> General Science. 2021 Feb 08;2(2):038-041. doi: 10.37871/jbres1185.
Nodular Pulmonary Amyloidosis: A Case over Fourteen Years
Ekladious A1*, Fish L2, De Chaneet C3 and Cox C2
2Department of General Medicine, Bunbury Regional Hospital, Bunbury, Australia
3Department of General Medicine, St John of God Hospital, Bunbury, Australia
Abstract
A 75-year-old man presented with pleuritic chest pain, haemoptysis and dyspnoea. Imaging found multiple pulmonary nodules, concerning for malignancy. CT-guided biopsy was consistent with amyloid. The patient has a history of pulmonary amyloidosis, with a single nodule resected 14 years prior. This case allows comparison between imaging fourteen years apart, providing insight into the progressive nature of these benign nodules.
Nodular pulmonary amyloidosis is a rare condition with few case reports published. Of those published, few are of nodular amyloidosis in the absence of underlying neoplastic aetiology. This case presents a 14-year interval of a patient with nodular amyloidosis, allowing insight into disease progression which has not previously been well described.
A 75-year-old man presented to a regional hospital in Australia with right sided pleuritic chest pain, and a 2-week history of productive cough, haemoptysis, dyspnoea and reduced exercise tolerance. Further questioning revealed a 3-month history of worsening dyspnoea, haemoptysis and cough, with no orthopnoea, paroxysmal nocturnal dyspnoea or weight loss.
The patients’ past medical history included resection of an amyloid tumour from the left lower lobe of the lung in 2004, ex-smoker with a 30-pack year history, significant occupational asbestos exposure through work as a diesel mechanic, and hypercholesterolaemia. The patient lives at home with his partner and is independent with the activities of daily living.
On examination, the patient appeared well, in no respiratory distress. Observations were within normal limits, with oxygen saturations of 94% on room air, respiration rate of 18. On auscultation, there were late inspiratory crackles, as well as decreased hepatic dullness to percussion. There was a central trachea and no palpable cervical lymphadenopathy. Cardiovascular examination was unremarkable, and the patient was euvolaemic. Abdominal examination was unremarkable with no palpable organomegaly, and he did not have a rash or skin abnormalities seen. The initial investigations were conducted to investigate a potential lung malignancy underlying malignancy, based on the appearance on CT scan. The patient advised us that he had a previous diagnosis of primary pulmonary amyloidosis, first diagnosed 14 years ago. The diagnosis was made after a chest x-ray and CT showed a nodule, suspicious for cancer. Pulmonary function tests were performed and he was referred to a Cardiothoracic surgeon for consideration of a left lower lobectomy. He was treated with a wedge resection. Histopathology from this specimen showed primary pulmonary amyloidosis. There was a delay in the understanding of how the patient came to have this rare diagnosis, as it took five days for the medical records to be sourced from the archive.
Investigations this Admission and Results there are Listed Below
ECG
Normal sinus rhythm, no ischemic changes, no signs suggestive of right heart strain. CXR: Bilateral variously sized nodular opacities are indeterminate. Differential diagnosis includes, although not limited to metastases, granulomas, infective lesions etc.
CT pulmonary angiogram
No central pulmonary arterial thrombosis. Heterogenous enhancing consolidation right base is less pronounced in the left base. Disseminated nodular lesions throughout the lung fields some with central calcification. Differential diagnosis includes disseminated secondary phenomena with secondary consolidation.
Repeat CT chest 2 days later
No interval change in the size of the pulmonary nodules. Note this scan was done as the patient had been commenced on oral steroids and the Consultant Physician wanted to assess if the nodules had decreased in size.
CT brain, abdomen and pelvis
Normal with no evidence of primary metastatic process.
CT guided lung biopsy
Small aggregates of epithelial cells with slight nuclear enlargement, no pleomorphism or atypia. The majority of the core consists of homogenous amorphous eosinophilic material, which polarises apple green following staining with congo red, consistent with amyloid.
Pulmonary function tests
FVC 2.55 L (61% predicted), FEV1 2.06 L (65% predicted), FEV1/FVC 0.8, DLCO 22.9 mL/min/mmHg.
Based on the appearance on the initial CT chest, the primary diagnosis that needed to be further investigated was a cancer that had metastasised to the lungs. The differential diagnosis was progression of the patients’ previously diagnosis pulmonary amyloidosis, however this was not favoured initially as the natural history and expected progression of the disease was not well understood the treating team or local specialists. There was hesitancy to make this diagnosis due to concern about missing a malignancy. Other differentials included non-small cell lung cancer, sarcoidosis, and small vessel vasculitis.
The patients’ lower lobe pneumonia was treated with a 3-day course of Ceftriaxone and Azithromycin, stepped down to 5 days of oral cephalexin, in accordance with local guidelines. Prednisolone 50 mg was trialled for 3 days, but then ceased as it was not thought to be beneficial, following review of repeat CT chest.
In terms of treatment for his pulmonary amyloidosis, none was commenced during this admission as it was unclear as to the degree of the amyloidosis contributing to his current symptoms, and what if any treatment could be implemented. Instead, the patient was referred for outpatient Haematology follow up.
The Patient was Referred to a Respiratory Physician and Haematologist
Further investigations were conducted, including a myeloma screen. Free kappa light chains were mildly elevated at 20.3 mg/L (normal range 3.3 - 19.4), with all other investigations normal: full blood picture, troponin, urea and electrolytes, quantitative electrophoresis, serum free light chains, complement C3 and C4, IgG, LDH, phosphate, aPTT, Ca, BNP, beta-2 macroglobulin, TFTs, HbA1c, urine protein:creatinine ratio.
Follow up with a Haematologist after 2 months, when amyloid subtyping was completed, confirmed the diagnosis of nodular amyloidosis, with no features of tracheobronchial or alveolar septal amyloid. No further specification of subtyping was evident on further investigation. Echocardiogram and cardiac MRI to further investigate potential cardiac involvement are pending. On discussion with the patient, repeat bone biopsy was not pursued as it was thought to be low yield. Currently, the patient is not on active treatment, with follow up booked in 3 months.
Amyloidosis is a condition secondary to the abnormal deposition of protein monomers produced by monoclonal immunoglobulins, and can affect the heart, liver, kidney, spleen, lung, gastrointestinal tract, endocrine organs or the autonomic nervous system [1]. There are four histopathological subtypes that can affect the lung - focal deposits in the tracheobronchial tree; diffuse parenchymal deposits; single or multiple parenchymal nodules; and senile [1]. The amyloid deposits in pulmonary amyloidosis can be composed of Amyloid Light Chain (AL), which accounts for 65-80% of cases, or amyloid A protein [2].
Nodular pulmonary amyloidosis primarily affects older patients aged 60 and above, with routine initial detection on chest X-ray, at which time the nodules may be confused with neoplasms, pulmonary oedema, diffuse interstitial fibrosis or tuberculosis [2].
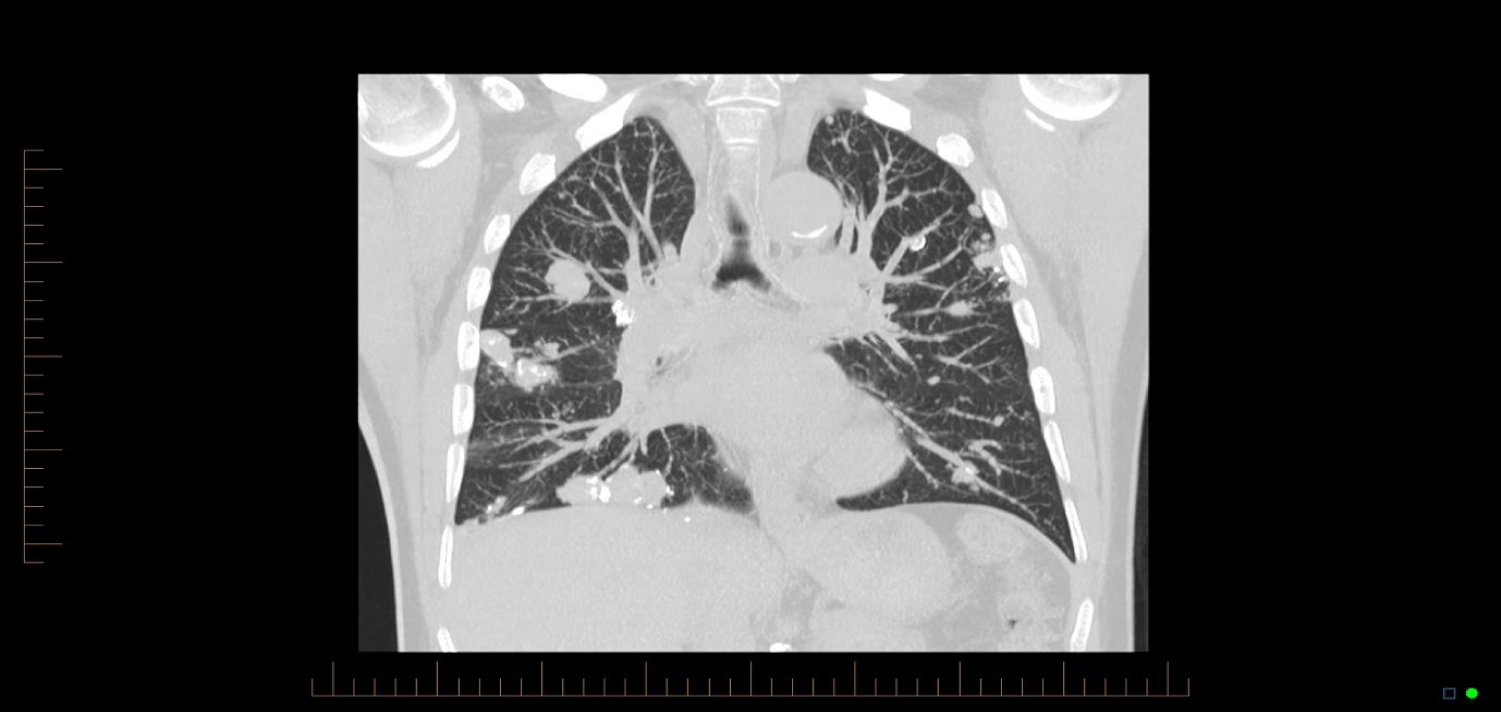
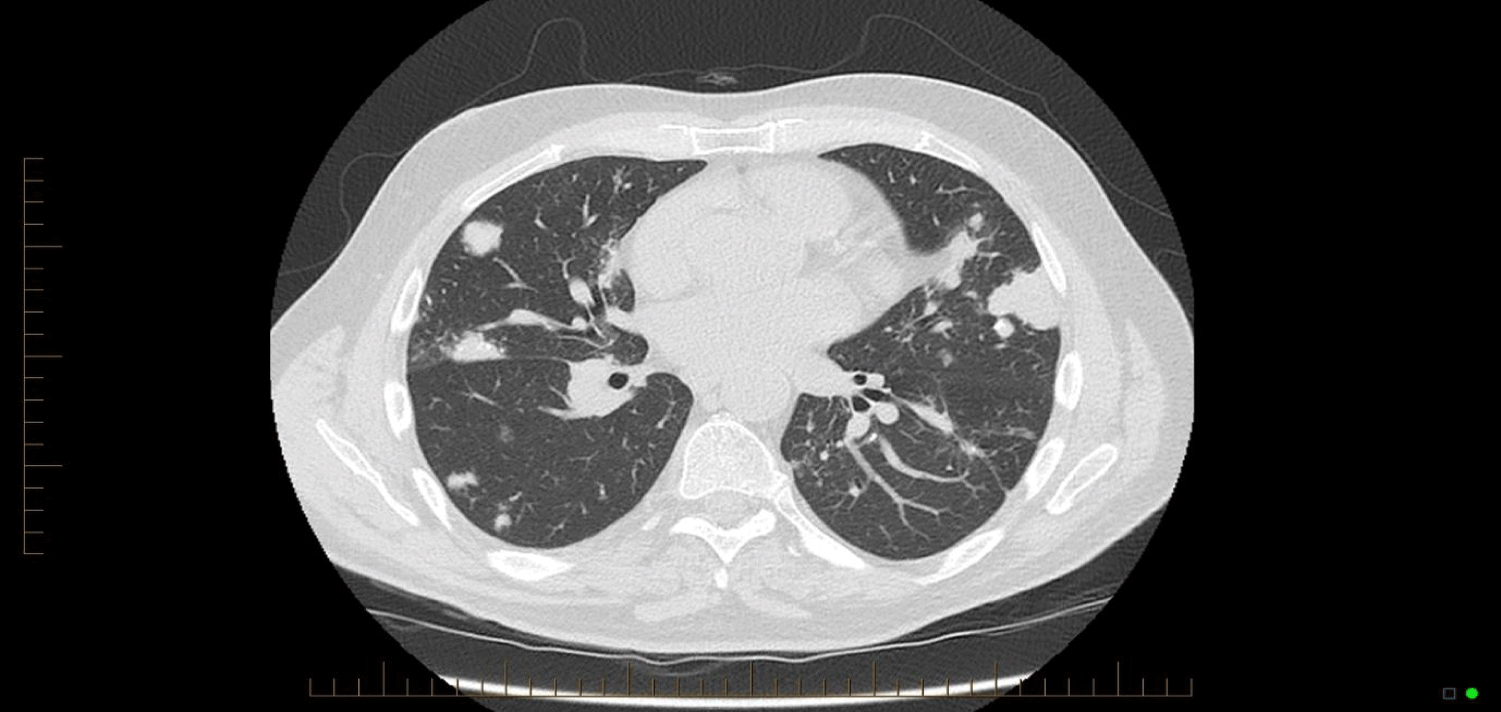
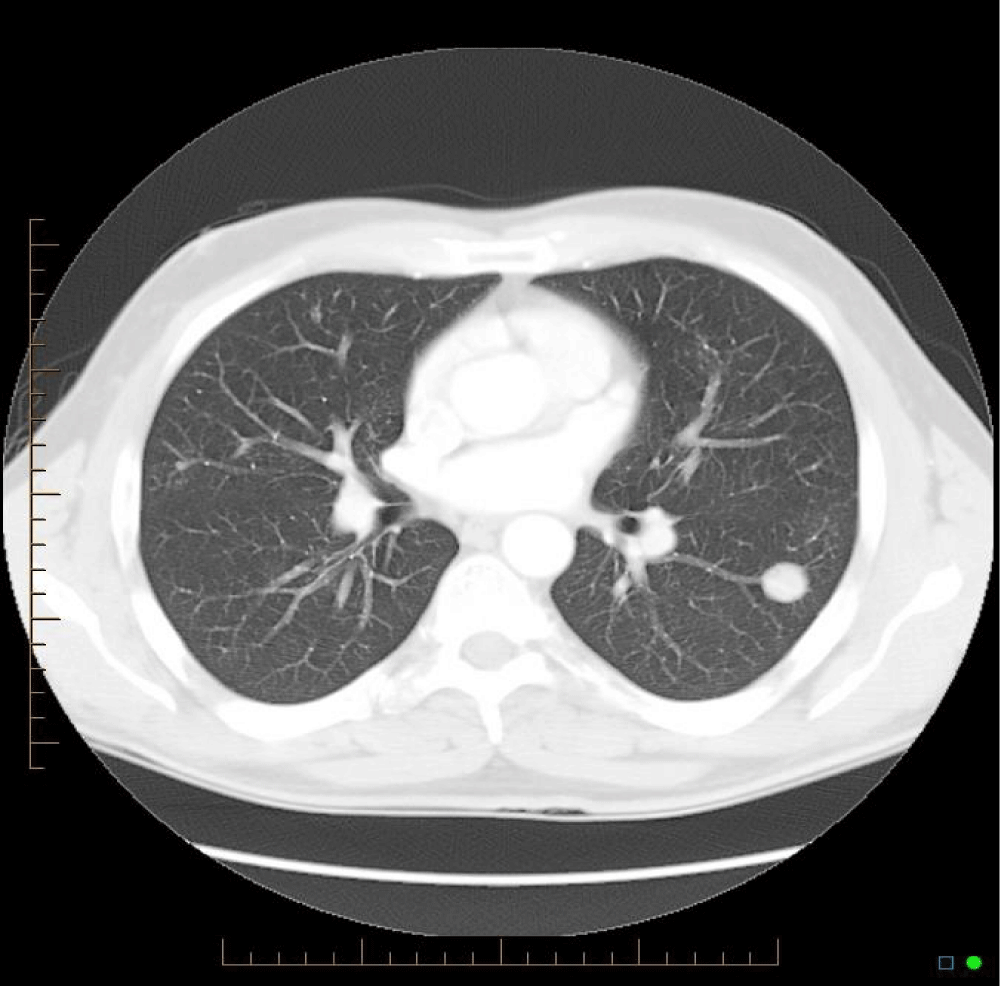
Similar cases have been published, with Bhavsar, et al. [3] presenting a case of a 59 year old woman who presented with 5 pulmonary nodules. Hui, et al. [2] presented 48 cases of lower respiratory tract amyloidosis, of which fourteen were tracheobronchial, six with a diffuse interstitial pattern, and twenty-eight with single or multiple pulmonary nodules. The majority of cases have been associated with underlying myeloma or Mucosa-Associated Lymphoid Tissue (MALT) lymphoma, and subtle analysis identifies clonal B-cell colonies in most cases [4-6]. Those with endobronchial or nodular amyloidosis generally had good long-term survival, while those with systemic or interstitial pulmonary amyloidosis had progressive disease and poor survival [7]. Mayo clinic data from 1980 to 1993 shows 55 patients who were found to have pulmonary amyloidosis - of these, 35 had primary systemic amyloidosis. In these, the median survival after diagnosis was 16 months, but in those with nodular pulmonary amyloidosis without systemic disease, there was benign progress [8] (Figures 1-3).
There are currently no clear diagnostic or treatment guidelines for nodular pulmonary amyloidosis, however there are guidelines for the treatment of AL amyloidosis from the British Journal of Haematology [9]. The clinical course of nodular pulmonary amyloidosis is generally benign, with patients remaining stable for long periods [10]. Surgery is often involved in the diagnosis, with specimens taken for histopathological analysis, as fine needle aspiration has low yield, as the amyloid proteins do not have a consistency that is able to be aspirated [11]. The importance in understanding the clinical course of amyloidosis is in the ability to differentiate it from the other conditions, such as neoplasms or infectious diseases, that present with the same radiological appearances.
Comparison of the CT images below provide insight into the progression of this patients’ pulmonary nodules. There is progression from a singular nodule to diffuse nodular process. The patient presented in this case provides insight into the progression of the disease over a fourteen year period, which has not previously been evident in the literature, and provides a case without a known underlying neoplastic cause.
This case highlighted some key learning points. The first is that Nodular pulmonary amyloidosis is a progressive benign condition, which can lead to diagnostic uncertainty when patients present late in their disease process. Secondly, patients with rare disease diagnoses are often more aware of their disease than their treating team. In this case, the treating Physician was not convinced that this patients’ lung pathology was in fact progression of his chronic disease, which lead to further investigation that may have bee avoided, had the progression of amyloidosis been understood. With this added investigation comes added emotional strain on the patient, who was once again being told they may have cancer.
References
- Cordier JF, Loire R, Brune J. Amyloidosis of the lower respiratory tract. Clinical and pathologic features in a series of 21 patients. Chest. 1986 Dec;90(6):827-31. doi: 10.1378/chest.90.6.827. PMID: 3780328.
- Hui AN, Koss MN, Hochholzer L, Wehunt WD. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med. 1986 Mar;110(3):212-8. PMID: 3753854.
- Bhavsar T, Huang Y, Gaughan C, Inniss S, Thomas R. Bilateral pulmonary nodular amyloidosis: A case report and review of the literature. World J Respirol. 2012 Apr 28;2(2):6-8. doi: 10.5320/wjr.v2.i2.6.
- Grogg KL, Aubry MC, Vrana JA, Theis JD, Dogan A. Nodular pulmonary amyloidosis is characterized by localized immunoglobulin deposition and is frequently associated with an indolent B-cell lymphoproliferative disorder. Am J Surg Pathol. 2013 Mar;37(3):406-12. doi: 10.1097/PAS.0b013e318272fe19. PMID: 23282974.
- Ross P Jr, Magro CM. Clonal light chain restricted primary intrapulmonary nodular amyloidosis. Ann Thorac Surg. 2005 Jul;80(1):344-7. doi: 10.1016/j.athoracsur.2004.03.075. PMID: 15975406.
- Miyamoto T, Kobayashi T, Makiyama M, Kitada S, Fujishima M, Hagari Y, Mihara M. Monoclonality of infiltrating plasma cells in primary pulmonary nodular amyloidosis: detection with polymerase chain reaction. J Clin Pathol. 1999 Jun;52(6):464-7. doi: 10.1136/jcp.52.6.464. PMID: 10562817; PMCID: PMC501436.
- Howard ME, Ireton J, Daniels F, Langton D, Manolitsas ND, Fogarty P, McDonald CF. Pulmonary presentations of amyloidosis. Respirology. 2001 Mar;6(1):61-4. doi: 10.1046/j.1440-1843.2001.00298.x. PMID: 11264765.
- Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med. 1996 Feb 15;124(4):407-13. doi: 10.7326/0003-4819-124-4-199602150-00004. PMID: 8554249.
- Wechalekar AD, Gillmore JD, Bird J, Cavenagh J, Hawkins S, Kazmi M, Lachmann HJ, Hawkins PN, Pratt G; BCSH Committee. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015 Jan;168(2):186-206. doi: 10.1111/bjh.13155. Epub 2014 Oct 10. PMID: 25303672.
- Hiroshima K, Ohwada H, Ishibashi M, Yamamoto N, Tamiya N, Yamaguchi Y. Nodular pulmonary amyloidosis associated with asbestos exposure. Pathol Int. 1996 Jan;46(1):66-70. doi: 10.1111/j.1440-1827.1996.tb03535.x. PMID: 10846552.
- Guidelines Working Group of UK Myeloma Forum; British Commitee for Standards in Haematology, British Society for Haematology. Guidelines on the diagnosis and management of AL amyloidosis. Br J Haematol. 2004 Jun;125(6):681-700. doi: 10.1111/j.1365-2141.2004.04970.x. PMID: 15180858.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.