Environmental Sciences 2025 June 09;6(6):611-641. doi: 10.37871/jbres2116.
Toxicity of Mercury and Its Compounds: Impacts on Human and Animal Health, Ecosystems, Mechanisms, and Bioremediation Strategies - A Systematic Review
Suresh R Naik1* and Dipesh Gamare2
2Yashwantrao Bhonsale College of Pharmacy, Sawantwadi, India
- Mercury toxicity
- Environmental pollution
- Bioaccumulation
- Biomagnification
- Toxic effects
- Organ dysfunction
- Remediation strategies
Abstract
Mercury is a pervasive, environmentally toxic global pollutant that significantly impacts ecosystems and human health. Its chemical and toxicological properties contribute to environmental contamination through natural processes like volcanic eruptions and human activities such as industrial emissions and agriculture. Over the past century, mercury levels in the environment have nearly doubled due to rapid industrialization, leading to bioaccumulation and biomagnification in food chains, especially in aquatic ecosystems, which significantly impact human food safety.
This review examines mercury's toxic effects on multiple organ systems through acute and chronic pathways. Mercury induces neurotoxicity, nephrotoxicity, immune dysfunction, cardiovascular damage, and endocrine disruption, including cancer promotion in both humans and animals. Mercury compounds and mercury-containing xenobiotics interfere with cellular and molecular mechanisms, leading to oxidative stress, DNA damage, mitochondrial damage, apoptosis, epigenetic alterations, and impaired signaling pathways.
The review also highlights mercury's environmental persistence, affecting biodiversity by impairing various species' growth, reproduction, and survival, from microorganisms to higher vertebrates and invertebrates. It explores mercury's broader ecological footprint, including its role in disrupting food web dynamics, soil health, and plant productivity, ultimately impacting global biodiversity and ecosystem stability. The potential for mercury-induced bioaccumulation in aquatic life is emphasized, highlighting its cascading effects on human food safety. Addressing these challenges, the review discusses advances in remediation strategies, such as chelation therapy, natural antioxidants of phytochemical origin, and innovative biotechnological interventions. By incorporating documented research findings, this review aims to inspire future research and policymaking to mitigate mercury's adverse health effects and ensure environmental sustainability.
Review Highlights:
- Mercury is a toxic pollutant harming ecosystems and human health globally.
- It bioaccumulates in food chains, impacting food safety and biodiversity.
- Mercury causes neuro, nephro, hepato, cardio, and endocrine toxicity, harming organs.
- It disrupts DNA, mitochondria, and cell signaling, leading to severe toxicity.
- Advances in remediation include chelation, antioxidants, and biotech solutions.
Introduction
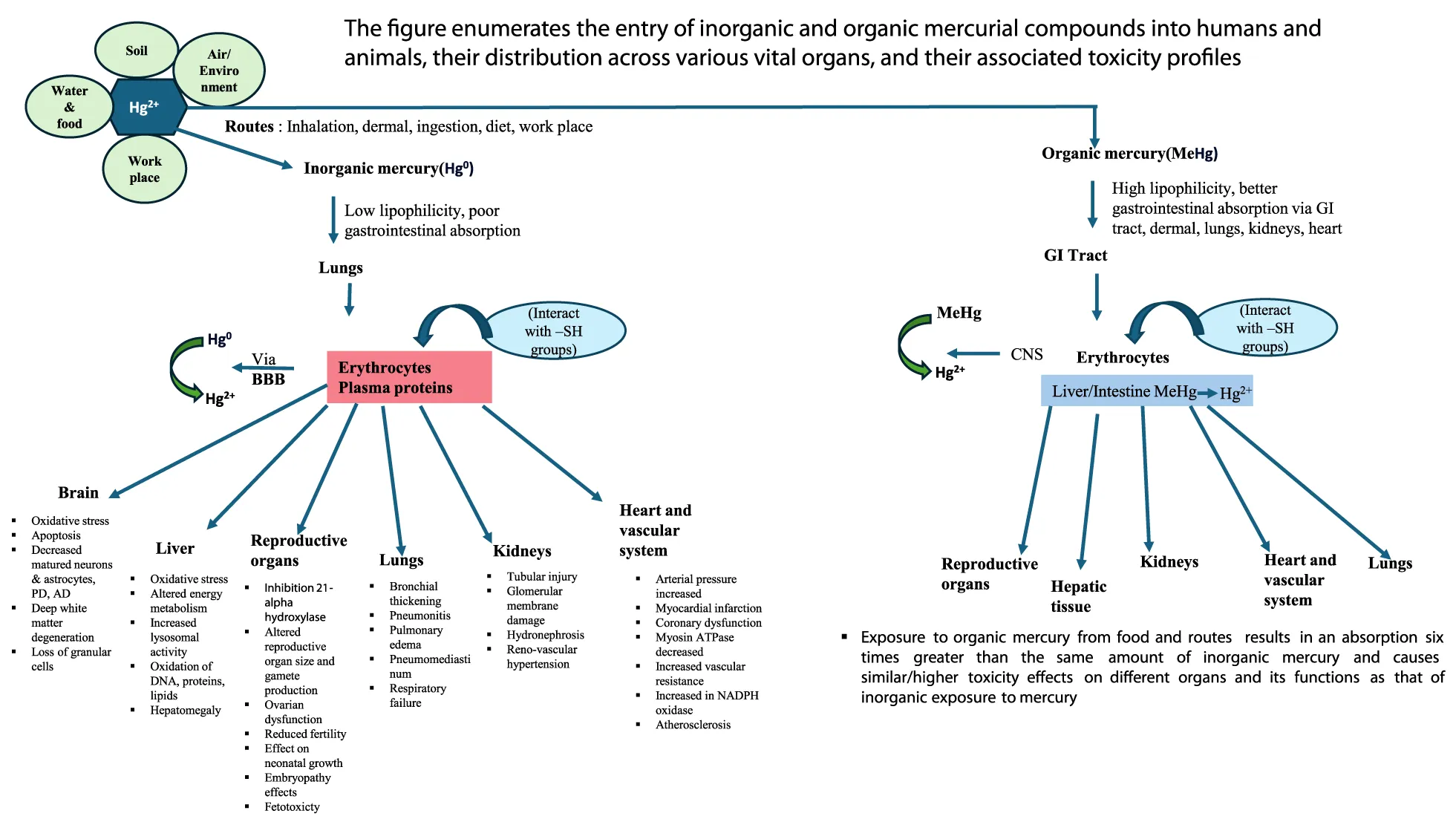
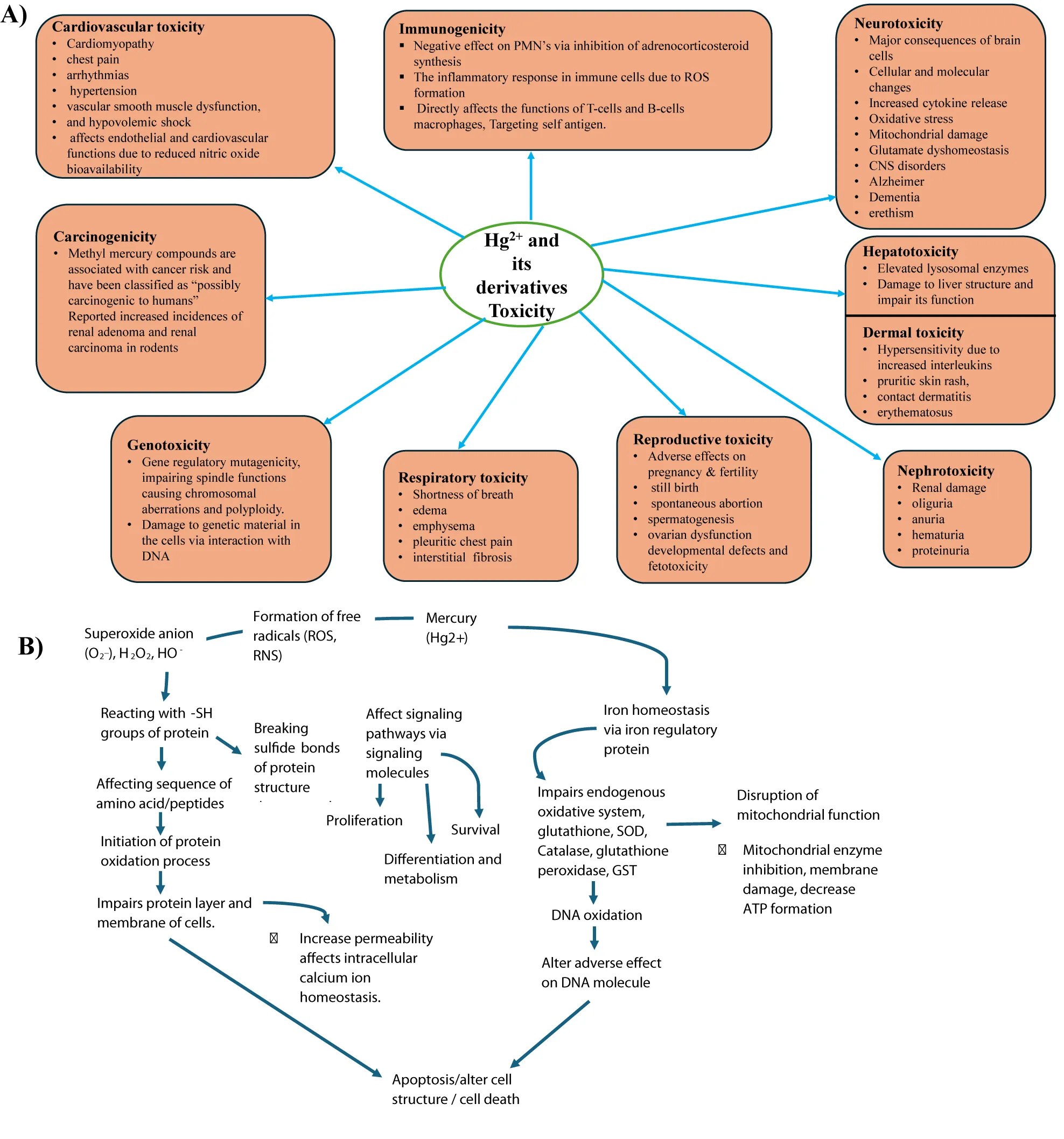
Heavy metals (metals and metalloids) are defined as metallic elements of relatively higher density as compared to water and are major pollutants in the environment (also known as xenobiotic metals), metal toxicity is both acute (shorter period) and chronic (long period) exposure (maybe months or years) lead to health disorders like other chemical and gaseous environmental pollutants [1,2]. Among metals, mercury has a unique feature and physical properties that confer its specific toxicological effects via metal accumulation in different tissues and organs known to interact with accumulated mercury [3](Figure 1b).
The possible clinical symptoms of mercury both organic and inorganic compounds poisoning reported are: headache, disequilibrium, incoordination (gait impairment), tremors muscle weakness “pins and needles” feelings usually in hands, feet, and around the mouth, atypical movements, paresthesia in the distal part of extremities, impairment of speech, hearing, walking, hallucination, seizures, disruption of attention, fine motor function and verbal memory and death [4].
Hence, mercury contamination is a serious public health and environmental problem. In the USA, the EPA (Environmental Protection Agency) and the Agency for Toxic Substances and Disease Registry (ATSDR) top 20 hazardous substances in their priority list in 2001. Among these toxic substances are As, Pb, and Hg in the 1st, 2nd, and 3rd positions, respectively, and Cd in the 7th place. Hence, As, Pb, Hg, and Cd metals are considered the most hazardous substances found on the surface of the earth and are toxic to humans, animals, and the environment [5]. During the past 150 years, human activities (increased industrialization) have almost doubled the natural amount of mercury in the environment (soil, water, and air), constituting a grave threat to human health and the ecosystem [6] .Mercury exposure can occur via inhalation of inorganic metallic mercury, ingestion of inorganic complexed mercury compounds, ingestion of organic forms of mercury, or dermal contact [1,7,8]. Further, mercury bioaccumulation occurs especially in aquatic organisms as they take MeHg from water/ingest prey containing MeHg. Such a process leads to higher concentrations of Hg in marine organisms at higher trophic levels due to biomagnification [9].
The major sources of heavy metals, including mercury, in the environment are
- Natural catastrophic events such as biomagnification, volcanic eruptions, atmospheric depositions, and metal evaporations from water resources to soil and groundwater have also contributed significantly (biogeochemical cycle of mercury movement and transformation via various environmental compartments) [10].
- Metal processing in refineries, coal burning in power plants, and petroleum combustion led to further complex environmental pollution and also increased massive exposure to humans and animals [1].
- Industrial (coal combustion and industrial processes) and agricultural activities, drugs, and pharmaceuticals, including radioisotopes and nanoparticles, Indigenous medicines, magico-ritualistic practices, domestic effluents, and atmospheric sources [11]. EPA rules indicate the maximum allowable level limit of mercury in water is 2 ppm, and the safe dose of mercury in food is 0.1 μg/kg of body weight [12]. A maximum inhalation concentration is 0.3 μg/m3 for atmospheric Hg0 is acceptable [13]. The acceptable level of mercury in the soil is 720 ppm with a safety factor of 10, the limit would be reduced to 72 ppm [14] (Table 1).
| Table 1: Major sources of mercury pollution in the environment. | |||
| Natural sources | Industrial activities | Industrial products | Diet/food/medicines |
| Anthropogenic sources, weathering and volcanic eruption release from oceans, earth crust volcanoes, forest fires, evaporation from natural bodies, atmospheric deposition, principal earth reservoir of mercury comprises of atmosphere, terrestrial ecosystems, and aquatic compartments, sunlight, radiation | Fossil fuels, burning of coal-fired power plants, boilers in industries, petroleum combustion, heavy metal pollution, mining, and precious metal extraction, Chloro-alkali process, industries: steel and cement plants, plastic, paper, and pulp industries, incinerations of medical waste and electronic waste | Fungicides, pesticides, batteries, thermometers and barometers, dental amalgam, disinfectants, paints, fluorescent bulbs, switches, and medical devices. | Rice, seafood, grains, vegetables, vegetable oils, wine, skin creams, herbal medicines, herbal tea, high fructose syrup, vaccines |
Mercury's chemical and physical state determines its pharmacokinetics and biotransformation pattern. Acute and especially chronic toxicological studies of metallic elemental mercury and its compounds, both organic and inorganic, are known to elicit effects on different body organs such as the gastrointestinal tract, renal system, central nervous system, hematopoietic, hepatic, cardiovascular, respiratory, immune, and reproductive systems, including cancer promotion [15-17] (Figure 1a) Toxic mercury especially (methyl mercury) is recorded to be a significant threat to marine wildlife (both inside and outside) of the oceans particularly dolphins, whales, seals, and predatory fish (tuna and swordfish)[18-20]. The reproductive problems in birds suffering from mercury poisoning have also been documented. The isolated adverse effects of mercury in avians and mammals are hepatic, renal damage, and neurobehavioral alterations [21].
The present review deals with the toxic/adverse effects of mercury and its environmental pollutants (both organic and inorganic) on human and animal health and briefly refers to its effects on other ecosystems. It also discusses the manifestation of clinical symptoms and adverse toxic effects on different organ systems, and physiological functions with an underlying profile of mechanisms both at cellular and molecular levels, which are assessed with the help of advanced analytical methods and state-of-the-art techniques. The review also elucidates mercury toxicity to human health in detail, covering neurotoxicity, nephrotoxicity, cardiac toxicity, respiratory disorders, immune toxicity, adverse effects on endocrine organs, pregnancy and reproductive system, ecosystem, and cancer promotion. Furthermore, it includes different remedial approaches to mitigate the adverse effects of mercury strategies to overcome mercury toxicity using the new generation chelating agents, and probable therapeutic interventions from natural product origins based on the toxicity mechanisms of Hg. Finally, to provide future perspectives by updating the compendium of mercury toxicity that may be useful for further research and development, and consultation reference sources from documented studies.
The Effect of Mercurial Compounds and Mercury-Contaminated Xenobiotics on Various Organs and their Functions, along with the Clinical Symptoms and Underlying Mechanisms of Action, has been Explained Under Different Sections Below
Nephrotoxicity
Mercurial compounds are absorbed through inhalation, ingestion, or dermal exposure and transported via the bloodstream to the kidneys. Mercury accumulates in the proximal tubular epithelial cells due to active uptake by Organic Anion Transporters (OATs) and metallothionein [23].
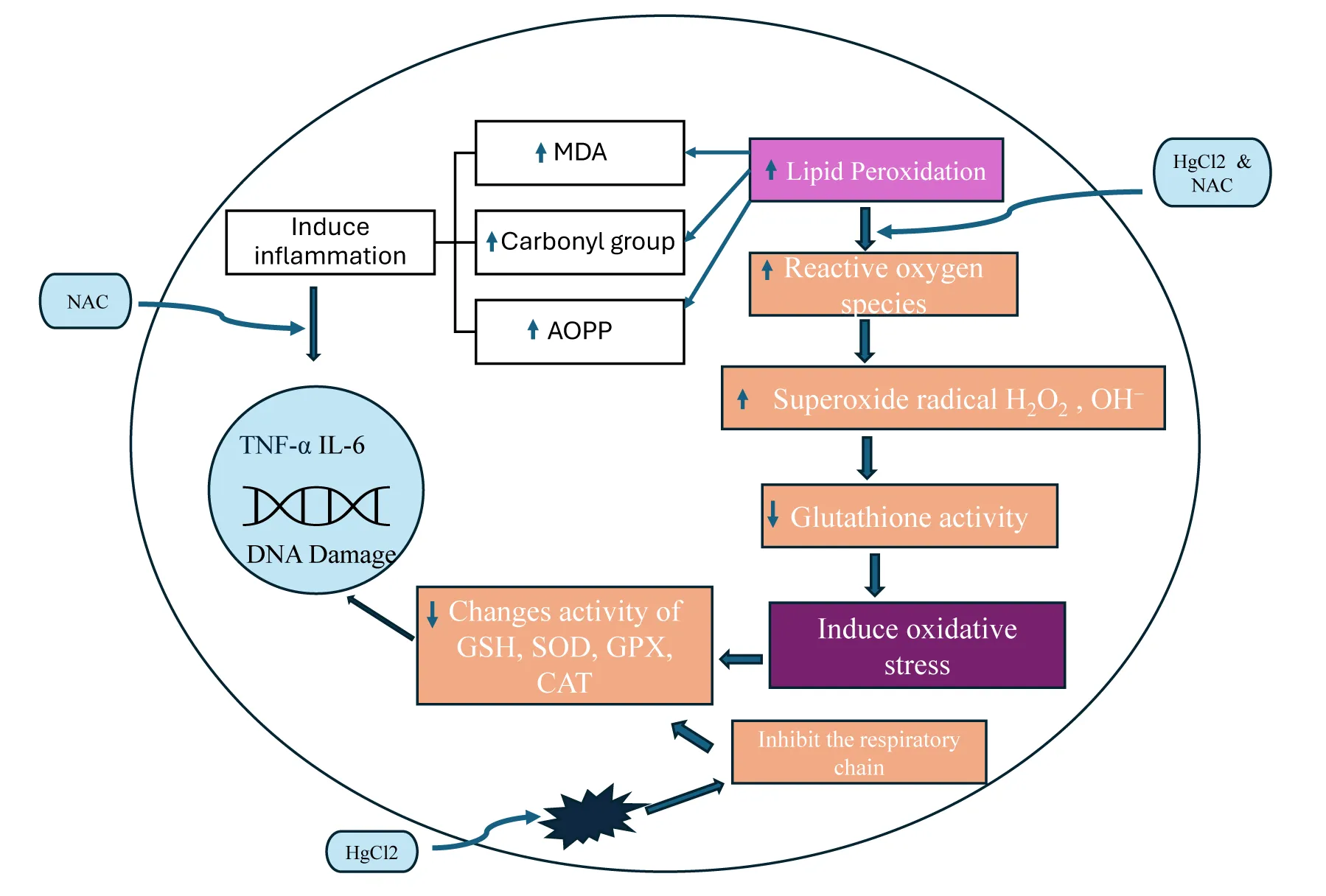
Accumulated mercurial compounds exert their effects on organelles and cellular components, namely mitochondria, nuclei, lysosomes, cell membranes, enzymes, and other biomolecules such as amino acids, peptides, proteins, and dissolved organic matter [24]. Mercury, like other heavy metals, interacts with the proteins and DNA, thereby inhibiting protein synthesis, disrupting and inhibiting enzymatic activity, and nucleic acid functions, impairing DNA repairs, and altering DNA stability [25]. It also induces Protein Phosphatase 2A (PP2A), which enhances the formation of Reactive Oxygen Species (ROS), leading to oxidative stress and irreversible inhibition of thioredoxin reductase, (a selenoenzyme) which is known to play a role in redox regulation, protein expression, selenium metabolism and restore vitamin C and vitamin E, including the increased membrane permeability [26,27]. In biological pathways, Hg affects both oxidation and reduction processes, eliciting its speciation and affecting its biological uptake. Furthermore, mercury is known to alter cellular homeostasis, Nuclear factor Kappa-light chain enhancer activation (NF-kB) driving inflammatory responses, activation of tumor protein (p53) and c-Jun N-terminal kinases (JNK) pathways (JNK also known as Stress-Activated Protein Kinase (SAPK) (all leading to apoptosis[28]. Mercuric ion Hg2+ has the greatest affinity for binding thiol groups that lead to activation of NF-kB, which results in the release of cytokines (e.g. IL-6, TNF-α) and is thus responsible for the manifestation of chronic inflammation, exacerbation of renal damage and fibrosis[28,29]. Furthermore, DNA binding at extremely low concentrations of Hg in renal tissues induces damage to the cytoskeleton structure, and tight junctions compromise epithelial integrity. These effects are mainly observed in proximal tubule cells with segments affecting primarily S2 and S3 [30,31]. In addition, mercury exposure has been shown to alter stress-responsive genes, such as those encoding metallothioneins and heat proteins, which are involved in the regulation of endogenous defense mechanisms [32]. It has also been reported that Hg alters gene expression by modifying DNA methylation and histone acetylation [33].
Kidney dysfunction following exposure to mercury inhalation in humans manifests in the form of proteinuria, increased blood urea and plasma creatinine, decreased alkaline and acid phosphatase, glutathione-S-transferase, increased thiobarbituric acid reactive substances thereby clearly indicating increased free radicals formation mainly due to accumulations of high levels of mercury compounds in kidney tissues accompanied by proximal tubular and glomerular changes [31,34]. Clinical studies in humans have reported that plasma mercury is a reliable biomarker after acute and high-level exposure. In contrast, the urine mercury level is a dependable biomarker in chronic exposure to both elemental and inorganic mercury, and also an indicator of the body's burden of mercury [35]. A person died following acute poisoning of alkyl Hg, revealing necrosis of the tubule epithelium, swollen granular protoplasm, and non-stainable nuclei in the kidneys [36,37]. Glomerular kidney functions are also altered in humans who are exposed to u-Hg or Hg0, and the same has been confirmed by urine levels of creatinine and alpha-1-microglobulin, and Gamma-Glutamyl Transferase (GGT) in the luminal brush border of the proximal tubule [31]. In addition, chronic exposure of persons at occupation is known to cause kidney damage and glomerular proteinuria, assessed by excretion of albumin, IgG, and transferrin in urine [38]. However, recent developments in proteomics profiling have emerged as a major detection tool that has facilitated tracing of the early targets of Hg in kidney structural damage and can be a novel biomarker in nephrotoxicity studies [39].
In mice, orally administered MeHg is excreted in the urine largely as the conjugate of cysteine (Cys-MeHg). Among the most predictive, reliable biomarkers, bismethylmercury sulphide (MeHg-S) and Cys-MeHg are the most critical parameters for detecting impaired kidney functional status before their irreversible damage [40,41]. In addition, determining the urinary enzyme, Gamma-Glutamyl Transferase (GGT) in the luminal brush border of the proximal tubule is also considered an important biomarker in predicting kidney damage [42]. Experimental research studies in animals indicate that nephrotoxicity and cell death progression are the reflection of the release of intracellular enzymes, namely, Lactic Acid Dehydrogenase (LDH), N-acetyl-beta-D-Glucose-amidase (NDG), and are subsequently excreted in the urine [43]. Furthermore, alpha-1, beta-2 microglobulin, and Retinol-Binding Protein (RBP) are also found to be reliable biomarkers of tubular dysfunction [44]. Animal experimental studies also indicated that Kidney Injury Molecule-1 (KIM-1) is closely associated with nephrotoxicity [45]. Studies in rodents showed dose-dependent renal damage, including tubular necrosis, glomerular injury, and interstitial inflammation, which agrees with clinical reports of renal system damage in humans exposed to Hg2+[46].
Mercury chloride (HgCl2), a hazardous, stable industrial toxin enters the body through various routes mentioned earlier and via circulation accumulates in renal and hepatic tissues and is bio-transformed into toxic metabolites and induces biochemical, cellular alterations, and generates oxidative stress that brings out conformational and cytoskeletal structural changes in kidney cells [47]. Also, known to elicit membrane nephropathy, histologically tubular necrosis, interstitial nephritis, vacuolation, onset of nephritic syndrome, secondary focal segmental glomerulosclerosis, syncretistic nephrotic syndrome, and membrane glomerulonephritis [48]. As described earlier, the characteristic property of mercury binding to the sulfhydryl group (mainly GSH) forms a glutathione mercury complex, which leads to oxidative stress. This is evidenced by the depletion of endogenous antioxidants, viz. GGT, GSH-reductases, reduced GSH, Catalase (CAT), Glutathione-S-Transferase (GST), and upregulation of expression of KIM-1 mRNA (by many folds), largely due to mercury accumulation in tubules and glomeruli of kidneys (Table 2). Furthermore, mercury chloride renal damage initiates (ERK ½) extracellular signal transducer, which in turn activates Signal Transducers and Activator of Transcription-3 (STAT-3) (Figure 3). Subsequently, interacts with the (KIM-1) Kidney Injury Molecule-1 promoter and induces the elevation of mRNA and protein levels as demonstrated by the histopathology and immunohistochemistry techniques [49]. HgCl2 treatment is known to produce renal damage through abnormal autophagy pathway: a) promoting apoptosis via excessive degradation of cellular components by worsening tissue injury b) accumulation of damaged organelles and proteins, contributing to fibrosis and inflammation, and correlated to oxidative stresss [50,51], c)HgCl2 activates the mechanistic Target of Rapamycin(mTOR) pathway, which negatively regulate autophagy [52].
| Table 2: Renal toxicity studies of short-term and long-term mercury compound exposure in Rats and mice [Table content adopted from (Francis Y, et al. [53]]). | ||||
| Animal species | Duration of study | NOAEL mg/kg/day | LOAEL mg/kg/day | Physio-pathological and pharmacological effects |
| Rat | 3-12 weeks | None | 0.84 | Fibrosis, inflammation |
| 12 weeks | None | 0.08 | Cytoplasmic masses in proximal tubules | |
| 2 years | 0.02 | 0.01 | Increased renal weight, decreased renal enzymes | |
| 0-21 days of age | None | 1.0 | Altered renal function and renal hypertrophy | |
| 2 years | None | 0.4 | Nephrosis | |
| Mouse | 26 weeks | 0.15 | 0.6 | Degeneration of proximal tubules |
| - | 0.03(Males) 0.13(Females) | 0.14(Males) 0.6(Females) | - | |
| once | 8 Males 24 females | 16(Males) 32(Females) | Decreased phenolsulfonapthalein excretion Increased serum creatinine, swollen tubular epithelium | |
Some of the documented studies suggest that exposure to nephron toxicants (mercurial compounds from the environment) to individuals with compromised renal functions (due to aging, disease, or a combination of aging and disorders) are particularly more susceptible and may be detrimental to these elderly and aged individuals [34,54]. Mercury impairs mitochondrial respiration by inhibiting electron transport chain complexes, decreasing ATP production, and causing energy deficits in renal cells. Mitochondrial damage triggers intrinsic apoptosis pathways [55]. It is now established by multiple studies that suggest that exposure of individuals with compromised renal function, whether due to aging or disease, or combined effects of both, makes them particularly susceptible to the toxic effects of mercurial xenobiotics. Even animal studies also confirmed that the kidneys of aged rats (characterized by reduced renal mass) are more prone to the adverse impacts of mercury compounds/other mercurial xenobiotics [46,56].
Neurotoxicity
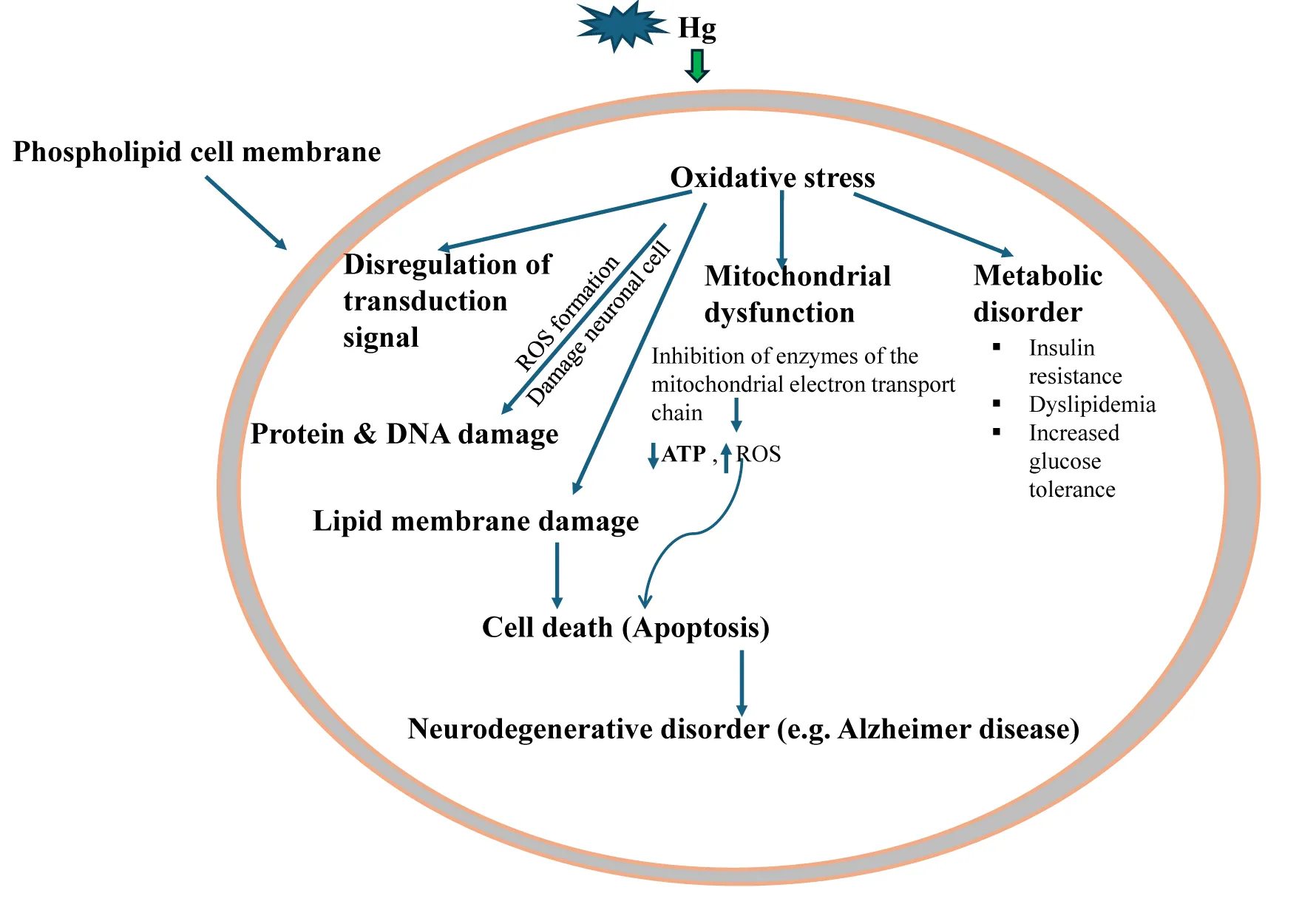
The neurotoxic effects associated with the exposure of mercury vapors, MeHg, EtHg, and mercurial xenobiotics are a well-documented public health concern, affecting both humans and animals [57]. These effects primarily arise from the ability of mercury species to cross the Blood-Brain Barrier (BBB) and interfere with the central and peripheral nervous system [58]. Inhalation of mercury vapor causes severe neurotoxic effects mainly due to high lipid solubility and penetration into the BBB [59]. Long-term exposure to mercury vapors causes mercurialism, and some of its deposition elicits effects on CNS exhibiting a wide variety of clinical symptoms related to delayed motor skill development and coordination, behavioral disorders such as autism-like symptoms, hyperactivity, anxiety, tremors, irritability, neuromuscular effects, impaired cognitive functions including reduced IQ and learning abilities and many more. These effects are associated with multifactorial biological processes, like oxidative stress, neuroinflammation, and disruption of synaptic and neuronal signaling pathways [60-62]. The most important and critical effect of mercury on the nervous system is interference with the production of energy, which adversely affects the cellular detoxification process, leading to the death of a cell or survival in a state of chronic malnutrition [57]. The mercury compounds are known to interact with the electrophilic and nucleophilic residues of proteins (e.g., Sulfahydryl groups and Selenol), which are critical factors for disruption of redox homeostasis that causes the formation of ROS, leading to lipid peroxidation and neuronal apoptosis [63-65]. Mercury inhibits enzymes in the mitochondrial electron transport chain, decreasing ATP synthesis, increasing ROS levels, impairing calcium homeostasis in neurons, signaling pathways essential for cell survival and synaptic activity [66-68]. Such complex biochemical alterations adversely affect neurotransmitter systems, including glutamate, GABA, dopamine, serotonin, and acetylcholine pathways [69,70]. Astrocytes are the major site for MeHg accumulation and thus, inhibit cystine transport (sulfhydryl-containing amino acid), severely impair the redox status, and lead to depletion of glutathione levels. Such biochemical and cellular alterations inhibit astrocytic glutamate, including aspartate uptake, and enhanced efflux effects, which result in the elevation of glutamate and aspartate levels in extracellular fluid [71]. Accumulated glutamate leads to excitotoxicity, characterized by activation of NMDA and AMPA receptors, increased intracellular calcium, and neuronal damage [71-74]. Reduced GABA levels impair inhibitory neurotransmission, exacerbating excitotoxic damage and contributing to conditions like epilepsy or anxiety disorders [75]. Thus, it appears astrocytic predisposition is also a major factor in MeHg-induced neurological disorders [76]. The available documented toxicity profile points out that methyl mercury exposure to brain cells induces neurotoxicity which is reflected in physiological, cellular, and molecular alterations along with cytokines release, oxidative stress, impaired mitochondrial function, calcium and glutamate dyshomeostasis, and ultimately manifest apoptosis [77,78]. Mercury exposure activates microglia, leading to chronic neuroinflammation, which exacerbates neuronal damage [79]. Some researchers have demonstrated that methyl mercury upregulates both phospholipase-D and phospholipase-A2, which trigger the release of arachidonic acid, which ultimately inhibits glutamate transporter, thereby facilitating an uninterrupted cytotoxic cycle [80-83].
The promotion of oxidative stress, as well as systemic inflammation and potentially influencing brain function in MeHg-induced neurotoxicity, has been confirmed unequivocally both in animal and human research studies with evidence of depletion of the antioxidant defense system and neuroinflammation [84,85]. The disruption of redox due to the formation of Reactive Species (ROS), leading to the inhibition of endogenous antioxidant defence mechanisms, results in cumulative oxidative stress [86]. Endogenous antioxidant molecules such as reduced Glutathione (GSH), Oxidized Glutathione (GSSG), Thioredoxin Reductase (TrxRs), Glutathione Peroxidase (GPx), glutathione reductase (GR), and nuclear factor erythroid 2 related factor (Nrf2) are established major endogenous factors and involved in the maintenance of redox balance in the CNS due to the high energy demand of brain tissues[84,87]. Among these molecules, GSH mainly acts as a ROS scavenger in an endogenous environment, thus minimizing the availability of MeHg for interaction with other biomolecules [88]. Dietary supplements such as selenium, cysteine, special proteins, fibrous foods, antioxidant vitamins, and phytochemicals are also reported to ameliorate oxidative stress and enhance the excretion of mercury in urine[89]. Thus, documented research findings show that co-exposure to antioxidants such as GSH, DHA, and Se is are strong confounder, and their interactions with MeHg may significantly modify cellular oxidative status. Also reported treatment with trolex, a soluble analog of vitamin E, acts as a scavenger of free radicals and chelation of MeHg, thereby significantly elevating antioxidants such as GSH and mitochondrial enzymes [90]. In an experimental rat model, trolex treatment reduced neurobehavioral deficits, oxidative stress biomarkers, and lipid peroxidation in the brain following chronic MeHg exposure. Human clinical studies reported that trolex co-administration during pregnancy reduced fetal neurotoxicity and oxidative damage caused by maternal MeHg exposure, mainly through diet (fish). Thus, different types of antioxidants can be an effective complementary treatment approach to the existing therapies, particularly in reducing CNS damage and amelioration oxidative stress. A well-designed experimental study in animals, with MeHg treatment, induced the depletion of intracellular antioxidants [91,92] inhibition of antioxidant enzymes[93–95] modulation of activity of transporters, neurotransmitters, and neuro-modulatory receptors' activity[96–98]. In addition, experimental studies in animals also established that low levels of MeHg exposure modify gene expression and induce long-lasting cell cycling signaling (specifically glutamate signaling), which has been explained earlier [99].
The very recent observations suggest that neurotoxicity in the CNS is largely due to increased activity of microglia and astrocytes, leading to chronic inflammation and release of cytokines (e.g., IL-6, TNF-α) that contribute to neuronal injury. With such experimental findings, authors have emphasized the need for extensive research to evaluate low levels of MeHg exposure on the activation of immune cells in CNS and neuronal differentiation, and their effects on the toxicity of neurodevelopmental aspects such as autism and others [99-101]. A glaringly reported clinical case suggests that chronically exposed workers showed mutational changes in the TBK-1 gene linked to ALS, providing (indicating a possible link between mercury exposure and neurodegenerative diseases [102]. Using animal models, authors provided preliminary information on the potential effects of early-developmental exposure to MeHg on the functional status by conducting exploratory activity and swimming ability at one month of age and immune effects after one year of age. Findings suggest delayed neurotoxicity may be due to increased functional impairment with aging, thus, the health-related risks associated with early MeHg exposure could last a lifetime [103,104]. Thus, mercury and mercury containing xenobiotics as critical neurotoxic agents with wide-reaching impacts on human and animal health.
Cardiovascular toxicity
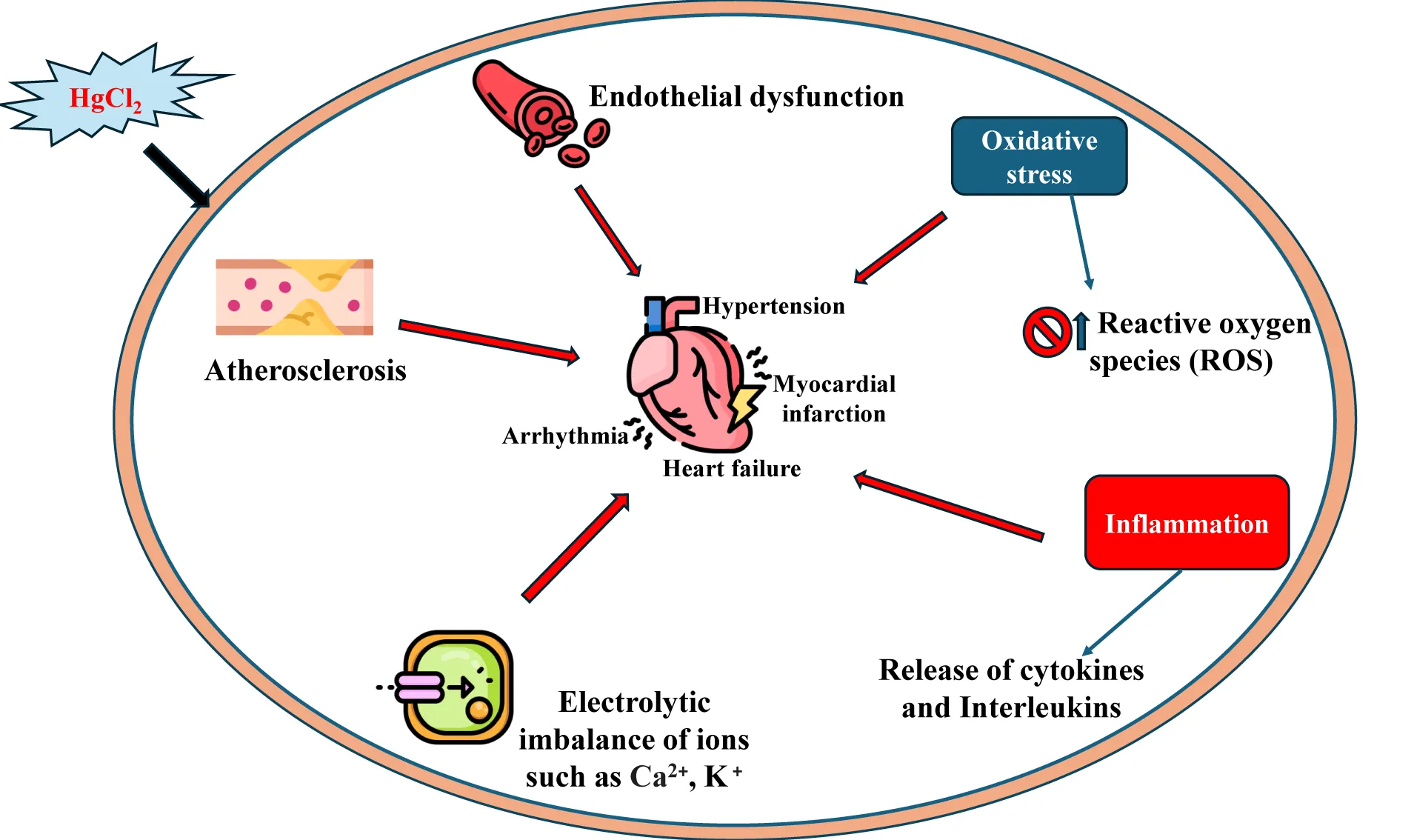
Mercurial compounds, including Methyl mercury (MeHg) and inorganic mercury (Hg2+), can induce cardiovascular toxicity through several mechanisms. Accumulation of MeHg, Hg2+ in cardiac tissue, and very high mercury levels lead to death due to idiopathic dilation of cardiomyopathy [105]. The symptoms of cardiovascular toxicity of mercury poisoning reported are hypertension, chest pain, angina, arrhythmia, myocardial infarction, and atherosclerosis. Such clinical symptoms are directly correlated to mercury concentrations in blood and hair [106,107].
Acute inorganic mercury exposure in vivo promotes a decrease in myocardial force development and depletion of ATPase activity, increased vascular resistance, and hypertension, leading to coronary heart diseases and myocardial infarction [108]. Mercury oxidizes Low-Density Lipoprotein (LDL), and LDL particles are closely related to the genesis of atherosclerosis, thereby increasing the risk of stroke, acute coronary insufficiency, and myocardial infarction [109]. The underlying mechanism(s) of cardiotoxicity of mercury are attributed to the inhibition or inactivation of the enzyme, paraoxonase, which acts endogenously as a controller or reducer of the LDL oxidation process and is known as an endogenous anti-atherosclerotic biomolecule [110]. Chronic mercury exposure enhances ROS production (free radicals) due to the Fenton reaction, leading to lipid peroxidation and inhibition of endogenous antioxidants (glutathione peroxidase, selenol) [36]. Glutathione peroxidase is a selenium-dependent enzyme, and mercury adversely affects selenium levels. The principal antioxidant enzymes, catalase and superoxide dismutase, are also reduced due to increased ROS formation by mercury intoxication. Such physiological changes increase the risk of developing hypertension, coronary heart disease, myocardial infarction, and cardiac arrhythmia, promoting a decrease in myocardial force development, carotid intima-media thickness, and carotid artery obstruction [111].
Exposure to low concentrations of mercury shown to induce dysfunction of endothelial function and conductance of vessels by impairing nitric oxide (NO) bioavailability which enhances the formation of scavengers like superoxide anion production from NADPH oxidase, which is involved in the expression of SOD-2, NOX-1, and NOX-4 (two major isoforms of NADPH oxidase), results in vasoconstriction, increased vascular resistance and hypertension [112,113]. Chronic exposure to mercurial compounds enhances free radical formation, leading to lipid peroxidation, DNA damage, and endothelial dysfunction (described earlier). The impact of such vascular oxidative stress damages vascular smooth muscle cells and compromises vascular integrity [114,115].
Mercury interacts with mitochondrial enzymes, leading to ROS overproduction, creating a vicious cycle of oxidative stress and facilitating MAPK-mediated cellular damage. Inhibition of antioxidant enzymes increases reliance on MAPK pathways to manage stress, which becomes detrimental when overactivated[88]. Mercury disrupts intracellular calcium signaling, which is tightly connected to MAPK activation, exacerbating cardiomyocyte dysfunction and arrhythmias [116-118].
Furthermore, mercury exposure can activate phospholipase-D through phospholipase-A2 and lipid oxygenase via oxidative stress and alterations in thiol redox induce COX formation and LOX-derived eicosanoids disrupt endothelial homeostasis [119,120]. Mercury also alters the functions of the renin-angiotensin system via the activation of Angiotensin-Converting Enzyme (ACE), leading to renal hypertension [121].
In animal experiments, parental administration of HgCl2 elicits cardiac diastolic failure and pulmonary hypertension. Experimental findings demonstrated that low doses of mercury chloride exposure to rats produced increased heart rate, blood pressure, and vascular reactivity to catechol amines, and these effects have been correlated with the increased formation of free radicals [122]. Long-time exposure produces structural changes in the arterial walls and myocardial fibrosis in rodents [123,124]. Chronic exposure for 30 days at low concentration of mercury in animals induces a negative inotropic effect which is explained based on atherosclerosis and calcium handling mechanisms, associated with protein expressions of Sarcoendoplasmic Reticulum Calcium ATPase (SERCA), sodium- potassium ATPase (NKA) and sodium-calcium ion (NCX) channel disruption and reduction in beta-adrenergic stimulation [125]. Mercurial compounds exposure to rodents induces hyperhidrosis, tachycardia, and ptyalism (hypersalivation), and such adverse effects have been attributed to the inhibition of catecholamine metabolism due to the reduction of S-adenosyl methionine levels, leading to the accumulation of epinephrine [126].
Prenatal exposure to mercury leads to developmental cardiovascular defects in offspring and highlights teratogenic risks [127]. Mercury disrupts mitochondrial respiration by binding to thiol groups in mitochondrial proteins, impairing ATP production and exacerbating energy deficits in cardiac tissues [128]. Thus, mechanistic insight from both human and animal studies suggests that chronic exposure to low doses of mercury produces toxic effects on the cardiovascular system and heart tissues through multiple mechanisms [16]. The other effects on the inflammatory pathways of mercury are the activation of inflammatory cytokines (IL-6, TNF-α) and CRP that can promote atherosclerosis, thrombosis, and myocardial injury. Thus, it has been established beyond doubt in both human and animal studies that mercury exposure is a major risk factor for developing hypertension, myocardial infarction, coronary dysfunction, and atherosclerosis [126] (Figure 4).
Effect on the reproductive and endocrine system of mercury and mercurial salts
The endocrine system is one of three major integrating and regulating systems in the human body. The important endocrine glands are the hypothalamus, pituitary, thyroid, adrenal gland, pancreas, and gonads (ovary and testis) [129]. In Laboratory animal studies, however, mercury was found to elicit a series of impairments of all endocrine glands in a variety of animal models [130]. The endocrine-disrupting effects of mercury have recently become increasingly important public problems.
Mercury exposure (acute, subacute, or chronic) induced pathophysiological alterations by disrupting the hypothalamic-pituitary-adrenal and hypothalamic-gonadal axis, which in turn adversely impacts reproductive functions. These disruptions interfere mainly with circulating hormones, such as FSH and LH, further impacting steroids (estrogens and progesterone androgens). Mercury primarily affects adrenal function and hormone production by inhibiting 21-alpha hydroxylase and also impacts reproductive organs, including gonad size, and gamete production [131,132]. Mercury exposure negatively impacts quality, including alterations in sperm parameters such as sperm DNA and abnormal sperm morphology. Suppression of testosterone levels is due to mercury's inhibitory effects on Leydig cells (closely associated with the process of spermatogenesis) and LH signaling. Oxidative stress induced by mercury on reproductive tissues can lead to disruption of mitochondrial functions (in mitochondria, steroidogenesis occurs due to a) impairment of cholesterol conversion to pregnenolone by the enzyme, cholesterol side-chain cleavage enzyme impaired; b) affect ATP production, and c) disrupting the import and processing of cholesterol into mitochondria by targeting steroidogenic Acute Regulatory Protein, a key regulator of steroid biosynthesis, and cellular apoptosis.) Hair mercury levels have been linked to lower sperm concentration, reduced counts, and decreased progressive mortality. The other reproductive effects associated with mercury exposure are the effects on neonatal growth, altered hormonal levels, placental weight, and gestation period, particularly in women with high seafood consumption [133-135]. Mercury exposure can reduce fertility among dental assistants due to occupational mercury exposure. In men, exposure to mercury-contaminated xenobiotics induces spermatogenesis, epididymal sperm counts, testicular weight, and, in some individuals, erectile dysfunction. In women, mercury inhibits Follicle Stimulating Hormone (FSH) release from the anterior pituitary, disrupting estrogen and progesterone levels which can lead to ovarian dysfunction, painful or irregular menstruation, premature menopause, and retroverted uterus [26,132,136,137]. The influence of mercury on the binding of hormones by their receptors is also a participating factor in the initiation of mercury endocrine toxicity. HgCl2 was shown to inhibit estradiol binding to rat uterine nuclear receptors, and the presence of divalent ions enhanced the binding [138]. Mercury exposure in pregnant women induces adverse effects on reproductive organs and can lead to miscarriage, spontaneous abortions, stillbirth, and low birth weight. Mercury's oxidative stress in ovarian follicles causes apoptosis and diminished ovarian reserve. In pregnant women exposed to MeHg mainly via diet and or inhalation of environmental pollution, the absorbed MeHg crosses the placenta and accumulates in fetal tissues, which can cause neural tube defects, craniofacial malformations, and delayed fetal growth [139]. Furthermore, MeHg induces embryopathic effects that include cerebellar hyperplasia, decreased nerve cell population in the cerebral cortex, reduction in total brain weight, abnormal neural migration, and disorganized brain layer structure [140,141]. Epigenetic alterations are induced by MeHg such as DNA methylation, and histone modifications in steroidogenic tissues causing the suppression of critical genes like (CYP1A19) aromatase and results in the disruption of conversion of androgens into estrogens that contribute to hormonal imbalance which a profound effect on long-term reproductive dysfunction [142,143]. Thus, these multifaceted mechanisms create a cascade of disruptions in steroid metabolism, leading to hormonal imbalances, reproductive dysfunction, and broader endocrine pathologies [144].
Mercury binds to sulfur sites of insulin, impairing its normal biological functions and affecting blood glucose levels [145]. It disrupts the endocrine system in humans by impacting the pituitary, thyroid, adrenal glands, and pancreas, possibly through binding to or inhibiting key enzymes and critical steps in adrenal steroid biosynthesis (e.g, inhibition of 21-alpha-hydroxylase) [146]. It has been observed in humans that mercury largely accumulates in the thyroid and pituitary glands due to its high affinity following chronic exposure in working workplace. Accumulated mercury blocks thyroid hormones by occupying iodine binding sites, which can cause damage to the thyroid and alter thyroid hormone action, leading to impairment of body temperature regulation, hypothyroidism, thyroid inflammation, and mental depression [147,148].
In animal experiments, mercury chloride (20mg/kg) administered intramuscularly to rats elicits a marked increase in the thyroid peroxidase activity, with increased T3 levels and decreased T4 levels resulting high T3/T4 ratio, which leads to the onset of acute hyperthyroidism. Also, demonstrated that a small dose of mercury chloride (0.26mg/kg) administered orally daily for 6 weeks to rodents produces thyroid hypertrophy and decreased iodine uptake, elevated protein-bound iodine. All these events in the thyroid gland led to coupling defects in the synthesis of T3 but not T4 [149,150]. Such an effect on T3 synthesis has been correlated to mercury's effect on 5-deiodinase. However, the authors suggested that it may be necessary to investigate the effect of mercury on serum thyroxine levels to understand the exact underlying mechanism of action of mercury chloride on the thyroid gland [151].
Chronic exposure to catfish with methyl mercuric chloride for 180 days or emisan6 resulted in hyperplasia, lymphocyte infiltration, fibrosis even necrosis in the area of the adrenal gland, accompanied by a reduction in plasma cortisol levels. Also, pituitary cells are hypertrophied and degranulated, indicating hypersecretion of ACTH. Further, chronic methyl mercury exposure affects the sensitivity of the adrenal cortex to stress stimulation of ACTH and impairs adrenal steroid production and metabolism (in vivo). Mercury's toxic effects on bioplasm (cellular protoplasm) and its broad-spectrum enzyme inhibition can be attributed to mercury's high affinity for thiol group enzymes and proteins. Similar findings are also reported in adrenal and gonadal glands in the experimental seal model [152,153]. The testicular toxicities and steroidogenesis have also been observed in different laboratory animal species (e.g., fish, fowls, rodents, and primates) [154,155]. The effects of mercury on female gonad function were also reported in fish, mice, and hamsters. Fish exposed to mercury-polluted water had decreased gonadal steroidogenesis and pituitary gonadotropin-releasing, inhibited ovarian maturation, decreased ovarian weight, and decreased the number and diameter of oocytes. The meiosis of ova was affected by mercury in mice and hamsters [130,156-158].
The key mechanisms of mercury toxicity can be attributed to its interference with the intracellular calcium mechanisms in the endocrine system, as well as in the organ functional systems. Mercuric chloride is likely to inhibit the release of vasopressin via disruption of the calcium pumping mechanism in the neurohypophysis. Endocrine gland damage also occurs due to oxidative stress (lipid peroxidation, increased ROS formation, and redox-sensitive signaling pathways that regulate the expression of steroidogenic genes) [159].
The toxicological impact of mercurial compounds and mercurial xenobiotics on the endocrine and reproductive system underscores the need for preventive measures, stricter enforcement of regulatory controls, and in-depth research to understand and unfold mechanisms of toxicity to mitigate health risks [11].
Effects of Mercurial compounds and mercurial xenobiotics exposure on the immune system
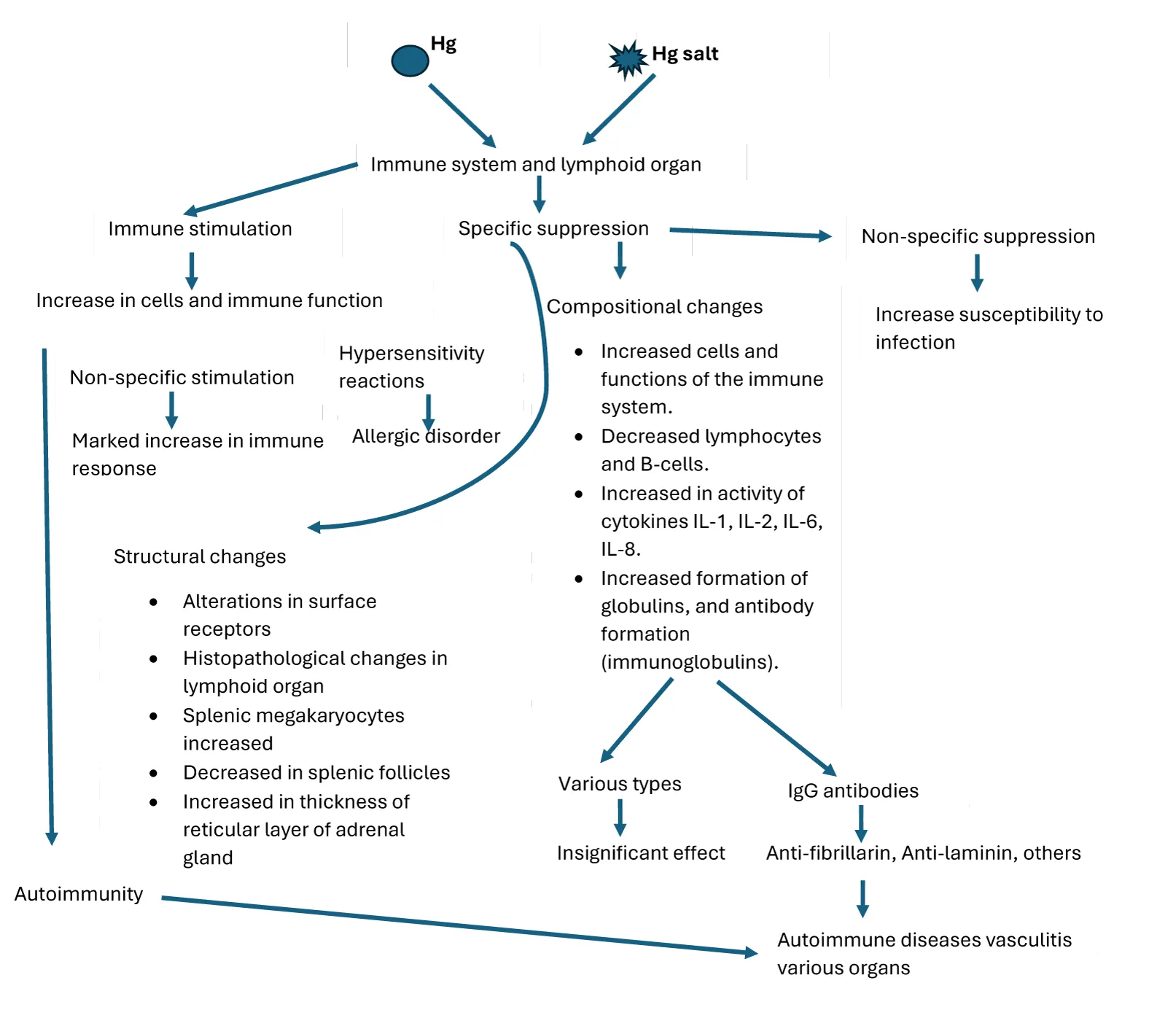
Documented findings indicate that elemental mercury and its organic or inorganic salts (known immune toxicants), including those derived from environmental exposure, exert significant and multifaceted effects on both the innate and adaptive arms of the immune system, leading to immunosuppression, autoimmunity, and inflammation [160-162]. These effects stem from mercury's ability to interact with immune cells (macrophages, dendritic cells, T cells, and B cells) and modulate immune signaling pathways, often leading to the deregulation of both innate and adaptive immunity [163-166]. Experimentally, it has been shown that mercurial compounds impair the immune system and its functions, mainly through negative effects on polymorphonuclear cells (PMNs) due to the suppression of adrenocortical steroid synthesis, which prevents the normal stimulation of PMN function and inhibits their ability to destroy foreign substances [167,168]. Both short and long-term exposure to mercury in the workplace environment or seafood consumption, and usage of skin-lightening creams containing mercury can interact with immune cells leading to stimulation of macrophages (the release of excessive ROS and pro-inflammatory mediators such as cytokines(TNF-α, IL-6 and IL-1β via activation of Toll-like receptors 4, nuclear factor kappa B signaling pathways) causing tissue damage; inhibits the function dendritic cells and major histocompatibility molecules results in a reduction in the efficiency of antigen presentation)[161].
The ability of metal ions to stimulate inflammatory reactions in the absence of a mitogen is well documented. The main cellular mechanism of inflammation is due to stimulation of the innate immune system, due to a) cell death, b) participation of pattern recognition receptors, ability to present antigens to secondary lymphoid organs, leading to activation of the adaptive immune system (associated with TNF-α producing Th1 CD4+ T cells). Mercurial-containing xenobiotics exposure triggers T-helper cell polarization, specifically Th2 and Th17 subsets, promoting autoantibody production and inflammatory responses [169,170]. Thus, adaptive immunity associated with mercury toxicity also acts as a complimentary to the innate immune system. This is supported by the fact that the adaptive immune cells, particularly activated T cells, produce cytokines like IFN-γ, which enhance innate immune responses. All these cellular events in metal-induced immune cell activation are likely to play a significant role in the initiation of inflammation and lead to subsequent immunopathology [171]. Dendritic cells or other antigen-presenting cells exposed to mercury or mercury salts enhance the maturation of dendritic cells, up-regulation of co-stimulatory molecules (CD80, CD86), and present major mercury-modified peptides (histocompatibility complex) to T cells [172]. Thus, activation of various cells such as T cells and B cells, macrophages, increased cytokine production, impaired phagocytosis, and suppression of lymphocyte proliferation depend on the chemical form, dose, exposure duration, and individual genetic predisposition [173] (Figure 5).
Overactive dendritic cells can skew T-cell differentiation toward auto-reactive phenotypes. Decreased cytotoxicity of NK cells occurs due to adverse effects on calcium signalling and immunity. [174-176]. Mercury exposure can induce DNA Methylation, histone modification, and microRNA dysregulation in immune cells, altering gene expression related to immune responses. Such epigenetic modifications subsequently influence long-term immune dysfunction. From the documented data, it appears that inorganic mercury exposure affects the likely myeloid and lymphoid linkage formation and has a greater impact on inflammatory and immune responses [99,143,177]. In animal experiments (mice, rats), administration of mercury chloride salts was shown to induce chromosomal aberrations in peripheral lymphocytes and bone marrow cells, as well as micronucleus formation in reticulocytes[178-180].
Mercury chloride in vitro induces lymphoproliferative activity in guinea pigs, rats, rabbits, and mice [181]. The micromolar concentration of mercury chloride negatively affects neutrophil functional activities but increases chemical luminescence, H2O2 formation, and enhanced release of lysosomal enzymes within the shortest period [168,182].
Mercury exposure alters immune responses and potentially targets self-antigens in a manner akin to autoimmune reactions and molecular mimicry, most probably due to bio similarities within mercury-containing structures leading to autoimmune diseases such as eczema, Amyotrophic Lateral Sclerosis (ALS), autoimmune thyroiditis, etc[11,183-185]. Experimental findings in mercury-induced autoimmunity mouse model throw more insights into the initial modification of antigen fibrillarin by mercury and then followed by a Th2 dominant responses such as increased production of IL-4, IL-5, and IL-13, which may cause allergic and autoimmune responses mainly driven by the modified fibrillarin [185-188]. Mercury-induced cell death results in proteolytic cleavage of fibrillarin to a 19-kDa fragment. As a result of the proteolysis of fibrillarin, the leakage of cysteine occurs, hence unable to bind to mercury and induces fragmentation, which is capable of producing fibrillarin autoantibodies. Such molecular interactions suggest that cell death is likely to generate new types of regimens as a source of antigenic determinants for self-reactive T-lymphocytes. In addition, mercury is shown to alter immunological signaling pathways, such as nitrogen-activated protein kinase and nuclear factor kappa B (NF-kB) [161]. Dysregulation of these molecular signaling pathways points out the possibility of the involvement of specific genes in deriving such immunological responses [189]. In an experimental study in mice, mercury chloride (5 mg/kg) treatment intraperitoneally induced spleen damage within 24 hrs [190]. Splenic transcription analysis demonstrated that 3334 genes (2134 upregulated and 1200 downregulated) are expressed differently in mercury chloride-induced spleen damage in mice models. Notably, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment indicated phosphatidylinositol 3 kinase (PI3K) AKT might be a key signaling pathway in mercury-induced spleen damage. In an animal experimental study, mercury chloride-induced autophagic cell death has been explained based on increased protein expression of PI3K, AKT, LC3-II, and p62, and the number of apoptotic cells [191].
Mercury pollutants of environmental origin interact with the various components of the immune system and significantly alter/modify immune responses [192]. Furthermore, mercury is known to trigger immunological responses in the CNS, modify the formation and function of immune cells, and modulate IFN-γ levels [193,194]. These events consequently escalate the production of anti-nuclear antibodies while diminishing anti-inflammatory cytokines. Thus, there seems to exist a complex bidirectional relationship between the CNS and immune system via receptors for neuropeptides, neurotransmitters, and hormones in the lymphoid organs, which may be involved in modulating immune functions [195].
Other experimental observations with the animal study demonstrated that mercury chloride treatment in ASW mice elicited an increase in serum IgE and IgG levels, whereas C57BI/6 mice treated with mercuric chloride exhibited an increase only in serum IgE with no change in IgG levels[196]The lack of response in the DBA/2 is due to the absence of inflammation at the site of exposure, coincident with reduced proinflammatory cytokines(IL-1β, TNF-alpha, and IFN-γ) levels, which impair the recruitment and activation of immune cells, thereby dampening the immune response. Reduced activity of cathepsin B likely hinders antigen presentation and reduces the activation of the inflammasome complex, further suppressing inflammation [197].
However, mercury-induced murine autoimmunity (mHgIA) studies with different approaches demonstrated that it increases the gene expression of CD40 ligand (CD40L) on T cells and may be deregulated, intensifying the immune response and contributing to autoimmune phenomena via increased cytokine production [198,199]. Mercury's effect on CD-28 signaling can also lead to abnormal T-cell activation [200,201]. Alternatively, anergic states undermine immune tolerance and are likely to promote the autoimmunity process. This creates a vicious cycle of autoantibody production and immune complex deposition, contributing to tissue damage and autoimmune pathology [201,202].
Such chronic effects of mercury on immune function may lead to vulnerability to infections, prolonged inflammatory responses, and altered vaccine responses. Further, the modifications to self-antigen immune complexes may lead to the immune system misidentifying normal tissue as foreign, thus triggering an autoimmune reaction. Hence, further research is needed to fully understand the exact mechanism by which mercury impacts immune function and also to establish a clear cause-and-effect relationship [164].
Effects of mercury on Gastrointestinal (GI) and Haematological systems
Most human exposures generally occur due to contaminated seafood, outgassing from dental amalgam, or occupational exposure. Mercury exposure can be absorbed and methylated/demethylated, and HgCl2 in the gut. The impacts of Hg have multiple detrimental effects on the GI system involving a range of mechanisms impacting its structure, function, and gut microbiota. Trillions of microbes inhabit animals and humans and are known to play as the biological barrier in the gut [203]. Both Hg and MeHg were found to cause intestinal microbial disorders, abnormal metabolite production, tight junction damage, and immune responses in the gut. Mercury absorbed via epithelial cells when ingested causes irritation, inflammation in neurons of the enteric nervous system ulceration, which can be manifested as symptoms like abdominal pain, nausea, vomiting, and diarrhoea (due to mercury accumulation in neurons of the enteric nervous system) [203-205].
Mercury interacts with (–SH) groups in proteins, and digestive enzymes leading to loss of catalytic activity of enzymes and rendering them non-functional thereby affecting carbohydrate and fat digestion and protein breakdown; and structural damage in the mucosal epithelial lining contributes to inflammation and promotes pathogenic bacteria[206-209]. Chronic exposure is associated with atrophy and degeneration of intestinal villi and impaired nutrient absorption [210]. Oxidative stress and lipid peroxidation lead to impairment of the GI tract antioxidant defense system and compromise the integrity of cellular membranes, causing cell death, and increasing intestinal permeability (leaky gut), allowing toxins and allergens to enter the bloodstream [211]. Chronic mercury exposure activates immune cells in gut-associated lymphoid tissue, resulting in inappropriate inflammation (e.g., colitis, mimicking autoimmune-like syndrome) [212]. Furthermore, mercurial compounds are also known to interfere through direct interaction with their active sites, alteration of redox homeostasis, and structural modification of the function of xanthine oxidase and dipeptidyl peptidase-4 [7,213,214].
Mercury disrupts the balance of gut microbiota (dysbiosis), causing an increase in undigested food products in the bloodstream that can lead to immune-mediated reactions and resistance to pathogenesis (pathogenic infections), and also impair blood glucose concentration by binding to the sulfur sites of insulin [137,204,215-217].
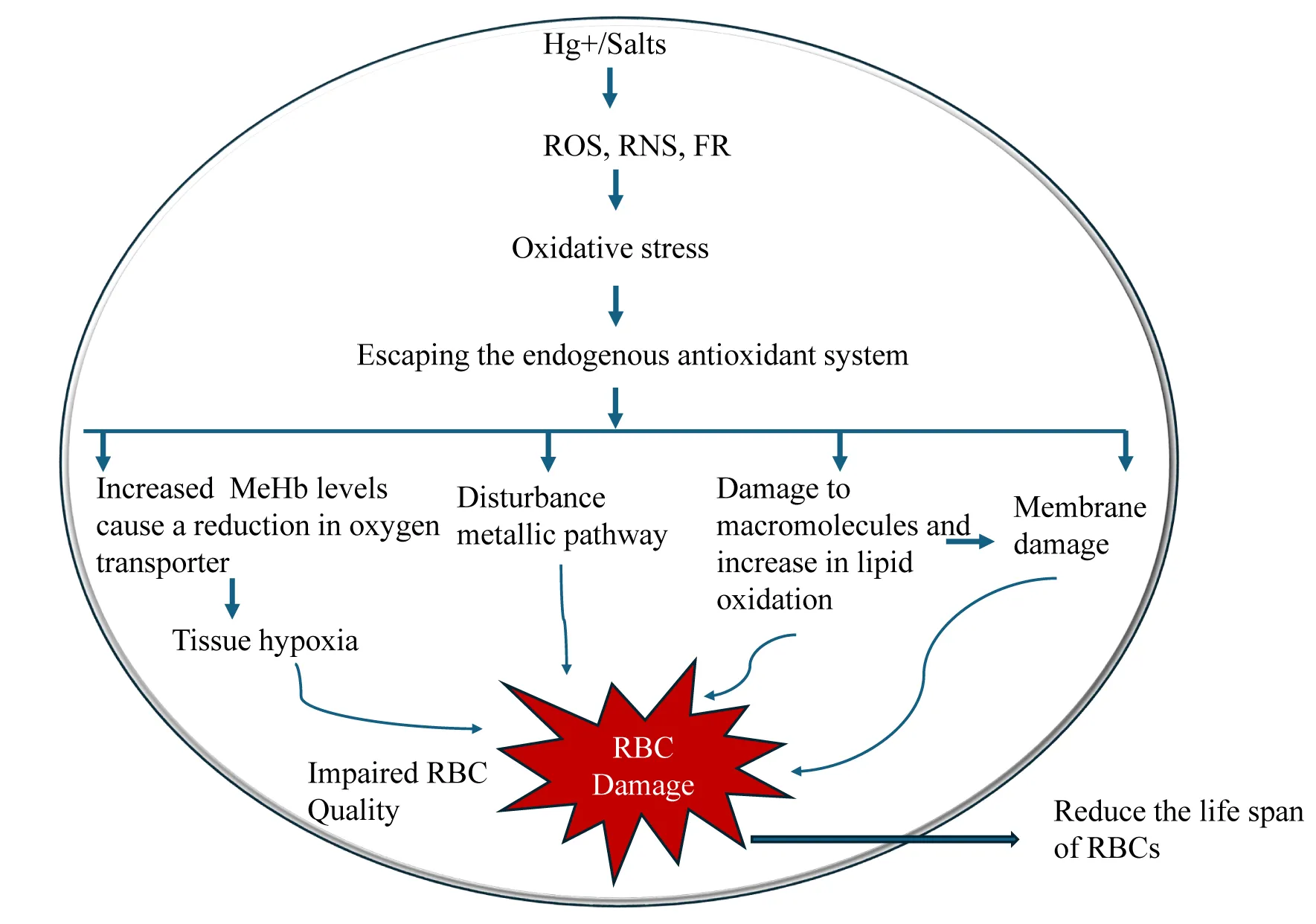
Mercurial compounds (both organic and inorganic) affect significantly the hematologic or blood system due to oxidative stress formation of free radicals, (ROS, RNS, FR) leading to various toxic effects: a) particularly in organic forms, can induce hemolysis largely due to the affinity for sulfide groups in red blood cells disrupting cell membrane integrity and causing cell lysis resulting in anaemia (haemolytic anaemia and aplastic anaemia)[218,219]. In addition, mercury can impair erythropoiesis in the bone marrow and oxidize haemoglobin (Methyl mercury haemoglobin), thereby impairing oxygen-carrying capacity. b) Mercury exposure affects lymphocytes, neutrophils, and monocytes. (Figure 6) Experimental studies have demonstrated that mercury compounds can reduce lymphocytes and impair immune responses, making the body vulnerable to infection. Oxidative stress of mercury can alter leukocyte function and adversely affect normal surveillance and inflammatory responses. Enzymes required for platelet adhesion and aggregation are inhibited, leading to the prolongation of bleeding time and the wound healing process [220–223].
Carcinogenic promoting effect of mercurial compounds
It has been documented that worker at occupational exposure chloralkali workers, dentist and dental nurses, nuclear weapons workers, gold mining workers, and other industries and people eating kinds of fish and shellfish, too mercurial compounds especially methyl mercury and inorganic compounds (as determined by their mercury levels in toenail, hair, and plasma/serum) shown to be at risk of cancer and mortality [224]. The toxic manifestations of mercury vary depending on its speciation of bioaccessibility, bioavailability, and kinetic pattern. Some of the case reports revealed that farmers using mercury-based fungicides and seed dressings develop risk factors for lymphocytic leukemia, and people show higher mercury levels in blood, 3.67 μg/L, likely to promote renal cancer [11]. However, low dose of mercury exposure induces proliferative responses both in normal and cancerous cells, accompanied by cytotoxicity. The carcinogenic effects of mercury have been linked to its potential to disrupt cellular homeostasis, induce genetic and epigenetic alterations, and promote chronic inflammation. In experimental animal models, a high dose of mercury exposure has been linked to interference with estrogen receptors, ERK-1/2, JNK, NADPH-oxidase, and potentially Nrf2 signaling. Mercury can mimic or antagonize hormonal activity, such as estrogen receptors. Such endocrine disruptors can promote hormone-sensitive cancers like breast and prostate) [225]. Hormonal imbalance likely creates situations favorable for carcinogenesis, such as an increased rate of proliferation of hormone-dependent tissues or organs [226]. Genetic damage and mutagenesis, such as oxidative DNA damage (8-oxo-dG and chromosomal aberration), genotoxicity, and epigenetic effects of Hg, may be collectively involved in cancer promotion [227].
Further, combined with decreased apoptosis and pro-survival signaling upon low-dose mercury exposure, accumulation of DNA lesions in cells may predispose to the risk of malignant transformation [11]. The interference of epigenetics (via gene modification by various molecular processes such as DNA methylation (both hyper and hypo patterns and histone modifications have been documented) is likely to cause abnormal gene expression, and that may be a major driving factor or hallmark of molecular interaction of mercury for carcinogenic effects. Hence, it has been proposed that mercury may be a potential epigenetic metal. All these above-described epigenetic changes modify gene expression without altering DNA sequences, facilitating abnormal cell proliferation, immune evasion, and angiogenesis in the tumor microenvironment (sustained inflammatory signaling creates a microenvironment conducive to tumor initiation). In animal experiments, at very high doses, some mercurial compounds are reported to produce several types of tumors in rats and mice and are considered highly carcinogenic [6]. Although mercury exposure is associated with cancer risk, many available data are contradictory, hence, mercury may be a promoter of carcinogenic risk through modulation of cell proliferation. In a broader sense, mercury might be an example of an epidemic tumor promoter, a mutagenic effector associated with multifaceted complex mechanisms with probable carcinogenic actions of mercurial compounds [228,229].
Ecotoxicity
Mercurial compounds and mercurial xenobiotics can persist in natural environments and accumulate through the food chain, causing toxicity in organisms, disrupting ecological balance, and impacting biodiversity [9]. In plants, low concentrations of mercury improved germination rate, root length, early blooming, plant height, pollen viability, and chlorophyll content. On the contrary, at higher concentrations, mercury slows down and restrains the plant, including the yield of biomass production, impaired photosynthesis, nutritional deficiency, and growth [230]. This impacts the entire food chain. This has been attributed to soil microorganisms, largely influencing the bioavailability of nutrients required for plant growth. Microorganisms are susceptible to heavy metal stress [231].
Mercury's impact on aquatic life, especially in fish, affects development, reproduction, and behavior, reducing populations and disturbing ecosystem dynamics [9,232]. Also influences growth, production, and enzymatic processes in organisms like molluscs and crustaceans, which are key to food webs. Thus, mercury disrupts food web dynamics by altering predator-prey relationships and reducing species diversity. Such events can lead to cascading effects throughout the ecosystem, disturbing ecological balance.
Exposure of invertebrates, arthropods, worms, and Drosophila to mercury/mercury xenobiotics produces deleterious and severe impacts such as locomotion, growth, eating behaviour, poor prey acquisition, and promotion of developmental defects in embryos. The effect of exposure to methyl mercury also developed slowly and was exhibited with patterning, positioning, and maturation of neurons and glia. Thus, it appears from the documented research findings that invertebrates are more vulnerable to mercury exposure in their early life stages, such as eggs, embryos, and Larvae. The adverse effects reported among vertebrate species (amphibians, birds, fishes, reptiles, and mammals with the mercurial compounds, methyl mercury, include endocrine-disrupting activity and impairment of functions of vital organs such as the liver, kidney, CNS, embryos, and changes in the reproductive habits. Mercury is reported to interfere with the reproductive success of birds by causing eggshell thinning, decreasing hatchability, by altering hormone levels. Birds exposed to high mercury levels result in reduced fertility and developmental abnormalities in embryos. Further, birds show weakened immune responses, making them more susceptible to infections and diseases. Cardiotoxicity (such as abnormal heart rhythms, compromised heart structure, and decreased capability to oxygenate tissues), particularly in migratory birds, has been reported. Such adverse toxic effects hinder their survival rates and impact population dynamics. Thus, mercurial xenobiotics pose a threat to biodiversity, as they reduce the productive success and survival rates of sensitive species [9,232-239].
Available Avenues for Mitigating Mercury Toxicity in Humans
As described earlier, mercury is a pervasive environmental toxin with significant health implications, affecting almost all vital organs. Advancements in mitigating human mercury toxicity have concentrated on enhancing chelation therapies. Chelators normally bind to mercury ions, facilitating their excretion from the body. The chelators used are: Dimercaptosuccinic acid (DMSA), 2,3-Dimercapto-1-Propanesulfonic acid (DMPS), dimercaprol (BAL), and NBMI [N, N′-bis(2-mercaptoethyl)isophthalamide][25,240-243]. Their efficacy and safety profiles of these chelators by making esters (e.g., mono isoamyl ester) increased their bioavailability and mercury binding capability compared to the parent molecules. Furthermore, chelators are combined with antioxidants like alpha-lipoic acid. This combination approach helps to mitigate oxidative stress associated with mercury toxicity and is used in conventional and alternative medicine [244]. The German environmental agency (Umweltbundesamt) listed DMSA and DMPS as the two most useful tools and reasonably safe chelating agents. These agents, during the chelating process, also remove vitamin C&E, hence these need to be supplemented [240,244-246]. The side effects of chelation therapy include dehydration, low blood calcium, harm to kidneys, and liver enzymes are elevated, and allergic reactions [247]. The protective effects of Indigenous plants (phytochemicals) and recognizing traditional medicinal system (Ayurveda) significantly elevated mercury-induced toxicity in animal models and in-vitro experimental effects are largely attributed to their antioxidant properties (alleviating oxidative stress induced by mercury), which enhances endogenous antioxidant defenses, reduce cellular damage and some of them may chelate mercury ions, facilitating their excretion from the body [248]. These phytochemicals, along with beta-carotene and alpha-tocopherol tocopherol showed a more prominent ameliorating effect, probably by recuperating oxidative stress, indicating its usefulness in clinical regimens. Thus, these phytochemicals of natural product origin, preclinical mercury toxicity alleviative effects, reveal innate antioxidant properties by repression of mercury-induced oxidative stress by multimodal elevation of endogenous enzymatic and non-enzymatic fortification systems, leading to mitigation of mercury-induced toxicity in experimental animals. These preclinical studies could serve as a pivot for further discovery of potential agents that may be useful in the clinical management of mercury toxicity. Bio-medicinal plants, Carica papaya leaves (ethanolic extract), Adansonia polygamus, Curcuma longa, flax seeds, Rheum turkestanicum, and Tribulus terrestris alleviate inorganic mercury-induced kidney damage evaluated by biochemical and molecular alterations [49].
The selenium treatment/supplementation in diet supplement has been used because selenium interacts with mercury and forms biological inert complexes (mercury selenide) thereby reducing mercury’s affinity for thiol groups in proteins and mitigating its toxic effects (e.g. the mechanism associated in selenium protection is due to amelioration/reduction mitochondrial injury and DNA damage, cellular calcium dyshomeostasis, pro-demethylation of methyl mercury, sequestering of mercury via mercury selenium complexes, and redistribution of mercury inside organisms) via inhibitory effects of selenium on the action of mercury. Selenium and acetylcysteine administration together for 4 days reduced kidney and liver mercury concentration by 70% and 80%, respectively, and restored histopathological architecture in mercury chloride, methyl mercury, and dimethyl mercury-treated rats [249].
Remediation approaches for environmental mercury pollution
The different traditional biological methods employed to remediate mercury with bacteria. Some of them have been mentioned briefly below:
The utilization of mercury-resistant bacteria, such as Pseudomonas, Bacillus, and Enterobacter, possesses a mercury reductase enzyme that converts toxic methyl mercury to less toxic Hg0 through enzymatic reduction [250]. This may lower the burden of mercury exposure through food chains, especially fish containing methylmercury. Bioabsorption is the ability of microorganisms to capture mercury by increasing their biomass. In this process, the mercury capture is retained largely in the bacterial cell wall without the need for intracellular bioaccumulation [251-253]. The application of yeast cells and whole-cell biosensors has also been adapted as a bioremediation technique for mercury contamination in the atmosphere (soil and water). Mercury-volatilizing bacteria inherit the Mer operon, which enables the reduction of Hg²⁺ to volatile Hg⁰. The Mer operon encodes several proteins: a mercury reductase (MerA), an organomercurial lyase (MerB), a periplasmic mercury-binding protein (MerP), inner membrane proteins involved in Hg²⁺ transport (MerT, MerC, MerE, MerF, and MerG), and regulatory proteins that control operon expression (MerR and MerD) [254].
The phytoextraction process removes heavy metals, including mercury, through plants. Environmental pollutants, such as mercury, induce oxidative stress in plants, leading to the overproduction of Reactive Oxygen Species (ROS)[255]. These ROS include Hydrogen peroxide (H₂O₂), Hydroxyl radicals (HO•), molecular oxygen (O₂), and superoxide anions (O₂⁻) [86]. To counteract oxidative damage, plants produce various antioxidant enzymes, such as Superoxide Dismutase (SOD), Glutathione Reductase (GR), catalase, and Ascorbate Peroxidase (APx), which catalyze and degrade ROS into less harmful molecules [256]. Mercury exposure has been observed to enhance the cellular production of APx and other enzymes in certain plant species. This surge in enzyme activity is interpreted as a protective or defensive response to ROS, triggered by the intracellular presence of mercury [257].
Traditional remediation techniques, such as soil excavation, removal, and containment or capping of contaminated sites, are often disruptive and costly. Recent research has explored innovative materials, including synthetic corals made from aluminum oxide nanoparticles that effectively absorb heavy metals like mercury from water sources. Similarly, modern nano-biotechnological techniques (affordable and cost-effective) such as gold-decorated titanium dioxide nanotubes have demonstrated excellent mercury ion removal capabilities through a photocatalytic process, making them efficient for cleaning contaminated water bodies and transposon-mediated in-vitro molecular breeding (ISMoB) (process associated horizontal gene transfer which involves basic mechanisms like transformation, transduction and conjugation process) for effective removal of mercury pollution. The above mentioned techniques seem to be more efficient in the removal of mercury [253,258]. To advance mercury remediation efforts, it is imperative to develop new technologies that leverage microbial species in bioremediation. This requires a deeper understanding of the mechanisms that enhance microbial activity and pollutant metabolism under various ecological conditions.
Conclusion
Mercury and its compounds' toxicity remains a global concern, impacting human and animal health, ecosystems, and biogeochemical cycles. Its multifaceted effects disrupt multiple organ systems, including cardiovascular, renal, endocrine, and immune, and have profound effects on fertility and reproductive processes. Its molecular mechanisms involve oxidative stress, genetic disruptions, hormonal dysregulation, and immune dysregulation. An urgent need for interdisciplinary approaches to develop innovative mitigation strategies coupled with a forward-looking policy vision to curb mercury exposure and safeguard biodiversity, public health, and future reproductive potential. This may ensure safer, sustainable coexistence with our environment.
CRediT Authorship Contribution Statement
Suresh Naik: Supervision, Conceptualization, Formal analysis, Visualization, original draft, review, editing. Dipesh Gamare: Writing – original draft, review, editing, & Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to acknowledge Ms. Amisha Bhopatrao (Technological University of the Shannon, Midlands-Midwest, Athlone, Ireland) for her technical assistance throughout this manuscript writing.
Data Availability
The authors do not have permission to share data.
References
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133-64. doi: 10.1007/978-3-7643-8340-4_6. PMID: 22945569; PMCID: PMC4144270.
- Naik SR, Gamare D, Bhopatrao A. Chemical health hazards and toxicity of environmental pollutants on humans, animals and others: An overview. Journal of Toxicological Studies. 2024;2:1135. doi: 10.59400/jts.v2i1.1135.
- Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020 Sep 8;6(9):e04691. doi: 10.1016/j.heliyon.2020.e04691. PMID: 32964150; PMCID: PMC7490536.
- James AK, Nehzati S, Dolgova NV, Sokaras D, Kroll T, Eto K, O'Donoghue JL, Watson GE, Myers GJ, Krone PH, Pickering IJ, George GN. Rethinking the Minamata Tragedy: What Mercury Species Was Really Responsible? Environ Sci Technol. 2020 Mar 3;54(5):2726-2733. doi: 10.1021/acs.est.9b06253. Epub 2020 Feb 12. PMID: 31951385.
- ATSDR. 1999.
- Mercury emissions: The Global Context. USEPA 2024.
- Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014 Mar;47(2):74-83. doi: 10.3961/jpmph.2014.47.2.74. Epub 2014 Mar 31. PMID: 24744824; PMCID: PMC3988285.
- Ali H, Khan E, Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J Chem. 2019;2019:1-14. doi: 10.1155/2019/6730305.
- Ward DM, Nislow KH, Folt CL. Bioaccumulation syndrome: Identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann N Y Acad Sci. 2010 May;1195:62-83. doi: 10.1111/j.1749-6632.2010.05456.x. PMID: 20536817; PMCID: PMC2977981.
- Veeraswamy D, Subramanian A, Mohan D, Ettiyagounder P, Selvaraj PS, Ramasamy SP, Veeramani V. Exploring the origins and cleanup of mercury contamination: a comprehensive review. Environ Sci Pollut Res Int. 2024 Sep;31(41):53943-53972. doi: 10.1007/s11356-023-30636-z. Epub 2023 Nov 14. PMID: 37964142.
- Wu YS, Osman AI, Hosny M, Elgarahy AM, Eltaweil AS, Rooney DW, Chen Z, Rahim NS, Sekar M, Gopinath SCB, Mat Rani NNI, Batumalaie K, Yap PS. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega. 2024 Jan 22;9(5):5100-5126. doi: 10.1021/acsomega.3c07047. PMID: 38343989; PMCID: PMC10851382.
- Rice DC. The US EPA reference dose for methylmercury: Sources of uncertainty. Environ Res. 2004;95(3):406-13. doi: 10.1016/j.envres.2003.08.013. PMID: 15220074.
- Health-based air concentrations for mercury vapor health-based guidance values (Air Concentrations) for Mercury Vapor. New York State Department of Health. 2008.
- Revis NW, Osborne TR, Holdsworth G, Hadden C. Mercury in soil: A method for assessing acceptable limits. Arch environ contam toxicol. 1990;19:221-6. doi: 10.1007/BF01056090.
- Gazwi HSS, Yassien EE, Hassan HM. Mitigation of lead neurotoxicity by the ethanolic extract of Laurus leaf in rats. Ecotoxicol Environ Saf. 2020 Apr 1;192:110297. doi: 10.1016/j.ecoenv.2020.110297. Epub 2020 Feb 13. PMID: 32061979.
- Fernandes Azevedo B, Barros Furieri L, Peçanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simões M, Fiorim J, Rossi de Batista P, Fioresi M, Rossoni L, Stefanon I, Alonso MJ, Salaices M, Valentim Vassallo D. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol. 2012;2012:949048. doi: 10.1155/2012/949048. Epub 2012 Jul 2. PMID: 22811600; PMCID: PMC3395437.
- DesMarais TL, Costa M. Mechanisms of Chromium-Induced Toxicity. Curr Opin Toxicol. 2019 Apr;14:1-7. doi: 10.1016/j.cotox.2019.05.003. Epub 2019 May 17. PMID: 31511838; PMCID: PMC6737927.
- Evers DC, Ackerman JT, Åkerblom S, Bally D, Basu N, Bishop K, Bodin N, Braaten HFV, Burton MEH, Bustamante P, Chen C, Chételat J, Christian L, Dietz R, Drevnick P, Eagles-Smith C, Fernandez LE, Hammerschlag N, Harmelin-Vivien M, Harte A, Krümmel EM, Brito JL, Medina G, Barrios Rodriguez CA, Stenhouse I, Sunderland E, Takeuchi A, Tear T, Vega C, Wilson S, Wu P. Global mercury concentrations in biota: their use as a basis for a global biomonitoring framework. Ecotoxicology. 2024 Jul;33(4-5):325-396. doi: 10.1007/s10646-024-02747-x. Epub 2024 Apr 29. PMID: 38683471; PMCID: PMC11213816.
- Barone G, Storelli A, Meleleo D, Dambrosio A, Garofalo R, Busco A, Storelli MM. Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics. 2021 Feb 20;9(2):39. doi: 10.3390/toxics9020039. PMID: 33672494; PMCID: PMC7923435.
- Nigro M, Leonzio C. Intracellular storage of mercury and selenium in different marine vertebrates. Mar Ecol Prog Ser. 1996;135:137-43. doi: 10.3354/meps135137.
- Scheuhammer A, Braune B, Chan HM, Frouin H, Krey A, Letcher R, Loseto L, Noël M, Ostertag S, Ross P, Wayland M. Recent progress on our understanding of the biological effects of mercury in fish and wildlife in the Canadian Arctic. Sci Total Environ. 2015 Mar 15;509-510:91-103. doi: 10.1016/j.scitotenv.2014.05.142. Epub 2014 Jun 14. PMID: 24935263.
- Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012 Nov;45(6):344-52. doi: 10.3961/jpmph.2012.45.6.344. Epub 2012 Nov 29. PMID: 23230464; PMCID: PMC3514464.
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13(5):385-410. doi: 10.1080/10937401003673750. PMID: 20582853; PMCID: PMC6943924.
- Wyatt LH, Luz AL, Cao X, Maurer LL, Blawas AM, Aballay A, Pan WK, Meyer JN. Effects of methyl and inorganic mercury exposure on genome homeostasis and mitochondrial function in Caenorhabditis elegans. DNA Repair (Amst). 2017 Apr;52:31-48. doi: 10.1016/j.dnarep.2017.02.005. Epub 2017 Feb 13. PMID: 28242054; PMCID: PMC5394729.
- Mitra S, Chakraborty AJ, Tareq AM, Emran T Bin, Nainu F, Khusro A, Abubakr M. Idris, Mayeen Uddin Khandaker, Hamid Osman, Fahad A. Alhumaydhi, Jesus Simal-Gandara. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. 2022;34:101865. doi: 10.1016/j.jksus.2022.101865.
- Chen C, Yu H, Zhao J, Li B, Qu L, Liu S, Zhang P, Chai Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ Health Perspect. 2006 Feb;114(2):297-301. doi: 10.1289/ehp.7861. PMID: 16451871; PMCID: PMC1367848.
- Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Amiri RJ, Pirzadeh M, Moghadamnia AA. Aluminum Poisoning with Emphasis on Its Mechanism and Treatment of Intoxication. Emerg Med Int. 2022 Jan 11;2022:1480553. doi: 10.1155/2022/1480553. PMID: 35070453; PMCID: PMC8767391.
- Wu YS, Osman AI, Hosny M, Elgarahy AM, Eltaweil AS, Rooney DW, Chen Z, Rahim NS, Sekar M, Gopinath SCB, Mat Rani NNI, Batumalaie K, Yap PS. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega. 2024 Jan 22;9(5):5100-5126. doi: 10.1021/acsomega.3c07047. PMID: 38343989; PMCID: PMC10851382.
- Lash LH. Mercury nephrotoxicity. Encyclopedia of Metalloproteins, New York, NY: Springer New York; 2013:1357-62. doi: 10.1007/978-1-4614-1533-6_327.
- Ellingsen DG, Efskind J, Berg KJ, Gaarder PI, Thomassen Y. Renal and immunologic markers for chloralkali workers with low exposure to mercury vapor. Scand J Work Environ Health. 2000 Oct;26(5):427-35. doi: 10.5271/sjweh.564. PMID: 1110384.
- Diamond GL, Zalups RK. Understanding renal toxicity of heavy metals. Toxicol Pathol. 1998 Jan-Feb;26(1):92-103. doi: 10.1177/019262339802600111. PMID: 9502391.
- Saydam N, Adams TK, Steiner F, Schaffner W, Freedman JH. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. J Biol Chem. 2002 Jun 7;277(23):20438-45. doi: 10.1074/jbc.M110631200. Epub 2002 Mar 28. PMID: 11923282.
- Lee HT, Oh S, Ro DH, Yoo H, Kwon YW. The Key Role of DNA Methylation and Histone Acetylation in Epigenetics of Atherosclerosis. J Lipid Atheroscler. 2020 Sep;9(3):419-434. doi: 10.12997/jla.2020.9.3.419. Epub 2020 Sep 21. PMID: 33024734; PMCID: PMC7521974.
- Bridges CC, Zalups RK. The aging kidney and the nephrotoxic effects of mercury. J Toxicol Environ Health B Crit Rev. 2017;20(2):55-80. doi: 10.1080/10937404.2016.1243501. Epub 2017 Feb 7. PMID: 28339347; PMCID: PMC6088787.
- Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012 Nov;45(6):344-52. doi: 10.3961/jpmph.2012.45.6.344. Epub 2012 Nov 29. PMID: 23230464; PMCID: PMC3514464.
- Ni M, Li X, Marreilha dos Santos AP, Farina M, Teixeira da Rocha JB, Avila DS, et al. Mercury. Reproductive and developmental toxicology, Elsevier; 2011. p.451-9. doi: 10.1016/B978-0-12-382032-7.10035-9.
- Dhanapriya J, Gopalakrishnan N, Arun V, Dineshkumar T, Sakthirajan R, Balasubramaniyan T, Haris M. Acute kidney injury and disseminated intravascular coagulation due to mercuric chloride poisoning. Indian J Nephrol. 2016 May-Jun;26(3):206-8. doi: 10.4103/0971-4065.164230. PMID: 27194836; PMCID: PMC4862267.
- Bernard A, Lauwerys R. Epidemiological application of early markers of nephrotoxicity. Toxicol Lett. 1989 Mar;46(1-3):293-306. doi: 10.1016/0378-4274(89)90137-9. PMID: 2705200.
- Branco V, Caito S, Farina M, Teixeira da Rocha J, Aschner M, Carvalho C. Biomarkers of mercury toxicity: Past, present, and future trends. J Toxicol Environ Health B Crit Rev. 2017;20(3):119-154. doi: 10.1080/10937404.2017.1289834. Epub 2017 Apr 5. PMID: 28379072; PMCID: PMC6317349.
- Zalups RK, Barfuss DW, Kostyniak PJ. Altered intrarenal accumulation of mercury in uninephrectomized rats treated with methylmercury chloride. Toxicol Appl Pharmacol. 1992 Aug;115(2):174-82. doi: 10.1016/0041-008x(92)90321-i. PMID: 1641852.
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13(5):385-410. doi: 10.1080/10937401003673750. PMID: 20582853; PMCID: PMC6943924.
- Donadio C, Tramonti G, Lucchesi A, Giordani R, Lucchetti A, Bianchi C. Gamma-glutamyltransferase is a reliable marker for tubular effects of contrast media. Ren Fail. 1998 Mar;20(2):319-24. doi: 10.3109/08860229809045117. PMID: 9574458.
- Bernard A, Lauwerys R. Epidemiological application of early markers of nephrotoxicity. Toxicol Lett. 1989 Mar;46(1-3):293-306. doi: 10.1016/0378-4274(89)90137-9. PMID: 2705200.
- Franko A, Budihna MV, Dodic-Fikfak M. Long-term effects of elemental mercury on renal function in miners of the Idrija Mercury Mine. Ann Occup Hyg. 2005 Aug;49(6):521-7. doi: 10.1093/annhyg/mei022. Epub 2005 Jun 17. PMID: 15964879.
- Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008 Jan;101(1):159-70. doi: 10.1093/toxsci/kfm260. Epub 2007 Oct 13. PMID: 17934191; PMCID: PMC2744478.
- Bridges CC, Zalups RK. The aging kidney and the nephrotoxic effects of mercury. J Toxicol Environ Health B Crit Rev. 2017;20(2):55-80. doi: 10.1080/10937404.2016.1243501. Epub 2017 Feb 7. PMID: 28339347; PMCID: PMC6088787.
- Urbano T, Malavolti M, Vinceti M, Filippini T. Mercuric chloride (HgCl2). Encyclopedia of Toxicology, Elsevier; 2024. p.117-22. doi: 10.1016/B978-0-12-824315-2.00084-1.
- Miller S, Pallan S, Gangji AS, Lukic D, Clase CM. Mercury-associated nephrotic syndrome: a case report and systematic review of the literature. Am J Kidney Dis. 2013 Jul;62(1):135-8. doi: 10.1053/j.ajkd.2013.02.372. Epub 2013 Apr 18. PMID: 23602193.
- Maria Francis Y, Karunakaran B, Ashfaq F, Yahia Qattan M, Ahmad I, Alkhathami AG, Idreesh Khan M, Varadhan M, Govindan L, Ponnusamy Kasirajan S. Mercuric Chloride Induced Nephrotoxicity: Ameliorative Effect of Carica papaya Leaves Confirmed by Histopathology, Immunohistochemistry, and Gene Expression Studies. ACS Omega. 2023 Jun 5;8(24):21696-21708. doi: 10.1021/acsomega.3c01045. Retraction in: ACS Omega. 2025 Feb 28;10(9):9808. doi: 10.1021/acsomega.5c01751. PMID: 37360438; PMCID: PMC10286259.
- Nath KA, Croatt AJ, Likely S, Behrens TW, Warden D. Renal oxidant injury and oxidant response induced by mercury. Kidney Int. 1996 Sep;50(3):1032-43. doi: 10.1038/ki.1996.406. PMID: 8872981.
- Wu YS, Osman AI, Hosny M, Elgarahy AM, Eltaweil AS, Rooney DW, Chen Z, Rahim NS, Sekar M, Gopinath SCB, Mat Rani NNI, Batumalaie K, Yap PS. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega. 2024 Jan 22;9(5):5100-5126. doi: 10.1021/acsomega.3c07047. PMID: 38343989; PMCID: PMC10851382.
- Alhusaini A, Alghilani S, Alhuqbani W, Hasan IH. Vitamin E and Lactobacillus Provide Protective Effects Against Liver Injury Induced by HgCl2: Role of CHOP, GPR87, and mTOR Proteins. Dose Response. 2021 Apr 26;19(2):15593258211011360. doi: 10.1177/15593258211011360. PMID: 33994889; PMCID: PMC8083003.
- Francis YM, Vijayakumar J, Raghunath G, Vijayalakshmi S, Sivanesan S, Vijayaraghavan R, Ethirajan S. Protective effect of carica papaya leaf extract against mercuric chloride-induced nephrotoxicity in wistar rats. Pharmacogn Mag. 2020;16:379. doi: 10.4103/pm.pm_11_20.
- Song Y, Lee CK, Kim KH, Lee JT, Suh C, Kim SY, Kim JH, Son BC, Kim DH, Lee S. Factors associated with total mercury concentrations in maternal blood, cord blood, and breast milk among pregnant women in Busan, Korea. Asia Pac J Clin Nutr. 2016;25(2):340-9. doi: 10.6133/apjcn.2016.25.2.16. PMID: 27222418.
- Zhang X, Agborbesong E, Li X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int J Mol Sci. 2021 Oct 19;22(20):11253. doi: 10.3390/ijms222011253. PMID: 34681922; PMCID: PMC8537003.
- Dybiec J, Szlagor M, Młynarska E, Rysz J, Franczyk B. Structural and Functional Changes in Aging Kidneys. Int J Mol Sci. 2022 Dec 6;23(23):15435. doi: 10.3390/ijms232315435. PMID: 36499760; PMCID: PMC9737118.
- Kazantzis G. Mercury exposure and early effects: an overview. Med Lav. 2002 May-Jun;93(3):139-47. PMID: 12197264.
- Wu YS, Osman AI, Hosny M, Elgarahy AM, Eltaweil AS, Rooney DW, Chen Z, Rahim NS, Sekar M, Gopinath SCB, Mat Rani NNI, Batumalaie K, Yap PS. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega. 2024 Jan 22;9(5):5100-5126. doi: 10.1021/acsomega.3c07047. PMID: 38343989; PMCID: PMC10851382.
- Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990 Summer;14(2):169-76. doi: 10.1016/s0149-7634(05)80217-9. PMID: 2190116.
- Fernandes Azevedo B, Barros Furieri L, Peçanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simões M, Fiorim J, Rossi de Batista P, Fioresi M, Rossoni L, Stefanon I, Alonso MJ, Salaices M, Valentim Vassallo D. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol. 2012;2012:949048. doi: 10.1155/2012/949048. Epub 2012 Jul 2. PMID: 22811600; PMCID: PMC3395437.
- Oliveira CS, Nogara PA, Ardisson-Araújo DMP, Aschner M, Rocha JBT, Dórea JG. Neurodevelopmental Effects of Mercury. Adv Neurotoxicol. 2018;2:27-86. doi: 10.1016/bs.ant.2018.03.005. Epub 2018 May 24. PMID: 32346667; PMCID: PMC7188190.
- Chang LW. Neurotoxic effects of mercury--a review. Environ Res. 1977 Dec;14(3):329-73. doi: 10.1016/0013-9351(77)90044-5. PMID: 338298.
- Karri V, Schuhmacher M Professor, Kumar V. A systems toxicology approach to compare the heavy metal mixtures (Pb, As, MeHg) impact in neurodegenerative diseases. Food Chem Toxicol. 2020 May;139:111257. doi: 10.1016/j.fct.2020.111257. Epub 2020 Mar 14. PMID: 32179164.
- Karri V, Ramos D, Martinez JB, Odena A, Oliveira E, Coort SL, Evelo CT, Mariman ECM, Schuhmacher M, Kumar V. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: Deepening into the molecular mechanism of neurodegenerative diseases. J Proteomics. 2018 Sep 15;187:106-125. doi: 10.1016/j.jprot.2018.06.020. Epub 2018 Jul 12. PMID: 30017948.
- Ajsuvakova OP, Tinkov AA, Aschner M, Rocha JBT, Michalke B, Skalnaya MG, Skalny AV, Butnariu M, Dadar M, Sarac I, Aaseth J, Bjørklund G. Sulfhydryl groups as targets of mercury toxicity. Coord Chem Rev. 2020 Aug 15;417:213343. doi: 10.1016/j.ccr.2020.213343. Epub 2020 May 7. PMID: 32905350; PMCID: PMC7470069.
- Kang B, Wang J, Guo S, Yang L. Mercury-induced toxicity: Mechanisms, molecular pathways, and gene regulation. Sci Total Environ. 2024 Sep 15;943:173577. doi: 10.1016/j.scitotenv.2024.173577. Epub 2024 Jun 7. PMID: 38852866.
- Roos D, Seeger R, Puntel R, Vargas Barbosa N. Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J Biomed Biotechnol. 2012;2012:248764. doi: 10.1155/2012/248764. Epub 2012 Jul 3. PMID: 22927718; PMCID: PMC3425894.
- Cheng H, Yang B, Ke T, Li S, Yang X, Aschner M, Chen P. Mechanisms of Metal-Induced Mitochondrial Dysfunction in Neurological Disorders. Toxics. 2021 Jun 17;9(6):142. doi: 10.3390/toxics9060142. PMID: 34204190; PMCID: PMC8235163.
- Wu T, Cai W, Chen X. Epigenetic regulation of neurotransmitter signaling in neurological disorders. Neurobiol Dis. 2023 Aug;184:106232. doi: 10.1016/j.nbd.2023.106232. Epub 2023 Jul 20. PMID: 37479091.
- Wang W, Chen F, Zhang L, Wen F, Yu Q, Li P, Zhang A. Neurotransmitter disturbances caused by methylmercury exposure: Microbiota-gut-brain interaction. Sci Total Environ. 2023 May 15;873:162358. doi: 10.1016/j.scitotenv.2023.162358. Epub 2023 Feb 21. PMID: 36822423.
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000 Aug-Sep;37(2-3):199-206. doi: 10.1016/s0197-0186(00)00023-1. PMID: 10812205.
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-3Hglutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909-19. doi: 10.1523/JNEUROSCI.05-11-02909.1985. PMID: 2865341; PMCID: PMC6565182.
- Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379-87. doi: 10.1016/0165-6147(90)90184-a. PMID: 2238094.
- Guo C, Ma YY. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front Neural Circuits. 2021 Aug 17;15:711564. doi: 10.3389/fncir.2021.711564. PMID: 34483848; PMCID: PMC8416103.
- Aroniadou-Anderjaska V, Figueiredo TH, De Araujo Furtado M, Pidoplichko VI, Lumley LA, Braga MFM. Alterations in GABAA receptor-mediated inhibition triggered by status epilepticus and their role in epileptogenesis and increased anxiety. Neurobiol Dis. 2024 Oct 1;200:106633. doi: 10.1016/j.nbd.2024.106633. Epub 2024 Aug 6. PMID: 39117119.
- Sasaki S, Negishi T, Tsuzuki T, Yukawa K. Methylmercury-induced reactive oxygen species-dependent and independent dysregulation of MAP kinase-related signaling pathway in cultured normal rat cerebellar astrocytes. Toxicology. 2023 Mar 15;487:153463. doi: 10.1016/j.tox.2023.153463. Epub 2023 Feb 20. PMID: 36813253.
- Kang B, Wang J, Guo S, Yang L. Mercury-induced toxicity: Mechanisms, molecular pathways, and gene regulation. Sci Total Environ. 2024 Sep 15;943:173577. doi: 10.1016/j.scitotenv.2024.173577. Epub 2024 Jun 7. PMID: 38852866.
- Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011 Oct 10;89(15-16):555-63. doi: 10.1016/j.lfs.2011.05.019. Epub 2011 Jun 13. PMID: 21683713; PMCID: PMC3183295.
- Martínez-Hernández MI, Acosta-Saavedra LC, Hernández-Kelly LC, Loaeza-Loaeza J, Ortega A. Microglial Activation in Metal Neurotoxicity: Impact in Neurodegenerative Diseases. Biomed Res Int. 2023 Jan 31;2023:7389508. doi: 10.1155/2023/7389508. PMID: 36760476; PMCID: PMC9904912.
- Verity MA, Sarafian T, Pacifici EH, Sevanian A. Phospholipase A2 stimulation by methyl mercury in neuron culture. J Neurochem. 1994 Feb;62(2):705-14. doi: 10.1046/j.1471-4159.1994.62020705.x. PMID: 8294933.
- Brookes PC, mcgrath sp. Effect of metal toxicity on the size of the soil microbial biomass. Journal of soil science 1984;35:341-6. doi: 10.1111/j.1365-2389.1984.tb00288.x.
- Peltz A, Sherwani SI, Kotha SR, Mazerik JN, O'Connor Butler ES, Kuppusamy ML, Hagele T, Magalang UJ, Kuppusamy P, Marsh CB, Parinandi NL. Calcium and calmodulin regulate mercury-induced phospholipase D activation in vascular endothelial cells. Int J Toxicol. 2009 May-Jun;28(3):190-206. doi: 10.1177/1091581809338077. PMID: 19546257.
- Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di Lisa F, Bernardi P. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004 Jun 11;279(24):25219-25. doi: 10.1074/jbc.M310381200. Epub 2004 Apr 7. PMID: 15070903.
- Krishna Chandran AM, Christina H, Das S, Mumbrekar KD, Satish Rao BS. Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ Toxicol Pharmacol. 2019 Oct;71:103224. doi: 10.1016/j.etap.2019.103224. Epub 2019 Jul 23. PMID: 31376681.
- Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011 Nov 1;256(3):405-17. doi: 10.1016/j.taap.2011.05.001. Epub 2011 May 9. PMID: 21601588; PMCID: PMC3166649.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014 May 19;24(10):R453-62. doi: 10.1016/j.cub.2014.03.034. PMID: 24845678; PMCID: PMC4055301.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016 Jul 25;15(1):71. doi: 10.1186/s12937-016-0186-5. PMID: 27456681; PMCID: PMC4960740.
- Fujimura M, Usuki F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants (Basel). 2020 Oct 16;9(10):1004. doi: 10.3390/antiox9101004. PMID: 33081221; PMCID: PMC7602710.
- Chapman L, Chan HM. The influence of nutrition on methyl mercury intoxication. Environ Health Perspect. 2000 Mar;108 Suppl 1(Suppl 1):29-56. doi: 10.1289/ehp.00108s129. PMID: 10698722; PMCID: PMC1637774.
- Kaur P, Evje L, Aschner M, Syversen T. The in vitro effects of Trolox on methylmercury-induced neurotoxicity. Toxicology. 2010 Sep 30;276(1):73-8. doi: 10.1016/j.tox.2010.07.006. Epub 2010 Jul 15. PMID: 20637824.
- Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol. 2007 Dec;20(12):1919-26. doi: 10.1021/tx7002323. Epub 2007 Oct 19. PMID: 17944542.
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res. 2007 Apr;11(3-4):241-60. doi: 10.1007/BF03033570. PMID: 17449462.
- Grotto D, Valentini J, Fillion M, Passos CJ, Garcia SC, Mergler D, Barbosa F Jr. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci Total Environ. 2010 Jan 15;408(4):806-11. doi: 10.1016/j.scitotenv.2009.10.053. Epub 2009 Nov 14. PMID: 19914681.
- Rocha JB, Freitas AJ, Marques MB, Pereira ME, Emanuelli T, Souza DO. Effects of methylmercury exposure during the second stage of rapid postnatal brain growth on negative geotaxis and on delta-aminolevulinate dehydratase of suckling rats. Braz J Med Biol Res. 1993 Oct;26(10):1077-83. PMID: 8312839.
- Wagner C, Sudati JH, Nogueira CW, Rocha JB. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. Biometals. 2010 Dec;23(6):1171-7. doi: 10.1007/s10534-010-9367-4. Epub 2010 Aug 18. PMID: 20717703.
- Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci. 2003 May;73(1):135-40. doi: 10.1093/toxsci/kfg058. Epub 2003 Apr 15. PMID: 12700422.
- Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005 May 1;204(3):343-54. doi: 10.1016/j.taap.2004.11.013. PMID: 15845423.
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007 Feb 2;1131(1):1-10. doi: 10.1016/j.brainres.2006.10.070. Epub 2006 Dec 19. PMID: 17182013; PMCID: PMC1847599.
- Cediel-Ulloa A, Lindner S, Rüegg J, Broberg K. Epigenetics of methylmercury. Neurotoxicology. 2023 Jul;97:34-46. doi: 10.1016/j.neuro.2023.05.004. Epub 2023 May 8. PMID: 37164037.
- Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020 Nov 26;9(1):42. doi: 10.1186/s40035-020-00221-2. PMID: 33239064; PMCID: PMC7689983.
- Solt I, Bornstein J. Childhood vaccines and autism--much ado about nothing?. Harefuah. 2010 Apr;149(4):251-5, 260. Hebrew. PMID: 20812501.
- Bittencourt LO, Chemelo VS, Aragão WAB, Puty B, Dionizio A, Teixeira FB, Fernandes MS, Silva MCF, Fernandes LMP, de Oliveira EHC, Buzalaf MAR, Crespo-Lopez ME, Maia CDSF, Lima RR. From Molecules to Behavior in Long-Term Inorganic Mercury Intoxication: Unraveling Proteomic Features in Cerebellar Neurodegeneration of Rats. Int J Mol Sci. 2021 Dec 22;23(1):111. doi: 10.3390/ijms23010111. PMID: 35008538; PMCID: PMC8745249.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1-24. doi: 10.3109/10408449509089885. PMID: 7734058.
- Akagi H, Malm O, Kinjo Y, Harada M, Branches FJP, Pfeiffer WC, Hiroo Kato.
Methylmercury pollution in the Amazon, Brazil. Science of the Total Environment. 1995;175:85-95. doi: 10.1016/0048-9697(95)04905-3. - Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, Cellini C, Possati GF, Maseri A. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J Am Coll Cardiol. 1999 May;33(6):1578-83. doi: 10.1016/s0735-1097(99)00062-5. PMID: 10334427.
- Massaroni L, Oliveira EM, Stefanon I, Vassallo DV. Effects of mercury on the mechanical and electrical activity of the Langendorff-perfused rat heart. Braz J Med Biol Res. 1992;25(8):861-4. PMID: 1342624.
- Rhee HM, Choi BH. Hemodynamic and electrophysiological effects of mercury in intact anesthetized rabbits and in isolated perfused hearts. Exp Mol Pathol. 1989 Jun;50(3):281-90. doi: 10.1016/0014-4800(89)90038-5. PMID: 2721650.
- Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury Exposure and Heart Diseases. Int J Environ Res Public Health. 2017 Jan 12;14(1):74. doi: 10.3390/ijerph14010074. PMID: 28085104; PMCID: PMC5295325.
- Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppänen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):228-33. doi: 10.1161/01.ATV.0000150040.20950.61. Epub 2004 Nov 11. PMID: 15539625.
- Halbach S. Mercury compounds: lipophilicity and toxic effects on isolated myocardial tissue. Arch Toxicol. 1990;64(4):315-9. doi: 10.1007/BF01972992. PMID: 2386431.
- Magos L, Clarkson TW. Overview of the clinical toxicity of mercury. Ann Clin Biochem. 2006 Jul;43(Pt 4):257-68. doi: 10.1258/000456306777695654. PMID: 16824275.
- Lemos NB, Angeli JK, Faria Tde O, Ribeiro Junior RF, Vassallo DV, Padilha AS, Stefanon I. Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS One. 2012;7(11):e49005. doi: 10.1371/journal.pone.0049005. Epub 2012 Nov 7. PMID: 23145049; PMCID: PMC3492199.
- Omanwar S, Fahim M. Mercury Exposure and Endothelial Dysfunction: An Interplay Between Nitric Oxide and Oxidative Stress. Int J Toxicol. 2015 Jul-Aug;34(4):300-7. doi: 10.1177/1091581815589766. Epub 2015 Jun 9. PMID: 26060268.
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. Epub 2017 Jul 27. PMID: 28819546; PMCID: PMC5551541.
- Kibel A, Lukinac AM, Dambic V, Juric I, Selthofer-Relatic K. Oxidative Stress in Ischemic Heart Disease. Oxid Med Cell Longev. 2020 Dec 28;2020:6627144. doi: 10.1155/2020/6627144. PMID: 33456670; PMCID: PMC7785350.
- Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circ Res. 2017 Jun 9;120(12):1969-1993. doi: 10.1161/CIRCRESAHA.117.310083. PMID: 28596175; PMCID: PMC5607780.
- Fujimura M, Usuki F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants (Basel). 2020 Oct 16;9(10):1004. doi: 10.3390/antiox9101004. PMID: 33081221; PMCID: PMC7602710.
- Ai X, Yan J, Carrillo E, Ding W. The Stress-Response MAP Kinase Signaling in Cardiac Arrhythmias. Rev Physiol Biochem Pharmacol. 2016;172:77-100. doi: 10.1007/112_2016_8. PMID: 27848025; PMCID: PMC6791713.
- Aviram M, Rosenblat M. Phospholipase A2 and phospholipase D are involved in macrophage NADPH oxidase-mediated oxidation of low density lipoprotein. Isr J Med Sci. 1996 Sep;32(9):749-56. PMID: 8865831.
- Petan T, Manček-Keber M. Half is enough: Oxidized lysophospholipids as novel bioactive molecules. Free Radic Biol Med. 2022 Aug 1;188:351-362. doi: 10.1016/j.freeradbiomed.2022.06.228. Epub 2022 Jun 30. PMID: 35779690.
- Vassallo DV, Simões MR, Giuberti K, Azevedo BF, Ribeiro Junior RF, Salaices M, Stefanon I. Effects of Chronic Exposure to Mercury on Angiotensin-Converting Enzyme Activity and Oxidative Stress in Normotensive and Hypertensive Rats. Arq Bras Cardiol. 2019 Apr;112(4):374-380. doi: 10.5935/abc.20180271. Epub 2019 Jan 7. PMID: 30624528; PMCID: PMC6459440.
- Raymond LJ, Ralston NVC. Mercury: selenium interactions and health implications. Neurotoxicology. 2020 Dec;81:294-299. doi: 10.1016/j.neuro.2020.09.020. Epub 2020 Oct 14. PMID: 35587137.
- Arbi S, Bester MJ, Pretorius L, Oberholzer HM. Adverse cardiovascular effects of exposure to cadmium and mercury alone and in combination on the cardiac tissue and aorta of Sprague-Dawley rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021;56(6):609-624. doi: 10.1080/10934529.2021.1899534. Epub 2021 Mar 15. PMID: 33720805.
- Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury Exposure and Heart Diseases. Int J Environ Res Public Health. 2017 Jan 12;14(1):74. doi: 10.3390/ijerph14010074. PMID: 28085104; PMCID: PMC5295325.
- Furieri LB, Fioresi M, Junior RF, Bartolomé MV, Fernandes AA, Cachofeiro V, Lahera V, Salaices M, Stefanon I, Vassallo DV. Exposure to low mercury concentration in vivo impairs myocardial contractile function. Toxicol Appl Pharmacol. 2011 Sep 1;255(2):193-9. doi: 10.1016/j.taap.2011.06.015. Epub 2011 Jun 24. PMID: 21723307.
- Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich). 2011 Aug;13(8):621-7. doi: 10.1111/j.1751-7176.2011.00489.x. Epub 2011 Jul 11. PMID: 21806773; PMCID: PMC8108748.
- Wang C, Pi X, Yin S, Liu M, Tian T, Jin L, Liu J, Li Z, Wang L, Yuan Z, Wang Y, Ren A. Maternal exposure to heavy metals and risk for severe congenital heart defects in offspring. Environ Res. 2022 Sep;212(Pt C):113432. doi: 10.1016/j.envres.2022.113432. Epub 2022 May 6. PMID: 35533713.
- Baldissera MD, Souza CF, Grings M, Descovi SN, Henn AS, Flores EMM, Aleksandro S. da Silva, Guilhian Leipnitz, Bernardo Baldisserotto. Exposure to methylmercury chloride inhibits mitochondrial electron transport chain and phosphotransfer network in liver and gills of grass carp: Protective effects of diphenyl diselenide dietary supplementation as an alternative strategy for mercury toxicity. Aquaculture 2019;509:85-95. doi: 10.1016/j.aquaculture.2019.05.012.
- Overview of the endocrine system. US EPA. 2024.
- Zhu X, Kusaka Y, Sato K, Zhang Q. The endocrine disruptive effects of mercury. Environ Health Prev Med. 2000 Jan;4(4):174-83. doi: 10.1007/BF02931255. PMID: 21432482; PMCID: PMC2723593.
- Rowland AS, Baird DD, Weinberg CR, Shore DL, Shy CM, Wilcox AJ. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occup Environ Med. 1994 Jan;51(1):28-34. doi: 10.1136/oem.51.1.28. PMID: 8124459; PMCID: PMC1127897.
- Schrag SD, Dixon RL. Occupational exposures associated with male reproductive dysfunction. Annu Rev Pharmacol Toxicol. 1985;25:567-92. doi: 10.1146/annurev.pa.25.040185.003031. PMID: 2408559.
- Fossato da Silva DA, Teixeira CT, Scarano WR, Favareto AP, Fernandez CD, Grotto D, Barbosa F Jr, Kempinas Wde G. Effects of methylmercury on male reproductive functions in Wistar rats. Reprod Toxicol. 2011 May;31(4):431-9. doi: 10.1016/j.reprotox.2011.01.002. Epub 2011 Jan 22. PMID: 21262343.
- Heath JC, Abdelmageed Y, Braden TD, Goyal HO. The effects of chronic ingestion of mercuric chloride on fertility and testosterone levels in male Sprague Dawley rats. J Biomed Biotechnol. 2012;2012:815186. doi: 10.1155/2012/815186. Epub 2012 Jul 4. PMID: 22829750; PMCID: PMC3398687.
- Mocevic E, Specht IO, Marott JL, Giwercman A, Jönsson BA, Toft G, Lundh T, Bonde JP. Environmental mercury exposure, semen quality and reproductive hormones in Greenlandic Inuit and European men: a cross-sectional study. Asian J Androl. 2013 Jan;15(1):97-104. doi: 10.1038/aja.2012.121. Epub 2012 Dec 10. PMID: 23223027; PMCID: PMC3739114.
- Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, El Feki A. Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol. 2009 Jul;28(1):81-9. doi: 10.1016/j.reprotox.2009.03.011. Epub 2009 Apr 5. PMID: 19427169.
- Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol. 2006 Aug;19(8):1080-5. doi: 10.1021/tx0600705. PMID: 16918248.
- Kimura T. Effects of hypophysectomy and ACTH administration on the level of adrenal cholesterol side-chain desmolase. Endocrinology. 1969 Sep;85(3):492-9. doi: 10.1210/endo-85-3-492. PMID: 4389419.
- Yoshida M. Placental to fetal transfer of mercury and fetotoxicity. Tohoku J Exp Med. 2002 Feb;196(2):79-88. doi: 10.1620/tjem.196.79. PMID: 12498319.
- Dack K, Fell M, Taylor CM, Havdahl A, Lewis SJ. Prenatal Mercury Exposure and Neurodevelopment up to the Age of 5 Years: A Systematic Review. Int J Environ Res Public Health. 2022 Feb 10;19(4):1976. doi: 10.3390/ijerph19041976. PMID: 35206164; PMCID: PMC8871549.
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain. J Neuropathol Exp Neurol 1978;37:719-33. doi:10.1097/00005072-197811000-00001.
- Hertz DL, Henry NL, Rae JM. Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics. 2017 Apr;18(5):481-499. doi: 10.2217/pgs-2016-0205. Epub 2017 Mar 27. PMID: 28346074; PMCID: PMC6219438.
- Khan F, Momtaz S, Abdollahi M. The relationship between mercury exposure and epigenetic alterations regarding human health, risk assessment and diagnostic strategies. J Trace Elem Med Biol. 2019 Mar;52:37-47. doi: 10.1016/j.jtemb.2018.11.006. Epub 2018 Nov 14. PMID: 30732897.
- Patisaul HB. REPRODUCTIVE TOXICOLOGY: Endocrine disruption and reproductive disorders: impacts on sexually dimorphic neuroendocrine pathways. Reproduction. 2021 Oct 5;162(5):F111-F130. doi: 10.1530/REP-20-0596. PMID: 33929341; PMCID: PMC8484365.
- McGregor AJ, Mason HJ. Occupational mercury vapour exposure and testicular, pituitary and thyroid endocrine function. Hum Exp Toxicol. 1991 May;10(3):199-203. doi: 10.1177/096032719101000309. PMID: 1678950.
- Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009 Mar;12(3):206-23. doi: 10.1080/10937400902902062. PMID: 19466673.
- Chen A, Kim SS, Chung E, Dietrich KN. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007-2008. Environ Health Perspect. 2013 Feb;121(2):181-6. doi: 10.1289/ehp.1205239. Epub 2012 Nov 16. PMID: 23164649; PMCID: PMC3569681.
- Pamphlett R, Doble PA, Bishop DP. Mercury in the human thyroid gland: Potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PLoS One. 2021 Feb 9;16(2):e0246748. doi: 10.1371/journal.pone.0246748. PMID: 33561145; PMCID: PMC7872292.
- Ghosh N, Bhattacharya S. Thyrotoxicity of the chlorides of cadmium and mercury in rabbit. Biomed Environ Sci. 1992 Sep;5(3):236-40. PMID: 1449659.
- Goldman M, Blackburn P. The effect of mercuric chloride on thyroid function in the rat. Toxicol Appl Pharmacol. 1979 Mar 30;48(1 Pt 1):49-55. doi: 10.1016/s0041-008x(79)80007-1. PMID: 452043.
- Barregård L, Lindstedt G, Schütz A, Sällsten G. Endocrine function in mercury exposed chloralkali workers. Occup Environ Med. 1994 Aug;51(8):536-40. doi: 10.1136/oem.51.8.536. PMID: 7951778; PMCID: PMC1128033.
- Freeman HC, Sangalang G, Uthe JF, Ronald K. Steroidogenesis in vitro in the harp seal (Pagophilus groenlandicus) without and with methyl mercury treatment in vivo. Environ Physiol Biochem. 1975;5(6):428-39. PMID: 1213032.
- Veltman JC, Maines MD. Alterations of heme, cytochrome P-450, and steroid metabolism by mercury in rat adrenal. Arch Biochem Biophys. 1986 Aug 1;248(2):467-78. doi: 10.1016/0003-9861(86)90500-x. PMID: 2943220.
- Chowdhury AR, Arora U. Toxic effect of mercury on testes in different animal species. Indian J Physiol Pharmacol. 1982 Jul-Sep;26(3):246-9. PMID: 6217157.
- Mohamed MK, Burbacher TM, Mottet NK. Effects of methyl mercury on testicular functions in Macaca fascicularis monkeys. Pharmacol Toxicol. 1987 Jan;60(1):29-36. doi: 10.1111/j.1600-0773.1987.tb01715.x. PMID: 3562387.
- Dey S, Bhattacharya S. Ovarian damage to Channa punctatus after chronic exposure to low concentrations of Elsan, mercury, and ammonia. Ecotoxicol Environ Saf. 1989 Apr;17(2):247-57. doi: 10.1016/0147-6513(89)90044-4. PMID: 2737117.
- Reddy PS, Tuberty SR, Fingerman M. Effects of cadmium and mercury on ovarian maturation in the red swamp crayfish, Procambarus clarkii. Ecotoxicol Environ Saf. 1997 Jun;37(1):62-5. doi: 10.1006/eesa.1997.1523. PMID: 9212337.
- Mukherjee D, Kumar V, Chakraborti P. Effect of mercuric chloride and cadmium chloride on gonadal function and its regulation in sexually mature common carp Cyprinus carpio. Biomed Environ Sci. 1994 Mar;7(1):13-24. PMID: 8024715.
- Zhu X, Kusaka Y, Sato K, Zhang Q. The endocrine disruptive effects of mercury. Environ Health Prev Med. 2000 Jan;4(4):174-83. doi: 10.1007/BF02931255. PMID: 21432482; PMCID: PMC2723593.
- Drago G, Aloi N, Ruggieri S, Longo A, Contrino ML, Contarino FM, Cibella F, Colombo P, Longo V. Guardians under Siege: Exploring Pollution's Effects on Human Immunity. Int J Mol Sci. 2024 Jul 16;25(14):7788. doi: 10.3390/ijms25147788. PMID: 39063030; PMCID: PMC11277414.
- Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH. Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta Gen Subj. 2019 Dec;1863(12):129299. doi: 10.1016/j.bbagen.2019.02.001. Epub 2019 Feb 10. PMID: 30742953; PMCID: PMC6689266.
- Wada H, Cristol DA, McNabb FM, Hopkins WA. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol. 2009 Aug 1;43(15):6031-8. doi: 10.1021/es803707f. PMID: 19731714.
- Kim SH, Sharma RP. Cytotoxicity of inorganic mercury in murine T and B lymphoma cell lines: involvement of reactive oxygen species, Ca(2+) homeostasis, and cytokine gene expression. Toxicol In Vitro. 2003 Aug;17(4):385-95. doi: 10.1016/s0887-2333(03)00040-7. PMID: 12849721.
- Hui LL, Chan MHM, Lam HS, Chan PHY, Kwok KM, Chan IHS, Li AM, Fok TF. Impact of fetal and childhood mercury exposure on immune status in children. Environ Res. 2016 Jan;144(Pt A):66-72. doi: 10.1016/j.envres.2015.11.005. Epub 2015 Nov 9. PMID: 26562044.
- Shenker BJ, Pankoski L, Zekavat A, Shapiro IM. Mercury-induced apoptosis in human lymphocytes: caspase activation is linked to redox status. Antioxid Redox Signal. 2002 Jun;4(3):379-89. doi: 10.1089/15230860260196182. PMID: 12215206.
- Shenker BJ, Pankoski L, Zekavat A, Shapiro IM. Mercury-induced apoptosis in human lymphocytes: caspase activation is linked to redox status. Antioxid Redox Signal. 2002 Jun;4(3):379-89. doi: 10.1089/15230860260196182. PMID: 12215206.
- Perrone P, Ortega-Luna R, Manna C, Álvarez-Ribelles Á, Collado-Diaz V. Increased Adhesiveness of Blood Cells Induced by Mercury Chloride: Protective Effect of Hydroxytyrosol. Antioxidants (Basel). 2024 Dec 20;13(12):1576. doi: 10.3390/antiox13121576. PMID: 39765902; PMCID: PMC11673208.
- Contrino J, Marucha P, Ribaudo R, Ference R, Bigazzi PE, Kreutzer DL. Effects of mercury on human polymorphonuclear leukocyte function in vitro. Am J Pathol. 1988 Jul;132(1):110-8. PMID: 3394794; PMCID: PMC1880613.
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006 Jun;1069:62-76. doi: 10.1196/annals.1351.006. PMID: 16855135.
- Patel DD, Kuchroo VK. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity. 2015 Dec 15;43(6):1040-51. doi: 10.1016/j.immuni.2015.12.003. PMID: 26682981.
- Han B, García-Mendoza D, van den Berg H, van den Brink NW. Modulatory Effects of Mercury (II) Chloride (HgCl2 ) on Chicken Macrophage and B-Lymphocyte Cell Lines with Viral-Like Challenges In Vitro. Environ Toxicol Chem. 2021 Oct;40(10):2813-2824. doi: 10.1002/etc.5169. Epub 2021 Sep 2. PMID: 34288095; PMCID: PMC9291928.
- Magee CN, Boenisch O, Najafian N. The role of costimulatory molecules in directing the functional differentiation of alloreactive T helper cells. Am J Transplant. 2012 Oct;12(10):2588-600. doi: 10.1111/j.1600-6143.2012.04180.x. Epub 2012 Jul 3. PMID: 22759274; PMCID: PMC3459149.
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014 Oct 7;5:491. doi: 10.3389/fimmu.2014.00491. PMID: 25339958; PMCID: PMC4188125.
- Poggi A, Carosio R, Spaggiari GM, Fortis C, Tambussi G, Dell'Antonio G, Dal Cin E, Rubartelli A, Zocchi MR. NK cell activation by dendritic cells is dependent on LFA-1-mediated induction of calcium-calmodulin kinase II: inhibition by HIV-1 Tat C-terminal domain. J Immunol. 2002 Jan 1;168(1):95-101. doi: 10.4049/jimmunol.168.1.95. PMID: 11751951.
- Barreira da Silva R, Münz C. Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals. Cell Mol Life Sci. 2011 Nov;68(21):3505-18. doi: 10.1007/s00018-011-0801-8. Epub 2011 Aug 23. PMID: 21861182; PMCID: PMC11114903.
- SSI 2017. SSI 2017 44th Annual meeting of the scandinavian society of immunology stockholm, sweden 17-20 october 2017. Scand J Immunol. 2017;86:249-350. doi: 10.1111/sji.12587.
- Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol Rev. 2010 Nov;238(1):37-46. doi: 10.1111/j.1600-065X.2010.00963.x. PMID: 20969583; PMCID: PMC2975965.
- Vianna ADS, Matos EP, Jesus IM, Asmus CIRF, Câmara VM. Human exposure to mercury and its hematological effects: a systematic review. Cad Saude Publica. 2019 Feb 11;35(2):e00091618. doi: 10.1590/0102-311X00091618. PMID: 30758455.
- Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI. Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro. 2001 Dec;15(6):649-54. doi: 10.1016/s0887-2333(01)00081-9. PMID: 11698165.
- Rozgaj R, Kasuba V, Blanusa M. Mercury chloride genotoxicity in rats following oral exposure, evaluated by comet assay and micronucleus test. Arh Hig Rada Toksikol. 2005 Mar;56(1):9-15. PMID: 15969203.
- Pollard KM, Landberg GP. The in vitro proliferation of murine lymphocytes to mercuric chloride is restricted to mature T cells and is interleukin 1 dependent. Int Immunopharmacol. 2001 Mar;1(3):581-93. doi: 10.1016/s1567-5769(00)00034-5. PMID: 11367541.
- Baginski B. Effect of mercuric chloride on microbicidal activities of human polymorphonuclear leukocytes. Toxicology. 1988 Aug;50(3):247-56. doi: 10.1016/0300-483x(88)90042-x. PMID: 3394153.
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res. 2010 May;110(4):345-54. doi: 10.1016/j.envres.2010.02.001. Epub 2010 Feb 21. PMID: 20176347; PMCID: PMC2873228.
- Habibi MA, Nezhad Shamohammadi F, Rajaei T, Namdari H, Pashaei MR, Farajifard H, Ahmadpour S. Immunopathogenesis of viral infections in neurological autoimmune disease. BMC Neurol. 2023 May 23;23(1):201. doi: 10.1186/s12883-023-03239-x. PMID: 37221459; PMCID: PMC10203689.
- Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Leung PSC, Ansari AA, Gershwin ME, Anaya JM. Molecular mimicry and autoimmunity. J Autoimmun. 2018 Dec;95:100-123. doi: 10.1016/j.jaut.2018.10.012. Epub 2018 Oct 26. PMID: 30509385.
- Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002 Oct;110 Suppl 5(Suppl 5):877-81. doi: 10.1289/ehp.02110s5877. PMID: 12426151; PMCID: PMC1241265.
- Kono DH, Balomenos D, Pearson DL, Park MS, Hildebrandt B, Hultman P, Pollard KM. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol. 1998 Jul 1;161(1):234-40. PMID: 9647229.
- Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015 Sep;75(1):25-37. doi: 10.1016/j.cyto.2015.05.008. Epub 2015 Jun 11. PMID: 26073683; PMCID: PMC5118948.
- Gardner RM, Nyland JF, Evans SL, Wang SB, Doyle KM, Crainiceanu CM, Silbergeld EK. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009 Dec;117(12):1932-8. doi: 10.1289/ehp.0900855. Epub 2009 Aug 19. PMID: 20049214; PMCID: PMC2799469.
- Huang YL, Lin TH. Effect of acute administration of mercuric chloride on the disposition of copper, zinc, and iron in the rat. Biol Trace Elem Res. 1997 Jul-Aug;58(1-2):159-68. doi: 10.1007/BF02910676. PMID: 9363330.
- Zhao G, Qi L, Wang Y, Li X, Li Q, Tang X, Wang X, Wu C. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol Environ Saf. 2021 Oct 1;222:112529. doi: 10.1016/j.ecoenv.2021.112529. Epub 2021 Jul 19. PMID: 34293585.
- Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol. 2013 May 21;47(10):4967-83. doi: 10.1021/es305071v. Epub 2013 May 3. PMID: 23590191; PMCID: PMC3701261.
- Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020 Sep 29;8:49. doi: 10.1186/s40364-020-00228-x. PMID: 33005420; PMCID: PMC7526126.
- Clark DN, Begg LR, Filiano AJ. Unique aspects of IFN-γ/STAT1 signaling in neurons. Immunol Rev. 2022 Oct;311(1):187-204. doi: 10.1111/imr.13092. Epub 2022 Jun 3. PMID: 35656941; PMCID: PMC10120860.
- ThyagaRajan S, Priyanka HP. Bidirectional communication between the neuroendocrine system and the immune system: relevance to health and diseases. Ann Neurosci. 2012 Jan;19(1):40-6. doi: 10.5214/ans.0972.7531.180410. PMID: 25205962; PMCID: PMC4117073.
- Pietsch P, Vohr HW, Degitz K, Gleichmann E. Immunological alterations inducible by mercury compounds. II. HgCl2 and gold sodium thiomalate enhance serum IgE and IgG concentrations in susceptible mouse strains. Int Arch Allergy Appl Immunol. 1989;90(1):47-53. PMID: 2509376.
- Cauvi DM, Cauvi G, Toomey CB, Jacquinet E, Pollard KM. From the Cover: Interplay Between IFN-γ and IL-6 Impacts the Inflammatory Response and Expression of Interferon-Regulated Genes in Environmental-Induced Autoimmunity. Toxicol Sci. 2017 Jul 1;158(1):227-239. doi: 10.1093/toxsci/kfx083. PMID: 28453771; PMCID: PMC6075572.
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009 May;229(1):152-72. doi: 10.1111/j.1600-065X.2009.00782.x. PMID: 19426221; PMCID: PMC3826168.
- Häggqvist B, Hultman P. Effects of deviating the Th2-response in murine mercury-induced autoimmunity towards a Th1-response. Clin Exp Immunol. 2003 Nov;134(2):202-9. doi: 10.1046/j.1365-2249.2003.02303.x. PMID: 14616778; PMCID: PMC1808855.
- Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191-212. doi: 10.1146/annurev.iy.11.040193.001203. PMID: 8386518.
- Colombo M, Hamelin C, Kouassi E, Fournier M, Bernier J. Differential effects of mercury, lead, and cadmium on IL-2 production by Jurkat T cells. Clin Immunol. 2004 Jun;111(3):311-22. doi: 10.1016/j.clim.2004.02.005. PMID: 15183152.
- Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 2015 Jun;125(6):2194-202. doi: 10.1172/JCI78084. Epub 2015 May 4. PMID: 25938780; PMCID: PMC4497746.
- Tian X, Lin X, Zhao J, Cui L, Gao Y, Yu YL, Li B, Li YF. Gut as the target tissue of mercury and the extraintestinal effects. Toxicology. 2023 Jan 15;484:153396. doi: 10.1016/j.tox.2022.153396. Epub 2022 Dec 13. PMID: 36521575.
- Pinto DV, Raposo RS, Matos GA, Alvarez-Leite JI, Malva JO, Oriá RB. Methylmercury Interactions With Gut Microbiota and Potential Modulation of Neurogenic Niches in the Brain. Front Neurosci. 2020 Nov 3;14:576543. doi: 10.3389/fnins.2020.576543. PMID: 33224022; PMCID: PMC7670038.
- Rodríguez-Viso P, Domene A, Vélez D, Devesa V, Monedero V, Zúñiga M. Mercury toxic effects on the intestinal mucosa assayed on a bicameral in vitro model: Possible role of inflammatory response and oxidative stress. Food Chem Toxicol. 2022 Aug;166:113224. doi: 10.1016/j.fct.2022.113224. Epub 2022 Jun 11. PMID: 35700822.
- Rupa SA, Patwary MAM, Matin MM, Ghann WE, Uddin J, Kazi M. Interaction of mercury species with proteins: towards possible mechanism of mercurial toxicology. Toxicol Res (Camb). 2023 May 30;12(3):355-368. doi: 10.1093/toxres/tfad039. PMID: 37397928; PMCID: PMC10311172.
- Gupta PK, Sastry KV. Effect of mercuric chloride on enzyme activities in the digestive system and chemical composition of liver and muscles of the catfish, Heteropneustes fossilis. Ecotoxicol Environ Saf. 1981 Dec;5(4):389-400. doi: 10.1016/0147-6513(81)90013-0. PMID: 6459225.
- Sastry KV, Gupta PK. In vitro inhibition of digestive enzymes by heavy metals and their reversal by chelating agent: Part I. Mercuric chloride intoxication. Bull Environ Contam Toxicol. 1978 Dec;20(6):729-35. doi: 10.1007/BF01683593. PMID: 107987.
- Zhao Y, Wang X, Qin Y, Zheng B. Mercury (Hg2+) effect on enzyme activities and hepatopancreas histostructures of juvenile Chinese mitten crab eriocheir sinensis. Chinese Journal of Oceanology and Limnology. 2010;28:427-34. doi: 10.1007/s00343-010-9030-2.
- Posin SL, Kong EL, Sharma S. Mercury Toxicity. 2023 Aug 8. In: StatPearls Internet. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 29763110.
- Ghosh S, Nukavarapu SP, Jala VR. Effects of heavy metals on gut barrier integrity and gut microbiota. Microbiota and Host. 2023;2. doi: 10.1530/MAH-23-0015.
- Rodríguez-Viso P, Domene A, Vélez D, Devesa V, Monedero V, Zúñiga M. Mercury toxic effects on the intestinal mucosa assayed on a bicameral in vitro model: Possible role of inflammatory response and oxidative stress. Food Chem Toxicol. 2022 Aug;166:113224. doi: 10.1016/j.fct.2022.113224. Epub 2022 Jun 11. PMID: 35700822.
- Jerrold B. Leikin, ahmed mian, esther jolanda van zuuren, dan randall. Mercury poisoning. DynaMedex. 2023.
- Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003 Sep-Dec;16(3):189-99. doi: 10.1177/039463200301600302. PMID: 14611720.
- Tsai TL, Kuo CC, Pan WH, Wu TN, Lin P, Wang SL. Type 2 diabetes occurrence and mercury exposure - From the National Nutrition and Health Survey in Taiwan. Environ Int. 2019 May;126:260-267. doi: 10.1016/j.envint.2019.02.038. Epub 2019 Feb 27. PMID: 30825744.
- He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes Care. 2013 Jun;36(6):1584-9. doi: 10.2337/dc12-1842. Epub 2013 Feb 19. PMID: 23423697; PMCID: PMC3661833.
- Roy C, Tremblay PY, Ayotte P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ Res. 2017 Jul;156:747-760. doi: 10.1016/j.envres.2017.04.038. Epub 2017 May 5. PMID: 28482296.
- Pyszel A, Wróbel T, Szuba A, Andrzejak R. Wpływ narazenia na metale, benzen, pestycydy i tlenek etylenu na układ krwiotwórczy Effect of metals, benzene, pesticides and ethylene oxide on the haematopoietic system. Med Pr. 2005;56(3):249-55. Polish. PMID: 16218139.
- Robinson MM. Dermatitis medicamentosa simulating Hodgkin's disease due to mercury compounds. Ann Allergy. 1952 Jan-Feb;10(1):21-3. PMID: 14894984.
- Alam RTM, Abu Zeid EH, Khalifa BA, Arisha AH, Reda RM. Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ Sci Pollut Res Int. 2021 Jun;28(24):31391-31402. doi: 10.1007/s11356-021-13014-5. Epub 2021 Feb 19. PMID: 33606169.
- Hilmy AM, Shabana MB, Said MM. Haematological responses to mercury toxicity in the marine teleost, Aphanius dispar (Rüpp). Comp Biochem Physiol C Comp Pharmacol. 1980;67C(2):147-58. doi: 10.1016/0306-4492(80)90010-6. PMID: 6108182.
- Vianna ADS, Matos EP, Jesus IM, Asmus CIRF, Câmara VM. Human exposure to mercury and its hematological effects: a systematic review. Cad Saude Publica. 2019 Feb 11;35(2):e00091618. doi: 10.1590/0102-311X00091618. PMID: 30758455.
- Silva-Filho R, Santos N, Santos MC, Nunes Á, Pinto R, Marinho C, Lima T, Fernandes MP, Santos JCC, Leite ACR. Impact of environmental mercury exposure on the blood cells oxidative status of fishermen living around Mundaú lagoon in Maceió - Alagoas (AL), Brazil. Ecotoxicol Environ Saf. 2021 Aug;219:112337. doi: 10.1016/j.ecoenv.2021.112337. Epub 2021 May 21. PMID: 34029837.
- Langworth S, Elinder CG, Göthe CJ, Vesterberg O. Biological monitoring of environmental and occupational exposure to mercury. Int Arch Occup Environ Health. 1991;63(3):161-7. doi: 10.1007/BF00381563. PMID: 1917065.
- Skalny AV, Aschner M, Sekacheva MI, Santamaria A, Barbosa F, Ferrer B, Aaseth J, Paoliello MMB, Rocha JBT, Tinkov AA. Mercury and cancer: Where are we now after two decades of research? Food Chem Toxicol. 2022 Jun;164:113001. doi: 10.1016/j.fct.2022.113001. Epub 2022 Apr 18. PMID: 35447290.
- Malarkey DE, Hoenerhoff MJ, Maronpot RR. Carcinogenesis. Fundamentals of Toxicologic Pathology, Elsevier; 2018. p.83-104. doi: 10.1016/B978-0-12-809841-7.00006-X.
- Russo MT, De Luca G, Palma N, Leopardi P, Degan P, Cinelli S, Pepe G, Mosesso P, Di Carlo E, Sorrentino C, Musiani P, Crebelli R, Bignami M, Dogliotti E. Oxidative Stress, Mutations and Chromosomal Aberrations Induced by In Vitro and In Vivo Exposure to Furan. Int J Mol Sci. 2021 Sep 7;22(18):9687. doi: 10.3390/ijms22189687. PMID: 34575853; PMCID: PMC8465244.
- Pelch KE, Tokar EJ, Merrick BA, Waalkes MP. Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicol Appl Pharmacol. 2015 Aug 1;286(3):159-67. doi: 10.1016/j.taap.2015.04.011. Epub 2015 Apr 25. PMID: 25922126; PMCID: PMC4461502.
- Virani S, Rentschler KM, Nishijo M, Ruangyuttikarn W, Swaddiwudhipong W, Basu N, Rozek LS. DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere. 2016 Feb;145:284-90. doi: 10.1016/j.chemosphere.2015.10.123. Epub 2015 Dec 11. PMID: 26688266; PMCID: PMC5047795.
- Chakraborty D, Choudhury B. TOXIC effects of mercury on crop plants and its physiological and biochemical responses - a review. Int J Adv Res (Indore). 2023;11:168-74. doi: 10.21474/IJAR01/16236.
- Vasilachi IC, Stoleru V, Gavrilescu M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture. 2023;13:1983. doi: 10.3390/agriculture13101983.
- Bijay Kumar Das, Avi Kush, Sumit Kumar, Khushbu Kumari. Impacts of mercury toxicity in aquatic ecosystem: a review. European Chemical Bulletin. 2023;12:1476–82.
- Silliman BR, Angelini C. Trophic cascades across diverse plant ecosystems. Nature Education Knowledge. 2012:44.
- Pace ML. Trophic cascades. Encyclopedia of Biodiversity, Elsevier; 2013. p.258-63. doi: 10.1016/B978-0-12-384719-5.00396-8.
- Moreno‐Jiménez E, Peñalosa JM, Esteban E, Carpena‐Ruiz RO. Mercury accumulation and resistance to mercury stress in rumex induratus and marrubium vulgare grown in perlite. Journal of Plant Nutrition and Soil Science. 2007;170:485-94. doi: 10.1002/jpln.200625238.
- Rand MD, Dao JC, Clason TA. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology. 2009 Sep;30(5):794-802. doi: 10.1016/j.neuro.2009.04.006. Epub 2009 May 4. PMID: 19409416; PMCID: PMC2774130.
- Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio. 2007 Feb;36(1):12-8. doi: 10.1579/0044-7447(2007)3612:eoemot2.0.co;2. PMID: 17408187.
- Frederick P, Jayasena N. Altered pairing behaviour and reproductive success in white ibises exposed to environmentally relevant concentrations of methylmercury. Proc Biol Sci. 2011 Jun 22;278(1713):1851-7. doi: 10.1098/rspb.2010.2189. Epub 2010 Dec 1. PMID: 21123262; PMCID: PMC3097836.
- Wolfe MF, Schwarzbach S, Sulaiman RA. Effects of mercury on wildlife: A comprehensive review. Environ Toxicol Chem. 1998;17:146-60. doi: 10.1002/etc.5620170203.
- George GN, Prince RC, Gailer J, Buttigieg GA, Denton MB, Harris HH, Pickering IJ. Mercury binding to the chelation therapy agents DMSA and DMPS and the rational design of custom chelators for mercury. Chem Res Toxicol. 2004 Aug;17(8):999-1006. doi: 10.1021/tx049904e. PMID: 15310232.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. doi: 10.1155/2012/460508. Epub 2011 Dec 22. PMID: 22235210; PMCID: PMC3253456.
- Schutzmeier P, Focil Baquerizo A, Castillo-Tandazo W, Focil N, Bose-O'Reilly S. Efficacy of N,N'bis-(2-mercaptoethyl) isophthalamide on mercury intoxication: a randomized controlled trial. Environ Health. 2018 Feb 14;17(1):15. doi: 10.1186/s12940-018-0358-1. PMID: 29444690; PMCID: PMC5813329.
- Gerhardsson L, Aaseth J. Guidance for clinical treatment of metal poisonings-Use and misuse of chelating agents. Chelation Therapy in the Treatment of Metal Intoxication, Elsevier. 2016, p. 313-41. doi: 10.1016/B978-0-12-803072-1.00007-9.
- Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010 Jul;7(7):2745-88. doi: 10.3390/ijerph7072745. Epub 2010 Jun 28. PMID: 20717537; PMCID: PMC2922724.
- Cao Y, Skaug MA, Andersen O, Aaseth J. Chelation therapy in intoxications with mercury, lead and copper. J Trace Elem Med Biol. 2015;31:188-92. doi: 10.1016/j.jtemb.2014.04.010. Epub 2014 May 14. PMID: 24894443.
- Blaurock-Busch E, Busch YM. Comparison of chelating agents dmps, dmsa and edta for the diagnosis and treatment of chronic metal exposure. Br J Med Med Res. 2014;4:1821-35.
- Kosnett MJ. Chelation for heavy metals (arsenic, lead, and mercury): protective or perilous? Clin Pharmacol Ther. 2010 Sep;88(3):412-5. doi: 10.1038/clpt.2010.132. Epub 2010 Jul 21. PMID: 20664538.
- Bhattacharya S. Medicinal plants and natural products can play a significant role in mitigation of mercury toxicity. Interdiscip Toxicol. 2018 Dec;11(4):247-254. doi: 10.2478/intox-2018-0024. Epub 2019 Oct 18. PMID: 31762676; PMCID: PMC6853017.
- Spiller HA, Hays HL, Casavant MJ. Rethinking treatment of mercury poisoning: The roles of selenium, acetylcysteine, and thiol chelators in the treatment of mercury poisoning: a narrative review. Toxicol Commun. 2021;5:19-59. doi: 10.1080/24734306.2020.1870077.
- Yadav V, Manjhi A, Vadakedath N. Mercury remediation potential of mercury-resistant strain Rheinheimera metallidurans sp. nov. isolated from a municipal waste dumping site. Ecotoxicol Environ Saf. 2023 Jun 1;257:114888. doi: 10.1016/j.ecoenv.2023.114888. Epub 2023 Apr 17. PMID: 37075645.
- Jing X, Lu T, Sun F, Xie J, Ma D, Wang X, Dong Q. Microbial transformation to remediate mercury pollution: strains isolation and laboratory study. International Journal of Environmental Science and Technology. 2023;20:3039-48. doi: 10.1007/s13762-022-04158-z.
- Vijayaraghavan K, Yun YS. Bacterial biosorbents and biosorption. Biotechnol Adv. 2008 May-Jun;26(3):266-91. doi: 10.1016/j.biotechadv.2008.02.002. Epub 2008 Feb 15. PMID: 18353595.
- Kumari S, Amit, Jamwal R, Mishra N, Singh DK. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ Nanotechnol Monit Manag. 2020;13:100283. doi: 10.1016/j.enmm.2020.100283.
- Gionfriddo CM, Stott MB, Power JF, Ogorek JM, Krabbenhoft DP, Wick R, Holt K, Chen LX, Thomas BC, Banfield JF, Moreau JW. Genome-Resolved Metagenomics and Detailed Geochemical Speciation Analyses Yield New Insights into Microbial Mercury Cycling in Geothermal Springs. Appl Environ Microbiol. 2020 Jul 20;86(15):e00176-20. doi: 10.1128/AEM.00176-20. PMID: 32414793; PMCID: PMC7376564.
- Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front Plant Sci. 2020 Apr 30;11:359. doi: 10.3389/fpls.2020.00359. PMID: 32425957; PMCID: PMC7203417.
- Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants (Basel). 2020 Jul 29;9(8):681. doi: 10.3390/antiox9080681. PMID: 32751256; PMCID: PMC7465626.
- González-Reguero D, Robas-Mora M, Probanza A, Jiménez PA. Evaluation of the oxidative stress alleviation in Lupinus albus var. orden Dorado by the inoculation of four plant growth-promoting bacteria and their mixtures in mercury-polluted soils. Front Microbiol. 2022 Sep 29;13:907557. doi: 10.3389/fmicb.2022.907557. PMID: 36246290; PMCID: PMC9556840.
- González-Reguero D, Robas-Mora M, Probanza Lobo A, Jiménez Gómez PA. Bioremediation of environments contaminated with mercury. Present and perspectives. World J Microbiol Biotechnol. 2023 Jul 13;39(9):249. doi: 10.1007/s11274-023-03686-1. PMID: 37438584; PMCID: PMC10338569.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.