the skin rash. IgG levels usually remain low even in successfully treated patients, while IgM typically drops off to low levels 6 months after infectious onset. Because IgG and IgM antibodies may persist for years after treatment, a positive IgM test alone is likely a false-positive after 1 month of active disease and should not be used to diagnose LD after this period [9]. That’s probably what happened in the reported case, once that several months after successful treatment, the IgM kept positive. Neither ELISA nor Western blot testing can distinguish active from inactive infection [9].
Oral antibiotic therapy is efficient for most cases of early localized or disseminated LD. For late stages, antibiotics are less efficient due to a direct effect of bacteria and immunological changes and, for that reason, NB typically requires intravenous therapy [9].
In adult, the prognosis of FP secondary to LD is generally good, with an improvement of FN function in about 90% of patients [5].
The presented case report represents an involvement of right VII and VIII cranial nerves in the context of NB, causing PFP, hearing loss and vestibular hypofunction. Initially, the clinical condition was interpreted as idiopathic but, given the absence of clinical improvement after 2 cycles of corticosteroids, it was raised the suspicion of a neurological or infectious disease. The patient was treated with doxycycline based on fact that on European guidelines about LD [10], the use of oral doxycycline showed equal effectiveness as intravenous ceftriaxone in the treatment of NB without involvement of CNS.
Although Bb IgM have remained positive months after treatment, the fact that there was a significant clinical improvement of VII and VIII nerves functions after antibiotic therapy shows that the prognosis is favourable. The patient will be followed up in the ENT and infectious diseases consultations for several months or even years.
Conclusion
The present case illustrates the difficulties and the challenge in diagnosing Lyme disease, especially when classical migrant erythema or painful meningo-polyneuritis are absent and when neurologic symptoms predominate. It’s important that physicians maintain a high level of clinical suspicion for LM and NB as a prevalent cause of secondary FP, especially in endemic areas. Peripheric FP significantly and negatively affects the patient’s quality of life, whose prognosis varies according to the etiology in question, so a timely diagnosis and therapeutic implementation are fundamental for the resolution of the clinical situation.
References
- Basić-Kes V, Dobrota VD, Cesarik M, Matovina LZ, Madzar Z, Zavoreo I, Demarin V. Peripheral facial weakness (Bell’s palsy). Acta Clin Croat. 2013 Jun;52(2):195-202. PMID: 24053080.
- Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4-30. PMID: 12482166.
- Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, Deckard NA, Dawson C, Driscoll C, Gillespie MB, Gurgel RK, Halperin J, Khalid AN, Kumar KA, Micco A, Munsell D, Rosenbaum S, Vaughan W. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg. 2013 Nov;149(3 Suppl):S1-27. doi: 10.1177/0194599813505967. PMID: 24189771.
- Zajkowska J, Czupryna P, Kuśmierczyk J, Ciemerych A, Ciemerych M, Kondrusik M, Pancewicz S, Grygorczuk S, Hermanowska-Szpakowicz T. Analiza postaci klinicznych neuroboreliozy wśród pacjentów hospitalizowanych w Klinice Chorób Zakaźnych i Neuroinfekcji Akademii Medycznej w Białymstoku w latach 2000-2005 [Clinical forms of neuroborreliosis--the analysis of patients diagnosed in department of infectious diseases and neuroinfection medical academy in Bialystok between 2000-2005]. Przegl Epidemiol. 2007;61(1):59-65. Polish. PMID: 17702440.
- Kaiser R. Neuroborreliosis. J Neurol. 1998;245(5):247-55.
- Djukic M, Schmidt-Samoa C, Lange P, Spreer A, Neubieser K, Eiffert H, Nau R, Schmidt H. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J Neurol. 2012 Apr;259(4):630-6. doi: 10.1007/s00415-011-6221-8. Epub 2011 Sep 6. PMID: 21898139; PMCID: PMC3319903.
- Fleming S. Pathophysiology: Clinical concepts of disease processes. S. A. Price and L. M. Wilson (Eds). McGraw-Hill, New York, J Pathol. 1986 Nov 1;150(3):223.
- Steere AC. Lyme borreliosis. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Logo DL, Jameson, JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. NewYork, NY: McGraw-Hill. 2001:1061-1065.
- Smith IS, Rechlin DP. Delayed diagnosis of neuroborreliosis presenting as bell palsy and meningitis. J Am Osteopath Assoc. 2010 Aug;110(8):441-4. PMID: 20805550.
- Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I; European Federation of Neurological Societies. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010 Jan;17(1):8-16, e1-4. doi: 10.1111/j.1468-1331.2009.02862.x. Epub 2009 Nov 23. PMID: 19930447.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.