2025 June 30;6(6):815-828. doi: 10.37871/jbres2134.
Trans - Resveratrol as a Food Ingredient Safety Assessment
Díaz López Cristina*
- Resveratrol
- Dietary Supplements
- Food Security
Abstract
Resveratrol is a polyphenolic compound naturally found in various plants, notably in red grapes, and initially isolated from the roots of white hellebore. Due to its potential health-promoting properties, it has gained increasing attention in the fields of nutrition, cosmetics, and food technology. Its growing popularity as a dietary supplement has highlighted the need for a comprehensive safety assessment to ensure proper regulatory oversight.
This narrative review evaluates the safety of Trans-resveratrol as a food ingredient by addressing the four key stages of risk assessment: Hazard identification, hazard characterization, exposure assessment, and risk characterization. A literature search was conducted using PubMed and ScienceDirect, focusing on studies that examine bioavailability and toxicity in vitro, in animal models and in human clinical trials. Reports from food safety agencies such as AESAN and EFSA, as well as European regulatory documents, were also consulted.
Current scientific evidence suggests that Trans-resveratrol exerts beneficial effects-including antioxidant, anti-inflammatory, antiestrogenic, and antibacterial properties-with relatively low toxicity. However, its pharmacokinetics and bioavailability are significantly influenced by factors such as dose, gut microbiota, and food matrix, resulting in high interindividual variability. Regulatory authorities recommend a maximum supplemental dose of 150 mg/day. Although it is also present in food, dietary intake contributes minimally to overall exposure.
Despite promising results, concerns remain regarding its long-term safety. Adverse effects—primarily gastrointestinal—have been reported at high doses. Therefore, generalized high-dose consumption is not currently supported by sufficient evidence. Accurate labeling and adherence to regulatory limits are essential for consumer safety. Further research is needed to better understand Trans-resveratrol’s metabolism, safety, and therapeutic potential in humans, particularly in the context of long-term use.
Abbreviations
AESAN: Spanish Agency for Food Safety and Nutrition; APTT: Activated Partial Thromboplastin Time; APP: Amyloid Precursor Protein; BMD: Benchmark Dose; CTCAE: Common Terminology Criteria for Adverse Events; COX: Cyclooxygenase; CYP450: Cytochrome P450; DAFNE: The European Food Data Network Project; EFSA: European Food Safety Authority; ENIDE: The Spanish National Survey of Dietary Intake; EPIC: European Prospective; Investigation into Cancer and Nutrition; EU: European Union; FAO: Organization of the United Nations; GLP: Good Laboratory Practices; GST: Glutation S- Transferase; Hb: Hemoglobin; IL – 17: Interleukin-17; MOE: Margin of Exposure; MRP2: Multidrug Resistance-Associated Protein 2; NOAEL: No Adverse Effect Level; NSAIDS: Non-Steroidal Anti-Inflammatory Drugs; NF- κB: Nuclear Factor Kappa B; OAT1/OAT3: Organic Anion Transporter 1/3; OECD: Organisation for Economic Co-operation and Development; ROS: Reactive Oxygen Species; RWD: Red Blood Cell Distribution Width; TNF-α: Tumor Necrosis Factor α; UGT 1A1: UDP - glucuronylTransferase 1A1; UVC: Ultraviolet Light Type C
Introduction
Historical perspectives
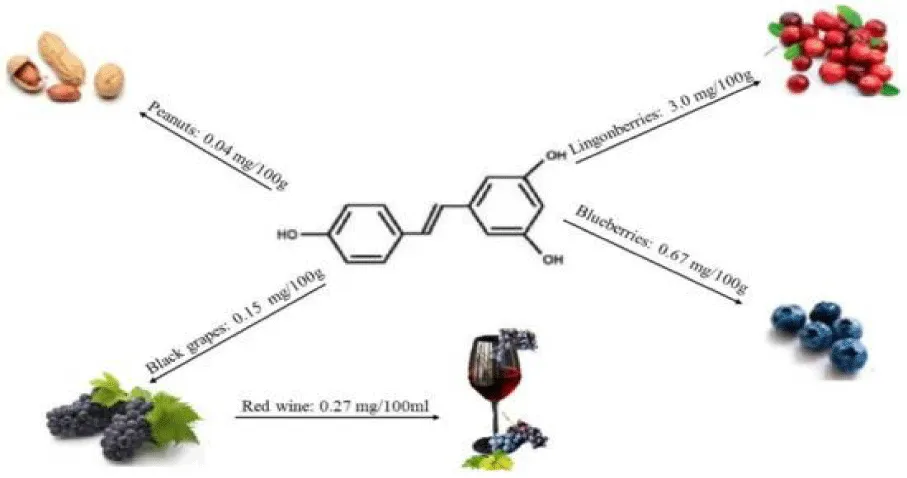
Resveratrol (Trans -3,5,4′-trihydroxystilbene) is a compound of the polyphenol family first isolated in 1939 by Takaoka from the roots of the white hellebore (Veratrum grandiflorum). Furthermore, it belongs to a group of substances known as phytoalexins, which are low molecular weight secondary metabolites produced by plants as a defensive response to microbial damage, fungal infection or abiotic stress [1]. Since its discovery, this compound has aroused great scientific interest and has been isolated from more than 70 species of the plant kingdom in response to stressful situations such as ultraviolet radiation or fungal infections [2]. The best-known sources of this polyphenol are red grapes (Vitis vinifera) and muscatel grapes (Vitis rotundifolia), and therefore, it can be found in derivatives of these foods, such as wines and juices. Even so, the highest concentration of resveratrol has been isolated from the skin of these fresh grapes. Other food sources where this compound can be found in abundance are peanuts and their derivatives (oil and butter), blackberries and their derivatives (jams and juices), blueberries, cocoa or pistachios [3].
The first claims about the beneficial properties of resveratrol arose in the early 1990s, when it was discovered that moderate red wine intake in the French population was associated with a lower incidence of heart disease and obesity, a phenomenon known as the "French paradox." Today, this famous finding is believed to be the result of multiple factors, but after several decades of research, numerous studies associate resveratrol with antioxidant, anticancer, antidiabetic, estrogenic, vasodilatory, anti-inflammatory, and neuroprotective properties, among others [4]. However, resveratrol remains a major challenge for the pharmaceutical industry due to its low solubility and bioavailability, as well as its adverse effects. Classification, physical-chemical properties and uses.
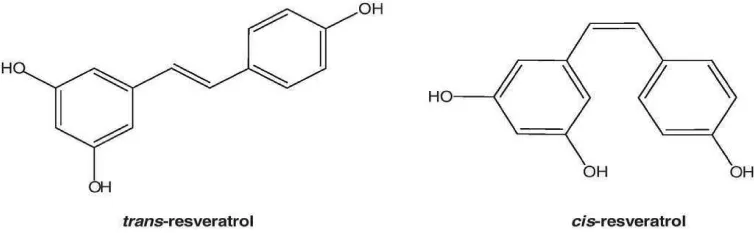
This phytochemical belongs to the stilbenes group, a type of polyphenol. Its chemical structure is characterized by two phenolic rings joined by an ethylene bridge. Certain studies have emphasized the structure-function relationship of this compound, from which its multiple mechanisms of action in living organisms derive. Resveratrol is, therefore, a molecule that can undergo numerous chemical reactions due to the presence of hydroxyl groups, the benzene ring, and the C-C double bond in its structure [5]. Naturally, resveratrol coexists in its two isomeric forms; cis and Trans, the transform being the predominant one and from which the most potent therapeutic benefits derive due to the lower steric hindrance of its side chains (Figure 1) [2]. Isomerization occurs when Trans - resveratrol is at a pH higher than 11 or by UV radiation at wavelengths of 260 nm. The trans isomer represents the predominant and most stable natural form. Furthermore, its bioavailability is relatively higher compared to the cis form (Figure 2) [5].
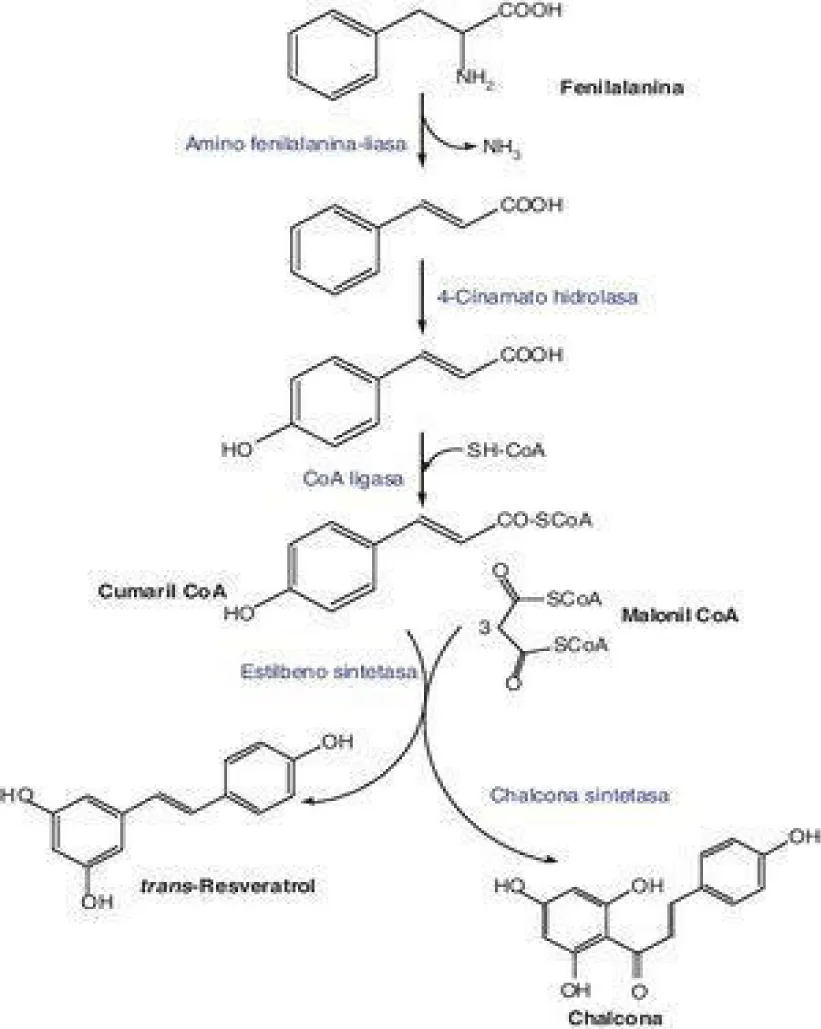
Trans - resveratrol biosynthesis pathway (Figure 3) occurs through the shikimate pathway, also known as the phenylpropanoid pathway. This compound is produced de novo by plants in plastid organelles. This pathway is initiated from phenylalanine and takes place through the condensation of a coumaryl-CoA molecule with 3 malonyl-CoA molecules. This reaction is catalyzed by resveratrol synthetase, an enzyme that belongs to the Stilbene Synthetase (STS) family. Resveratrol synthesis regularly decreases with the ripening of fruits such as grapes, due to a lower induction of STS gene expression [6]. Due to its potential beneficial properties, resveratrol is a bioactive compound that has aroused great interest not only in the field of nutrition (both due to its natural presence in certain foods and its commercialization in the form of supplements), but it is also being used in cosmetics (since it has been shown that formulations enriched with resveratrol can stimulate fibroblast proliferation or protect cells against photoaging) [7] or in the food industry for food packaging, given its antimicrobial nature [5].
Resveratrol metabolism
Resveratrol has an oral absorption rate of 75% in humans, being absorbed at the enterocyte level. Even so, only a small portion of this compound ingested through diet or supplements reaches the bloodstream and body tissues. Furthermore, due to its complex structure and high molecular weight, the proper functioning of resveratrol metabolism in the liver and intestine results in an oral bioavailability of approximately 12% of Trans -resveratrol [1]. Studies in humans and animals reveal that resveratrol absorption occurs by passive diffusion or by Transporters across the apical membrane of enterocytes, rapidly metabolized to glucuronides or sulfates. It is worth noting that glucuronidation of the cis form is 5 to 10 times faster than that of the trans form , which would lead to a lower bioavailability of the cis form . Furthermore, approximately 90% of ingested resveratrol reaches the colon intact, where it undergoes intestinal fermentation processes. Once absorbed through the portal vein, the polyphenolic metabolites produced enter the liver where they are further methylated, glucuronidated, or sulfated. Subsequently, these metabolites will penetrate the systematic circulation and reach the target tissues and cells, where they can exert their potential beneficial actions [3].
Mechanisms of action
Resveratrol is a biomolecule that exhibits multiple and complex mechanisms of action, from which the potential beneficial effects described to date in published studies derive.
Resveratrol as an antioxidant: The antioxidant activity of resveratrol derives from its chemical structure and, consequently, from the arrangement of functional groups within the nucleus. Therefore, the configuration, substitution, and total number of hydroxyl groups substantially influence several mechanisms of its potential antioxidant activity, such as free radical scavenging and the ability to chelate metal ions [8]. As previously discussed, the antioxidant potential of resveratrol has been attributed to its ability to neutralize Reactive Oxygen Species (ROS) and positively regulate the antioxidant defenses of cells. However, depending on the conditions, some studies indicate that Trans- resveratrol, depending on the dose and chemical conditions of the medium, can be oxidized to generate semiquinones and a relatively stable 4′-phenoxyl radical, which ultimately leads to the production of ROS, behaving as a pro-oxidant [4].
Resveratrol and cancer: Numerous studies have shown that resveratrol has antitumor action, making it a likely candidate for the treatment and prevention of several types of cancer. In addition to acting as a chemopreventive, it also has chemotherapeutic properties linked to its anti-inflammatory, antioxidant, pro-apoptosis, and antiproliferative actions. In fact, resveratrol is believed to target certain components of intracellular signaling pathways, such as regulators of cell survival and apoptosis or proinflammatory mediators, by modulating different Transcription factors, kinases, and their corresponding regulators [9].
Resveratrol and cardiovascular protection: Resveratrol is known, among other aspects, for its protective action on blood vessels against inflammation, thrombus formation, and platelet deterioration. At physiological concentrations, resveratrol may act as a potential vasodilator, exerting preventive effects in hypertension and other cardiovascular diseases. Furthermore, animal studies have shown that it protects cardiomyocytes from oxidative stress, cardiac fibrosis, and apoptosis, showing a beneficial effect in heart failure by improving left ventricular function, decreasing cardiac hypertrophy, contractile dysfunction, and remodeling [8]. Furthermore, other studies have shown that resveratrol modulates the Transcription of genes involved in insulin response and fatty acid metabolism, exerting a protective effect against the development of type 2 diabetes mellitus and obesity [9].
Resveratrol as a neuroprotector: Resveratrol may have several neuroprotective functions in various neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and alcohol-induced neurodegenerative disorders. It has been shown that the protective effects of resveratrol are not limited to anti-inflammatory and antioxidant activity but also improves mitochondrial functions and biogenesis through the SIRT1/AMPK/PGC1α pathway, exerting a preventive effect on the harmful effects of oxidative stress. In addition, resveratrol can decrease cholinergic neuroTransmission, promote the clearance of β-amyloid peptides and anti-amyloidogenic cleavage of amyloid precursor protein (APP), and reduce neuronal apoptosis [4].
Resveratrol as an anti-inflammatory: There are numerous studies detailing the effects of resveratrol within inflammatory processes. Although there are numerous mechanisms involved, they are generally mediated by its modulatory actions on the Transcription factor NF-κB, the cytochrome P450 isoenzyme CYP1A1, cyclooxygenases (COX), Fas/FasL-mediated apoptosis, p53, mTOR or cyclins. Furthermore, Trans - resveratrol induces apoptosis of activated T cells and reduces the production of tumor necrosis factor α (TNF-α), interleukin-17 (IL-17) and other proinflammatory molecules [8].
Resveratrol as an antimicrobial: The capacity of resveratrol as an inhibitor of the growth of some pathogenic microorganisms, including bacteria, viruses and fungi, has also been studied. After demonstrating antibacterial action against gram-positive bacteria, antifungal action against Candida albicans and antiviral action against the pseudorabies virus, the mechanisms of action against these microbial agents are not well understood and it is not clear whether it is due to its antioxidant and anti-inflammatory action or for other reasons, which is why they are subject to controversy and it is concluded that further studies would be necessary [4].
Resveratrol as an estrogen analogue: Based on its structural similarity to diethylstilbestrol, a synthetic estrogen, resveratrol may act as a phytoestrogen, exerting agonist actions at estrogen receptors. In the studies conducted, resveratrol was found to act as a superagonist, while in others it produced equal or lesser activation than estradiol, acting as an antagonist at higher concentrations. Therefore, resveratrol exhibits dual dose-dependent behavior, and further studies are needed to clarify these results [4].
Drug interactions
Interaction with cytochrome P450: resveratrol may trigger certain interactions with several members of the cytochrome P450 family, especially when taken in high doses [10]. In vitro inhibition mechanisms of CYP3A4 have been described when Trans - resveratrol was administered in high doses. This finding has also been observed in human studies, mainly when this polyphenol was used in supplement form [11]. Therefore, special caution must be taken with those drugs that are metabolized by this pathway, as it can lead to therapeutic failure [4].
Interaction with Transporters: resveratrol has been shown to be a potent inhibitor of P-glycoprotein, Multidrug Resistance-associated Protein 2 (MRP2), and organic anion transporter 1/3 (OAT1/OAT3) [12]. However, these interactions are not yet fully understood, and further studies are needed [4]. Studies indicate that resveratrol is involved in platelet aggregation processes in vitro [13,14]. It is believed that high intake of resveratrol in the form of supplements may increase the risk of both bruising and bleeding when taken with anticoagulant medications, drugs with antiplatelet action and even Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Given its rapid absorption, low bioavailability and aqueous solubility, and potential adverse effects and drug interactions, the application of resveratrol as an active ingredient remains a challenge for the pharmaceutical industry today[4].
Toxicological information
There are currently numerous studies that demonstrate the beneficial properties of resveratrol. However, all the mechanisms described above seem to indicate that Trans -resveratrol has multiple positive effects and these have been investigated in both animal and human studies. Even so, other published reviews do not precisely attribute potential benefits to this compound and point out that the possible harmful effects that it could trigger depend on the matrix where the polyphenol is contained (food or supplement), doses used, exposure time and age at which the intake of this molecule is established [2,4].
Studies in animal models, cell cultures and culture media
The great interest that Trans- resveratrol has aroused in the scientific community due to its multiple benefits has led to numerous studies conducted in cell cultures and animals to test these positive effects. Table 1 lists some of the studies that have shown that this compound can have dual actions depending on the dose. Particularly noteworthy is its action as a pro-oxidant agent when administered at high doses [4,15].
| Table 1: Adverse effects derived from the use of trans-resveratrol in cell cultures, modified media and animals. | ||||
| Type of study | Duration | Dose | Observed adverse effects | Authors |
| Cell culture with and without astrocytes | 2.5 hours | 25 μM | Accumulation of H202 in the absence of astrocytes | [29] |
| Culture of human tumor cells | 72 hours | 0.1 to 100.0 μM/ml | Low doses of resveratrol (0.1–1 μM/ml) increased cell proliferation. Higher doses (>1 μM/ml) induced apoptosis. | [30] |
| Animal models in mice | 4 weeks | 300, 1000 and 3000 mg/kg/day | Nephrotoxicity, weight loss and food intake in mice at doses of 3000 mg | [24] |
| Animal models in mice | 4 months | 1800 mg/day | Death | [31] |
| Animal models in rabbits with hypercholesterolemia | 60 days | 1 mg/kg body weight/day | Development of atherosclerosis | [32] |
| Cell culture. HT-29 colorectal adenocarcinoma cells | 96 h | 1 to 100.0 μM/l | Lower doses (1 and 10 μmol/L) → increase in cell number. Doses of 50 or 100 μmol/L → reduction in cell number and increase in the percentage of apoptotic or necrotic cells. | [33] |
| Abbreviations:H202: Hydrogen Peroxide | ||||
Toxicity data in humans
It has been shown that the results derived from the administration of Trans -resveratrol in humans are influenced by numerous factors. These include the food matrix containing it (whether it is ingested through food), the dose, the frequency of administration, concomitant use with certain medications, preexisting conditions, and the feeding/fasting conditions of the subjects. Table 2 compiles several studies where, in some cases, certain adverse effects derived from the administration of Trans- resveratrol were observed. It should be noted that in most cases, these are mild gastrointestinal problems and generally occur when administered at high doses. Other studies describe drug interaction phenomena with certain groups of drugs [4,15].
| Table 2: Adverse effects resulting from the administration of trans-resveratrol, human studies. | |||||
| Characteristics of the subjects | Dose | Number of subjects | Duration of follow-up | Observed adverse effects | Authors |
| Healthy volunteers | 0.5, 1, 2.5, 5 grams/day | 40 | 29 days | Doses of 2.5 and 5 grams/day caused mild to moderate gastrointestinal symptoms. | [34] |
| Healthy volunteers | 1 gram/day | 42 | 4 weeks | Drug interactions due to inhibition of various proteins of the CYP450 family. Mild gastrointestinal symptoms | [11] |
| Patients with colorectal cancer and liver metastasis | 5 grams/day | 7 | 14 days | Gastrointestinal problems | [35] |
| Healthy subjects | 2 grams twice a day | 8 | 4 days | Diarrhea | [36] |
| Healthy obese men | 150 mg once a day | 24 | 4 weeks | Gastrointestinal problems | [37] |
| Healthy adults | 25, 50, 100 or 150 mg, six times a day | 10 | 2 days | Mild gastrointestinal problems | [38] |
Unfortunately, given the disparate results obtained, further short- and long-term human studies are needed to accurately define the therapeutic or toxic effects of the suggested doses of Trans -resveratrol in the different studies. Furthermore, the gaps in dosage are even more evident when it comes to Trans- resveratrol metabolites, whose potential effects are unknown given their low bioavailability.
Evaluation of the safety of Trans - resveratrol
Problem statement
Food supplements are foods intended to complement the normal diet and consist of concentrated sources of nutrients (vitamins and minerals) or other substances that have a nutritional or physiological effect, in simple or combined form [16]. The provision of a concentrated amount of nutrients or other substances may pose a risk of overconsumption by the population consuming them. Furthermore, in the case of pregnant or lactating women, children, the elderly, and the sick, the use of food supplements should only be undertaken if justified, since the safety assessment for their use refers to adults with a normal physiological status [16]. One of the most fascinating aspects of resveratrol for its future development as a promising drug is that it does not appear to have debilitating or toxic side effects. A wide range of resveratrol doses have been used in various in vivo and in vitro studies. However, determining the most appropriate dose and route of administration is imperative. Furthermore, resveratrol has been documented to induce cell death in tumor tissues with relatively little effect on adjacent normal tissues [4].
A study was conducted to analyze the potential nephrotoxic effects of resveratrol. Rats were orally administered 3000 mg of Trans -resveratrol per kg of body weight for 28 days. Lesions were identified whose pathogenesis could be increased by the concentration of resveratrol (or its potential metabolites) based on renal osmotic concentration gradients, resulting in toxic levels in the renal pelvis. This would result in necrosis, obstruction of the renal tubules, and therefore, dilation of the tubules behind the obstructed region. Indeed, inflammation and hyperplasia of the pelvic epithelium are expected responses to the presence of necrotic tissue. Thus, administration of 1000 or 300 mg of resveratrol per kg of body weight/day did not produce nephrotoxic findings. Resveratrol has been reported to reduce cell growth and induce apoptosis in normal cells, when administered at high doses, confirming its biphasic effects across the spectrum from low to high concentrations [17]. Furthermore, consumption of resveratrol at modest doses results in an increased lifespan in 1-year-old mice. However, when mice consumed higher doses of resveratrol (1800 mg/kg), the animals were shown to die within 3–4 months. Studies on the steady-state pharmacokinetics and tolerability of 2000 mg of Trans -resveratrol, administered twice daily with food, quercetin, and alcohol (ethanol), showed that healthy subjects tolerated Trans -resveratrol well, although diarrhea was frequently observed [4]. Most of the existing studies in the scientific literature on Trans-resveratrol, both in animals and humans, associate the molecule with low toxicity. On the other hand, human studies indicate that long-term consumption of high doses of this substance was associated with adverse effects such as gastrointestinal discomfort, but with little clinical relevance [18].
Trans -resveratrol concentrations used in the scientific literature
Resveratrol does not appear to have any side effects at short-term doses less than 1 gram/day. However, doses of 2.5 g or more per day can produce side effects such as nausea, vomiting, diarrhea, and liver dysfunction in patients with non-alcoholic fatty liver disease. Interestingly, no major side effects were reported in long-term clinical trials. In fact, resveratrol has been found to be safe and well-tolerated at doses up to 5 g/day, either as a single dose or as part of a multi-day dosing schedule. However, it is imperative to mention that these studies were conducted in healthy populations, and this may vary in sick patients. Our understanding of the dose and route dependence of resveratrol is further complicated by the fact that orally administered resveratrol is metabolized by the gut microbiota, making it difficult to determine which effects are due to resveratrol alone or to resveratrol and its metabolites [4].
According to the reports of the Scientific Committee of AESAN published on its website, one of them is based on the relationship between the application of ultraviolet light type C (UVC) for the induction of bioactive compounds in grapes, from which relative information is extracted from various factors that can affect the content and quality of polyphenols [19]. The polyphenol content of fruits, vegetables, and their derivatives (juices, wine, jams, etc.) is affected by various factors. These factors can increase polyphenol content when they induce anabolic enzyme activity; they can also promote associations and lead to new compounds; or they can cause a decrease when the predominant activity is catabolic (oxidative enzymes), or when spontaneous degradation occurs related to temperature, pH, etc. [19].
The use of UVC light is a well-studied procedure for inducing the production of stilbenes in grapes and preserving their quality. In this sense, the application of UVC light as a technology for the induction of bioactive compounds in harvested grapes can increase the content of phytoalexins and stilbenes, mainly resveratrol [19]. No studies have documented the presence of other metabolites in grapes as a result of UVC treatment, with the exception of stilbenes. Likewise, no scientific evidence has been documented to support the presence of adverse effects in animals following the use of resveratrol [19]. On January 22, 2021, Implementing Regulation (EU) 2021/51 was published, a document authorizing changes to the conditions for the use of Trans -resveratrol as a novel food. According to this regulation, the marketing of this compound is permitted in the form of tablets or capsules intended solely for the adult population, with a maximum dose of 150 mg per day. Table 3 shows the specifications that this compound must meet in order to be marketed within the framework of food supplements [20].
| Table 3: Commission implementing regulation (EU) 2021/51 of 22 January 2021. Specifications for trans-resveratrol as a novel food [20]. | |
| Chemical name : 5-[(E)-2-(4-hydroxyphenyl)ethyl]benzene-1,3-diol Chemical formula: C 14 H 12 O 3 Molecular weight 228.25 Da Description: Trans - resveratrol consists of whitish to beige crystals. | |
| Purity | No less than 99% |
| Total by-products (related substances) | No more than 0.5% |
| Any individual related substance | No more than 0.1% |
| Lead | No more than 1 ppm |
| Mercury | No more than 0.1 ppm |
| Cadmium | No more than 0.5 ppm |
| Arsenic | No more than 1 ppm |
| Loss due to desiccation | No more than 0.5% |
| Sulfated ash | No more than 0.1% |
| Diisopropylamine | No more than 50 mg/kg |
Studies indicate that the most important source of resveratrol in Spanish subjects belonging to the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort was wine (98.4%), followed by grapes and grape juices (1.6%), while peanuts, pistachios and berries contributed less than 0.01%. The highest intake of polyphenols included in the European Food Safety Authority (EFSA) database was 2.93 mg/day consumed by very elderly subjects [18]. Therefore, following various clinical studies, it is considered that resveratrol for human consumption as a new food ingredient is not disadvantageous from a nutritional point of view. Furthermore, it is considered that the data provided do not pose safety problems with respect to the amount ingested by society through food, because it is very difficult to reach the maximum recommended dose [18].
Selection of contaminants or waste of interest
The increasing use of natural products is common among patients taking conventional medications, leading to a potential risk of interactions between these natural products and medications. As previously discussed, resveratrol can interact with several medications. It can cause interactions with several Cytochrome P450 (CYP450) enzymes, especially when taken at high doses. In fact, people taking medications, such as tamoxifen, whose efficacy is highly specific and dependent on CYP enzymes, could be particularly affected. Therefore, caution should be exercised when using supplemental doses of resveratrol for health benefits [4] (Table 4).
| Table 4: Natural sources of trans-resveratrol. Analytical method of determination and concentrations [6]. | |||
| Natural sources | Variety/type | Analytical method of determination | Resveratrol content (unit weight/gram of food) |
| Purple blueberry | Raw blueberry | LC-MS/MS | 49.3 - 140 pmol/g |
| Cocoa | Cocoa powder | HPLC | 1.25–2.27 µg/g |
| Black chocolate | HPLC | 0.25–0.43 µg/g | |
| Milk chocolate | HPLC | 0.05–0.17 µg/g | |
| Cranberry | Raw blueberry | HPLC-DAD | 712.2 ng/g |
| Cranberry juice | HPLC-DAD | 533.4–598.2 ng/g | |
| Grape | Red grape | HPLC | 1,348–15,472 mg/kg |
| White grapes | LC-MS/MS | 0.011–0.030 μg/mg | |
| Juice of grapes | HPLC | 0.05–0.67 mg/l | |
| Red wine | HPLC - DAD | 0.13–0.64 mg/l | |
| White wine | HPLC-DAD | 0.04–0.34 mg/l | |
| Peanuts | Raw peanuts | HPLC-DAD | 0.03–1.92 μg/g |
| Peanut butter | HPLC | 0.148–0.504 μg/g | |
| Pistachio | Raw pistachios | HPLC-DAD | 1.04–1.67 μg/g |
| Abbreviations: HPLC-DAD: High-Performance Liquid Chromatography with Diode Array Detector; LC-MS, Liquid Chromatography-Mass Spectrometry | |||
Few clinical studies have been conducted to determine transporter-mediated interactions between resveratrol and drugs. On the other hand, it is also speculated that higher doses of resveratrol compete with other polyphenols for transporters, reducing both their absorption and potential synergistic effects. Furthermore, the absorption, distribution, renal excretion, and/or hepatic clearance of resveratrol's active ingredients in humans are not as well explored as required for the realistic prediction of resveratrol-drug interactions. Therefore, the modulatory effects of resveratrol on transporter-drug interactions warrant further investigation [4]. Resveratrol has been reported to impair human platelet aggregation in vitro. Presumably, high intakes of resveratrol in supplement form may increase the risk of both bruising and bleeding when taken with anticoagulant medications, antiplatelet medications, and even Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [4].
Hazard characterization
Relationship between exposure or dose and adverse health effects.
Resveratrol is rapidly absorbed and metabolized after oral ingestion. This is why its efficacy in mammals in vivo is questionable. Its oral bioavailability is very low, and its plasma half-life is 8-14 minutes. This is due to its extensive interaction with Phase I and II metabolic enzymes. In a study conducted in 42 healthy individuals, in whom the basal activity of Phase I and Phase II metabolic enzymes was measured, it was observed that resveratrol at a dose of 1 g per day for 1 month changes the phenotypic expression of these enzymes as follows: it induces CYP1A2 activity, inhibits CYP3A4, CYP2D6, CYP2C9 activity, and has minimal effect on the activity of Glutathione S - Transferase (GST) and UDP - glucuronyltransferase 1A1 (UGT1A1). However, it was observed that if individuals had low baseline activity of these latter two enzymes, an induction did occur, which likely represents a return to normal activity levels. These effects are important to consider, since, for example, CYP3A4 inhibition can result in increased toxicity of drugs metabolized by this isoenzyme, including immunosuppressive drugs, protease inhibitors, statins, and chemotherapy drugs. Similarly, inhibition of CYP2C9, which is responsible for metabolizing many Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), oral anticoagulants, and oral hypoglycemic agents, can lead to increased toxicity [21].
Inhibition of CYP2D6, which is responsible for metabolizing tamoxifen to its most potent metabolite endoxifen, may result in a higher rate of breast cancer recurrence [21]. In this same study, it was observed that the maximum plasma concentration of resveratrol was reached approximately 1 hour after oral intake and was 72.7 ng/mL. Plasma concentrations of resveratrol metabolites were significantly higher than those of resveratrol [21]. Administration of this dose of resveratrol for 4 weeks was generally well tolerated by the subjects, and the main adverse effects observed during the study that are probably attributed to resveratrol due to temporal proximity are all mild to moderate according to the CTCAE (Common Terminology Criteria for Adverse Events) version 4.0 classification, and include diarrhea, heartburn, increased appetite, mood swings, menstrual disorders, vivid dreams, hot flashes, insomnia, decreased appetite, flatulence, nausea, abdominal pain, and dysuria. No alterations in blood count and blood chemistry were observed [21].
All polyphenols exhibit tissue-specific characteristics, and the production of their active metabolites depends on liver function and can therefore be affected by factors such as age. The complex kinetics of resveratrol, in addition to the wide range of doses used in animal studies, make it difficult to establish the recommended dose for human use. To date, the doses studied have not demonstrated serious long-term adverse effects in animals; however, further studies are required [21]. Resveratrol is a polyphenol present in the human diet and has a wide variety of potential therapeutic properties. However, it is not possible to absorb the recommended therapeutic doses of resveratrol by drinking wine or through dietary sources. Furthermore, to date, most of the beneficial effects have only been established in preclinical models. One of the greatest challenges in resveratrol research is determining whether the observed health benefits are Transferable to humans. Therefore, clinical trials are urgently needed to determine the effective dosage regimen for the therapy of specific diseases [21].
These trials should be conducted with well-standardized resveratrol formulations to allow for comparison of the results obtained. Because previous human studies have consistently shown that resveratrol bioavailability after oral intake is quite low, the development of resveratrol formulations with improved pharmacological properties remains a challenging task. Furthermore, structural optimization and the development of new galenic resveratrol formulations, such as resveratrol-encapsulated nanoparticles, should help physiologically increase resveratrol activity and overall bioavailability, reduce the required dose, prevent unwanted side effects during therapy, and direct resveratrol activity to specific targets [21]. Resveratrol-enriched supplements could be suitable for daily intake of therapeutically relevant doses (currently assumed to be 1 g) that cannot be obtained from conventional foods or beverages. Furthermore, resveratrol could be used as a supplement to improve the growth or quality of crops grown in different agricultural and aquaculture environments, thus developing health-promoting effects [22].
Toxicological evaluation
A 90-day study was conducted with synthetic Trans -resveratrol produced by the applicant using the above process. Groups of 10 Wistar rats of each sex were fed diets containing Trans -resveratrol at concentrations designed to provide an intake of 0, 120, 300, and 750 mg/kg of body weight per day for 90 days. Other groups of five rats of each sex were fed control or high-dose (750 mg) diets for 90 days, followed by at least 4 weeks on the control diet (recovery animals). The study was conducted in accordance with Good Laboratory Practice (GLP) and was based on Organisation for Economic Co-operation and Development (OECD) Guideline 408. Dietary concentrations were adjusted weekly. The doses achieved were lower than expected (males 0, 118, 285, 668 mg/kg, females 0, 123, 300, 729 mg/kg body weight per day). The level of animal exposure was assessed from blood samples taken from rats of each sex per group at weeks 4, 8, 13 and 15 of recovery. Animals were housed in groups of 5 and examined for clinical signs at least once a day [23].
Observations included a functional observation battery, ophthalmoscopy, body weight, food consumption, water consumption, and estrous cycle determination. Blood was drawn from all animals before necropsy, and all hematological parameters were examined. Urine was collected from all animals overnight and examined for a range of endpoints, and the sediment was examined [18]. Histopathological examination was limited to control and high-dose animals, along with any abnormal tissue from intermediate groups. Measurement of total and free resveratrol in serum confirmed a dose-related level of both that remained constant from 4 weeks to 13 weeks of treatment. One high-dose male died on day 30, but the cause of death could not be established. There were no treatment-related differences in clinical signs, functional observations, ophthalmoscopy, urinalysis, semen analysis, or estrous cycle. Body weight and weight gain of high-dose animals were lower than those of controls throughout the study for males and from week 4 for females and remained lower during the recovery period. Reductions were approximately 10% but were not always statistically significant [18].
The body weight gain in the intermediate group (300 mg/kg bw/day) was slightly lower (approximately 3%), although not statistically significant, than in the controls for both sexes, and the body weight of females in this group was lower from week 4 onwards. Food consumption returned to control levels during the recovery period. No effect was observed on body weight and food consumption in the 120 mg/kg bw/day treatment group. Hematological results showed a statistically significant increase in platelet count, Activated Partial Thromboplastin Time (APTT), and Red blood cell Distribution Width (RDW) test, and a reduction in Hemoglobin (Hb) in males at 300 mg/kg bw/day, but not at the highest dose. There were no comparable differences in females and no dose-related differences in hematological outcomes [18]. Organ weights and relative organ weights were generally similar across all groups, with no evidence of dose-related differences. Microscopic examination of tissues showed no treatment-related differences in the incidence of most histopathological findings. A slight increase in tubular basophilia was observed in the kidneys of high-dose females. This would not normally be considered relevant; however, in light of previous findings with resveratrol, the applicant undertook further investigation. An expert review of renal pathology in all treatment groups was conducted and concluded that there was no evidence of a treatment-related renal effect in this study [24].
The effects on body weight and food intake at the highest dose indicate that the dose tested is close to the maximum tolerated by this route. The study authors argue that the lower weight gain observed at the highest dose represents a lack of palatability. However, lower weight gain was also reported at the highest dose for both sexes and for females at 1,000 mg/kg bw per day in a 28-day study in 20 male- to-male Charles River rats given 0, 300, 1,000, and 3,000 mg/kg bw of Trans - resveratrol (99.7% purity) by gavage [24]. In the 3,000 mg/kg bw dose group, there was also a significantly increased incidence and severity of nephropathy. Feed consumption was significantly reduced only in the 3,000 mg group. Although the dose at which a reduction in body weight was observed is higher than in the recent 90-day study, it confirms that this is an effect of Trans-resveratrol, even when administered by gavage; therefore, the reduction in weight gain observed in the subchronic rat study cannot be fully explained by lack of palatability. The Scientific Committee therefore considers that the intermediate dose in this subchronic study (300 mg/kg body weight per day), which caused slight reductions in body weight and weight gain, should be the NOAEL for the novel food in this study [18].
Trans- resveratrol were reviewed and a number of studies were summarized that used doses of up to 3,000 mg/kg of body weight per day (28-day studies in rats, dogs, and rabbits), but very little information was given on the experimental design. The authors stated that this formulation of resveratrol had greater bioavailability due to its greater stability and micronization [18].
Toxicity parameters based on reference values.
Chronic toxicity/carcinogenicity studies conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Guidelines 451 and 452 or 453 were not conducted with Trans -resveratrol. Therefore, in response to the concern about the carcinogenicity risk of this food supplement, a 6-month study was conducted in p53-knockout mice [25]. In this study, 99.5% pure synthetic Trans -resveratrol produced by another manufacturer was administered by gavage to groups of 10 male and 10 female animals per dose group (i.e., 1,000 mg/kg, 2,000 mg/kg, and 4,000 mg/kg body weight) for the safety of synthetic Trans -resveratrol daily. Histopathology identified the kidney and urinary bladder as target tissues for resveratrol toxicity. No effects on body weight were observed in this study in either dose group. Despite the limitations of the p53 knockout mouse model for carcinogenicity testing, this study does not indicate a carcinogenic effect [18].
Exposure estimation
Resveratrol is a natural polyphenol present in numerous plants and fruits such as peanuts, blueberries, and, to a greater extent, in grapes and red wine. The best-known sources are red grapes or European grapes (Vitis vinifera) and muscatel grapes (V. rotundifolia). The resveratrol content of different foods can be summarized in the following table [5]. Trans - resveratrol content of red wine ranges from 13 to 64 mg/L. There is no increase in Trans - resveratrol absorption when consumed with alcohol or other phenolic compounds. Accurately estimating dietary exposure to Trans - resveratrol can be challenging. Very few epidemiological studies have estimated daily resveratrol intake using questionnaires on consumption of polyphenol-rich foods such as red wine [6].
The Spanish National Survey of Dietary Intake (ENIDE) quantified in 2011 the average daily consumption of polyphenols in the Spanish diet with the information reflected in table 5 [26]. The European Food Data Network Project (DAFNE), the Food and Agriculture Organization of the United Nations (FAO) Food Balance Sheets (FBS) estimate that the highest average per capita intake of natural Trans - resveratrol among European Union countries is 0.46 mg/day [26].
| Table 5: Average intake per person per day of polyphenols in the foods (by fresh weight) most consumed in the Spanish population [26]. | ||
| Average intake g ml/person/day | Polyphenol intake mg polyphenols/day | |
| Drinks (total) | 357.3 mg/p/day | |
| Came | 35.9 | 118.4 |
| Beer | 86.2 | 24 |
| Coffee | 56.9 | 152 |
| Tea | 30.8 | 51.3 |
| Fruits (total) | 294 mg/p/day | |
| Orange | 34.6 | 96.5 |
| Grape | 3.0 | 36.3 |
| Pear | 18.2 | 83.2 |
| Kiwi | 1.1 | 12.8 |
Risk Characterization
As discussed above, several studies have been conducted to assess toxicity, however, No Adverse Effect Level (NOAEL) of Trans - resveratrol was observed for doses of 200 or 300 mg/kg of body weight per day. The toxicity of the marketed supplement called ResVida® was studied, where an acceptable daily intake was determined to be 450 mg/day and no adverse effects were observed at doses of 750 mg/kg/day for 13 weeks. Reference has been made in the literature to developmental toxicity with Trans - resveratrol and administration to pregnant female rats where, again, no evidence of embryofetal toxicity was observed [18].
A reference dose-response model was developed to calculate the Benchmark Dose (BMD) for Trans - resveratrol based on eight animal experiments, concluding that the reference dose is 344 mg/kg/day in rats. The Margin Of Exposure (MOE) was calculated for both substances as an indicator of whether intake can achieve effective doses. For the ingestion of a 100 ml glass of wine, the average MOE was found to be 4.1 for ethanol and 459.937 for resveratrol. In the best-case scenario for resveratrol (e.g., very high content) the MOE would be at least 111, meaning that 111 glasses of wine should be consumed per day to reach the reference dose [27]. Since the required dose cannot be achieved through food, the pharmaceutical industry has developed alternatives to meet these requirements, since resveratrol does not have a relevant nutritional role in the human diet. When marketing a supplement in capsule or tablet form at a daily dose of 150 mg/day, the target population is adults. Consumption of a maximum supplemental intake of 150 mg/day in supplement form is 50 times higher than the dietary intake [27].
Conclusion
As previously discussed, Trans - resveratrol is a promising molecule due to its potential beneficial effects in the treatment of certain diseases or even as a chemo preventive agent. All the findings collected on this substance were of such relevance that in 2010, a working group on resveratrol came to the following general conclusion: "The published evidence is not strong enough to justify a recommendation for the administration of resveratrol to humans beyond the dose that can be obtained from dietary sources," but "in animals the data are promising in the prevention of several types of cancer, coronary heart disease and diabetes, which clearly indicates the need for clinical trials in humans" [28].
It would be risky to recommend widespread consumption of Trans -resveratrol at the doses stipulated in the scientific literature, even though it presents very promising beneficial effects in numerous pathologies. Although this compound has been shown to possess antioxidant, antiestrogenic, anti-inflammatory, and antibacterial properties in multiple studies, occasional or chronic consumption is not without risk, and many authors point out that further short- and long-term studies are needed. Furthermore, the observed side effects appear to be dependent on multiple factors, generally occur at high doses, and typically cause gastrointestinal discomfort. In this paper, after reviewing animal and human studies, we conclude that the bioavailability and pharmacokinetics of Trans -resveratrol depend on the ingested dose, particle size, gut microbiota, circadian variation, and dietary matrix, and therefore, there is interindividual variability.
Due to the wide variety of commercially available dietary supplements containing Trans -resveratrol, either alone or in combination with other bioactive molecules, it would be necessary to first ensure that the product is correctly labeled. Even so, the authorized dose of this compound in supplement form would be 150 mg/day, as recommended by the relevant authorities. Furthermore, diet could also be a source of exposure to this substance, but the concentrations found in food are much lower than those found in commercially available supplements. On the other hand, certain scientific articles indicate that Trans -resveratrol may interact with certain drugs, so if you decide to consume dietary supplements containing this molecule and you are taking any type of medication or have a cardiovascular or immune system-related condition, it is advisable to consult a specialist physician beforehand.
To date, most of the studies in the scientific literature on Trans -resveratrol in cell cultures, animal models, and clinical trials associate this molecule with potential and multiple beneficial effects, along with low toxicity. However, there are studies in humans and animal models where this compound does not appear to be as beneficial, and may be pro-oxidant or cause gastrointestinal discomfort, but this generally occurs at high doses. Despite numerous studies that point to its potential benefits, further research into this polyphenol and its metabolites is needed to understand its bioavailability, metabolic pathways, and toxicity in humans, in order to establish suitable therapeutic doses and use it in clinical practice.
In addition, future human trials should also study the health benefits of Trans -resveratrol when combined with other supplements or drugs, and even with diet and exercise.
References
- Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules. 2021 Jan 5;26(1):229. doi: 10.3390/molecules26010229. PMID: 33406713; PMCID: PMC7795473.
- Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020 Mar 18;21(6):2084. doi: 10.3390/ijms21062084. PMID: 32197410; PMCID: PMC7139367.
- Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, Gallelli L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human microRNA modulation. Molecules. 2020 Jan 1;25(1):63. doi: 10.3390/molecules25010063. PMID: 31878082; PMCID: PMC6983040.
- Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, et al. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018 Sep 9;6(3):91. doi: 10.3390/biomedicines6030091. PMID: 30205574; PMCID: PMC6164842.
- Tian B, Liu J. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. 2020 Mar;100(4):1392–404. doi: 10.1002/jsfa.10152. PMID: 31756276.
- Riccio BVF, Spósito L, Carvalho GC, Ferrari PC, Chorilli M. Resveratrol isoforms and conjugates: a review from biosynthesis in plants to elimination from the human body. Arch Pharm (Weinheim). 2020 Dec;353(12):e2000146. doi: 10.1002/ardp.202000146. PMID: 32974977.
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019 Apr;21(2):84–90. doi: 10.1080/14764172.2018.1469767. PMID: 29723052.
- Repossi G, Das UN, Eynard AR. Molecular basis of the beneficial actions of resveratrol. Arch Med Res. 2020 Feb;51(2):105–14. doi: 10.1016/j.arcmed.2020.03.002. PMID: 32204999.
- Zhang LX, Li CX, Kakar MU, Khan MS, Wu PF, Amir RM, et al. Resveratrol (RV): a pharmacological review and call for further research. Biomed Pharmacother. 2021 Mar;143:112164. doi: 10.1016/j.biopha.2021.112164. PMID: 33388241.
- Detampel P, Beck M, Krähenbühl S, Huwyler J. Drug interaction potential of resveratrol. Drug Metab Rev. 2012 Sep;44(3):253–65. doi: 10.3109/03602532.2012.712598. PMID: 22901046.
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010 Sep;3(9):1168–75. doi: 10.1158/1940-6207.CAPR-10-0054. PMID: 20713696.
- Zha W. Transporter-mediated natural product-drug interactions for the treatment of cardiovascular diseases. J Food Drug Anal. 2018 Apr;26(2 Suppl):S32–44. doi: 10.1016/j.jfda.2018.01.001. PMID: 29703552; PMCID: PMC5937027.
- Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17(1):1–3. PMID: 7772694.
- Shen MY, Hsiao G, Liu CL, Fong TH, Lin KH, Chou DS, et al. Inhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol. 2007 May;139(3):475–85. doi: 10.1111/j.1365-2141.2007.06840.x. PMID: 17854384.
- Novelle MG, Wahl D, Diéguez C, Bernier M, de Cabo R. Resveratrol supplementation: where are we now and where should we go? Aging Res Rev. 2015 Mar;21:1–15. doi: 10.1016/j.arr.2014.10.004. PMID: 25445574; PMCID: PMC4303791.
- European Union. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off J Eur Communities. 2002 Jul 12;L183:51–7.
- Ferry-Dumazet H, Garnier O, Matsuda MM, Vercauteren J, Belloc F, Billiard C, et al. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002 Aug;23(8):1327–33. doi: 10.1093/carcin/23.8.1327. PMID: 12151356.
- Safety of synthetic trans-resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016;14(1):4368. doi: 10.2903/j.efsa.2016.4368.
- Informe del comité científico de la AESAN sobre la aplicación de luz UVC para la introducción de compuestos bioactivos en uvas. AESAN-2007-009. 2007;15–30.
- Commission Implementing regulation (EU) 2021/51 of 22 January 2021 authorizing a change of the conditions of use of the novel food 'trans-resveratrol' under Regulation (EU) 2015/2283. Off J Eur Communities. 2021 Jan 22;L23:10–12.
- Masís BA, Vega SM, Sánchez VJP. El resveratrol y sus posibles usos como nueva terapia farmacológica. Rev Med Costa Rica Centroam. 2013;70(608):679-84.
- Weiskirchen S, Weiskirchen R. Resveratrol: how much wine do you have to drink to stay healthy? Adv Nutr. 2016 Jul;7(4):706–18. doi: 10.3945/an.115.011627. PMID: 27422505; PMCID: PMC4942868.
- Edwards J, Stannard D, Burger D, Hofmann P. Resveratrol embryo-fetal study in the CD rat by dietary administration. DSM Nutr Products. 2007;25:210.
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004 Dec;82(2):614–9. doi: 10.1093/toxsci/kfh263. PMID: 15329443.
- Horn TL, Cwik MJ, Morrissey RL, Kapetanovic I, Crowell JA, Booth TD, et al. Oncogenicity evaluation of resveratrol in p53(+/-) mice. Food Chem Toxicol. 2007 Jan;45(1):55–63. doi: 10.1016/j.fct.2006.07.015. PMID: 16965847; PMCID: PMC1855246.
- Navarro I, Periago MJ, García FJ. Estimación de la ingesta diaria de compuestos fenólicos en la población española. Rev Esp Nutr Hum Diet. 2017;21(4):320-6. doi: 10.14306/renhyd.21.4.357.
- Lachenmeier DW, Godelmann R, Witt B, Riedel K, Rehm J. Can resveratrol in wine protect against the carcinogenicity of ethanol? A probabilistic dose-response assessment. Int J Cancer. 2014 Jan 1;134(1):144–53. doi: 10.1002/ijc.28229. PMID: 23784940.
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, et al. What is new for resveratrol? Is a new set of recommendations necessary? Ann N Y Acad Sci. 2013 Apr;1290(1):1–11. doi: 10.1111/nyas.12160. PMID: 23855460.
- Erlank H, Elmann A, Kohen R, Kanner J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic Biol Med. 2011 Dec 15;51(12):2319–27. doi: 10.1016/j.freeradbiomed.2011.09.027. PMID: 21964393.
- Szende B, Tyihák E, Király-Véghely Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med. 2000 Jun 30;32(2):88–92. doi: 10.1038/emm.2000.13. PMID: 10901164; PMCID: PMC3055234.
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008 Aug;8(2):157–68. doi: 10.1016/j.cmet.2008.06.011. PMID: 18599363.
- Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996;59(1):PL15–21. doi: 10.1016/0024-3205(96)00260-3. PMID: 8684261.
- San Hipólito-Luengo Á, Alcaide A, Ramos-González M, Cercas E, Vallejo S, Romero A, et al. Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutr Cancer. 2017;69(7):1019–27. doi: 10.1080/01635581.2017.1359324. PMID: 28937798.
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010 Nov 15;70(22):9003–11. doi: 10.1158/0008-5472.CAN-10-2364. PMID: 20935227; PMCID: PMC2982884.
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila). 2011 Sep;4(9):1419–25. doi: 10.1158/1940-6207.CAPR-11-0148. PMID: 21774592; PMCID: PMC3173869.
- La Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, et al. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010 Jul;49(7):449–54. doi: 10.2165/11531820-000000000-00000. PMID: 20545556.
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial. Diabetes. 2013 Apr;62(4):1186–95. doi: 10.2337/db12-0975. PMID: 23349451; PMCID: PMC3609591.
- Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009 May;53 Suppl 1:S7–15. doi: 10.1002/mnfr.200800177. PMID: 19194969.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.