Medicine Group 2025 June 08;6(6):580-585. doi: 10.37871/jbres2113.
Duodenal Exclusion in the Regulation of Glycemia in Type 2 Diabetic Patients Submitted to Gastrectomy with Roux-en-Y by Gastric Cancer: Cohort Study
Maurice Youssef Franciss1, Carol Viviana Serna González2, Leandro Cardoso Barchi1, Myriam Boueri3*, Marcus Fernando Kodama Pertille Ramos4, Rodrigo Moises de Almeida Leite5 and Bruno Zilberstein5
2Gastromed Clinic, Zilberstein Institute, São Paulo, SP, Brazil
3Medical Student at the Lebanese American University Medical Center Rizk Hospital LAUMCRH, Beirut, Lebanon
4Cancer Institute, Hospital das Clínicas, Department of Gastroenterology, FMUSP School of Medicine, University of São Paulo, São Paulo, SP, Brazil
5São Leopoldo Mandic College, Campinas, Brazil
- Blood glucose
- Diabetes mellitus
- Stomach neoplasms
- Bariatric surgery
- Gastrectomy
- Roux-en-Y anastomosis
Abstract
Background: The effect of the duodenal exclusion in glycemic regulation has yet to be defined. Individuals with type 2 Diabetes Mellitus (T2DM) operated for other reasons than obesity, represent an adequate model to analyze clinical outcomes of duodenal exclusion.
Objective: To analyze the changes in glycemia and pharmacotherapy for T2DM in patients undergoing gastrectomy with Roux-in-Y derivation for gastric cancer.
Methods: An observational study was conducted in 2018 on patients who were submitted to surgery from 2001 to 2016. Medical records of 129 patients’ cohort operated in two public hospitals were analyzed retrospectively before the surgery (T0) and one year after (T1). The research protocol was approved by the ethics committee. The final sample was mainly represented by women (50.5%) with a mean age of 65.5 years, and a mean body mass index of 26.5 kg/m2 SD 4.30.
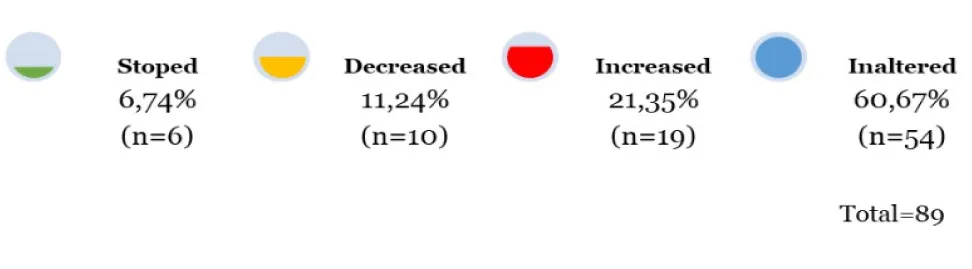
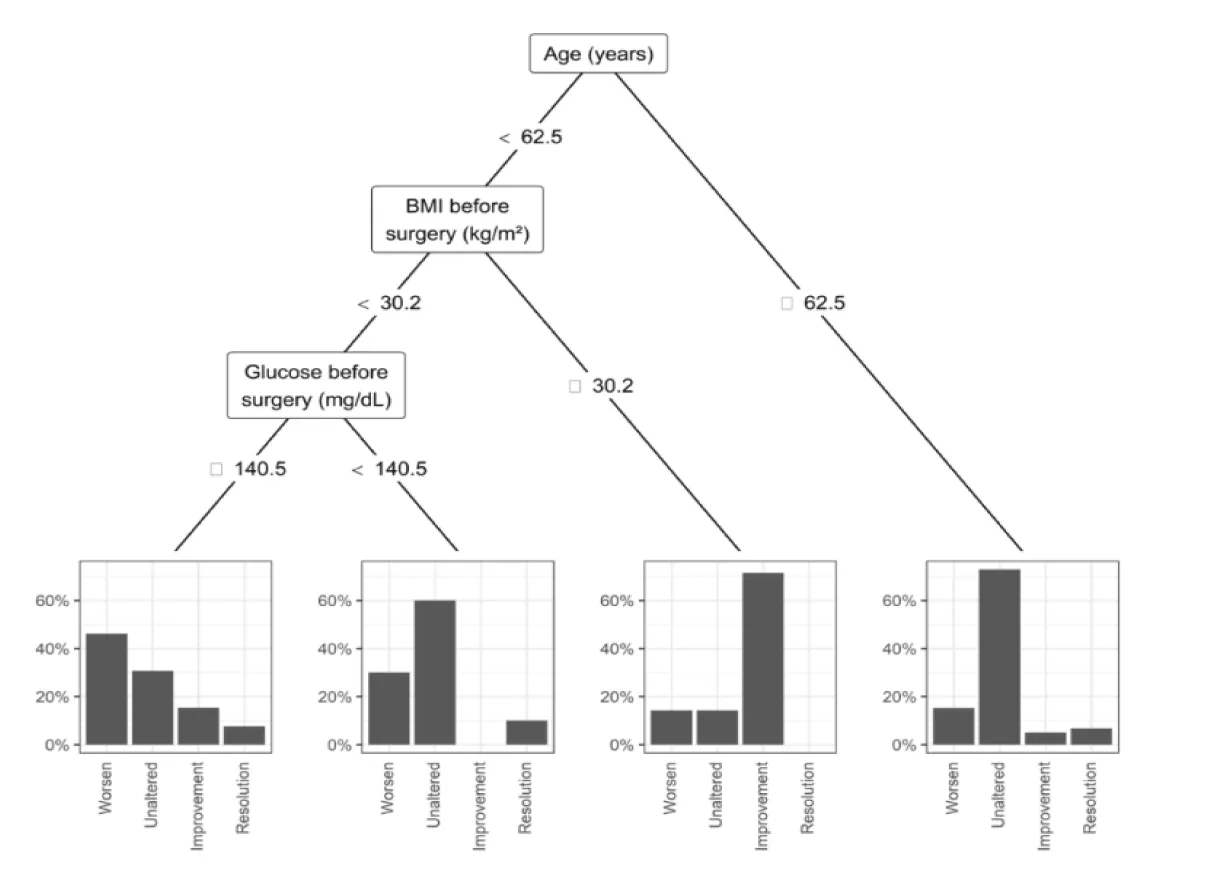
Results: One year later, mean glucose levels of the entire sample decreased (p=0.046), but 70% of patients with glycemia> 100 at T0, remained with the same value in T1. Glycated hemoglobin had no significant change (p=0.988). Regarding the pharmacotherapy for T2DM, 60.7% of the sample had no change. However, 6.7% had discontinuation of the medication with the improvement of T2DM. The multivariate model by classification and decision tree method (CART) found as predictors of change in DM2 medication, age (< 62.5 years) and a body mass index (> 30.2 kg/m2) with a predictive value of 71.4%.
Conclusion: There was no improvement of glycemia and pharmacotherapy in patients with T2DM who underwent gastrectomy with Roux-en-Y reconstruction, with a body mass index below 30 kg/m2
Abbreviations
RYGB : Roux-En-Y Gastric Bypass Surgery; T2DM : Type 2 Diabetes Mellitus
Introduction
Obesity has become a global epidemic in the 21st century, with Type 2 Diabetes Mellitus (T2DM) being one of its most serious and costly consequences [1]. In 2019, an international consensus recognized metabolic and bariatric surgery as a viable long-term treatment for T2DM. Procedures such as Roux-en-Y gastric bypass (RYGB) have demonstrated effectiveness in improving glycemic control, suggesting mechanisms beyond mere weight loss [2-4].
Studies have proposed that enhanced glycemic regulation may result from increased secretion of incretins in the distal intestine, stimulated by the accelerated transit of nutrients through this region [5,6]. Alternatively, some authors have hypothesized that exclusion of the proximal intestine plays a critical role in diabetes improvement [7]. A 2017 meta-analysis concluded that surgical interventions for T2DM yield superior remission rates and reduced risks of microvascular and macrovascular complications, as well as lower mortality, compared to non-surgical treatments [8]. Patients undergoing gastrointestinal surgery for conditions unrelated to obesity represent an important model for examining the isolated impact of duodenal exclusion on T2DM outcomes [9,10]. Accordingly, the aim of this study was to evaluate changes in glycemic control in diabetic patients who underwent total or subtotal gastrectomy with Roux-en-Y reconstruction for gastric cancer.
Methods
This observational, longitudinal cohort study employed a retrospective design and adhered to STROBE guidelines [11]. The study was conducted in two high-complexity public tertiary care hospitals affiliated with the University of São Paulo, Brazil, following ethical approval (Protocol No. 1.486.299).
The institutions included one general hospital providing gastrointestinal surgical care and one cancer center specializing in gastric oncology. All patients received multidisciplinary care including endocrinology, oncology, neurology, nursing, and physical therapy.
Inclusion criteria
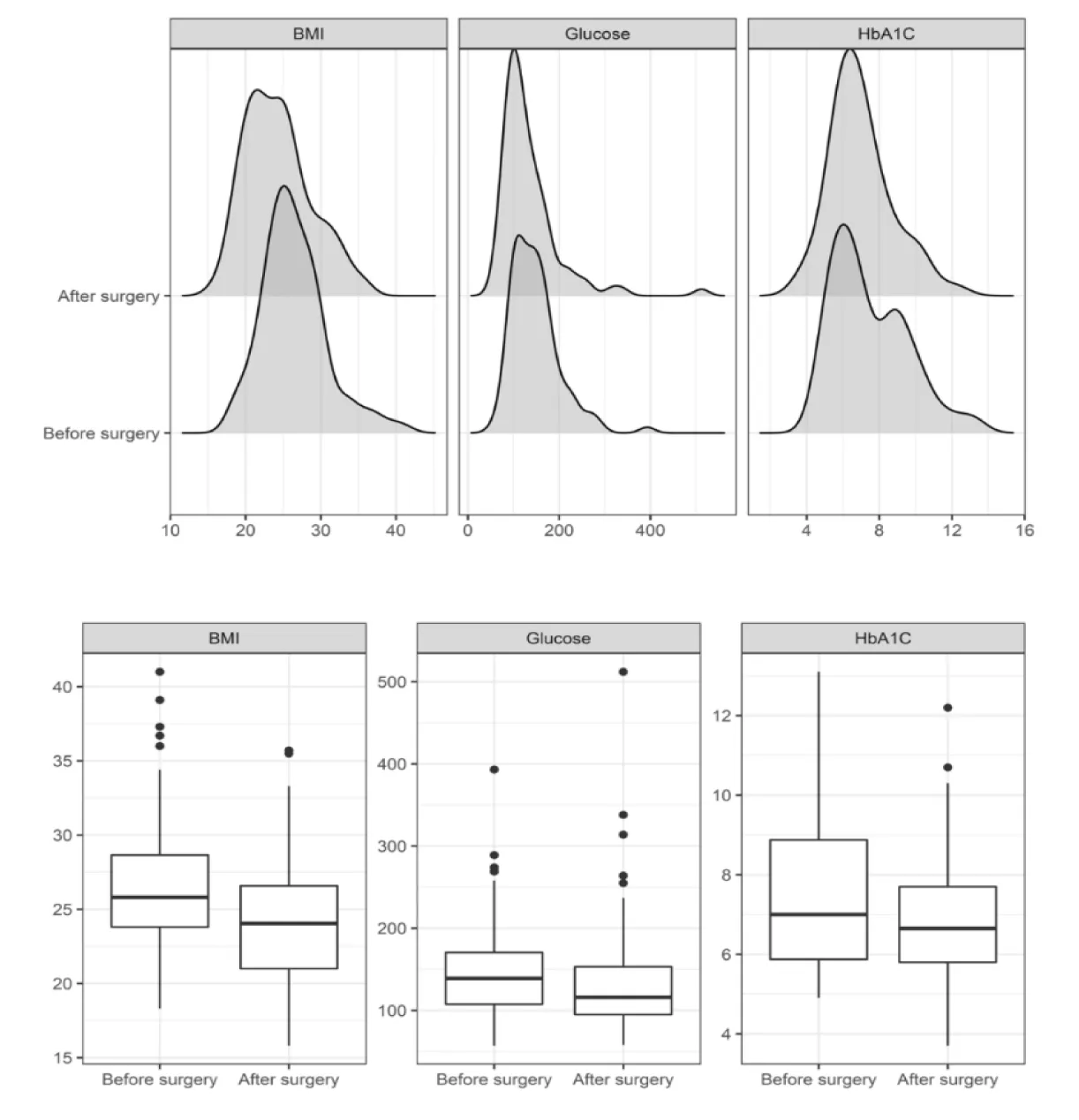
Were adults diagnosed with T2DM (defined by HbA1c and fasting glucose levels) who underwent gastrectomy for gastric adenocarcinoma. Roux-en-Y limbs lengths were standardized between 40-50 cm. Medical records were reviewed for demographic (age, sex) and clinical variables (comorbidities, BMI, fasting glucose, HbA1c, antidiabetic medication type and dose) at baseline (T0) and one year postoperatively (T1) (Figure 1). From an initial cohort of 129 patients treated between 2001 and 2016, 26 were excluded due to death or incomplete follow-up. The final analysis included 103 patients (50.5% female; mean age 65.5 ± 9.57 years, range 41-89) (Table 1). Baseline BMI was <25 kg/m² in 38.8%, 25-30 kg/m² in 44.7%, and >30 kg/m² in 16.5%. Subtotal gastrectomy was performed in 79.6% (n = 82), and total gastrectomy in 20.4% (n = 21); 71% of surgeries occurred after 2010 (Figure 2).
Primary outcomes
| Table 1: Sample Characterization. | |
| Sex | Female 52 (50.5%) / Male 51 (49.5%) |
| Mean Age | 65,5 ± 9,57 years (min. 41 e max. 89 years) |
| Comorbidities | 66% with Arterial Hypertension |
| Gastrectomy Type | Subtotal: 79,6% (n=82) Total: 20,4% (n=21) |
| BMC Distribution | <25 kg/m2 3.8,8% (n=40) 25 a 30 kg/m2: 44,7% (n=46) >30 kg/m2: 16,5% (n=17) |
| Gastric Adenocarcinoma TNM 2010 | IA (36,9%, n=38) / IB (10,7%, n=11) IIA (14,6%, n=15) / IIB (7,8%, n=8) IIIA (10,7%, n=11) / IIIB (8,7%, n=9) IIIC (7,8%, n=8) / IV (2,9%, n=3) |
| Clavicn-Dindo Classification (Moreira, 2016) | Grau I: 1,9% (n=3) Grau II: 8,7% (n=9) Grau IIIb: 13,6% (n=14) Grau IVa: 2,9% (n=3) Grau IVb: 1% (n=1) |
Included changes in BMI, fasting glucose, HbA1c, and antidiabetic medication usage from T0 to T1. Statistical analysis involved descriptive statistics, paired Student’s t-tests, and a Classification and Regression Tree (CART) model [12] to identify independent predictors of changes in antidiabetic therapy, accounting for potential confounding factors. A p <0.05 was considered statistically significant.
Results
At one year postoperatively, mean fasting glucose decreased significantly from 147.6 mg/dL (T0) to 134 mg/dL (T1) (p = 0.046), though 70% of patients with baseline glucose > 100 mg/dL remained above that threshold at T1. HbA1c did not significantly change (7.5% at T0 vs. 7.0% at T1; p = 0.988). BMI declined significantly from a mean of 26.5 to 24.3 kg/m² (p < 0.001), with the greatest reductions observed in patients undergoing total gastrectomy. Regarding antidiabetic medication use, 6.7% (n = 6) discontinued treatment, 11.2% (n = 10) reduced medication doses, 60.7% (n = 54) remained unchanged, and 21.4% (n = 19) experienced a worsening of glycemic control. The subgroup with BMI between 30-35 kg/m² showed the most favorable response. CART analysis identified age < 62.5 years and BMI > 30.2 kg/m² as predictors of medication reduction, with a predictive value of 71.4%.
Discussion
The mechanisms underlying T2DM improvement following metabolic surgery remain incompletely understood. While the "distal intestine hypothesis" - incretin stimulation via rapid nutrient exposure to the ileum - is well-supported, the role of proximal intestinal exclusion ("foregut hypothesis") remains controversial. In lean T2DM patients (BMI < 25 kg/m²), preserving nutrient absorption via the proximal intestine is preferred to avoid long-term nutritional deficits. These patients typically do not require significant weight loss, but rather glycemic stabilization and nutritional maintenance. Thus, evaluating the isolated effect of proximal intestinal exclusion is crucial in this population (Figure 3). While bariatric surgery consistently improves T2DM in patients with obesity [13], the present study found no significant glycemic benefit in patients with BMI < 30 kg/m² undergoing gastrectomy. The modest BMI reduction (2.14 units) and limited improvement in glycemic markers support the notion that duodenal exclusion alone may not be sufficient for T2DM resolution.
Notably, some patients achieved diabetes improvement, particularly those with BMI > 30 kg/m². This suggests weight loss and baseline insulin resistance remain key contributors to glycemic improvement, consistent with previous findings [14]. Although GIP levels were not measured, all patients underwent duodenal exclusion, and many had total gastrectomy with fundus resection, reducing ghrelin secretion. This hormonal milieu likely influenced metabolic outcomes. Huh YJ, et al. [14] demonstrated in a rodent model that only conventional Roux-en-Y with a 15 cm biliopancreatic limb improved glycemic parameters and reduced GIP levels [15]. Similar effects were not definitively observed here, but the possibility of enterohormonal modulation remains. Prior studies, including a prospective cohort from São Paulo, found significant GLP-1 increases and insulin resistance reduction shortly after Roux-en-Y, regardless of duodenal transit [16]. These findings further question the centrality of the proximal intestine hypothesis.
Moreover, in the current sample - composed primarily of cancer patients with low BMI - treatment goals often prioritized nutritional status over glycemic optimization, which may have confounded the analysis [17]. Cancer-related factors, including limited treatment adherence to diabetes care, may further explain the modest metabolic outcomes observed.
Limitations
Include the retrospective design, reliance on medical record data, and inability to determine T2DM duration prior to surgery. These factors may have influenced both baseline severity and responsiveness to surgical intervention. We also lack information such as entero-hormones dosing and comparison of total vs subtotal gastrectomy. Future studies should include prospective, controlled trials assessing the metabolic impact of proximal bowel exclusion in lean T2DM patients, ideally with hormonal profiling and comparison to techniques such as intestinal bipartition and vertical gastrectomy with jejunoileal bypass.
Conclusion
In this retrospective cohort of predominantly lean patients with gastric cancer and T2DM, total or subtotal gastrectomy with Roux-en-Y reconstruction did not result in significant glycemic improvement. The findings do not support the foregut exclusion hypothesis as a primary mechanism for diabetes remission in this population. However, improvements were more likely in younger individuals and those with BMI >30 kg/m², suggesting weight-related metabolic factors still play an important role.
Grant Support
This project was funded by the Clinica Gastromed, Instituto Zilberstein, São Paulo, SP, and Brazil. This work was also partially supported by Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) - Finance Code 001.
References
- Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res. 2016 May 27;118(11):1723-35. doi: 10.1161/CIRCRESAHA.115.306825. PMID: 27230638; PMCID: PMC4887150.
- Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE; Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a Joint Statement by International Diabetes Organizations. Obes Surg. 2017 Jan;27(1):2-21. doi: 10.1007/s11695-016-2457-9. PMID: 27957699.
- Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE; Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a Joint Statement by International Diabetes Organizations. Obes Surg. 2017 Jan;27(1):2-21. doi: 10.1007/s11695-016-2457-9. PMID: 27957699.
- Campos J, Ramos A, Szego T, Zilberstein B, Feitosa H, Cohen R. THE ROLE OF METABOLIC SURGERY FOR PATIENTS WITH OBESITY GRADE I AND TYPE 2 DIABETES NOT CONTROLLED CLINICALLY. Arq Bras Cir Dig. 2016;29 Suppl 1(Suppl 1):102-106. doi: 10.1590/0102-6720201600S10025. PMID: 27409057; PMCID: PMC5064276.
- Pais R, Gribble FM, Reimann F. Stimulation of incretin secreting cells. Ther Adv Endocrinol Metab. 2016 Feb;7(1):24-42. doi: 10.1177/2042018815618177. PMID: 26885360; PMCID: PMC4740941.
- Holst JJ, Gasbjerg LS, Rosenkilde MM. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology. 2021 Jul 1;162(7):bqab065. doi: 10.1210/endocr/bqab065. PMID: 33782700; PMCID: PMC8168943.
- Guner A, Cho M, Son T, Kim HI, Noh SH, Hyung WJ. Improved glycemic control with proximal intestinal bypass and weight loss following gastrectomy in non-obese diabetic gastric cancer patients. Oncotarget. 2017 Nov 1;8(61):104605-104614. doi: 10.18632/oncotarget.22262. PMID: 29262665; PMCID: PMC5732831.
- Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L. The Long-Term Effects of Bariatric Surgery on Type 2 Diabetes Remission, Microvascular and Macrovascular Complications, and Mortality: a Systematic Review and Meta-Analysis. Obes Surg. 2017 Oct;27(10):2724-2732. doi: 10.1007/s11695-017-2866-4. PMID: 28801703.
- Koliaki C, Liatis S, le Roux CW, Kokkinos A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr Disord. 2017 Aug 10;17(1):50. doi: 10.1186/s12902-017-0202-6. PMID: 28797248; PMCID: PMC5553790.
- Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001 Nov 10;323(7321):1123-4. doi: 10.1136/bmj.323.7321.1123. PMID: 11701584; PMCID: PMC1121605.
- Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. New York: Routledge. 2017.
- Associação Brasileira para Estudo da Obesidade e da Síndrome Metabólica. Diretrizes brasileiras de obesidade. São Paulo: ABESO; 2016.
- Hershey DS. Importance of Glycemic Control in Cancer Patients with Diabetes: Treatment through End of Life. Asia Pac J Oncol Nurs. 2017 Oct-Dec;4(4):313-318. doi: 10.4103/apjon.apjon_40_17. PMID: 28966959; PMCID: PMC5559941.
- Huh YJ, Son YG, Kim TH, Park JH, Oh TJ, Choi B, Min J, Cho YM, Yang HK, Lee HJ. Effect and Mechanisms of Diabetes Resolution According to the Range of Gastric Resection and the Length of Anastomosis in Animal Models: Implication for Gastric Cancer Surgery in Patients with Diabetes Mellitus. World J Surg. 2018 Apr;42(4):1056-1064. doi: 10.1007/s00268-017-4228-8. PMID: 28929278.
- Fernandes G. Avaliação do metabolismo glicêmico e perfil entero-hormonal no pós-operatório precoce em pacientes obesos graves diabéticos submetidos à gastroplastia em Y de Roux. Universidade de São Paulo; 2017.
- Pok EH, Lee WJ. Gastrointestinal metabolic surgery for the treatment of type 2 diabetes mellitus. World J Gastroenterol. 2014 Oct 21;20(39):14315-28. doi: 10.3748/wjg.v20.i39.14315. PMID: 25339819; PMCID: PMC4202361.
- Zilberstein B, Silveira-Filho AS, Ferreira JA, Carvalho MH, Bussons C, Joaquim H. Gastroplastia vertical com desvio jejunoileal: novo procedimento técnico. ABCD Arq Bras Cir Dig. 2014;27(3):199-201.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.