> Biology. 2020 August 07;1(4):055-063. doi: 10.37871/jels1121.
Effects of Root Bioaccumulation of Arsenic and Mercury on the Expression of the Nramp2b Gene in Prosopis alba (Griseb)
Sergio Loyola1#, Claudia Cárcamo1#, Francisca Concha1, Cristian Becerra1,2, Luis Pouchucq1,2*
2Departamento de Biotecnología, Universidad Tecnológica Metropolitana, Santiago, Chile
#Both authors contributed in the equally for the development of this manuscript
- Phytostabilization
- NRAMP metal transporter
- Fluorescence Rx quantification
- Gene expression
- Bioaccumulation
Finding of vegetal species suitable for ecologic restoration in arid climates is a primary goal of most of the governmental and private companies for fighting against the desertification and the recovery of contaminated soils. The Prosopis genus, a desert woody leguminous, with a wide distribution around the world, represents a very interesting source of highly tolerant and adaptable trees for revegetation and bioremediation of soils contaminated with metal and metalloids (metal(loid)s). The aim of the present work was to evaluate the South American representative of this genus, P. alba, as a possible solution for restoration of soils contaminated with mercury and arsenic. For this, we assessed possible tolerance mechanisms against both metal(loid)s by means of the evaluation of bioaccumulation behaviors and expression changes in metal(loid) tolerance genes. The results revealed that P. alba was able to tolerate high metal(loid) concentrations, related with the accumulation of high quantities of arsenic and mercury in the roots, with bioaccumulation factors of 2, 8 and 3, 1 respectively. Moreover, changes in the expression levels of the gene codifying for the metal transporter NRAMP2b was also observed after the exposure to the metal(loid)s, decreasing ten times for arsenic and three times for mercury. All of these results revealed the existence of cellular mechanisms that allow P. alba to tolerate and accumulate high quantities of arsenic and mercury diluted into the substrate, making it a promising prospect for the treatment of contaminated soils.
Notable among the desert woody plants are those belonging to the Prosopis genus (Fabaceae subfamily Mimosoideae), which is widely distributed in arid and semiarid areas worldwide [1,2], where they take on a great importance in social, cultural, food and economic terms. In South America, the nutritious pods produced by plants of this genus have been used as human and animal food since pre-Columbian times [3]. The species of this genus occupy soils overlying a variety of geological formations with no specific affinities [4]. The American representatives of Prosopis genus are poorly selective, tolerating a wide variety of soil types, pH and high salinity. Moreover, since its ability of nitrogen fixation, Prosopis plants can grow in soils with very low nutrient levels [5].
It has been established that different Prosopis species are tolerant and can even accumulate metal(loid)s diluted into the soil, such as fluoride [6], chromium [7-10], lead, cadmium [9,11], and arsenic [12]. In particular, P. juliflora (Swarts) DC and P. laevigata (Humb. and Bonpl. ex Willd M.C. Johnston) evidence notable metal accumulation abilities which may have biotechnological importance in the recovery of contaminated soils. Prosopis alba (Griseb) is one of the most outstanding representatives of this genus in South America, commonly named as "algarrobo blanco". The endemic distribution of this specie extends across the arid and semiarid regions of South America, ranging from the south of Perú and Bolivia to the north of Chile and northwest of Argentina [2,3,13,14], especially associated with the "Gran Chaco" biogeographic region [15]. The P. alba seedlings and plants have shown a high tolerance to desiccation and hyper-salinity conditions [16] and probably represent an ideal candidate for ecosystem restoration in arid and semiarid regions.
Restricted location or accumulation of metal(loid)s in different plants organ such as roots is the most common resistance trait, however, true tolerance mechanisms require the development of one or more precise physiological mechanisms with a genetic basis [17]. This necessarily implies a gene regulation of metal uptake and its coordination with detoxification and storage mechanisms, most of them related to the activity of metal transporter proteins. Several gene families which codified for metal transporters proteins have been characterized in these terms such as Natural Resistance-Associated Macrophage Protein (NRAMP), Zinc Transporter Protein (ZIP), Cation Diffusion Facilitator (CDF), Heavy Metal-Associated Protein (HMA), Copper Transporter Protein (COP), among others [18-21]. In the present work the researchers evaluate a possible tolerance and accumulation mechanism of mercury and arsenic in P. alba, relating it to changes in the expression levels of different metal transporter genes.
Plant germination and cultivation
Prosopis alba seeds were collected from a valley of the Andes in the Atacama Desert of the northern Chile (27°03'18.0"S 69°18'47.7"W). The seeds were extracted from the pods and selected according to their size (i.e. seeds between 3 to 5 mm of diameter). The selected seeds were treated with 97% sulfuric acid for 20 minutes and neutralized with sodium bicarbonate before being sowed. Two kinds of substrate were used for tolerance bioassays: a) commercial substrate (Biocompost Anasac® Chile: pH 6.8 ± 0.5; <3000 S/m; >20% of organic matter; 30-45% of humidity; >50 C/N ratio) and b) Murashige & Skoog (MS) plant growth media (Phyto Technologies Laboratories®). Seeds were aseptically placed over the sterilized substrate contained within glass flasks for commercial substrate (10g in a 200-cc flask), or Petri dishes for MS media (20 mL in 9 cm Petri dish). The trials were prolonged during 7 days into germination chambers (ArchiClima® Temuco-Chile) set at 16:8 photoperiod and 22 ± 2°C. After exposure period two parameters were measured, the percentage of the germinated seeds (% of germination) and the total length of the principal root (Root Length) as a morphological parameter. LD50 values were calculated by using the Probity Analysis [22]. For metal(loid) accumulation assays, the trials were prolonged during 60 days, P. alba seeds were planted aseptically in sterilized commercial substrate contained within plastic plant cultivation holders (250g in a 500-cc holder). 1 mL of distillated water was applied per 5 g of substrate every three days. After the exposure period the plants were carefully washed three times with a brush in abundant Nano pure Infinity™ water (Branstead® US), and the root length were measured. Then, the above-ground organs (shoots + leaves) and roots were separated for subsequent metal(loid) measurements. Each experiment was performed in three independents biological replicates, in where at least three seeds were sowed by each replicate, giving a total number of at least nine seedlings per treatment (n = 9). Control groups were treated in the same way without the addition of metal(loid)s.
Metal(loid)s testing
For substrate contamination trials previously diluted salts of sodium arsenite (As(III); NaAsO2) and mercury acetate (Hg(II)); Hg (CH3COO)2) were used. All the reagents applied were obtained from MERK® - Germany and were diluted in purified nanopure water using a magnetic stirrer.
Mercury and arsenic measurements in plant tissues
Energy dispersive X-ray fluorescence (ED-XRF) technique was used for determination of arsenic and mercury concentrations in plant tissues [23,24]. After the exposure period, 60-day-old plant tissues were dried in an oven at 60°C until reaching constant mass, and then homogenized in a mortar until obtaining a fine powder. This fine powder was directly measured for metal(loid)s concentrations in energy dispersive S1 Titan BRUKER® equipment using a configuration for heavy and light elements (45kV and 15kV, respectively; method 5015LD_ plastic). Low detection limits for the method were 6 mg k-1 for Hg and 3 mg k-1 for as [24]. Bioaccumulation and translocation factors were calculated as described by Buendía-Gonzalez, et al. [9].
Measurements of chlorophyll a and b
P. alba plants were cultivated in 60 days trials under the conditions described above. After the exposure period, all plant leaves were collected, weighted, rinsed with nanopure water, and macerated in an agate mortar using 5 mL of cold acetone (80%) solution for chlorophyll extraction following the methodology described by Bruinsma [25]. Measurements were performed by absorption spectroscopy in a Perkin-Elmer 8452A equipment (Massachusetts, U.S.).
RT-PCR and Quantitative PCR (qPCR)
For qPCR experiments, seedlings were aseptically germinated and cultured for 7 days in Murashige & Skoog Medium (MS) supplemented with Hg (100 ppm) and as (100 ppm). For root mRNA extraction and RT-PCR, the RNeasy Plant Mini Kit and One-Step RT-PCR Kit (QIAGEN®) were used, respectively. RNA concentrations were estimated using an Epoch BioTech® instrument. 2 µg of total RNA was used to synthesize cDNA. A fraction (1/20) of the synthesized cDNA was used to amplify gene transcripts by qPCR. For this, specific primers were designed against pa-rRNA ITS2, pa-atpase, pa-nramp, pa-cdf and pa-zip genes, according the (Table 1). All primers were synthesized by Integrated DNA Technologies (IDT®, Coralville - Iowa, U.S.). qPCR was performed using Fast SYBR®Green Master Mix from Applied Bio system reagent in an Applied Bio system qPCR instrument (Foster City - California, U.S.). Melting curves were recuperated in order to estimate the quality of the amplification process. All PCR products were verified by agarose gel electrophoresis (2%). Table 1 Specifications of RT-PCR and qPCR primers designed to detect and quantify the expression of ribosomal RNA ITS2, pa-zip, pa-nramp2b, pa-hma8 and pa-cdf genes in P. alba roots tissue. The targeted gene sequences were obtained from P. alba and P. chilensis annotated sequences with the respective accession number.
Table 1: Specifications of RT-PCR and qPCR primers designed. |
|||
| Primer | Gene | Accession | Sequence |
| ITS2-Pc-Fw | Pa-5,8s + internal transcribed spacer2 (ITS2) | AY145693.1 XR_003737815.1 JX139106.1 |
5´-GAA CCA TCG AGT CTT TGA AC-3´ |
| ITS2-Pc-Rv | 5´-CGC ATC TCA TTC ACT CAT C-3´ | ||
| Zip-Pa-Fw | Pa-zip | GAOO01006042 | 5´-CTC ATC GTC TCC GAA TCC AT-3´ |
| Zip-Pa-Rv | 5´-GTC AGA GTC GGG TGT GGA AT-3´ | ||
| Nramp-Pa-Fw | Panramp2b | GAOO01007665 | 5´-CAC AGG CCC TGG TTT TCT TA-3´ |
| Nramp-Pa-Rv | 5´-ACT CCT CCC TGC AGA GTT CA-3´ | ||
| Atpase-Pa-Fw | Pa-hma8 (atpase paa) | GAOO01005873 | 5´-ACC CTT CTG CTG GAG TCT CA-3´ |
| Atpase-Pa-Rv | 5´-GTT GGT TGC AGT TGG TTC CT-3´ | ||
| Cdf-Pa-Fw | Pa-cdf | GAOO01010410 | 5´-AAG CAA GTG TGC AGC ATC AG-3´ |
| Cdf-Pa-Rv | 5´-TGG GGG AAG TAA GCT TTG TG-3´ | ||
Tolerance of Prosopis alba seedlings to As(III) and Hg(II) exposure
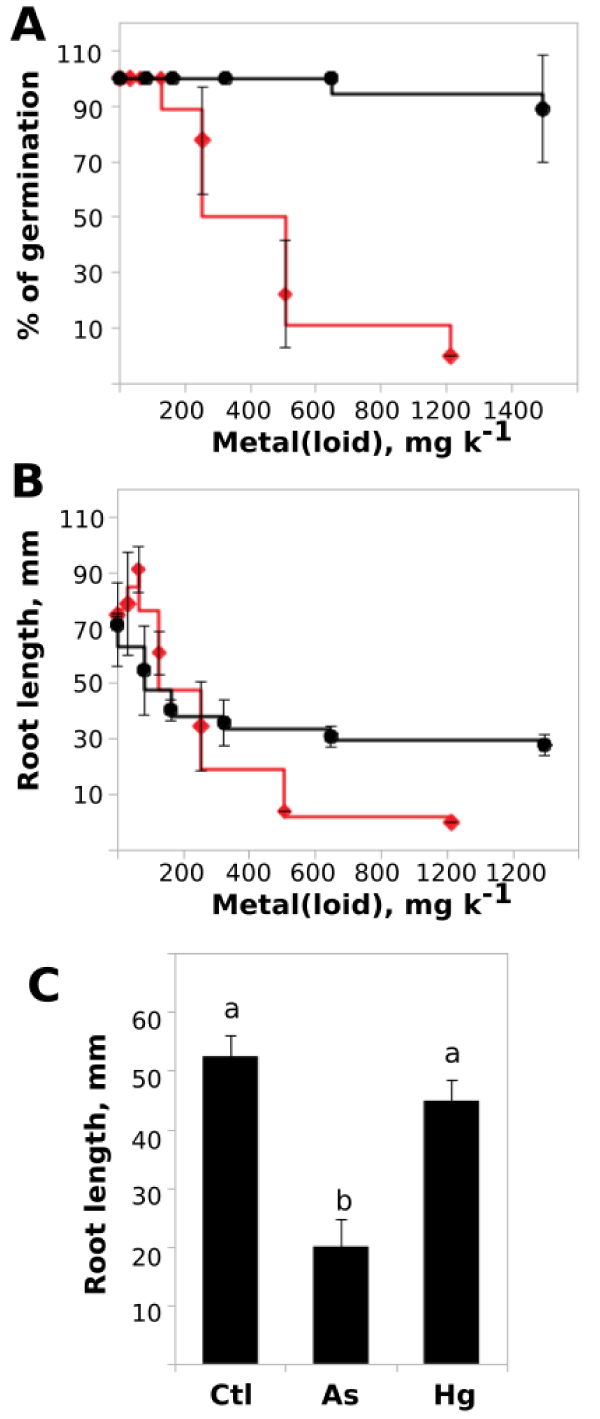
Bioassay trials were performed in order to quantify the tolerance levels of P. alba seedlings to As(III) and Hg(II) exposure. In this trial, P. alba seedlings were germinated in commercial substrate supplemented with increasing concentrations of metal(oid)s. As shown in (Figure 1A), the germination rate was poorly affected by Hg(II), remained above 88%, even with contaminated soils over 1000 mg k-1 of Hg(II). Nevertheless, the As(III) exposed seedlings showed a toxic effect over the germination rate with an LC50 = 385.7 mg k-1 (Figure 1A). Despite the fact that it appeared a toxic effect with As(III), it occurred at very high concentrations, revealing a tolerance behavior.
In terms of morphological analysis, the principal root length shown to be an informative parameter for evidence toxic effects. (Figure 1B) shows that despite the tolerance observed in germination rate, Hg(II) exposure produced a significant decrease to almost one half of the root length (LC50 = 429.7 mg k-1). However, with higher concentrations the seedlings remained alive and the root length parameter also remained at the half of the control value (Figure 1B). By other way, high tolerance was also observed against As(III), which at low concentrations even induced a slight increment in the principal root length. However, as the concentration increased, the root length was negatively affected (Figure 3B), reaching zero over 400 mg k-1 of As(III) (LC50 = 251.7 mg k-1). Concordant results were obtained in artificial Medium (MS) as shown in (Figure 1C) in where the root length were significantly lower in As(III) exposed seedlings.
Accumulation of As and Hg in Prosopis alba plants
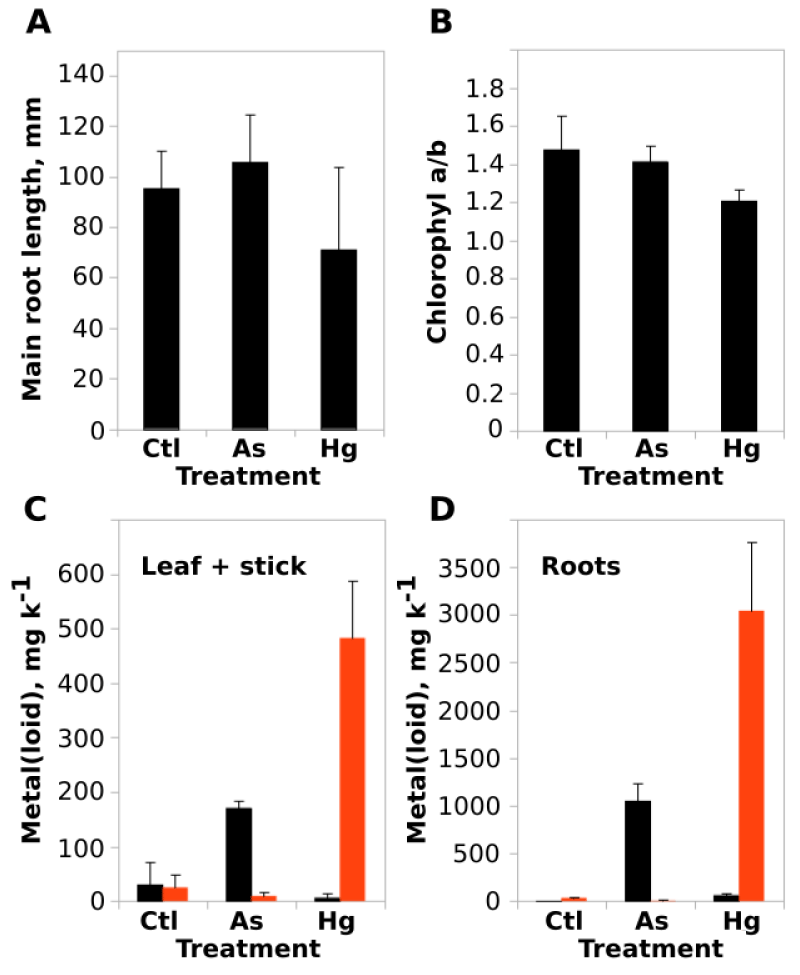
A possible accumulation mechanism of As and Hg related to the tolerance behavior were evaluated. For this, P. alba plants were exposed during 60 days to As(III) = 380 mg and Hg(II) = 971 mg per kg of the substrate. Morphological and physiological evaluations were done together with metal(loid)s quantification in the plant tissues by FRx techniques. As shown in (Figure 2A), only the exposure to Hg(II) induced a non-significant decrease in principal root length, which is also evidenced by an increase in the dispersion (deviation standard) of root length in plants exposed to the metal. In the same way, chlorophyll-a/chlorophyll-b content ratio did not show significant changes, despite the slight decrease observed for Hg(II) exposure, discarding the existence of a serious oxidative stress effect over the photosynthetic apparatus of the exposed plants (Figure 2B).
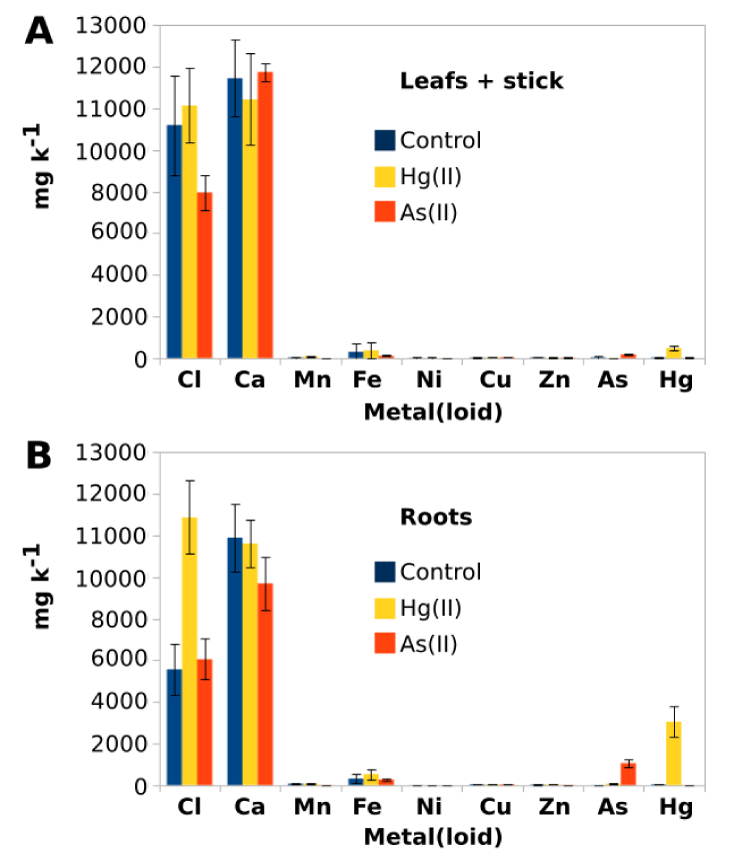
Unexpectedly, together with all these observed tolerance to metal(loid)s, P. alba plants showed high levels of accumulation of As = 1051.5 ± 188.5 mg k-1 and Hg = 3043.4 ± 88 mg k-1 at the roots (Figure 2D). Meanwhile, the quantified values of As and Hg concentrations in the above ground organs (leafs + stick) were considerably lower, but still high, 171 ± 13.8 mg k-1 and 482.7 ± 104.3 mg k-1 respectively (Figure 2C). Figure 3 offer a general view of the metal(loid) (and other elements) composition of the treated plants. At above-ground organs, the Cl deficiency was observed only in As(III) exposed plants (Figure 3A). However, it is possible to observe a significant increase in the Cl uptake at the roots of the Hg(II) treated plants compared to the controls (Figure 3B). No significant changes were observed in other analyzed ions, including Ca, Mn and Fe. Notably, As and Hg became in an abundant metal(loid) in the root tissue composition, surpassing even the Fe concentration but still lower than Cl and Ca.
As shown in (Table 2), the calculated bioaccumulation factors indicated that the roots of P. alba uptake Hg and As in around three times over the substrate concentrations (of dried substrate). These results suggested that probably the metal(loid)s were actively incorporated and accumulated into the tissues by some molecular mechanism. By other way, in above-ground organs the obtained bioaccumulation factors were below 1.0, indicating that the metal(loid)s were not accumulated in these organs. Concomitantly, the calculated translocation factor values (root-to-shoot) indicated that the metal(loid)s accumulated in the roots were poorly transported to above ground anatomy (Table 2).
Table 2: Metal(loid)s bioaccumulation (BF) and translocation (TF) factors of As and Hg in Prosopis alba plantsexposed during 60 days to Hg (971mg k-1 ) and As (380 mg k-1) diluted into the substrate. (±): standard deviation of three biological replicates. BF and TF were calculated from dried substrate and vegetal tissue. |
|||
| Bioaccumulation factors | Leafs + stick | Roots | Transference factor |
| Hg(II) | 0.5 ± 0.1 | 3.1 ± 0.7 | 0.17 ± 0.06 |
| As(III) | 0.45 ± 0.04 | 2.8 ± 0.5 | 0.16 ± 0.02 |
Expression changes in genes related to metal uptake and tolerance in Prosopis alba seedlings
In order to evaluate a possible cellular and genetic mechanism related with the tolerance to As(III) and Hg(II), we performed a molecular study of the gene expression by quantitative PCR (qPCR). Sequence analysis of the Prosopis alba transcripome (GAOO00000000.1) as published by Torales et al. [26] revealed the presence of four genes belonging to four different gene families broadly related to metal uptake and tolerance in plants: pa-hma8, pa-nramp2b, pa-zip and pa-cdf. A set of qPCR primers was designed in order to quantify mRNA expression levels of each one of these genes, including a couple of primers against the polycistronic precursor of the ribosomal RNA 47S (ITS2) as a reference gene, designed from transcriptomic information of P. alba and genomic information from a close relative: P chilensis (see table 1). The criteria beneath the design of the reference gen primer was to avoid the rRNA processing steps [27], amplifying only the ribosomal RNA primary transcript which has been shown high expression stability in plants [28].
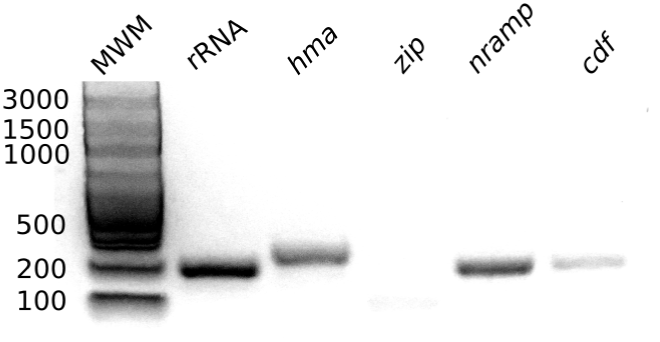
Seedlings of P. alba were germinated in artificial Media (MS) supplemented with previously dissolved metal(loid)s solutions (i.e. As(III) = 100 mg L-1 and Hg(II) = 100 mg L-1, at a final concentration). Seven days after germination the trials were stopped and the mRNA was purified from the roots and subjected to a retro transcription procedure in order to synthesize cDNA. As shown in (Figure 4), amplification by conventional RT-PCR resulted positive for all transcripts except pa-zip, which could not be amplified under the tested conditions. The obtained PCR products showed the expected size, i.e. rRNA ITS2=204 bp, pa-hma- = 244 bp, pa-cdf =202 bp and pa-nramp = 203 bp.
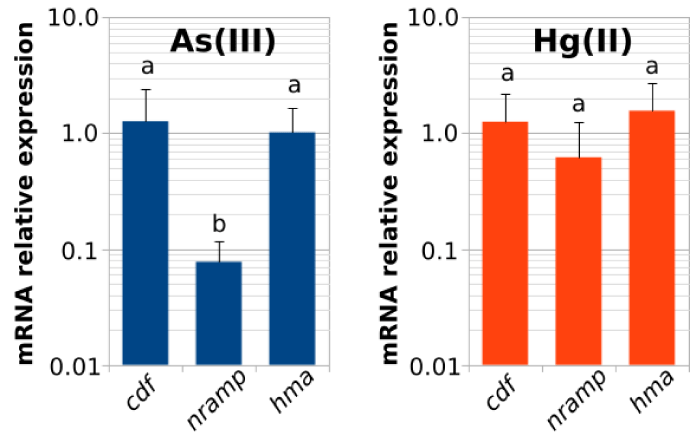
Relative mRNA expression of pa-nramp2b, pa-hma8, and pa-cdf genes were calculated by RT-qPCR using the ribosomal RNA as a reference gene in arsenic and mercury exposed seedlings. The expression levels were calculated as reported by Schmittgen, et al. [29] and potted in bars as shown in the figure 5. The results indicate that the expression of pa-nramp2b gene suffered a dramatic decrease, in around ten folds, following As(III) exposure. Meanwhile, a less significant decrease was also observed under Hg(II) exposure conditions. The expression of pa-hma8 and pa-cdf genes did not show any significant expression changes related to metal(oid)s exposure despite the slight variations observed. This notable down regulation of pa-nramp2b gene could reveal a genetic mechanism related to the tolerance to metal(loid) accumulation in P. alba individuals.
Tolerance to metals and metal(loid)s in plants relies on confinement and detoxification in a controlled way [30], which are manifested as a battery of cellular and molecular mechanisms that joined together allows plants to establish in places where metal contamination levels are extremely high. The results presented in this work suggest the existence of a molecular mechanism in Prosopis alba that gives a high tolerance to metal(loid)s diluted into the soil. This mechanism appeared to be related to the accumulation of high quantities of metal(loid)s into the roots. qPCR gene expression studies allowed to link this tolerance and accumulation of P. alba to As(III) and Hg(II) with the differential regulation of the gene pa-nramp2b belonging to divalent cation transporter family NRAMP.
This down-regulation of gene expression appears consistent with previous data reported for Gylicine max L., in where the gene gm-nramp2b was down regulated in response to Fe(II) and Cu(II) exposure [31]. Other members of this gene family has been related to the uptake and hypersensitivity to divalent metals [32,33] and it have been hypothesized to function in the mobilization of the micronutrients Fe(II), Mn(II) and Zn(II) from the vacuole to the cytoplasm [20]. In the same order, it has been reported that NRAMP proteins localize to the plant vacuolar membrane in where, depending on the NRAMP type, participate in the efflux from the vacuole to the cytoplasm of a wide variety of divalent cations[34]. Moreover, indirect evidence suggests that the transport activities of NRAMPs differ in a species-specific manner [34] probable expanding the specificity to different cations, as As(III). All these data combined with the present results are consistent with a cellular mechanism of cytoplasmic depletion of toxic metal(loid)s, that favors the metal accumulation at the vacuole level due to the pa-nramp2b down-regulation.
In a comparison with other highly tolerant and hyper-accumulating plant species, P. alba showed an accumulation of As = 1051,5 mg k-1 at the roots, a value comparable to the 1000 ~ 8331 mg k-1 reported for the hyperaccumulator Pteris vitata [35-37]; In the same way, the amount of Hg(II) that P. alba was able to tolerate and accumulate was surprising. In literature, plants accumulation of Hg tent to be lower than the 3043.4 mg k-1 of tissue that we quantified in the P. alba roots; 397.23 mg k-1 in the leaves of Cyrtomium macrophyllum [38]; 43.5 mg k-1 in leaves of Cynoglossum cheirifolium; 3.6 mg k-1 in the roots of Erato polymnioides [39,40]. As expected, when tolerance limits were exceeded, toxic symptoms that included root growth inhibition and impairment of photosynthesis was observed, concomitantly with previously reported information [41].
Phytostabilization through the use of colonizer hyper-tolerant plants is a promising alternative, particularly if these plants present natural tolerance to salinity, lack of nutrients, poor soil structure, and extreme pH [42]. Moreover, when metal(loid)s contaminated soils are located in arid or semiarid regions, limited water resources further restrict successful plant establishment, thus drastically reducing the list of feasible plants that can be used in phytoremediation [42]. The trees belonging to the genus Prosopis appear as a good alternative for these conditions. This genus comprises of more than 44 species [43] distributed worldwide in arid and semiarid regions [4]. Several of their representatives have been explored for potential use in metal(loid)s decontamination, such as P. laevigata, which evidences chromium accumulation [9,10]; P. juliflora and P. pubescens (Benth) accumulate copper, mainly in the roots [44,45]; lead accumulation was described in P. juliflora [11]. Similar to the results obtained in this work, accumulation of arsenic has been observed for other Prosopis species [12]. Nevertheless, up to our knowledge, this is the first time that one Prosopis genus representative has been related with mercury tolerance and accumulation. On the other hand, this would correspond to the first experimental study directed to determinate the metal(loid)s phytoremediation potential of P. alba.
Importantly, the evaluation and selection of plants for phytoremediation purposes entirely depend on the end-goal of remediation [35,46-48], i.e. whether contaminants will be extracted or stabilized to a safe state. In this context, the translocation factors to the aboveground organs would by the most important factor to consider [35,42]. Consequently, P. alba appears to be an unsuitable phytoextraction candidate due to the poor translocation factors obtained. But in contrast, the great tolerance and accumulation of Hg(II) and As(III) in the roots could promote P. alba for phytostabilization purposes. This would ensure the safe underground stabilization of dangerous contaminants as As(III) and Hg(II) in contaminated soils form arid and semiarid soils.
P. alba seedlings showed high tolerance to As(III) and Hg(II) diluted in the substrate, with LC50 of 251.7 mg k-1 and 429.7 mg k-1 respectively. Furthermore, P. alba plants showed a bioaccumulation behavior of As and Hg at the root level, with a bioaccumulation factors close to 3 for both metal(loid)s. Finally, exposure to As(III) induced the down-regulation in the expression of the metal transporter gene nramp2b in P. alba seedlings, suggesting the presence of a cellular mechanism of metal(loid) tolerance and detoxification.
This research was primarily undertaken in the Programa de Biotecnología Vegetal y Ambiental Aplicada, Universidad Tecnológica Metropolitana. This work was funded by FPA-NAC-083-2014 Fondo de Protección Ambiental - Ministerio del Medio Ambiente -Chile and by the Initiation R&D Program, year 2014, code L1-02 and year 2015 code L2-08-2015, Universidad Tecnológica Metropolitana.
- Villagra P, Vilela A, Giordano C, Alvarez J. Ecophysiology of prosopis species from the arid lands of Argentina: What do we know about adaptation to stressful environments. Desert Plants Biology and Biotechnology. Springer-Verlag. Berlin Heidelberg. 2010. DOI: https://doi.org/10.1007/978-3-642-02550-1
- Shackleton R, Le Maitre D, Pasiecznik N, Richardson D. Prosopis: A global assessment of the biogeography, benefits, impacts and management of one of the world's worst woody invasive plant taxa. AoB Plants. 2014; 6. DOI: https://doi.org/10.1093/aobpla/plu027
- McRostie V, Gayo E, Santoro C, De Pol-Holz R, Latorre C. The pre-columbian introduction and dispersal of Algarrobo (Prosopis, Section Algarobia) in the Atacama Desert of northern Chile. PLoS One. 2017; 12: e0181759. DOI: https://doi.org/10.1371/journal.pone.0181759
- Pasiecznik N, Felker P, Harris P, Harsh L, Cruz G, Tewari J, et al. The Prosopis juliflora - Prosopis pallida Complex: A Monograph, Hdra; Coventry UK. 2001; 172. https://bit.ly/2ENTqdZ
- Geesing D, Felker P, Bingham R. Influence of mesquite (Prosopis glandulosa) on soil nitrogen and carbon development: Implications for global carbon sequestration. J Arid Environ. 2000; 47: 157-180. DOI: https://doi.org/10.1006/jare.2000.0661
- Saini P, Khan S, Baunthiyal M, Sharma V. Organ-wise accumulation of fluoride in Prosopis juliflora and its potential for phytoremediation of fluoride contaminated soil. Chemosphere. 2012; 89: 633-635. DOI: https://doi.org/10.1016/j.chemosphere.2012.05.034
- Arias J, Peralta-Videa J, Ellzey J, Ren M, Viveros M, Gardea-Torresdey J, et al. Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot. 2010; 68: 139-148. DOI: https://doi.org/10.1016/j.envexpbot.2009.08.009
- Arias J, Peralta-Videa J, Ellzey J, Viveros M, Ren M, Mokgalaka-Matlala N, et al. Plant growth and metal distribution in tissues of Prosopis Juliflora-velutina grown on chromium contaminated soil in the presence of Glomus deserticola. Environ Sci Technol. 2010; 44: 7272-7279. DOI: https://doi.org/10.1021/es1008664
- Buendía-González L, Orozco-Villafuerte J, Cruz-Sosa F, Barrera-Díaz C, Vernon-Carter E. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010; 101: 5862-5867. DOI: https://doi.org/10.1016/j.biortech.2010.03.027
- Aldrich M, Gardea-Torresdey J, Peralta-Videa J, Parsons J. Uptake and reduction of Cr (VI) to Cr (III) by mesquite (Prosopis spp.): Chromate-plant interaction in hydroponics and solid media studied using XAS. Environ Sci Technol. 2003; 37: 1859-1864. DOI: https://doi.org/10.1021/es0208916
- Varun M, D'Souza R, Pratas J, Paul M. Phytoextraction potential of Prosopis juliflora (Sw.) DC. with specific reference to lead and cadmium. Bull. Environ. Contam. Toxicol. 2011; 87: 45-49. DOI: https://doi.org/10.1007/s00128-011-0305-0
- Aldrich MV, Peralta-Videa JR, Parsons JG, Gardea-Torresdey JL. Examination of arsenic (III) and (V) uptake by the desert plant species mesquite (Prosopis spp.) using X-ray absorption spectroscopy. Sci Total Environ. 2007; 379: 249-255. DOI: https://doi.org/10.1016/j.scitotenv.2006.08.053
- Ferreyra L, Bessega C, Vilardi JC, Saidman B. Consistency of population genetics parameters estimated from isozyme and RAPDs dataset in species of genus Prosopis (Leguminosae, Mimosoideae). Genetica. 2007; 131: 217–230. DOI: https://doi.org/10.1007/s10709-006-9132-3
- Pasiecznik N, Harris PJC, Smith JS. Identifying Tropical Prosopis Species: A Field Guide. Hdra; Coventry UK. 2004; 44: 1-30. https://bit.ly/2DM8kkk
- Morales M, Oakley L, Sartori A, Mogni V, Atahuachi M, Vanni R, et al. Diversity and conservation of legumes in the Gran Chaco and biogeograpical inferences. PLoS ONE. 2019; 14: e0220151. DOI: https://doi.org/10.1371/journal.pone.0220151
- Meloni D, Gulotta M, Silva D, Arraiza M. Effects of salt stress on germination, seedling growth, osmotic adjustment, and chlorophyll fluorescence in Prosopis alba G. Rev. La Fac. Ciencias Agrar. 2019; 51: 69-78. https://bit.ly/3a2q6vO
- Pulford I, Watson C. Phytoremediation of heavy metal-contaminated land by trees - A review Environ Int. 2003; 29: 529-540. DOI: https://doi.org/10.1016/S0160-4120(02)00152-6
- Memon AR, Schröder P. Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res Int. 2009; 16: 162-175. DOI: https://doi.org/10.1007/s11356-008-0079-z
- Krämer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Lett. 2007; 581: 2263-2272. DOI: https://doi.org/10.1016/j.febslet.2007.04.010
- Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006; 88: 1707-1719. DOI: https://doi.org/10.1016/j.biochi.2006.07.003
- Yang X, Feng Y, He Z, Stoffella P. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol. 2005; 18: 339-353. DOI: https://doi.org/10.1016/j.jtemb.2005.02.007.
- Finney DJ. Probit Analysis, 2nd ed., Cambridge University Press, New York, 1952. DOI: https://doi.org/10.1002/jps.3030411125
- Gei V, Erskine PD, Harris HH, Echevarria G, Mesjasz-Przybyłowicz J, Barnabas A, et al. Tools for the discovery of hyperaccumulator plant species and understanding their ecophysiology. Agromining: Farming for Metals. 2018; 117-133. DOI: https://doi.org/10.1007/978-3-319-61899-9
- Fellin M, Negri M, Zanuttini R. Multi-elemental analysis of wood waste using Energy Dispersive X-ray Fluorescence (ED-XRF) analyzer. Eur J Wood & Wood Prod. 2014; 72: 199-211. DOI: https://doi.org/10.1007/s00107-013-0766-4
- Bruinsma J. The quantitative analysis of chlorophylls a and b in plant extracts, photochem. Photobiol. 1963; 2: 241-249. DOI: https://doi.org/10.1111/j.1751-1097.1963.tb08220.x
- Torales SL, Rivarola M, Pomponio MF, Gonzalez S, Acuña CV, Fernández P, et al, De novo assembly and characterization of leaf transcriptome for the development of functional molecular markers of the extremophile multipurpose tree species Prosopis alba. BMC Genomics. 2013; 14: 705. DOI: https://doi.org/10.1186/1471-2164-14-705
- Micol-Ponce R, Sarmiento-Mañús R, Ruiz-Bayón A, Montacié C, Sáez-Vasquez J, Ponce M. Arabidopsis ribosomal RNA processing is required for 18s rRNA maturation. Plant Cell. 2018; 30: 2855-2872. DOI: https://doi.org/10.1105/tpc.18.00245
- Wang X, Fu Y, Ban L, Wang Z, Feng G, Li J, et al. Selection of reliable reference genes for quantitative real-time RT-PCR in alfalfa. Genes Genet Syst. 2015; 90: 175-180. DOI: https://doi.org/10.1266/ggs.90.175
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal Biochem. 2000; 285: 194-204. DOI: https://doi.org/10.1006/abio.2000.4753
- Lin Y, Aarts MG. The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci. 2012; 69: 3187-3206. DOI: https://doi.org/10.1007/s00018-012-1089-z
- Qin L, Han P, Chen L, Walk T, Li Y, Hu X, et al. Genome-wide identification and expression analysis of NRAMP Family Genes in Soybean (Glycine Max L.). Front Plant Sci. 2017; 8: 1436. DOI: https://doi.org/10.3389/fpls.2017.01436
- Thomine S, Wang R, Ward JM, Crawford N, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. PNAS. 2000; 97: 4991-4996. DOI: https://doi.org/10.1073/pnas.97.9.4991
- Bereczky Z, Wang H, Schubert V, Ganal M, Bauer P. Differential Regulation of NRAMP and IRT Metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem. 2003; 278: 24697-24704. DOI: https://doi.org/10.1074/jbc.M301365200
- Sharma SS, Dietz KJ, Mimura T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016; 39: 1112-1126. DOI: https://doi.org/10.1111/pce.12706
- Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals - concepts and applications. Chemosphere. 2013; 91: 869-881. DOI: https://doi.org/10.1016/j.chemosphere.2013.01.075
- De Oliveira L, Ma L, Santos J, Guilherme L, Lessl J. Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ Pollut. 2014; 184: 187-192. DOI: https://doi.org/10.1016/j.envpol.2013.08.025.
- Koller CE, Patrick WJ, Rose RJ, Offler CE, MacFarlane GR. Arsenic and heavy metal accumulation by Pteris vittata L. and P. umbrosa R. Br. Bull. Environ Contam Toxicol. 2008; 80: 128-133. DOI: https://doi.org/10.1007/s00128-007-9330-4.
- Xun Y, Feng L, Li Y, Dong H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere. 2017; 189: 161-170. DOI: https://doi.org/10.1016/j.chemosphere.2017.09.055
- Chamba I, Rosado D, Kalinhoff C, Thangaswamy S, Sánchez-Rodríguez A, Gazquez M. Erato polymnioides – A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere. 2017; 188: 633-641. DOI: https://doi.org/10.1016/j.chemosphere.2017.08.160
- Millán R, Gamarra R, Schmid T, Sierra M, Quejido AJ, Sánchez DM, et al. Mercury content in vegetation and soils of the Almadén mining area (Spain). Sci Total Environ. 2006; 368: 79-87. DOI: https://doi.org/10.1016/j.scitotenv.2005.09.096
- Hernández LE, Sobrino-Plata J, Montero-Palmero M, Carrasco-Gil S, Flores-Cáceres M, Ortega-Villasante C, et al. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J Exp Bot. 2015; 66: 2901-2911. DOI: https://doi.org/10.1093/jxb/erv063
- Mendez MO, Maier RM. Phytostabilization of mine tailings in arid and semiarid environments - An emerging remediation technology. Environ Health Perspect. 2008; 116: 278-283. DOI: https://doi.org/10.1289/ehp.10608
- Burkart A. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). Catalogue of the recognized species of Prosopis. J Arnold Arbor. 1976; 57: 450-525.
- Senthilkumar P, Prince WSPM, Sivakumar S, Subbhuraam CV. Prosopis juliflora - A green solution to decontaminate heavy metal (Cu and Cd) contaminated soils, Chemosphere. 2005; 60: 1493-1496. DOI: https://doi.org/10.1016/j.chemosphere.2005.02.022
- Zappala MN, Ellzey JT, Bader J, Peralta-Videa JR, Gardea-Torresdey J. Prosopis pubescens (screw bean mesquite) seedlings are hyperaccumulators of copper. Arch Environ Contam Toxicol. 2013; 65: 212-223. DOI: https://doi.org/10.1007/s00244-013-9904-6
- Mahar A, Wang P, Ali A, Awasthi M, Lahori A, Wang Q, et al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review, Ecotoxicol. Environ Saf. 2016; 126: 111-121. DOI: https://doi.org/10.1016/j.ecoenv.2015.12.023
- Padmavathiamma P, Li L. Phytoremediation technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007; 184: 105-126. DOI: https://doi.org/10.1007/s11270-007-9401-5
- Thakur S, Singh L, Wahid Z, Siddiqui M, Atnaw S, Din M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ Monit Assess. 2016; 188: 206. DOI: https://doi.org/10.1007/s10661-016-5211-9.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.