> Medicine Group. 2021 Jan 30;2(1):034-037. doi: 10.37871/jbres1184.
Investigation of the Mass Attenuation Coefficients, Effective Atomic Numbers and Electron Densities for Compounds of Painkiller
Tekerek Saniye*
- Mass absorption coefficient
- Cross section
- Effective electron densitiy
- WinXCom
In this study the effects of gamma radiations with compounds are an important subject in the field of medicine, radiation shielding and radiation physics. With technological advances the using of radiation has increased in the medicine in the last century. The mass absorpsion coefficient (µ/ρ) is the fundamental a quantity characterizing gamma ray and is of major importance in radiation shielding. In this study, the mass absorption coefficient of painkillers named Ketoprofen, Flurbiprofen, Etodolac, Ibuprofen, Meloxicam, Diclofenac and Aspirin were calculated at energy range from 4.65 keV to 59.543 keV using the WinXCom data programme. In addition total atomic (σta), moleculer (σtm), electronic cross-section (σte), effective atomic number (Zeff), effective electron density (Neff) were calculated.
With the increasing use of radiation sources in areas related to human health such as nuclear medicine, radiotherapy and radiology, it was necessary to examine the parameters that are considerable in the interaction of photon with material. In radiation dose calculations, the effective atomic number information of the samples absorption of beam in the samples were required [1,2]. The mass absorption coefficient, which is a characteristic feature for materials is a very significant parameter in agriculture, pharmacy, radiation dosimetry, biology, nuclear and radiation physics [3]. The accurate reliable values of mass attenuation coefficients were required to provide essential data in varied fields such as nuclear diagnostics, radiation protection, nuclear medicine, radiation biophysics and etc. [4]. The effective atomic number physically enables the characteristic of the compound to be understandable with the help of a single atomic number [5]. µ/ρ was also used to calculate and control the material thickness because radiation can change the characteristics of material. Since this may cause various undesirable results, it was very important to examine the interaction of many materials, alloys and compounds with radiation and to know what changes they cause in the material [6]. In the literature, the effects of radiation material interactions at different energies on Zeff and Neff were studied. Zeff were calculated in the energy range of 15.746-40.930 keV using the mixing rule depending on the Ni contribution in CuCoNi alloys, and it was stated that the Zeff value increased with the increase in Ni ratio [7]. Zeff and Neff between at various energies of essential amino acids were calculated theoretically [8]. The Zeff of composite materials at various energy ranges of 280 to 1115 keV were obtaned [9]. Experimental and theoretical µ/ρ values of vitamins in some photon energies were calculated [10]. The mass attenuation coefficient for compounds or compositions was calculated with the help of WinXCom program. WinXCom is a data programme base the "mixing rule". In this rule, the elements in the ingredient are considered independent from each other and their interaction with each other is neglected [11].
In this present work were calculated the mass attenuation coefficients for some painkiller compounds such as Ketoprofen (C16H14O3), Flurbiprofen (C15H13FO2), Etodolac (C17H21NO3), Ibuprofen (C13H18O2), Meloxicam (C14H13N3O4S2), Diclofenac (C14H11C12NO2) and Aspirin (C9H8O4) in the energy range from 4.65 keV to 59.543 keV using the WinXCom data. Zeff and Neff parameters were calculated with the help of the data obtained. It has been observed that Neff and Zeff values are interrelated and parallel to each other. The computational results of the pain relief compounds μ/ρ, Zeff and Neff presented in this study can be referenced as guiding in a variety of medical applications and fields. In the paper, calculated datas were published on the study of mass attenuation coefficient calculate on painkiller compounds in the photon energy 4.65 to 59.543 keV through which radiation interaction of the drug can be defined.
The substances used in around the world to address various pains are listed in table 1. Use of aspirin, ibuprofen were said to show beneficial synergistic effects by combatting pain at multiple sites of action [12]. Flurbiprofen was used in the treatment of pain or inflammation in humans. Flurbiprofen is indicated for the management of excimer laser photorefractive keratectomy, and ocular gingivitis. Recent reports suggest use of flurbiprofen in radioprotection, the inhibition of colon tumor, the protection of post irradiation myelosuppression and peridontal surgery [13]. Etodolac drug is an effective drug in the treatment of osteoarthritis, rheumatoid arthritis and spinal rheumatism.Since these painkillers was used for different purposes in the field of medicine [14]. It was considered necessary theoretically to examine their reactions to high energy rays.
| Table1: Painkiller coumpounds. | |
| Ketoprofen | C16H14O3 |
| Flurbiprofen | C15H13O2 |
| Etodolac | C17H21O3 |
| İbuprofen | C13H18O2 |
| Meloxicam | C14H13O4S2 |
| Diclofenac | C14H11C12NO2 |
| Aspirin | C9H8O4 |
The μ/ρ for a compound material is the total of the mass attenuation coefficients of every element according to "mixing rule" [15]. According to the WinXcom program, μ/ρ is calculated with the help of formula (1).
In equation (1), ωi is fractional weight of the i atom.
Theoretical μ/ρ is calculated for compound materials using the WinXcom. Using the equation (2) σtm values were calculated [16].
Ai is the atomic weight, ni is the number of atoms, NA is Avogadro's number.
σta is calculated using the following Equation (3) [16].
In formula (3), σta is the total atomic cross section, σtm is the total molecular cross section, ni is the total number of atoms. The unit of σta is cm2/atom.
The electronic cross section was obtaned by using the mass attenuation coefficient of each element in the samples. σte was calculated using Formula (4) [16].
Zi atomic weight and fi is abundance fraction.
Zeff was calculated using formula (5) [5].
Neff was calculated using the following formula (6) [17].
WinXCom program determines the μ/ρ of the compounds consisting of more than single element for any sample, taking into account "mixture rule" while calculating the mass absorption coefficients. According to the this rule, μ/ρ of the compound is the sum of the mass absorption coefficients of each element [18]. μ/ρ, Zeff and Neff values calculated at the specified energies of the compounds are given in tables 2-4.
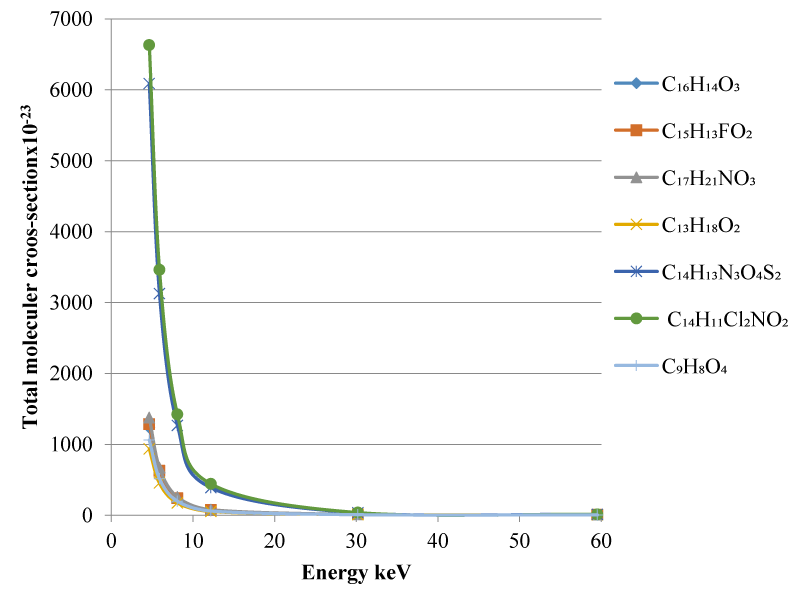
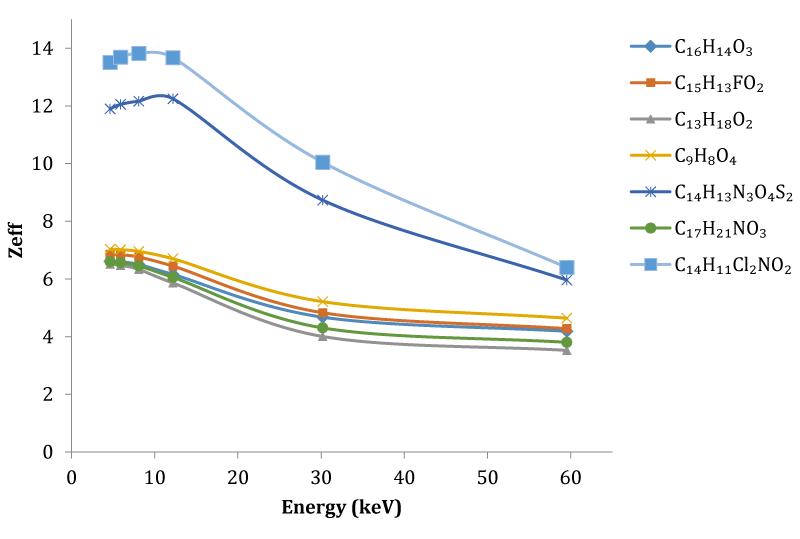
As can be seen from the table 2, with increasing energy a decrease is observed in the µ/ρ values and accordingly the Zeff values. The highest mass absorption coefficient of 134.8 belongs to the compound C14H11C12NO2 called Diclofenac at 4.65 keV energy. The mass attenuation coefficient value of C14H11C12NO2 compound named Diclofenac was found as 0.248 at the highest energy calculated at 59.543 keV. It can be seen from Table 2 that the lowest mass attenuation coefficient of the calculated energy ranges belongs to the C13H18O2 compound.
It seems obvious that the total moleculer cross-section values decrease with increasing photon energies as shown in the figure 1. It can be seen from figure 1 that the lowest total moleculer cross-section value of the calculated energy ranges belongs to the İbuprofen C13H18O2 compound.
| Table 2: The mass attenuation coefficients µ/ρ (cm2/gr) for painkiller compounds at different photon energies. | ||||||
| Compounds | 4.65 keV |
5.9 keV |
8.1 keV |
12.2 keV |
30.2 keV |
59.543 keV |
| C13H18O2 | 27.33 | 13.28 | 5.11 | 1.579 | 0.282 | 0.191 |
| C17H21O3 | 28.84 | 14.01 | 5.388 | 1.656 | 0.284 | 0.189 |
| C16H14O3 | 29.29 | 14.23 | 5.468 | 1.667 | 0.282 | 0.187 |
| C15H13O2 | 31.7 | 15.44 | 5.941 | 1.813 | 0.290 | 0.187 |
| C9H8O4 | 35.48 | 17.28 | 6.643 | 2.011 | 0.301 | 0.188 |
| C14H13O4S2 | 104.3 | 53.56 | 21.71 | 6.686 | 0.618 | 0.228 |
| C14H11C12NO2 | 134.8 | 70.37 | 28.93 | 9.009 | 0.782 | 0.248 |
| Table 3: The effective atomic number (Zeff) for painkiller compounds at different photon energies. | ||||||
| Compounds | 4.65 keV |
5.9 keV |
8.1 keV |
12.2 keV |
30.2 keV |
59.543 keV |
| C13H18O2 | 6.510 | 6.470 | 6.333 | 5.861 | 4.006 | 3.524 |
| C17H21O3 | 6.600 | 6.567 | 6.454 | 6.055 | 4.303 | 3.804 |
| C16H14O3 | 6.611 | 6.590 | 6.505 | 6.157 | 4.675 | 4.186 |
| C15H13O2 | 6.853 | 6.838 | 6.759 | 6.446 | 4.829 | 4.278 |
| C9H8O4 | 7.031 | 7.017 | 6.954 | 6.700 | 5.213 | 4.641 |
| C14H13O4S2 | 11.899 | 12.051 | 12.163 | 12.247 | 8.732 | 5.963 |
| C14H11C12NO2 | 13.503 | 13.687 | 13.818 | 13.672 | 10.045 | 6.396 |
| Table 4: The effective Electron Density (Neffx1023) (electron/gram) for painkiller compounds at different photon energies. | ||||||
| Compounds | 4.65 keV |
5.9 keV |
8.1 keV |
12.2 keV |
30.2 keV |
59.543 keV |
| C14H11C12NO2 | 8.234 | 8.347 | 8.427 | 8.337 | 6.125 | 3.900 |
| C14H13O4S2 | 7.339 | 7.432 | 7.501 | 7.553 | 5.385 | 3.678 |
| C13H18O2 | 6.269 | 6.232 | 6.099 | 5.647 | 3.859 | 3.395 |
| C17H21O3 | 5.807 | 5.778 | 5.679 | 5.328 | 3.786 | 3.347 |
| C16H14O3 | 5.164 | 5.148 | 5.082 | 4.808 | 3.653 | 3.277 |
| C15H13O2 | 5.236 | 5.224 | 5.164 | 4.925 | 3.689 | 3.269 |
| C9H8O4 | 4.934 | 4.924 | 4.880 | 4.701 | 3.658 | 3.257 |
It is clearly seen that the mass attenuation coefficient depends on the photon energy and on the chemical structure of the compounds. The µ/ρ values decrease with increasing photon energies as shown in the table 1. With the presence of Cl in the compound, Diclofenac (C14H11C12NO2) compound has the largest Zeff value. Simultaneously while the effective atomic number for the C14H11C12NO2 compound increased, a parallel increase was observed in the effective electron density. Although there is not much change in low energies in compounds with multiple elements, a decrease in the effective atomic numbers has been observed due to the increase in energy. Ibuprofen (C13H18O2) is a pain reliever with the lowest mass attenuation coefficient in the energies studied. In general, as the number of quantity elements forming the compound increases, μ/ρ and Zeff values increase.
The effective electron dentisity value of C14H11C12NO2 compound named Diclofenac was found as 3.900 at the highest energy calculated at 59.543 keV. It can be seen from table 4 that the lowest effective electron dentisity value of the calculated energy ranges belongs to the C13H18O2 compound.
In this study, µ/ρ at various energies were calculated for some compounds belonging to painkillers. By using µ/ρ values, σtm, σta, σte, Zeff, Neff values were calculated. Absorption of the photon at various energies occurs depending on the properties of the material under study. Therefore, beam permeability data at different energies provide information about the absorption and shielding properties of the material. Radiation absorption properties at low energies give better results for pain relief compounds. As can be seen from the results of the calculations, the radiation energy and the mass absorption coefficient vary, and it is understood that the materials cause changes in the attenuation parameters of the material withal increasing radiation. The data obtained from this study is important in terms of guiding researchers working in this field. The data obtained in the light of these values will be used in various fields and it is thought to be suitable for use as shielding material in order to protect from radiation in low and medium energy beams.
- Gerward L, Guilbert N, Jensen, KB, Levring H. X-ray absorption in matter Reengineering XCOM. Radiation Physics and Chemistry. 2001 Jan;60:23-24. doi: 10.1016/S0969-806X(00)00324-8
- Chantler CT. Theoretical form factor, attenuation and scattering tabulation for Z=1-92 from E=1-10 eV to E=0.4-1.0 MeV. J Phys Chem. 1995;24:71-643. doi: 10.1063/1.555974
- Singh VP, Medhat ME, Badiger NM, Rahman AZMS. Radiation shielding effectiveness of newly developed superconductors. Radiation Physics and Chemistry. 2015 Jan;106:175-183. doi: 10.1016/j.radphyschem.2014.07.013
- Phelps ME, Hoffman EJ, Ter-Pogossian MM. Attenuation coefficients of various body tissues, fluids, and lesions at photon energies of 18 to 136 keV. Radiology. 1975 Dec;117(3 Pt 1):573-83. doi: 10.1148/117.3.573. PMID: 810827.
- Singh K, Singh H, Sharma V, Nathuram R, Khanna A, Kumar R, Bhatti SS, Sahota HS. Gamma-ray attenuation coefficients in bismuth borate glasses. Nuclear Instruments and Methods in Physics Research Section B. 2002 July;194(1):1-6. doi: 10.1016/S0168-583X(02)00498-6
- Han I, Demir L. Determination of mass attenuation coefficients, effective atomic and electron numbers for Cr, Fe and Ni alloys at different energies. Nuclear Instruments and Methods in Physics Research Section B. 2009 Jan;267:3-8. doi: 10.1016/j.nimb.2008.10.004
- İçelli O, Erzeneoglu S, Karahan IH, Çankaya G. Effective atomic numbers for CoCuNi alloys using transmission experiments. Journal of Quant. & Radiative Transfer. 2005 April 1;91(4):485-91. doi: 10.1016/j.jqsrt.2004.07.006
- Manohara SR, Hanagodimath SM. Studies on effective atomic numbers and electron densities of essential amino acids in the energy range 1 keV–100 GeV, Nuclear Instruments and Methods in Physics Research Section B. 2007 May;258(2):321-328. doi: 10.1016/j.nimb.2007.02.101
- Kumar PS, Manjunathagura V, Umesh TK. Effective atomic numbers of some H-, C-, N-, and O-based composite materials derived from differential incoherent scattering cross-sections. Journal Physics. 2010 Apr 28;74(4):555-562. doi: 10.1016/j.jqsrt.2004.07.006
- Demir D, Turşucu A. Studies on mass attenuation coefficient, mass energy absorption coefficient and kerma of some vitamins. Annals of Nuclear Energy. 2012 oct;48:17-20. doi: 10.1016/j.anucene.2012.05.013
- Kerur BR, Thontadarya SR, Hanumaıah B. Anomalous x-Ray Attenuation Coefficients Around the Absorption Edges Using Mn Kα, and Cu Kα X-Rays. Appl. Radiat. 1994 Feb;45(2):159-163. doi: 10.1016/0969-8043(94)90005-1
- Mehlisch DR. The efficacy of combination analgesic therapy in relieving dental pain. J Am Dent Assoc. 2002 Jul;133(7):861-71. doi: 10.14219/jada.archive.2002.0300. PMID: 12148679.
- Abdel-Aziz AA, Al-Badr AA, Hafez GA, Flurbiprofen. Profiles Drug Subst Excip Relat Methodol. 2012;37:113-81. doi: 10.1016/B978-0-12-397220-0.00004-0. Epub 2012 Mar 19. PMID: 22469318.
- Tas C, Ozkan Y, Okyar A, Savaser A. In vitro and ex vivo permeation studies of etodolac from hydrophilic gels and effect of terpenes as enhancers. Drug Deliv. 2007 Oct;14(7):453-9. doi: 10.1080/10717540701603746. PMID: 17994363.
- Hubbell JH, Seltzer SM. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z=1 to 92 and 48 Additional Substances of Dosimetric Interest, National Institute of Standards and Physics Laboratory, NISTIR. 1995 Jan 1;5632.
- Singh MP, Sandhu BS, Singh B. Measurement of effective atomic number of composite materials using scattering of γ-rays. Nuclear Instruments and Methods in Physics Research. 2007 Sep;580(1):50-53. doi: 10.1016/j.nima.2007.05.037
- Manohara SR, Hanagodimath SM, Thind KS , Gerward L. On the effective atomic number: A comprehensive set of formulas for all types of materials. Nucl. Instrum. Methods Phys Res B at press. 2008 Sep;266(18):3906-3912. doi: 10.1016/j.nimb.2008.06.034
- Morabad RB, Kerur BR. Mass attenuation coefficients of X-rays in different medicinal plants. Appl Radiat Isot. 2010 Feb;68(2):271-4. doi: 10.1016/j.apradiso.2009.10.033. Epub 2009 Oct 24. PMID: 19910203.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.