2025 June 30;6(6):792-807. doi: 10.37871/jbres2132.
The Abiwaijiwuli-Mufasili Attenuates Cartilage Damage and Ameliorates Osteoarthritis In vivo and In vitro By Regulating Serpina3-Mediated Wnt/b-Catenin Pathway
Qin Ma#1, Xinyi Liu2, Xiaomin Wang2, Rui Liu2, Jingjing Li3, Jialin Wu2, Zhe Xu2, Peipei Wang3 and Wei Chen1#*
2Xinjiang Huashidan Pharmaceutical Research Co., Ltd., China 835000
3Pharmacy College of Xinjiang Medical University, China 835000
#These authors have contributed equally
- Osteoarthritis
- Aibiwajiwuli-mufasili
- Cartilage rehabilitation
- Serpina3
Abstract
Introduction: Aibiwajiwuli-Mufasili (ABYJ), an ancient classic formula of Uyghur medicine, is used to treat osteoarthritis (OA) in clinical practice in China, yet the underlying mechanism remains obscure. We aimed to propose a novel therapeutic effect of ABYJ on the MIA-induced OA model and ATDC5 cell inflammatory model, and further demonstrated the cartilage renovation of OA through the Serpina3-mediated Wnt/β-catenin pathway.
Methods: In this study, the chemical composition of ABYJ were elucidated using optimized ultrahigh-performance liquid chromatography coupled with quadrupole exactive-orbitrap mass spectrometry (UPLC-Q-Exactive-orbitrap-MS). In an in vivo experiment, rats were randomly divided into 6 groups, the OA model in rats were induced by intraarticular injection with sodium iodoacetate (MIA) solution (60mg·mL-1), behavior of model animals were evaluated by Lequesne MG, and Micro-CT imaging was used for morphological evaluation, Hematoxylin-Eosin (HE) and Safranin O-fast green (SF) staining were used for histology assessment. In addition, 4D Label-free quantification proteomics analysis was used to further explore proteins differentially expressed after different treatment. The expression of Serpina3 and proteins on the Wnt/β-catenin signaling pathway in the rats OA models were determined by Western blot. In vitro, a LPS-induced ATDC5 cells inflammation model was used to validate the possible active ingredients and signaling pathway.
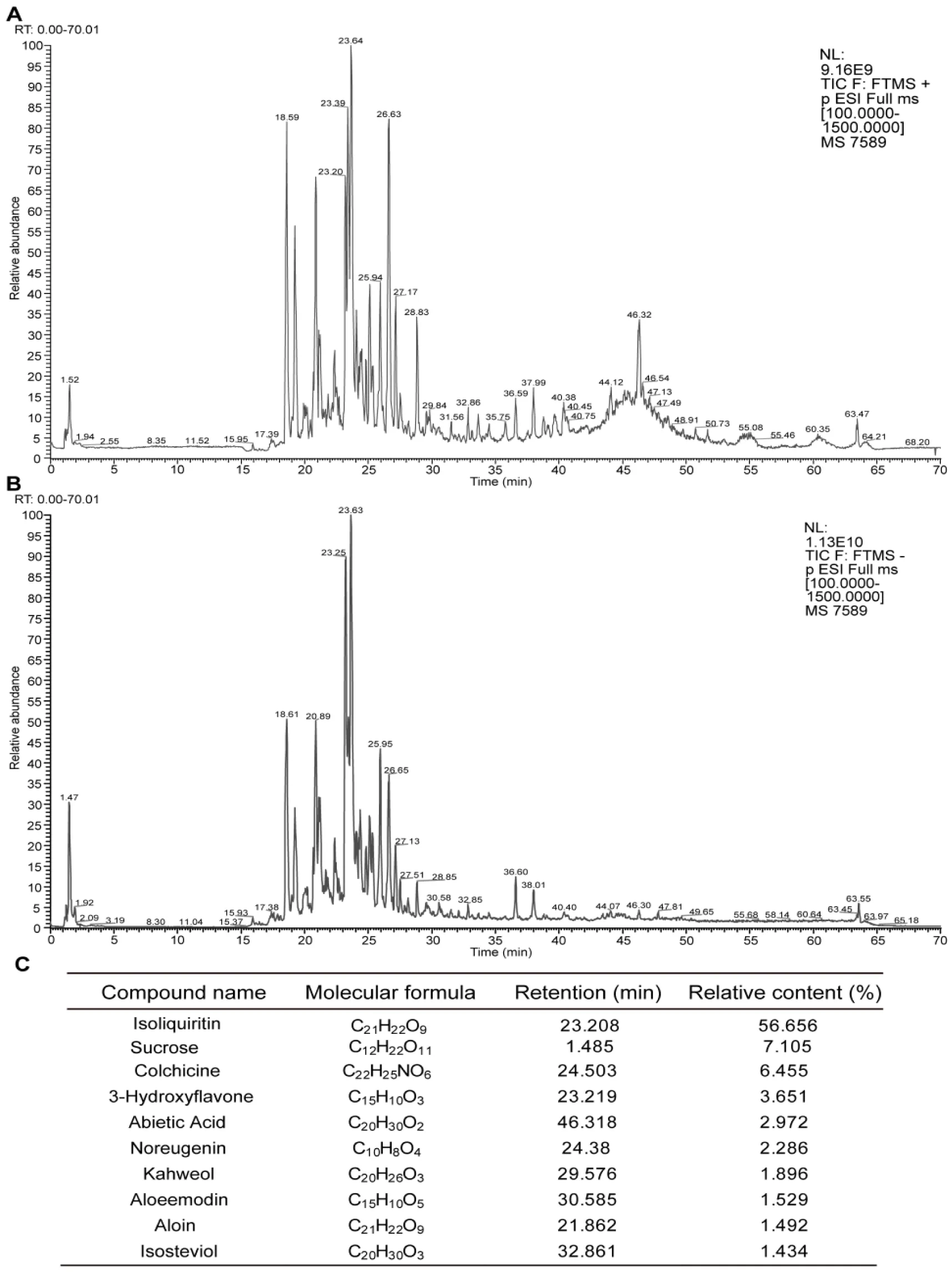
Result: Our studies had shown that a total of 75 active ingredients of ABYJ formula were identified by UPLC-Q-Exactive-orbitrap-MS, Ten marker compounds were identified using orthogonal partial least-squares discrimination analysis, which indicated colchicine and aloin may be the material basis for ABYJ. ABYJ reversed MIA-induced behavioral characteristics, articular inflammatory lesions, the pathological changes and micro structure of knee joint tissue, balance conversion of cartilage cell in rat and reduced the protein expression level of wnt5a, β-catenin and MMP13, while increased Serpina3. A similar rule was demonstrated, the result in vitro suggested ABYJ could inhibit LPS-induced overproduction of pro-inflammatory mediators in ATDC5 cells, including IL-1β, TNF-α and increased the expression level of Serpina3, the increasing expression of Serpina3 would induce [Ca2+] i influx, accordingly, chondrocyte apoptosis was inhibited.
Conclusion: These findings suggest that ABYJ exerts therapeutic effects on joint inflammation and cartilage damage by regulating the wnt5a/β-catenin signaling pathway and modulating the expression of key proteins such as Serpina3, highlighting its potential as a promising candidate for the treatment of joint-related diseases.
Abbreviations
ABYJ: Aibiwajiwuli-Mufasili; OA: Osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic, degenerative and disabling disease characterized by structural defects of articular cartilage, subchondral bone loss, synovial tissue hyperplasia, and instability of the tendons and ligaments [1]. The global incidence rate of OA has exhibited an upward trend over the past two decades. This can be attributed to the aging population and a growing prevalence of severe obesity. Consequently, OA has progressively evolved into a prevalent disabling condition among the elderly [2]. Cellular senescence [3], low-grade inflammation [4], mechanical stress and systemic progresses [5] are imporant factors contributing to joint damage in the progression of OA. The high incidence and disability rate of OA have imposed a significant burden on both the psychological well-being of patients and the socioeconomic landscape. Currently, the pharmacological treatment for early and mid-stage OA primarily consists of symptom-modifying drugs such as acetaminophen and nonsteroidal anti-inflammatory drugs, as well as disease-modifying drugs such as glucosamine sulfate and chondroitin sulfate. However, they may cause serious adverse reactions, such as liver and kidney injury, nausea, vomiting, diarrhea, and bone pain [6]. Currently, there are no preventive or regenerative available therapies for degenerative joint diseases [7]. For patients who do not experience improvement in their symptoms following oral medication and intra-articular injection treatment, surgical intervention is typically required. However, surgical treatment is associated with certain drawbacks, including high costs, perioperative complications, and the risk of prosthesis-related infections that may necessitate joint replacement revision for patients [8]. Clinical trials have demonstrated promising results for compounds that halt the structural progression of osteoarthritis, such as cathepsin K inhibitors, Wnt inhibitors, and anabolic growth factors. Furthermore, the use of nerve growth factor inhibitors could also reduce OA pain [9].
The Wnt/β-Catenin pathway is a highly conserved signaling pathway that plays a crucial role in embryonic development. Additionally, it regulates cell proliferation, cell proliferation, differentiation, and tissue repair and regeneration during adulthood. This pathway controls various biological processes by managing the intracellular stability and nuclear translocation of β-catenin [10]. The Wnt pathway plays a crucial role in maintaining the homeostasis of articular cartilage and bone, regulating chondrogenesis in various ways at different stages [11]. For instance, Wnt5a and Wnt5b have been showed to promote chondrogenesis in vitro, as well as to stimulate chondrogenesis and regulate proliferation and differentiation by modulating the expression of cell cycle regulators p130 and cyclin D1 [12]. Furthermore, Wnt5a has been found to promote early chondrogenesis also by activating noncanonical Wnt signaling pathways [13]. There is mounting evidence indicating that aberrant chondrocyte metabolism can contribute to the onset and progression of OA [14]. Traditional Chinese medicine formulas can also be utilized for the treatment of OA [15,16]. Ethnic Chinese medicines play a significant role in traditional Chinese medicine, making invaluable contributions to the prevention and treatment of numerous diseases. By incorporating relevant theories of traditional Chinese medicine and foreign medicine, Uygur medicine is gradually evolving into an ethnic medicine with distinctive characteristics and strong regional influences. The uyghur medicine prescription ABYJ is an ancient classic formula, first recorded in 1750. It is now included in the “Catalogue of Ancient Classic Prescriptions (Second Batch)” Volume of the Uyghur Medicine published by the State Administration of Traditional Chinese Medicine. This formula is composed of five medicinal herbs: Aloevera, Colchicum autumnale, Terminalia chebula, Rosa rugosa and Pistacia lentiscus.
In clinical practice for treating osteoarthritis, this formula has shown definite efficacy. However, its active ingredient and therapeutic mechanism are not yet clear. Therefore, the aim of this study was to investigate the therapeutic effect and mechanism of ABYJ in the treatment of OA through both in vitro and in vivo experiments.
| Table 1: Three-dimensional strategy for description of Proof of Principle (PoP), Real-World Effectiveness (RWE), and Value, i.e. the three answers to the three questions of Sir Archibald Cochrane. Non-exp. RWC: Non-experimental Real-World Condition. Preliminary versions of this table have been published [2-5,20]. | |||
| Cochrane questions | Can it work? | Does it work? | Is it worth it? |
| Outcome dimensions |
Efficacy or Proof of Principle (objective PoP) |
Real-World Effective-ness (objective RWE) | Value individual or societal (subjective) |
| Study conditions | Experimental study condition (ESC) |
Non-exp., RWC with systematic evaluation of data | Non-exp., RWC without systematic but individual evaluation of data |
| Perspectives | Clinical Research | Health services research | Economic Research |
| Forms (structures) | Explanatory or interventional study |
Pragmatic or observational study | Complete economic analysis |
| Functions | Demonstration of Proof of Principle | Confirmation of Real-Word Effectiveness | Comparison of costs and consequences |
| Tool | Randomised Controlled Trial (RCT) | Pragmatic Controlled Trial (PCT) | Cost Effectiveness Analysis |
Materials and Methods
Drugs, chemicals and reagents
AV (No.K30038803) and RR (No.H30018002) were purchased from Xinjiang Madison Pharmaceutical Co, Ltd (Hetian, China). CA (No.230301) was purchased from Anhui Xintai Pharmaceutical Co, Ltd (Bozhou, China). TC (No.YL-180-2207013) was purchased from Anhui Xinsheng Traditional Chinese Medicine Decoction Co, Ltd (Bozhou, China), and PL (No.230301) from Bozhou Qingfengyutang Traditional Chinese Medicine Decoction Co, Ltd. (Bozhou, China). MIA (No.J26GS149832) was purchased from Shanghai Yuanye Bio-Technology Co, Ltd. (Shanghai, China), GS (No.7TT0202) was purchase from Taiwan Biotech Co, Ltd. (Taoyuan, China). The primary antibody against β-actin, Wnt5a, β-catenin, and MMP-13 was purchased from BOSTER Biological Technology Co, Ltd. (Wuhan, China) and Serpina3 from Servicebio Technology Co., Ltd. (Wuhan, China).
Preparation of ABYJ suspention and UPLC-Q-Exactive-orbitrap-MS analysis
The five herbs of ABYJ were prepared according to the following dosage, AV:CA:TC:RR:PL = 6:4:4:1:1. The dried sample was pulverized to a uniform size and passed through a 100-mesh sieve. A 200 mg sample was accurately weighed and sonicated with 10 mL of a 50% methanol aqueous solution for 30 min. Subsequently, the sample was centrifuged at 14000 rpm for 5 min, and the supernatant was passed through a 0.22 μm filter membrane finally, qualitative analysis was conducted using UPLC-Q-Exactive-orbitrap-MS.
UPLC-Q-Exactive-orbitrap-MS analysis was conducted using an U3000 UHPLC (ThermoFisher Scientific, Waltham, MA, USA) connected to Q Exactive Orbitrap high resolution mass spectrometer (ThermoFisher Scientific, Waltham, MA, USA). The separation was performed using an ACQUITY UPLC HSS T3 Column (2.1×100 mm, 1.8 μm) at a tempurature of 35℃ and a flow rate of 0.3 mL/min. The optimal mobile phases consisted of 0.1: 100 (v/v) HCOOH/H2O (solvent A) and 0.1:100 (v/v) HCOOH/acetonitrile (solvent B). The optimized UPLC elution conditions were as follows: 0 to 10 min, 0 to 30% B; 10 to 45 min, 30 to 100% B; and 45 to 60 min, 100% B. The injection volume was set at 10 μL. The mass spectrum data was acquired in both positive and negative ion mode through Full MS-ddMS2 mode by the Q Exactive Orbitrap high resolution mass spectrometer. The scan parameters were set as follows: scan range, 100 to 1200 m/z; MS1 resolution, 70000; MS2 resolution, 17500; ion source voltage, 320V; capillary temperature, 320℃; auxiliary gas heater temperature, 350℃; Sheath gas flow rate, 40 L/min; AGC target, 1×105; TopN, 5. The Normalized Collision Energy (NCE) was set at 30, 40, and 50 V.
Establishment of OA model and drug treatment
Sixty 8-week-old male SD rats were obtained from the Animal Center of Xinjiang Medical University (Urumqi, China). The rats were kept in a temperature-controlled room with a 12 h light-dark cycle and provided with ad libitum access to food and water. All animal experiments were conducted in accordance with the guidelines approved by the Experimental Animal Ethics Committee of Xinjiang Huashidan Pharmaceutical Research Co., Ltd. (20230007-HSD).
After a one-week adjustment period, all rats were randomly divided into control (n = 10) and treatment (n = 50) groups. Rats in the treatment group underwent OA modeling, wherein they received an intraarticular injection of 50 μL sodium iodoacetate (MIA, 60 mg/mL) in their left knee joints to establish MIA-induced arthritis model. Rats in the control group were injected with an equal volume of normal saline in the left knee joint. Fourteen days after the final injection, rats in the treatment group were randomly divided into five groups (n = 10 per group): OA, Glucosamine Sulfate (GS, 157.5 mg/kg), low-dose ABYJ (L-A, 0.84 g/kg), medium-dose ABYJ (M-A, 2.52 g/kg) and high-dose ABYJ group (H-A, 5.04 g/kg). The same volume of saline was given to control and OA groups. Each rat was administrated by oral gavageonce once per day for 21 days. After the drug treatment, rats were euthanized by cervical dislocation. The fascia, fat and muscle tissue around the knee joint were stripped. A portion of knee articular cartilages were fixed with 10% formaldehyde solution for Micro-computed tomography (micro-CT) and histopathology experiment. The rest were frozen at -80℃ until subsequent analysis.
Evaluation of Lequesne MG
Lequesne MG scoring criteria including degree of pain, gait change, joint swelling, and joint range of motion in rats. 1) Degree of pain: no pain (0 points); the knee joint will contract (1 point); the limb will spasm and there will be a mild systemic reaction (2 points); the limb will cramp and the whole body will shake (3 points). 2) Gait: normal walking (0 points); limping slightly and pushing hard (1 point); walking but with a pronounced limp (2 points); unable to walk properly (3 points). 3) Joint swelling: no swelling, bone marks clearly visible (0 points); mild swelling, shallow bone marks (1 point); obvious swelling, bone makers are gone (2 points). 4) Joint range of motion: over 90° (0 points); 45°-90° (1 point); 15°-45° (2 points); blow15° (3 points). The Lequesne MG score is the sum of four indicators.
Micro-CT analysis
Rat knee joints were fixed in 10% neutral buffered formalin. The joints microstructure was analyzed using a micro-CT scanner (SkyScan 1176; Bruker, Kontich, Belgium). Additionly, the Bone Mineral Density (BMD), total bone volume/total tissue volume (BV/TV), trabecular number (Tb.N) and separation (Tb.Sp) were also analyzed as 3D structural parameters with a builtin program. Furthermore, 3D reconstruction images were acquired with CTVOX software.
Histopathologic analysis
The knee joints were immersed in a 10% formaldehyde solution for 48 h and then decalcified using 10% EDTA for an additional 2 months. After decalcification, the knee joints underwent dehydration through a series of ethanol and xylol baths before being embedded in paraffin wax. Sagittal slices with a thickness of 5 µm were stained with HE and SF for observation under a microscope.
4D Label-free quantification proteomics analysis
The protein samples from the control, OA and H-A groups were injected into a nano-HPLC (nanoElute, Bruker Daltonics) using 150 mm × 75 μm ID pulled emitter columns (IonOptiks) packed with 1.7 μm C18-particles and heated at 60℃ in a column oven. The samples were separated by a 22 min stepped gradient ranging from 3.5 to 95% B at a flow rate of 500 nL/min. Mobile phase A consisted of 0.1% formic acid in water, while mobile phase B consisted of 80% acetonitrile containing 0.1% formic acid. A 22-min elution gradient was performed by increasing mobile phase B from 3.5% B to 32% over a period of 17 min, from 32% B to 95% over 1 min. The mobile phase was then maintained at 95% for 2 min before being equilibrated at 1% for an additional 2 min. MS acquisition was conducted in Data-Dependent Acquisition (DDA) mode with PASEF. The accumulation time in the TIMS tunnel was set to 100 ms, and the capillary voltage was set to 1.6 kV. Mass spectrometry ranged from 100 to 1700 m/z in MS and MS/MS, and the mobility ranged from 0.6 to 1.6 cm2/ (vs). Dynamic exclusion was activated for ions within a mass of 0.015 m/z and a mobility of 0.015 cm2/ (vs) and released after 0.4 min. The total cycle time was 1.1 s with 10 PASEF cycles. The obtained data were analyzed using FragPipe and proteins with a Fold Change (FC) > 1.5 (or < 0.6667) and p < 0.05 were considered as Differentially Expressed Proteins (DEPs).
Cell culture
The murine ATDC5 chondrocyte cell line was purchased from Henan Engineering Research Center of Industrial Microbiology (Zhengzhou, China). The cells were then placed in a CO2 constant-temperature incubator with DMEM-High Glucose Culture Media (Hyclone, Logan, Utah, USA) containing 10% FBS and 1% Penicillin Streptomycin solution for cultivation.
Cell viability assay In vitro
The concentration of ABYJ in the cell experiments was confirmed using the MTT kit. ATDC5 cells were seeded at a density of 104 cells per-well in a 96-well plate, and then incubated with 100 μL of serum-free medium containing different concentrations of ABYJ, aloin and colchicine for an additional 12 hours. Subsequently, 20 μL MTT solution was added to each well and incubated for 4 h followed by addition of 150 μL DMSO. Finally, the optical density at 490 nm was measured using an enzyme labeling instrument. Three replicated wells were used for each experiment.
Enzyme linked immunosorbent assay
The secretion of inflammatory cytokines IL-1β and TNF-α in the cell culture medium was detected using the ELISA technique. ATDC5 cells were inoculated at a density of 5×104 cells per-well in a 6-well plate for 12 h. Subsequently, the cells were stimulated with LPS (1 μg/mL), different concentrations of ABYJ (125, 250, 500 μg/mL), Aloin (150 μg/mL) and Colchicine (12.5 μg/mL) were stimulated for another 24 hours, the levels of IL-1β and TNF-α were measured based on the standard curves of samples obtained from the cell culture medium.
Fluorescence labeling calcium ion experiment
The calcium ion levels of chondrocytes were detected using a Fluo-4 AM Fluorescence Calcium Ion Detection Kit (Servicebio, Wuhan, China). ATDC5 cells were seeded with the density of 104 cells per-well in a 96-well plate and then incubated LPS (1 μg/mL) along with different concentrations of ABYJ, aloin and colchicine for another 12 h. The original cell culture medium was removed and 100 μL Fluo-4 AM detection working fluid was added to each well, followed by a 30-min incubated at 37℃. After incubation, calcium ion detection buffer was used for incubation for an additional 15 min before using fluorescence microscopy to detect and analyze calcium ion levels.
DAPI analysis
First, ATDC5 cells were inoculated on slides in 6-well plate and cultured for 12 h. ABYJ, aloin, colchicine and/or LPS were then added and the cells were cultured further. After incubation for 24 h, the cells were fixed with carnoy fixed solution (methanol: acetic acid = 3: 1), washed with PBS and finally stained with DAPI (Boster, Wuhan, China). The staining was observed under a fluorescence microscope.
Western blot analysis
The total proteins of rat knee cartilage and chondrocytes were extracted with RIPA cell lysate, and the protein concentration was measured with a BCA protein assay kit. All the sample proteins were separated using 10% SDS-PAGE, then transferred to a polyvinylidene fluoride membrane (PVDF), and sealed with 5% skimmed milk powder overnight at 4℃. The primary antibody was incubated at 37℃ for 2 h, followed by the secondary antibody which was incubated at room temperature for another 2 h. The protein bands were detected with an ECL luminescence reagent in the imager. The β-actin was used as internal controls.
Statistical analysis
All experimental data were expressed as mean ± Standard Deviation (SD) and analyzed using SPSS 27.0.1 statistical software. Differences among groups were assessed by one-way analysis of variance (ANOVA), followed by Tukey’s test for between-group comparison. Statistical significance was considered when p < 0.05.
Results
Identification of components in ABYJ formula
UPLC-Q-Exactive-orbitrap-MS analysis was utilized to investigate the intricate chemical components of ABYJ formula. Compound Discover 3.2 software were employed to extract characteristic peaks from raw Raw mass spectrometry data, an mzcloud online database as well as a locally built mzVault traditional Chinese medicine natural product database were utilized for characteristic peaks identification. The mass spectra were collected in both positive and negative ion modes, with the total ion chromatograms are presented in figure 1(A-B). Through comparison of names, molecular weights, molecular formulas, retention times and specific fragment ions with the reported data, a total of 75 compounds within the ABYJ formula were identified. The top 10 compounds are displayed in figure 1C.
Effect of ABYJ on body weight and behavior of the rats
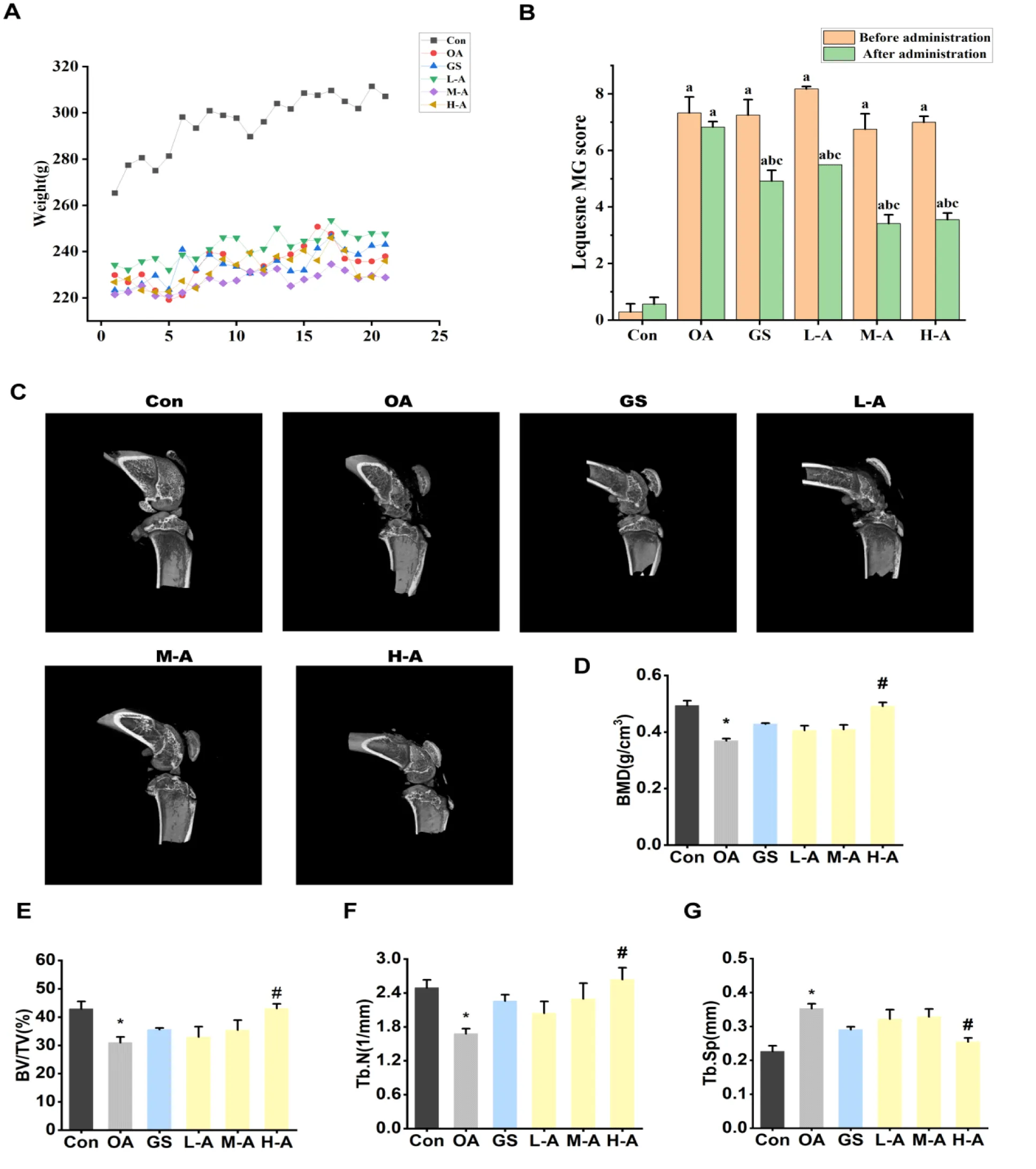
The rats were weighed daily to determine the dosage, and the weight changes of the rats were recorded after 21 days of administration. After 1 day of modeling, severe swelling was observed at the injection site, accompanied by significantly reduced mobility. The affected limb was unable to bend or contract on the ground. In contrast, the control group injected with physiological saline did not exhibit these symptoms and displayed high levels of activity. After 21 days of administration, symptoms such as claudication and pain improved to a certain extent, and the degree of joint swelling decreased in each treatment group. Six groups of rats were evaluated for Lequesne MG knee joint evaluation level before intervention treatment and 3 weeks after intervention treatment. The Lequesne MG knee joints evaluation index of model group was significantly high than those of the control group after modeling (p < 0.01). After treatment, the of Lequesne MG knee joints indexes in the high, medium and high dose groups of ABYJ were significantly lower than those in the model group (p < 0.05) (Figure 2).
ABYJ improved microstructure of cartilage and bone in OA rats
3D reconstructed images obtained from micro-CT analysis revealed that the severity of cartilage damage in the OA group was greater compared to the control group. Furthermore, when compared to the control group, the model group exhibited a significant reduction in BMD, BV/TV, and Tb.N values (p < 0.05), along with a significant increase in Tb.Sp value (p < 0.05). After the modeling progress, there was a decreased in bone mass and strength of bone trabecular, leading to damage in the bone microstructure. In comparison to the model group, ABYJ showed improvement in the pathological changes in the cartilage and bone microstructure of the OA rats. The values of BMD, BV/TV, Tb.N were significantly increased, while the value of Tb. Sp was decreased in high dose (p < 0.05).
Effect of ABYJ on histologic evaluation of articular cartilage
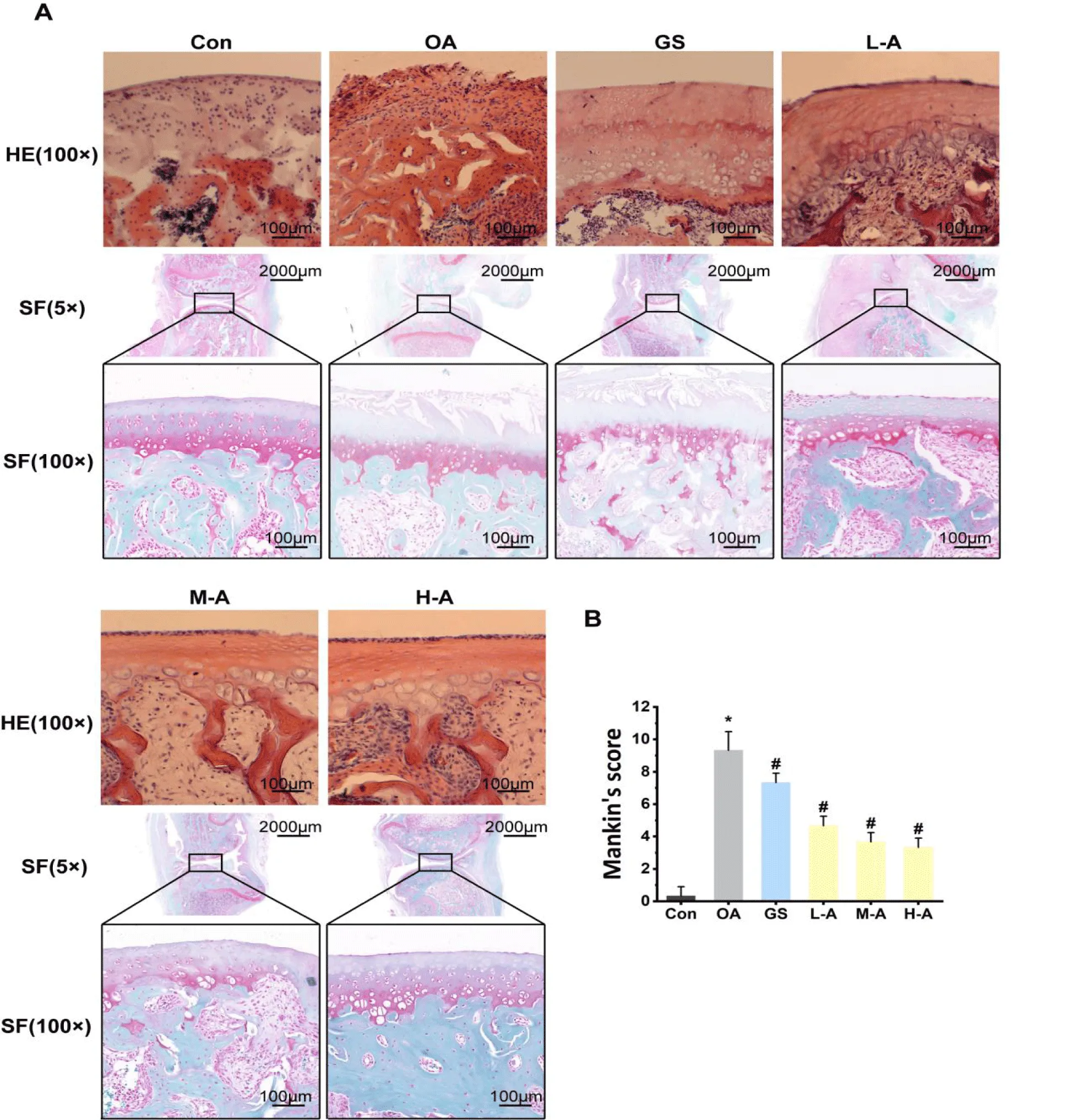
The histological examination (HE and SF staining) of articular cartilage is presented in figure 3. The findings revealed that the surface of the cartilage remained relatively intact with the appropriate amount of chondrocytes in the control group. In contrast, the OA group exhibited thinner cartilage layers with irregular arrangement of chondrocytes, as well as marked defects in cartilage surface. However, treatment with ABYJ protected the articular cartilage from loss of chondrocytes. Furthermore, compared to the control group, the OA group showed significantly higher Mankin’s scoring (p < 0.01). Nevertheless, ABYJ treatment notably ameliorated cartilage lesions and matrix degradation, leading to a obvious decrease in Mankin’s scores (p < 0.05).
Proteomic Analyses showed ABYJ regulated Serpin in OA rats
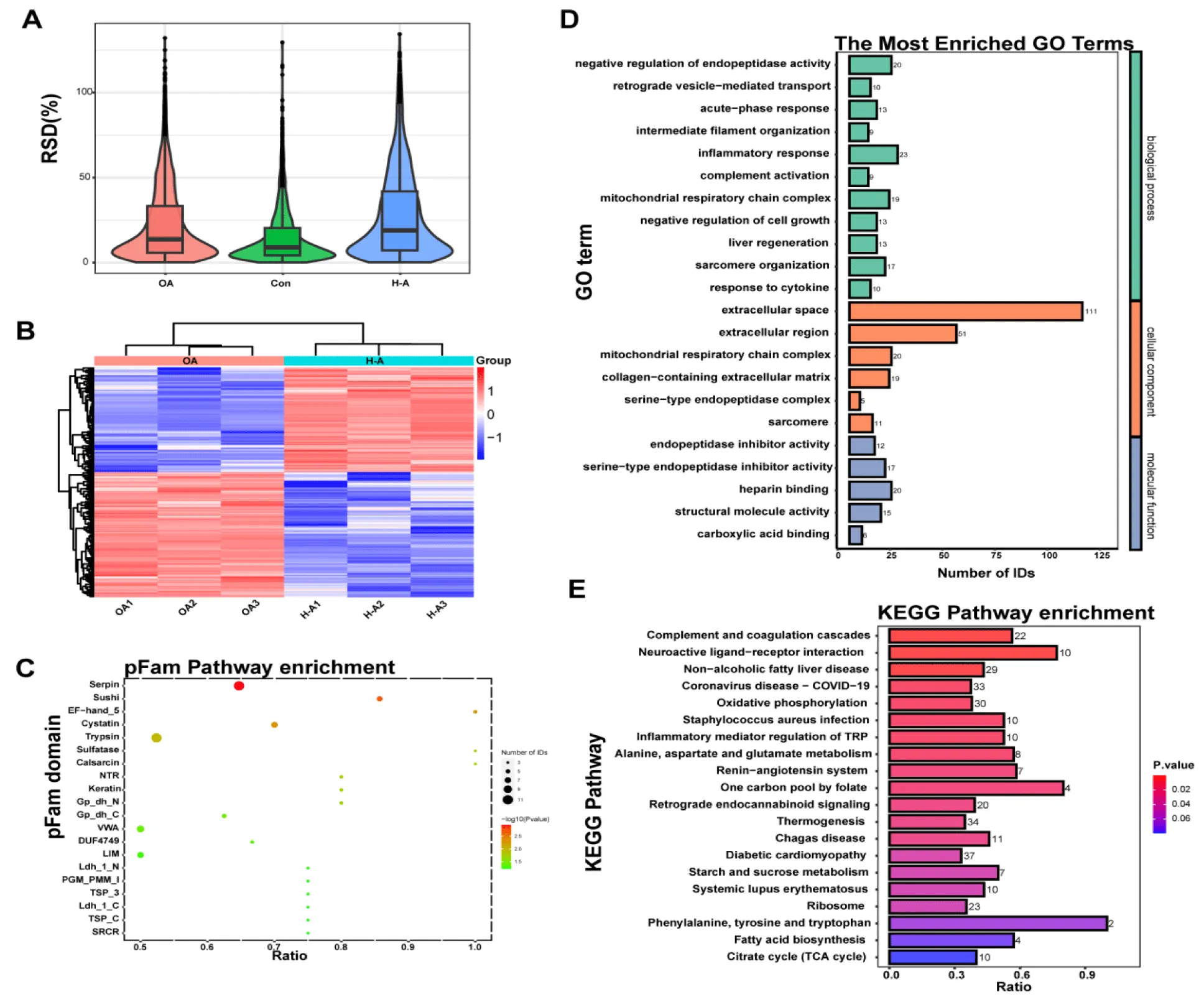
Protein extracts from Control, OA, H-ABYJ group were collected for 4D Label-free analysis (Figure 4A). In total, we found that ABYJ group had 612 DEPs compared with OA group, of which 279 were upregulated while 333 were downregulated (Figure 4B). Among these DEPs, The Serpin domain showed a remarkable change (p = 0.001) as depicted in figure 4C. DEPs were categorized according to Gene Ontology annotation into three categories: biological process, cellular component, and molecular function. The GO analysis revealed that the highly expressed proteins exhibited significant enrichment in “endopeptidase inhibitor activity” (p = 4.38E-04) and “serinetype endopeptidase inhibitor activity” (p = 7.93E-04), indicating the inhibition of serine protease function may be the target of ABYJ in the treatment of OA. In terms of biological progress, these proteins were involved in “negative regulation of endopeptidase activity” (p = 2.90E-07) and “retrograde vesicle-mediated transport” (p = 5.54E-04). Additionally, GO analysis of the cellular components indicated that most proteins were annotated as extracellular space (Figure 4D). Furthermore, KEGG pathway analysis demonstrated the involvement of differential proteins in the “complement and coagulation cascade” (p = 3.13E-05) following ABYJ treatment (Figure 4E).
Effect of ABYJ and its active ingredients on relative cell viability
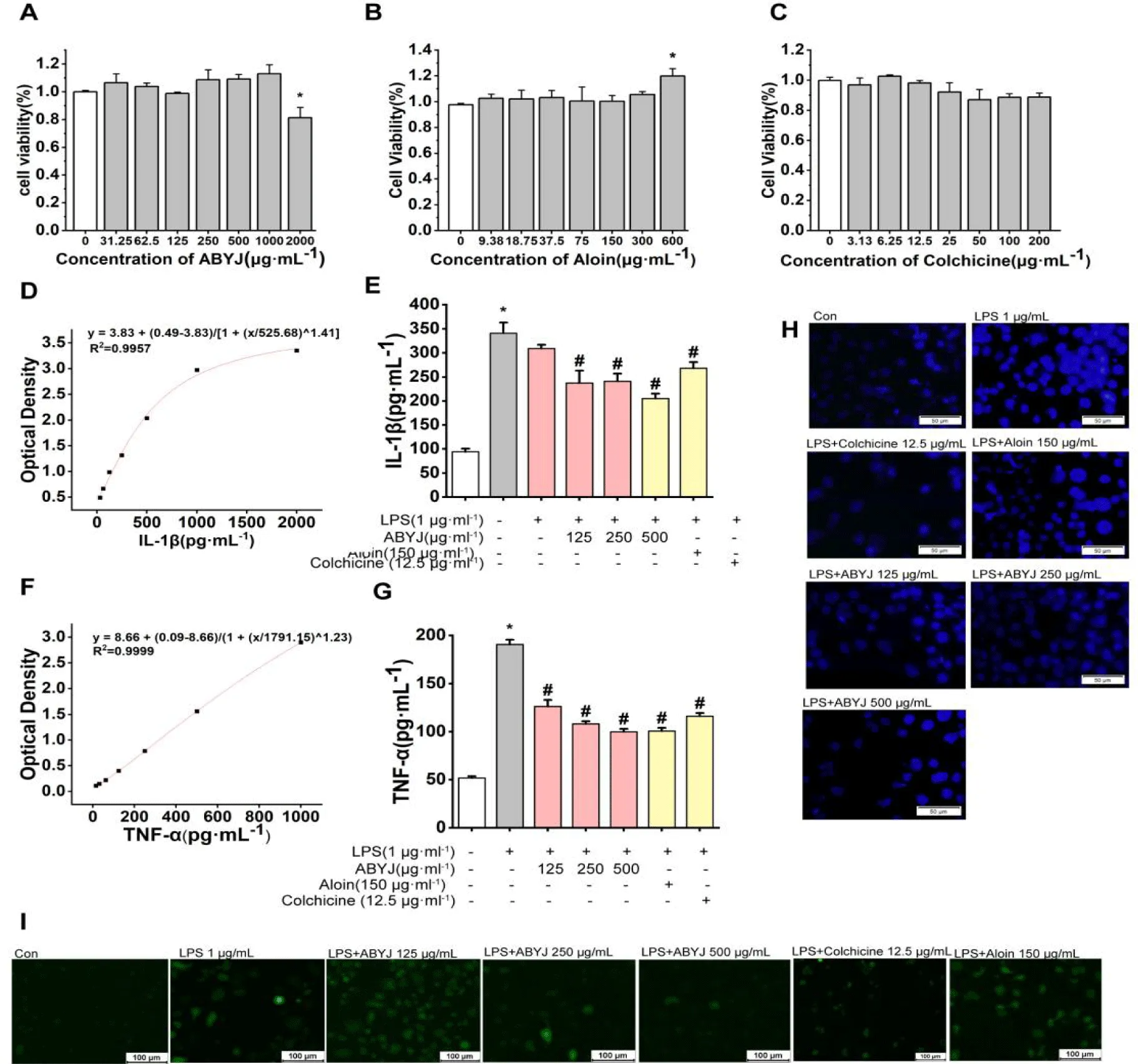
The MTT assay was utilized to assess the cytotoxicity of ABYJ at concentrations of 2000, 1000, 500, 250, 125, 62.5 and 31.25 μg/mL . Additionally, Aloin was tested at concentrations of 600, 300, 150, 75, 37.5, 18.75 and 9.38 μg/mL while colchicine was tested at concentrations of 200, 100, 50, 25, 12.5, 6.25 and 3.13 μg/mL in ATDC5 cells. No significant differences were observed in relative cell viability compared with the control group except for 2000 μg/mL ABYJ, which exhibited a significant decrease in relative cell viability (p < 0.05; Figure 5A). Therefore, concentrations of 125, 250, 500 μg/mL were selected as the optimal concentrations for further study. At a concentration of 600 μg/mL, aloin was found to promote the proliferation of ATDC5 cells, but had no significant effect at other concentrations (Figure 5B). Colchicine reduced cell viability after administration, although the difference was not statistically significant (Figure 5C).
ABYJ inhibited LPS-induced chondrocyte inflammatory factor secretion and cell apoptosis
We conducted an analysis to assess the impact of ABYJ, Aloin and Colchicine on the expression of IL-1β, TNF-α and calcium ion level in chondrocytes induced by LPS. Our findings revealed that ABYJ effectively reduced the release of inflammatory factors. As illustrated in figure 5(D-G), levels of IL-1β and TNF-α were significantly elevated in the LPS-induced group compared with the control group (p < 0.01). However, following treatment with ABYJ, aloin and colchicine, all aforementioned indexes exhibited a reversal trend with a noticeable decrease in the expression of IL-1β, TNF-α (p < 0.01). In addition, ABYJ inhibited cell apoptosis and decreased intracellular calcium ion concentration.
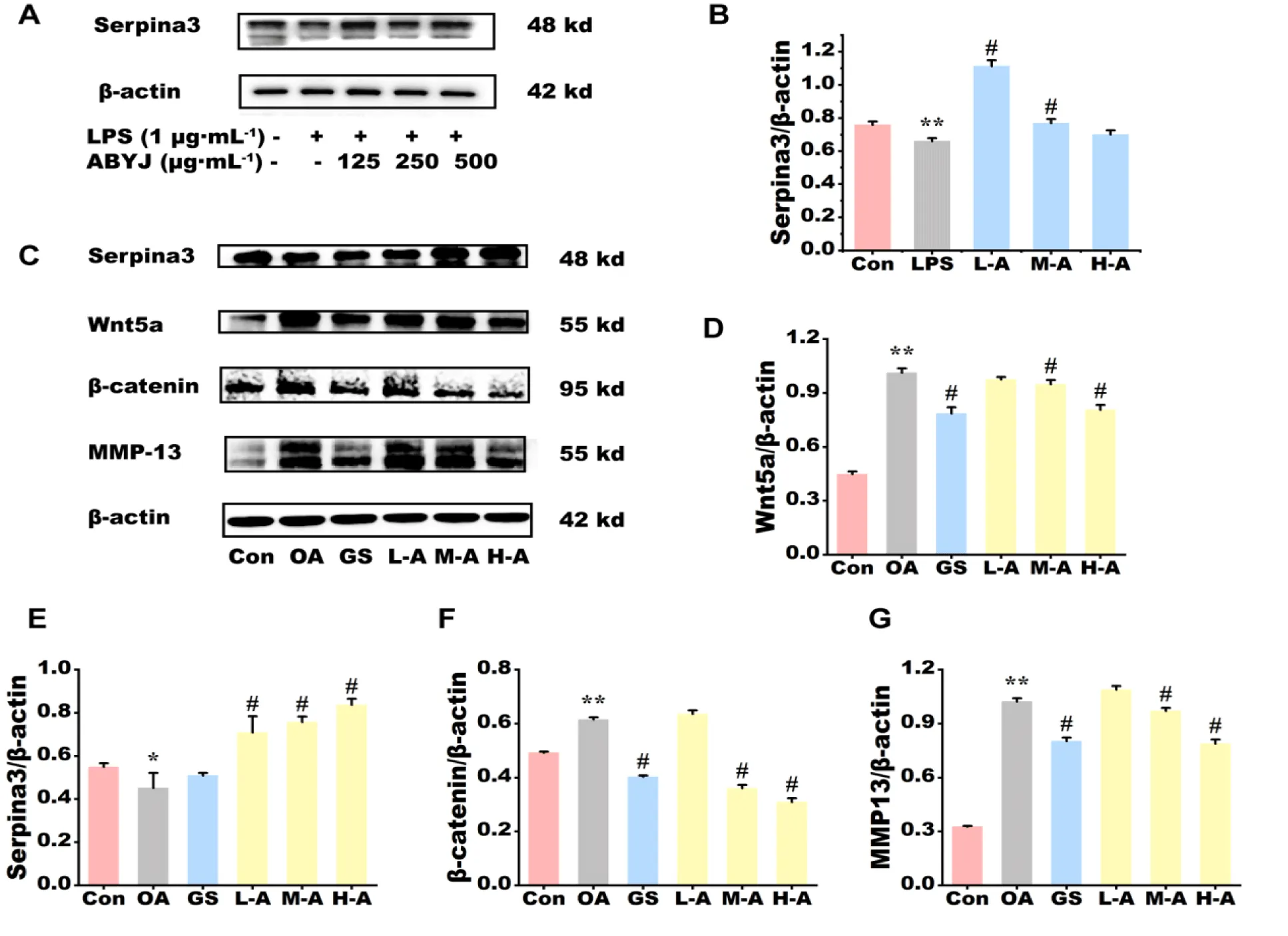
Effect of ABYJ on protein expression of articular cartilage in OA rats and in chondrocytes
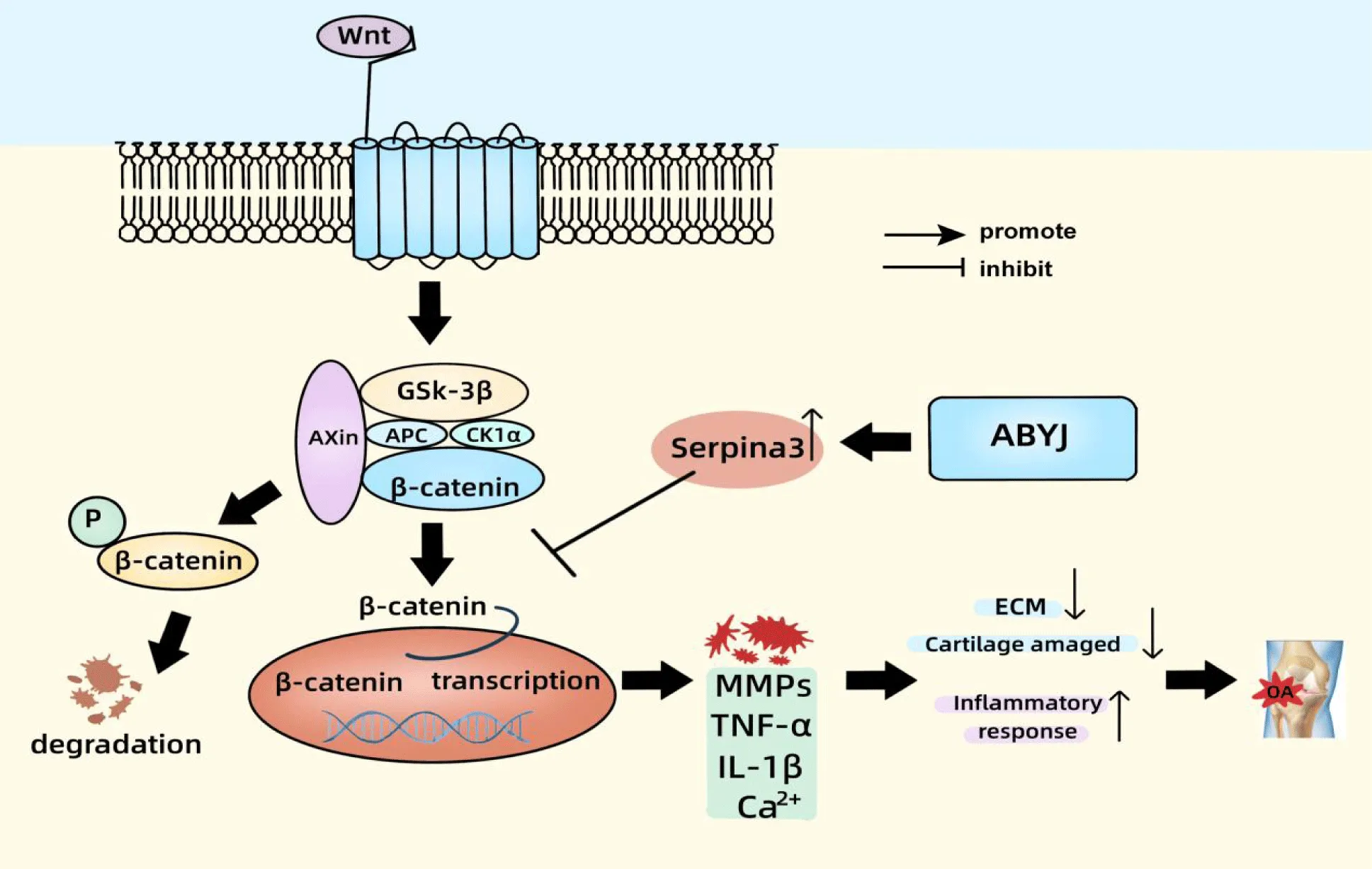
Protein expression levels of serpina3, Wnt-5a, β-catenin and MMP-13 in OA rats and chondrocytes were assessed using western blot analysis (Figure 6). The findings revealed a significant increase in the protein expression of Wnt5a, β-catenin and MMP-13 in the OA group compared with to the control group (p < 0.01). The protein expression of Serpina3 was significantly lower in both the OA group of rats and LPS-induced chondrocyte compared with the control group (p < 0.05 and p < 0.01). H-ABYJ demonstrated significantly lower protein expression of Wnt5a, β-catenin, MMP-13 and higher expression of serpina3 with the OA group (p < 0.05). L-ABYJ and M-ABYJ exhibited significantly increased protein expression of serpina3 in LPS induced cells compared with the control group (p < 0.05). Our experimental findings suggest that ABYJ can protect cartilage by increasing the expression level of Serpina3. ABYJ inhibits the activation of Wnt5a/β-catenin pathway and reduces the release of inflammatory and damaging factors, such as IL-1β, TNF-α, MMP-13 and Ca2+, which may be associated with the involvement of Serpina3 in regulation (Figure 7).
Discussion
OA is a prevalent disease among middle-aged and elderly individual globally, and its high incidence has garnered the significant attention. Articular cartilage destruction, as a key characteristic of OA, is now widely recognized to be mediated by Metalloproteinases (MMPs), which are responsible for the essentially irreversible destruction of cartilage collagen [17]. Studies have indicated that the expression levels of MMP-1, MMP-3, MMP-9 and MMP-13 in the articular cartilage and synovium of OA patients are significantly increased [18]. Moreover, the critical enzyme responsible for degrading type II collagen, MMP-13, demonstrates a high level of expression in the damaged articular cartilage of patients with OA [19]. Numerous pro-inflammatory cytokines are accountable for the gradual degradation and restructuring of joints via MMPs [20]. Inflammation plays a pivotal role in the degeneration of joint cartilage. This inflammation process is triggered by an imbalance between pro-inflammatory and anti-inflammatory factors, leading to a cascade reaction that ultimately results in the damage of cartilage and other intra-articular structures through the activation of MMPs [21]. An excessive increase in MMPs can result in an imbalance between MMPs and TIMPs, which subsequently disrupts the integrity of chondrocytes and ECM, this disruptsion accelerates ECM degradation, ultimately leading to cartilage damage [22]. TNF-α and IL-1β are the primary pro-inflammatory cytokines responsible for the degradation of articular cartilage. The production and accumulation of these inflammatory molecules in the cells lead to an increase in the production of NO in chondrocytes, which in turn results in mitochondrial respiratory chain damage ultimately leads to chondrocyte apoptosis [23]. For example, in the articular chondrocytes of patients with OA, there is an abnormal increase in the expression level of TNF-α. This lead to excessive production of MMPs by chondrocytes, thereby exacerbating the occurrence and development of osteoarthritis [24]. The biological functions of chondrocytes are influenced by their environment, which includes mechanical stimulation, electrical stimulation, osmotic pressure, and the regulation of chemical factors [25,26]. Changes in the structure and composition of ECM and the levels of inflammatory factors during the process of cartilage degeneration may result in alterations within the Ca2+ concentration of OA chondrocytes [27]. Our experiments have confirmed that MIA is capable of inducing a joint inflammatory reaction, increasing the level of MMPs, and causing the degradation of the ECM. Additionally, LPS has been shown to induce the inflammation and of chondrocytes and increase calcium ion concentration. After the ABYJ treatment, it was observed that there was an improvement in the microstructure of joint and a restoration of the morphology of the cartilage layer, additionally, there was a reduction in inflammatory reactions, intracellular Ca2+ levels, and degradation of the ECM. As a result, there was an overall improvement in the progress of OA.
Serine proteinases perform crucial functions in the progression of MMPs-driven cartilage proteolysis. They are involved in pro MMP activation, direct ECM degradation, cytokine regulation and receptor activation. A decrease in the levels of inhibitory serpins may promote cartilage degradation by increasing the abundance of serine proteinases [28,29]. Serpina3, also known as α-1-antichymotrypsin (AACT, ACT), is a significant member of serine protease inhibitor superfamily. The main function of Serpina3 is to inhibit serine proteases by binding them in a stable complex, thereby preventing their proteolytic activity and leading to consequent changes in the ECM [30]. Serpina3 was previously categorized as a typical acute-phase inflammatory response protein due to its increased concentration in response to inflammation [31]. However, further study have revealed inconsistent findings. It has been discovered that Serpina3 down-regulate the inflammatory response and inhibit infammation by inhabiting cathepsin G, a type of pro-infammatory enzyme [32]. For example, the upregulation of Serpina3 has been shown to alleviate CYP-induced interstitial cystitis/bladder pain syndrome, by reducing pro-infammatory factor levels, indicating that Serpina3 exerts an anti-inflammatory effect [33]. Furthermore, it is worth noting that Serpina3, is highly expressed in chondrocytes [34], and plays an important role in promoting cartilage formation and repair. For example, Sinensetin, a type of plant-derived polymethoxy flavonoid, has been shown to reduce the inflammatory response of IL-1β-induced human OA chondrocytes and alleviate the progress of OA in rats. This is achieved by up-regulating the expression of Serpina3 [35]. Based on our proteomics experimental findings, we observed significant changes in Serpin following the administration of ABYJ in the OA rat’s model. The Western Blot analysis revealed a notable increase in the expression level of Serpina3 in the group treated with ABYJ, indicating that promoting the upregulation of Serpina3 holds promising therapeutic potential for managing OA.
In this study, we have identified that the mechanism of ABYJ treatment for osteoarthritis may be associated with the Wnt/β-Catenin signaling pathway, which is activated by Serpina3. Additionally, it has been observed that Serpinh1, a member of the serine proteinase inhibitors, plays a regulatory role in the epithelial-mesenchymal transition of gastric cancer cells and gastric cancer metastasis through modulation of the Wnt/β-Catenin signaling pathway [36]. Serpina3, another member of the Serpins, has been proven to inhibit retinal inflammation and block the activation of Wnt signaling pathway in diabetic rats. This exerts a unifying effect of anti-inflammatory and anti-angiogenic properties [37]. We discovered that serpina3 may induce the activation of β-catenin. In the OA group, the expression of β-catenin significantly increased, while Serpina3 decreased. Conversely, in the ABYJ treatment group, opposite results were observed. Considering the regulatory role of Wnt in β-catenin signaling, it is plausible to hypothesize serpina3-induced activation of β-catenin signaling may be mediated through Wnt/β-Catenin signaling. On the basis of the pathological improvement of ABYJ on OA, we evaluated mechanisms related to the Wnt pathway. Results suggested that ABYJ regulates and inhibits the Wnt/β-catenin signaling pathway through up-regulation of Serpina3, thus inhibiting the release of inflammatory factors and MMPs, reducing the inflammatory response and alleviating the progress of OA.
ABYJ is a traditional Uyghur medicine formula consisting of five herbs and complex chemical components. Despite its extensive clinical history in traditional Chinese medicine, the exact composition of its medicinal ingredients remains ambiguous. In our study, we conducted an analysis of the chemical components of Aibiwaijiwuli-Mufasili using UPLC-MS/MS and identified 75 chemical components. These components are primarily comprised of terpenoids, carboxylic acid, flavonoids, and alkaloids. Colchicine and aloin are likely to be the main active ingredients among these compounds. Previous studies have demonstrated the anti-inflammatory properties of colchicine and aloin. Aloin, an anthraquinone glycoside derived from the Aloe species, has been found to possess anti-inflammatory activities. It has been shown to significantly reduce serum concentration of TNF-α, IL-1β and IL-6 in Nrf2 KO mice [38], as well as exhibit anti-inflammatory activity in RAW264.7 cells [39]. Colchicine modulates multiple inflammatory pathways, including the prevention of microtubule assembly, this disruption leads to the inhibition of inflammasome activation, inflammatory cell chemotaxis, generation of leukotrienes and cytokines, as well as phagocytosis [40]. In addition, both colchicine and aloin have to be reported a protective effect on joint cartilage. Colchicine can inhibit MMP13 expression and consequent cartilage degradation by abrogateing phosphorylation of PLC-γ1 [41]. Aloin protectcts anabolism and catabolism of the ECM in IL-1β-induced chondrocytes,by suppressing the PI3K/Akt/NF-κB signalling pathway cascades to ameliorate the progression of osteoarthritis [42]. The results of our in vitro analysis are consistent with the above reports, highlighting that both aloin and colchicine are important active ingredients for its anti-inflammatory effect within ABYJ.
Conclusion
In conclusion, our findings provide evidence supporting the use of the traditional Chinese herbal formula ABYJ in the treatment of OA in rats. We have confirmed that ABYJ can reduce the inflammatory response of chondrocytes and alleviate the progress of OA in rats, by up-regulating the expression of Serpina3 and inhibiting the nuclear translocation of Wnt/β-catenin. Aloin and Colchicine may important active ingredients in ABYJ.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank the Science and Technology Innovation Talent Program of Xinjiang Tianshan Talented Youth Top Talent Project (Wei Chen), Project of Xinjiang Traditional Chinese Medicine and Ethnic Medicine Substance - Basis Research Innovation Team (Grant No. 2023CB002), Key Talents of the Xinjiang Corps Talent Support Program (Qin Ma), Science Technology Tackling Projects of the Xinjiang Corps (Grant No. 2023AB044), Innovative Key Talent Project of the Xinjiang Corps for their support (Grant No. 2024DB053).
References
- Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, Zhong Y, He T, Chen S, Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023 Feb 3;8(1):56. doi: 10.1038/s41392-023-01330-w. PMID: 36737426; PMCID: PMC9898571.
- Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020 Nov 28;396(10264):1711-1712. doi: 10.1016/S0140-6736(20)32230-3. Epub 2020 Nov 4. PMID: 33159851.
- Xie J, Wang Y, Lu L, Liu L, Yu X, Pei F. Cellular senescence in knee osteoarthritis: molecular mechanisms and therapeutic implications. Ageing Res Rev. 2021 Sep;70:101413. doi: 10.1016/j.arr.2021.101413. Epub 2021 Jul 21. PMID: 34298194.
- Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016 Oct;12(10):580-92. doi: 10.1038/nrrheum.2016.136. Epub 2016 Aug 19. PMID: 27539668; PMCID: PMC5500215.
- Visser AW, de Mutsert R, le Cessie S, den Heijer M, Rosendaal FR, Kloppenburg M; NEO Study Group. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015 Oct;74(10):1842-7. doi: 10.1136/annrheumdis-2013-205012. Epub 2014 May 20. PMID: 24845389..
- Geng R, Li J, Yu C, Zhang C, Chen F, Chen J, Ni H, Wang J, Kang K, Wei Z, Xu Y, Jin T. Knee osteoarthritis: Current status and research progress in treatment (Review). Exp Ther Med. 2023 Aug 25;26(4):481. doi: 10.3892/etm.2023.12180. PMID: 37745043; PMCID: PMC10515111.
- Joiner DM, Less KD, Van Wieren EM, Zhang YW, Hess D, Williams BO. Accelerated and increased joint damage in young mice with global inactivation of mitogen-inducible gene 6 after ligament and meniscus injury. Arthritis Res Ther. 2014 Mar 27;16(2):R81. doi: 10.1186/ar4522. PMID: 24670222; PMCID: PMC4060238.
- Gunaratne R, Pratt DN, Banda J, Fick DP, Khan RJK, Robertson BW. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J Arthroplasty. 2017 Dec;32(12):3854-3860. doi: 10.1016/j.arth.2017.07.021. Epub 2017 Jul 21. PMID: 28844632.
- Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021 Feb 9;325(6):568-578. doi: 10.1001/jama.2020.22171. PMID: 33560326; PMCID: PMC8225295.
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012 Jun 8;149(6):1192-205. doi: 10.1016/j.cell.2012.05.012. PMID: 22682243.
- Gan L, Deng Z, Wei Y, Li H, Zhao L. Decreased expression of GEM in osteoarthritis cartilage regulates chondrogenic differentiation via Wnt/β-catenin signaling. J Orthop Surg Res. 2023 Oct 4;18(1):751. doi: 10.1186/s13018-023-04236-z. PMID: 37794464; PMCID: PMC10548561.
- Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003 Mar;130(5):1003-15. doi: 10.1242/dev.00324. PMID: 12538525.
- Bradley EW, Drissi MH. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol Endocrinol. 2010 Aug;24(8):1581-93. doi: 10.1210/me.2010-0037. Epub 2010 Jun 23. PMID: 20573686; PMCID: PMC5417459.
- Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021 Mar;66:101249. doi: 10.1016/j.arr.2020.101249. Epub 2020 Dec 29. PMID: 33383189.
- Xia H, Cao D, Yang F, Yang W, Li W, Liu P, Wang S, Yang F. Jiawei Yanghe decoction ameliorates cartilage degradation in vitro and vivo via Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2020 Feb;122:109708. doi: 10.1016/j.biopha.2019.109708. Epub 2019 Dec 30. PMID: 31918279.
- Xu X, Wan Y, Gong L, Ma Z, Xu T. Chinese herbal medicine Yanghe decoction for knee osteoarthritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020 Aug 21;99(34):e21877. doi: 10.1097/MD.0000000000021877. PMID: 32846845; PMCID: PMC7447488.
- Rowan AD, Litherland GJ, Hui W, Milner JM. Metalloproteases as potential therapeutic targets in arthritis treatment. Expert Opin Ther Targets. 2008 Jan;12(1):1-18. doi: 10.1517/14728222.12.1.1. PMID: 18076366.
- Özler K, Aktaş E, Atay Ç, Yılmaz B, Arıkan M, Güngör Ş. Serum and knee synovial fluid matrix metalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late-stage osteoarthritis. Acta Orthop Traumatol Turc. 2016;50(3):356-61. doi: 10.3944/AOTT.2015.15.0115. PMID: 27130394.
- Hu Q, Ecker M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int J Mol Sci. 2021 Feb 9;22(4):1742. doi: 10.3390/ijms22041742. PMID: 33572320; PMCID: PMC7916132.
- van den Bosch MHJ, van Lent PLEM, van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage. 2020 May;28(5):532-543. doi: 10.1016/j.joca.2020.01.016. Epub 2020 Feb 7. PMID: 32044352.
- Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, Rod E, Čukelj F, Vrdoljak T, Vidović D, Starešinić M, Sabalić S, Dobričić B, Petrović T, Antičević D, Borić I, Košir R, Zmrzljak UP, Primorac D. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021 Aug 26;22(17):9208. doi: 10.3390/ijms22179208. PMID: 34502117; PMCID: PMC8431625.
- Chen C, Zhang C, Cai L, Xie H, Hu W, Wang T, Lu D, Chen H. Corrigendum to "Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models" [Int. Immunopharmacol. 52 (2017) 218-226]. Int Immunopharmacol. 2021 Jun;95:107546. doi: 10.1016/j.intimp.2021.107546. Epub 2021 Mar 16. Erratum for: Int Immunopharmacol. 2017 Nov;52:218-226. doi: 10.1016/j.intimp.2017.09.017. PMID: 33735716.
- Shen J, Abu-Amer Y, O'Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017 Jan;58(1):49-63. doi: 10.1080/03008207.2016.1208655. Epub 2016 Jul 7. PMID: 27389927; PMCID: PMC5266560.
- Wang C, Al-Ani MK, Sha Y, Chi Q, Dong N, Yang L, Xu K. Psoralen Protects Chondrocytes, Exhibits Anti-Inflammatory Effects on Synoviocytes, and Attenuates Monosodium Iodoacetate-Induced Osteoarthritis. Int J Biol Sci. 2019 Jan 1;15(1):229-238. doi: 10.7150/ijbs.28830. PMID: 30662362; PMCID: PMC6329921.
- Leong DJ, Li YH, Gu XI, Sun L, Zhou Z, Nasser P, Laudier DM, Iqbal J, Majeska RJ, Schaffler MB, Goldring MB, Cardoso L, Zaidi M, Sun HB. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011 Jan;25(1):182-91. doi: 10.1096/fj.10-164277. Epub 2010 Sep 8. PMID: 20826544; PMCID: PMC3005439.
- Zhang Y, Li R, Zhong Y, Zhang S, Zhou L, Shang S. Fuyuan Decoction Enhances SOX9 and COL2A1 Expression and Smad2/3 Phosphorylation in IL-1β-Activated Chondrocytes. Evid Based Complement Alternat Med. 2015;2015:821947. doi: 10.1155/2015/821947. Epub 2015 Dec 7. PMID: 26770254; PMCID: PMC4685114.
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691-713. doi: 10.1146/annurev.bioeng.2.1.691. PMID: 11701528.
- Wilkinson DJ, Desilets A, Lin H, Charlton S, Del Carmen Arques M, Falconer A, Bullock C, Hsu YC, Birchall K, Hawkins A, Thompson P, Ferrell WR, Lockhart J, Plevin R, Zhang Y, Blain E, Lin SW, Leduc R, Milner JM, Rowan AD. The serine proteinase hepsin is an activator of pro-matrix metalloproteinases: molecular mechanisms and implications for extracellular matrix turnover. Sci Rep. 2017 Dec 1;7(1):16693. doi: 10.1038/s41598-017-17028-3. PMID: 29196708; PMCID: PMC5711915.
- Wilkinson DJ, Arques MDC, Huesa C, Rowan AD. Serine proteinases in the turnover of the cartilage extracellular matrix in the joint: implications for therapeutics. Br J Pharmacol. 2019 Jan;176(1):38-51. doi: 10.1111/bph.14173. Epub 2018 Mar 30. PMID: 29473950; PMCID: PMC6284380.
- de Mezer M, Rogaliński J, Przewoźny S, Chojnicki M, Niepolski L, Sobieska M, Przystańska A. SERPINA3: Stimulator or Inhibitor of Pathological Changes. Biomedicines. 2023 Jan 7;11(1):156. doi: 10.3390/biomedicines11010156. PMID: 36672665; PMCID: PMC9856089.
- Baker C, Belbin O, Kalsheker N, Morgan K. SERPINA3 (aka alpha-1-antichymotrypsin). Front Biosci. 2007 May 1;12:2821-35. doi: 10.2741/2275. PMID: 17485262.
- Wilkinson DJ. Serpins in cartilage and osteoarthritis: what do we know? Biochem Soc Trans. 2021 Apr 30;49(2):1013-1026. doi: 10.1042/BST20201231. PMID: 33843993; PMCID: PMC8106492.
- Fang W, Song Q, Lv T, Lv J, Cai Z, Wang Z, Song X, Ji X, Huang J. Serpina3n/serpina3 alleviates cyclophosphamide-induced interstitial cystitis by activating the Wnt/β-catenin signal. Int Urol Nephrol. 2023 Dec;55(12):3065-3075. doi: 10.1007/s11255-023-03726-7. Epub 2023 Aug 18. PMID: 37594700; PMCID: PMC10611603.
- Ajekigbe B, Cheung K, Xu Y, Skelton AJ, Panagiotopoulos A, Soul J, Hardingham TE, Deehan DJ, Barter MJ, Young DA. Identification of long non-coding RNAs expressed in knee and hip osteoarthritic cartilage. Osteoarthritis Cartilage. 2019 Apr;27(4):694-702. doi: 10.1016/j.joca.2018.12.015. Epub 2019 Jan 3. PMID: 30611906; PMCID: PMC6444060.
- Liu Z, Liu R, Wang R, Dai J, Chen H, Wang J, Li X. Sinensetin attenuates IL-1β-induced cartilage damage and ameliorates osteoarthritis by regulating SERPINA3. Food Funct. 2022 Oct 3;13(19):9973-9987. doi: 10.1039/d2fo01304e. PMID: 36056701.
- Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging (Albany NY). 2020 Feb 24;12(4):3574-3593. doi: 10.18632/aging.102831. Epub 2020 Feb 24. PMID: 32091407; PMCID: PMC7066881.
- Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, He X, Ma JX. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6900-5. doi: 10.1073/pnas.0906764107. Epub 2010 Mar 29. PMID: 20351274; PMCID: PMC2872418.
- Xu Q , Fan Y , Loor JJ , Liang Y , Lv H , Sun X , Jia H , Xu C . Aloin protects mice from diet-induced non-alcoholic steatohepatitis via activation of Nrf2/HO-1 signaling. Food Funct. 2021 Jan 21;12(2):696-705. doi: 10.1039/d0fo02684k. Epub 2021 Jan 7. PMID: 33410857.
- Ma Y, Tang T, Sheng L, Wang Z, Tao H, Zhang Q, Zhang Y, Qi Z. Aloin suppresses lipopolysaccharide‑induced inflammation by inhibiting JAK1‑STAT1/3 activation and ROS production in RAW264.7 cells. Int J Mol Med. 2018 Oct;42(4):1925-1934. doi: 10.3892/ijmm.2018.3796. Epub 2018 Jul 31. PMID: 30066904; PMCID: PMC6108888.
- Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014 Oct 1;36(10):1465-79. doi: 10.1016/j.clinthera.2014.07.017. Epub 2014 Aug 21. PMID: 25151572.
- Takeuchi K, Ogawa H, Kuramitsu N, Akaike K, Goto A, Aoki H, Lassar A, Suehara Y, Hara A, Matsumoto K, Akiyama H. Colchicine protects against cartilage degeneration by inhibiting MMP13 expression via PLC-γ1 phosphorylation. Osteoarthritis Cartilage. 2021 Nov;29(11):1564-1574. doi: 10.1016/j.joca.2021.08.001. Epub 2021 Aug 20. PMID: 34425229; PMCID: PMC8542595.
- Zhang C, Shao Z, Hu X, Chen Z, Li B, Jiang R, Bsoul N, Chen J, Xu C, Gao W. Inhibition of PI3K/Akt/NF-κB signaling by Aloin for ameliorating the progression of osteoarthritis: In vitro and in vivo studies. Int Immunopharmacol. 2020 Dec;89(Pt B):107079. doi: 10.1016/j.intimp.2020.107079. Epub 2020 Oct 20. PMID: 33096361.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.