Medicine Group 2025 April 15;6(4):320-37. doi: 10.37871/jbres2087.
Long-Term Efficacy of a Single Intravenous MSC Infusion on HbA1c Reduction in Type 2 Diabetic Mellitus
Matsuoka T1,3*, Hara Y3, Itohara T1, Ueda N3, Nishimura K2, Ryugo A2, Kanai S2 and Kobayashi N1
2Stemcell Co.Ltd, Japan
3Helene Japanese Day Surgery Center Dubai, Dubai
Abstract
Background: Mesenchymal Stem Cell (MSC) therapy has garnered increasing attention as a novel therapeutic strategy for Type 2 Diabetes Mellitus (T2DM), owing to its immunomodulatory and regenerative properties. However, the long-term efficacy of a single intravenous MSC infusion on glycemic control remains insufficiently explored.
Objective: This study aimed to evaluate the long-term impact of a single intravenous infusion of MSCs on HbA1c reduction in patients with moderate and severe T2DM and to investigate potential dose-dependent responses.
Methods: A total of 61 patients diagnosed with T2DM (HbA1c ≥ 6.2%) were enrolled. Patients received a single intravenous infusion of MSCs derived from umbilical cord tissue, bone marrow, or adipose tissue, with doses ranging from 100 million to 2 billion cells, tailored to individual clinical conditions. Patients were categorized into moderate (HbA1c 6.2-8%) and severe (HbA1c > 8%) diabetes groups. Follow-up assessments were conducted over a median duration of 35 months (range: 6-68 months), with primary endpoints including changes in HbA1c levels. Statistical analyses were performed using paired t-tests, and correlation analyses evaluated the relationship between MSC dose and glycemic outcomes.
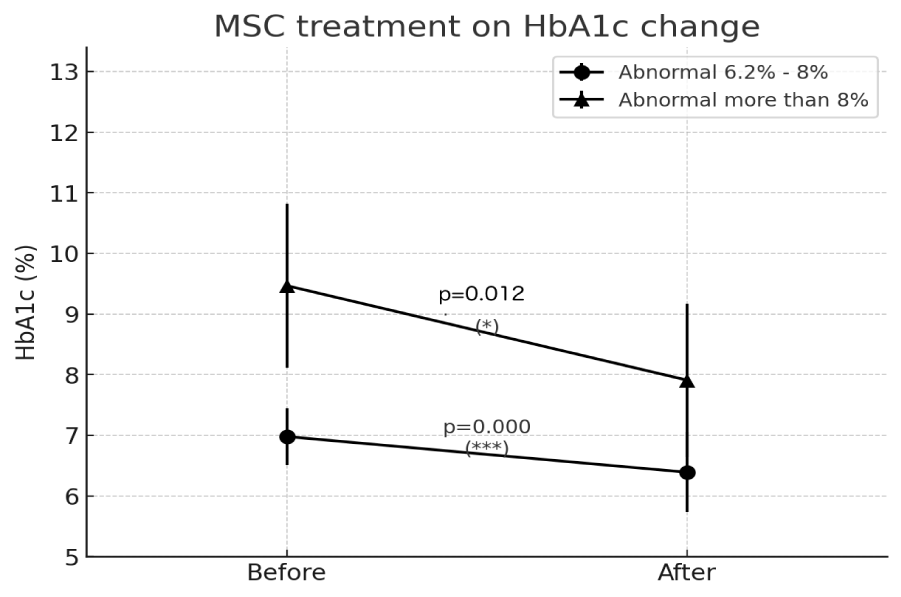
Results: The mean baseline HbA1c level was 7.49%, which significantly decreased to 6.7% post-treatment (p < 0.0001). In the moderate diabetes group, the mean HbA1c reduction was 0.58% (p < 0.0000002), while the severe diabetes group exhibited a more pronounced mean reduction of 1.55% (p = 0.0122). Subgroup analysis suggested a trend toward greater HbA1c reduction in patients receiving higher MSC doses (≥1 billion cells), though this did not reach statistical significance. No serious adverse events were reported during the follow-up period.
Conclusion: A single intravenous infusion of MSCs resulted in significant and sustained HbA1c reduction, particularly in patients with severe diabetes, with effects persisting for over three years. These findings highlight the potential of MSC therapy as a disease-modifying intervention in T2DM management. Further randomized controlled trials are warranted to validate these results and optimize dosing strategies.
Introduction
Diabetes mellitus remains a global health challenge, with HbA1c levels serving as a critical marker for long-term glycemic control. Conventional treatments, including insulin and oral hypoglycemic agents, often require lifelong management and do not address the underlying disease pathology. MSC therapy, with its potential immunomodulatory and regenerative effects, has shown promise in preclinical and clinical studies [1]. Studies, including those referenced in the reference [2], indicate that MSCs can modulate immune responses, reduce inflammation, and enhance beta-cell function, thereby improving glycemic control.
A recent comprehensive review summarized 22 clinical studies, [3-24], investigating the effects of intravenous stem cell therapy in both type 1 and type 2 diabetes patients. Across these studies, a consistent trend of glycemic improvement was reported. Specifically, 18 of the 22 studies documented significant reductions in HbA1c levels, with reductions ranging from 1.31% to 1.36% at 48 weeks. Several studies [25,26] also reported marked increases in C-peptide levels, indicating enhanced endogenous beta-cell activity. Notably, some studies demonstrated reduced insulin requirements, with insulin independence achieved in up to 59% of patients.
These studies utilized a variety of stem cell types, including autologous Hematopoietic Stem Cells (HSCs), allogeneic umbilical cord-derived MSCs, bone marrow-derived MSCs, and cord blood-derived stem cells. Treatment protocols varied, employing both single and multiple infusion strategies, with doses often tailored based on body weight (e.g., 1 × 10^6 cells/kg). Importantly, the safety profile across these studies was favorable, with most adverse events being mild and transient. Some studies identified factors influencing treatment response, such as shorter disease duration, lower BMI, and higher baseline beta-cell function.
The accumulation of evidence from these 22 studies, [3-24], underscores the broad potential of stem cell therapies in diabetes management. These findings support the rationale for further exploration of MSC therapy, particularly its long-term efficacy and safety in diverse patient populations. Building upon these insights, the present study aims to assess the real-world impact of a single MSC infusion on long-term HbA1c reduction and its implications for diabetes management.
Methods
The methodology for this study follows the protocol established in our previous research [1], with modifications tailored to evaluate the long-term effects of MSC therapy on diabetes management. This treatment is a treatment approved under the Japanese Act on the Safety of Regenerative Medicine, with approval number PB3220010, and regulations require that the total number of cases be reported to the Ministry of Health, Labour and Welfare in Japan once a year. Patients with HbA1c levels classified as moderate (6.2-8%) or severe (>8%) diabetes were enrolled and received a single intravenous infusion of MSCs. The MSCs were derived from umbilical cord tissue, bone marrow, or adipose tissue, processed under Good Manufacturing Practice (GMP) standards to ensure sterility, viability, and potency.
The treatment protocol involved a single intravenous administration of MSCs at doses ranging from 100 million to 2 billion cells, determined based on patient body weight and clinical condition. The infusion procedure followed standard aseptic techniques, with patients monitored for immediate adverse reactions.
Patient selection
Patients were recruited based on inclusion criteria such as confirmed diagnosis of type 2 diabetes, HbA1c levels of at least 6.2%, and failure to achieve adequate glycemic control with conventional therapy. Exclusion criteria included a history of malignancy, active infections, or autoimmune diseases that could interfere with MSC function.
MSC preparation and characterization
MSC isolation and culture followed previously established protocols. The cells were expanded in a GMP-compliant facility, and quality control measures included viability testing, immunophenotyping (CD73, CD90, CD105 positive; CD34, CD45 negative), and functional assays for differentiation potential.
MSC administration protocol
The prepared MSCs were suspended in normal saline and infused intravenously over 30-60 minutes. Patients were monitored for immediate adverse reactions, including fever, chills, or infusion-related complications. No serious adverse events were reported in our cohort.
Follow-up and outcome measures
Patients underwent scheduled follow-ups at 3, 6, 12 months, and annually for up to 5 years. Primary outcome measures included changes in HbA1c, fasting blood glucose, and insulin sensitivity indices. Secondary outcomes assessed systemic inflammation markers (CRP, TNF-α, IL-6) and pancreatic beta-cell function.
Statistical analysis
Data analysis was performed using Excel application. Paired t-tests were used to compare pre- and post-treatment HbA1c levels, and Pearson correlation analysis examined associations between MSC dose, treatment duration, and metabolic improvements. A p <0.05 was considered statistically significant. In addition to paired t-tests, linear regression analysis was performed to evaluate the association between MSC dose and HbA1c reduction, adjusting for baseline HbA1c, age, and duration of follow-up. This analysis aimed to better characterize potential dose-dependent treatment effects, which were not fully captured by stratified subgroup analysis alone.
Results
A total of 61 patients diagnosed with type 2 diabetes were included in this study. The cohort comprised 42 male and 16 female patients, with a mean age of 63.3 years (range: 42 to 89 years). All patients underwent a single intravenous infusion of Mesenchymal Stem Cells (MSCs), with administered doses ranging from 100 million to 2 billion cells. The median dose was 2 billion cells, reflecting the preference for high-dose MSC administration across the cohort.
Before treatment, the mean HbA1c level was 7.49%, with values ranging from 6.3% to 12.4%. Following MSC therapy, the mean HbA1c level decreased to 6.7%, with a post-treatment range of 4.6% to 10.9%. The median follow-up duration after MSC infusion was 35 months, with individual follow-up periods ranging from 6 to 68 months. These patient characteristics underscore the inclusion of a broad spectrum of diabetic severity and provide a solid foundation to evaluate the long-term efficacy of MSC therapy across diverse clinical backgrounds (Table 1, figure 1).
| Table 1: Baseline characteristics and treatment details of type 2 diabetes patients undergoing Mesenchymal Stem Cell (MSC) therapy. | |||||
| Sex | Age | Stem cell Dose x million cells |
HbA1c Before treatment | HbA1c After Treatment | Observed Month |
| M | 66 | 1000 | 7.5 | 6.4 | 27 |
| M | 66 | 2000 | 7.2 | 4.6 | 51 |
| M | 71 | 100 | 7.6 | 6.7 | 45 |
| M | 61 | 2000 | 7.1 | 6.5 | 18 |
| M | 76 | 2000 | 6.8 | 7.7 | 68 |
| M | 66 | 2000 | 6.9 | 6.5 | 13 |
| M | 79 | 1000 | 7.1 | 5.7 | 64 |
| M | 55 | 1000 | 6.8 | 5.9 | 18 |
| M | 72 | 1000 | 6.3 | 5.9 | 64 |
| M | 89 | 400 | 7.4 | 6.3 | 18 |
| F | 42 | 1000 | 7.2 | 8 | 62 |
| F | 60 | 1000 | 6.3 | 5.9 | 7 |
| M | 66 | 500 | 9.3 | 8.5 | 16 |
| M | 61 | 1000 | 7.2 | 6.9 | 53 |
| M | 52 | 2000 | 6.9 | 5.7 | 54 |
| M | 59 | 1000 | 6.4 | 5.1 | 53 |
| M | 63 | 2000 | 6.3 | 5.7 | 45 |
| F | 52 | 2000 | 6.4 | 5.7 | 47 |
| M | 69 | 2000 | 12.4 | 6.5 | 51 |
| M | 61 | 2000 | 7 | 6.9 | 46 |
| F | 53 | 2000 | 8.2 | 7.9 | 37 |
| M | 63 | 2000 | 7.9 | 7.2 | 45 |
| M | 66 | 2000 | 6.4 | 5.8 | 51 |
| F | 59 | 2000 | 10.3 | 7.4 | 44 |
| M | 69 | 2000 | 6.3 | 6.8 | 60 |
| F | 60 | 2000 | 7.3 | 6.3 | 40 |
| M | 64 | 2000 | 6.7 | 6.3 | 40 |
| M | 63 | 2000 | 6.8 | 6.3 | 40 |
| M | 64 | 2000 | 6.5 | 6.3 | 60 |
| F | 71 | 2000 | 8.7 | 7.4 | 39 |
| M | 65 | 2000 | 7.8 | 7.9 | 44 |
| M | 62 | 2000 | 8.7 | 8.5 | 45 |
| M | 47 | 2000 | 6.4 | 6.2 | 35 |
| F | 61 | 2000 | 7.5 | 6.7 | 42 |
| F | 57 | 100 | 8.5 | 7.4 | 14 |
| F | 58 | 2000 | 11.9 | 8.4 | 16 |
| F | 77 | 2000 | 7.5 | 6.4 | 19 |
| M | 59 | 2000 | 6.7 | 6.4 | 27 |
| F | 71 | 2000 | 6.9 | 7.4 | 17 |
| M | 48 | 2000 | 7.2 | 6.6 | 15 |
| F | 70 | 2000 | 6.4 | 5.9 | 17 |
| F | 60 | 2000 | 9.3 | 10.9 | 22 |
| M | 62 | 2000 | 8 | 6.6 | 25 |
| M | 66 | 2000 | 7.1 | 6.4 | 6 |
| M | 59 | 2000 | 6.9 | 6.4 | 11 |
| M | 67 | 400 | 8.2 | 8.2 | 9 |
| M | 45 | 2000 | 7.8 | 6.1 | 12 |
| M | 72 | 1000 | 7.7 | 7.2 | 14 |
| M | 68 | 2000 | 6.9 | 7 | 14 |
| M | 80 | 2000 | 6.5 | 5.8 | 14 |
| M | 76 | 2000 | 6.4 | 5.5 | 15 |
| M | 56 | 1000 | 9.8 | 8.4 | 16 |
| F | 74 | 700 | 6.8 | 6.6 | 7 |
| M | 46 | 2000 | 7.1 | 6.3 | 41 |
| M | 67 | 2000 | 7.3 | 6.1 | 48 |
| M | 64 | 2000 | 8.3 | 5.4 | 36 |
| F | 43 | 500 | 6.9 | 6.5 | 9 |
| M | 72 | 500 | 6.9 | 6.6 | 15 |
- Moderate Diabetes Group (HbA1c 6.2 - 8%):
- Mean HbA1c reduction: 0.58%
- Statistical significance: p < 0.0000002 (highly significant)
- Severe Diabetes Group (HbA1c > 8%):
- Mean HbA1c reduction: 1.55%
- Statistical significance: p = 0.0122 (significant)
- Sustained Effect Duration:
- Median follow-up duration: 35.5 months
- Interquartile range: 15.25 - 45.75 months
- Correlation between duration and HbA1c reduction: 0.041 (no significant correlation)
Additional subgroup analysis indicated that patients with higher baseline HbA1c levels experienced a more pronounced reduction, suggesting that the initial disease severity influences treatment response. Patients receiving higher doses of MSCs (≥1 billion cells) demonstrated a trend toward greater HbA1c reduction, though this did not reach statistical significance (p > 0.05). Notably, patients who received the highest MSC doses (≥1.5 billion cells) showed a mean HbA1c reduction of 1.72%, compared to 1.12% in those who received doses below 1 billion cells. This suggests a potential dose-dependent response, though variability among individuals highlights the need for further dose-optimization studies.
Discussion
According to Systematic review regarding Insulin Requirements, many studies reported reductions in insulin requirements following stem cell therapy, with some patients achieving insulin independence.
Key findings include:
- Reported that 59% of patients achieved insulin independence within 6 months, with 32% remaining insulin-free at the last follow-up [5].
- Observed a significant reduction in insulin requirements, with 32.3% of patients achieving insulin withdrawal [2].
- Found that 14 out of 15 patients became insulin-free for varying durations [10].
- Reported a significant reduction in insulin requirements, with a higher percentage of insulin reduction in the UC-MSCs group compared to placebo [15].
- Observed that all patients became independent of exogenous insulin after treat- ment, although one patient resumed low-dose insulin after 7 months [4].
The degree of insulin reduction varied across studies, with some patients experiencing complete insulin independence and others showing partial reductions in insulin requirements. The durability of these effects also varied, with some studies reporting sustained benefits and others noting a gradual return to insulin dependence in some patients.
The results indicate a greater HbA1c reduction in patients with severe diabetes, supporting the hypothesis that MSC therapy has a stronger impact in more advanced cases. The long-term efficacy, with a median effect duration exceeding three years, is particularly noteworthy. These findings align with prior research 2), which suggests that MSCs may exert their effects through paracrine signaling, immune modulation, and potential beta-cell regeneration.
Our analysis also suggests a possible dose-response relationship, where patients receiving higher doses of MSCs (particularly ≥1 billion cells) exhibited greater reductions in HbA1c. However, this trend did not reach statistical significance (p > 0.05), likely due to the small sample size in the lower-dose group (n = 6 for <1 billion cells). To further explore this relationship, we conducted a linear regression analysis adjusting for baseline HbA1c, age, and follow-up duration. The regression coefficient remained directionally consistent with a dose-dependent effect, although statistical significance was not achieved. This limitation has been acknowledged, and future prospective studies are planned to include stratified randomization by dose range to validate the optimal dosing strategy. The findings from broader clinical trials reinforce this observation, indicating that a tailored MSC dosing strategy could further enhance treatment efficacy. Future studies should investigate whether multiple doses or repeated infusions could further enhance therapeutic outcomes.
The results of this study demonstrate a significant and sustained reduction in HbA1c levels following a single intravenous infusion of MSCs, particularly in patients with severe diabetes. Notably, the median follow-up duration exceeded three years, yet the correlation analysis revealed no significant relationship between follow-up duration and the magnitude of HbA1c reduction (correlation coefficient: 0.041). This suggests that the therapeutic effect is not transient but rather maintained over several years after only one administration.
Such a long-lasting effect is unprecedented in the field of diabetes management. Conventional antidiabetic agents, including insulin, GLP-1 receptor agonists, and oral hypoglycemic agents, typically require continuous and lifelong administration to maintain glycemic control. In contrast, our findings indicate that a single MSC infusion yields durable improvements in glycemic parameters, reducing HbA1c levels significantly without ongoing therapy. This highlights a unique, disease-modifying potential of MSC treatment that differentiates it from standard pharmacological approaches.
Additionally, the absence of a significant decline in therapeutic efficacy over time implies that MSC therapy may induce long-term modulation of the immune environment, reduction of systemic inflammation, or enhancement of endogenous beta-cell function. These mechanisms align with previous studies suggesting that MSCs exert their beneficial effects through paracrine signaling (e.g., secretion of IGF-1, TGF-β, and VEGF), immunomodulation (e.g., increased Treg cell activity and decreased levels of IL-6 and TNF-α), and promotion of pancreatic islet regeneration. Although our present study did not include molecular or cellular assays to directly verify these pathways, multiple clinical trials have reported increased C-peptide levels and decreased systemic inflammatory markers following MSC therapy. To address this limitation, future trials from our group will incorporate longitudinal immune profiling and cytokine assays to directly assess these mechanistic effects. Importantly, our findings are consistent with multiple clinical trials summarized in the recent systematic review, which reported sustained HbA1c reductions and increased C-peptide levels over extended periods following stem cell therapy. However, unlike those studies where multiple infusions were often required, our results underscore the efficacy of a single high-dose MSC treatment in achieving long-term glycemic control.
These observations suggest a paradigm shift in diabetes management, offering the possibility of achieving lasting metabolic improvement with minimal therapeutic burden. Future research should focus on elucidating the precise biological mechanisms responsible for this sustained effect and verifying these findings in larger, randomized controlled trials.
Conclusion
In conclusion, a single intravenous infusion of Mesenchymal Stem Cells (MSCs) resulted in a significant and durable reduction in HbA1c levels in patients with type 2 diabetes, with the effect being particularly pronounced in those with severe diabetes. Remarkably, the median follow-up duration exceeded three years, and no significant correlation was observed between follow-up duration and the magnitude of HbA1c reduction. This suggests that the glycemic improvement achieved after a single MSC infusion is sustained long-term without the need for repeated treatments-an outcome that is unprecedented compared to conventional antidiabetic therapies, which typically require continuous administration.
The findings of this study support the concept that MSC therapy may possess disease-modifying properties, potentially through immune modulation, reduction of systemic inflammation, and enhancement of endogenous beta-cell function. Our results, together with evidence from prior clinical trials, indicate that MSC therapy has the potential to fundamentally change the management paradigm for diabetes by offering long-lasting metabolic benefits with minimal therapeutic burden.
Further large-scale randomized controlled trials and mechanistic studies are warranted to validate these findings, clarify the underlying biological mechanisms, and determine optimal dosing strategies to maximize therapeutic efficacy.
Author Contributions
All authors contributed to the study conception and design. T.M., T.I., N.U., Y.H. and N.K. treated the patients as attending physicians and contributed to the collection of clinical data on patients. K.N., A.R. and S.K. organised data on medical record information. T.M. prepared the materials and analyses, wrote the first draft of the manuscript, and commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Helene Ethical Committee (approval number: HCS-20140601 on 1 June 2014). Written informed consent was obtained from all study participants.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
Not applicable.
Conflicts of Interest
All authors declare no financial or non-financial competing interests.
References
- Matsuoka T, Itohara T, Hara Y, Kobayashi N. Systematic Intravenous Administration of Autologous Mesenchymal Stem Cells Is Safe. J Clin Med. 2024 Dec 7;13(23):7460. doi: 10.3390/jcm13237460. PMID: 39685916; PMCID: PMC11642704.
- Hu J, Wang Y, Gong H, Yu C, Guo C, Wang F, Yan S, Xu H. Long term effect and safety of Wharton's jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med. 2016 Sep;12(3):1857-1866. doi: 10.3892/etm.2016.3544. Epub 2016 Jul 26. PMID: 27588104; PMCID: PMC4997981.
- Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, Sachdeva N, Sharma RR, Marwaha N, Khandelwal N. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23(9):1075-85. doi: 10.3727/096368913X665576. PMID: 23561959.
- Snarski E, Milczarczyk A, Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K, Franek E, Wiktor-Jedrzejczak W. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant. 2011 Apr;46(4):562-6. doi: 10.1038/bmt.2010.147. Epub 2010 Jun 28. PMID: 20581881.
- D'Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes. 2014 Sep;63(9):3041-6. doi: 10.2337/db14-0295. Epub 2014 Jun 19. PMID: 24947362.
- Penaforte-Saboia JG, Montenegro RM Jr, Couri CE, Batista LA, Montenegro APDR, Fernandes VO, Akhtar H, Negrato CA, Malmegrim KCR, Moraes DA, Dias JBE, Simões BP, Gomes MB, Oliveira MC. Microvascular Complications in Type 1 Diabetes: A Comparative Analysis of Patients Treated with Autologous Nonmyeloablative Hematopoietic Stem-Cell Transplantation and Conventional Medical Therapy. Front Endocrinol (Lausanne). 2017 Nov 23;8:331. doi: 10.3389/fendo.2017.00331. PMID: 29218029; PMCID: PMC5703738.
- Gan J, Wang Y, Zhou X. Stem cell transplantation for the treatment of patients with type 1 diabetes mellitus: A meta-analysis. Exp Ther Med. 2018 Dec;16(6):4479-4492. doi: 10.3892/etm.2018.6769. Epub 2018 Sep 19. PMID: 30542397; PMCID: PMC6257425.
- Markmann J, Naji A, Rickels M, Alba GM. Marigowda, Ross L, Wang C, Pagliuca F, Sanna B, Leslie KS, Peters LA, Witkowski P, Ricordi C. Stem cell–derived, fully differentiated islet cells for type 1 diabetes. 259-OR: Diabetes. 2022;71(S1):259-OR. doi: 10.2337/db22-259-OR.
- Skyler JS, Fonseca VA, Segal KR, Rosenstock J; MSB-DM003 Investigators. Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care. 2015 Sep;38(9):1742-9. doi: 10.2337/dc14-2830. Epub 2015 Jul 7. PMID: 26153271; PMCID: PMC4542273.
- Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007 Apr 11;297(14):1568-76. doi: 10.1001/jama.297.14.1568. PMID: 17426276.
- Hu J, Wang Y, Gong H, Yu C, Guo C, Wang F, Yan S, Xu H. Long term effect and safety of Wharton's jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med. 2016 Sep;12(3):1857-1866. doi: 10.3892/etm.2016.3544. Epub 2016 Jul 26. PMID: 27588104; PMCID: PMC4997981.
- Lu J, Shen SM, Ling Q, Wang B, Li LR, Zhang W, Qu DD, Bi Y, Zhu DL. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res Ther. 2021 Jun 10;12(1):340. doi: 10.1186/s13287-021-02417-3. PMID: 34112266; PMCID: PMC8194026.
- Nguyen LT, Hoang DM, Nguyen KT, Bui DM, Nguyen HT, Le HTA, Hoang VT, Bui HTH, Dam PTM, Hoang XTA, Ngo ATL, Le HM, Phung NY, Vu DM, Duong TT, Nguyen TD, Ha LT, Bui HTP, Nguyen HK, Heke M, Bui AV. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl Med. 2021 Sep;10(9):1266-1278. doi: 10.1002/sctm.20-0506. Epub 2021 Jun 3. PMID: 34080789; PMCID: PMC8380443.
- Zang L, Li Y, Hao H, Liu J, Zhang Q, Gao F, Wang H, Chen Y, Gu W, Du J, Meng J, Zhang S, Lyu Z, Dou J, Mu Y. Efficacy of Umbilical Cord-Derived Mesenchymal Stem Cells in the Treatment of Type 2 Diabetes Assessed by Retrospective Continuous Glucose Monitoring. Stem Cells Transl Med. 2023 Dec 18;12(12):775-782. doi: 10.1093/stcltm/szad060. PMID: 37738447; PMCID: PMC10726406.
- Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B, Yin Y, Zhang Q, Gao F, Wang H, Gu S, Li J, Lin F, Zhu Y, Tian G, Chen Y, Gu W, Du J, Chen K, Guo Q, Yang G, Pei Y, Yan W, Wang X, Meng J, Zhang S, Ba J, Lyu Z, Dou J, Han W, Mu Y. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther. 2022 May 3;13(1):180. doi: 10.1186/s13287-022-02848-6. PMID: 35505375; PMCID: PMC9066971.
- Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W, Zhang N, Zhuge YZ, Chen B, Xu J, Zhu D. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab. 2012 May;97(5):1729-36. doi: 10.1210/jc.2011-2188. Epub 2012 Mar 14. PMID: 22419704.
- Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, Rabbani A, Kompani F, Hedayati Asl AA, Abbasi Kakroodi F, Jaroughi N, Mohseni Meybodi MA, Setoodeh A, Abbasi F, Hosseini SE, Moeini Nia F, Salman Yazdi R, Navabi R, Hajizadeh-Saffar E, Baharvand H. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022 Jun 20;13(1):264. doi: 10.1186/s13287-022-02941-w. PMID: 35725652; PMCID: PMC9208234.
- Carlsson P, Espes D, Davies L, Svahn M. Dose-dependent preservation of beta-cell function in type i diabetes by mesenchymal stromal cells. Cytotherapy. 2020;22(5):S192-S193. doi: 10.1016/j.jcyt.2020.04.053.
- Chen P, Huang Q, Xu XJ, Shao ZL, Huang LH, Yang XZ, Guo W, Li CM, Chen C. [The effect of liraglutide in combination with human umbilical cord mesenchymal stem cells treatment on glucose metabolism and β cell function in type 2 diabetes mellitus]. Zhonghua Nei Ke Za Zhi. 2016 May 1;55(5):349-54. Chinese. doi: 10.3760/cma.j.issn.0578-1426.2016.05.004. PMID: 27143183.
- Thakkar UG, Trivedi HL, Vanikar AV, Dave SD. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015 Jul;17(7):940-7. doi: 10.1016/j.jcyt.2015.03.608. Epub 2015 Apr 11. PMID: 25869301.
- Sood V, Bhansali A, Mittal BR, Singh B, Marwaha N, Jain A, Khandelwal N. Autologous bone marrow derived stem cell therapy in patients with type 2 diabetes mellitus - defining adequate administration methods. World J Diabetes. 2017 Jul 15;8(7):381-389. doi: 10.4239/wjd.v8.i7.381. PMID: 28751962; PMCID: PMC5507836..
- Li W, Jiao X, Song J, Sui B, Guo Z, Zhao Y, Li J, Shi S, Huang Q. Therapeutic potential of stem cells from human exfoliated deciduous teeth infusion into patients with type 2 diabetes depends on basal lipid levels and islet function. Stem Cells Transl Med. 2021 Jul;10(7):956-967. doi: 10.1002/sctm.20-0303. Epub 2021 Mar 4. PMID: 33660433; PMCID: PMC8235136.
- Gu X, Yu X, Zhao C, Duan P, Zhao T, Liu Y, Li S, Yang Z, Li Y, Qian C, Yin Z, Wang Y. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell Physiol Biochem. 2018;49(1):40-52. doi: 10.1159/000492838. Epub 2018 Aug 22. PMID: 30134223.
- Lian XF, Lu DH, Liu HL, Liu YJ, Han XQ, Yang Y, Lin Y, Zeng QX, Huang ZJ, Xie F, Huang CH, Wu HM, Long AM, Deng LP, Zhang F. Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus. World J Diabetes. 2022 Oct 15;13(10):877-887. doi: 10.4239/wjd.v13.i10.877. PMID: 36312002; PMCID: PMC9606793.
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Zhou H, Yin Z, Chen Y, Zhang Y, Wang S, Shen J, Thaker H, Jain S, Li Y, Diao Y, Chen Y, Sun X, Fisk MB, Li H. Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: phase I/II clinical trial. BMC Med. 2013 Jul 9;11:160. doi: 10.1186/1741-7015-11-160. PMID: 23837842; PMCID: PMC3716981.
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, Li H, Zhang Y, Diao Y, Li Y, Chen Y, Sun X, Fisk MB, Skidgel R, Holterman M, Prabhakar B, Mazzone T. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012 Jan 10;10:3. doi: 10.1186/1741-7015-10-3. PMID: 22233865; PMCID: PMC3322343.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.