> Medicine Group. 2021 Mar 24;2(3):206-212. doi: 10.37871/jbres1212.
Cord Blood Haematological Parameters Reference Range Difference in Urban and Rural Population of West Bengal as Compared to Global Scenario
Sayak Manna, Indranil Dhar, Tapan Kumar Naskar and Sujata Law*
- Cord blood
- Reference range
- Complete blood count
- Urban
- Rural
- Normal delivery
- Caesarean delivery
Abstract
Background: Human umbilical cord blood is often discarded as a biomedical waste. We aimed to standardise a local cord blood reference range for the West Bengal population. The cord blood haematological values differ depending on ethnic, regional and genealogical groups, so we primarily focussed our study on the international and intra-national differences of cord blood parameters. Comparison between the cord blood values of urban and the rural population along with normal and caesarean deliveries was not recorded before.
Methods: Umbilical cord blood was procured from consenting mothers (average age: 26.1 and 38-40 weeks gestation period), post-delivery at Medical College, Kolkata. Cord blood parameters were divided into two major groups: urban and rural population, each group was further divided into two sub-groups depending on the mode of delivery: normal and caesarean delivery. Comparison was also drawn on the basis of other international groups having different ethnic backgrounds and inter-state individuals having similar ethnic background.
Results: Our results showed West Bengal’s cord blood WBC value was higher and monocyte count was much less when compared globally. The neutrophil value was higher in rural as compared to urban and both the lymphocyte and platelet values of urban were recorded more than rural. Slight differences were recorded among urban-normal, urban-caesarean, rural normal and rural caesarean groups.
Conclusion: A new angle in terms of urban and rural population study is introduced in cord blood analysis. The standardisation of cord blood reference range for the West Bengal population is a new step for neonatal studies.
Introductıon
Umbilical cord blood is rich in hematopoietic stem/progenitor cells [1] and an effective mode of transplantation [2] for its reduced or depressed immunological responses [3] and high availability. The cells with their immense ability of self-renewal and differentiation into multi hematopoietic lineages [4] can lead to the opening of various avenues including researches in translational medicine and other therapeutic measures [5,6]. The advantages of procurement of cord blood for hematopoietic cells include easy and harmless collection procedure, which is safe for both the mother and the new born, reduced graft-versus-host reactivity, low viral contamination [7] and a painless procedure [8]. When cord blood is procured through proper guidelines availability of sample is high and risk of transmissible infections is low [9]. Studies revealed umbilical cord blood can also be considered as an excellent alternative to neonatal blood for the evaluation of sepsis in infants [10,11].
Studies revealed extensive research work is already done to standardize reference values of human peripheral blood but work on haematological reference value of cord blood is still in progress [12,13]. Ethnicity differs on the basis of geographical, socio-cultural and genealogical aspects and one can certainly expect differences in haematological parameters in people belonging from different race and nationality. Some of the previous works were done to establish reference range of cord blood values focussing mostly on Caucasian populations [14-16] and few South East Asian populations [17,18]. Since ethnicity ensures difference in blood parameters we expected to unearth the difference in cord blood haematology profile of Indian population.
Human umbilical cord blood is a biological waste and in most cases it is discarded. The target of the present study was to obtain important information by analysing cellular components of cord blood and to assess the ethnic difference in Indian population (focussing Bengal) when compared to the Caucasian and other populations of the world. Emphasis was also given to study the haematological difference on the basis of area i.e. urban and rural population of Bengal and mode of delivery i.e. normal and caesarean. To avoid overlapping of data, four groups were made consisting of urban and rural population as two major divisions and mode of delivery as sub-divisions. Thus the groups formed were urban-normal, urban-caesarean, rural normal and rural caesarean. Through this study we targeted both international and intra national difference in cord blood parameters and anticipated to establish a reference value for further scientific studies.
Materials and Methods
Procurement of cord blood
Umbilical cord blood was collected from mothers (average age: 26.1 and 38-40 weeks gestation period) as per the ethical guidelines and permission obtained from Clinical Research Ethics Committee (CREC) of Calcutta School of Tropical Medicine and Medical College, Kolkata. An average of 40-45 ml of cord blood was directly collected from the transected cord in sterile heparinised 50ml Falcon Tube (Tarson, India), immediately after the separation of the infant from the mother at Eden Hospital, Medical College with the help of doctors. Mothers having diabetes mellitus, hepatitis, respiratory problems, blood infections, genitourinary disease, eclampsia and previous cases of miscarriages were not taken into consideration. After the blood collection, the samples were taken to the Dept. of Biochemistry and Medical Biotechnology laboratory, School of Tropical medicine within 10-15 mins in 4°C-8°C ice containers. Cord blood procurement was followed by the informed consent of the mothers and patient parties. Patient details were collected from the daily medical reports of the hospital for scientific analysis. The mothers were from different districts of southern part of West Bengal that includes major cities, towns and rural areas.
Analysis of cord blood for haemogram and biochemical parameters
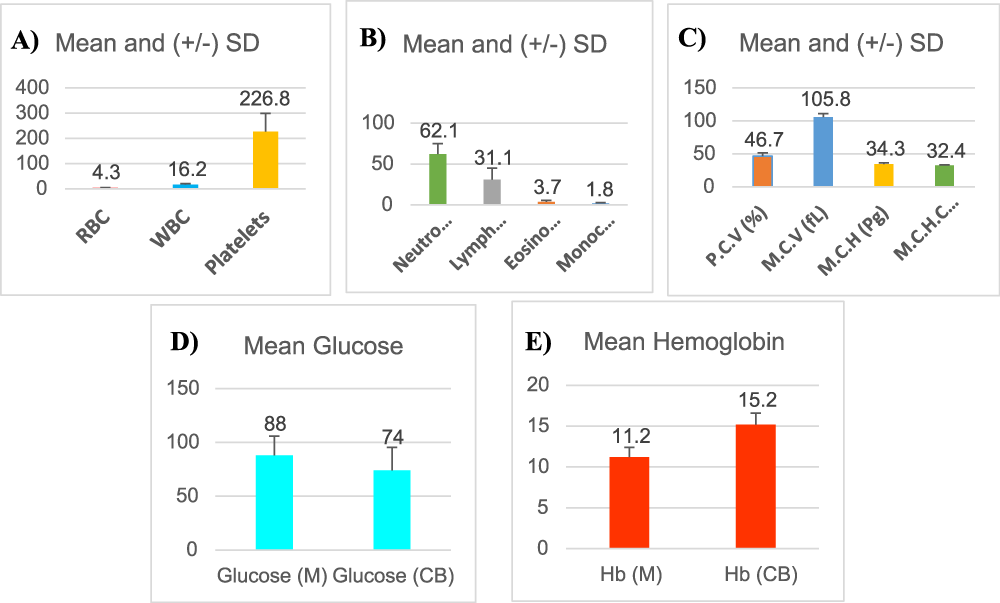
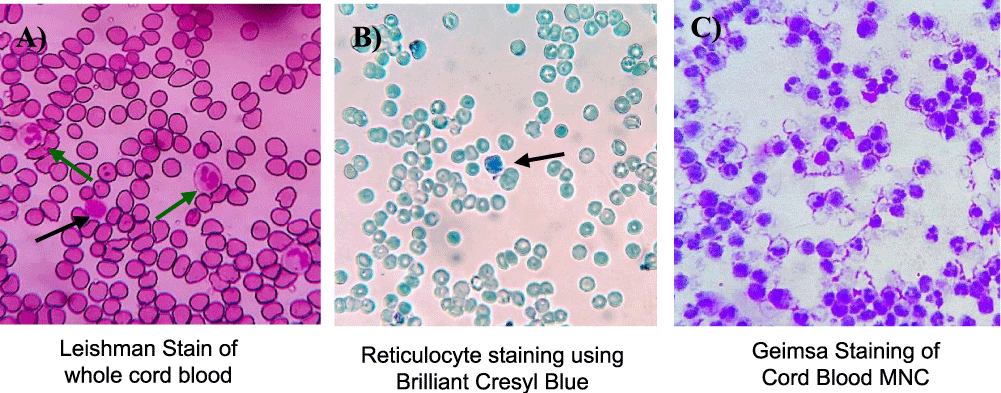
A total of 10-12 ml of the collected undiluted cord blood sample was used for the blood haemogram and biochemical parameter analysis at the laboratory. Haematological analyses were performed using both the manual and automatic multiparameter hematology analyser. Complete Blood Count (CBC) was done including Haemoglobin concentration (Hb), Red Blood Cells (RBC), White Blood Cells (WBC), Packed Cell Volume (PCV), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC), Platelets Count, WBC Differential Count (Figure 1A-C).
Glucose and haemoglobin level estimation were done for both peripheral blood of mothers and cord blood samples (Figure 1D & 1E).
Reticulocyte count was done separately by staining unfixed cord blood with Brilliant Cresyl Blue (Himedia S066, India) solution in 1:1 ratio in an Eppendorf tube and incubated for 45 minutes in room temperature. After incubation the stained blood was smeared on a glass slide and observed under the binocular microscope. The juvenile red cells or reticulocytes contain remnants of basophilic ribonucleoproteins. The supra-vital stain reacts with remnants of riboproteins to form a bluish black precipitate of granules or filaments. Approximately 1000 cells were counted under the microscope (Olympus CH20i, Japan) and among them total number of reticulocytes were determined (Figure 2A & 2B).
Isolation of Mononuclear Cells (MNC) and cord blood plasma factors
The isolation of MNC from cord blood was done using density-gradient centrifugation within 30 mins of procurement. Preparation of the blood for centrifugation was performed in a highly sterilised environment, within Laminar Air Flow hood. The cord blood (preferably stored in 4°C) was first diluted with equal volume (1:1 ratio) of cold sterilised 0.9% Sodium Chloride Solution (NaCl) or Phosphate Buffered Saline Solution (PBS) (pH: 7.4). A total of 4ml of cold diluted cord blood was carefully topped or layered over 3ml of HiSep LSM 1077 solution (Himedia, India) (room temperature) in a 15 ml centrifuge tube (Tarson, India). Careful measures were taken so that the HiSep solution was not disturbed and the blood was topped on the solution without mixing. The centrifuge tubes were then placed in (REMI R8C, India) centrifuge machine and centrifuged at 500 g for 25 minutes [19].
After centrifugation the content of the tube was separated in 4 broad layers. The topmost supernatant contains plasma, consisting of platelets and Cord Blood Plasma Factors (CBPF). At the interphase of plasma and clear HiSep solution was thin cloudy buffy coat consisting of mononuclear cells. The bottom-most layer right below the HiSep layer consists of erythrocytes and granulocytes. The plasma layer consisting of plasma factors was pipetted out without disturbing the buffy coat and was stored in -20°C for further analysis. The MNC layer was prudently pipetted out and was washed twice with chilled 0.9% NaCl by centrifugation for 5 mins at 200 g. The supernatant was discarded and the pellet was resuspended in 0.9% NaCl for further analysis.
Staining of isolated mononuclear cells
The freshly isolated MNCs were stained using Geimsa stain. The Geimsa stain was diluted using distilled water in the ratio 1:9 (1 ml stain and 9 ml dist. water). The suspended mononuclear cells were smeared on a clean glass slide, Geimsa solution was added after air-drying. The stained cells were incubated in room temperature, covered and away from air for 10 mins and then washed with tap water. The stained slide was then observed under microscope (Olympus CH20i, Japan) to study the cellular morphology (Figure 2C).
Results
The Mean ± SD of participant’s peripheral blood data like glucose and haemoglobin was estimated. The blood sugar of mother’s peripheral blood was 88.0 ± 17.9 and the haemoglobin was 11.2 ± 1.2. The cord blood’s estimated glucose and haemoglobin value was 74.0 ± 21.5 and 15.2 ± 1.4 (Figure 1D & 1E). The cord blood haemoglobin value was within the standard European range 11.0-18.0 g/dL but more than the South East Asian which is within 11.2 g/dL.
The estimated Mean ± SD values of total cord blood RBC, PCV (%), MCV (fL), MCH (Pg) and MCHC (g/dL) were 4.3 ± 0.6, 46.7 ± 4.8, 105.8 ± 5.3, 34.3 ± 2.3 and 32.4 ± 0.9. The estimated values of total cord blood WBC, neutrophil (%), lymphocyte (%), eosinophil (%) and monocyte (%) were 16.2 ± 4.7, 62.1 ± 12.8, 31.1 ± 13.8, 3.7 ± 1.8 and 1.8 ± 0.9. The total cord blood platelet count was 226.8 ± 73. To compare the observed values of cord blood RBC, Hb (g/dL), PCV (%), MCV (fL), MCH (Pg), MCHC (g/dL), WBC, neutrophil (%), lymphocyte (%), eosinophil (%), monocyte (%) and platelet count of urban-normal, urban caesarean, rural-normal and rural caesarean groups were taken separately (Table 1).
| Table 1: Table representing cord blood parameter differences in urban and rural population and mode of delivery via normal and caesarean delivery. | ||||
| Blood Parameters | West Bengal | |||
| Urban | Rural | |||
| Normal | Caesarean | Normal | Caesarean | |
| RBC | 4.5 ± 0.3 | 4.3 ± 0.6 | 4.6 ± 0.5 | 4.5 ± 0.6 |
| Hb (g/dL) | 15.9 ± 1.2 | 15.1 ± 1.9 | 15.2 ± 1.0 | 14.4 ± 0.8 |
| P.C.V (%) | 48.5 ± 4.1 | 45.5 ± 5.8 | 47.9 ± 3.7 | 45.0 ± 3.9 |
| M.C.V (fL) | 106.1 ± 1.9 | 106.7 ± 5.9 | 104.8 ± 8.0 | 104.4 ± 6.2 |
| M.C.H (Pg) | 34.3 ± 1.0 | 34.5 ± 2.4 | 34.0 ± 3.6 | 33.9 ± 2.9 |
| M.C.H.C (g/dL) | 32.4 ± 0.7 | 32.4 ± 0.8 | 32.1 ± 1.4 | 32.3 ± 1.2 |
| WBC | 16.5 ± 6.0 | 15.6 ± 4.3 | 15.4 ± 2.7 | 17.1 ± 5.1 |
| Neutrophil (%) | 63.3 ± 10.2 | 56.9 ± 15.3 | 66.8 ± 12.8 | 63.0 ± 11.2 |
| Lymphocyte (%) | 29.6 ± 11.5 | 37.2 ± 16.4 | 25.0 ± 12.0 | 30.7 ± 12.7 |
| Eosinophil (%) | 4.9 ± 2.1 | 3.2 ± 1.4 | 4.5 ± 1.6 | 2.6 ± 1.2 |
| Monocyte (%) | 1.8 ± 0.9 | 1.2 ± 1.0 | 2.3 ± 0.8 | 2.0 ± 0.5 |
| Platelets | 254.7 ± 66.8 | 218.0 ± 78.2 | 216.8 ± 103.0 | 195.6 ± 45.3 |
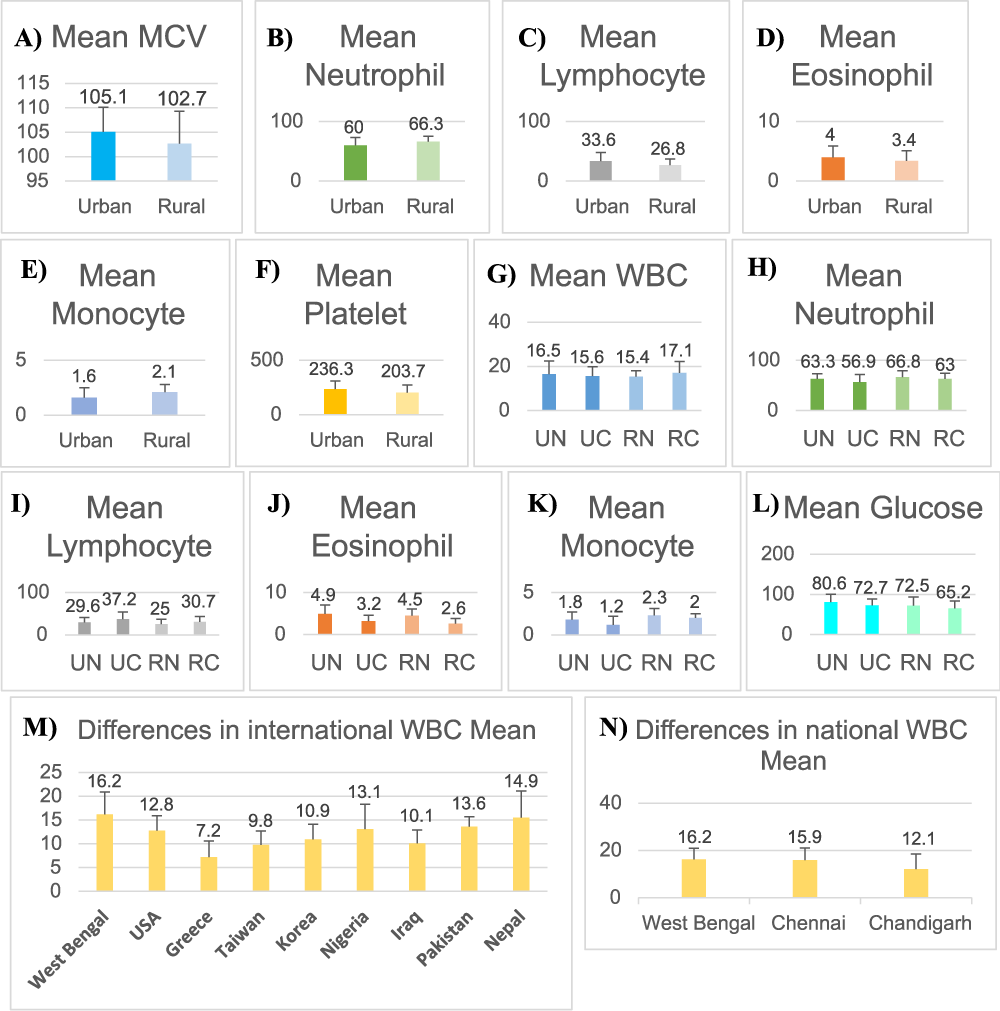
Since our study primarily focusses on the difference in haematological parameters of urban and rural population along with mode of delivery, we separately estimated Mean ± SD values of MCV (fL), neutrophil (%), lymphocyte (%), eosinophil (%), monocyte (%) and platelets count of urban and rural groups only. It was noticed that the estimated MCV value of urban population was 105.1 ± 5.0 which was higher than the rural estimated range 102.7 ± 6.6. The mean value of neutrophil in urban population of West Bengal was 60.0 ± 13.3 which was lesser than the rural population’s estimated mean 66.3 ± 9.0. As expected the mean value of lymphocyte in urban population of West Bengal was 33.6 ± 14.5 which was higher than the rural population’s estimated mean 26.8 ± 10.4 and platelet count of urban was 236.3 ± 73.5 which was much higher than the rural population 203.7 ± 69.6. There were slight differences noticed in the observed values of eosinophil and monocytes but other parameters like RBC and WBC did not show much difference (Figure 3A-F). The scores obtained of each haematological parameter and glucose level from the four groups Urban-Normal (UN), Urban Caesarean (UC), Rural-Normal (RN) and Rural Caesarean (RC) were non-normally distributed and hence descriptive statistics were done using SPSS version 16. Graphical estimations of Mean and SD values of the blood parameters from four groups were done (Figure 3G-L).
The results showed difference in haematological parameters of West Bengal when studied internationally or in perspective to global scenario. It was noticed that the estimated mean value of total WBC population was higher when compared to Caucasian population like USA and Greece. The value was also higher than South East Asian population like Korea and Taiwan, African population like Nigeria, Middle Eastern like Iraq, East Asian population like Pakistan and Nepal (Figure 3M). When intra-national comparison was done, it was noticed that the value of West Bengal’s WBC was similar to Chennai but was higher than Chandigarh (Figure 3N). The neutrophil count in West Bengal was found to be higher than USA, Korea, Taiwan as well as Chennai and Chandigarh of India. The estimated value of lymphocyte in West Bengal was found to be equal to USA but lesser than Taiwan and slightly lesser than Korea and much lesser in values when compared to other countries like Iraq, Pakistan and Nepal. It was also less when compared to other Indian regions like Chennai and Chandigarh. The eosinophil mean value of West Bengal was equal to USA but higher than other international countries as well as higher than the two Indian cities. The Interesting observation was the mean value of monocyte population in West Bengal was marginally more than Nepal but far less than countries like USA, Greece, Taiwan, Korea, Iraq and Pakistan even it was lesser than Chennai (Table 2).
| Table 2: Table representing cord blood parameter differences of different countries and Indian States when compared to West Bengal. | |||||||||||
| West Bengal | USA (38-41 weeks) | Greece | Taiwan | Korea | Nigeria | Iraq | Pakistan | Nepal | Chennai | Chandigarh | |
| RBC | 4.3 ± 0.6 | 4.2 | 2.4 ± 0.8 | 3.2 ± 0.4 | _ | 4.0 ± 0.5 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.3 ± 0.6 | 4.1 ± 0.4 | _ |
| Hb (g/dL) | 15.2 ± 1.4 | 14.4 | 8.8 ± 2.9 | 11.2 ± 1.5 | 11.8 ± 1.4 | 13.2 ± 1.5 | 13.7 ± 1.4 | 14.9 ± 1.4 | 15.2 ± 1.9 | 14.9 ± 1.7 | _ |
| P.C.V (%) | 46.7 ± 4.8 | 45.9 | 25.9 ± 8.8 | 36.9 ± 4.6 | _ | 44.8 ± 5.7 | 44.4 ± 4.7 | 45.6 ± 4.8 | 48.7 ± 6.8 | 44.6 ± 5.3 | _ |
| M.C.V (fL) | 105.8 ± 5.3 | 109 | 105 ± 6 | 115 ± 6.8 | _ | 110.3 ± 11.8 | 111.5 ± 6.0 | 105.8 ± 6.2 | 101.2 ± 6.0 | 108.1 ± 4.8 | _ |
| M.C.H (Pg) | 34.3 ± 2.3 | 34.2 | 35.8 ± 3.1 | 34.9 ± 1.9 | _ | 32.6 ± 4.1 | 34.4 ± 2.3 | 34.9 ± 2.1 | 33.9 ± 2.2 | 36 ± 1.7 | _ |
| M.C.H.C (g/dL) | 32.4 ± 0.9 | 31.4 | 34.3 ± 7.3 | 30.3 ± 1.2 | _ | 29.7 ± 1.6 | 30.9 ± 1.9 | 32.4 ± 2.1 | 33.2 ± 1.5 | 33.3 ± 0.8 | _ |
| WBC | 16.2 ± 4.7 | 12.8 | 7.2 ± 3.4 | 9.8 ± 2.9 | 10.9 ± 3.2 | 13.1 ± 5.2 | 10.1 ± 2.8 | 13.6 ± 4.2 | 14.9 ± 4.4 | 15.9 ± 5.1 | 12.1 ± 6.4 |
| Neutrophil (%) | 62.1 ± 12.8 | 54.2 | _ | 44.9 ± 14.7 | 57.2 ± 8.0 | _ | 51 ± 11.24 | 50.1 ± 12.4 | 63.0 ± 11.6 | 50.3 ± 12.2 | 52.5 ± 16 |
| Lymphocyte (%) | 31.1 ± 13.8 | 31.2 | _ | 33.8 ± 10.9 | 30.9 ± 7.4 | _ | 39.8 ± 10.17 | 39.8 ± 12.2 | 35.2 ± 11.5 | 35.9 ± 12.2 | 41.6 ± 16.2 |
| Eosinophil (%) | 3.7 ± 1.8 | 3 | 1.22 ± 0.97 | 2.6 ± 2.3 | 2.9 ± 1.7 | _ | 1.22 ± 0.97 | 3.3 ± 2.1 | 1.2 ± 1.5 | 2.9 ± 2 | 2.3 ± 1.9 |
| Monocyte (%) | 1.8 ± 0.9 | 10.5 | 7.85 ± 2.77 | 8.8 ± 4.4 | 8.5 ± 2.0 | _ | 7.8 ± 2.77 | 6.5 ± 2.8 | 0.48 ± 0.7 | 8.9 ± 3.1 | _ |
| Platelets | 226.8 ± 73 | 173 | 160 ± 59 | 217 ± 45 | 208 ± 39 | 225.0 ± 72.2 | 267.6 ± 60.6 | 256.2 ± 76.5 | 226.8 ± 61.2 | 215 ± 67 | 199.2 ± 56.6 |
Discussion
The present study was conducted for the characterization and establishment of a standardized reference range of cord blood cells in West Bengal population and to assess the ethnic difference in Indian population (focussing West Bengal) when compared to various other populations of the world and to analyse the difference in cellular components of cord blood, procured from urban and rural population of West Bengal. Previous studies already showed works on cord blood in respect of gender, weight, mode of delivery and gestation period but little or no work is done on the difference of cord blood parameters when compared to urban and rural population. Since ethical variations play a vital role in constructing socio-cultural aspects that might have an effect on the differences in haematological parameters, the design of the experiment was framed in a manner where comparison can be drawn on both international and intra-national level (Supplementary figure).
In both the urban and rural population there was an increase in neutrophil and monocyte mean values in normal delivery when compared to caesarean delivery. In case of lymphocyte count the values in caesarean delivery was more when compared to normal delivery. Our observed descriptive statistics was in sync with a previous study which showed statistically significant results [20]. The total WBC value in case of urban population was similar with the previous work but the WBC of rural population showed a slight different result. It was observed both eosinophil and platelet count showed higher values in normal delivery when compared to caesarean delivery. Our study also showed the higher value of neutrophil count in rural areas and high lymphocyte count in urban areas which is an interesting result. The range of PVC was higher than Greece and Taiwan but almost similar to other countries and two Indian states. The MCH, MCV and MCHC of Indian population was within the European and South East Asian standard reference range.
The Mean and SD value of RBC was within the standard reference range 4.0-6.2 (106 cells/ul) but the value for WBC was more than the usual Caucasians, South East Asians (Taiwan and Korea), and Nigerian population [21], Iraq [22], Pakistan [23], Nepal [24] and even higher when compared to other Indian cities [25,26], which is a unique finding. The standard European reference value is 4.00-11.0 (103 cells/ul) which is similar to South East population and Nigerian but lesser than the estimated Indian value [17,27,28]. Our studies showed the values of Neutrophils, Lymphocytes and Monocytes in Bengal population were within the reference range of South Asian population which are 11.0-69.0 (Neutrophils), 17.0-56.0 (Lymphocytes) and 1-17 (Monocytes) but when the values of urban and rural groups were observed with the normal and caesarean delivery, the ranges within the groups showed some differences. The mean neutrophil value of rural-caesarean group was lesser than the urban-normal, rural-normal and rural caesarean group, eventually making the lymphocyte value higher than the three mentioned groups. The eosinophil value of urban-normal and rural-normal were recorded to be higher than the other groups. As we succeeded to standardise a local reference range and reported the difference in urban and rural population in cord blood parameters for the first time focussing Bengal, we assume this can be an important study. Though there is no obvious explanation for higher difference in WBC population in Bengal as compared to global scenario and differences in cord blood parameters between urban and rural population, we expect further studies can unveil the possible reasoning.
Conclusion
The analyses ensures a difference in haematological parameters in Indian population as and when compared to the usual standard reference values of other ethnic communities like Caucasians, South East Asians and Nigerians. It is also noticed that urban and rural population of Southern part of West Bengal shows difference in neutrophil, lymphocyte and eosinophil count. The study ascertains its contribution to the standardization of reference value of cord blood parameters in Indian population, focusing West Bengal.
Acknowledgement
We are thankful to the Department of Biotechnology, Government of West Bengal for the financial assistance [59(Sanc.)-BT/P/Budget/RD-03/2017]. We are grateful to Dr. Tapan Kumar Naskar of Department of Gynaecology and Obstetrics, Medical College Kolkata, Dr. Indranil Dhar of Department of Lab-Medicine, Calcutta School of Tropical Medicine and the Director of the Calcutta School of Tropical Medicine (Kolkata) for his encouragement and support for the successful completion of this work.
References
- Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16(3):153-65. doi: 10.1002/stem.160153. PMID: 9617891.
- Rocha V, Sanz G, Gluckman E. Umbilical cord blood transplantation. Current Opinion in Hematology. 2004. doi: 10.1097/01.moh.0000145933.36985.eb.
- Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998 Jul 1;92(1):11-8. PMID: 9639493.
- Lu L, Xiao M, Shen RN, Grigsby S, Broxmeyer HE. Enrichment, characterization, and responsiveness of single primitive CD34 human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. 1993 Jan 1;81(1):41-8. PMID: 7678069.
- Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283. Epub 2016 Jul 19. PMID: 27516776; PMCID: PMC4969512.
- Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155-62. doi: 10.1007/s12015-006-0022-y. PMID: 17237554; PMCID: PMC3753204.
- Tse W, Laughlin MJ. Umbilical cord blood transplantation: a new alternative option. Hematology Am Soc Hematol Educ Program. 2005:377-83. doi: 10.1182/asheducation-2005.1.377. PMID: 16304407.
- Costakos DT, Walden J, Rinzel MT, Dahlen L. Painless blood testing to prevent neonatal sepsis. WMJ. 2009 Sep;108(6):321-2. PMID: 19813501.
- Lanfranchi A, Porta F, Chirico G. Stem cells and the frontiers of neonatology. Early Hum Dev. 2009 Oct;85(10 Suppl):S15-8. doi: 10.1016/j.earlhumdev.2009.08.005. PMID: 19836907.
- Carroll PD, Nankervis CA, Iams J, Kelleher K. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J Perinatol. 2012 Feb;32(2):97-102. doi: 10.1038/jp.2011.60. Epub 2011 May 12. PMID: 21566570; PMCID: PMC3891501.
- Hansen A, Forbes P, Buck R. Potential substitution of cord blood for infant blood in the neonatal sepsis evaluation. Biol Neonate. 2005;88(1):12-8. doi: 10.1159/000083946. Epub 2005 Feb 10. PMID: 15711036.
- Walka MM, Sonntag J, Kage A, Dudenhausen JW, Obladen M. Complete blood counts from umbilical cords of healthy term newborns by two automated cytometers. Acta Haematol. 1998;100(4):167-73. doi: 10.1159/000040898. PMID: 9973637.
- Pranke P, Failace RR, Allebrandt WF, Steibel G, Schmidt F, Nardi NB. Hematologic and immunophenotypic characterization of human umbilical cord blood. Acta Haematol. 2001;105(2):71-6. doi: 10.1159/000046537. Erratum in: Acta Haematol 2001;105(4):251. PMID: 11408707.
- Forestier F, Daffos F, Catherine N, Renard M, Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood. 1991 Jun 1;77(11):2360-3. PMID: 2039818.
- Noguera NI, Detarsio G, Pérez SM, Bragós IM, Lanza O, Rodríguez JH, Acosta I, Davoli R, Milani AC. Hematologic study of newborn umbilical cord blood. Medicina (B Aires). 1999;59(5 Pt 1):446-8. PMID: 10684163.
- Redźko S, Przepieść J, Zak J, Urban J, Wysocka J. Influence of perinatal factors on hematological variables in umbilical cord blood. J Perinat Med. 2005;33(1):42-5. doi: 10.1515/JPM.2005.007. PMID: 15841613.
- Lee HR, Shin S, Yoon JH, Kim BJ, Hwang KR, Kim JJ, Roh EY. [Complete blood count reference values of donated cord blood from Korean neonates]. Korean J Lab Med. 2009 Jun;29(3):179-84. Korean. doi: 10.3343/kjlm.2009.29.3.179. PMID: 19571613.
- Jan RH, Wen SH, Shih WL, Chang YH, Chen SH. Evaluation of biological features of cord blood units in different Taiwan ethnics. Acta Paediatr Taiwan. 2007 Mar-Apr;48(2):57-61. PMID: 17626603.
- Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625-34. doi: 10.1634/stemcells.22-4-625. PMID: 15277708.
- Glasser L, Sutton N, Schmeling M, Machan JT. A comprehensive study of umbilical cord blood cell developmental changes and reference ranges by gestation, gender and mode of delivery. J Perinatol. 2015 Jul;35(7):469-75. doi: 10.1038/jp.2014.241. Epub 2015 Jan 29. PMID: 25634517.
- Adewumi A, Titilope A A, Akinsegun AA, Abidoye G, Ebele U, Sulaimon AA. Cord blood full blood count parameters in Lagos, Nigeria. Pan Afr Med J. 2014 Mar 13;17:192. doi: 10.11604/pamj.2014.17.192.3680. PMID: 25396018; PMCID: PMC4228999.
- Al-Marzoki JM, Al-Maaroof ZW, Kadhum AH. Determination of reference ranges for full blood count parameters in neonatal cord plasma in Hilla, Babil, Iraq. J Blood Med. 2012;3:113-8. doi: 10.2147/JBM.S35895. Epub 2012 Oct 1. PMID: 23055785; PMCID: PMC3464059.
- Qaiser DH, Sandila MP, Ahmed ST, Kazmi T. Haematological reference values for full-term, healthy, newborns of Karachi, Pakistan. J Pak Med Assoc. 2009 Sep;59(9):618-22. PMID: 19750858.
- Basnet S, Singh SK, Sathian B, Mishra R. Reference ranges for hematological values in umbilical cord blood in Pokhara, Nepal. J Nepal Paediatr Soc. 2016;36(2):160-164. doi: 10.3126/jnps.v36i2.15605.
- Suman FR, Raj RS, Priyathersini N, Rajendran R, Rajendran R, Ramadoss U. Biological Reference Interval for Hematological Profile of Umbilical Cord Blood: A Study Conducted at A Tertiary Care Centre in South India. J Clin Diagn Res. 2015 Oct;9(10):SC07-9. doi: 10.7860/JCDR/2015/14713.6675. Epub 2015 Oct 1. PMID: 26557584; PMCID: PMC4625303.
- Marwaha N, Marwaha RK, Narang A, Thusu K, Garewal G, Bhakoo ON. Routine hematological values in term newborns. Indian Pediatr. 1992 Sep;29(9):1095-9. PMID: 1452304.
- Katsares V, Paparidis Z, Nikolaidou E, Karvounidou I, Ardelean KA, Drossas N, Grigoriadis N, Grigoriadis J. Reference ranges for umbilical cord blood hematological values. Lab Med. 2009;40(7):437-439. doi: 10.1309/LMWCY2YGYCF9EEMQ.
- Chang YH, Yang SH, Wang TF, Lin TY, Yang KL, Chen SH. Complete blood count reference values of cord blood in Taiwan and the influence of gender and delivery route on them. Pediatr Neonatol. 2011 Jun;52(3):155-60. doi: 10.1016/j.pedneo.2011.03.007. Epub 2011 May 6. PMID: 21703558.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.