> Biology. 2020 August 01;1(4):051-054. doi: 10.37871/jels1120.
-
Subject area(s):
- Toxicology
Heavy Metal Accumulation in Veli Lake Sediments - Kerala Coast of India
Leena Grace Beslin*
- Trace elements
- Heavy metals
- Copper
- Nickel
- Zinc
- Lead
- Manganese
Organisms inhabiting in water require little amount of trace elements. At finest level they are favorable and further than that edge it affected the ecosystem. Continuous dumping of impurities like heavy metals from various supplies in significant quantities are constantly discharged into the rivers reaches estuaries and oceans. For the reason of this unwanted human liberation oceanic biosphere is recognized to hold heaviness of heavy metals. For most of the heavy metals estuarine sediments form the ultimate catch. The metals entering the system through various sources may associate with the sediment particles either by absorption are gradually sink with sediment or they may get released when mixed with marine water due to altered physico-chemical environment. Veli Lake is polluted by different means which is situated in the south west coast of India (Kerala). The additional quantities of heavy metals come from agricultural, industrial and domestic wastes. Hence sediments are indicators of the quality of overlying water, for the present study five different heavy metals were analyzed from the sediments of Veli Lake. Monthly collection of sediments and analysis were carried out for a period of one year (April-2017 to March-2018). The five metals analyzed were copper, lead, manganese, nickel and zinc. Low levels of copper were observed in all the stations while comparing other metals. The seasonal variations were also noted for all the five metals and discussed.
Pollution of an aquatic system in general is classified into two, one type is pollution by the poisonous substances like heavy metals or pesticides and the other type is pollution by the nutrients or organic materials. The former is responsible for the toxic effect. The final results in both the cases lead to deterioration of water quality; intern seriously threatens the survivability of the existing flora and fauna [1]. Sediments can play an important role in estuarine ecosystems by buffering the environment. The sediments showed wide geographic variability in their metal content, complexion, sorption and biochemical transformation [2].
Heavy metals are potentially hazardous particularly in estuaries and near shore water [3]. Knowledge of elemental fluxes from rivers to the oceans is essential for calculating their possible sinks and sources. The trace metals are found to be absorbed into clay particles due to their high surface area to volume ratio and surface charges [4]. When concentration of metals exceeds the acceptable limit, it creates environmental hazards. Many aquatic organisms are known to accumulate and concentrate metal at relatively higher concentration than the surrounding environment [5].
The Veli Lake is the smallest among the backwaters of Kerala. It is situated 8 km North West of Trivandrum city at 8° 28’ N latitude and 76° 57’ E longitude. The system widens from the bar mouth to the eastern part and is 2km long and 0.3 km broad. It is connected to the Kadinamkulam backwater through Parvathiputhanar canal. On the southern side it is connected with the Chackai canal and Aakulam Lake. The three stations selected for the present study are Valiyaveli, Madavapuram and Pozhikara.
Sampling of sediments was done during the fresh hours monthly for a period of 12 months from April 2017 to March 2018.
Heavy metals of the sediment were measured by the method of Ahmad et al [1]. The oven dried and grained samples were measured and digested with a combination of concentrated nitric acid and perchloric acid in the ratio of 5:1. The digested samples were washed with distilled water and centrifuged as 3000 rpm for 5 minutes. The made up solutions were transferred to polythene bottles and analyzed the metals in atomic absorption spectrophotometer (GBC 932 AA).
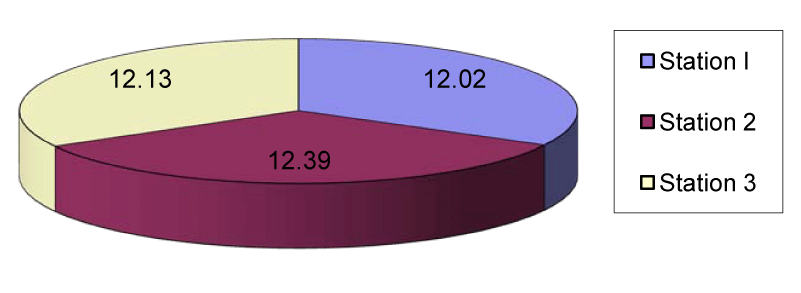
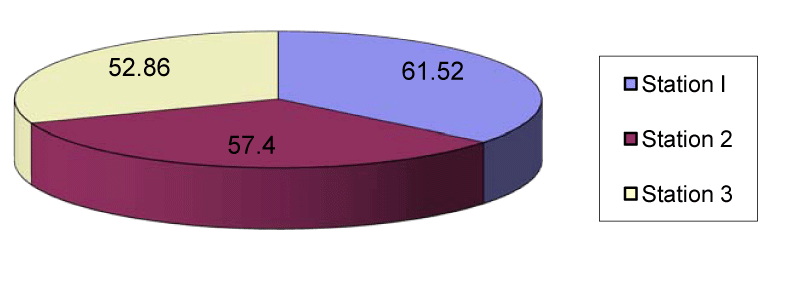
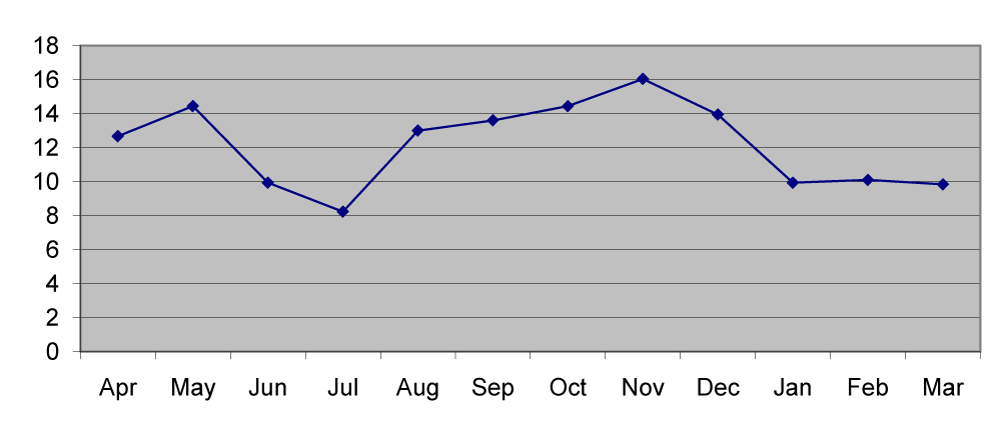
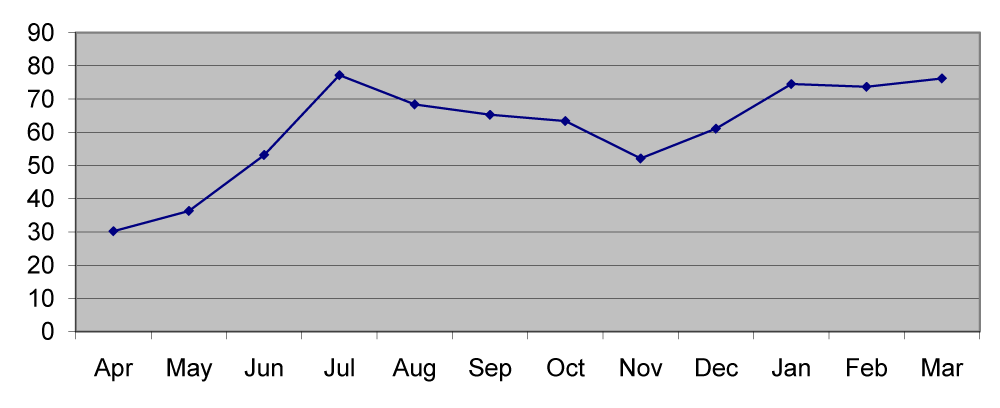
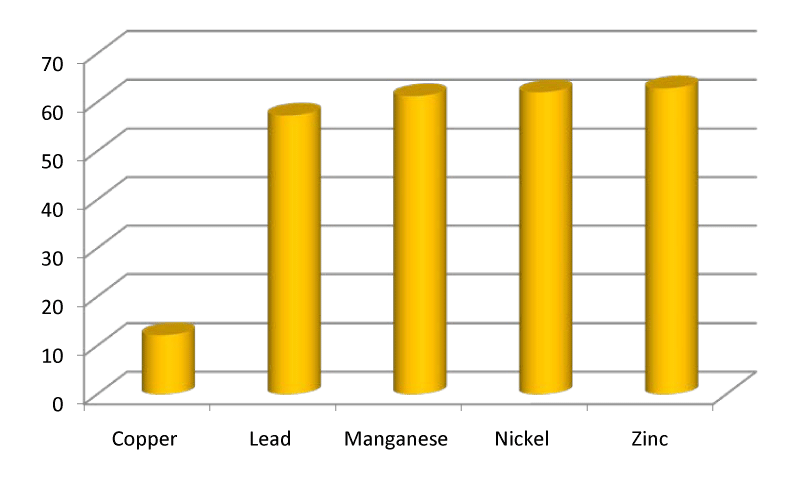
The copper content of station1 ranged from 8.1µg/g in July to 15.1µg/g in November with the mean value of 12.02µg/g. In station 2 the mean copper in sediment was 12.39μg/g which ranged from 8.4µg/g in July to 17.2µg/g in November. In station 3 the copper content ranged from 8.2µg/g in July to 15.18µg/g in November with the mean value of 12.13µg/g (Figures 1,6). The lead content of station 1 sediment ranged from 30.1µg/g in April to 96.5µg/g in March with the mean value of 61.52µg/g. In station 2 the mean lead content was 57.4µg/g ranged between 20.6µg/g in April and 89.6µg/g in August. In station 3 the mean lead content was 52.86µg/g which ranged from 15.8µg/g in may to 82.7µg/g in January (Figures 2,7).
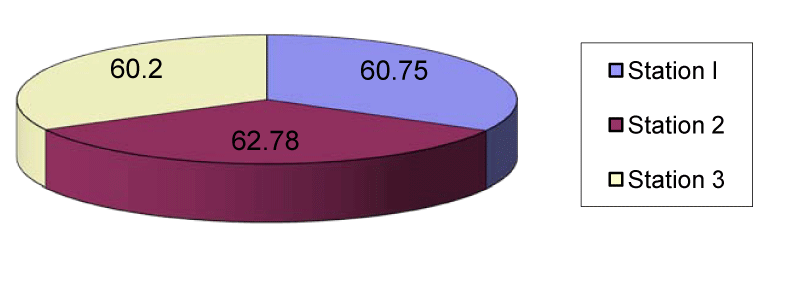
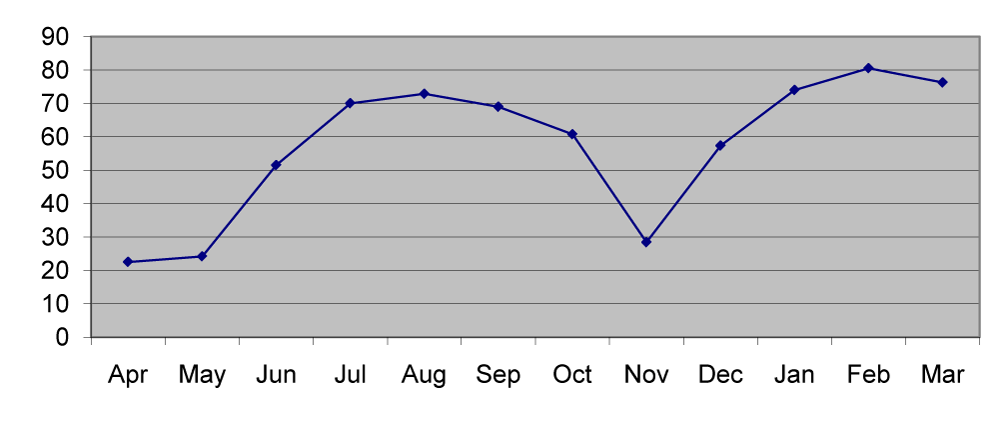
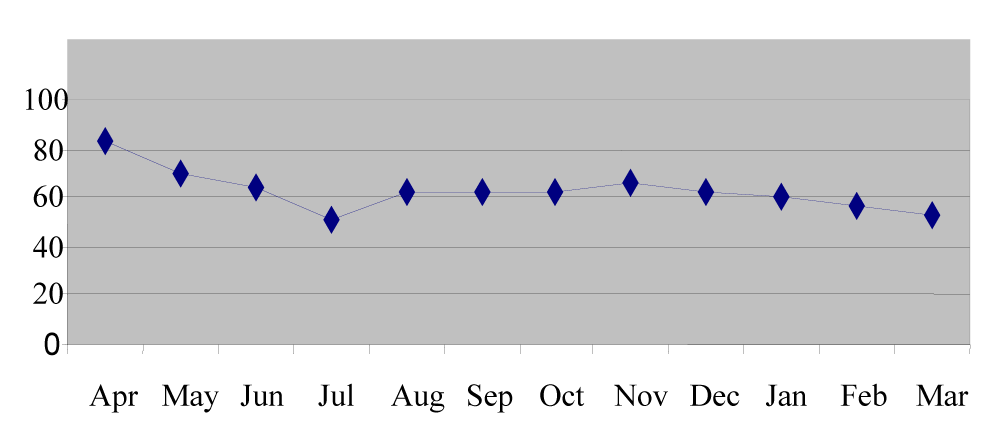
The manganese content of station 1 sediment ranged from 27.7µg/g in April to 80.9µg/g in March with the mean value of 60.75µg/g. In station 2 the mean manganese content was 62.78µg/g ranged between 32.4µg/g in April and 77.7µg/g in July. In station 3 the mean manganese content was 60.2µg/g that ranged from 30.6µg/g in April to 76.5µg/g in July (Figures 3,8).
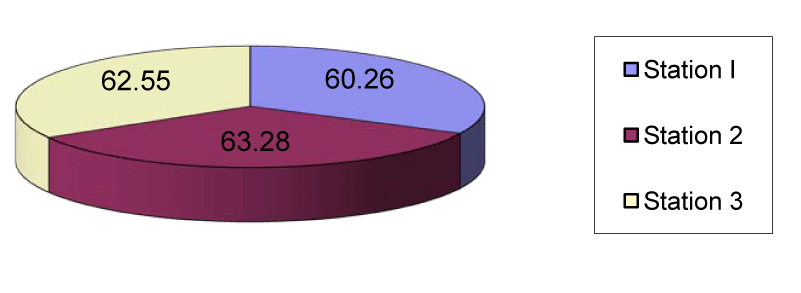
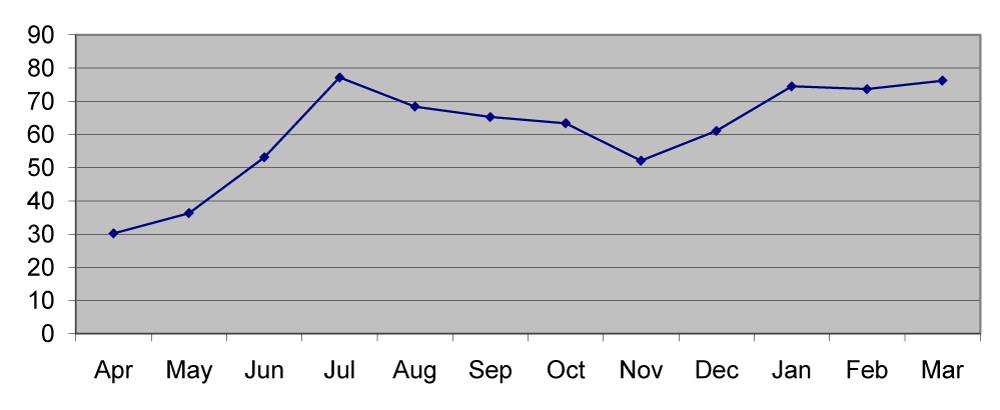
In station 1 the mean nickel was 60.26µg/g which ranged between 54µg/g in August and 70.5µg/g in April. In station 2 the mean nickel was 63.28µg/g which ranged from 54.6µg/g in August to 80.2µg/g in April. In station 3 the mean nickel was 62.55µg/g that ranged from 55.2µg/g in September to 70.7µg/g in April (Figures 4,9).
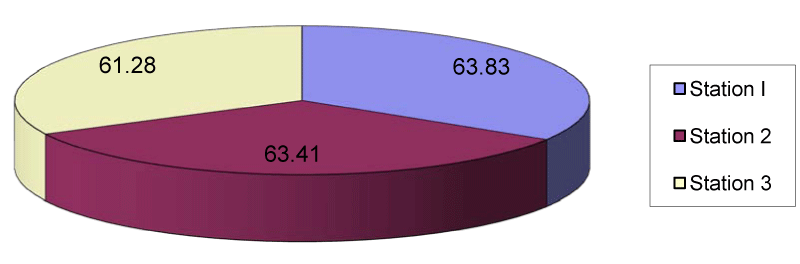
The zinc content of station 1 ranged between 51.7µg/g in July and 85.7µg/g in April with the mean value of 63.83µg/g. In station 2 the mean zinc was 63.41µg/g which ranged from 50.1µg/g in July to 83.7µg/g in April. In station 3 the mean zinc content was 61.28µg/g which ranged from 55.2µg/g in July to 78.6µg/g in April (Figures 5,10). Figure 11 depicted the entire view of the trace metals analyzed and showed the level of each heavy metal.
The differences in metal deposition in sediments were explained on the basis of textural features of the sediment. The finer particles contain more metal than the coarse particles [6-8]. A biologically important and widely distributed metal occurring in low concentrations is copper [9]. In the present study low concentration of copper was due to the lack of copper discharging industries in the surrounding area. The station wise copper distribution showed a clear pattern of seasonal distribution [8]. In general, monsoon season showed the lowest and the pre monsoon with highest concentration. However in the present study highest concentration in post monsoon (Figure 6,7) reported more copper during estuarine mixing and the seasonal variation depends on the location of the station.
The periodical decline of copper may be due to the remobilization from shelf sediments and subsequent diffusion into the overlying waters and release of copper from organisms during degradation decay and their involvement in biogeochemical cycles [10]. Lead is a toxic element and its increased concentration is due to the discharge of industrial wastes. In the present study irregular pattern of distribution was noticed in different seasons (Figure 7). External input of lead in the estuary resulted in the increase of their particulate and dissolved concentrations [11]. Grace [6] stated that the increase in lead may be due to the high amount of metal carried into the estuary through river discharge during heavy rain.
Soil manganese acts as a mineralizing agent for organic matter productivity [12]. The minimum concentration was recorded in pre monsoon and the maximum concentration in monsoon and post monsoon seasons [Figure 8]. This may be due to the heavy runoff and absorption during monsoon months [13]. Large inputs of dissolved manganese were attributed to reductive solubility and diffusion from the sediment water interface. Excess particulate manganese concentrations in the sediments were due to the oxidation of dissolved manganese [14]. The particulate metal concentrations of nickel were inversely related to salinity although the relationship was less pronounced at times of high fluvial inputs and increased tidal amplitude [15]. A regular pattern of seasonal distribution was noticed in nickel distribution. Maximum concentration in pre-monsoon was due to adsorption and less concentration in monsoon was due to leaching and desorption [16,17] from the sediments (Figure 9).
High concentrations of zinc were noticed during premonsoon due to the deposition of zinc to adsorption. Low concentration was seen during monsoon months by leaching from the sediments [Figure 10]. Moreover flocculation and coagulation of the sediments lead to increased concentration since phytoplankton is known to concentrate large amounts of Zn; it is possible that diatoms can play an important role in biogeochemical cycling [16,18-20] suggested that higher levels of zinc may be due to more discharge of zinc containing fertilizers.
Due to the constant turbulent nature of water by wave action, near shore environment becomes more oxygenated which in turn increases the deposition of precipitate along with adsorption of different elements [19]. When comparing estuarine and near shore environments the slight reduction of concentration of these elements in the estuarine environment is due to the fact that desorption of heavy metals from the surface because of the development of a reducing condition of the sediment water interface along with a high pH [21]. The distribution of heavy metals observed from the present study reflects the presence of these metals in the waste discharges into the estuary and seasonal fluctuation of the environment.
- Ahmad MKS Islam, S Rahman, MR Haque, Islam MM. Heavy metals in water, sediment and some fishes of buriganga river, Bangladesh. IJER. 2010; 4: 321-332. DOI: 10.22059/IJER.2010.24
- Clarice R Perryman, Jochen Wirsing, Kathryn A Bennett, Owen Brennick, Apryl L Perry, Nicole Williamson, Jessica G Ernakovich. Heavy metals in the Arctic: Distribution and enrichment of five metals in Alaskan soils. PLOS ONE. 2020; 15: e0233297 DOI: https://doi.org/10.1371/journal.pone.0233297
- Gao X Zhou, F Chen, CTA. Pollution status of the Bohai Sea: An overview of the environmental quality assessment related tracemetals. Environ Int. 2014; 62: 12-30. DOI: 10.1016/j.envint.2013.09.019
- Grace BL. Trace metal Concentration in the Sediments of Kadinamkulam Estuary-Southwest coast of India. Pollution Research. 2006; 25: 613-621.
- Grace BL. Concentration of Trace metals in the Sediments of Poonthura backwater-Southwest coast of India. Pollution Research. 2007a; 26: 807-810.
- Grace BL. Trace metal Concentration in the Sediments of three back waters at the Southwest coast of India. Pollution Research. 2007b; 26: 801-805.
- Grace BL. Water and Sediment Quality Assesment of Poonthura Backwater in the Southwest Coast of India. American Journal of Marine Science. 2014. 2: 43-46. DOI: 10.12691/marine-2-2-3
- Grace BL. Textural Features-Indicators of Pollution. J Environ Anal Toxicol. 2017; 7: 505. DOI: 10.4172/2161-0525.1000505
- Huang L, Pu X, Pan JF, Wang B. Heavy metal pollution status in surface sediments of Swan Lake lagoon and Rongcheng Bayin the northern Yellow Sea. Chemosphere. 2013; 93: 1957-1964. DOI: https://doi.org/10.1016/j.chemosphere.2013.06.080
- Kanat G, Ikizoglu B, Erguven GO, Akgun B. Determination of Pollution and Heavy Metal Fractions in Golden Horn Sediment Sludge (Istanbul, Turkey). Pol J Environ Stud. 2018; 27: 2605-2611. DOI: DOI: https://doi.org/10.15244/pjoes/80805
- Xuming Kang, Jinming Song, Huamao Yuan, Liqin Duan, Xuegang Li, Ning Li, et al. Speciation of heavy metals in different grain sizes of Jiaozhou Bay sediments: Bioavailability, ecological risk assessment and source analysis on a centennial timescale. Ecotoxicol Environ Sust. 2017; 143: 296-306. DOI: 10.1016/j.ecoenv.2017.05.036
- Liu B, Zhang W, Chi G. Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China. Minerals.2019 9: 111. DOI: https://doi.org/10.3390/min9020111
- MacDonald, DD Ingersoll, Berger TA. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology. 2000; 39: 20-31. DOI: https://doi.org/10.1007/s002440010075
- Mocanu R, Cucu Man S, Steinnes E. Heavy Metals Pollution: An Everlasting Problem. In: Environmental Simulation Chambers: Application to Atmospheric Chemical Processes,Barnes I, Rudzinski K (Eds), Springer, Netherlands. 2006; DOI: 10.1007/1-4020-4232-9
- Nour HE, El-Sorogy AS. Heavy metals contamination in seawater, sediments and seashells of the Gulf of Suez, Egypt. Environ Earth Sci.2020; 79: 274. DOI: https://doi.org/10.1007/s12665-020-08999-0
- Paiu M, Iancu OG, Breaban IG. Geochemical distribution of trace elements in an abandoned waste mine dump from Giumalau Mountains, Romania, Environmental Engineering and Management Journal.2017; 16: 847-857.
- Pan J, Pan JF, Wang M. Trace elements distribution and eco-logical risk assessment of seawater and sediments from DingziBay, Shandong Peninsula, North China, Mar Pollut Bull. 2014; 89: 427-434. DOI: 10.1016/j.marpolbul.2014.10.022
- Baoxiao Qu, Jinming Song, Huamao Yuan, Xuegang Li, Ning Li, Liqin Duan. Intensive anthropogenic activities had affected Daya Bay in South China Sea since the 1980s: Evidence from heavy metal contaminations. Mar Pollut Bull. 2018;135: 318-333. DOI: 10.1016/j.marpolbul.2018.07.011
- Tankere SP, Statham PJ, Prince NB. Biogeochemical cycling of Mn and Fe in an area affected by eutrophication; The Adriatic Sea. Estuar Coast Shelf Sci. 2000; 51: 491-506. DOI: https://doi.org/10.1006/ecss.2000.0688
- Vignati DAL, Secrieru D, Bogatova YI, Domnik J, Cereghino R, Berlinsky NA, Oaie G, et al. Trace element contamination in the arms of the Danube Delta (Romania/Ukraine): Current state of knowledge and future needs. Journal of Environmental Management.2013; 125: 169-178. DOI: 10.1016/j.jenvman.2013.04.007
- Wei J, Duan M, Li Y, Amechi S Nwankwegu, Yong Ji, Jie Zhang. Concentration and pollution assessment of heavy metals within surface sediments of the Raohe Basin, China. Sci Rep. 2019; 9: 13100. https://www.nature.com/articles/s41598-019-49724-7
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.