2025 June 23;6(6):775-783. doi: 10.37871/jbres2130.
Age and Disease activity related Biomarkers in Takayasuarteritis: Comparison with Giant Cell Arteritis
Andrea DG1,2, Lisa C2, Jennifer LC3, Harald B3,4 and Peter MV5*
2Department of Rheumatology and Immunology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
3Department of Radiology, Linden hospital, CH-3012 Bern, Switzerland
4Department of Radiology, Inselspital, Bern University Hospital, Bern, Switzerland
5Medical Center Monbijou, CH-3011 Bern, Switzerland
Abstract
Background and objective: Measuring Takayasu (TAK) disease activity remains a difficult task. The aims of this study were to identify biomarkers reflecting disease activity in TAK, to analyze the effects of treatment, to compare the findings of TAK with Giant Cell Arteritis (GCA) and to assess age-related differences.
Methods: Biomarkers in sera of 26 TAK patients (median age: 33 years) were analyzed and compared with sera of 18 GCA patients (median age: 72 years), meeting the respective 1990 ACR criteria, as well as with sera of age-matched Healthy Controls (HC). In a screening phase, sera levels of 48 biomarkers were quantified using Luminex technology. Biomarkers showing differences between groups and subgroups were confirmed by ELISA. Disease activity was scored using the EULAR criteria of 2018. Additionally, arterial wall signals in MRI were graded from 0 (normal) to 3 (intense late enhancement).
Results: 7/26 TAK patients showed active disease. MRI signals of 11/26 TAK patients indicated vessel wall inflammation. Serum levels of YKL-40 were elevated in TAK patients compared to HC. YKL-40 as well as sIL-2R and CD163 were lower in TAK patients compared to GCA patients; YKL-40 levels were elevated in GCA compared to HC. None of the molecules were significantly associated with clinical disease activity, neither with Magnetic Resonance Angiography (MRA) signal intensity. Analysis of HC showed a significant age correlation of YKL-40, CD163 and MMP-3, and an inverse correlation for Pentraxin-3.
Conclusions: YKL-40 reliably distinguished between TAK and healthy controls. Biomarkers in active GCA were higher compared to TAK. Interpretation of serological markers must consider age-related differences.
Abbreviations
TAK: Takayasu Arteritis; GCA: Giant Cell Arteritis; HC: Healthy Controls; aHC: aged Healthy Controls; LVV : Large Vessel Vasculitides; CRP : C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; MRA: MR-Angiography; TCZ: Tocilizumab; GUSTO: GCA treated with Ultra-Short glucocorticoids followed by TCZ; TNF: Tumor Necrosis Factor; MMP-3 (2,9): Matrix Metalloproteinase 3 (2,9); TIMP-1: Tissue Inhibitor of Metalloproteinases; sIL-2R: Soluble Interleukin-2-Receptor; IL-6: Ilnterleukin-6; CD163: Cluster of Differetiation; DMARD: Disease-modifying anti-rheumatic drug; TNFα inhibitors: Tumor Necrosis Factor α inhibitors; PTX-3: Pentraxin-3; IL-17: Interleukin-17; GC: Glucocorticoid
Introduction
Takayasu Arteritis (TAK) and Giant Cell Arteritis (GCA) are large vessel vasculitides with similarities in histology and pathogenesis [1]. However, TAK begins early in life and primarily affects women, whereas GCA is a disease of elderly and old-age persons of both sexes [2,3]. TAK typically leads to vascular stenosis whereas GCA eventually to life-threatening aneurysma formation. Systemic inflammation as measured with CRP is generally higher in active GCA than in active TAK. Taken together, these clinical differences together with pathogenetic similarities offer interesting comparisons regarding biomarkers in sera.
Disease activity of Large Vessel Vasculitides (LVV) is traditionally assessed by signs, symptoms and the inflammatory serological markers CRP and ESR. Recently, imaging techniques such as MR-Angiography (MRA) and PET-CT have been introduced for diagnosis, however their role in monitoring disease activity is debated. Studies have shown persisting wall enhancement in MR-Angiography (MRA) as well as in PET-CT of GCA patients in clinical and serological remission [4, 5]. Similarly, wall enhancement in TAK has been documented to persist in remission in many patients [6]. Regarding inflammatory markers, IL-6 blocking agents such as Tocilizumab (TCZ) suppress production of hepatic acute phase proteins and render CRP unreliable [7, 8]. The revised EULAR recommendations list signs and symptoms of active TAK, but as recently shown, even these cannot capture mild disease activity [9-11].
In conclusion, neither imaging techniques, nor clinical signs and symptoms nor conventional biomarkers help in capturing and quantifying ongoing disease. There is an obvious need for the identification of biomarkers which are not under the direct control of IL-6 and reflect subclinical disease activity.
After detailed analysis and description of our TAK patient cohort [10], we set out to analyze a broad range of biomarkers including regulatory molecules, cell-type specific parameters and catabolic enzymes in sera of patients with active disease as compared to remission, and we studied the effect of the different drug treatments on serum levels of these biomarkers. Furthermore, we compared the results with the sera of new-onset GCA patients included in the GUSTO study [12] and with age-and sex-matched controls. Finally, we searched for an age-dependency of serum concentration of the molecules of interest.
Patients and Methods
Patients and data collection
Sera of 26 TAK consecutive patients listed in the patient registry of the Clinical Trial Unit, University of Bern, Switzerland, were sampled at inclusion and in 19 patients at least once during follow-up (median follow-up time: 34 months). All patients fulfilled the 1990 ACR criteria for TAK [11]. Data were compared with anonymized sera of 18 patients with new-onset GCA fulfilling the 1990 ACR criteria and were evaluated for inclusion in the proof-of-concept study: GCA treated with Ultra-Short glucocorticoids followed by TCZ [GUSTO: ClinicalTrials.gov, NCT03745586] [12]. TAK patients were aged between 18 and 47 years, and GCA patients were aged between 63 and 91 years. Twenty-six age- and sex-matched healthy individuals were analyzed as controls for TAK and 18 unpaired healthy individuals (aged 59 – 92 years) as controls for GCA. Healthy controls did not take any regular medication nor anti-inflammatory agents, and they did not show signs of inflammation nor infection within 4 weeks of venipuncture. Immunosuppressive therapy (IS) refers to treatment with corticosteroids (prednisolone) as well as conventional DMARDs (e.g., methotrexate) or biologics (e.g., tocilizumab).
Assessment of biomarker levels
48 biomarkers representing T lymphocytes, regulatory T cells, B lymphocytes and monocytes, cytokines of the TNF and Interferon superfamily, catabolic enzymes such as MMPs, adipocytokines and inflammatory markers such as high sensitivity CRP were analyzed (Supplementary table S1). Serum samples were processed according to SOP (processing 30 minutes after venipuncture, centrifugation at 2730 r.p.m. for 10 min, storing in aliquots at -80°C). In a first step, biomarkers were assayed with Luminex technology (R&D Systems, Minneapolis, USA and Invitrogen, Carlsbad, USA). Samples were quantified on a multiplex system [Bio-Plex 100 array reader with Bio-Plex Manager software (version 6.1); Bio-Rad, Hercules, CA, USA]. In a second step, biomarkers showing different serum levels between the TAK groups and subgroups in three independent measurements were confirmed and further analyzed using commercial Enzyme-Linked Immunosorbent Assay (ELISA) kits according to the manufacturer’s protocol (R&D Systems, Minneapolis, USA and Invitrogen, Carlsbad, USA). If not specified, protein concentrations are given in pg/ml. C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR) were measured in the central laboratory of the University Hospital of Bern from the same blood samples.
Magnetic resonance angiography
MRA were performed in 19 TAK patients within 4 months of sera collection and vessel wall signals of the aorta were evaluated by two experience radiologists according to a published protocol [5]. In brief vessel wall signals scored from 0 to 3; 0 = no mural thickening (maximal vessel wall thickness <2.3 mm), no enhancement; 1 = no thickening, slight mural enhancement; 2 = mural thickening (>2.3 mm), significant mural enhancement; 3 = strong thickening (>3 mm), strong mural and perivascular enhancement. A score ≥ 2 was predefined as active mural inflammation.
Criteria for disease activity
The revised EULAR criteria were used to assess disease activity [9]. In addition to key symptoms and key findings, at least one of the following criteria had to be present: a) current activity on imaging b) ischemic complications attributed to large vessel vasculitis, c) persistently elevated inflammatory markers (after exclusion of other causes).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 software and IBM SPSS Statistics 25. Continuous and categorical variables are presented as median [lower quartile, upper quartile] or number and percentage of patients. In case of missing data, the resulting number of data is added, and the %-age refers to this number. Wilcoxon signed-rank test was used to calculate the significance of differences between paired sample groups, and the Mann–Whitney U test was used to calculate the significance of differences between unpaired sample groups. Effect sizes were estimated using the rank-biserial correlation (r₍b₎). To analyze the relationship between age and biomarker levels, simple linear regression was performed. Spearman’s rho was used for correlation between biomarkers and CRP/ESR. Missing data were handled via case-wise deletion. No imputation was performed. Only participants with complete data for each specific analysis were included.
Ethical Approval and Patient Informed Consent
The study was approved by the local ethics committee (Kantonale Ethikkommission Bern; numbers 2016-00338 and 2018–00845) and performed in accordance with the Declaration of Helsinki. All TAK patients gave written informed consent.
Results
Patient characteristics
The study cohort comprised 26 TAK patients of the registry and 18 GCA patients of the GUSTO study [10,12]. The female to male ratio was (13:1) in the TAK group and (1.6:1) in the GCA group. The median age was 33 (28, 43) in the TAK group and 71 (66, 73) in the GCA group. 19 TAK patients were followed-up for 7 to 44 months (median follow-up time 34 (26, 36) months). Seven of the TAK patients were clinically active according to the EULAR criteria at time of blood collection, while 2 patients showed signs of disease activity but did not fulfill the EULAR criteria and were therefore categorized as not active. All GCA patients had new-onset GCA with active disease. Twenty TAK patients received immunosuppressive therapy (Supplementary table S2). Twelve of the GCA patients were treatment naïve while the remaining 6 patients were treated with oral prednisone up to 1mg/kg/day for a maximum of 7 days. Demographics of TAK and GCA patients are summarized in table 1.
| Table 1: Demographic profiles of 26 TAK patients and 18 GCA patients at baseline. | ||
| TAK (median, quartiles) | GCA (median, quartiles) | |
| Age | 33 (28,43) | 71 (66,73) |
| Disease duration | 4.4 (1.7,12.1) years | < 4 weeks |
| TAK (number, percentages) | GCA (number, percentages) | |
| Female sex | 24 (92%) | 11 (61%) |
| Active disease at baseline or during follow up | 7 (27%) | 18 (100%) |
| Immunosuppressive therapy | 20 (77%)* | 6 (33%)** |
| *Prednisone, cDMARDs, biologics **Prednisone 1-7 days (median 3 days) | ||
Biomarker Levels of Tak Patients Compared with Age- and Sex-Matched Healthy Controls
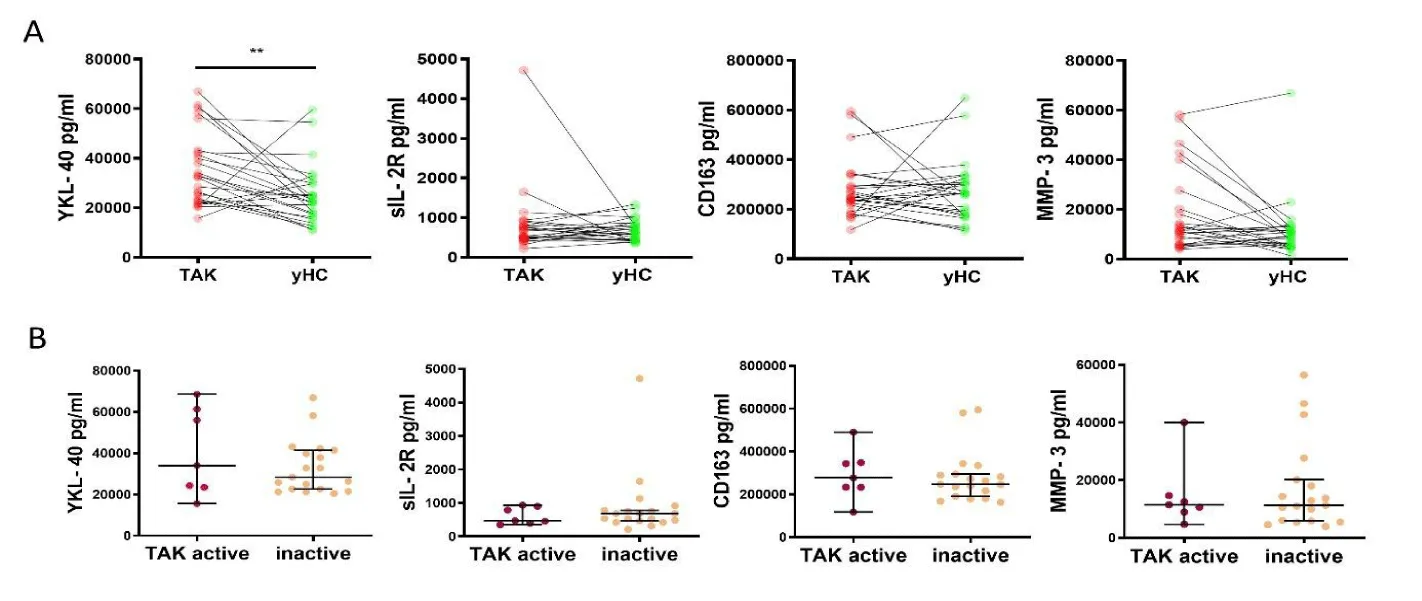
Upon three independent experiments measuring biomarker levels, only 5 of the 48 biomarkers remained of potential interest to answer the posed questions. YKL-40 concentrations were significantly elevated in TAK patients compared to healthy controls: TAK 32516 (22697, 42523) vs controls 22767 (17124, 30230) (p = 0.024) with r₍b₎= -0.66. In contrast, we could not observe different concentrations of MMP-3, sIL-2R and CD163 between the two groups: MMP-3 in TAK 11413 (5981, 22040) vs controls 9572 (5636, 12596), sIL-2R in TAK 612 (470, 816) vs controls 633 (444, 817), CD163 in TAK 246487 (210535, 304629) vs controls 264622 (177556, 316497) (figure 1A).
Biomarker levels of active, inactive and indeterminate TAK patients
YKL-40 concentrations showed a tendency to be higher in TAK patients with active disease compared to TAK patients in remission, but the differences did not reach significance (active patients 34078 (23532, 61387) vs inactive patients 28458 (22667, 41476)). Serum levels of MMP-3, sIL-2R and CD163 did not differ between TAK patients with active diseases and TAK patients in remission: MMP-3 in TAK active 11476 (8960, 14667) vs TAK inactive 111350(5881, 20163), sIL-2R in TAK active 473 (393, 902) vs TAK inactive 686 (464, 782), CD163 in TAK active 277181 (233317, 348119) vs inactive 246985 (2190910, 595260) (figure 1B).
Correlation of biomarker levels with arterial wall enhancement and with CRP and ESR levels
MRA signals of 11 TAK patients indicated vessel wall inflammation but none of the biomarkers showed a significant association with the MRA score (not shown).
Correlations between biomarker levels and inflammatory markers (CRP and ESR) were analyzed in 42 and 39 patients, respectively. No significant associations were observed between any of the four biomarkers and CRP or ESR levels. Specifically, the following and p-values and Spearman correlation coefficients (ρ) were found:YKL-40/CRP (p = 0.116, ρ = 0.246), YKL-40/ESR (p = 0.518, ρ = 0.107), sIL-2R/CRP (p = 0.878, ρ = -0.024), sIL-2R/ESR (p = 0.272, ρ = -0.180), CD163/CRP (p = 0.179, ρ = 0.212), CD163/ESR (p = 0.278, ρ = 0.178), MMP-3/CRP (p = 0.473, ρ = -0.114), MMP-3/ESR (p = 0.190, ρ = -0.185). As none of these correlations reached statistical significance, graphs are not shown.
Effect of immunosuppressive agents on biomarker levels
Glucocorticoids: The most pronounced effect was found on MMP-3 levels: TAK patients treated with glucocorticoids (GC) had higher concentrations (33848 (12271, 49059) vs TAK without GC 9956 (5453, 13354) (p = 0.0015)) with r₍b₎ = 0.71. A trend was observed for higher YKL-40 levels in patients treated with GC (TAK with GC 39726 (27226, 58755) vs TAK without GC 25917 (21393, 36941 p = 0.1057). Concentrations of sIL-2R and CD163 were not affected by GC treatment: sIL-2R TAK with GC 515 (470, 923) vs TAK without GC 687 (419, 785); CD163 TAK with GC 248229 (229257, 290659) vs TAK without GC 236414 (179636, 339498) (figure 1C).
Immunosuppressive (IS) therapy (with or without GC): CD163 concentrations were increased in TAK patients in drug-free remission compared to TAK patients treated with any kind of IS: TAK with IS 235031 (183108, 270366) vs TAK without IS 417112 (273207, 584025) (p = 0.0020) with r₍b₎ = 0.80. For YKL-40, sIL-2R and MMP-3, no differences were found between TAK patients with and without IS: YKL-40 with IS 32889 (22913, 43009) vs TAK patients without IS 25482 (22135, 43865), sIL-2R with IS 526 (466, 775) vs TAK patients without IS 830 (619, 2413), MMP-3 with IS 12728 (6431, 36935) vs TAK patients without IS 9984 (5651, 11959) (figure 1D).
Patients treated with TCZ showed similar levels of YKL-40 to untreated patients and significantly lower levels than patients treated with DMARDs and/or TNFα inhibitors; TAK with TCZ 27447 (21349, 39939) vs TAK with DMARDs and/or TNFα inhibitors 39726 (33168, 59851) (p = 0.0473) with r₍b₎=0.54, TAK without IS 25482 (22135, 43865). Treatment with TCZ or DMARDs and/or TNFα inhibitors lead to lower levels of CD163 compared to untreated patients; TAK with TCZ 203993 (170695, 259730) vs TAK without IS 417112 (273207, 584025) (p = 0.0032) with r₍b₎=0.83 and TAK with DMARDs and/or TNFα inhibitors 244446 (234340, 285158) vs TAK without IS 417112 (273207, 584025) (p = 0.0200) with r₍b₎ = 0.75. sIL-2R and MMP3 did not differ in serum levels between the three treatment groups; sIL-2R TAK with TCZ 533 (433, 738) vs TAK with DMARDs and/or TNFα inhibitors 515 (469, 886) vs TAK without IS 758 (418, 1645). MMP-3 TAK with TCZ 11035 (6141, 25258) vs TAK with DMARDs and/or TNFα inhibitors 17276 (7545, 45634) vs TAK without IS 11007 (6015, 13787) (Supplemental figure 1).
Correlation between age and biomarkers in healthy controls
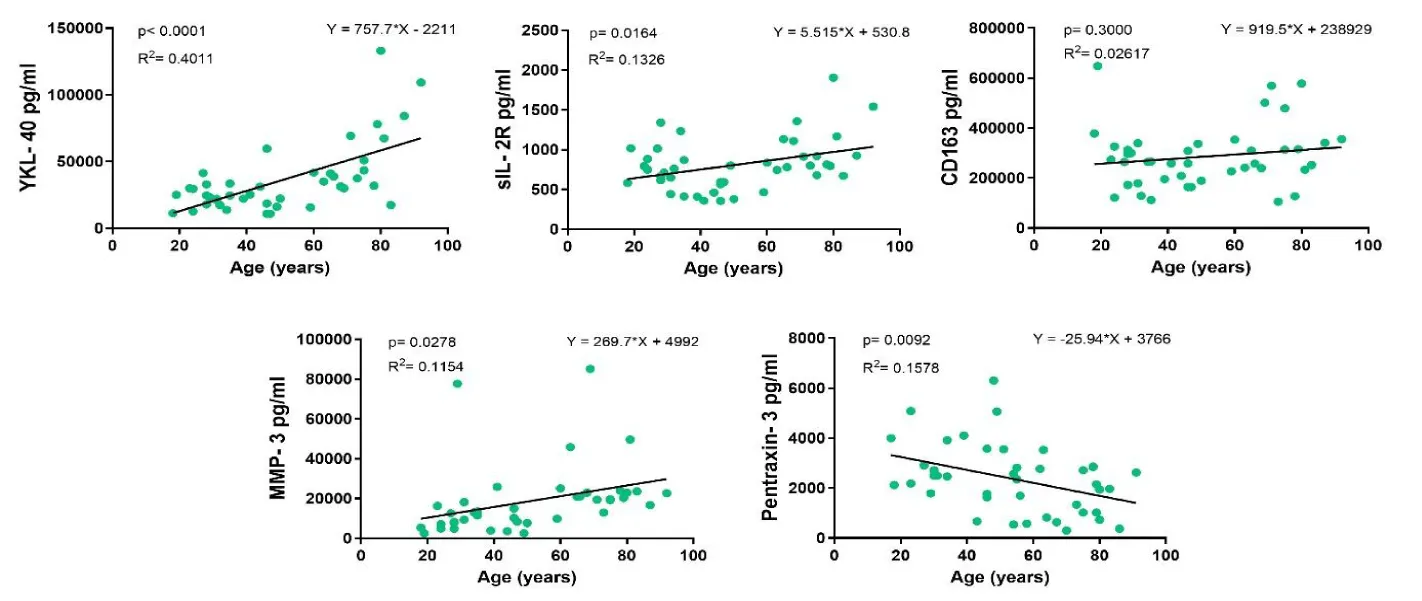
In 44 healthy individuals (18 to 92 years old), an age-related linear increase in YKL-40, MMP3 and sIL-2R concentrations was observed. The most significant association with age was found for YKL-40 (p < 0.0001), followed by sIL-2R (p = 0.012) and MMP-3 (p = 0.0278). Pentraxin-3, on the other hand, showed a decrease in concentration with age (p = 0.0092) (figure 2).
Biomarker concentrations in GCA patients compared with healthy controls
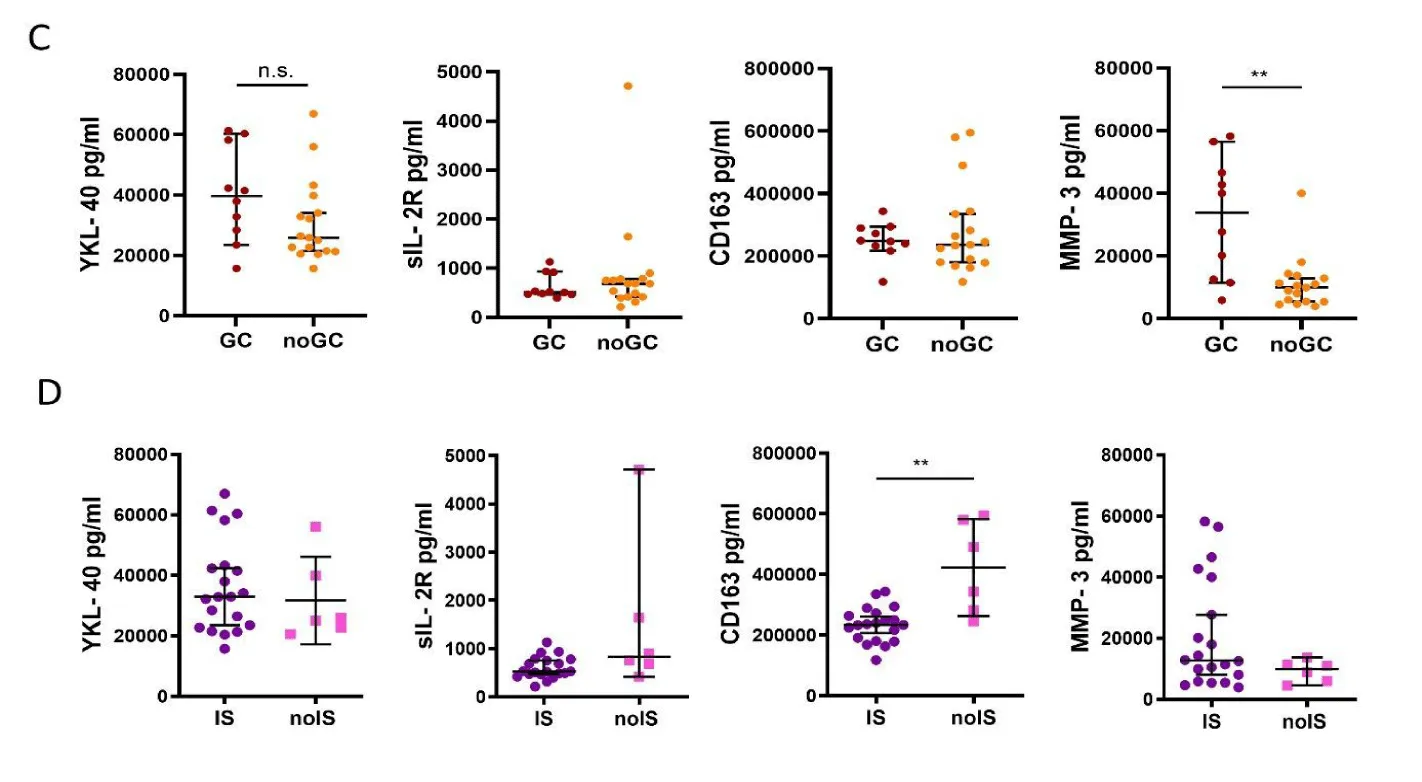
YKL-40 concentrations were higher in GCA patients compared with age-matched healthy controls: GCA 70286 (53944, 97511) vs controls 41443 (31857, 71517) (p = 0.0224) with r₍b₎=0.44. However, there was no difference in concentrations of MMP-3, sIL-2R and CD163 between the two groups: MMP-3 GCA 11767 (9183, 29220) vs controls 10776 (8110, 15624), sIL-2R GCA 1156 (822, 1328) vs controls 875 (712, 1141), CD163 GCA 360735 (257391, 535914) vs controls 312195 (238357, 386351) (figure 3A).
Biomarker levels in GCA as compared with TAK patients
GCA sera contained higher concentrations of YKL-40, sIL-2R and CD163 compared to TAK patients, while MMP-3 concentrations were equal in both groups: YKL-40 TAK 22697 (32516, 42523) vs GCA 70286 (53944, 97511) (p < 0.0001) with r₍b₎=0.80, sIL-2R TAK 612 (470, 816) vs GCA 1156 (822, 1328) (p = 0.0006) with r₍b₎ = 0.59, CD163 TAK 246487 (210535, 304629) vs GCA 360735 (257391, 535914) (p = 0.0245) with r₍b₎=0.40 and MMP-3 TAK 11413 (5981, 22040) vs GCA 11767 (9183,29220) (figure 3B).
Discussion
Our study set out to explore a broad range of biomarkers seeking to distinguish TAK from healthy controls and monitor disease activity. Among these biomarkers, many were either undetectable or showed no significant differences between samples and were therefore not included in further analysis. These low systemic biomarker levels probably reflect an immune response restricted to the vessel wall, characterized by localized cytokine release with limited spillover into the circulation. Such a pattern is particularly typical of chronic, low-grade inflammation in TAK, eventually leading to severe stenosis. Moreover, immunosuppressive therapies, especially corticosteroids and IL-6 inhibitors rapidly normalize a broad range of systemic markers such as CRP, ESR, and IL-6, despite persistent vascular inflammation [13, 14].
YKL-40 was the only one demonstrating modest yet statistically significant discrimination reliably differentiating between TAK and healthy controls. YKL-40 was shown to reflect disease activity in earlier studies, but it did not consistently reach significance in our experiments [15].
Previous studies have highlighted the complex role of YKL-40, also known as Chitinase-3-Like protein 1 (CHI3L1), in various physiological and pathological processes. YKL-40, a product of activated neutrophils, macrophages, and vascular smooth muscle cells, plays a central role in arterial inflammation, extracellular tissue remodeling, and fibrosis. These findings render YKL-40 a potential biomarker for detection of disease activity in TAK [15].
An increase of YKL-40 was previously shown, along with other inflammatory markers such as IL-6, PTX-3 and MMP-9 in active TAK according to NIH criteria for active disease [15]. While the individual markers could not segregate active and inactive disease states, a combination with inclusion of YKL-40 enhanced the sensitivity and specificity of differentiation. These findings resonate with our observation on the unique position of YKL-40 among the biomarkers tested.
The fact that YKL-40 was measurable in some patients with inactive disease (based on current definition) but reached values of age-matched healthy controls in lasting drug-free remission could best be explained by persistent localized vessel wall inflammation in the former group. Thus, YKL-40 could eventually qualify as a marker to detect subclinical disease activity and help guide therapy.
T lymphocyte markers such as sIL-2R, but also IL-17 and IFγ (not shown) remained very low and unchanged over time. This may primarily be due to the fact that T cells are recruited into the vessel wall where they act locally. Their cytokines act in paracrine fashion and do not spill-over into circulation in measurable quantities.
In contrast to regulatory molecules e.g. proinflammatory cytokines, effector molecules such as MMPs might better suit to measure disease activity. This is in line with recent publications showing promising results for MMP2, MMP9 and TIMP-1 [15-18]. In most studies, however, sera were collected after initiation of treatment, mostly treatment with GC. Therefore, it is notoriously difficult to differentiate between the effects of GC and the effects of disease. This has been exemplified by MMP3, which has been found to increase in response to GC [19]. Our data confirm further support this notion.
None of the biomarkers measured in active TAK showed a correlation with vessel wall activity as quantified with late enhancement in MRI. It corresponds to the lack of a predictive value of MRI signals in the aortic wall regarding relapse in GCA [20]. So far, it remains impossible to answer the question whether the lasting signals reflect persisting local inflammation of purely persistent hyperperfusion of neovascularized thickened vessel walls. However, it would be reasonable to assume that a change in localization or an increase of intensity of wall enhancement is of inflammatory origin. In these cases, a prompt increase in immune-suppressive therapy will eventually lead to an extinction of vessel wall activity. Indeed, some patients of our cohort showed lasting full remission as assessed by MRI. Of note, most of those were treated with infliximab.
As illustrated above GC treatment changes biomarker profiles, mainly in suppressing proinflammatory markers. However, GC use was also associated with elevated expression of molecules such, as MMP-3, and showed a non-significant trend toward increased YKL-40 levels. Interestingly, treatment with TCZ monotherapy but not with DMARDs or infliximab normalized levels of YKL-40, whereas the cell-specific molecules sIL-2R and CD163 did not differ between treatment groups. The suppression of sIL-2R and CD163 below levels in sera of patients in drug-free remission (Supplemental figure 1) might suggest overtreatment and may open up a new avenue in that biomarkers might not only help measuring disease activity but also help to titrate treatment intensity.
The comparison of TAK with CGA shows significant differences in YKL-40, sIL2R and CD163 concentrations. All three are higher in GCA, which might be explained by the higher local and systemic inflammation of GCA. Based on the design of our study with healthy controls for TAK as well as GCA patients, we could also analyze age-dependent differences in biomarker levels. Indeed, we could show for the first-time age-dependent differences for all presented molecules in healthy persons. These findings must be taken into account in interpreting the data of diseased persons.
Age is the most important risk factor for development of GCA, implicating age-related immunological and vascular changes as key contributors in the pathogenesis of GCA. Senescent cells undergo a phenotypic shift marked by the Senescence-Associated Secretory Phenotype (SASP), characterized by the release of proinflammatory cytokines and other mediators important for cell homeostasis. The observed age-dependent differences in circulating biomarker levels among healthy individuals in our study population support the notion of distinct secretory profiles shaped by immunosenescence [21]. The aging immune system is further characterized by a marked decline in both the number and function of CD8⁺ T cells, including CD8⁺ regulatory T cells (Tregs), which normally suppress vessel-resident CD4⁺ T cells through the release of NOX2-containing exosomes. In GCA, these cells fail prematurely due to abnormal NOTCH4 signaling, which disrupts endosomal transport and impairs exosome-mediated immune regulation, contributing to persistent vascular inflammation. Furthermore, GCA shows a proinflammatory shift in macrophage polarization amplifying IL-6 signaling, among others, driving systemic inflammation [22]. In contrast, TAK arises in younger individuals with a more stringently regulated immune responses and senescent cell burden [21]. This may, in some degree, account for the observed differences in systemic biomarker levels between TAK and GCA [21].These findings must be taken into account in interpreting the data of diseased persons. Of noteHowever, the measured differences between TAK and GCA were substantially higher than the age-associated changes.
The main weakness of our study is the small numbers. They precluded comparison of treated versus non-treated patients and limited the analysis treatment effects across different therapy modalities except monotherapy with TCZ. On the other hand, the data of both cohorts of patients were prospectively collected, analyzed and published (10, 12), the data of GCA in a formal study to analyze the effect of TCZ after ultra-short treatment with GC (GUSTO study). We selected the molecules to be measured in a hypothesis-driven approach. To identify novel molecules, it would be necessary to perform a proteome-analysis. Possibly this would lead to not yet unrecognized molecules or sets of molecules of diagnostic and prognostic value and/or suitable to monitor disease-activity and treatment intensity. Another limitation of our design is the lack of age- and sex-matched controls for the GCA group, which may lead to residual confounding factors. Future studies should include paired controls for both disease cohorts.
In summary, the prospectively collected data of two patient cohorts and healthy controls show higher biomarker levels for active GCA than TAK, a disease-independent age-correlated change in biomarker concentrations and a lack of correlation between biomarkers and vessel wall enhancement as measured with MRI. They support an inducing effect of GC on MMP3 expression and show a suppressing effect of immuno-therapy on CD-163 and sIL-2R below levels of patients in drug-free remission. Of 48 measured molecules, YKL-40 was the only one elevated in TAK and active TAK. Its value in diagnosing TAK and vascular disease activity merits further evaluation.
Competing Interests
A.G. none; L.C. research/non-financial support, advisory fee and stock ownership from Gebauer Stiftung, Gilead Sciences, F. Hoffmann-La Roche, Novartis, Pfizer, Bristol-Myers Squibb, Vifor, and Sanofi., J.C. and H.B. none; P.V. speaker, advisory fees and research support by Roche, MSD, Abbvie, Grünenthal, Drossapharm, Amgen, GSK, Vifor, AstraZeneca.
Funding
The study was funded by the Research Funds of the Department of Rheumatology, University Hospital of Bern.
References
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11. doi: 10.1002/art.37715. PubMed PMID: 23045170.
- Borchers AT, Gershwin ME. Giant cell arteritis: a review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun Rev. 2012;11(6-7):A544-54. Epub 2012/01/21. doi: 10.1016/j.autrev.2012.01.003. PubMed PMID: 22285588.
- Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919-29. doi: 10.7326/0003-4819-120-11-199406010-00004. PubMed PMID: 7909656.
- Salvarani C, Soriano A, Muratore F, Shoenfeld Y, Blockmans D. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16(11):1125-30. Epub 2017/09/09. doi: 10.1016/j.autrev.2017.09.007. PubMed PMID: 28899801.
- Reichenbach S, Adler S, Bonel H, Cullmann JL, Kuchen S, Bütikofer L, et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford). 2018;57(6):982-6. doi: 10.1093/rheumatology/key015. PubMed PMID: 29529280.
- Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G, et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60(4):1193-200. doi: 10.1002/art.24416. PubMed PMID: 19333926.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Glucocorticoid Dosages and Acute-Phase Reactant Levels at Giant Cell Arteritis Flare in a Randomized Trial of Tocilizumab. Arthritis Rheumatol. 2019;71(8):1329-38. Epub 2019/07/03. doi: 10.1002/art.40876. PubMed PMID: 30835950; PubMed Central PMCID: PMC6772126.
- Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R204. doi: 10.1186/ar4397. PubMed PMID: 24295403; PubMed Central PMCID: PMC3978585.
- Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2019. Epub 2019/07/03. doi: 10.1136/annrheumdis-2019-215672. PubMed PMID: 31270110.
- Gloor AD, Chollet L, Christ LA, Cullmann JL, Bonel HM, Villiger PM. Takayasu arteritis: Prevalence and clinical presentation in Switzerland. PLoS One. 2021;16(6):e0250025. Epub 20210618. doi: 10.1371/journal.pone.0250025. PubMed PMID: 34143786; PubMed Central PMCID: PMC8213155.
- Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129-34. PubMed PMID: 1975175.
- Christ L, Seitz L, Scholz G, Sarbu AC, Amsler J, Bütikofer L, et al. Tocilizumab monotherapy after ultra-short glucocorticoid administration in giant cell arteritis: a single-arm, open-label, proof-of-concept study. Lancet Rheumatol. 2021;3(9):e619-e26. Epub 20210702. doi: 10.1016/s2665-9913(21)00152-1. PubMed PMID: 38287611.
- Keser G, Aksu K, Direskeneli H. Discrepancies between vascular and systemic inflammation in large vessel vasculitis: an important problem revisited. Rheumatology (Oxford). 2018;57(5):784-90. doi: 10.1093/rheumatology/kex333. PubMed PMID: 28968895.
- Christ L, Gloor AD, Kollert F, Gaber T, Buttgereit F, Reichenbach S, et al. Serum proteomics in giant cell arteritis in response to a three-day pulse of glucocorticoid followed by tocilizumab monotherapy (the GUSTO trial). Front Immunol. 2023;14:1165758. Epub 20230523. doi: 10.3389/fimmu.2023.1165758. PubMed PMID: 37287970; PubMed Central PMCID: PMC10242646.
- Sun Y, Kong X, Wu S, Ma L, Yan Y, Lv P, et al. YKL-40 as a new biomarker of disease activity in Takayasu arteritis. Int J Cardiol. 2019;293:231-7. Epub 2019/07/04. doi: 10.1016/j.ijcard.2019.06.058. PubMed PMID: 31303395.
- Cui X, Qin F, Song L, Wang T, Geng B, Zhang W, et al. Novel Biomarkers for the Precisive Diagnosis and Activity Classification of Takayasu Arteritis. Circ Genom Precis Med. 2019;12(1):e002080. doi: 10.1161/CIRCGEN.117.002080. PubMed PMID: 30645172.
- Wu G, Mahajan N, Dhawan V. Acknowledged signatures of matrix metalloproteinases in Takayasu's arteritis. Biomed Res Int. 2014;2014:827105. Epub 2014/09/03. doi: 10.1155/2014/827105. PubMed PMID: 25276821; PubMed Central PMCID: PMC4167960.
- Sun Y, Ma L, Yan F, Liu H, Ding Y, Hou J, et al. MMP-9 and IL-6 are potential biomarkers for disease activity in Takayasu's arteritis. Int J Cardiol. 2012;156(2):236-8. Epub 20120212. doi: 10.1016/j.ijcard.2012.01.035. PubMed PMID: 22330005.
- Gloor AD, Yerly D, Adler S, Reichenbach S, Kuchen S, Seitz M, et al. Immuno-monitoring reveals an extended subclinical disease activity in tocilizumab-treated giant cell arteritis. Rheumatology (Oxford). 2018;57(10):1795-801. doi: 10.1093/rheumatology/key158. PubMed PMID: 29961816.
- Adler S, Reichenbach S, Gloor A, Yerly D, Cullmann JL, Villiger PM. Risk of relapse after discontinuation of tocilizumab therapy in giant cell arteritis. Rheumatology (Oxford). 2019. Epub 2019/03/26. doi: 10.1093/rheumatology/kez091. PubMed PMID: 30915462.
- Gloor AD, Berry GJ, Goronzy JJ, Weyand CM. Age as a risk factor in vasculitis. Semin Immunopathol. 2022. Epub 20220209. doi: 10.1007/s00281-022-00911-1. PubMed PMID: 35141865.
- Jiemy WF, van Sleen Y, Graver JC, Pringle S, Brouwer E, van der Geest KSM, et al. Indication of Activated Senescence Pathways in the Temporal Arteries of Patients With Giant Cell Arteritis. Arthritis Rheumatol. 2023;75(10):1812-8. Epub 20230628. doi: 10.1002/art.42525. PubMed PMID: 37057491.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.