Medicine Group 2025 May 09;6(5):407-416. doi: 10.37871/jbres2098.
Alpha-Chymotripsin Immobilization on Amino-Functionalized ZnO Nanoparticles to Prepare ZnO/PMMA Nanocomposites Coatings to Create Antimicrobial and Hydrophobic Surfaces on Cotton Fabric
Sorna Gowri Vijaya Kumar1*, Hideaki Morikawa2, Kim Ick Soo2, Gopiraman Mayakrishnan2, Jyoti Lodhi1 and Raju Khan1
2Shinshu University, 3151 Tokida Ueda, Nagano 3868567, Japan
- Polymer nanocomposites
- Enzyme
- Antimicrobial
- Cotton textile
- Hydrophobicity
Abstract
Incorporation of enzyme immobilized ZnO nanoparticles in to hydrophilic polymer Polymethyl metharcylate (PMMA) has been considered in this present study with the goal of developing antimicrobial hydrophobic textile coatings. The enzyme immobilized nanoparticles polymer coatings on textiles by dip coating, using the polymer Polymethyl methacrylate and proteolytic enzymes alpha chymotripsin immobilized green synthesized, amino functionalized ZnO nanoparticles. Immobilization of enzymes on nanoparticles with large surface to volume ratio provides obvious advantage. Alpha chymotripsin was immobilized on amino functionalized ZnO nanoparticles dispersed in n-proponal. The enzyme immobilized ZnO nanoparticles were used for making the ZnO/PMMA polymer nanocomposite coating. This coating was applied on cotton textile by dip coating and the coated textiles were evaluated for antimicrobial and hydrophobicity Properties.
Introduction
Antimicrobial textile is the functionalized textiles which inhibit the growth of microorganism during usage. Different methods were used for the preparation of antimicrobial textiles. Hydrophobically treated textile also help in keeping the surface clean. Among various methods using nanoparticles based coatings are very common to impart antimicrobial property to natural as well as synthetic textiles. Silver nanomaterials are very efficient in imparting self-cleaning and antimicrobial properties to textiles [1,2]. Other than silver many metal oxides and metal nanoparticles like Cu, Au, Zinc and tin have also been used for the development of medical textiles. CuO coated textiles have shown broad spectrum antimicrobial activity [3]. Nano selenium treated textiles exhibited activity against basicillus sublitis [4]. Prevention of agglomeration, desired morphology and uniform size of the nanoparticles are very challenging aspects for nanoparticles based antimicrobial treatment of textiles. Meanwhile selection of proper polymer matrices is very crucial for the development of polymer nanocomposites coatings for textiles [5].
Among various natural products proteolytic enzymes are found to be very interesting class of antimicrobial agents. They can destruct the microorganism structures and chemically degrade the molecules that could favour the adsorption of the microbes on textiles [6]. Application of enzymes is an effective alternative process to impart antimicrobial property to textiles [7]. As they are natural materials enzymes are environmentally safe to use as antimicrobial agent although microorganisms show diverse procedures to adhere to a substrate. Researchers have shown that common proteases reduce the adhering strength of the microorganisms to a surface and also to textiles [8-11].
When virus SARS-CoV-2pandemic happens causing the Corona Virus Disease 2019 (COVID-19), there was no specific treatment and gains an unexpected but predictable importance. Recycling non- reusable PPEs with antimicrobial properties has been an interesting area of research, particularly after the market shortage [12-14].
Antimicrobial textiles are essential to guard health workers from possible infections. Using silver nanoparticles (AgNPs) on polymeric fiber mats is a easy way to provide antimicrobial properties to textiles [15,16], while the antibacterial tests for AgNPs treated PPEs are generally carried out in solution, the performance of treated PPEs in a gaseous environment is uncertain. Organic macromolecules having nitrogen-halogen generally show intense oxidative properties through oxidative halonium ions release in an aqueous environment [17] and could be used onto fiber mats to impart antimicrobial properties [18,19]. N-halamine is one of the most commonly used macromolecule in spite of its weak intrinsic toxicity [17,20]. Unlike silver nanoparticles, ion release using N-halamine may take place in humid environment, which can be used for inhalation protection applications. In order to make the antimicrobial textiles bio safe and environment friendly, botanic extracts were used for textile functionalization [21,22].
There is no universal alternative for sterilizing textiles used for medical purpose. There are four methods of reprocessing exist namely, thermal, chemical, radiation and energetic [23,24]. Thermal treatments are commonly used for virus deactivation since it induces changes in virus protein structure [23]. These types of treatments may face scalability problems and deform fabrics [14,23].
During this period of pandemic diseases, a contamination-safe PPEs can be designed with the support of nanotechnology. Effective antimicrobial property can be achieved using copper, silver, and zinc nanostructures which have a controlled and long-lasting ionic release. However, frequently in the case of virus, nanoparticles are not able to completely destabilize the protein capsid of the virion. In this situation, the use of proteolytic enzymes which are able to digest the capsid proteins before the virus enters the target cell or to destabilize membrane proteins in bacteria have been successfully tested [24]. Nanoparticles are also used as carriers for enzyme immobilization and they were successfully employed in redox enzymes immobilization, stabilization, catalytic enhancement for bio-electrochemical sensor devices and biomedical applications [25,26]. There are basically two main concepts of antimicrobial surfaces involving nanotechnology: (i) Passive super hydrophobic microscopically rough surfaces that mimic the well-known lotus leaves effect repelling water and microorganisms; (ii) Active surfaces that can actively destabilize or destroy microorganism by metal or metal-oxide nanoparticles or enzymes as nanocatalyst [27]. However, rarely the both strategy are used at the same time and usually nanoparticles and enzymes are not present in the same nanocoatings. Moreover, enzymes incorporation or grafting into and onto metal NPs and textiles to prevent microbial colonization remains an area of experimental need. Using polymer matrices as dispersion medium for these nanoparticles not only imparts hydrophobicity and antiviral properties but also permanently bind these nanoparticles with textile surfaces [28].
Development of these multifunctional super hydrophobic and in-situ self-cleaning, antimicrobial and antiviral textiles with nanoparticles immobilized with proteolytic enzymes is a step ahead of the current state-of-the-art. The interdisciplinary research area combining nanotechnology, biotechnology and textile science is still in its initial stages. To our knowledge the approach proposed in this paper is unique and there is need to study the properties and performance of such bio-nanocomposites.
Materials and Methods
The metal oxide precursor, zinc acetate dihydrate (Zn (C2H3O2)2.2H2O), was purchased from Sigma-Aldrich, a-Chymotrypsin (from bovine pancreas), was obtained from Sigma Chemical Co. Poly (methyl methacrylate) (PMMA) (MW 996 kDa), was obtained from Aldrich. Commercial grade reagents and solvents were used.
Green synthesis of ZnO nanoparticles
To prepare neem leave extract 20 gm dried neem leaves were crushed and added to 50 ml deionised water. The mixture was stirred in a magnetic stirrer for 1 hour at 60°C. When the mixture became yellow colour, it was filtered. ZnO nanoparticles were prepared by using this extract. Further 21.94 gm of Zn(CH3COO)2.2H2O was added to 50 ml deionised water and kept under stirring for 20 minutes at 35⁰C. 4 gm of sodium hydroxide was mixed with 50 ml deionised water and kept under stirring for 20 minutes at 35⁰C separately. Both the solutions were stirred well in a stirrer. The neem leaf extract was added drop by drop, while stirring. The product obtained was filtered and dried at 80⁰C for 4h and then calcined at 250⁰C for 4h. After calcination, the product was ground well to get ZnO nanoparticles [29].
Amino-functionalization of ZnO Nanoparticles
Hydrothermal Method: Amine-functionalized zinc oxide nanoparticles were prepared by hydrothermal method. 200 mg ZnO NPs were mixed in 50 mL Dimethylformamide (DMF) and then sonicated for 30 minutes for complete dispersion of ZnO NPs. Subsequently, 1 g urea was mixed in the solutions and sonicated for another 10 minutes. Further, the mixed solutions were transferred in a Teflon-lined stainless-steel autoclave. The urea was used to functionalize the amine group on the ZnO NPs surface. Afterward, the solution was heated at 200°C for 24 h. When the reaction was completed, they were cooled at room temperature and washed three times using milli-Q water and dried at 25°C. Subsequently, the washed NPs were equilibrated with 0.1 M phosphate buffered (pH 7.4) and incubated with 1 mL of 25% glutaraldehyde for 2 hours at room temperature with continuous stirring. After the incubation, the NPs were washed several times with milli-Q water and dried at 60°C for 12h [30].
Chemical method: Amine-functionalization of ZnO nanoparticles was carried out by a chemical method. 200 mg ZnO NPs were mixed in 25 mL Dimethylformamide (DMF) and then sonicated for 30 minutes for complete dispersion of ZnO NPs. Subsequently, 1 g urea was mixed in the solutions and sonicated for another 10 minutes. The urea was used to functionalize the amine group on the ZnO NPs surface. Afterwards, the solution was stirred at 200°C for 24 h on a magnetic stirrer. When the reaction was completed, it was cooled to room temperature, and washed three times using milli-Q water and dried at 25°C. Subsequently, the washed NPs were equilibrated with 0.1 M phosphate buffered (pH 7.4) and incubated with 1 mL of 25% glutaraldehyde for 2 hours at room temperature with continuous stirring. After the incubation, the nanoparticles were washed with milli-Q water and dried at 60°C for 12h [26].
Immobilization of alpha Chymotrypsin on ZnO nanoparticles
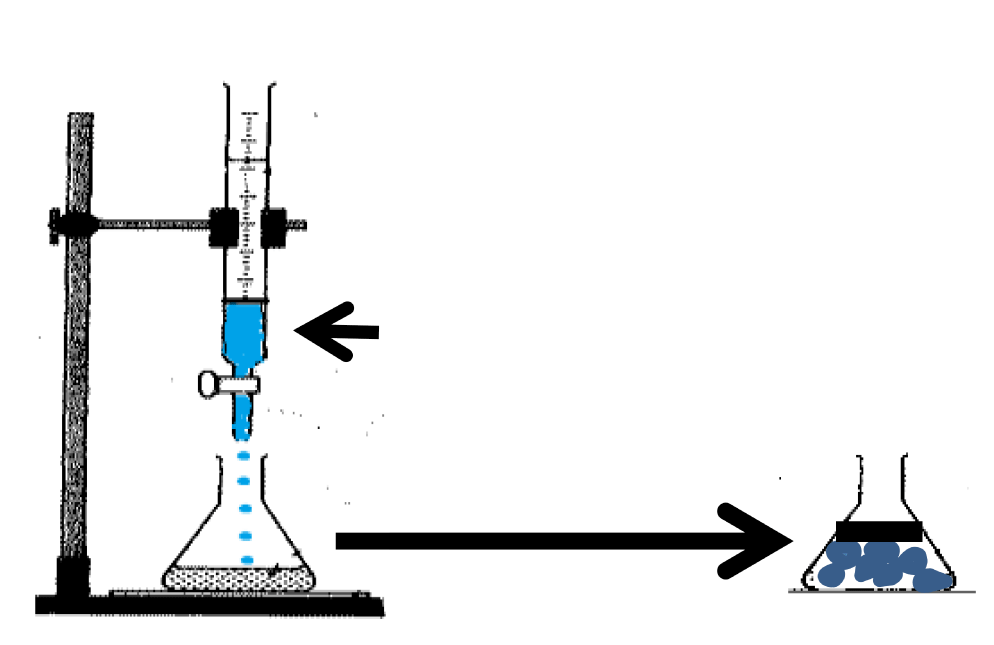
Precipitation of alpha chymotripsin over ZnO nanoparticles was carried out by mixing with an organic solvent [31]. The precipitation method is outlined in figure 1. This processes minimizes enzyme inactivation by noncovalent approaches. The reasons for selecting Alpha chymotrypsin are: (1) it has been widely used for diversified application in organic media like, peptide synthesis. (2) It has antimicrobial properties. To prepare an uniform suspension of enzyme immobilized nanoparticles, A weighed amount of ZnO nanoparticles was taken in a vial containing a fixed volume of n-propanol and placed a shaker. The alpha chymotrypsin solution was prepared with buffer and added to the above suspension (Figure 1). The mixing was done by adding of aqueous buffer to the organic solvent. This mode favours retention of original structure of alpha chymotrypsin. This method helps is to incorporate enzyme uniformly over the nanoparticles. These enzyme immobilised ZnO nanoparticles were filtered and used for making polymer nanocomposites with polymethyl methacrylate(PMMA) [32].
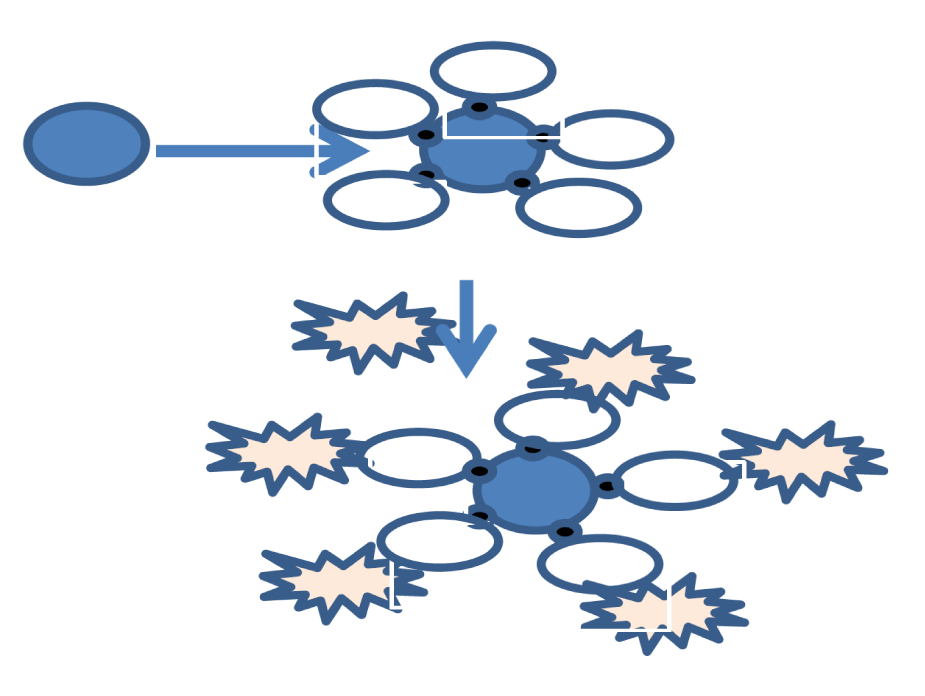
Following figure 2 shows the schematic representation of enzyme immobilasation on amonio modified ZnO nanoparticles Preparation of enzyme-ZnO/PMMA polymer nanocomposites coated cotton fabric. Cotton fabric was cleaned by washing the fabric with detergent and drying in the air before use.
Coating the cotton fabric with enzyme- ZnO–PMMA
5% stock solution of PMMA was prepared by dissolving (PMMA) in toluene. Zinc oxide nanoparticles of different percentages (0.1, 0.5, and 1) were mixed with PMMA. A homogeneous solution of the mixture was obtained by stirring it well in a stirrer. The fabric was cut into size of 30 X 30 cm and dipped in nano ZnO solutions with different concentrations of ZnO and kept under ultrasonic vibrations for 15 min and air dried. Then the fabric was rinsed with deionised water and air dried again [33].
Testing for antimicrobial properties of treated textiles
The treated cotton fabric with ZnO/PMMA polymer nanocomposite was evaluated for antibacterial activity at BTRA, Mumbai as per JIS L 1902-2015 Method against Staphylococcus aureus by ATCC 6538 and against Klebsiella pneumoniae by ATCC 4352.
Antibacterial Activity of Fabrics Assessment of Textile Materials–Parallel Streak Method- AATCC 147 to assess antimicrobial activity of the coated fabrics by Parallel Streak Method- AATCC 147; the coated fabrics was cut in to samples of size 25mmX 50mm. The test was conducted in Nutrient Agar media. The test organisms were Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae (ATCC 4352). The incubation of the textile samples was carried out at 37⁰C for 24 Hrs and antimicrobial activity was assessed.
Antimicrobial testing by plate count method
For quantitative measurement the samples were weighed to 0.4± 0.05 g. Tryptone Soya Agar was used as medium. The test organisms were Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352. The incubation conditions were37°C for 24-48 hrs. The samples were sterilised by autoclaving at 120⁰C for 15 minutes. Physiological saline was used as diluent. The culture density was 1-3x105 CFU/ml.
Characterization of ZnO nanoparticles enzyme-ZnO/PMMA coated cotton fabric
FT-IR analysis: FT-IR spectroscopic analysis was carried out with Nicolet Avatar 360 spectrophotometer for the chemical analysis of the prepared nanoparticles ZnO and ZnO–PMMA.
A high-resolution transmission electron microscope (HR-TEM) (Make: JEOL, Model: JEM-F200) was used for morphological analysis. The size and morphology of microscopy images of ZnO, amino functionalized ZnO were observed. HR-TEM samples were prepared by drop-casting 6 µl of nanoparticles solution onto carbon-coated copper grids. The developed film was dried at room temperature.
X- ray difractometer analysis
X-ray diffractometer (Model: Bruker D8 Discover) was used for XRD pattern analysis of nanoparticles using Cu Ka radiation at wavelength k = 0.15406 nm. The scan range was 2h = 20-90, at scanning rate = 0.02 deg/s (applied voltage 40 kV, current 20 mA).
Contact Angle Measurement
Contact angle measurements were carried out in Automatic Contact Angle Meter DMs-400, Kyowa Interface Science Co-Ltd, Japan using distilled water. Water droplets (5 µl) were delivered to different points of the textile specimen and at a height close to the textile surface. The height of the needle was adjusted in such a way the needle remains in contact with the water drop. After withdrawing the needle, the image of the drop was captured to measure the static contact angle.
Results
X-ray diffraction analysis of ZnO nanoparticles
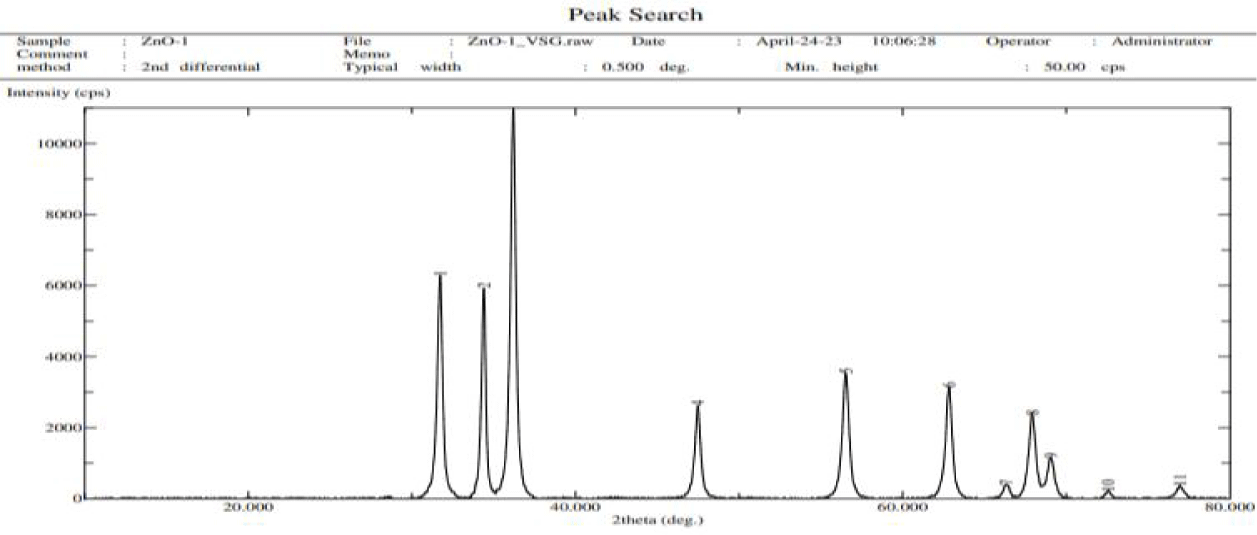
XRD spectrum of the ZnO nanoparticles (Figure 3) showed typical peaks of Zincite in ZnO having crystal structure. The diffract gram of ZnO nanoparticles have shown characteristic peaks of crystalline zinc oxide nanoparticles with 2θ° values: 31.8, 33.9, 36.8, 47.9, 57.9, 62.8, 66.4, 67.9, 68. 72.9, and 79.9 respectively.
FTIR analysis of ZnO nanoparticles
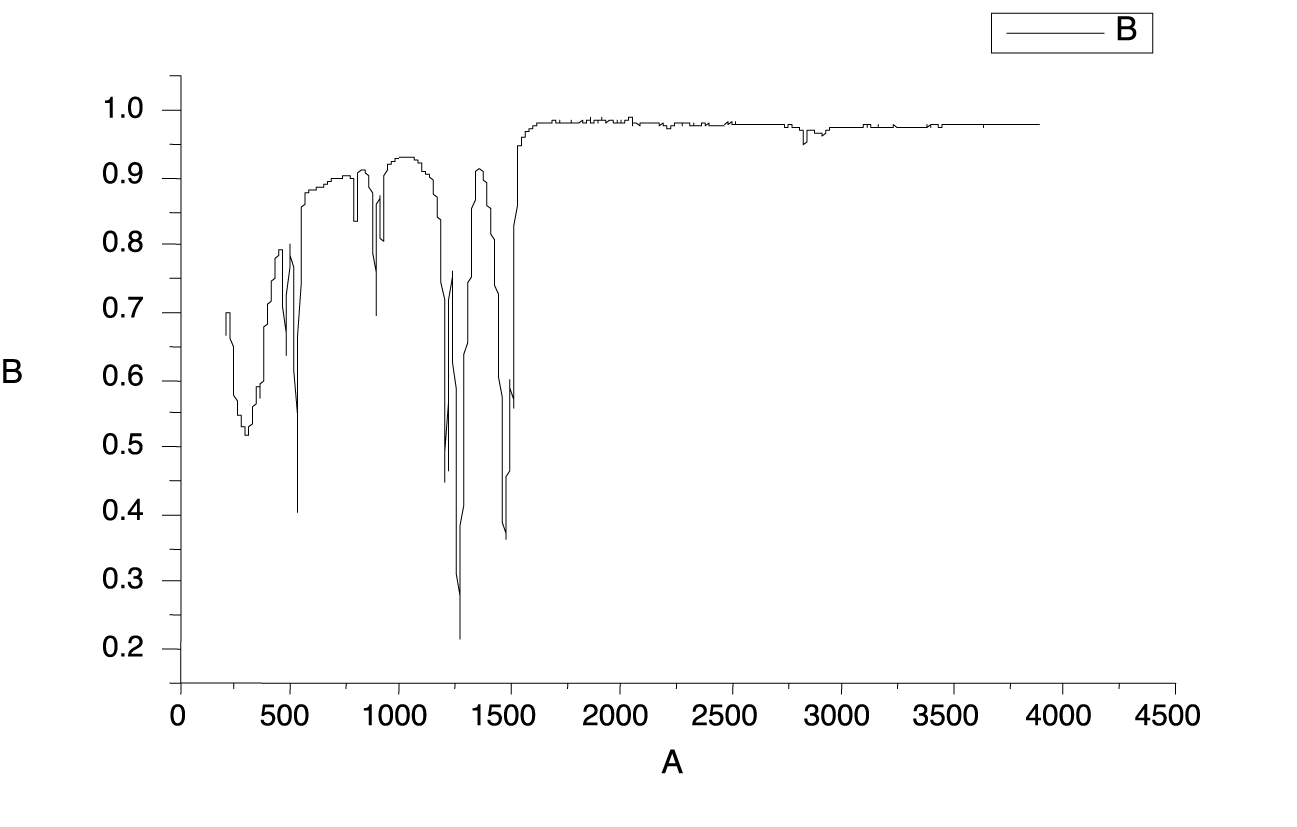
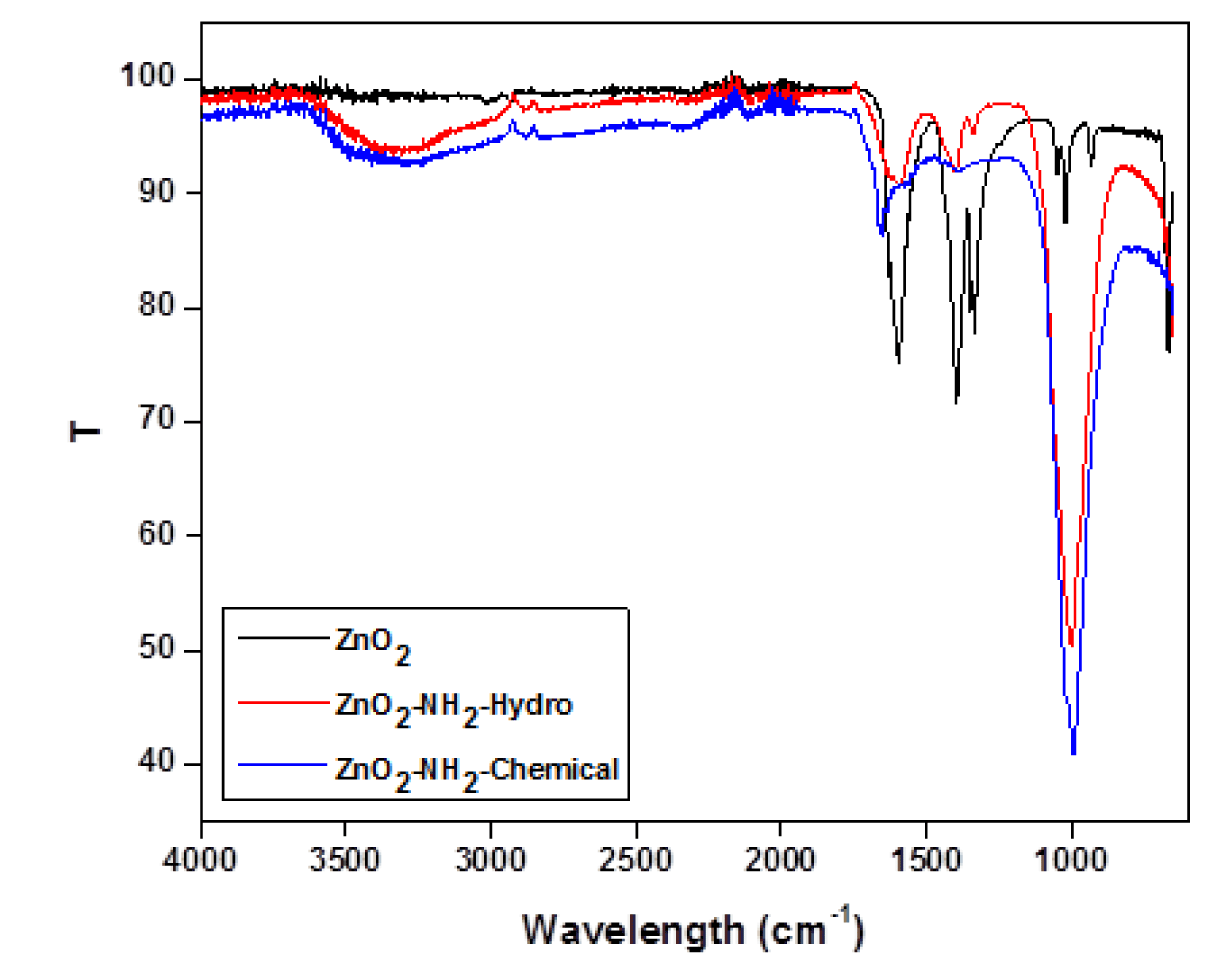
The FT-IR spectroscopy is very useful to for the structural analysis compounds. The FT-IR spectrum of the synthesized nano ZnO (Figure 4). Amine-functionalization of ZnO Nanoparticles has been carried out by both chemical ad hydrothermal methods.
Successful Amino functionalization of ZnO nanoparticles was confirmed by FTIR spectra as shown in figure 5.
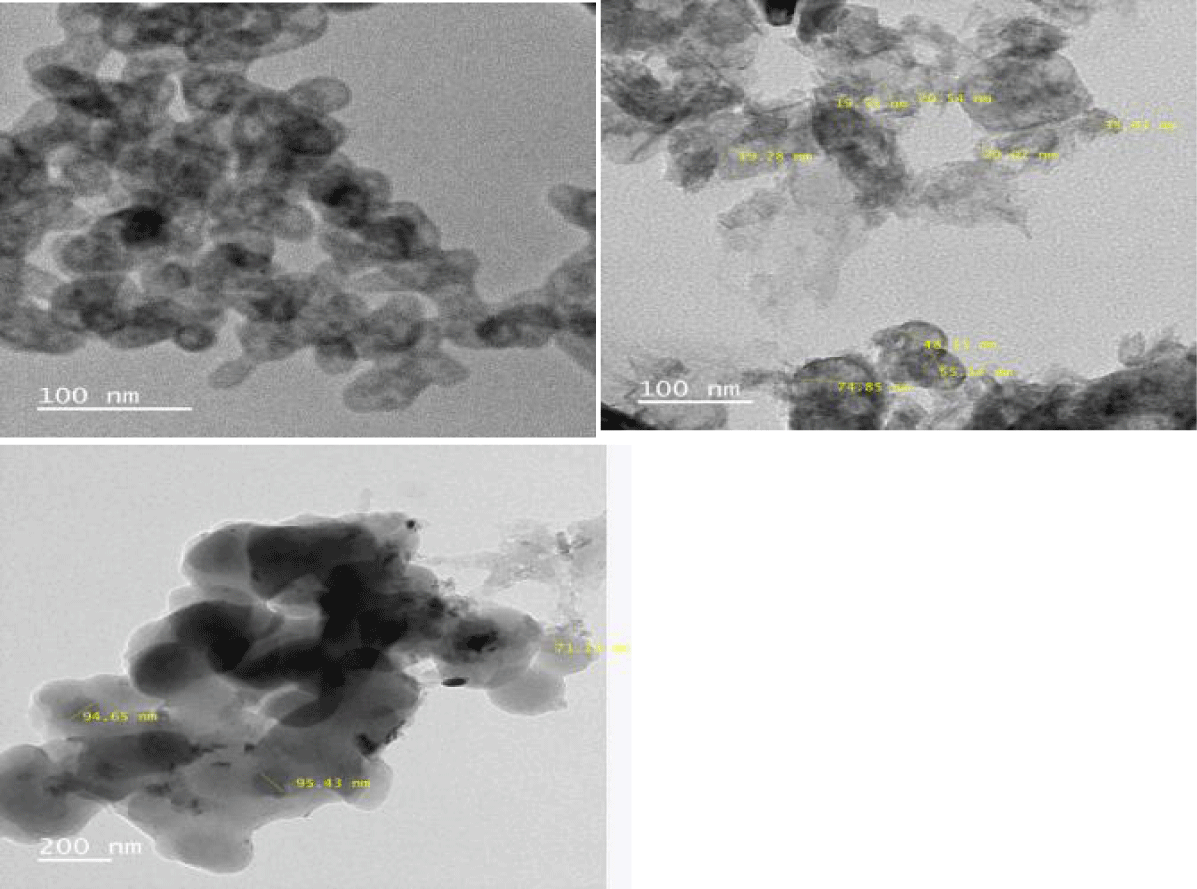
Figures 6a-c show the TEM analyisis of ZnO and amiono modification of ZnO by both the chemical and hydrothermal mehod. Both the products were compared for their shape and size distribution.
The TEM micrographs (Figure 6a) showed the Zinc Oxide nanoparticles were in network form. The TEM results are supported by XRD analysis. It has been observed that the synthesized Zinc Oxide nanoparticles were in spherical shape with 40 to 65 nanometers size.
Antimicrobial testing of treated textile samples
Cotton fabric coated with 1% ZnO/PMMA and enzyme -ZnO/PMMA polymer nanocomposites coatings were evaluated for antibacterial activity value tested at BTRA, Mumbai against Staphylococcus aureus by ATCC 6538 and against Klebsiella pneumoniae by ATCC 4352 as per JIS L 1902-2015 Method.
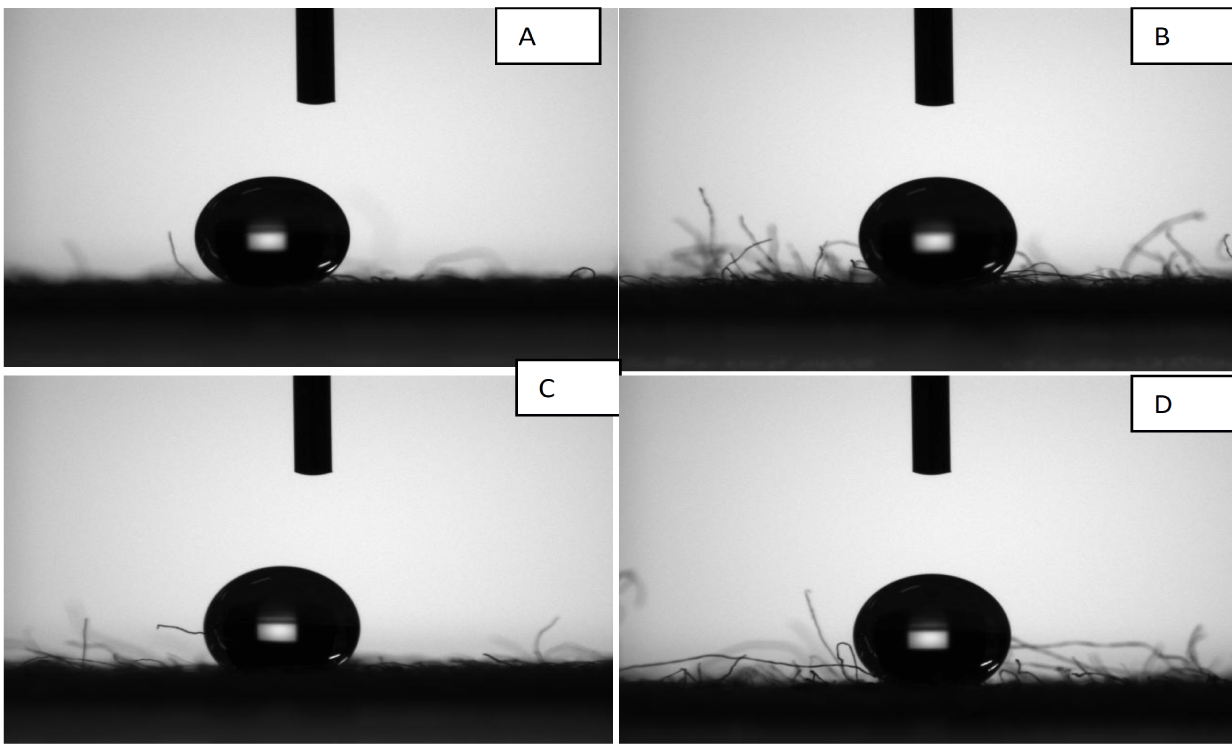
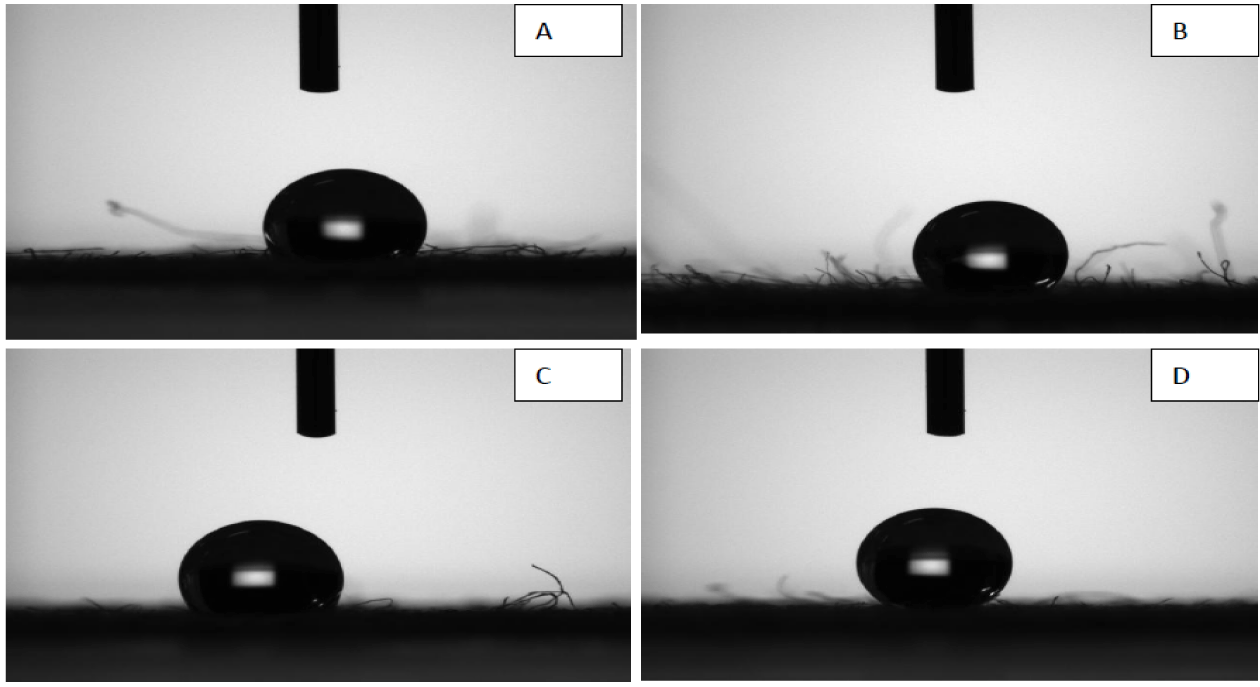
The sample in contact with individual test organisms were kept under observation for 18-24 hrs and the results by Parallel Streak Method is given in tables 1,2. The antimicrobial activity results by Plate Count Method are given in tables 3,4. It can be seen from figures 7,8 that the contact angle for water on the fabric coated with enzyme-ZnO/PMMA coating and ZnO/PMMA coatings, the contact angle increased with increase of the ZnO nanoparticles concentration in both the coatings.
| Table 1: Antimicrobial activity of ZnO/PMMA coated fabric by parallel streak method. | ||||||
| Sample | Klebsiellaa pneumoniae ATCC 4352 | Staphylococcus aureus ATCC 6538 | ||||
| Growth under specimen | Zone of inhibition | Zone of Inhibition (in mm) | Growth under specimen | Zone of inhibition | Zone of Inhibition (in mm) | |
| ZnO /PMMA coated textile | Absent | Absent | - | Absent | Absent | - |
| Table 2: Antimicrobial activity of chymotrypsin immobilized ZnO/PMMA coated fabric by parallel streak method. | ||||||
| Sample | Klebsiella pneumoniae ATCC 4352 | Staphylococcus aureus ATCC 6538 | ||||
| Growth under specimen | Zone of inhibition | Zone of Inhibition (in mm) | Growth under specimen | Zone of inhibition | Zone of Inhibition (in mm) | |
| Chymotrypsin immobilised ZnO /PMMA coated textile | Absent | Absent | - | Absent | Absent | - |
| Table 3: Antimicrobial activity of ZnO/PMMA coated textile by plate count method. | ||||
| Sample | Test organism | Growth value of control specimen | Growth value of test sample | Antibacterial Activity Value (A Value) |
| CFU/ml | CFU/ml | CFU/ml | ||
| ZnO /PMMA coated textile | Klebsiella pneumoniae ATCC 4352 | 2.00 | -0.98 | 2.98 |
| Staphylococcus aureus ATCC 6538 | 2.08 | -3.68 | 5.76 | |
| strong>Table 4: Antimicrobial activity of chymotrypsin immobilised ZnO/PMMA coated textile by plate count method. | ||||
| Sample No. | Test organism | Growth value of control specimen | Growth value of test sample | Antibacterial Activity Value (A Value) |
| (CFU/ml) | (CFU/ml) | (CFU/ml) | ||
| Chymotrypsin immobilised ZnO/PMMA coated textile | Klebsiella pneumoniae ATCC 4352 | 2.00 | -1.20 | 3.20 |
| Staphylococcus aureus ATCC 6538 | 2.08 | -4.44 | 6.52 | |
Discussion
The XRD spectrum values showed that the crystallinity of the synthesized ZnO. The broad diffraction maxima showed size of the crystallites are very small similar to the size of ZnO nanoparticles observed by the SEM. These peaks obtained were in well agreement with those reported in the literature and also very well consistent with the JCPDS file card number 01-075-0576, indicated that the synthesized nanoparticles were identical to hexagonal phase of ZnO. Particle size plays important role in peak broadening in XRD analysis.
The FT-IR spectrum of Zinc oxide showed absorption band at 438 cm-1. Peaks at 1635 cm-1 were due to the bending vibrations of water molecules. Bandwidth at 1500-1600 cm-1 represents the fingerprint region of ZnO nanoparticles. A small peak at 1650 cm-1 was due the N-H bending and weak peaks. Symmetric and asymmetric vibration of C=O showed peak at1508 cm-1 and 1641 cm-1. The broad peak around 3400 cm-1 was assigned to - OH stretching bond vibration which may be due to adsorption of water on the surface of Zinc Oxide.
Successful Amino functionalization of ZnO nanoparticles was confirmed by FTIR spectra. (Blue line) represents the FTIR spectra of amino functionalized Zinc oxide nanoparticles by chemical method with characteristic peaks at 3400 cm-1, 1632 cm-1. The weak peaks at 1400 cm0-1 and 1462 cm-1 were due to O-H and N-H bending, showing less amount of N-H group. The red line represents FTIR spectra of amine functionalized ZnO NP, prepared by hydrothermal method. It shows the presence of O–H stretching frequency along with –N-H stretching frequency of NH2 group at 3373 cm-1. Presence of a peak at 2930 cm-1 was due to the asymmetric C-H stretching frequency of the CH2 group. Peaks at 1580 cm-1 and 1470 cm-1 were due to NH2 scissoring of primary amine. A clear peak at 1411 cm-1 was due to low intensity -O-H bending. A peak between 2800 and 3800 cm-1 includes the symmetric stretching vibration of primary amino group( 3250-3450 cm-1), and 1025 cm-1 the sample showed new peak at about 2928 cm-1. The NH2 - ZnO peak at 1630 cm-1 shifted to 1548 cm-1 for bending mode of N-H bond. Amino functionalization by hydrothermal process showed very distinct peaks when compare to the amino functionalized ZnO by chemical method. So in the present study amino functionalized ZnO by hydrothermal process was used in the further studies. While comparing TEM analysis of both the products, ZnO nano particles by both the amino modification process, the modification by hydrothernal process (Figures 7,8) maintain the shape and size, when compare to the ZnO amino modified by Chemical method. In both the methods the avearage particle size is maintained within 100nm. Aminomodified ZnO by hydrothermal process was used for further processing in the present study.
In qualitative analysis for antimicrobial properties by parallel streak method, ZnO/PMMA coated Fabric showed Antibacterial Activity against Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352, when tested according to AATCC 147. Chymotrypsin immobilized ZnO/PMMA coated fabric showed Antibacterial Activity against Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352, when tested according to AATCC 147. In quantitative analysis by plate count method, ZnO/PMMA coated textile showed Antibacterial Activity value of 2.98 against Klebsiella pneumoniae ATCC 4352 and that of 5.76 against Staphylococcus aureus ATCC 6538, when tested as per JIS L 1902 Method.: The sample Chymotrypsin immobilized ZnO/PMMA coated textile showed Antibacterial Activity value of 3.20 against Klebsiella pneumonia ATCC 4352 and that of 6.52 against Staphylococcus aureus ATCC 6538, when tested as per JIS L 1902 Method. Chymotrypsin immobilized ZnO/PMMA coated textile showed full effect against Klebsiella pneumoniae ATCC 4352 and Staphylococcus aureus ATCC 6538 compared to untreated ZnO/PMMA coated textile samples.
The values of contact angle for enzyme-ZnO/PMMA treated cotton fabric are 138.7, 138.6, 143.8, and 144.9 for 0.2, 0.5, 0.8, and 1% of enzymes immobilized ZnO nanoparticles concentration respectively. The contact angle values of ZnO/PMMA coated textile samples are 134.1,135.6, 136,136.6 for 0.2, 0.5, 0.8, and 1% ZnO nanoparticles concentration respectively. The contact angle values of ZnO/PMMA coated were slightly lesser than the enzyme immobilized ZnO/PMMA coated textile samples, which may be due to hydrophobic nature of the chymotripsin, but still both the textiles samples showed significant hydrophobicity due to higher contact angle values. Both the ZnO/PMMA and enzyme immobilized ZnO/PMMA treated fabrics showed linear relationship in contact angle values [34].
Conclusion
The contact angle for water on the fabric coated with ZnO/PMMA coating and enzyme-ZnO/PMMA coatings increased with increase of the ZnO nanoparticles concentration in both the coatings and they found to be hydrophobic. The contact angle values of ZnO/PMMA coated were slightly lesser than the enzyme immobilized ZnO/PMMA coated textile samples, which may be due to the hydrophobic nature of the chymotripsin, but still both the textiles samples showed significant hydrophobicity. In qualitative analysis by parallel streak method, ZnO/PMMA coated Fabric showed Antibacterial Activity against Staphylococcus aureus. And Klebsiella pneumoniae. Chymotrypsin immobilized ZnO/PMMA coated fabric also showed Antibacterial Activity against Staphylococcus aureus and Klebsiella pneumoniae. In quantitative analysis by plate count method, ZnO/PMMA coated textile showed Antibacterial Activity value of 2.98 against Klebsiella pneumoniae and 5.76 against Staphylococcus aureus. And Chymotrypsin immobilized ZnO/PMMA coated textile showed Antibacterial Activity value of 3.20 against Klebsiella pneumoniae and 6.52 against Staphylococcus aureus. Chymotrypsin immobilized ZnO/PMMA coated textile showed full effect against Klebsiella pneumoniae and Staphylococcus aureus compared to untreated ZnO/PMMA coated textile samples. The results showed that coated textile with polymer nanocomposites with enzyme immobilized ZnO has shown good hydrophobicity and enhanced antimicrobial properties. These results offer exciting prospects for the development of health care products such as Personal Protective Equipments (PPEs), Sanitary napkins and wound dressings.
Funding
This research was funded by the Department of Science and Technology (DST), India and The Japan Society for the Promotion of Science (JSPS), Japan, grant number DST/INT/JSPS/P-370/2023(G).
References
- SI Shahid-ul-Islam, BS Butola and F Mohammad. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC. 2016;6: 44232-44247. doi: 10.1039/C6RA05799C.
- Jeremiah SS, Miyakawa K, Morita T, Yamaoka Y, Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun. 2020 Nov 26;533(1):195-200. doi: 10.1016/j.bbrc.2020.09.018. Epub 2020 Sep 11. PMID: 32958250; PMCID: PMC7486059.
- Román LE, Gomez ED, Solís JL, Gómez MM. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules. 2020 Dec 9;25(24):5802. doi: 10.3390/molecules25245802. PMID: 33316935; PMCID: PMC7764683.
- PSadalage PS, Nimbalkar MS, Sharma KKK, Patil PS, Pawar KD. Sustainable approach to almond skin mediated synthesis of tunable selenium microstructures for coating cotton fabric to impart specific antibacterial activity. J Colloid Interface Sci. 2020 Jun 1;569:346-357. doi: 10.1016/j.jcis.2020.02.094. Epub 2020 Feb 25. PMID: 32126347.
- M Zayed, M Bakr, H Ghazal. Recent developments in the utilization of polymer nanocomposites in textile applications. Journal of Textiles, Coloration and Polymer Science. 2023;20:147-170. doi: 10.21608/jtcps.2023.193744.1172.
- Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM. Antimicrobial enzymes: an emerging strategy to fight microbes and microbial biofilms. Biotechnol J. 2013 Jan;8(1):97-109. doi: 10.1002/biot.201200313. PMID: 23281326.
- Olsen SM, Pedersen LT, Laursen MH, Kiil S, Dam-Johansen K. Enzyme-based antifouling coatings: a review. Biofouling. 2007;23(5-6):369-83. doi: 10.1080/08927010701566384. PMID: 17852071.
- Pettitt ME, Henry SL, Callow ME, Callow JA, Clare AS. Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling. 2004 Dec;20(6):299-311. doi: 10.1080/08927010400027068. PMID: 15804714.
- Aldred N, Phang IY, Conlan SL, Clare AS, Vancso GJ. The effects of a serine protease, Alcalase, on the adhesives of barnacle cyprids (Balanus amphitrite). Biofouling. 2008;24(2):97-107. doi: 10.1080/08927010801885908. PMID: 18231899.
- Caro A, Humblot V, Méthivier C, Minier M, Salmain M, Pradier CM. Grafting of lysozyme and/or poly(ethylene glycol) to prevent biofilm growth on stainless steel surfaces. J Phys Chem B. 2009 Feb 19;113(7):2101-9. doi: 10.1021/jp805284s. PMID: 19166331.
- NA Ibrahim, M Gouda, AM Elshafei, OM Abdel-Fatah. Antimicrobial activity of cotton fabrics containing immobilized enzymes. Journal of Applied Polymer Science. 2007;104:1754-1761. doi: 10.1002/app.25821.
- Cadnum JL, Li DF, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of Ultraviolet-C Light and a High-Level Disinfection Cabinet for Decontamination of N95 Respirators. Pathog Immun. 2020 Apr 20;5(1):52-67. doi: 10.20411/pai.v5i1.372. PMID: 32363254; PMCID: PMC7192214.
- Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic - Case study from the Republic of Ireland. Sci Total Environ. 2020 Jul 10;725:138532. doi: 10.1016/j.scitotenv.2020.138532. Epub 2020 Apr 6. PMID: 32304970; PMCID: PMC7195029.
- Derraik JGB, Anderson WA, Connelly EA, Anderson YC. Rapid Review of SARS-CoV-1 and SARS-CoV-2 Viability, Susceptibility to Treatment, and the Disinfection and Reuse of PPE, Particularly Filtering Facepiece Respirators. Int J Environ Res Public Health. 2020 Aug 22;17(17):6117. doi: 10.3390/ijerph17176117. PMID: 32842655; PMCID: PMC7504573.
- CB Hiragond AS Kshirsagar, VV Dhapte, T Khanna, P Joshi, PV. More, enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. Vacuum. 2018;156:475-482. doi: 10.1016/j.vacuum.2018.08.007.
- Mokhena TC, Luyt AS. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr Polym. 2017 Jun 1;165:304-312. doi: 10.1016/j.carbpol.2017.02.068. Epub 2017 Feb 21. PMID: 28363554.
- F Hui and C Debiemme-Chouvy. Antimicrobial N-Halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules. 2013;14:585-601. doi: 10.1021/bm301980q.
- J Choi, SG Ryu, SGB Lee, WB Ying, KJ Lee, N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer. 138(2018) 146-155. doi: 10.1016/j.polymer.2018.01.066.
- P Tang, M Zhang, B Ji, T Yong, G Sun. Hierarchical nucleophilic nanofibrous membranes for fast, durable, and bare-eye visible detoxification of carcinogenic alkylating toxicants. Adv Funct Mater. 2019;29:1905990. doi: 10.1002/adfm.201905990.
- Demir B, Cerkez I, Worley SD, Broughton RM, Huang TS. N-Halamine-modified antimicrobial polypropylene nonwoven fabrics for use against airborne bacteria. ACS Appl Mater Interfaces. 2015 Jan 28;7(3):1752-7. doi: 10.1021/am507329m. Epub 2015 Jan 14. PMID: 25587845.
- Ghayempour S, Montazer M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J Microencapsul. 2016 Sep;33(6):497-510. doi: 10.1080/02652048.2016.1216187. Epub 2016 Oct 4. PMID: 27701985.
- N Kamrudi, S Akbari, M Haghighat Kish. Enhanced control release of thyme essential oils from electrospun nanofiber/polyamidoamine dendritic polymer for antibacterial platforms. Polym Adv Technol. 2020;31:1719-1731. doi: 10.1002/pat.4899.
- Kenney PA, Chan BK, Kortright KE, Cintron M, Russi M, Epright J, Lee L, Balcezak TJ, Havill NL, Martinello RA. Hydrogen peroxide vapor decontamination of N95 respirators for reuse. Infect Control Hosp Epidemiol. 2022 Jan;43(1):45-47. doi: 10.1017/ice.2021.48. Epub 2021 Feb 9. PMID: 33557979; PMCID: PMC8185421.
- Song P, Zhang X, Wang S, Xu W, Wang F, Fu R, Wei F. Microbial proteases and their applications. Front Microbiol. 2023 Sep 14;14:1236368. doi: 10.3389/fmicb.2023.1236368. PMID: 37779686; PMCID: PMC10537240.
- Khafaga DSR, Muteeb G, Elgarawany A, Aatif M, Farhan M, Allam S, Almatar BA, Radwan MG. Green nanobiocatalysts: enhancing enzyme immobilization for industrial and biomedical applications. PeerJ. 2024 Jul 8;12:e17589. doi: 10.7717/peerj.17589. PMID: 38993977; PMCID: PMC11238728.
- LS Dyshlyuk, MV Novoselova, TA Rozalenok. Immobilization of chymotrypsin on magnetic Fe3O4 nanoparticles. Foods and Raw Materials. 2013;1:85-88. doi: 10.12737/2060.
- Lishchynskyi O, Shymborska Y, Stetsyshyn Y, Raczkowska J, Skirtach AG, Peretiatko T, Budkowski A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem Eng J. 2022 Oct 15;446:137048. doi: 10.1016/j.cej.2022.137048. Epub 2022 May 18. PMID: 35601363; PMCID: PMC9113772.
- M Neha, K Vishaka. Application of zinc oxide nano particles using polymeric binders on cotton fabric. Research Journal of Textile and Apparel. 2022;26:310-322. doi: 10.1108/RJTA-02-2021-0018.
- MJ Haque, MM Bellah, MR Hassan, S Rahman. Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties. Nano Ex. 2020;1:010007. doi: 10.1088/2632-959X/ab7a43.
- AA Naser, HZ Al-Sawaad, AS Al-Mubarak. Novel graphene oxide functionalization by urea and thiourea, and their applications as anticorrosive agents for carbon steel alloy in acidic medium. J Mater Environ Sci. 2020;11:404-420.
- Mukherjee J, Gupta MN. Alpha chymotrypsin coated clusters of Fe3O4 nanoparticles for biocatalysis in low water media. Chem Cent J. 2012 Nov 8;6(1):133. doi: 10.1186/1752-153X-6-133. PMID: 23137100; PMCID: PMC3505189.
- A Kajbafvala, S Zanganeh, E Kajbafvala. Microwave-assisted synthesis of narcis-like Zinc Oxide nanostructures. Journal of Alloys and Compounds. 2010;497:325-329. doi: 10.1016/j.jallcom.2010.03.057.
- VS Gowri, L Almeida, MTP Amorim, C Noemia, E Fatima, S Pedro. Functional finishing of polyamide fabrics using ZnO-PMMA nanocomposites. J Mater Sci. 2010;45:2427-2435. doi: 10.1007/s10853-010-4210-4.
- JP Jose, J Abraham, H Maria, K Varughese, S Thomas. Contact angle studies in xlpe hybrid nanocomposites with inorganic nanofillers. Macromolecular Symposia. 2016;366:66-78. doi: 10.1002/masy.201650048.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.