2025 June 16;6(6):715-728. doi: 10.37871/jbres2125.
Emerging Combination Therapies Involving Photodynamic Therapy for Basal and Squamous Cell Carcinomas
Calista Persson1* and Akhil Gupta2
2Skin Center of Florida, North Palm Beach, FL
- Photodynamic therapy
- Basal cell carcinoma
- Squamous cell carcinoma
- Combination therapy
- Immunotherapy
- Photochemotherapy
- Nanotechnology
- Non-melanoma skin cancer
Abstract
Background: Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC) are the most prevalent non-melanoma skin cancers. Photodynamic Therapy (PDT) is an established, non-invasive treatment known for its favorable cosmetic results, especially in superficial lesions. However, its limited penetration restricts effectiveness against thicker or more aggressive tumors, prompting the development of combination strategies to enhance PDT's efficacy.
Methods: This narrative review examines studies published from 2015 to 2025 that explore combining PDT with other treatments for BCC and SCC. A systematic search identified clinical trials, cohort studies, and case reports focusing on combinations with immunotherapy, chemotherapy, physical debulking, nanotechnology, and pharmacologic agents. The analysis considered treatment outcomes, safety, mechanistic insights, and therapeutic synergy.
Results: Combination treatments showed improved tumor clearance rates, reduced recurrence, and enhanced immune responses. Immunomodulators and immune checkpoint inhibitors boosted the immune effects of PDT. Topical chemotherapy, like fluorouracil, enhanced photosensitizer uptake and lesion clearance. Mechanical techniques, such as curettage or laser ablation, improved cure rates for nodular BCC. Nanoparticle delivery systems enabled deeper tissue penetration, while agents like vitamin D analogues and mTOR inhibitors increased tumor sensitivity to PDT. Importantly, these combinations preserved cosmetic outcomes while expanding efficacy for more aggressive lesions.
Conclusion: Integrating PDT with immunological, chemical, physical, or nanotechnological approaches represents a significant advancement in non-melanoma skin cancer treatment. These combinations overcome traditional monotherapy limitations and maintain cosmetic benefits. Future large-scale trials are crucial to refine protocols, validate long-term outcomes, and encourage routine clinical implementation.
Abbreviations
BCC: Basal Cell Carcinoma; SCC: Squamous Cell Carcinoma; PDT: Photodynamic Therapy; ALA: 5-Aminolevulinic Acid; 5-FU: 5-Fluorouracil; ICB: Immune Checkpoint Blockade; cSCC: Cutaneous Squamous Cell Carcinoma; PD-1: Programmed Death-1; PD-L1: Programmed Death-Ligand 1; IFN-γ: Interferon-gamma; TNF-α: Tumor Necrosis Factor-alpha; DC: Dendritic Cell; NMSC: Non-Melanoma Skin Cancer; MAL: Methyl Aminolevulinate; PTT: Photothermal Therapy; mTORC: Mammalian Target of Rapamycin Complex 1; PDD: Ppix fluorescence; NSAIDs: Nonsteroidal Anti-Inflammatory Drugs
Introduction
The great bulk of cutaneous malignancies are non-melanoma skin cancers, mostly Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC). About eighty per cent of all skin malignancies are BCC alone. Although seldom metastatic, these carcinomas can be locally invasive and cause great morbidity. The gold-standard treatment is routine surgical excision since it provides high cure rates, often >95% for initial lesions [1]. For patients with many lesions, lesions in cosmetically sensitive places, or those unsuited for surgery, surgery may not be feasible, nevertheless. Under these circumstances, non-surgical treatments are quite useful substitutes. Especially in superficial subtypes of some BCC and SCC, Photodynamic Treatment (PDT) has become a useful non-invasive method. PDT generates reactive oxygen species that damage tumour cells by first administering a photosensitiser (such as topical 5-aminolevulinic acid, ALA), then lighting with visible light. It appeals to face lesions and regions where tissue-sparing is crucial since it provides outstanding cosmetic results with less scarring.
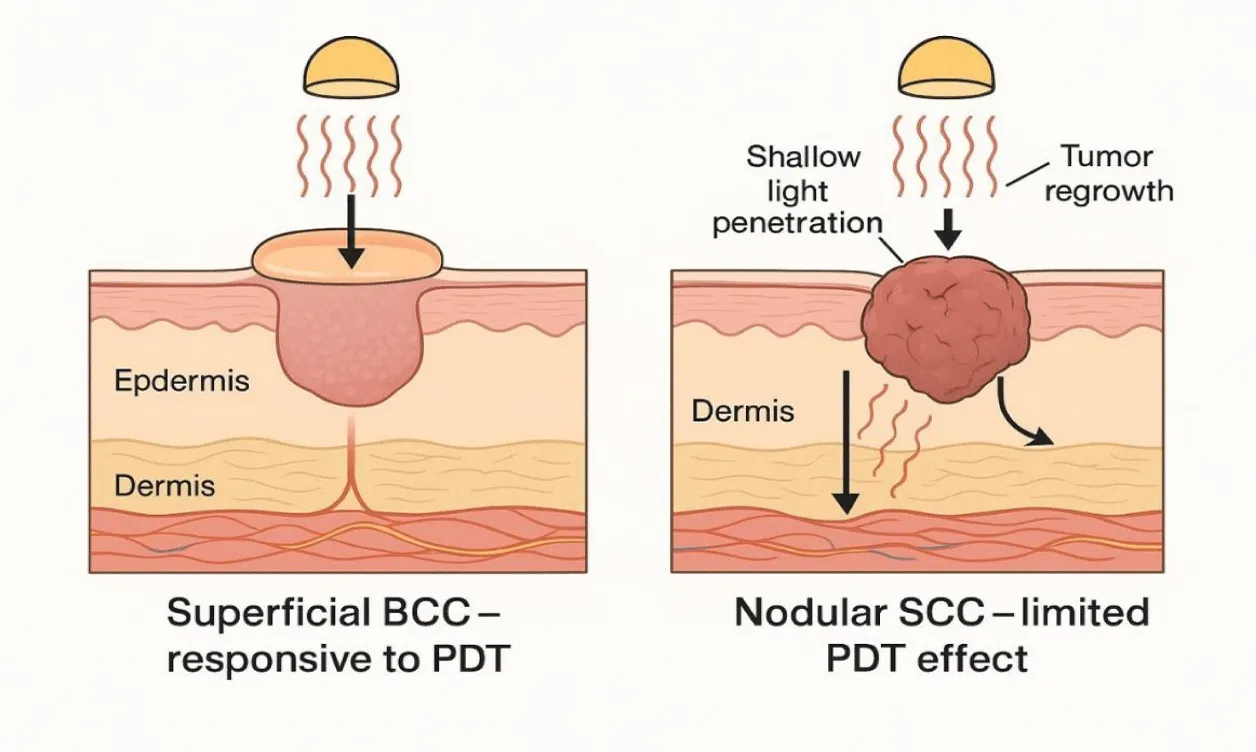
Conventional PDT as monotherapy has major restrictions, even if it offers benefits. Usually, at a depth of penetration of 2–3 mm, larger or nodular tumours react less fully. Although they are good, clinical response rates of PDT for low-risk BCC are still below those of surgery; for example, 1-year clearance rates for superficial BCC range from 80–90%, while excision yields >95%. While invasive SCC (particularly larger, poorly differentiated tumours) can be less sensitive and prone to recurrence, SCC in situ, Bowen's disease, responds well to PDT. Insufficient photosensitiser penetration, tumour hypoxia, and an immunosuppressive tumour microenvironment, allowing leftover cancer cells to survive, explain the less-than-ideal results in these situations. Therefore, there is a great justification to increase PDT with other treatments able to overcome these constraints [2] (Figure 1). Illustrates the differential response of PDT in superficial versus nodular non-melanoma skin cancers, underscoring the need for combination approaches that can address deeper or biologically resistant tumors.
Combining PDT with different therapy techniques marks a new horizon meant to increase efficacy and widen indications. One can accomplish synergistic anti-cancer effects by combining PDT's tumour-selective cytotoxicity with the complimentary mechanisms of a second modality [4]. Over the past ten years, several other combinations have been investigated. These include: (1) PDT with immunotherapy, to bolster anti-tumor immune responses; (2) PDT with chemotherapy or other drugs, to increase tumour photosensitiser uptake or sensitivity; (3) PDT with physical debulking methods like laser ablation or curettage, to reduce tumour thickness and improve light penetration; (4) PDT with nanotechnology-based delivery systems, to overcome physical limitations and add multimodal effects; and (5) PDT with biochemical modulators of tumour metabolism or survival pathways, to render cancer cells more vulnerable to photodynamic damage. Early studies combining PDT with some of these techniques go back years, but since 2015, there has been a boom in clinical and translational research assessing their effectiveness in BCC and SCC.
Crucially, many of these combinations have shown promise in obtaining lower recurrence and higher clearance rates than PDT by itself without appreciably sacrificing the aesthetic or safety benefits of PDT. Combining PDT with immune checkpoint inhibition, for example, has produced lasting systemic immunity in preclinical SCC models; applying topical 5-fluorouracil (5-FU) before PDT in clinical studies has cleared actinic keratoses and stopped SCC growth [5]. In dense, nodular BCC that would typically be PDT-resistant, combining PDT with a previous CO₂ laser or dermabrasion has produced full responses. In experimental models, nanoparticle-mediated PDT systems have shown simultaneous photothermal or immune-stimulating effects in addition to deeper tumour targeting. These developments show that while preserving its advantages, targeted combination therapy can reduce the shortcomings of PDT (such as shallow penetration or tumour immune evasion). Therefore, current agreement in the field indicates that mixed modality treatment is a major focus of study for increasing the relevance of PDT in Non-Melanoma Skin Cancer (NMSC) care [6].
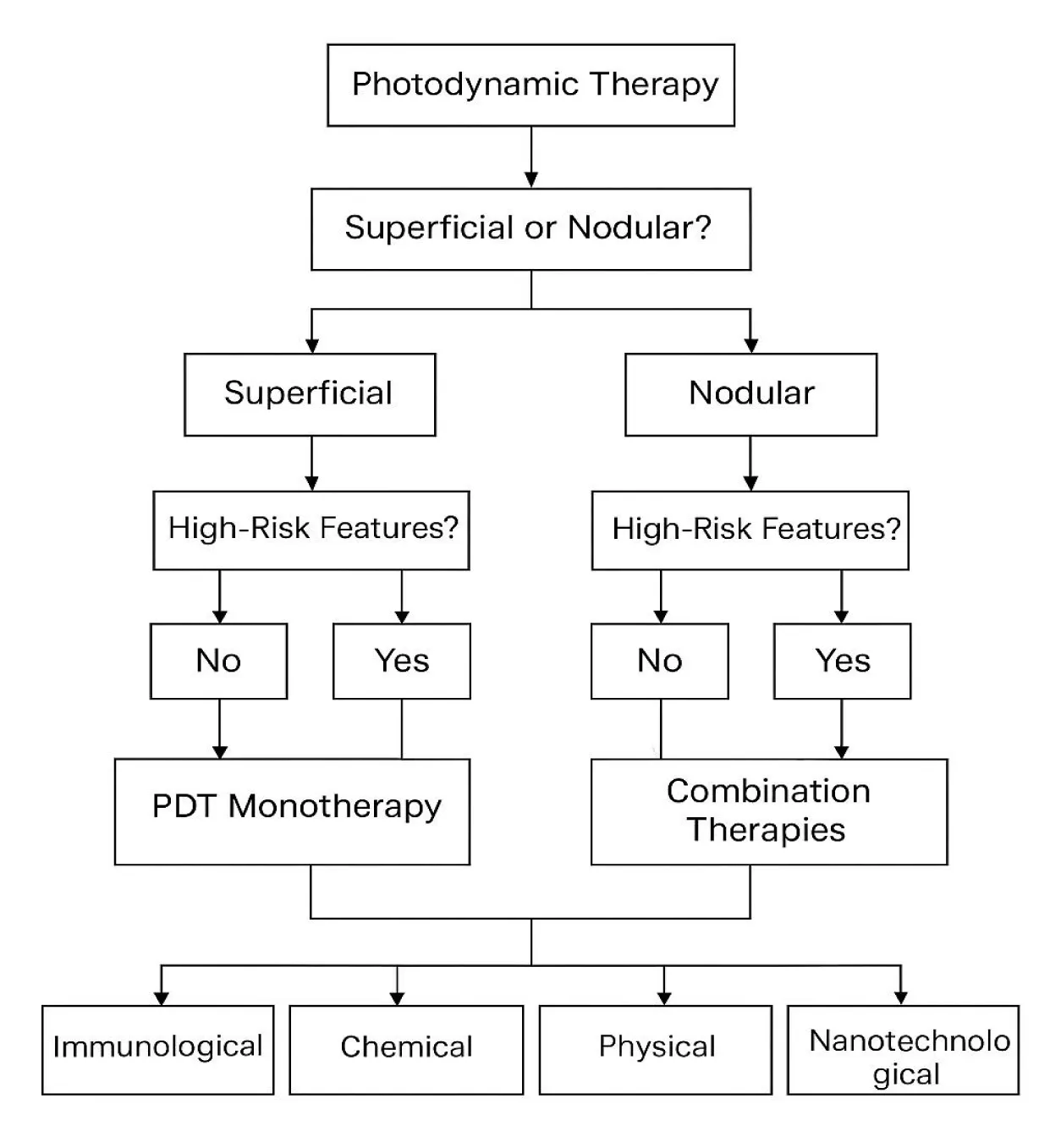
Emphasizing research from 2015 to 2025, this review provides a comprehensive overview of combination treatments, including PDT for BCC and SCC. We compile results from clinical investigations (randomized trials, uncontrolled series, and illustrated case reports) together with relevant preclinical research that clarifies mechanisms. Following a description of our literature search approach, we present the findings, organized by type of combination: Immunotherapy, chemotherapy, physical modalities, nanotechnology, and biochemical modulators. We then examine the clinical implications of these combination strategies, including their efficacy, safety, and practical considerations, and suggest how they can be integrated into therapy algorithms for BCC and SCC (Figure 2). Examining these novel approaches will help us demonstrate how combined PDT might advance the field and potentially set a new benchmark for treating some skin cancer patients. Ultimately, combining treatments could enable more patients to achieve surgical-level cure rates with a minimally invasive procedure, thereby marking a new frontier in the treatment of skin cancer.
Methods
Peer-reviewed research on combination treatments, including photodynamic therapy for BCC and SCC, was found in a literature search covering 2015 through 2025. Using terms including "photodynamic treatment, "basal cell carcinoma, "squamous cell carcinoma, "combination therapy," "immunotherapy," "chemotherapy," "laser," and "nanoparticle," the search method comprised PubMed, Scopus, and Web of Science databases. We concentrated on the main research papers evaluating PDT combined with another therapy modality, including randomised controlled trials, clinical trials, cohort and longitudinal studies, and case series or reports. Included selectively when they clarified mechanisms or proof-of-concept for combinations later used in clinical settings, were preclinical research (in vitro or animal). Except for background in the Introduction, review papers were not incorporated into the findings.
Studies were reviewed for outcomes related to treatment efficacy-such as tumor response and recurrence rates-as well as safety and relevance to non-melanoma skin cancers, specifically excluding melanoma. Eligible sources included peer-reviewed English-language publications and select conference proceedings with sufficient methodological detail. From over 100 relevant studies identified, approximately 30 high-quality, clinically significant papers were prioritized for detailed analysis. Extracted data included the type of therapeutic combination (e.g., photodynamic therapy with pharmacologic or procedural adjuncts), treatment protocols (photosensitizer type, light parameters, adjunct dosing regimens), subject characteristics, clinical outcomes, and reported mechanistic insights.
Due to the narrative nature of this review, no formal meta-analysis was performed. Instead, findings are synthesized qualitatively, with emphasis placed on higher-level evidence such as randomized controlled trials where available and on consistency across studies. The major therapeutic strategies are organized into thematic sub-sections: immunotherapy, chemotherapy, physical modalities, nanotechnology, and pharmacologic modulators. Within each section, clinical studies are presented first, followed by relevant mechanistic or translational research. This systematic framework provides a comprehensive and up-to-date overview of combination photodynamic therapy in the treatment of basal and squamous cell carcinoma.
Results
PDT combined with immunotherapy
Combining photodynamic therapy with immunotherapy is one of the leading strategies to boost systemic antitumor immune responses for treating non-melanoma skin cancers. PDT generates immunogenic cell death that causes inflammation in the tumor area. Still, the immune response it triggers lasts briefly and fails to clear remaining cancer cells, especially in tumors that suppress immune function or are less responsive to immune attack. The PDT process triggers danger signals and antigen release, which helps to bring dendritic cells and lymphocytes to the treatment area. Immunotherapeutic agents like immune checkpoint inhibitors and Toll-like receptor agonists enhance and prolong the immune response started by PDT. The combined treatment strategy increases tumor suppression capabilities, lowers recurrence rates, and establishes a long-lasting systemic immune response toward cancer cells [2,7,8].
Checkpoint inhibitors with PDT
The introduction of Immune Checkpoint Blockade (ICB) has transformed cancer treatment by increasing the effectiveness of T-cell responses against tumors. The treatment of advanced cutaneous Squamous Cell Carcinoma (cSCC) with PD-1 and PD-L1 inhibitors has proven to be highly effective therapeutically. Preclinical research indicates that using ICB in conjunction with photodynamic therapy generates enhanced antitumor responses through synergy. ALA-PDT and PD-L1 inhibition combination therapy demonstrated better tumor management than PDT alone in a UV-induced cSCC mouse model. The mice that received combined anti-PD-L1 therapy and PDT showed significant primary tumor regression and distant lesion suppression, reflecting systemic immune activation. The ability of these mice to resist subsequent tumor rechallenge demonstrated that they developed tumor-specific immunological memory. Immune potentiation was validated through mechanistic analysis, which showed greater cytotoxic T cell infiltration and higher serum levels of IFN-γ and TNF-α cytokines, thus demonstrating an adaptive immune response enhanced by checkpoint blockade. PDT induces immunogenic cell death, which works as a tumor vaccine in place, while ICB sustains T-cell activation and prevents exhaustion, thus allowing the elimination of residual or metastatic cancer cells [7,9].
The initial clinical observations reported so far uphold the results derived from experimental studies, even though clinical evidence remains insufficient. The 2019 study by Ferrara et al. detailed an elderly patient case that featured recurrent inoperable cSCC appearing as a malignant ulcer. After receiving intralesional ALA-PDT and systemic anti-PD-1 therapy through sintilimab, the lesion showed substantial regression and almost full resolution with a second treatment cycle [3]. The combined treatment showed good tolerability and clinical potential for refractory cases because no unexpected toxicities emerged beyond localized inflammation and photosensitivity.
Topical immunomodulation is a promising method to enhance Photodynamic Therapy. The immunomodulatory compound Imiquimod activates Toll-like receptor 7 to boost local immune responses and has undergone evaluation when used together with PDT. A study by Paolino G, et al. [10] found that sequential treatment with daylight PDT followed by imiquimod 5% cream produced a 91.3% complete response rate at 12 months for superficial basal cell carcinoma, which exceeded the 83.4% clearance rate achieved with PDT treatment alone. Those receiving the combination treatment experienced outstanding cosmetic results. The therapeutic approach gains an advantage through PDT-caused tumor breakdown and antigen exposure, enabling Imiquimod to activate the immune system and eliminate remaining tumor cells. Studies from randomized trials indicate that actinic keratosis patients achieved better histological clearance when treated with combined PDT and Imiquimod than when treated with either modality alone [11]. The research supports the benefits of combining immunologic approaches with PDT to effectively treat field cancerization and scattered cancer cells.
Cancer vaccines and cell therapies with PDT
Combining photodynamic therapy with cancer vaccines represents an innovative approach to immunotherapy. In studies conducted on mice, a dendritic cell (DC) vaccine pulsed with PDT-treated squamous cell carcinoma cells resulted in a robust anti-tumor immune response. When PDT was used alongside a DC vaccine, the growth of tumors was slower, and survival rates improved compared to treatments using PDT alone [2].
PDT generates immunogenic tumor cells that die in situ, supplying dendritic cells with a rich source of tumor antigens. When these antigen-laden dendritic cells are reintroduced into the mice, they trigger a systemic cytotoxic T cell response capable of targeting any remaining cancer cells. This combination effectively primes the immune system against the cancer [2,7].
The study by Zhang H, et al. [2] indicated that the ALA PDT DC vaccine can induce systemic antitumor responses to protect against cutaneous SCC in mice. Although this research is still experimental, it paves the way for developing therapeutic vaccines for patients with NMSC. For example, treating a primary tumor with PDT while simultaneously injecting a DC or peptide vaccine could potentially prevent new lesions in high-risk individuals, such as immunosuppressed patients who are prone to frequent SCCs [12].
All things considered, immunotherapy combined with PDT solves one of the main issues with non-melanoma skin cancers: subclinical microscopic tumour expansions or circulating tumour cells that evade local therapy. These combinations hope to accomplish not only local clearance but also the elimination of occult illness and long-term tumour management by increasing immune surveillance. Early clinical application of this idea can be demonstrated in procedures combining PDT with imiquimod for superficial BCC or Bowen's disease, producing better clearance than PDT by itself [13]. To formally measure synergy, ongoing clinical studies are looking at systemic ICB (such as cemiplimab, a PD-1 inhibitor authorised for metastatic cSCC) combined with PDT for locally advanced cSCC. Although one must remain alert for compounded inflammatory side effects (for example, heightened local erythema or immune-related events produced by tumour antigen release), adding contemporary immunotherapies to PDT is viable as their safety profiles are well defined. Combining treatment seems reasonable, thus far though. All things considered, immunotherapy-from vaccinations to topical immune stimulants to systemic checkpoint inhibitors-is a potent ally to PDT, able to transform a largely local treatment into a systemic one. This approach especially benefits SCC, which has more metastatic potential than BCC and so stands to help from immune-mediated tumour eradication [14].
PDT plus chemotherapy / Other drugs
PDT lesion priming through topical or intralesional agents leads to enhanced intratumoral PpIX buildup and tumor cell defense disruption while maintaining minimal systemic toxicity. Research demonstrates that a one-week treatment with 5-fluorouracil before ALA-based PDT leads to up to threefold higher PpIX production and better lesion removal for actinic keratoses and superficial basal or squamous cell carcinomas, achieving surgical excision-like results. According to studies by Maytin & Anand and Anand S, et al. [15,16], the side effects observed during treatment usually manifest as temporary superficial erosions.
While low-dose methotrexate, diclofenac, and ingenol mebutate exhibit moderate or investigational benefits when used with PDT, their adoption in standard medical practice remains rare [11]. Clinical studies have explored the use of curettage followed by intratumoral ALA-PDT to treat advanced squamous cell carcinoma as a less toxic alternative to systemic chemotherapy [17]. Debulking or sensitizing bulky tumors before PDT may be achieved through molecularly targeted agents like vismodegib, which inhibits Hedgehog signaling, and Epidermal Growth Factor Receptor blockers. Despite evidence confined to case series and early-phase trials, these strategies show potential for improving PDT results in difficult-to-treat lesions [16,18].
Adjunct therapy effectiveness is strongly influenced by treatment timing. Imiquimod and similar immune-modulating topical agents can be administered before or after PDT to strengthen local immune responses [10]. Patients typically receive cytotoxic chemotherapy with 5-FU or methotrexate multiple days before treatment to ensure proper photosensitizer accumulation, while intralesional injections are sometimes used at the same time as light exposure for deep SCC cases [19]. The strategic implementation of PDT treatment plans boosts therapeutic outcomes while expanding their practical application to various lesion dimensions.
PDT plus physical modalities
Techniques that physically thin or remove the overlaying tissue improve medication dispersion into the lesion and help overcome PDT's poor light penetration. For practically all nodular basal cell carcinomas, for instance, CO₂-laser debulking followed by Methyl-Aminolevulinate (MAL)-PDT leaves minimum scarring and essentially replaces several surgical excisions. Similarly, deep curettage before PDT yields about 90% long-term control across multiple BCC subtypes with excellent cosmetic results; dermabrasion-assisted PDT consistently eradicts vast fields of actinic keratoses or Bowen's disease and scattered superficial tumours, showing very few recurrences at three-year follow-up. Combining surgery with intra- or postoperative PDT can help to improve margins or minimise the final wound size in more difficult squamous or basal cell carcinoma situations. Pre-clinical studies of combined sonodynamic therapy and PDT point to synergistic "light-and-sound" methods that might improve treatment depth and efficacy even further [20].
PDT plus nanotechnology
Nanoparticle-based systems demonstrate potential as efficient solutions for photodynamic therapy's common issues of limited tissue penetration ability and reduced performance in low-oxygen tumor regions. These systems enhance therapeutic precision and immune activation by enabling multimodal tumor targeting. A single near-infrared light source activates plasmonic nanoparticles made from gold, copper sulfide, or iron oxide to produce both photodynamic and photothermal cytotoxic effects. According to research findings, the dual-action treatment method shows better tumor shrinkage and stronger immune reactions in mouse models of squamous cell carcinoma [12,21].
Catalytic liposomes and oxygen-generating nanocarriers successfully restore reactive oxygen species production inside tumors and enhance PDT effectiveness in hypoxic areas where conventional methods fail. The application of advanced biomimetic systems in which cancer-cell-membrane-coated nanoparticles deliver both photosensitizers and immune agonists like imiquimod enables specific tumor targeting with accumulation in draining lymph nodes, thus turning PDT into a functional in situ cancer vaccine [22]. Nanotechnology-based treatments that target specific pathways, such as rapamycin-based systems, which increase protoporphyrin IX levels and iron-chelating curcumin particles blocking NRF2/HO-1 antioxidant defenses, boost cell destruction but do not add systemic toxicity [23].
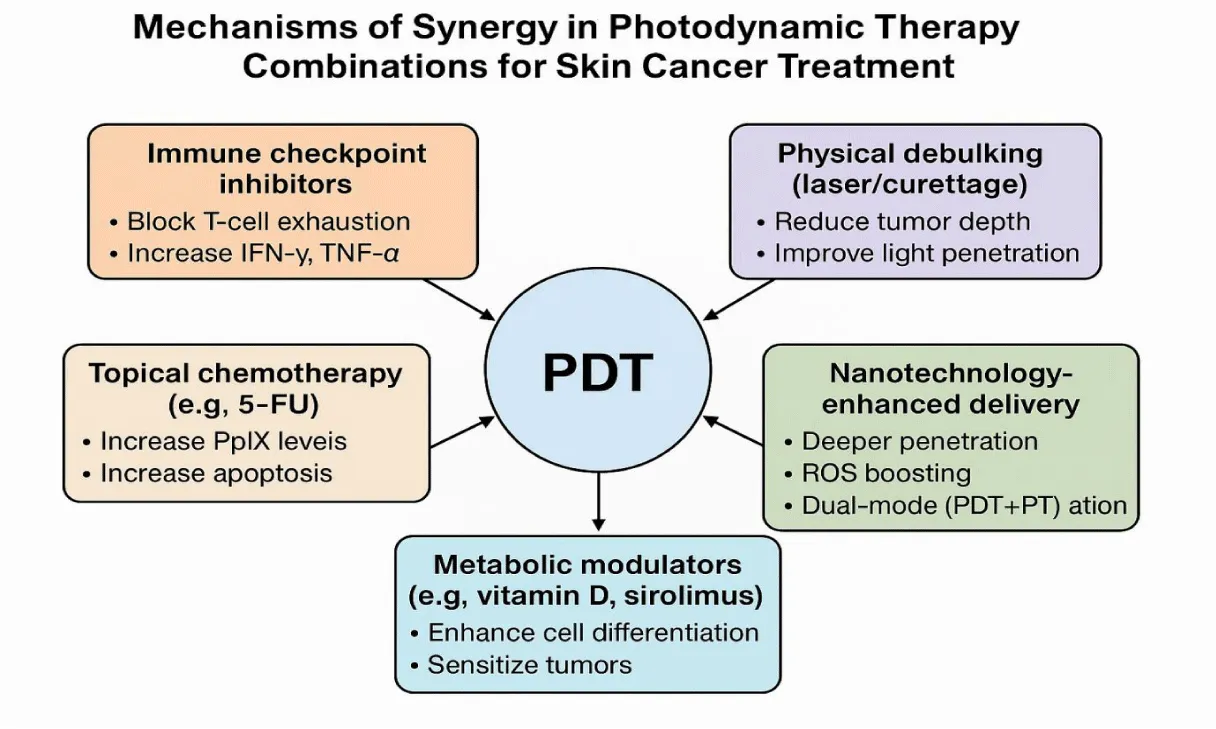
Photothermal therapy approved nanoshells represent simpler technology options that are poised to become the first nanomedicine applications adopted for regular dermatologic treatments. The evolution of nanomedicine continues to drive innovations that could transform PDT into a dual platform for tumor ablation and immunotherapy (Figure 3).
PDT plus biochemical modulators
When modulated by low-dose metabolic and signaling agents, the tumor microenvironment becomes more responsive to photodynamic therapy. Calcipotriol and other vitamin D derivatives produce better cellular differentiation while raising protoporphyrin IX levels within tumors, increasing the efficacy of PDT-induced cytotoxicity. Clinical and translational research has shown better clearance outcomes for actinic keratoses and superficial basal and squamous cell carcinomas [10,25]. Retinoids and histone deacetylase inhibitors enhance therapeutic outcomes by restoring keratinocyte differentiation and reducing hyperkeratotic plaque thickness, which improves light and drug penetration.
Synthetic ceramide analogues combined with iron-modulating compounds enhance photodynamic therapy in preclinical settings by raising oxidative stress levels and disrupting tumor antioxidant systems. The Mammalian Target of Rapamycin Complex 1 (mTORC1) inhibitor Sirolimus shows enhanced effects with PDT through reduced NRF2 and HO-1 signaling and increased tumor cell death [23]. Researchers are using advanced delivery systems like microneedles, fractional lasers, and permeation enhancers such as Dimethyl Sulfoxide to improve the absorption of photosensitizers. The field of photopharmacology and nanotechnology-based systems is advancing, which holds promise for achieving better precision in light-activated drug delivery mechanisms [26].
Table 1 summarizes the combination modalities used with PDT to address basal and squamous cell carcinoma. The treatment modalities consist of physical methods such as laser ablation and dermabrasion, along with topical treatments like vitamin D and imiquimod, and extend to systemic and nanotechnology-based therapies. The table demonstrates that these therapeutic strategies produce positive clinical and preclinical results by enhancing tumor removal efficiency and cosmetic results while occasionally triggering lasting immune responses.
| Table 1: Examples of combination therapies with PDT and their effects on BCC/SCC outcomes. | |||
| Combination modality paired with PDT | Tumor type & setting | Study design | Main improvement over PDT alone |
| Deep curettage (“attrition”) + intratumoral ALA‑PDT [1] | Advanced cutaneous SCC | Prospective phase‑II clinical series | Overall response 73% vs 47%; recurrence 17% vs 30 % |
| CO₂‑laser ablation + topical MAL‑PDT [2] | Nodular BCC | Clinical cohort (181 lesions) | Initial complete response 100%; 97% disease‑free at ≈11 months |
| Daylight ALA‑PDT followed by imiquimod 5 % cream [9] | Superficial BCC | Randomized split‑lesion trial | Twelve‑month clearance 91% vs 83% |
| CO₂‑laser debulking + repeat PDT [24] | Recurrent facial nodular BCC (case) | Illustrative clinical case | Durable 30‑month histologic cure after prior PDT failure |
| Dermabrasion + ALA‑PDT (“D‑PDT”) [3] | Mixed NMSC (nod‑BCC, inv‑SCC, Bowen, AK) | Multicenter retrospective (3 yr follow‑up) | Only 2 recurrences among 172 patients; 85% “excellent” cosmesis |
| CO₂‑laser channels + MAL‑PDT [12] | Recurrent nodular BCC (case) | Case report | Complete clinical and confocal clearance at 1 year |
| Calcipotriol (vitamin D) pretreatment + ALA‑PDT [14] | Superficial BCC & SCC in situ | Pilot split‑lesion human trial | PpIX ↑ six‑fold; greater necrosis; higher clearance trend |
| Sirolimus (mTORC1 inhibitor) pretreatment + MAL‑PDT [19] | Cutaneous SCC (cell lines & xenografts) | Translational in‑vitro/in‑vivo | Combo killed 70‑80% cells vs <30% with PDT alone |
| Gold nanocluster platform for combined PDT + Photothermal Therapy (PTT) [20] | Cutaneous SCC (mouse) | Pre‑clinical in‑vivo | Greater tumor regression and immune‑cell influx than single modes |
| Catalase‑loaded IR820 “oxygen‑generator” liposomes + NIR‑PDT/PTT [29] | Subcutaneous SCC (mouse) | Pre‑clinical in‑vivo | Higher ROS and superior tumor control vs standard PDT |
| PDD‑guided excision followed by PDT [30] | Locally advanced cutaneous SCC | Prospective case series | Tissue‑sparing excisions with high local control |
| Intralesional ALA‑PDT + systemic PD‑1 inhibitor [23] | Refractory cutaneous SCC (elderly) | Single‑patient clinical report | Rapid ulcer healing and durable control with manageable toxicity |
Discussion
Treating non-melanoma skin cancer using photodynamic therapy combined with other medications marks a significant departure in approach. The data above amply demonstrate that none of the techniques is perfect on its own; surgery can be too intrusive, PDT by itself may not be sufficient for thick tumours, immunotherapy by itself may not address localised disease, etc. By carefully combining any modality, clinicians can enhance its advantages and thereby minimise its drawbacks. Two malignancies with quite varying size, depth, and risk- BCC and SCC-this all-encompassing approach is particularly useful as one treatment regimen cannot fit all. Here we address the clinical consequences of various combinations, pragmatic problems for their application, and future directions of study and practice [27].
Enhanced efficacy and expanded indications
Combining treatments significantly increases efficacy indicators (complete response rates, lasting clearance) when compared to PDT alone. Adding a topical drug like 5-FU or imiquimod has pushed clearance rates for superficial BCC and Bowen's disease closer to those of excisional surgery, a great success for a non-surgical approach. Patients who choose PDT for its cosmetic or functional benefits therefore, no longer always have to sacrifice a reduced cure likelihood. Combining laser PDT with curettage PDT has made PDT feasible for nodular BCC and thicker lesions when otherwise it could have been contraindicated. Ten years ago, a nodular BCC > 4mm thick would not be taken into account for PDT; today, case series show similar tumours being eradicated with combination therapy. Combining treatments essentially extends the range of tumours treatable non-invasively. For patients with lesions in anatomically difficult locations-that instance, around the eyelids, nose, ears-where surgery could be disfiguring-this is particularly relevant. For patients with many lesions (field cancerisation), where a combined method may treat both the apparent tumours and surrounding precancerous area in one session – something surgery cannot readily accomplish, it also helps there [28].
Cosmetic and functional outcomes
Photodynamic therapy stands out for delivering positive cosmetic results, which are usually maintained when combined with other treatment techniques. Combining PDT with laser ablation produces enhanced cosmetic results by minimizing scarring compared to standard surgical excision. Recent research has established that aesthetic results are consistently positive when treatment combinations include laser-assisted PDT, PDT following curettage, and sequential therapies with PDT and topical immunomodulators like imiquimod [3,10,24].
The use of combined treatment methods can lead to stronger acute inflammatory responses. Patients must understand that standard PDT might cause mild crusting, while curettage-PDT typically leads to exudative wounds that need extended healing time. Final healed sites usually display minimal long-term scarring while preserving anatomical function, which proves essential in cosmetically sensitive regions such as the nasal rim or eyelid. The need for rigorous monitoring of wound healing processes and appropriate post-treatment management using dressings for laser-PDT or antibiotic ointments after curettage demonstrates clinical significance. Less invasive combination PDT methods provide a desirable treatment alternative from a quality-of-life viewpoint. Properly executing these techniques results in superior cosmetic and functional results, which frequently exceed outcomes from radiotherapy or Mohs surgery in specific situations [18].
Safety and tolerability
Safety-wise, most combinations have shown acceptable characteristics. Combining PDT's normal side effects-pain during illumination, localised redness/crusting, photosensitivity-can have an impact. Pretreatment with 5 FU or imiquimod, for example, will cause the treatment region to be more inflammatory, which might aggravate PDT discomfort; doctors note that 5 FU primed PDT is rather more painful than PDT on normal skin. Stronger analgesia or nerve blocks would help to control this, though [29]. Anti-PD-1 + PDT is one of the immunological-related combinations that might cause heightened immune responses; curiously, the few case studies did not record significant systemic immune adverse effects associated with combining with PDT [30]. Though further research is required to validate safety in bigger cohorts, it seems that the local effect of PDT may even assist in targeting the immune response to the tumour. One safety issue for physical combinations is infection risk when producing an open wound (laser ablation or curettage) and not mostly closing it, although infection rates in studies have been minimal, most likely because PDT itself has some antibacterial effects and wounds are maintained clean with bandages. Combining these modalities in the research to date has not revealed any new red-flag toxicities overall. This is positive, but constant awareness is crucial) [31]. Dermatologists using these approaches should constantly monitor patients to control any increased inflammatory reactions (e.g., short course prednisone or NSAIDS can help to control significant swelling following CO2 PDT on the face).
Patient selection & Individualized planning
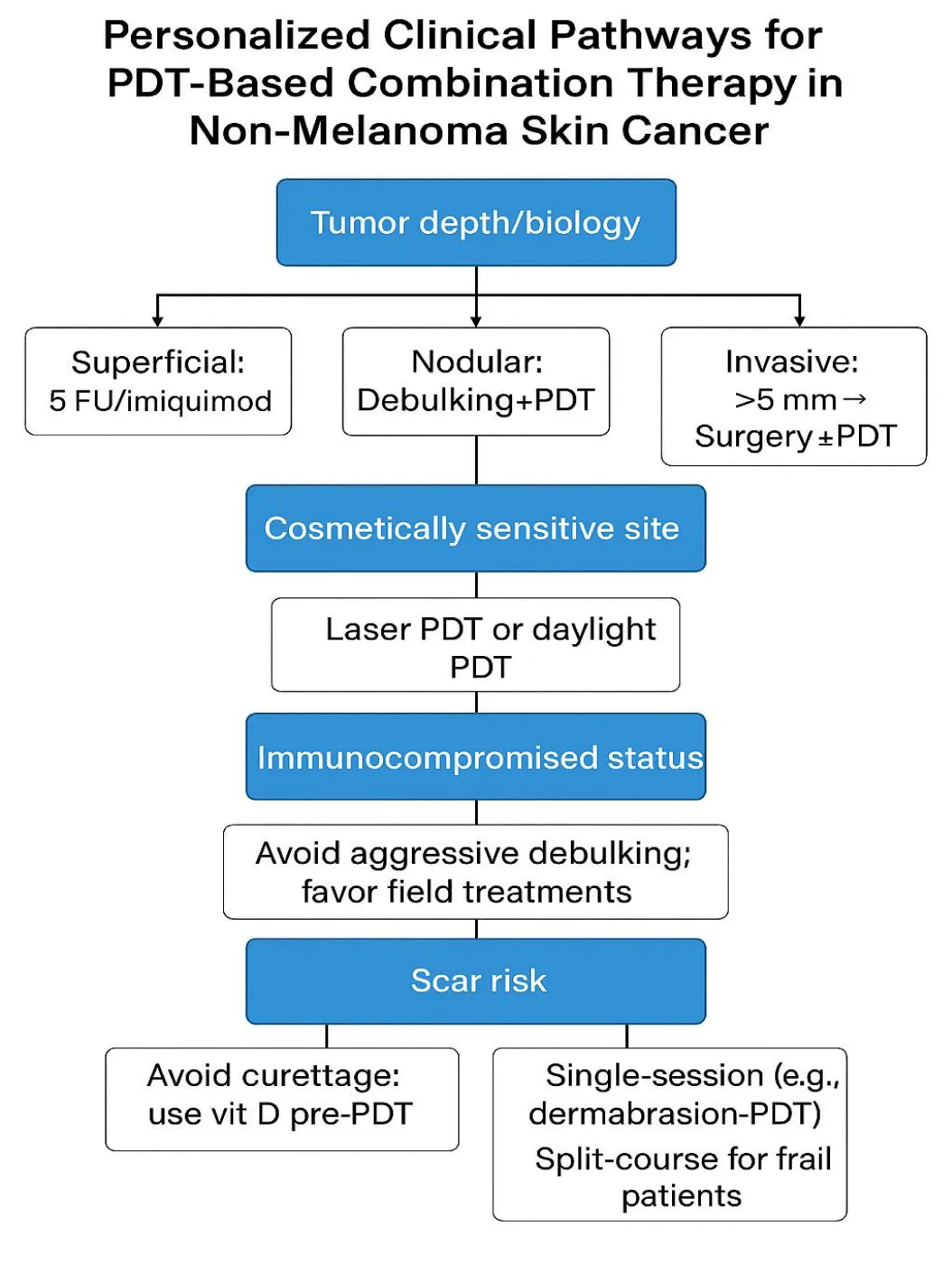
Achieving effective results with combination photodynamic therapy requires customized treatment plans that include detailed assessments of tumor properties along with patient-specific variables. Expanding therapeutic options through combinatorial strategies demands precise alignment with each patient's biological makeup and logistical circumstances to achieve optimal results. Superficial or field-based lesions show positive responses to 5-fluorouracil or imiquimod as pharmacologic adjuncts, whereas nodular tumors achieve better results through mechanical debulking techniques such as laser or curettage before PDT treatment [1,2]. Patient-focused factors, including cosmetic preferences and immune system status, determine which aggressive or conservative medical treatments are safe and acceptable. Elderly patients and those with immunosuppression should consider topical-only or fractionated treatment protocols, while younger patients who are highly motivated can choose intensive single-session approaches [25,26]. Shared decision-making considering tumor biology and other clinical and personal factors allows combination PDT to be effective and meet patient needs [22,24]. Table 2 details triage methods for personalized combination PDT treatments based on tumor characteristics, anatomical location, and patient-specific factors, including immunity status and potential scarring risk.
| Table 2: Clinical dimensions for selecting and personalizing PDT-based combination therapies. | |
| Dimension | Steering Strategy |
| Tumor biology |
• Nodular/thick ≤5 mm → mechanical debulk + PDT (laser‑PDT, curettage‑PDT). • >5 mm or perineural risk → consider staged surgery ± margin‑directed PDT [1] |
| Anatomy & cosmesis | |
| Host immunity | |
| Scar propensity | |
| Logistics & tolerance | • Fractionated or topical‑only sequences for frail or home‑bound individuals [25] |
| Patient preference | |
The visual algorithm displayed in (Figure 4) works together with (Table 2) to guide clinicians through individualized decision-making processes for PDT-based combination treatments, considering tumor traits, anatomical location, immune condition, scarring risk potential, treatment planning, and patient choices.
Integration into clinical practice
A few combinations are already, in a provisional form, following accepted practices. For thicker BCC, for example, recent European consensus notes PDT combined with pretreatments like curettage or laser as an alternative should surgery be contraindicated. The NCCN guidelines for SCC have started to recognise PDT's part in field treatment and note that topical treatments can be used adjunctively (albeit not particularly endorsing combinations, the inference is there) [3]. Dermatology clinics that provide PDT are progressively experimenting with combination treatments – e.g., using fractional laser prior to PDT for AK (a notion akin to dermabrasion-PDT) or employing daylight PDT followed by a short course of imiquimod [32]. More randomised controlled trials are required to persuade sceptics and precisely measure how much each combination improves outcomes if widespread acceptance is to be achieved. Fortunately, other trials are under way: one comparing MAL-PDT vs MAL-PDT following ablative fractional laser for superficial BCC; another comparing daylight PDT alone against daylight PDT + imiquimod for AK and superficial SCC (expanding on Paolino's work). Should these trials corroborate the advantages shown in smaller research, official advice should include combination PDT for suitable purposes [23].
Combination therapies represent a significant advancement in the treatment of non-melanoma skin cancer despite their existing limitations. The availability of specialized equipment needed for treatment, including fractional lasers and nanotechnology agents, is generally limited to tertiary care and academic institutions, which restricts their broader application [12,23]. The successful implementation of multimodal treatment regimens depends on specialized dermatologic surgical or photomedicine training, which remains inaccessible in many locations [33]. Some settings encounter cost barriers, particularly when using off-label or multi-agent treatment protocols, while other treatments, such as topical 5-FU and curettage tools, remain low-cost [16]. To ensure the safe and effective use of PDT combinations across various practice environments, medical professionals require standardized clinical protocols, along with comprehensive training programs.
Cost and practicality
Combining therapies raises cost or complexity, which is a factor healthcare systems take into account. Still, many of the additions-a tube of 5-FU, or a cheap curettage-are really low-cost. Should specialised equipment be required-a laser device or perhaps costly to manufacture nanoparticles-what can raise expenses? [12] One has to weigh this against possible savings from avoiding surgery or stopping recurrences. When considering operating room and pathology costs, a cost-analysis could show that, when low-risk BCC is treated with curettage-PDT, it is less expensive than Mohs surgery. Furthermore, combined therapy could cut the required treatment sessions-for instance, one vigorous session against several rounds of PDT-which would be more economical over time [16]. Practically speaking, dermatologists doing PDT should have the skills needed for these add-ons: e.g., being at ease with curettage or having laser access. Usually falling under the purview of procedural dermatology, training and protocol distribution will be especially important [33].
Clinical algorithms
Practically, a possible decision-making process could be: Consider standard PDT for a low-risk superficial BCC or SCC in situ; if the lesion is large or the patient wants more confidence of cure, add a pretreatment (5-FU or imiquimod). If the patient requests non-surgery for a nodular BCC (< 1 cm, low-risk location), take into account curettage-PDT or laser-PDT if available; if not, maybe schedule two sessions of PDT one week apart with curettage in between (some do curettage after the first PDT to remove any remaining tumour that's been softened by treatment). Consider PDT to the surface combined with systemic immunotherapy (in a multidisciplinary setting) for locally advanced SCC not suitable for surgery. Perhaps do dermabrasion-PDT to treat the whole field in one go for field cancerisation with multiple AKS and incipient SCCS (a technique that could be both curative and preventive). These are fictitious methods that will become proof as evidence mounts. Early adopters in dermatology clinics are already experimenting along similar lines, and sharing their results will help to improve these guidelines [21].
Combination therapies have broadened photodynamic therapy's clinical applications, allowing it to function as an essential part of multimodal treatment approaches in skin cancer rather than being limited to single therapy use. The transition focuses on reaching the best possible treatment results through the least invasive methods. These treatment strategies provide excellent tumor control while simultaneously minimizing patient morbidity and enhancing their quality of life. Clinical research indicates that PDT-based combination therapies produce improved clearance rates and better cosmetic results while helping to activate anti-tumor immune responses [7,10]. The immune system stimulation from this treatment acts like preventive immunization, which could provide enduring defense against tumor development while presenting significant benefits for individuals with chronic sun exposure or immunosuppression, since they face recurring lesions [2].
Because of these positive advances, oncologists and dermatologists must stay updated on new PDT combination protocols. Emerging PDT techniques such as laser-assisted PDT and immunomodulator-enhanced PDT will likely become established treatments for particular lesions shortly. The increasing use of PDT as adjuvant therapy for better surgical results or targeting hidden microscopic disease, neoadjuvant therapy to reduce tumors before systemic treatment, and as a primary treatment option in some instances demonstrates why PDT should be incorporated into multidisciplinary treatment approaches. The growing importance of collaborative care models necessitates that dermatologic surgeons, alongside medical and radiation oncologists, ensure PDT integration for optimal skin cancer treatment [18,22].
Future directions
The mixture of PDT terrain is probably going to keep growing. Interesting treatments like systemic immunotherapy plus PDT in clinical trials for locally advanced SCC (to investigate if organ preservation is feasible in instances that would otherwise need surgery or radiation) are on their way. An area of ongoing research is gene therapy or sirna given by nanoparticles to knock off resistance paths (like NRF2) during PDT, possibly allowing a very customised molecular combination. Since both photothermal therapy devices, such as those used for photothermal elimination of tumours with gold nanoshells, use light but distinct wavelengths, PDT in clinical practice could soon be coupled with them. A dual-wavelength laser might be employed consecutively, first an NIR pulse for photothermal ablation, then red light for PDT, so accomplishing what the gold nanocluster did, but with two independent phases. Another idea is fluorescence-guided PDT, in which residual tumour is identified after surgery or following an initial PDT pass using Ppix fluorescence (PDD), then re-treatment of those areas. This combines therapy with diagnostic imaging for a more all-encompassing treatment.
Crucially, long-term follow-up from these combo treatments is required to guarantee that recurrences are low at 5 or 10 years, not just 1–2 years. We also have to be alert for any unanticipated side effects resulting from higher patient counts; for example, may recurrent immune activation with PDT + checkpoint inhibitors cause autoimmunity in some individuals? Cases are isolated thus far, but larger investigations will address that.
All things considered, the combination of technologies-drugs, devices, biologics-with PDT marks a new front in skin cancer treatment. It represents individualised medicine, customising treatments to patient needs and tumour biology. Although optimising and standardising these techniques still presents difficulties, combination PDT is going from experimental to necessary. With continuous research and clinical experience, it has the potential to become a mainstream modality that provides patients with an efficient cure with minimum side effects - a real win-back in oncologic therapy.
Conclusion
For the treatment of basal and squamous cell carcinomas, combination therapies, including photodynamic therapy, have opened a bright new path. Clinicians can overcome several restrictions that traditionally limited PDT to only the simplest lesions by combining PDT's localised tumour-selectivity with additional modalities, from immune checkpoint inhibitors and vaccines to topical chemotherapy, lasers, and nanotechnology. The past decade (2015–2025) data show that these combined strategies can greatly enhance clinical results. Recurrence rates have reduced, tumour clearance rates have climbed, and hitherto untreatable patients with PDT, such as bigger tumours, are now displaying remarkable responses. Most importantly, these improvements preserve the main benefits of PDT: outstanding cosmetic results, tissue preservation, and outpatient feasibility.
Practically speaking, combined PDT treatments give patients more choices for customised treatment. Adding a brief topical or immunological therapy to their PDT treatment can help patients with low-risk superficial BCC or SCC in situ reach better cure probabilities. Those with nodular BCC who want to avoid surgery could get a combined laser-PDT or curettage-PDT treatment that completely removes the tumour with the least amount of scarring. Those with several lesions or significant field cancerisation can have combined treatments addressing both visible and occult lesions in one all-encompassing session. PDT can be combined with systemic treatments even in advanced situations to maximise tumour reduction and reduce morbidity. These chances show a paradigm change towards efficient but organ-sparing treatment of skin cancer.
From the clinician's vantage point, the expanding array of combination techniques calls for a deliberate, patient-specific approach. The choice of the best combination should be guided by elements including tumour size, depth, location, and patient immunological state. Clinical guidelines will probably include combination protocols as they grow more accepted; practitioners will also need instruction in these multi-modal techniques (such as learning to perform laser ablation before PDT, or controlling the inflammatory response of PDT-immune therapy combinations). Recognising the long-term cost-effectiveness of preventing surgeries and recurrences through somewhat more complex first treatments, health systems and payers could also have to adjust.
Future studies and clinical trials will help to clarify ideal procedures for combined PDT even more. Long-term follow-ups will reveal which combinations, in terms of preventing recurrence, really are timeless. New combinations, including targeted molecular inhibitors or more recent immunotherapies (e.g., cytokines, adoptive T-cells) with PDT, could show themselves. Furthermore, improving combo efficacy even more are technical developments like targeted light delivery (e.g., intralesional light probes).
Finally, the combination of photodynamic therapy with various treatment approaches is a new horizon that is fast changing into a productive terrain in the treatment of skin cancer. It embodies the medical synergy concept, whereby the whole is more than the sum of its components. We are now obtaining results that were once possible only with more aggressive measures by using the strengths of PDT (precision and cosmesis) in concert with the strengths of immunotherapy (systemic protection), chemotherapy (biochemical enhancement), and physical modalities (structural debulking). Effective, visually beautiful treatment for patients with BCC and SCC not only addresses current lesions but also, by immune activation, may lower future cancer risk.
In a time when patient quality of life is of great importance, combination PDT provides a means of cancer cure with light and complementary technologies instead of the knife by itself. It lays the stage for further developments and reflects the improvement in dermatologic oncology during the past ten years. Combining photodynamic therapy with greater data collecting and more clinics using these techniques would make it a pillar of non-melanoma skin cancer treatment, therefore realising the vision of a highly successful but least invasive new frontier in treatment.
References
- Peng J, Feng W, Luo X, Wang T, Xiang W, Dai Y, Zhu J, Zheng J. A Clinical Trial Using Attrition Combined with 5-Aminolevulinic Acids Based Photodynamic Therapy in Treating Squamous Cell Carcinoma. Med Sci Monit. 2017 Mar 18;23:1347-1354. doi: 10.12659/msm.900420. PMID: 28314866; PMCID: PMC5367852.
- Zhang H, Wang P, Wang X, Shi L, Fan Z, Zhang G, Yang D, Bahavar CF, Zhou F, Chen WR, Wang X. Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol Cancer Res Treat. 2018 Jan 1;17:1533033818785275. doi: 10.1177/1533033818785275. PMID: 30025490; PMCID: PMC6053869.
- Ferrara F, Lacava R, Barisani A, Messori S, Patrizi A, Bardazzi F, Vaccari S. Combined CO2 laser and photodynamic therapy enhances the efficacy of treatment of basal cell carcinomas. J Dtsch Dermatol Ges. 2019 Dec;17(12):1251-1256. doi: 10.1111/ddg.14004. Epub 2019 Dec 9. PMID: 31814292.
- Martins WK, Belotto R, Silva MN, Grasso D, Suriani MD, Lavor TS, Itri R, Baptista MS, Tsubone TM. Autophagy Regulation and Photodynamic Therapy: Insights to Improve Outcomes of Cancer Treatment. Front Oncol. 2021 Jan 20;10:610472. doi: 10.3389/fonc.2020.610472. PMID: 33552982; PMCID: PMC7855851.
- Boppana NB, Stochaj U, Kodiha M, Bielawska A, Bielawski J, Pierce JS, Korbelik M, Separovic D. C6-pyridinium ceramide sensitizes SCC17B human head and neck squamous cell carcinoma cells to photodynamic therapy. J Photochem Photobiol B. 2015 Feb;143:163-8. doi: 10.1016/j.jphotobiol.2015.01.001. Epub 2015 Jan 10. PMID: 25635908; PMCID: PMC4662378.
- Harris JC, Scully MA, Day ES. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers (Basel). 2019 Nov 21;11(12):1836. doi: 10.3390/cancers11121836. PMID: 31766360; PMCID: PMC6966582.
- Zeng Q, Yang J, Ji J, Wang P, Zhang L, Yan G, Wu Y, Chen Q, Liu J, Zhang G, Wang X. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. Oncoimmunology. 2022 Apr 3;11(1):2061396. doi: 10.1080/2162402X.2022.2061396. PMID: 35402079; PMCID: PMC8986186.
- Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022 Jan 1;12(1):434-458. doi: 10.7150/thno.67300. PMID: 34987658; PMCID: PMC8690913.
- Anand S, Shen A, Cheng CE, Chen J, Powers J, Rayman P, Diaz M, Hasan T, Maytin EV. Combination of vitamin D and photodynamic therapy enhances immune responses in murine models of squamous cell skin cancer. Photodiagnosis Photodyn Ther. 2024 Feb;45:103983. doi: 10.1016/j.pdpdt.2024.103983. Epub 2024 Jan 27. PMID: 38281610; PMCID: PMC11197882.
- Paolino G, Didona D, Scarnò M, Tallarico M, Cantoresi F, Calvieri S, Mercuri SR, Piccolo D, Bottoni U, Kyriakou A, Cantisani C. Sequential treatment of daylight photodynamic therapy and imiquimod 5% cream for the treatment of superficial basal cell carcinoma on sun exposed areas. Dermatol Ther. 2019 Mar;32(2):e12788. doi: 10.1111/dth.12788. Epub 2018 Dec 21. PMID: 30499211.
- Lucena SR, Salazar N, Gracia-Cazaña T, Zamarrón A, González S, Juarranz Á, Gilaberte Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int J Mol Sci. 2015 Oct 28;16(10):25912-33. doi: 10.3390/ijms161025912. PMID: 26516853; PMCID: PMC4632833.
- Liu P, Yang W, Shi L, Zhang H, Xu Y, Wang P, Zhang G, Chen WR, Zhang B, Wang X. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J Mater Chem B. 2019 Nov 28;7(44):6924-6933. doi: 10.1039/c9tb01573f. Epub 2019 Oct 22. PMID: 31638633.
- Yu N, Luo X, Wei T, Zhang Q, Yu J, Wu L, Su J, Chen M, Huang K, Li F, Xie Y, Fang F, Zhang L, He R, Chen X, Zhao S, Bu W. Dermabrasion combined with photodynamic therapy: a new option for the treatment of non-melanoma skin cancer. Lasers Med Sci. 2022 Mar;37(2):1255-1263. doi: 10.1007/s10103-021-03381-3. Epub 2021 Aug 8. PMID: 34365550.
- Yang N, Yang Y, Zhang W, Li X, Jiang H, Kou H, Zhang J, Wang Y, Tan L, Lu Y. Efficacy of PDD-guided tumor excision combined with photodynamic therapy in cutaneous squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2025 Feb;51:104469. doi: 10.1016/j.pdpdt.2024.104469. Epub 2025 Jan 1. PMID: 39753196.
- Maytin EV, Anand S. Combination photodynamic therapy using 5-fluorouracil and aminolevulinate enhances tumor-selective production of protoporphyrin IX and improves treatment efficacy of squamous skin cancers and precancers. Proc SPIE. 2016. doi: 10.1117/12.2218138.
- Anand S, Hasan T, Maytin EV. Treatment of nonmelanoma skin cancer with pro-differentiation agents and photodynamic therapy: Preclinical and clinical studies (Review). Photochem Photobiol. 2024 Nov-Dec;100(6):1541-1560. doi: 10.1111/php.13914. Epub 2024 Feb 4. PMID: 38310633; PMCID: PMC11297983.
- Yu N, Luo X, Wei T, Zhang Q, Yu J, Wu L, Su J, Chen M, Huang K, Li F, Xie Y, Fang F, Zhang L, He R, Chen X, Zhao S, Bu W. Dermabrasion combined with photodynamic therapy: a new option for the treatment of non-melanoma skin cancer. Lasers Med Sci. 2022 Mar;37(2):1255-1263. doi: 10.1007/s10103-021-03381-3. Epub 2021 Aug 8. PMID: 34365550.
- Collier NJ, Rhodes LE. Photodynamic Therapy for Basal Cell Carcinoma: The Clinical Context for Future Research Priorities. Molecules. 2020 Nov 18;25(22):5398. doi: 10.3390/molecules25225398. PMID: 33218174; PMCID: PMC7698957.
- Zhang M, Zhao Y, Ma H, Sun Y, Cao J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics. 2022 May 29;12(10):4629-4655. doi: 10.7150/thno.72465. PMID: 35832074; PMCID: PMC9254244.
- Valerio TI, Furrer CL, Sadeghipour N, Patrock SX, Tillery SA, Hoover AR, Liu K, Chen WR. Immune modulations of the tumor microenvironment in response to phototherapy. J Innov Opt Health Sci. 2023 May;16(3):2330007. doi: 10.1142/s1793545823300070. Epub 2023 Apr 27. PMID: 38550850; PMCID: PMC10976517.
- Adnane F, El-Zayat E, Fahmy HM. The combinational application of photodynamic therapy and nanotechnology in skin cancer treatment: A review. Tissue Cell. 2022 Aug;77:101856. doi: 10.1016/j.tice.2022.101856. Epub 2022 Jun 15. PMID: 35759978.
- Chen Y, Zhi S, Ou J, Gao J, Zheng L, Huang M, Du S, Shi L, Tu Y, Cheng K. Cancer Cell Membrane-Coated Nanoparticle Co-loaded with Photosensitizer and Toll-like Receptor 7 Agonist for the Enhancement of Combined Tumor Immunotherapy. ACS Nano. 2023 Sep 12;17(17):16620-16632. doi: 10.1021/acsnano.3c02724. Epub 2023 Aug 22. PMID: 37606341.
- Nicolás-Morala J, Mascaraque-Checa M, Gallego-Rentero M, Barahona A, Abarca-Lachen E, Carrasco E, Gilaberte Y, González S, Juarranz Á. The m-TORC1 inhibitor Sirolimus increases the effectiveness of Photodynamic therapy in the treatment of cutaneous Squamous Cell Carcinoma, impairing NRF2 antioxidant signaling. Int J Biol Sci. 2024 Aug 6;20(11):4238-4257. doi: 10.7150/ijbs.94883. PMID: 39247827; PMCID: PMC11379070.
- Alma A, Pongetti L, Clementi A, Chester J, Toccaceli M, Ciardo S, Zappia E, Manfredini M, Pellacani G, Greco M, Bennardo L, Farnetani F. Combined Carbon Dioxide Laser with Photodynamic Therapy for Nodular Basal Cell Carcinoma Monitored by Reflectance Confocal Microscopy. Medicina (Kaunas). 2023 Dec 24;60(1):30. doi: 10.3390/medicina60010030. PMID: 38256291; PMCID: PMC10821002.
- Xu M, Kong L, Jamil M. Advancements in skin cancer treatment: focus on photodynamic therapy: a review. Am J Cancer Res. 2024 Oct 25;14(10):5011-5044. doi: 10.62347/JOUT3260. PMID: 39553219; PMCID: PMC11560809.
- Zeng L, Gowda BHJ, Ahmed MG, Abourehab MAS, Chen ZS, Zhang C, Li J, Kesharwani P. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol Cancer. 2023 Jan 12;22(1):10. doi: 10.1186/s12943-022-01708-4. PMID: 36635761; PMCID: PMC9835394.
- Lultschik S, Tran J, Sapra S, Sharma K, Dong K. Non-Invasive Treatment of Basal Cell Carcinoma: Photodynamic Therapy Following Curettage. J Drugs Dermatol. 2023 May 1;22(5):481-485. doi: 10.36849/JDD.7133. PMID: 37133481.
- Zeng Q, Yang J, Ji J, Wang P, Zhang L, Yan G, Wu Y, Chen Q, Liu J, Zhang G, Wang X. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. Oncoimmunology. 2022 Apr 3;11(1):2061396. doi: 10.1080/2162402X.2022.2061396. PMID: 35402079; PMCID: PMC8986186.
- Champeau M, Vignoud S, Mortier L, Mordon S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J Photochem Photobiol B. 2019 Aug;197:111544. doi: 10.1016/j.jphotobiol.2019.111544. Epub 2019 Jul 2. PMID: 31295716.
- Tian D, Teng C, Lv Y, Duan X, Wang Y, Song X, Jiang Q, Huang D, Xin T, Yang Y, Li L. Photodynamic therapy with intratumoral injection of photosensitizers combined with immunotherapy brings hope to patients with refractory cutaneous malignant ulcers. Discov Oncol. 2024 Oct 4;15(1):522. doi: 10.1007/s12672-024-01342-0. PMID: 39365490; PMCID: PMC11452570.
- Kobauri P, Dekker FJ, Szymanski W, Feringa BL. Rational design in photopharmacology with molecular photoswitches. Angew Chem Int Ed Engl. 2023;62(30):e202300681. doi: 10.1002/anie.202300681.
- Baioco KS, Pereira R, Ferreira-Gonçalves T, Coelho JMP, Gaspar MM, Reis CP. Combining Phototherapy and Gold-Based Nanomaterials: A Breakthrough in Basal Cell Carcinoma Treatment. Int J Mol Sci. 2024 Oct 26;25(21):11494. doi: 10.3390/ijms252111494. PMID: 39519051; PMCID: PMC11545837.
- Rollakanti K, Anand S, Maytin EV. Topical calcitriol prior to photodynamic therapy enhances treatment efficacy in non-melanoma skin cancer mouse models. Proc SPIE Int Soc Opt Eng. 2015 Mar 2;9308:93080Q. doi: 10.1117/12.2077296. PMID: 25983370; PMCID: PMC4429796.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.